Survival Trends for Uterine Sarcomas from a Tertiary Center: The Oxford Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Considerations
2.2. Data Collection
2.3. Endpoints and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
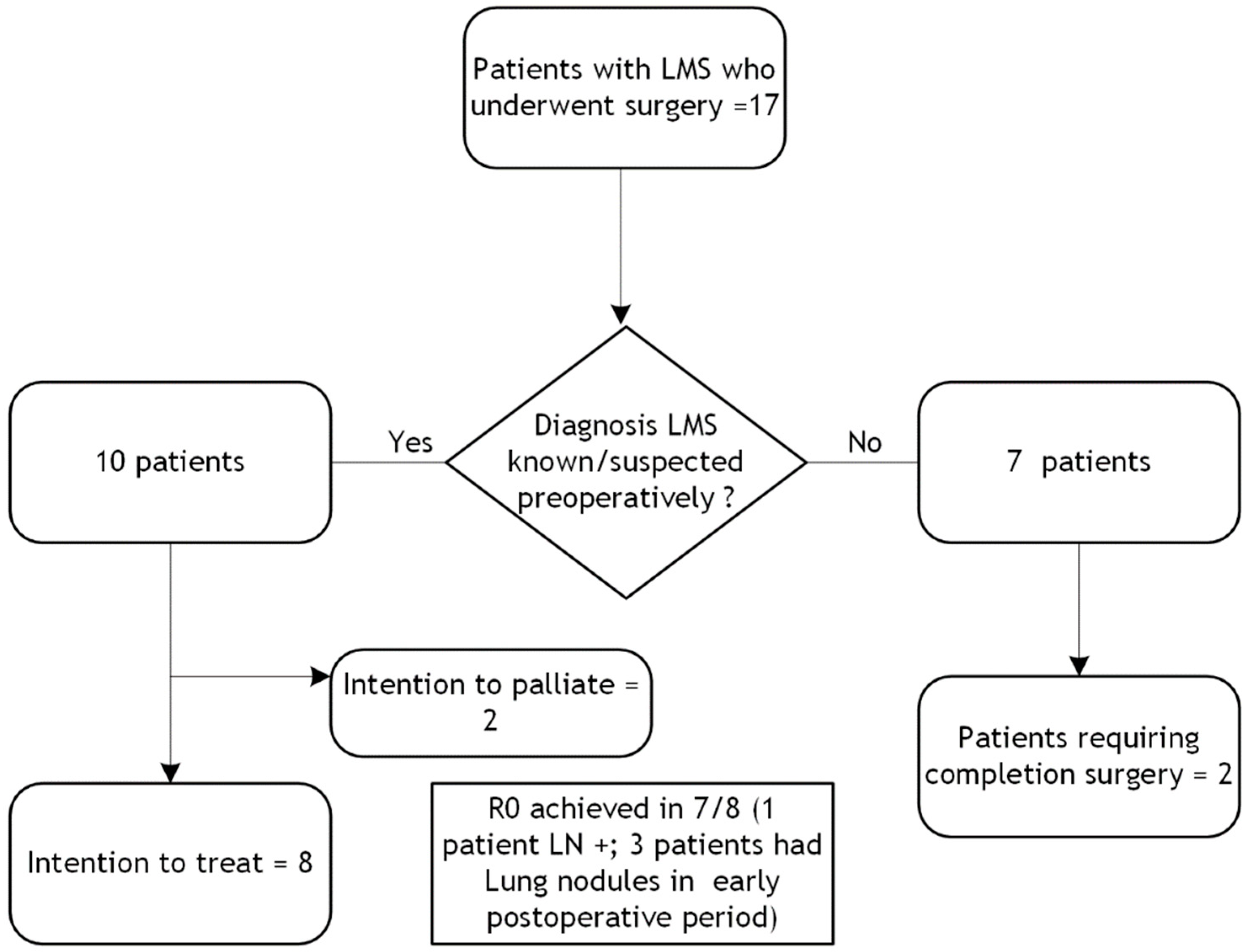
3.2. Management and Follow-Up for Leiomyosarcoma
3.3. Management and Follow-Up for Adenosarcoma
3.4. Management and Follow-Up for Endometrial Stromal Sarcoma
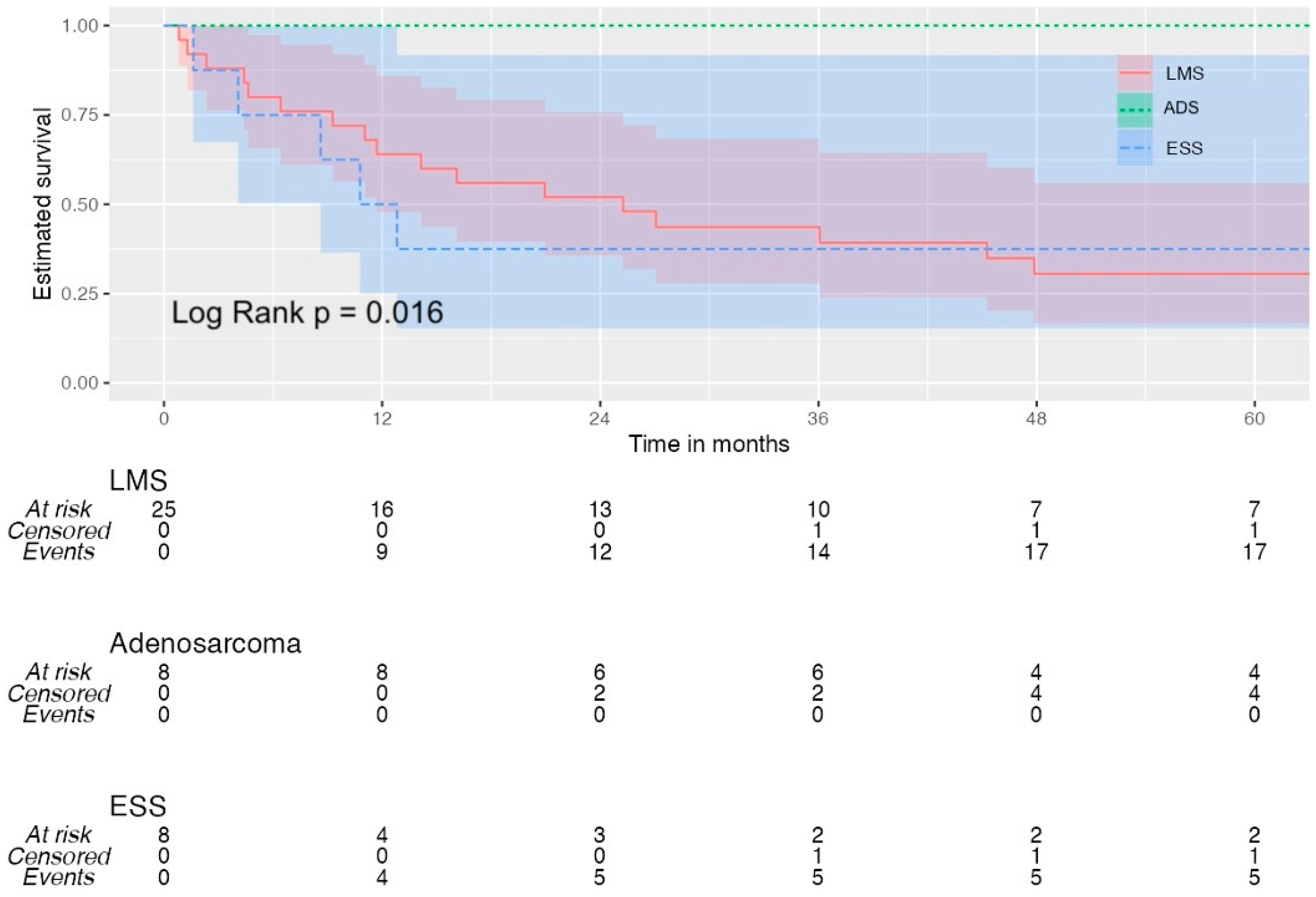
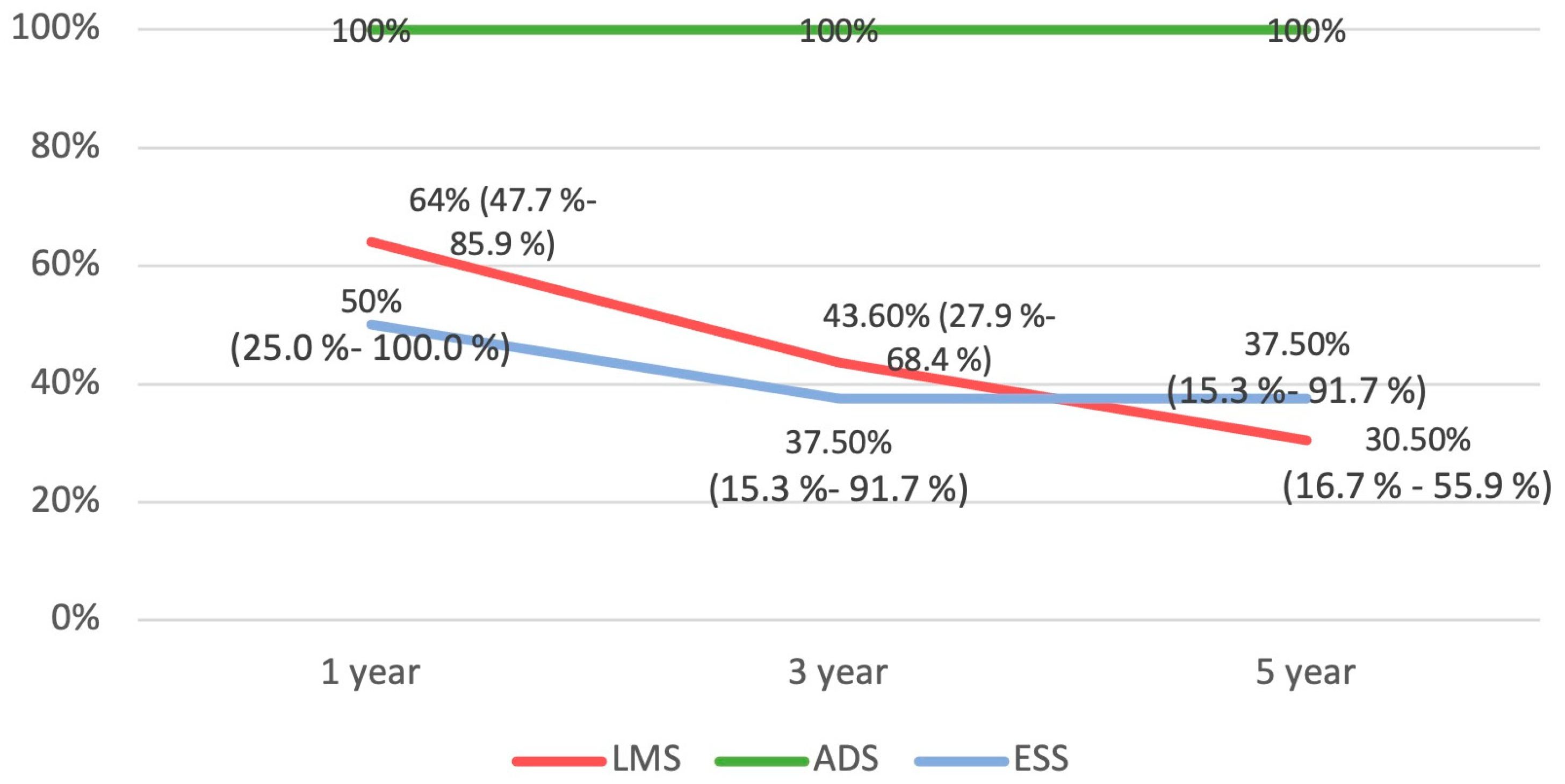
3.5. Survival Analysis
4. Discussion
4.1. General Characteristics of Uterine Sarcomas
4.2. Follow-Up Regimens
4.3. Limitations of This Study
4.4. Future Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Angelo, E.; Prat, J. Uterine Sarcomas: A Review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Uterine Myomas: An Overview of Development, Clinical Features, and Management. Obstet. Gynecol. 2005, 105, 216–217. [Google Scholar] [CrossRef]
- Brooks, S.E.; Zhan, M.; Cote, T.; Baquet, C.R. Surveillance, Epidemiology, and End Results Analysis of 2677 Cases of Uterine Sarcoma 1989–1999. Gynecol. Oncol. 2004, 93, 204–208. [Google Scholar] [CrossRef]
- Major, F.J.; Blessing, J.A.; Silverberg, S.G.; Morrow, C.P.; Creasman, W.T.; Currie, J.L.; Yordan, E.; Brady, M.F. Prognostic Factors in Early-stage Uterine Sarcoma: A Gynecologic Oncology Group Study. Cancer 1993, 71, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Mbatani, N.; Olawaiye, A.B.; Prat, J. Uterine Sarcomas. Int. J. Gynaecol. Obstet 2018, 143 (Suppl. S2), 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hanby, A.M.; Walker, C. Tavassoli FA, Devilee P: Pathology and Genetics: Tumours of the Breast and Female Genital Organs. WHO Classification of Tumours Series-Volume IV. Lyon, France: IARC Press. Breast Cancer Res. 2004, 6, 133. [Google Scholar] [CrossRef]
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO Classification: Female Genital Tract Tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef]
- Parkash, V.; Aisagbonhi, O.; Riddle, N.; Siddon, A.; Panse, G.; Fadare, O. Recent Advances in the Classification of Gynecological Tract Tumors: Updates from the 5th Edition of the World Health Organization “Blue Book”. Arch. Pathol. Lab. Med. 2023, 147, 1204–1216. [Google Scholar] [CrossRef]
- Santos, P.; Cunha, T.M. Uterine Sarcomas: Clinical Presentation and MRI Features. Diagn. Interv. Radiol. 2015, 21, 4–9. [Google Scholar] [CrossRef]
- Puliyath, G.; Nair, M.K. Endometrial Stromal Sarcoma: A Review of the Literature. Indian J. Med. Paediatr. Oncol. 2012, 33, 1–6. [Google Scholar] [CrossRef]
- Borella, F.; Mancarella, M.; Preti, M.; Mariani, L.; Stura, I.; Sciarrone, A.; Bertschy, G.; Leuzzi, B.; Piovano, E.; Valabrega, G.; et al. Uterine Smooth Muscle Tumors: A Multicenter, Retrospective, Comparative Study of Clinical and Ultrasound Features. Int. J. Gynecol. Cancer 2023, 34, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in Gynecological Disease (15): Clinical and Ultrasound Characteristics of Uterine Sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Tropé, C.G.; Abeler, V.M.; Kristensen, G.B. Diagnosis and Treatment of Sarcoma of the Uterus. A Review. Acta Oncol. 2012, 51, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, V.; Bizzarri, N.; Guida, F.; Vascone, C.; Costantini, B.; Scambia, G.; Fagotti, A. Role of Surgery in Gynaecological Sarcomas. Oncotarget 2019, 10, 2561–2575. [Google Scholar] [CrossRef]
- Cantú De León, D.; González, H.; Pérez Montiel, D.; Coronel, J.; Pérez-Plasencia, C.; Villavicencio-Valencia, V.; Soto-Reyes, E.; Herrera, L.A. Uterine Sarcomas: Review of 26 Years at The Instituto Nacional de Cancerologia of Mexico. Int. J. Surg. 2013, 11, 518–523. [Google Scholar] [CrossRef][Green Version]
- Giuntoli, R.L.; Garrett-Mayer, E.; Bristow, R.E.; Gostout, B.S. Secondary Cytoreduction in the Management of Recurrent Uterine Leiomyosarcoma. Gynecol. Oncol. 2007, 106, 82–88. [Google Scholar] [CrossRef]
- Oliva, E. Cellular Mesenchymal Tumors of the Uterus: A Review Emphasizing Recent Observations. Int. J. Gynecol. Pathol. 2014, 33, 374–384. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; Von Elm, E.; Langan, S.M. RECORD Working Committee The REporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Coosemans, A.; Debiec-Rychter, M.; Timmerman, D.; Vergote, I. Clinical Management of Uterine Sarcomas. Lancet Oncol. 2009, 10, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Minaya, M.; Mendizabal-Vicente, E.; Vasquez-Jimenez, W.; Perez-Burrel, L.; Aracil-Moreno, I.; Agra-Pujol, C.; Bernal-Claverol, M.; Martínez-Bernal, B.L.; Muñoz-Fernández, M.; Morote-Gonzalez, M.; et al. Retrospective Analysis of Patients with Gynaecological Uterine Sarcomas in a Tertiary Hospital. J. Pers. Med. 2022, 12, 222. [Google Scholar] [CrossRef]
- Bogani, G.; Cliby, W.A.; Aletti, G.D. Impact of Morcellation on Survival Outcomes of Patients with Unexpected Uterine Leiomyosarcoma: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2015, 137, 167–172. [Google Scholar] [CrossRef]
- Ricci, S.; Stone, R.L.; Fader, A.N. Uterine Leiomyosarcoma: Epidemiology, Contemporary Treatment Strategies and the Impact of Uterine Morcellation. Gynecol. Oncol. 2017, 145, 208–216. [Google Scholar] [CrossRef]
- Nano, O.; Nieto, M.J.; Saif, M.W.; Tarabichi, M. Retrospective Analysis of Patients with Gynecological Uterine Sarcomas: Leiomyosarcomas and Other Histological Subtypes at a Single Institution from 1996 to 2015. Cancer Med. J. 2020, 3 (Suppl. S2), 30–37. [Google Scholar]
- Kapp, D.S.; Shin, J.Y.; Chan, J.K. Prognostic Factors and Survival in 1396 Patients with Uterine Leiomyosarcomas: Emphasis on Impact of Lymphadenectomy and Oophorectomy. Cancer 2008, 112, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Kyriazoglou, A.; Liontos, M.; Ziogas, D.C.; Zagouri, F.; Koutsoukos, K.; Tsironis, G.; Tsiara, A.; Kaparelou, M.; Zakopoulou, R.; Thomakos, N.; et al. Management of Uterine Sarcomas and Prognostic Indicators: Real World Data from a Single-Institution. BMC Cancer 2018, 18, 1247. [Google Scholar] [CrossRef]
- Abeler, V.M.; Røyne, O.; Thoresen, S.; Danielsen, H.E.; Nesland, J.M.; Kristensen, G.B. Uterine Sarcomas in Norway. A Histopathological and Prognostic Survey of a Total Population from 1970 to 2000 Including 419 Patients. Histopathology 2009, 54, 355–364. [Google Scholar] [CrossRef]
- Kostov, S.; Kornovski, Y.; Ivanova, V.; Dzhenkov, D.; Metodiev, D.; Watrowski, R.; Ivanova, Y.; Slavchev, S.; Mitev, D.; Yordanov, A. New Aspects of Sarcomas of Uterine Corpus—A Brief Narrative Review. Clin. Pract. 2021, 11, 878–900. [Google Scholar] [CrossRef]
- Horng, H.-C.; Wen, K.-C.; Wang, P.-H.; Chen, Y.-J.; Yen, M.-S.; Ng, H.-T. Taiwan Association of Gynecology Systematic Review Group Uterine Sarcoma Part II-Uterine Endometrial Stromal Sarcoma: The TAG Systematic Review. Taiwan J. Obstet. Gynecol. 2016, 55, 472–479. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft Tissue and Visceral Sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Cui, R.R.; Wright, J.D.; Hou, J.Y. Uterine Leiomyosarcoma: A Review of Recent Advances in Molecular Biology, Clinical Management and Outcome. BJOG 2017, 124, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Liau, J.-Y.; Huang, W.-J.; Chang, Y.-T.; Chang, M.-C.; Lee, J.-C.; Tsai, J.-H.; Su, Y.-N.; Hung, C.-C.; Jeng, Y.-M. Targeted Next-Generation Sequencing of Cancer Genes Identified Frequent TP53 and ATRX Mutations in Leiomyosarcoma. Am. J. Transl. Res. 2015, 7, 2072–2081. [Google Scholar] [PubMed]
- An, Y.; Wang, S.; Li, S.; Zhang, L.; Wang, D.; Wang, H.; Zhu, S.; Zhu, W.; Li, Y.; Chen, W.; et al. Distinct Molecular Subtypes of Uterine Leiomyosarcoma Respond Differently to Chemotherapy Treatment. BMC Cancer 2017, 17, 639. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Fucà, G.; Maltese, G.; Ditto, A.; Martinelli, F.; Signorelli, M.; Chiappa, V.; Scaffa, C.; Sabatucci, I.; Lecce, F.; et al. Efficacy of Adjuvant Chemotherapy in Early Stage Uterine Leiomyosarcoma: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2016, 143, 443–447. [Google Scholar] [CrossRef]
- Roque, D.R.; Taylor, K.N.; Palisoul, M.; Wysham, W.Z.; Milam, B.; Robison, K.; Gehrig, P.A.; Raker, C.; Kim, K.H. Gemcitabine and Docetaxel Compared with Observation, Radiation, or Other Chemotherapy Regimens as Adjuvant Treatment for Stage I-to-IV Uterine Leiomyosarcoma. Int. J. Gynecol. Cancer 2016, 26, 505–511. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Dima, S.; Brasoveanu, V.; Popescu, I. Prognostic Factors and Survival in Patients Treated Surgically for Primary and Recurrent Uterine Leiomyosarcoma: A Single Center Experience. Anticancer Res. 2015, 35, 2229–2234. [Google Scholar]
- Koh, W.-J.; Greer, B.E.; Abu-Rustum, N.R.; Apte, S.M.; Campos, S.M.; Cho, K.R.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S.; et al. Uterine Sarcoma, Version 1.2016: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2015, 13, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.J.; Lecumberri, M.J.; Varela, S.; Alarcón, J.; Ortega, M.E.; Gaba, L.; Espinós, J.; Calzas, J.; Barretina, P.; Ruiz, I.; et al. Efficacy and Safety of Trabectedin in Metastatic Uterine Leiomyosarcoma: A Retrospective Multicenter Study of the Spanish Ovarian Cancer Research Group (GEICO). Gynecol. Oncol. Rep. 2020, 33, 100594. [Google Scholar] [CrossRef]
- Hardman, M.P.; Roman, J.J.; Burnett, A.F.; Santin, A.D. Metastatic Uterine Leiomyosarcoma Regression Using an Aromatase Inhibitor. Obstet. Gynecol. 2007, 110, 518. [Google Scholar] [CrossRef]
- Roy, M.; Musa, F.; Taylor, S.E.; Huang, M. Uterine Sarcomas: How to Navigate an Ever-Growing List of Subtypes. Am. Soc. Clin. Oncol. Educ. Book 2022, 49, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, S.; Cai, J.; Yang, L.; Lv, G.; Yang, Q. Uterine Adenosarcoma: A Case Report and Review of the Literature. Am. J. Nucl. Med. Mol. Imaging 2023, 13, 70–76. [Google Scholar] [PubMed]
- Arend, R.; Bagaria, M.; Lewin, S.N.; Sun, X.; Deutsch, I.; Burke, W.M.; Herzog, T.J.; Wright, J.D. Long-Term Outcome and Natural History of Uterine Adenosarcomas. Gynecol. Oncol. 2010, 119, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Bernard, B.; Clarke, B.A.; Malowany, J.I.; McAlpine, J.; Lee, C.-H.; Atenafu, E.G.; Ferguson, S.; Mackay, H. Uterine Adenosarcomas: A Dual-Institution Update on Staging, Prognosis and Survival. Gynecol. Oncol. 2013, 131, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.L.; Covens, A.; Glasspool, R.M.; Hilpert, F.; Kristensen, G.; Kwon, S.; Selle, F.; Small, W.; Witteveen, E.; Russell, P. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Mullerian Adenosarcoma of the Female Genital Tract. Int. J. Gynecol. Cancer 2014, 24, S78–S82. [Google Scholar] [CrossRef]
- Ulrich, U.A.; Denschlag, D. Uterine Adenosarcoma. Oncol. Res. Treat. 2018, 41, 693–696. [Google Scholar] [CrossRef]
- Pinto, A.; Howitt, B. Uterine Adenosarcoma. Arch. Pathol. Lab. Med. 2016, 140, 286–290. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Z.; Peng, Z.; Liu, H.; Yang, K.; Yao, X. The Diagnosis and Treatment of Mullerian Adenosarcoma of the Uterus. Aust. NZJ Obstet. Gynaecol. 2008, 48, 596–600. [Google Scholar] [CrossRef]
- Sundar, S.; Balega, J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; et al. BGCS Uterine Cancer Guidelines: Recommendations for Practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 71–97. [Google Scholar] [CrossRef]
- Astolfi, A.; Nannini, M.; Indio, V.; Schipani, A.; Rizzo, A.; Perrone, A.M.; De Iaco, P.; Pirini, M.G.; De Leo, A.; Urbini, M.; et al. Genomic Database Analysis of Uterine Leiomyosarcoma Mutational Profile. Cancers 2020, 12, 2126. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Chavan, S.S.; Solit, D.B.; Murali, R.; Soslow, R.; Chiang, S.; Jungbluth, A.A.; Bandlamudi, C.; Srinivasan, P.; Tap, W.D.; et al. Genomic Landscape of Uterine Sarcomas Defined Through Prospective Clinical Sequencing. Clin. Cancer Res. 2020, 26, 3881–3888. [Google Scholar] [CrossRef] [PubMed]

| All Uterine Sarcomas (n = 41) | LMS (n = 25) | ADS (n = 8) | ESS (n = 8) | |
|---|---|---|---|---|
| Age (mean ± SD) | 56.82 (±13.12) | 53.76 (±12.45) | 54.75 (±9.08) | 68.50 (±12.02) |
| Charlson comorbidity Index | ||||
| 0–3 | 16 (39%) | 8 (32%) | 6 (75%) | 2 (25%) |
| 4–6 | 16 (39%) | 10 (40%) | 2 (25%) | 4 (50%) |
| >7 | 9 (21.9%) | 7 (28%) | 0 | 2 (25%) |
| Postmenopausal | 25 (61%) | 12 (48%) | 6 (75%) | 8 (100%) |
| FIGO Stage | ||||
| Stage I | 8 (32%) | 8 (100%) | 4 (50%) | |
| Stage II | 0 | 0 | 0 | |
| Stage III | 3 (12%) | 0 | 1 (12.5%) | |
| Stage IV | 14 (56%) | 0 | 3 (37.5%) | |
| BMI (mean ± SD) | 29.7 (±7.037) | 30.1 (±6.35) | 26.12 (±3.21) | 32.12 (±9.89) |
| Vaginal bleeding | 20 (34.1%) | 6 (24%) | 8 (100%) | 6 (75%) |
| Bulking pelvic mass | 17 (41.4%) | 16 (64%) | - | 1 (12.5%) |
| Systemic symptoms | - | 3 (12%) | - | - |
| Other symptoms | - | - | - | 1 (12.5%) |
| Histological/IHC Characteristics | Percentage of Sample Expressing the Characteristic |
|---|---|
| Desmin | 68% |
| Smooth muscle actin | 84% |
| Vimentin | 64% |
| Caldesmon | 48% |
| Ki 67 proliferation index high | 40% |
| p16 | 32% |
| CD10 | 20% |
| p53 | 20% |
| WT-1 | 8% |
| Necrosis | 60% |
| Mitosis >/= 30/100HPF | 28% |
| Mitosis counts 15–30/100HPF | 68% |
| Average tumor size * | 133 mm (30–250 mm) |
| ER-positive | 40% |
| PR-positive | 32% |
| All Uterine Sarcomas (n = 41) | LMS (n = 25) | ADS (n = 8) | ESS (n = 8) | |
|---|---|---|---|---|
| Treatment modality used | ||||
| Surgery alone | 20 (48.7%) | 7 (28%) | 8 (100%) | 5 (62.5%) |
| Surgery and adjuvant CT | 11 (26.8%) | 10 (40%) | 0 | 1 (12.5%) |
| Adjuvant CT modality * | ||||
| Single drug adjuvant CT | 2 (4.8%) | 1 (4%) | 0 | 1 (1.2%) |
| Multi drug adjuvant CT | 9 (21.9%) | 9 (36%) | 0 | 0 |
| Palliative CT | 7 (17.1%) | 7 (28%) | 0 | 0 |
| Surgery and adjuvant EBRT | 1 (2.4%) | 0 | 0 | 1 (1.2%) |
| Palliative radiotherapy | 2 (4.8%) | 1 (4%) | 0 | 1 (1.2%) |
| Disease-free patients | 18 (43.9%) | 6 (24%) | 8 (100%) | 4 (50%) |
| Recurrences ** | 8 (44.4%) | 6 (100%) | 0 | 2 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, A.; Ferrari, F.; Zouridis, A.; Kehoe, S.; Pratap, S.; Gozzini, E.; Soleymani Majd, H. Survival Trends for Uterine Sarcomas from a Tertiary Center: The Oxford Experience. Diseases 2024, 12, 200. https://doi.org/10.3390/diseases12090200
Aggarwal A, Ferrari F, Zouridis A, Kehoe S, Pratap S, Gozzini E, Soleymani Majd H. Survival Trends for Uterine Sarcomas from a Tertiary Center: The Oxford Experience. Diseases. 2024; 12(9):200. https://doi.org/10.3390/diseases12090200
Chicago/Turabian StyleAggarwal, Aakriti, Federico Ferrari, Andreas Zouridis, Sean Kehoe, Sarah Pratap, Elisa Gozzini, and Hooman Soleymani Majd. 2024. "Survival Trends for Uterine Sarcomas from a Tertiary Center: The Oxford Experience" Diseases 12, no. 9: 200. https://doi.org/10.3390/diseases12090200
APA StyleAggarwal, A., Ferrari, F., Zouridis, A., Kehoe, S., Pratap, S., Gozzini, E., & Soleymani Majd, H. (2024). Survival Trends for Uterine Sarcomas from a Tertiary Center: The Oxford Experience. Diseases, 12(9), 200. https://doi.org/10.3390/diseases12090200








