Supporting Post-ICU Recovery: A Narrative Review for General Practitioners
Abstract
1. Introduction
| Category | References | |
|---|---|---|
| Physical | Neuromuscular Muscle weakness Fatigue and reduced endurance Joint and nerve pain Neuromyopathies Ventilator-induced diaphragm weakness Frailty Chronic pain Joint contractures and ectopic ossifications Speech difficulties (related to neuromuscular impairment) | Herridge et al., 2023 [28] Appleton et al., 2015 [29] Koch et al., 2014 [30] Clavet et al., 2015 [31] Latronico et al., 2023 [32] Amacher et al., 2024 [33] Nakamura et al., 2021 [34] Skoretz et al., 2010 [35] Macht et al., 2011 [36] Schefold et al., 2017 [37] |
| Respiratory Persistent breathlessness (dyspnea) Lung fibrosis (especially in ARDS patients) Chronic hypoxia requiring oxygen therapy Respiratory muscle weakness Tracheal stenosis (after prolonged intubation) | ||
| Cardiovascular Persistent tachycardia Stress-induced cardiomyopathy New-onset arrhythmias Autonomic dysfunction (e.g., postural hypotension) Increased risk of heart disease (heart failure, atherosclerosis) Increased risk of thrombosis (DVT, PE) | ||
| Gastrointestinal and Metabolic System Difficulty swallowing (dysphagia) Nutritional deficiencies and metabolic dysfunction Weight loss and sarcopenia New-onset diabetes and glucose dysregulation Chronic diarrhea and constipation (gut microbiome disruption, opioid use) | ||
| Renal System Chronic kidney disease Electrolyte imbalances Increased risk of hypertension | ||
| Endocrine System Adrenal insufficiency Hypothyroidism and non-thyroidal illness syndrome Hypogonadism (testosterone suppression, menstrual irregularities) | ||
| Skin, Soft Tissue, and Wound Healing Scarring caused by invasive procedures Procedure-related complications Oral injuries | ||
| Cognitive | Memory deficits Difficulty with attention and concentration Impaired executive function (problem solving, decision making) Reduced processing speed Brain fog or confusion Speech difficulties Delayed cognitive recovery (especially in ARDS and sepsis survivors) | Calsavara et al., 2018 [38] Semmler et al., 2013 [39] Hopkins et al., 2005 [40] Pandharipande et al., 2013 [41] Iwashyna et al., 2010 [42] |
| Psychological | Anxiety Depression Post-traumatic stress disorder Sleep disturbances Emotional lability (mood swings) Panic attacks Paranoia Guilt Decreased libido Social withdrawal Loss of motivation Complicated grief (PICS-family) | Patel et al., 2016 [43] Jones et al., 2004 [44] Desai et al., 2011 [45] Griffiths et al., 2006 [46] Broomhead et al., 2002 [47] Wells et al., 1989 [48] Zatzick et al., 2008 [49] Dowdy et al., 2008 [50] Bjørnøy et al., 2023 [51] Hatch et al., 2018 [52] |
2. Methods
3. Post-Intensive Care Syndrome (PICS) and PICS Family
3.1. Definition and Scope of PICS
| PICS Component | Modifiable Risk Factors | Non-Modifiable Risk Factors | References |
|---|---|---|---|
| PICS (General) | Prolonged ICU stay, hypoglycemia, hypoxemia, inadequate communication with ICU staff, restricted visitation policies. | Advanced age, pre-existing medical conditions, previous ICU admission, history of anxiety or depression requiring medication. | Needham et al., 2012 [59] Rawal et al., 2017 [60] Inoue et al., 2019 [58] Lee et al., 2020 [61] |
| Physical | Prolonged immobility, bed rest, catabolic state, microvascular ischemia, extended mechanical ventilation, hyperglycemia, use of glucocorticoids and neuromuscular blocking agents, sleep disturbances. | Acute respiratory distress dyndrome (ARDS), advanced age, hyperoxia, vasopressor administration. | Hopkins et al., 1999 [62] Lee et al., 2020 [61] Fan et al., 2014 [63] Stevens et al., 2007 [64] |
| Cognitive | ICU delirium, prolonged mechanical ventilation, hypoxia, dysglycemia, use of psychotropic medications, blood transfusions, blood pressure fluctuations. | Advanced age, comorbidities, lower education level, pre-existing cognitive impairment (e.g., dementia), presence of APOE allele, severity of illness. | Pandharipande et al., 2013 [41] Jackson et al., 2010 [65] Girard et al., 2010 [56] Iwashyna et al., 2010 [42] |
| Psychological | Duration of ICU delirium, distressing ICU memories, prolonged sedation, opioid and benzodiazepine dosage, nightmares, breathlessness, alcohol consumption. | Female sex, pre-existing depressive symptoms, poor pre-ICU physical functioning, lower education level, history of anxiety or depression. | Lee et al., 2020 [61] Parker et al., 2015 [66] |
| PICS-F (Family-Related) | Poor communication with ICU staff, restricted visitation, financial stress, inadequate caregiver education, lack of support systems. | Advanced age, prolonged ICU stay of a loved one, prior experience with ICU care, history of anxiety or depression requiring medication. | Cameron et al., 2016 [67] Shirasaki et al., 2024 [68] |
3.2. Physical Impairments in PICS
3.3. Cognitive Dysfunction in PICS
3.4. Psychological and Emotional Distress in PICS
3.5. PICS-F (PICS Family)
4. ICU-Specific Complications Beyond PICS
4.1. Cardiovascular Complications
4.2. Respiratory Complications
4.3. Gastrointestinal and Metabolic Complications
4.4. Renal Complications
4.5. Endocrine Dysfunction
4.6. Hematologic and Immunologic Complications
4.7. Overall Increased Long-Term Mortality
4.8. Socioeconomic Sequelae and Return to Work
5. Complications Related to Invasive Procedures
| Procedure | Complication | Clinical Presentation | Diagnostic Tools | Management | References |
|---|---|---|---|---|---|
| Intubation/Tracheostomy | Tracheal Stenosis | Delayed cough, secretion retention, dyspnea, stridor, wheezing | CT scan, laryngotracheoscopy, bronchoscopy | Laser resection, balloon dilation, stenting, surgical resection | Bello et al., 2016 [87] Zouket al., 2021 [88] |
| Tracheomalacia | Expiratory wheeze, secretion retention, cough | Dynamic CT, bronchoscopy, spirometry | Humidification, physiotherapy, CPAP, stenting, surgery | ||
| Tracheoesophageal Fistula (TEF) | Aspiration, cough with swallowing, fever, increased secretions | Chest X-ray, CT, barium swallow, endoscopy | Endoscopic stenting, sealing agents, surgical repair | Kim et al., 2020 [89] Dhiwakar et al., 2020 [90] | |
| Tracheostomy-Related Hemorrhage | Bleeding from tracheostomy, sentinel bleeding, massive hemorrhage | Clinical diagnosis | Emergency intubation, overinflation of cuff, surgery | Gilbey 2012 [91] | |
| Local Infection/Tracheocutaneous Fistula | Redness, discharge, persistent opening at stoma site | Clinical evaluation | Local wound care, antibiotics, surgical closure if needed | Jarosz et al., 2017 [92] | |
| Central Venous Catheters (CVC/PICC) | Catheter-Related Thrombosis | Limb swelling, pain, venous congestion | Doppler ultrasound, CT venography | Anticoagulation, catheter removal, thrombolysis in selected cases | Rajasekhar et al., 2017 [93] Evans et al., 2018 [94] Kucher 2011 [95] |
| Central Venous Stenosis | Edema, pain, superior vena cava syndrome, facial swelling, rare bleeding complications | CT/MR venography, contrast venography | Angioplasty, stenting, surgical bypass (rare) | Hussein et al., 2008 [96] Sonavane et al., 2015 [97] | |
| Post-Thrombotic Syndrome (PTS) | Chronic limb pain, heaviness, edema, skin changes, ulcers | Duplex ultrasound, clinical assessment | Compression therapy, anticoagulation, thrombolysis, lifestyle modification | Kahn 2016 [98] Tie et al., 2015 [99] Strijkers et al., 2017 [100] | |
| ECMO (VA/Peripheral Cannulation) | Arterial Ischemia/Stenosis | Limb ischemia, claudication, PAD exacerbation | ABI measurement, duplex ultrasound, wound inspection | Vascular surgery consult, endovascular intervention, PAD treatment | Banks et al., 2024 [101] Shin et al., 2024 [102] |
| Infection/Delayed Bleeding | Wound infection, signs of sepsis, bleeding at cannulation site | Clinical evaluation, imaging if abscess suspected | Antibiotics, wound care, surgical debridement | Banks et al., 2024 [101] |
5.1. Airway Complications
5.2. Central Venous Access Complications
5.3. Arterial Complications and ECMO-Related Sequelae
6. Prevention and Treatment of ICU Complications and PICS
6.1. Preventive Strategies
| Component | Description | Rationale |
|---|---|---|
| A: Assess, Prevent, and Manage Pain | Regular pain assessment and appropriate pain management strategies to enhance patient comfort and recovery. | Uncontrolled pain significantly increases delirium rates. |
| B. Spontaneous Awakening and Breathing Trials | Encouraging spontaneous awakening and breathing trials to reduce sedation duration and promote ventilator weaning. | “Sedation vacations” help limit delirium incidence, reduce mechanical ventilation duration, and shorten ICU stay. |
| C: Choice of Sedation and Analgesia | Using light sedation strategies and appropriate analgesia to minimize cognitive decline and delirium risk. | Benzodiazepines and excessive sedation increase delirium rates and prolong ICU stay. |
| D: Delirium: Assess, Prevent, and Manage | Routine delirium screening, implementation of non-pharmacological interventions, and management of contributing factors. | Delirium is associated with increased mortality, prolonged ICU and hospital stay, and long-term cognitive decline. |
| E: Early Mobility and Exercise | Promoting early mobilization, physical therapy, and rehabilitation to prevent muscle atrophy and improve functional outcomes. | Supported by RCTs; muscle weakness is linked to cognitive and psychological sequelae. |
| F: Family Engagement and Empowerment | Involving family members in the care process, educating them about the patient’s condition, and providing emotional support. | Reduces the incidence and severity of PICS-F. |
| G: Good Sleep Hygiene | Encouraging sleep-promoting practices, reducing nighttime disturbances, and optimizing circadian rhythms to enhance recovery. | Prevents delirium and helps reduce ICU length of stay. |
| H: Handout Materials and Follow-Up | Providing educational resources and ensuring post-discharge follow-up to monitor and manage PICS-related complications. | Reduces stress and anxiety, informs family members, and positively impacts PICS-F outcomes. |
6.2. Treatment Strategies for PICS and ICU-Related Complications
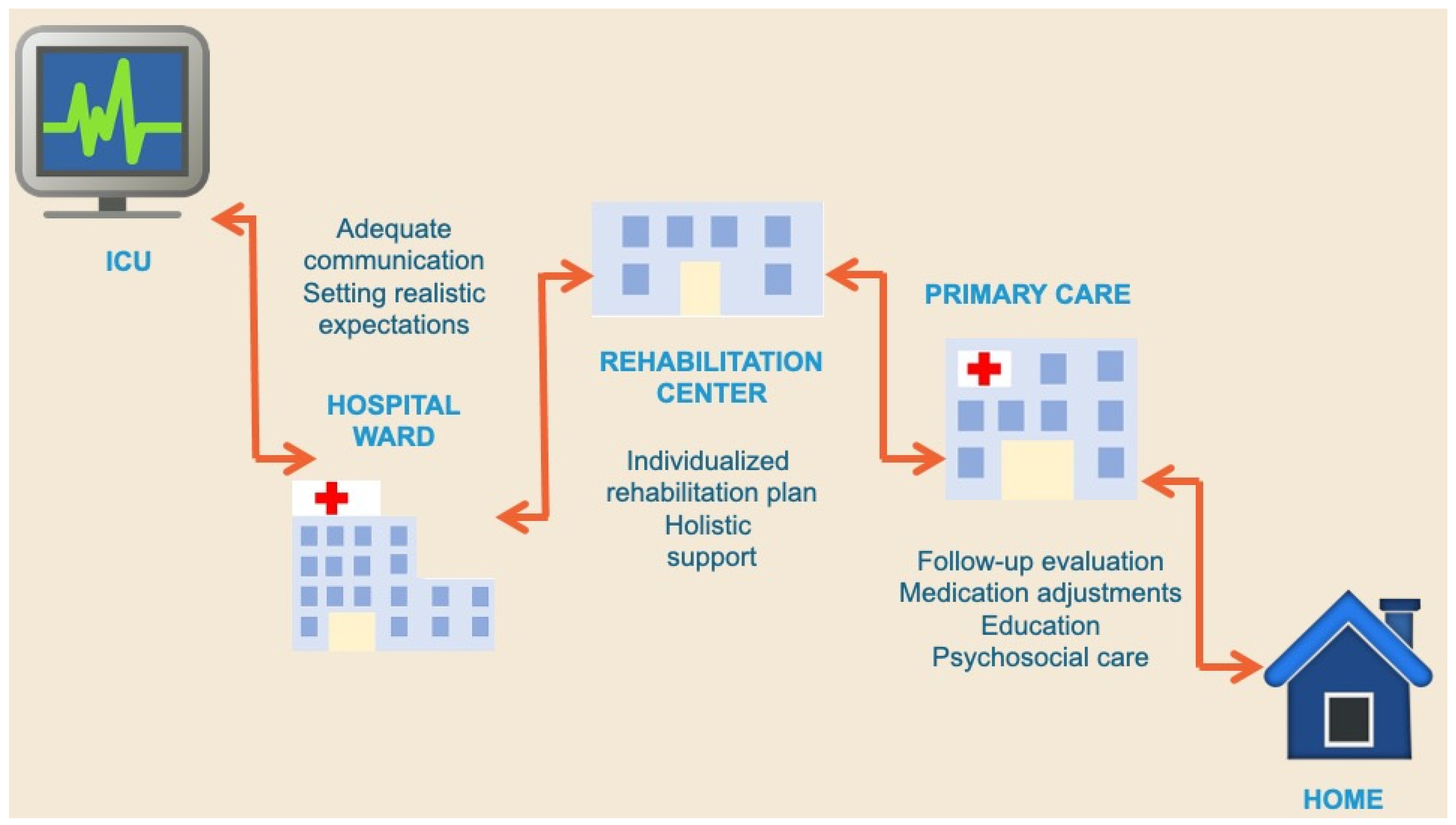
7. The Challenge of Post-ICU Follow-Up
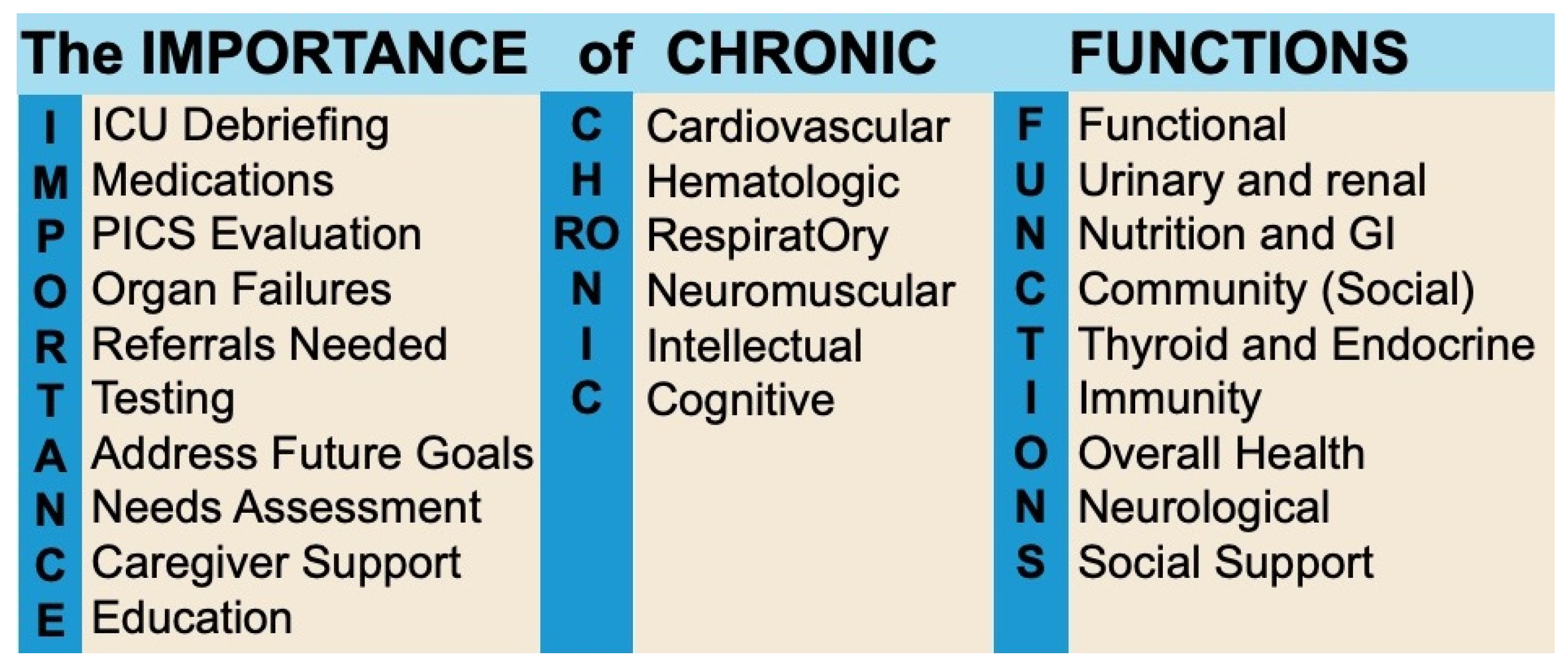
8. The Role of the General Practitioner
8.1. Barriers to Effective GP-Led Post-ICU Care
| System | Key Screening Tests and Referrals | When to Refer/Suggested Interventions | References |
|---|---|---|---|
| Cardiovascular | Blood pressure (sitting/standing) ECG (arrhythmias, QT prolongation) Echocardiogram (if signs of heart failure) Troponin (if chest pain or cardiac history) | Refer to cardiology if persistent arrhythmia, heart failure symptoms, or abnormal ECG findings Consider beta-blockers if autonomic dysfunction (e.g., POTS) is present | Prescott et al., 2014 [132] Schmidt et al., 2021 [155] |
| Respiratory | Dyspnea scale (e.g., modified Borg, mMRC) Pulmonary function tests Chest X-ray/CT (if persistent dyspnea, pulmonary fibrosis suspicion) | Refer to pulmonology if pulmonary function tests show restrictive/obstructive changes or if persistent hypoxia Prescribe pulmonary rehab for decreased exercise tolerance | Desai et al., 2011 [45] Vrettou et al., 2021 [22] Herridge et al., 2023 [28] Schmidt et al., 2021 [155] |
| Neuromuscular | Grip strength test Gait assessment (Timed Up and Go Test) 6-minute walk test Electromyography if persistent weakness | Refer to physiotherapy if significant muscle wasting or mobility impairment Consider nerve conduction studies for suspected critical illness polyneuropathy | Connolly et al., 2015 [156] Jolley et al., 2016 [157] Stevens et al., 2007 [64] |
| Endocrine | HbA1c (if ICU hyperglycemia or diabetes risk) Cortisol (morning sample if adrenal insufficiency suspected) Thyroid function (TSH, free T4) | Refer to endocrinology if adrenal or thyroid dysfunction Consider hydrocortisone trial for suspected adrenal insufficiency | Herridge et al., 2023 [28] Schmidt et al., 2021 [155] |
| Renal | Serum creatinine and eGFR (monitor for post-ICU AKI recovery) Electrolytes (K+, Na+, Mg2+) | Refer to nephrology if persistent eGFR <60 or electrolyte abnormalities | Herridge et al., 2023 [28] Schmidt et al., 2021 [155] |
| Gastrointestinal | Dysphagia screening test Nutritional status (BMI, albumin, pre-albumin) GI symptoms assessment (GERD, diarrhea) | Refer to speech therapy if dysphagia Prescribe proton pump inhibitors for stress-related GI issues | Herridge et al., 2023 [28] Schmidt et al., 2021 [155] |
| Hematologic | Complete blood count (check anemia, leukocytosis, thrombocytopenia) Coagulation panel (D-dimer, INR/PTT) Ferritin (if suspected iron deficiency) | Refer to hematology for unexplained cytopenias or hypercoagulability | Iba et al., 2020 [158] Schmidt et al., 2021 [155] |
| Infectious Risk (Asplenic) | Vaccination review (pneumococcal, meningococcal, Hib) | Provide missing vaccines and emergency ID/passport | Lefebvre et al. 2008 [159] |
| Cognitive | Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam (MMSE) | Refer to neuropsychology if cognitive impairment affects daily living, ocupational therapy, caregiver guidance | Hopkins et al. 2005 [40] Herridge et al. 2023 [28] Schmidt et al. 2021 [155] |
| Psychological | PTSD and depression screening (GAD-7, PHQ-9, IES-6) | Start antidepressants/CBT for persistent PTSD or depression | Bienvenu et al. 2012 [160] Hosey et al. Crit Care 2020 [161] |
| Functional | Frailty Index (Rockwood, Fried Frailty Criteria) Barthel Index (assess daily living independence) Physical rehab assessment | Refer to occupational therapy/rehab medicine if frailty or functional decline Consider home modifications for elderly ICU survivors | Griffiths et al. 2007 [162] Herridge et al. 2023 [28] Schmidt et al. 2021 [155] |
| Social | Caregiver burden assessment Work/financial hardship evaluation Social reintegration support | Connect with social workers for financial/workplace support Refer to peer support groups for ICU survivors | Schmidt et al. 2021 [155] Griffiths et al. 2007 [162] Kamdar et al. 2020 [86] Mantziou et al. 2022 [163] |
8.2. Strategies to Improve GP Involvement in Post-ICU Care
9. Conclusions
Funding
Conflicts of Interest
References
- Wilhelms, S.B.; Wilhelms, D.B. Emergency Department Admissions to the Intensive Care Unit—A National Retrospective Study. BMC Emerg. Med. 2021, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. Critical Illness Is an Iatrogenic Disorder. Crit. Care Med. 2010, 38, S582–S589. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Tong, D.-M.; Wang, S.-D.; Ye, S.; Xu, B.-W.; Yang, C.-X. Acute Spontaneous Intracerebral Hemorrhage and Traumatic Brain Injury Are the Most Common Causes of Critical Illness in the ICU and Have High Early Mortality. BMC Neurol. 2018, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, D.; Zhang, H.; Ding, X.; Wang, X. Focus on Host/Organ Unregulated Response: A Common Cause of Critical Illness. Chin. Med. J. 2023, 136, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Tanaka Gutiez, M.; Ramaiah, R. Demand versus Supply in Intensive Care: An Ever-Growing Problem. Crit. Care 2014, 18, P9. [Google Scholar] [CrossRef]
- Kikutani, K.; Nishikimi, M.; Emoto, R.; Matsui, S.; Ohbe, H.; Ogura, T.; Hashimoto, S.; Kushimoto, S.; Takeda, S.; Ohshimo, S.; et al. Increased National Critical Care Demands Were Associated with a Higher Mortality of Intubated COVID-19 Patients in Japan: A Retrospective Observational Study. J. Intensive Care 2024, 12, 46. [Google Scholar] [CrossRef]
- Bravata, D.M.; Perkins, A.J.; Myers, L.J.; Arling, G.; Zhang, Y.; Zillich, A.J.; Reese, L.; Dysangco, A.; Agarwal, R.; Myers, J.; et al. Association of Intensive Care Unit Patient Load and Demand With Mortality Rates in US Department of Veterans Affairs Hospitals During the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2034266. [Google Scholar] [CrossRef]
- Christensen, M.; Liang, M. Critical Care: A Concept Analysis. Int. J. Nurs. Sci. 2023, 10, 403–413. [Google Scholar] [CrossRef]
- Johnson, C. Design, Organization and Staffing of the Intensive Care Unit. Surg. (Oxf.) 2018, 36, 159–165. [Google Scholar] [CrossRef]
- Marshall, J.C.; Bosco, L.; Adhikari, N.K.; Connolly, B.; Diaz, J.V.; Dorman, T.; Fowler, R.A.; Meyfroidt, G.; Nakagawa, S.; Pelosi, P.; et al. What Is an Intensive Care Unit? A Report of the Task Force of the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 2017, 37, 270–276. [Google Scholar] [CrossRef]
- Rai, S.; Brace, C.; Ross, P.; Darvall, J.; Haines, K.; Mitchell, I.; Van Haren, F.; Pilcher, D. Characteristics and Outcomes of Very Elderly Patients Admitted to Intensive Care: A Retrospective Multicenter Cohort Analysis*. Crit. Care Med. 2023, 51, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Lilly, C.M.; Swami, S.; Liu, X.; Riker, R.R.; Badawi, O. Five-Year Trends of Critical Care Practice and Outcomes. Chest 2017, 152, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.-F.; Prescott, H.C.; Brett, S.J.; Weiss, B.; Azoulay, E.; Creteur, J.; Latronico, N.; Hough, C.L.; Weber-Carstens, S.; Vincent, J.-L.; et al. Long-Term Outcomes after Critical Illness: Recent Insights. Crit. Care 2021, 25, 108. [Google Scholar] [CrossRef]
- Doherty, Z.; Kippen, R.; Bevan, D.; Duke, G.; Williams, S.; Wilson, A.; Pilcher, D. Long-Term Outcomes of Hospital Survivors Following an ICU Stay: A Multi-Centre Retrospective Cohort Study. PLoS ONE 2022, 17, e0266038. [Google Scholar] [CrossRef]
- Van Houwelingen, F.; Van Dellen, E.; Visser-Meily, J.M.A.; Valkenet, K.; Heijnen, G.H.; Vernooij, L.M.; Kerckhoffs, M.C.; Slooter, A.J.C. Mental, Cognitive and Physical Outcomes after Intensive Care Unit Treatment during the COVID-19 Pandemic: A Comparison between COVID-19 and Non-COVID-19 Patients. Sci. Rep. 2023, 13, 14414. [Google Scholar] [CrossRef]
- McPeake, J.; Auriemma, C.L.; Harhay, M.O. Understanding the Impact of Critical Illness on Families: A Call for Standardization of Outcomes and Longitudinal Research. Ann. Am. Thorac. Soc. 2021, 18, 1783–1785. [Google Scholar] [CrossRef]
- Morgan, A. Long-Term Outcomes from Critical Care. Surg. (Oxf.) 2021, 39, 53–57. [Google Scholar] [CrossRef]
- Needham, D.M.; Dinglas, V.D.; Morris, P.E.; Jackson, J.C.; Hough, C.L.; Mendez-Tellez, P.A.; Wozniak, A.W.; Colantuoni, E.; Ely, E.W.; Rice, T.W.; et al. Physical and Cognitive Performance of Patients with Acute Lung Injury 1 Year after Initial Trophic versus Full Enteral Feeding. EDEN Trial Follow-Up. Am. J. Respir. Crit. Care Med. 2013, 188, 567–576. [Google Scholar] [CrossRef]
- Davidson, J.E.; Jones, C.; Bienvenu, O.J. Family Response to Critical Illness: Postintensive Care Syndrome-Family. Crit. Care Med. 2012, 40, 618–624. [Google Scholar] [CrossRef]
- Pochard, F.; Azoulay, E.; Chevret, S.; Lemaire, F.; Hubert, P.; Canoui, P.; Grassin, M.; Zittoun, R.; Le Gall, J.-R.; Dhainaut, J.F.; et al. Symptoms of Anxiety and Depression in Family Members of Intensive Care Unit Patients: Ethical Hypothesis Regarding Decision-Making Capacity. Crit. Care Med. 2001, 29, 1893–1897. [Google Scholar] [CrossRef]
- Azoulay, E.; Pochard, F.; Chevret, S.; Lemaire, F.; Mokhtari, M.; Le Gall, J.R.; Dhainaut, J.F.; Schlemmer, B.; French FAMIREA Group. Meeting the Needs of Intensive Care Unit Patient Families: A Multicenter Study. Am. J. Respir. Crit. Care Med. 2001, 163, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, C.S.; Mantziou, V.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Post-Intensive Care Syndrome in Survivors from Critical Illness Including COVID-19 Patients: A Narrative Review. Life 2022, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Rosa, R.G. Unmasking the Hidden Aftermath: Postintensive Care Unit Sequelae, Discharge Preparedness, and Long-Term Follow-Up. Crit. Care Sci. 2024, 36, e20240265en. [Google Scholar] [CrossRef]
- Nakanishi, N.; Liu, K.; Kawakami, D.; Kawai, Y.; Morisawa, T.; Nishida, T.; Sumita, H.; Unoki, T.; Hifumi, T.; Iida, Y.; et al. Post-Intensive Care Syndrome and Its New Challenges in Coronavirus Disease 2019 (COVID-19) Pandemic: A Review of Recent Advances and Perspectives. J. Clin. Med. 2021, 10, 3870. [Google Scholar] [CrossRef]
- Voiriot, G.; Oualha, M.; Pierre, A.; Salmon-Gandonnière, C.; Gaudet, A.; Jouan, Y.; Kallel, H.; Radermacher, P.; Vodovar, D.; Sarton, B.; et al. Chronic Critical Illness and Post-Intensive Care Syndrome: From Pathophysiology to Clinical Challenges. Ann. Intensive Care 2022, 12, 58. [Google Scholar] [CrossRef]
- Richardson, B.R.; Decavèle, M.; Demoule, A.; Murtagh, F.E.M.; Johnson, M.J. Breathlessness Assessment, Management and Impact in the Intensive Care Unit: A Rapid Review and Narrative Synthesis. Ann. Intensive Care 2024, 14, 107. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Herridge, M.S.; Azoulay, É. Outcomes after Critical Illness. N. Engl. J. Med. 2023, 388, 913–924. [Google Scholar] [CrossRef]
- Appleton, R.T.; Kinsella, J.; Quasim, T. The Incidence of Intensive Care Unit-Acquired Weakness Syndromes: A Systematic Review. J. Intensive Care Soc. 2015, 16, 126–136. [Google Scholar] [CrossRef]
- Koch, S.; Wollersheim, T.; Bierbrauer, J.; Haas, K.; Mörgeli, R.; Deja, M.; Spies, C.D.; Spuler, S.; Krebs, M.; Weber-Carstens, S. Long-Term Recovery In Critical Illness Myopathy Is Complete, Contrary to Polyneuropathy. Muscle Nerve 2014, 50, 431–436. [Google Scholar] [CrossRef]
- Clavet, H.; Doucette, S.; Trudel, G. Joint Contractures in the Intensive Care Unit: Quality of Life and Function 3.3 Years after Hospital Discharge. Disabil. Rehabil. 2015, 37, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Rasulo, F.A.; Eikermann, M.; Piva, S. Illness Weakness, Polyneuropathy and Myopathy: Diagnosis, Treatment, and Long-Term Outcomes. Crit. Care 2023, 27, 439. [Google Scholar] [CrossRef] [PubMed]
- Amacher, S.A.; Sahmer, C.; Becker, C.; Gross, S.; Arpagaus, A.; Urben, T.; Tisljar, K.; Emsden, C.; Sutter, R.; Marsch, S.; et al. Post-Intensive Care Syndrome and Health-Related Quality of Life in Long-Term Survivors of Cardiac Arrest: A Prospective Cohort Study. Sci. Rep. 2024, 14, 10533. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kawasaki, A.; Suzuki, N.; Hosoi, S.; Fujita, T.; Hachisu, S.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y. Grip Strength Correlates with Mental Health and Quality of Life after Critical Care: A Retrospective Study in a Post-Intensive Care Syndrome Clinic. J. Clin. Med. 2021, 10, 3044. [Google Scholar] [CrossRef]
- Skoretz, S.A.; Flowers, H.L.; Martino, R. The Incidence of Dysphagia Following Endotracheal Intubation: A Systematic Review. Chest 2010, 137, 665–673. [Google Scholar] [CrossRef]
- Macht, M.; Wimbish, T.; Clark, B.J.; Benson, A.B.; Burnham, E.L.; Williams, A.; Moss, M. Postextubation Dysphagia Is Persistent and Associated with Poor Outcomes in Survivors of Critical Illness. Crit. Care 2011, 15, R231. [Google Scholar] [CrossRef]
- Schefold, J.C.; Berger, D.; Zürcher, P.; Lensch, M.; Perren, A.; Jakob, S.M.; Parviainen, I.; Takala, J. Dysphagia in Mechanically Ventilated ICU Patients (DYnAMICS): A Prospective Observational Trial. Crit. Care Med. 2017, 45, 2061–2069. [Google Scholar] [CrossRef]
- Calsavara, A.J.C.; Costa, P.A.; Nobre, V.; Teixeira, A.L. Factors Associated With Short and Long Term Cognitive Changes in Patients With Sepsis. Sci. Rep. 2018, 8, 4509. [Google Scholar] [CrossRef]
- Semmler, A.; Widmann, C.N.; Okulla, T.; Urbach, H.; Kaiser, M.; Widman, G.; Mormann, F.; Weide, J.; Fliessbach, K.; Hoeft, A.; et al. Persistent Cognitive Impairment, Hippocampal Atrophy and EEG Changes in Sepsis Survivors. J. Neurol. Neurosurg. Psychiatry 2013, 84, 62–69. [Google Scholar] [CrossRef]
- Hopkins, R.O.; Weaver, L.K.; Collingridge, D.; Parkinson, R.B.; Chan, K.J.; Orme, J.F. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 340–347. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-Term Cognitive Impairment and Functional Disability among Survivors of Severe Sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Jackson, J.C.; Morandi, A.; Girard, T.D.; Hughes, C.G.; Thompson, J.L.; Kiehl, A.L.; Elstad, M.R.; Wasserstein, M.L.; Goodman, R.B.; et al. Incidence and Risk Factors for Intensive Care Unit–Related Post-Traumatic Stress Disorder in Veterans and Civilians. Am. J. Respir. Crit. Care Med. 2016, 193, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Skirrow, P.; Griffiths, R.D.; Humphris, G.; Ingleby, S.; Eddleston, J.; Waldmann, C.; Gager, M. Post-Traumatic Stress Disorder-Related Symptoms in Relatives of Patients Following Intensive Care. Intensive Care Med. 2004, 30, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.V.; Law, T.J.; Needham, D.M. Long-Term Complications of Critical Care. Crit. Care Med. 2011, 39, 371–379. [Google Scholar] [CrossRef]
- Griffiths, J.; Gager, M.; Alder, N.; Fawcett, D.; Waldmann, C.; Quinlan, J. A Self-Report-Based Study of the Incidence and Associations of Sexual Dysfunction in Survivors of Intensive Care Treatment. Intensive Care Med. 2006, 32, 445–451. [Google Scholar] [CrossRef]
- Broomhead, L.R.; Brett, S.J. Clinical Review: Intensive Care Follow-up--What Has It Told Us? Crit. Care 2002, 6, 411–417. [Google Scholar] [CrossRef]
- Wells, K.B. The Functioning and Well-Being of Depressed Patients: Results From the Medical Outcomes Study. JAMA 1989, 262, 914. [Google Scholar] [CrossRef]
- Zatzick, D.; Jurkovich, G.J.; Rivara, F.P.; Wang, J.; Fan, M.-Y.; Joesch, J.; Mackenzie, E. A National US Study of Posttraumatic Stress Disorder, Depression, and Work and Functional Outcomes after Hospitalization for Traumatic Injury. Ann. Surg. 2008, 248, 429–437. [Google Scholar] [CrossRef]
- Dowdy, D.W.; Dinglas, V.; Mendez-Tellez, P.A.; Bienvenu, O.J.; Sevransky, J.; Dennison, C.R.; Shanholtz, C.; Needham, D.M. Intensive Care Unit Hypoglycemia Predicts Depression during Early Recovery from Acute Lung Injury*. Crit. Care Med. 2008, 36, 2726–2733. [Google Scholar] [CrossRef]
- Bjørnøy, I.; Rustøen, T.; Mesina, R.J.S.; Hofsø, K. Anxiety and Depression in Intensive Care Patients Six Months after Admission to an Intensive Care Unit: A Cohort Study. Intensive Crit. Care Nurs. 2023, 78, 103473. [Google Scholar] [CrossRef] [PubMed]
- Hatch, R.; Young, D.; Barber, V.; Griffiths, J.; Harrison, D.A.; Watkinson, P. Anxiety, Depression and Post Traumatic Stress Disorder after Critical Illness: A UK-Wide Prospective Cohort Study. Crit. Care 2018, 22, 310. [Google Scholar] [CrossRef]
- Jackson, J.C.; Pandharipande, P.P.; Girard, T.D.; Brummel, N.E.; Thompson, J.L.; Hughes, C.G.; Pun, B.T.; Vasilevskis, E.E.; Morandi, A.; Shintani, A.K.; et al. Depression, Post-Traumatic Stress Disorder, and Functional Disability in Survivors of Critical Illness in the BRAIN-ICU Study: A Longitudinal Cohort Study. Lancet Respir. Med. 2014, 2, 369–379. [Google Scholar] [CrossRef]
- Sakusic, A.; O’Horo, J.C.; Dziadzko, M.; Volha, D.; Ali, R.; Singh, T.D.; Kashyap, R.; Farrell, A.M.; Fryer, J.D.; Petersen, R.; et al. Potentially Modifiable Risk Factors for Long-Term Cognitive Impairment After Critical Illness: A Systematic Review. Mayo Clin. Proc. 2018, 93, 68–82. [Google Scholar] [CrossRef]
- Kotfis, K.; Williams Roberson, S.; Wilson, J.E.; Dabrowski, W.; Pun, B.T.; Ely, E.W. COVID-19: ICU Delirium Management during SARS-CoV-2 Pandemic. Crit. Care 2020, 24, 176. [Google Scholar] [CrossRef]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a Predictor of Long-Term Cognitive Impairment in Survivors of Critical Illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef]
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-Intensive Care Syndrome: Its Pathophysiology, Prevention, and Future Directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving Long-Term Outcomes after Discharge from Intensive Care Unit: Report from a Stakeholders’ Conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Rawal, G.; Yadav, S.; Kumar, R. Post-Intensive Care Syndrome: An Overview. J. Transl. Intern. Med. 2017, 5, 90–92. [Google Scholar] [CrossRef]
- Lee, M.; Kang, J.; Jeong, Y.J. Risk Factors for Post-Intensive Care Syndrome: A Systematic Review and Meta-Analysis. Aust. Crit. Care 2020, 33, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.O.; Weaver, L.K.; Pope, D.; Orme, J.F.; Bigler, E.D.; Larson-Lohr, V. Neuropsychological Sequelae and Impaired Health Status in Survivors of Severe Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Dowdy, D.W.; Colantuoni, E.; Mendez-Tellez, P.A.; Sevransky, J.E.; Shanholtz, C.; Himmelfarb, C.R.D.; Desai, S.V.; Ciesla, N.; Herridge, M.S.; et al. Physical Complications in Acute Lung Injury Survivors: A Two-Year Longitudinal Prospective Study. Crit. Care Med. 2014, 42, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.D.; Dowdy, D.W.; Michaels, R.K.; Mendez-Tellez, P.A.; Pronovost, P.J.; Needham, D.M. Neuromuscular Dysfunction Acquired in Critical Illness: A Systematic Review. Intensive Care Med. 2007, 33, 1876–1891. [Google Scholar] [CrossRef]
- Jackson, J.C.; Girard, T.D.; Gordon, S.M.; Thompson, J.L.; Shintani, A.K.; Thomason, J.W.W.; Pun, B.T.; Canonico, A.E.; Dunn, J.G.; Bernard, G.R.; et al. Long-Term Cognitive and Psychological Outcomes in the Awakening and Breathing Controlled Trial. Am. J. Respir. Crit. Care Med. 2010, 182, 183–191. [Google Scholar] [CrossRef]
- Parker, A.M.; Sricharoenchai, T.; Raparla, S.; Schneck, K.W.; Bienvenu, O.J.; Needham, D.M. Posttraumatic Stress Disorder in Critical Illness Survivors: A Metaanalysis. Crit. Care Med. 2015, 43, 1121–1129. [Google Scholar] [CrossRef]
- Cameron, J.I.; Chu, L.M.; Matte, A.; Tomlinson, G.; Chan, L.; Thomas, C.; Friedrich, J.O.; Mehta, S.; Lamontagne, F.; Levasseur, M.; et al. One-Year Outcomes in Caregivers of Critically Ill Patients. N. Engl. J. Med. 2016, 374, 1831–1841. [Google Scholar] [CrossRef]
- Shirasaki, K.; Hifumi, T.; Nakanishi, N.; Nosaka, N.; Miyamoto, K.; Komachi, M.H.; Haruna, J.; Inoue, S.; Otani, N. Postintensive Care Syndrome Family: A Comprehensive Review. Acute Med. Surg. 2024, 11, e939. [Google Scholar] [CrossRef]
- HealthManagement.org Radiology Management, ICU Management, Healthcare IT, Cardiology Management, Executive Management. Available online: https://healthmanagement.org/c/icu/IssueArticle/optimising-nutrition-for-recovery-after-icu (accessed on 31 March 2025).
- Azoulay, E.; Pochard, F.; Kentish-Barnes, N.; Chevret, S.; Aboab, J.; Adrie, C.; Annane, D.; Bleichner, G.; Bollaert, P.E.; Darmon, M.; et al. Risk of Post-Traumatic Stress Symptoms in Family Members of Intensive Care Unit Patients. Am. J. Respir. Crit. Care Med. 2005, 171, 987–994. [Google Scholar] [CrossRef]
- Anderson, W.G.; Arnold, R.M.; Angus, D.C.; Bryce, C.L. Posttraumatic Stress and Complicated Grief in Family Members of Patients in the Intensive Care Unit. J. Gen. Intern. Med. 2008, 23, 1871–1876. [Google Scholar] [CrossRef]
- Choi, J.; Lingler, J.H.; Donahoe, M.P.; Happ, M.B.; Hoffman, L.A.; Tate, J.A. Home Discharge Following Critical Illness: A Qualitative Analysis of Family Caregiver Experience. Heart Lung 2018, 47, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Verceles, A.C.; Corwin, D.S.; Afshar, M.; Friedman, E.B.; McCurdy, M.T.; Shanholtz, C.; Oakjones, K.; Zubrow, M.T.; Titus, J.; Netzer, G. Half of the Family Members of Critically Ill Patients Experience Excessive Daytime Sleepiness. Intensive Care Med. 2014, 40, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Kosyakovsky, L.B.; Angriman, F.; Katz, E.; Adhikari, N.K.; Godoy, L.C.; Marshall, J.C.; Ferreyro, B.L.; Lee, D.S.; Rosenson, R.S.; Sattar, N.; et al. Association between Sepsis Survivorship and Long-Term Cardiovascular Outcomes in Adults: A Systematic Review and Meta-Analysis. Intensive Care Med. 2021, 47, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.B.; Werdan, K.; Müller-Werdan, U. Autonomic Dysfunction in the ICU Patient. Curr. Opin. Crit. Care 2001, 7, 314–322. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Mayr, F.; Kellum, J.A.; Weissfeld, L.; Kaynar, A.M.; Young, T.; Irani, K.; Angus, D.C.; the GenIMS Investigators. Elevated Hemostasis Markers after Pneumonia Increases One-Year Risk of All-Cause and Cardiovascular Deaths. PLoS ONE 2011, 6, e22847. [Google Scholar] [CrossRef]
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit. Care Clin. 2017, 33, 245–258. [Google Scholar] [CrossRef]
- Lawler, P.R.; Berg, D.D.; Park, J.-G.; Katz, J.N.; Baird-Zars, V.M.; Barsness, G.W.; Bohula, E.A.; Carnicelli, A.P.; Chaudhry, S.-P.; Jentzer, J.C.; et al. The Range of Cardiogenic Shock Survival by Clinical Stage: Data From the Critical Care Cardiology Trials Network Registry. Crit. Care Med. 2021, 49, 1293–1302. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Xu, Z.; Gu, H.; Chen, Z.; Ding, Y. Incidence of and Risk Factors for Post–Intensive Care Syndrome among Chinese Respiratory Intensive Care Unit Patients: A Cross-Sectional, Prospective Study. Aust. Crit. Care 2023, 36, 464–469. [Google Scholar] [CrossRef]
- Kim, G.S.; Moon, H.I.; Ham, J.A.; Ma, M.K. Relationship between Generalized Sarcopenia and the Severity of Dysphagia after a Stroke. J. Korean Dysphagia Soc. 2022, 12, 24–34. [Google Scholar] [CrossRef]
- Martín-Vicente, P.; López-Martínez, C.; Lopez-Alonso, I.; López-Aguilar, J.; Albaiceta, G.M.; Amado-Rodríguez, L. Molecular Mechanisms of Postintensive Care Syndrome. Intensive Care Med. Exp. 2021, 9, 58. [Google Scholar] [CrossRef]
- Haines, R.W.; Powell-Tuck, J.; Leonard, H.; Crichton, S.; Ostermann, M. Long-Term Kidney Function of Patients Discharged from Hospital after an Intensive Care Admission: Observational Cohort Study. Sci. Rep. 2021, 11, 9928. [Google Scholar] [CrossRef] [PubMed]
- Jivanji, C.J.; Asrani, V.M.; Windsor, J.A.; Petrov, M.S. New-Onset Diabetes After Acute and Critical Illness: A Systematic Review. Mayo Clin. Proc. 2017, 92, 762–773. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN Guideline on Clinical Nutrition in Hospitalized Patients with Acute or Chronic Kidney Disease. Clin. Nutr. 2021, 40, 1644–1668. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation and Immunosuppression: A Common Syndrome and New Horizon for Surgical Intensive Care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Kamdar, B.B.; Suri, R.; Suchyta, M.R.; Digrande, K.F.; Sherwood, K.D.; Colantuoni, E.; Dinglas, V.D.; Needham, D.M.; Hopkins, R.O. Return to Work after Critical Illness: A Systematic Review and Meta-Analysis. Thorax 2020, 75, 17–27. [Google Scholar] [CrossRef]
- Bello, G.; Di Muzio, F.; Antonelli, M. Quality of Life and Complications After Percutaneous Tracheostomy. In Percutaneous Tracheostomy in Critically Ill Patients; Servillo, G., Pelosi, P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 131–147. ISBN 978-3-319-22300-1. [Google Scholar]
- Zouk, A.N.; Batra, H. Managing Complications of Percutaneous Tracheostomy and Gastrostomy. J. Thorac. Dis. 2021, 13, 5314–5330. [Google Scholar] [CrossRef]
- Kim, H.S.; Khemasuwan, D.; Diaz-Mendoza, J.; Mehta, A.C. Management of Tracheo-Oesophageal Fistula in Adults. Eur. Respir. Rev. 2020, 29, 200094. [Google Scholar] [CrossRef]
- Dhiwakar, M.; Ronen, O.; Supriya, M.; Mehta, S. Surgical Repair of Mechanical Ventilation Induced Tracheoesophageal Fistula. Eur. Arch. Otorhinolaryngol. 2020, 277, 323–331. [Google Scholar] [CrossRef]
- Gilbey, P. Fatal Complications of Percutaneous Dilatational Tracheostomy. Am. J. Otolaryngol. 2012, 33, 770–773. [Google Scholar] [CrossRef]
- Jarosz, K.; Kubisa, B.; Andrzejewska, A.; Mrówczyńska, K.; Hamerlak, Z.; Bartkowska-Sniatkowska, A. Adverse Outcomes after Percutaneous Dilatational Tracheostomy versus Surgical Tracheostomy in Intensive Care Patients: Case Series and Literature Review. Ther. Clin. Risk Manag. 2017, 13, 975–981. [Google Scholar] [CrossRef]
- Rajasekhar, A.; Streiff, M.B. How I Treat Central Venous Access Device–Related Upper Extremity Deep Vein Thrombosis. Blood 2017, 129, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.S.; Ratchford, E.V. Catheter-Related Venous Thrombosis. Vasc. Med. 2018, 23, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N. Deep-Vein Thrombosis of the Upper Extremities. N. Engl. J. Med. 2011, 364, 861–869. [Google Scholar] [CrossRef]
- Hussein, F.A.; Mawla, N.; Befeler, A.S.; Martin, K.J.; Lentine, K.L. Formation of Downhill Esophageal Varices as a Rare but Serious Complication of Hemodialysis Access: A Case Report and Comprehensive Literature Review. Clin. Exp. Nephrol. 2008, 12, 407–415. [Google Scholar] [CrossRef]
- Sonavane, S.K.; Milner, D.M.; Singh, S.P.; Abdel Aal, A.K.; Shahir, K.S.; Chaturvedi, A. Comprehensive Imaging Review of the Superior Vena Cava. Radiographics 2015, 35, 1873–1892. [Google Scholar] [CrossRef]
- Kahn, S.R. The Post-Thrombotic Syndrome. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 413–418. [Google Scholar] [CrossRef]
- Tie, H.-T.; Luo, M.-Z.; Luo, M.-J.; Li, K.; Li, Q.; Wu, Q.-C. Compression Therapy in the Prevention of Postthrombotic Syndrome: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e1318. [Google Scholar] [CrossRef]
- Strijkers, R.H.; De Wolf, M.A.; Wittens, C.H. Risk Factors of Postthrombotic Syndrome before and after Deep Venous Thrombosis Treatment. Phlebology 2017, 32, 384–389. [Google Scholar] [CrossRef]
- Banks, C.A.; Blakeslee-Carter, J.; Nkie, V.; Spangler, E.L.; Still, S.A.; Eudailey, K.W.; McElwee, S.K.; Blood, M.S.; Novak, Z.; Beck, A.W. Occurrence, Predictors, and Management of Late Vascular Complications Following Extracorporeal Membrane Oxygenation. J. Vasc. Surg. 2024, 80, 864–872.e1. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, K.H.; Park, T.K.; Cho, Y.H.; Yang, J.H. Arterial Complications Assessed by Duplex Ultrasound After Decannulation of Peripheral Venoarterial Extracorporeal Membrane Oxygenation. Circ. J. 2024, CJ-24-0400. [Google Scholar] [CrossRef]
- Gelbard, A.; Francis, D.O.; Sandulache, V.C.; Simmons, J.C.; Donovan, D.T.; Ongkasuwan, J. Causes and Consequences of Adult Laryngotracheal Stenosis. Laryngoscope 2015, 125, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Pappal, R.B.; Burruss, C.P.; Witt, M.A.; Harryman, C.; Ali, S.Z.; Bush, M.L.; Fritz, M.A. Risk Factors for Developing Subglottic and Tracheal Stenosis from the Medical Intensive Care Unit. Laryngoscope Investig. Oto 2023, 8, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Achu, R.; Perry, A.; Yarrington, C.; Norris, M.; Tracy, L.; Spence, N.Z. Impact of Pregnancy on Airway Complications after Intubation for COVID-19 Infection: A Case Series. Am. J. Otolaryngol. 2022, 43, 103522. [Google Scholar] [CrossRef] [PubMed]
- Zubairi, A.B.S.; Dildar, B.; Husain, S.J.; Khan, M.F. Tracheal Stenosis Mimicking Severe Acute Asthma. Case Reports 2010, 2010, bcr1220092517. [Google Scholar] [CrossRef]
- Schaffer, S.A.; Manji, R.A.; Kirkpatrick, I.; Fang, T.; Arora, R.C.; Zieroth, S.; Jassal, D.S. Negative Pressure Pulmonary Edema in the Coronary Care Unit. Can. J. Cardiol. 2008, 24, S58–S59. [Google Scholar] [CrossRef]
- Peters, S.M. An Unexpected Cause of Wheezing and Shortness of Breath. JAAPA 2020, 33, 54–56. [Google Scholar] [CrossRef]
- Huyser, M.R.; Tukey, M.H.; Ely, S.; Lo, J.C.; Ray, A.L.; Ashiku, S.K. Transoral Minimally Invasive Tracheoplasty Technique for Cervical Tracheomalacia. Ann. Thorac. Surg. 2021, 112, e101–e102. [Google Scholar] [CrossRef]
- Tsai, J.-J.; Hsia, C.-C.; Tsai, D.-M.; Chen, W.-T.; Hsu, Y.-H. Epistaxis as a Rare Complication of Catheter-Related Central Venous Stenosis. Am. J. Kidney Dis. 2009, 53, 555–559. [Google Scholar] [CrossRef]
- Kim, C.Y.; Mirza, R.A.; Bryant, J.A.; Whiting, E.D.; Delong, D.M.; Spritzer, C.E.; Merkle, E.M. Central Veins of the Chest: Evaluation with Time-Resolved MR Angiography. Radiology 2008, 247, 558–566. [Google Scholar] [CrossRef]
- Rudd, K.M.; Roberts, K.K.; Hamilton, C.M. Improving Peripheral Artery Disease Screening and Treatment: A Screening, Diagnosis, and Treatment Tool for Use across Multiple Care Settings. J. Osteopath. Med. 2025, 125, 163–171. [Google Scholar] [CrossRef]
- Ely, E.W. The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit. Care Med. 2017, 45, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef]
- Mulkey, M.A.; Beacham, P.; McCormick, M.A.; Everhart, D.E.; Khan, B. Minimizing Post–Intensive Care Syndrome to Improve Outcomes for Intensive Care Unit Survivors. Crit. Care Nurse 2022, 42, 68–73. [Google Scholar] [CrossRef]
- Colbenson, G.A.; Johnson, A.; Wilson, M.E. Post-Intensive Care Syndrome: Impact, Prevention, and Management. Breathe 2019, 15, 98–101. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early Physical and Occupational Therapy in Mechanically Ventilated, Critically Ill Patients: A Randomised Controlled Trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- TEAM Study Investigators; Hodgson, C.; Bellomo, R.; Berney, S.; Bailey, M.; Buhr, H.; Denehy, L.; Harrold, M.; Higgins, A.; Presneill, J.; et al. Early Mobilization and Recovery in Mechanically Ventilated Patients in the ICU: A Bi-National, Multi-Centre, Prospective Cohort Study. Crit. Care 2015, 19, 81. [Google Scholar] [CrossRef]
- Morris, P.E.; Goad, A.; Thompson, C.; Taylor, K.; Harry, B.; Passmore, L.; Ross, A.; Anderson, L.; Baker, S.; Sanchez, M.; et al. Early Intensive Care Unit Mobility Therapy in the Treatment of Acute Respiratory Failure. Crit. Care Med. 2008, 36, 2238–2243. [Google Scholar] [CrossRef]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, F.E.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Schofield-Robinson, O.J.; Lewis, S.R.; Smith, A.F.; McPeake, J.; Alderson, P. Follow-up Services for Improving Long-Term Outcomes in Intensive Care Unit (ICU) Survivors. Cochrane Database Syst. Rev. 2018, 11, CD012701. [Google Scholar] [CrossRef]
- Haines, K.J.; Leggett, N.; Hibbert, E.; Hall, T.; Boehm, L.M.; Bakhru, R.N.; Bastin, A.J.; Butcher, B.W.; Eaton, T.L.; Harris, W.; et al. Patient and Caregiver-Derived Health Service Improvements for Better Critical Care Recovery. Crit. Care Med. 2022, 50, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Renner, C.; Jeitziner, M.-M.; Albert, M.; Brinkmann, S.; Diserens, K.; Dzialowski, I.; Heidler, M.-D.; Lück, M.; Nusser-Müller-Busch, R.; Sandor, P.S.; et al. Guideline on Multimodal Rehabilitation for Patients with Post-Intensive Care Syndrome. Crit. Care 2023, 27, 301. [Google Scholar] [CrossRef] [PubMed]
- The Role of Critical Care Nurses in Managing Post-Traumatic Stress Disorder (PTSD) Among Intensive Care Unit (ICU) Patients | PDF | Mental Health | Nursing. Available online: https://www.scribd.com/document/703299084/The-Role-of-Critical-Care-Nurses-in-Managing-Post-Traumatic-Stress-Disorder-PTSD-Among-Intensive-Care-Unit-ICU-Patients (accessed on 31 March 2025).
- Petrinec, A.; Wilk, C.; Hughes, J.W.; Zullo, M.D.; Chen, Y.-J.; Palmieri, P.A. Delivering Cognitive Behavioral Therapy for Post–Intensive Care Syndrome–Family via a Mobile Health App. Am. J. Crit. Care 2021, 30, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tronstad, O.; Flaws, D.; Churchill, L.; Jones, A.Y.M.; Nakamura, K.; Fraser, J.F. From Bedside to Recovery: Exercise Therapy for Prevention of Post-Intensive Care Syndrome. J. Intensive Care 2024, 12, 11. [Google Scholar] [CrossRef]
- Hiser, S.L.; Fatima, A.; Ali, M.; Needham, D.M. Post-Intensive Care Syndrome (PICS): Recent Updates. J. Intensive Care 2023, 11, 23. [Google Scholar] [CrossRef]
- Wilbur, J.; Rockafellow, J.; Shian, B. Post-ICU Care in the Outpatient Setting. Am. Fam. Physician 2021, 103, 590–596. [Google Scholar]
- Cuthbertson, B.H.; Roughton, S.; Jenkinson, D.; MacLennan, G.; Vale, L. Quality of Life in the Five Years after Intensive Care: A Cohort Study. Crit. Care 2010, 14, R6. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tansey, C.M.; Tomlinson, G.; Diaz-Granados, N.; Matté, A.; Barr, A.; Mehta, S.; Mazer, C.D.; Guest, C.B.; Stewart, T.E.; et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 538–544. [Google Scholar] [CrossRef]
- Prescott, H.C.; Langa, K.M.; Liu, V.; Escobar, G.J.; Iwashyna, T.J. Increased 1-Year Healthcare Use in Survivors of Severe Sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 62–69. [Google Scholar] [CrossRef]
- Gupta, L.; Subair, M.N.; Munjal, J.; Singh, B.; Bansal, V.; Gupta, V.; Jain, R. Beyond Survival: Understanding Post-Intensive Care Syndrome. Acute Crit. Care 2024, 39, 226–233. [Google Scholar] [CrossRef]
- Wojnar-Gruszka, K.; Wojtas, K.; Jędrocha, K. Post-Intensive Care Syndrome (PICS) –Complications after ICU Stay: Selected Issues. Nurs. Probl./Probl. Pielęgniarstwa 2023, 31, 166–173. [Google Scholar] [CrossRef]
- Castellanos-Ortega, Á.; Rothen, H.U.; Franco, N.; Rayo, L.A.; Martín-Loeches, I.; Ramírez, P.; Cuñat De La Hoz, J. Training in Intensive Care Medicine. A Challenge within Reach. Med. Intensiv. (Engl. Ed.) 2014, 38, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.; Freebairn, R.; Lee, R. College of Intensive Care Medicine: Changes to Intensive Care Medicine Training. Crit. Care Resusc. 2014, 16, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Bion, J.; Rothen, H.U. Models for intensive care training: A European perspective. Am. J. Respir. Crit. Care Med. 2014, 189, 256–262. [Google Scholar] [CrossRef]
- Devillers, L.; Friesse, S.; Caranta, M.; Tarazona, V.; Bourrion, B.; Saint-Lary, O. General Practice Undergraduate and Vocational Training: Ambulatory Teaching and Trainers’ Curriculum and Remuneration—A Cross-Sectional Study among 30 Member Countries of WONCA Europe. BMC Med. Educ. 2023, 23, 439. [Google Scholar] [CrossRef]
- Gupta, T.S.; Hays, R. Training for General Practice: How Australia’s Programs Compare to Other Countries. Aust. Fam. Physician 2016, 45, 18–21. [Google Scholar]
- McDonagh, S.T.; Reburn, C.; Smith, J.R.; Clark, C.E. Group-Delivered Interventions for Lowering Blood Pressure in Hypertension: A Systematic Review and Meta-Analysis. Br. J. Gen. Pract. 2025, 75, e266–e276. [Google Scholar] [CrossRef]
- Berger, P.; Braude, D. Post–Intensive Care Syndrome: A Crash Course for General Practice. Aust. J. Gen. Pract. 2021, 50, 647–649. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Still, M.; Anderson, B.J.; Bienvenu, O.J.; Brodsky, M.B.; Brummel, N.; Butcher, B.; Clay, A.S.; Felt, H.; Ferrante, L.E.; et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit. Care Med. 2020, 48, 1670–1679. [Google Scholar] [CrossRef]
- Johnson, C.C.; Suchyta, M.R.; Darowski, E.S.; Collar, E.M.; Kiehl, A.L.; Van, J.; Jackson, J.C.; Hopkins, R.O. Psychological Sequelae in Family Caregivers of Critically III Intensive Care Unit Patients. A Systematic Review. Ann. Am. Thorac. Soc. 2019, 16, 894–909. [Google Scholar] [CrossRef]
- Berger, P.; Braude, D. Post–Intensive Care Syndrome: Screening and Management in Primary Care. Aust. J. Gen. Pract. 2021, 50, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Liu, K.; Hatakeyama, J.; Kawauchi, A.; Yoshida, M.; Sumita, H.; Miyamoto, K.; Nakamura, K. Post-Intensive Care Syndrome Follow-up System after Hospital Discharge: A Narrative Review. J. Intensive Care 2024, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, S.; Leggett, N.E.; Deane, A.M.; Haines, K.J.; Abdelhamid, Y.A. Models of Intensive Care Unit Follow-up Care and Feasibility of Intervention Delivery: A Systematic Review. Aust. Crit. Care 2024, 37, 508–516. [Google Scholar] [CrossRef]
- Flores, L.; Barber, A.; Korona, R.B.; Bakhru, R.N. Post-ICU Clinic. CHEST Crit. Care 2024, 2, 100036. [Google Scholar] [CrossRef]
- Butcher, B.W.; Eaton, T.L.; Montgomery-Yates, A.A.; Sevin, C.M. Meeting the Challenges of Establishing Intensive Care Unit Follow-up Clinics. Am. J. Crit. Care 2022, 31, 324–328. [Google Scholar] [CrossRef]
- Haines, K.J.; Sevin, C.M.; Hibbert, E.; Boehm, L.M.; Aparanji, K.; Bakhru, R.N.; Bastin, A.J.; Beesley, S.J.; Butcher, B.W.; Drumright, K.; et al. Key Mechanisms by Which Post-ICU Activities Can Improve in-ICU Care: Results of the International THRIVE Collaboratives. Intensive Care Med. 2019, 45, 939–947. [Google Scholar] [CrossRef]
- Gehrke-Beck, S.; Gensichen, J.; Turner, K.M.; Heintze, C.; Schmidt, K.F. General Practitioners’ Views and Experiences in Caring for Patients after Sepsis: A Qualitative Interview Study. BMJ Open 2021, 11, e040533. [Google Scholar] [CrossRef]
- Schwarz, C.M.; Hoffmann, M.; Schwarz, P.; Kamolz, L.-P.; Brunner, G.; Sendlhofer, G. A Systematic Literature Review and Narrative Synthesis on the Risks of Medical Discharge Letters for Patients’ Safety. BMC Health Serv. Res. 2019, 19, 158. [Google Scholar] [CrossRef]
- Needham, D.M.; Sepulveda, K.A.; Dinglas, V.D.; Chessare, C.M.; Friedman, L.A.; Bingham, C.O.; Turnbull, A.E. Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study. Am. J. Respir. Crit. Care Med. 2017, 196, 1122–1130. [Google Scholar] [CrossRef]
- Girbes, A.R.; Beishuizen, A. Interfacing the ICU with the General Practitioner. Crit. Care 2010, 14, 172. [Google Scholar] [CrossRef]
- Etesse, B.; Jaber, S.; Mura, T.; Leone, M.; Constantin, J.-M.; Michelet, P.; Zoric, L.; Capdevila, X.; Malavielle, F.; Allaouchiche, B.; et al. How the Relationships between General Practitioners and Intensivists Can Be Improved: The General Practitioners’ Point of View. Crit. Care 2010, 14, R112. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Gehrke-Beck, S. Transitions to Primary Care. In Improving Critical Care Survivorship: A Guide to Prevention, Recovery, and Reintegration; Haines, K.J., McPeake, J., Sevin, C.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 207–227. ISBN 978-3-030-68680-2. [Google Scholar]
- Connolly, B.; Salisbury, L.; O’Neill, B.; Geneen, L.; Douiri, A.; Grocott, M.P.W.; Hart, N.; Walsh, T.S.; Blackwood, B.; ERACIP Group. Exercise Rehabilitation Following Intensive Care Unit Discharge for Recovery from Critical Illness. Cochrane Database Syst. Rev. 2015, 2015, CD008632. [Google Scholar] [CrossRef] [PubMed]
- Jolley, S.E.; Bunnell, A.E.; Hough, C.L. ICU-Acquired Weakness. Chest 2016, 150, 1129–1140. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The Unique Characteristics of COVID-19 Coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef]
- Writing Panel of Working Group; Lefebvre, S.L.; Golab, G.C.; Christensen, E.; Castrodale, L.; Aureden, K.; Bialachowski, A.; Gumley, N.; Robinson, J.; Peregrine, A.; et al. Guidelines for Animal-Assisted Interventions in Health Care Facilities. Am. J. Infect. Control 2008, 36, 78–85. [Google Scholar] [CrossRef]
- Bienvenu, O.J.; Colantuoni, E.; Mendez-Tellez, P.A.; Dinglas, V.D.; Shanholtz, C.; Husain, N.; Dennison, C.R.; Herridge, M.S.; Pronovost, P.J.; Needham, D.M. Depressive Symptoms and Impaired Physical Function after Acute Lung Injury: A 2-Year Longitudinal Study. Am. J. Respir. Crit. Care Med. 2012, 185, 517–524. [Google Scholar] [CrossRef]
- Hosey, M.M.; Leoutsakos, J.-M.S.; Li, X.; Dinglas, V.D.; Bienvenu, O.J.; Parker, A.M.; Hopkins, R.O.; Needham, D.M.; Neufeld, K.J. Screening for Posttraumatic Stress Disorder in ARDS Survivors: Validation of the Impact of Event Scale-6 (IES-6). Crit. Care 2019, 23, 276. [Google Scholar] [CrossRef]
- Griffiths, R.D.; Jones, C. Seven Lessons from 20 Years of Follow-up of Intensive Care Unit Survivors. Curr. Opin. Crit. Care 2007, 13, 508–513. [Google Scholar] [CrossRef]
- Mantziou, V.; Vrettou, C.S.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Family Burden of ICU Survivors and Correlations with Patient Quality of Life and Psychometric Scores—A Pilot Study. J Crit Care Med. (Targu Mures) 2022, 8, 242–248. [Google Scholar] [CrossRef]
- Plotnikoff, K.M.; Krewulak, K.D.; Hernández, L.; Spence, K.; Foster, N.; Longmore, S.; Straus, S.E.; Niven, D.J.; Parsons Leigh, J.; Stelfox, H.T.; et al. Patient Discharge from Intensive Care: An Updated Scoping Review to Identify Tools and Practices to Inform High-Quality Care. Crit. Care 2021, 25, 438. [Google Scholar] [CrossRef]
- Hauschildt, K.E.; Hechtman, R.K.; Prescott, H.C.; Iwashyna, T.J. Hospital Discharge Summaries Are Insufficient Following ICU Stays: A Qualitative Study. Crit. Care Explor. 2022, 4, e0715. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettou, C.S.; Mantelou, A.G. Supporting Post-ICU Recovery: A Narrative Review for General Practitioners. Diseases 2025, 13, 183. https://doi.org/10.3390/diseases13060183
Vrettou CS, Mantelou AG. Supporting Post-ICU Recovery: A Narrative Review for General Practitioners. Diseases. 2025; 13(6):183. https://doi.org/10.3390/diseases13060183
Chicago/Turabian StyleVrettou, Charikleia S., and Athina G. Mantelou. 2025. "Supporting Post-ICU Recovery: A Narrative Review for General Practitioners" Diseases 13, no. 6: 183. https://doi.org/10.3390/diseases13060183
APA StyleVrettou, C. S., & Mantelou, A. G. (2025). Supporting Post-ICU Recovery: A Narrative Review for General Practitioners. Diseases, 13(6), 183. https://doi.org/10.3390/diseases13060183







