Toxic Shock Syndrome in a 45-Year-Old Woman Possibly Associated with Tampon Use: A Case Report of Multiorgan Failure Due to Streptococcus agalactiae
Abstract
1. Introduction
2. Case Description
3. Discussion
3.1. Case-Based Analysis and Literature Context
3.2. Pathophysiological Considerations
3.3. Differential Diagnosis and Clinical Implications
3.4. Comparison with Previous Reports
3.5. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCoy, A.; Das, R. Reducing patient mortality, length of stay and readmissions through machine learning-based sepsis prediction in the emergency department, intensive care unit and hospital floor units. BMJ Open Qual. 2017, 6, e000158. [Google Scholar] [CrossRef] [PubMed]
- Coopersmith, C.M.M.; De Backer, D.M.; Deutschman, C.S.M.; Ferrer, R.; Lat, I.; Machado, F.R.; Martin, G.S.M.; Martin-Loeches, I.; Nunnally, M.E.M.; Antonelli, M.; et al. Surviving Sepsis Campaign: Research Priorities for Sepsis and Septic Shock. Crit. Care Med. 2018, 46, 1334–1356. [Google Scholar] [CrossRef]
- Ross, A.; Shoff, H.W. Toxic Shock Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Touaitia, R.; Ibrahim, N.A.; Almuqri, E.A.; Basher, N.S.; Idres, T.; Touati, A. Toxic Shock Syndrome Toxin-1 (TSST-1) in Staphylococcus aureus: Prevalence, Molecular Mechanisms, and Public Health Implications. Toxins 2025, 17, 323. [Google Scholar] [CrossRef]
- Dufresne, K.; Al, K.F.; Craig, H.C.; Coleman, C.E.M.; Kasper, K.J.; Burton, J.P.; McCormick, J.K. TSST-1 promotes colonization of Staphylococcus aureus within the vaginal tract by activation of CD8+ T cells. Infect. Immun. 2025, 93, e0043924. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, J.; Chen, T.; Xu, D.; Hou, F.; Huang, Q.; Peng, Y.; Ye, C.; Hu, D.-L.; Fang, R. Toxic Shock Syndrome Toxin 1 Induces Immune Response via the Activation of NLRP3 Inflammasome. Toxins 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Y.; Zhao, H.; Wang, X.; Rao, L.; Guo, Y.; Yi, X.; Hu, L.; Chen, S.; Han, L.; et al. Methicillin-resistant Staphylococcus aureus in China: A multicentre longitudinal study and whole-genome sequencing. Emerg. Microbes Infect. 2022, 11, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, B.; Jirholt, P.; Henning, P.; Lindholm, C.; Ohlsson, C.; McInnes, I.B.; Lerner, U.H.; Gjertsson, I. Antibiotics with Interleukin-15 Inhibition Reduce Joint Inflammation and Bone Erosions but Not Cartilage Destruction in Staphylococcus aureus-Induced Arthritis. Infect. Immun. 2018, 86, e00960-17. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Roller, R.J.; Kilgore, S.H.; Villarreal, M.; Klingelhutz, A.J.; Leung, D.Y.M. Staphylococcal TSST-1 Association with Eczema Herpeticum in Humans. mSphere 2021, 6, e0060821. [Google Scholar] [CrossRef]
- Sapugahawatte, D.N.; Li, C.; Yeoh, Y.K.; Dharmaratne, P.; Zhu, C.; Ip, M. Swine methicillin-resistant Staphylococcus aureus carrying toxic-shock syndrome toxin gene in Hong Kong, China. Emerg. Microbes Infect. 2020, 9, 1534–1536. [Google Scholar] [CrossRef]
- Armeftis, C.; Ioannou, A.; Lazarou, T.; Giannopoulos, A.; Dimitriadou, E.; Makrides, K.; Pana, Z.D. Staphylococcus epidermidis induced toxic shock syndrome (TSS) secondary to influenza infection. BMC Infect. Dis. 2023, 23, 1–6. [Google Scholar] [CrossRef]
- EUCAST. EUCAST Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. Available online: http://www.eucast.org (accessed on 13 November 2025).
- Zulaika, G.; Otieno, F.O.; Mason, L.; van Eijk, A.M.; Bhaumik, R.; Green, S.J.; Phillips-Howard, P.A.; Mehta, S.D. Menstrual cups to reduce bacterial vaginosis and STIs through reduced harmful sexual and menstrual practices among economically vulnerable women: Protocol of a single arm trial in western Kenya. BMC Public Health 2024, 24, 3089. [Google Scholar] [CrossRef]
- Hamilton, S.M.; Bayer, C.R.; Stevens, D.L.; Lieber, R.L.; Bryant, A.E. Muscle Injury, Vimentin Expression, and Nonsteroidal Anti-inflammatory Drugs Predispose to Cryptic Group A Streptococcal Necrotizing Infection. J. Infect. Dis. 2008, 198, 1692–1698. [Google Scholar] [CrossRef]
- Hochwalt, A.E.; Abbinante-Nissen, J.M.; Bohman, L.C.; Hattersley, A.M.; Hu, P.; Streicher-Scott, J.L.; Teufel, A.G.; Woeller, K.E. The safety assessment of tampons: Illustration of a comprehensive approach for four different products. Front. Reprod. Heal. 2023, 5, 1167868. [Google Scholar] [CrossRef]
- Chiaruzzi, M.; Barbry, A.; Muggeo, A.; Tristan, A.; Jacquemond, I.; Badiou, C.; Cluzeau, L.; Bourdeau, S.; Durand, T.; Engelmann, A.; et al. Vaginal Tampon Colonization by Staphylococcus aureus in Healthy Women. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Kaiser, R.; Bauer, J. Menstrual Cup-Associated Toxic Shock Syndrome. Eur. J. Case Rep. Intern. Med. 2020, 7, 001825. [Google Scholar] [CrossRef]
- Contou, D.; Colin, G.; Travert, B.; Jochmans, S.; Conrad, M.; Lascarrou, J.-B.; Painvin, B.; Ferré, A.; Schnell, D.; La Combe, B.; et al. Menstrual Toxic Shock Syndrome: A French Nationwide Multicenter Retrospective Study. Clin. Infect. Dis. 2021, 74, 246–253. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Gaitán, A.V.; Kilgore, S.H.; Roe, A.L.; Maukonen, J.; Lehtoranta, L.; Leung, D.Y.M.; Marsman, D.S. Inhibition of Toxic Shock Syndrome-Associated Staphylococcus aureus by Probiotic Lactobacilli. Microbiol. Spectr. 2023, 11, e0173523. [Google Scholar] [CrossRef] [PubMed]
- Lappin, E.; Ferguson, A.J. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 2009, 9, 281–290. [Google Scholar] [CrossRef]
- Taki, Y.; Watanabe, S.; Sato’o, Y.; Tan, X.-E.; Ono, H.K.; Kiga, K.; Aiba, Y.; Sasahara, T.; Azam, A.H.; Thitiananpakorn, K.; et al. The Association Between Onset of Staphylococcal Non-menstrual Toxic Shock Syndrome With Inducibility of Toxic Shock Syndrome Toxin-1 Production. Front. Microbiol. 2022, 13, 765317. [Google Scholar] [CrossRef] [PubMed]
- Font-Font, M.; Bellés-Bellés, A.; Fernández-Fernández, R.; Torres, C. Molecular characterization of Staphylococcus aureus causing menstrual toxic shock syndrome in a young woman. Enfermedades Infecc. y Microbiol. Clin. (English ed.) 2023, 41, 311–312. [Google Scholar] [CrossRef]
- Maduta, C.S.; McCormick, J.K.; Dufresne, K. Vaginal community state types (CSTs) alter environmental cues and production of the Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1). J. Bacteriol. 2024, 206, e0044723. [Google Scholar] [CrossRef] [PubMed]
- Billon, A.; Gustin, M.-P.; Tristan, A.; Bénet, T.; Berthiller, J.; Gustave, C.A.; Vanhems, P.; Lina, G. Association of characteristics of tampon use with menstrual toxic shock syndrome in France. eClinicalMedicine 2020, 21, 100308. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Everett, E.D.; Dellinger, P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft-Tissue Infections. Clin. Infect. Dis. 2005, 41, 1373–1406. [Google Scholar] [CrossRef]
- Tsuchihashi, Y.; Tamura, K.; Matsumoto, K.; Mitsushima, S.; Fujiya, Y.; Kuronuma, K.; Tanabe, Y.; Kasahara, K.; Maruyama, T.; Gotoh, K.; et al. Comparative analysis of streptococcal toxic shock syndrome caused by three β-hemolytic streptococcal species in Japan. Int. J. Infect. Dis. 2025, 158, 107962. [Google Scholar] [CrossRef]
- Inada, M.; Iwamoto, N.; Nomoto, H.; Tsuzuki, S.; Takemoto, N.; Fuwa, N.; Moriya, A.; Ohmagari, N. Characteristics of Streptococcal Toxic Shock Syndrome Caused by Different Beta-hemolytic Streptococci Species: A Single-center Retrospective Study. Open Forum Infect. Dis. 2024, 11, ofae486. [Google Scholar] [CrossRef]
- Kawai, S.; Miyoshi-Akiyama, T.; Katano, H.; Sunagawa, K. Invasive Streptococcus agalactiae (group B streptococcus) infection with toxic shock-like syndrome: A report of a fatal non-pregnant case and a review of the literature. J. Infect. Chemother. 2023, 30, 71–76. [Google Scholar] [CrossRef]
- Yun, A.E.; Johnson, L.B. Recurrent streptococcal toxic shock syndrome due to distinct episodes of Streptococcus dysgalactiae and Streptococcus agalactiae septic arthritis. BMJ Case Rep. 2024, 17, e260409. [Google Scholar] [CrossRef] [PubMed]
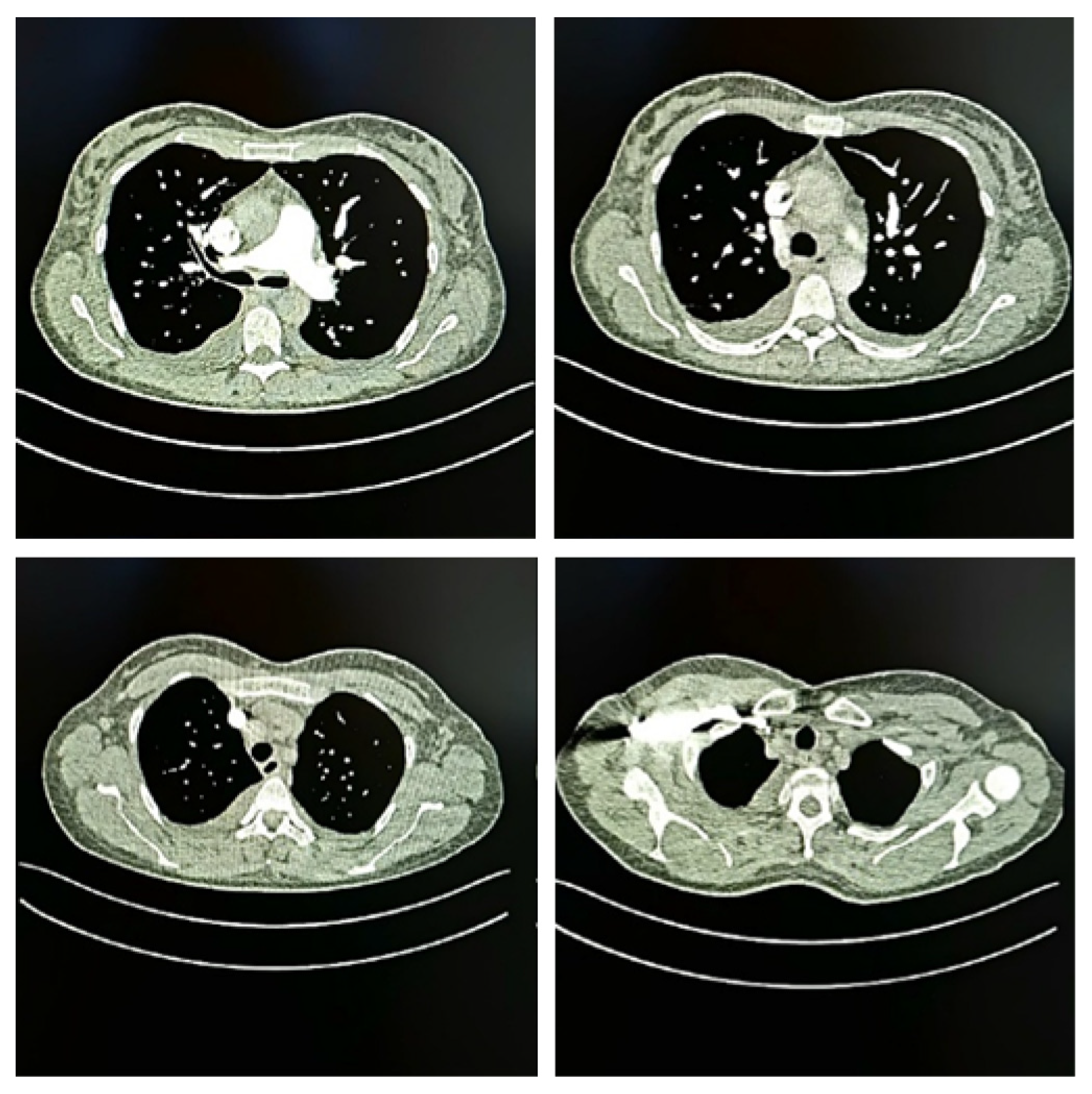
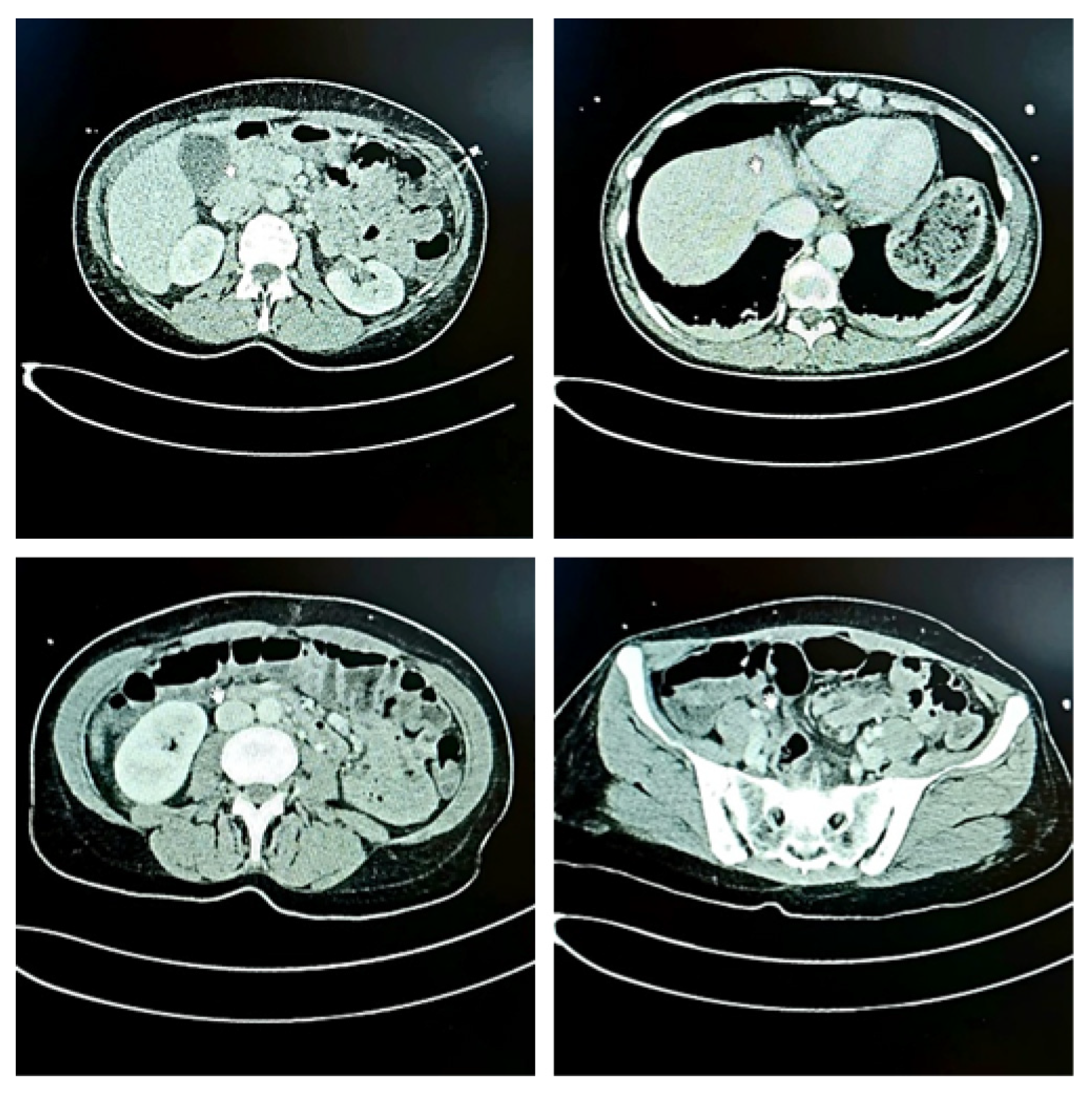
| Parameter | Value | Reference Range | Interpretation |
|---|---|---|---|
| Temperature | 39.6 °C | — | High fever |
| Blood pressure | 90/55 mmHg | >100/60 mmHg | Hypotension |
| Heart rate | 110 bpm | 60–100 bpm | Tachycardia |
| Platelets | 60 × 10⁹/L | 150–400 × 10⁹/L | Thrombocytopenia |
| CRP | 194.2 mg/L | <5 mg/L | Markedly elevated |
| PCT | 1.07 ng/mL | <0.05 ng/mL | Elevated |
| AST/ALT | 61/58 U/L | <40 U/L | Mild hepatic dysfunction |
| ALP/GGT | 364/275 U/L | <120/<40 U/L | Cholestatic elevation |
| eGFR | 90 mL/min | >90 mL/min | Normal renal function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavidić, T.; Dejhalla, E.; Zahirović, D. Toxic Shock Syndrome in a 45-Year-Old Woman Possibly Associated with Tampon Use: A Case Report of Multiorgan Failure Due to Streptococcus agalactiae. Diseases 2025, 13, 376. https://doi.org/10.3390/diseases13110376
Zavidić T, Dejhalla E, Zahirović D. Toxic Shock Syndrome in a 45-Year-Old Woman Possibly Associated with Tampon Use: A Case Report of Multiorgan Failure Due to Streptococcus agalactiae. Diseases. 2025; 13(11):376. https://doi.org/10.3390/diseases13110376
Chicago/Turabian StyleZavidić, Tina, Ema Dejhalla, and David Zahirović. 2025. "Toxic Shock Syndrome in a 45-Year-Old Woman Possibly Associated with Tampon Use: A Case Report of Multiorgan Failure Due to Streptococcus agalactiae" Diseases 13, no. 11: 376. https://doi.org/10.3390/diseases13110376
APA StyleZavidić, T., Dejhalla, E., & Zahirović, D. (2025). Toxic Shock Syndrome in a 45-Year-Old Woman Possibly Associated with Tampon Use: A Case Report of Multiorgan Failure Due to Streptococcus agalactiae. Diseases, 13(11), 376. https://doi.org/10.3390/diseases13110376





