The Effect of COVID-19 and COVID-19 Vaccination on Assisted Human Reproduction Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Study Quality
2.5. Statistical Analysis
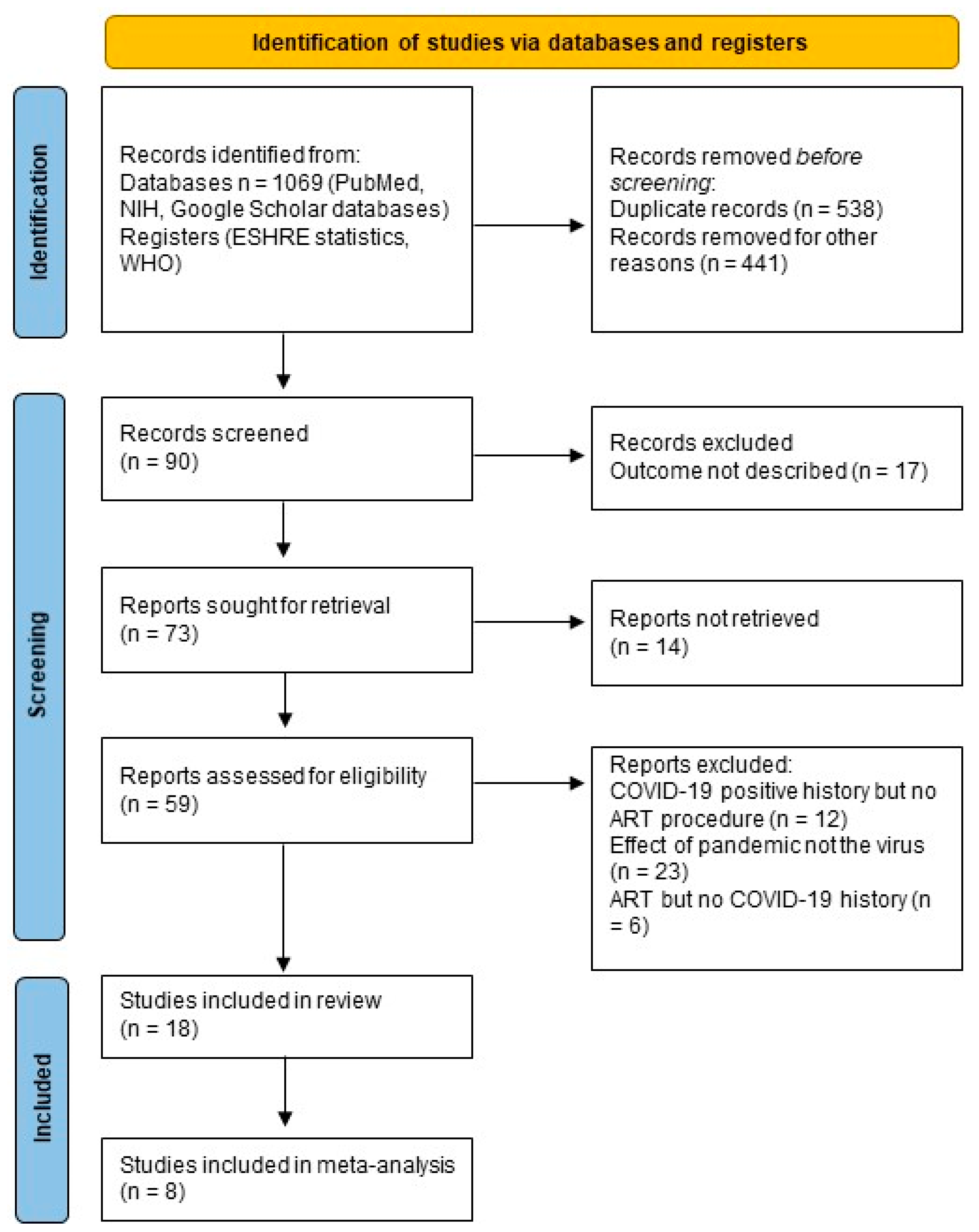
3. Results
3.1. Characteristics of the Reviewed Studies
| Study | Study Type | City, Country | Publication Date | No. COVID-19 Vaccinated Participants | Mean Age ± SD | Pregnancy | Birth | Miscarriage |
|---|---|---|---|---|---|---|---|---|
| Huang et al. [51] | retrospective cohort study | China | 9 February 2022 | 66 | 33.7 ± 5.6 | 39/66 (59.1) | ||
| Dong et al. [52] | prospective cohort study | China | 27 June 2022 | 735 infertile couples | 33.15 ± 3.55 | 70/132 (53.03) | ||
| Wang et al. [53] | cohort study | China | 16 December 2022 | 4185 couples | 31.49 (3.70) | 603 couples with clinical pregnancies | ||
| Chen et al. [54] | retrospective study | China | 11 January 2023 | 268 women with inactivated or recombinant COVID-19 | 33.32 ± 5.14 (inactivated) 33.00 ± 5.16 (recombinant) | (inactivated) 46/77 (57.94) (recombinant) 11/15 (3.33) | No data yet | (inactivated) 2/77 (2.60) (recombinant) 2/15 (13.33) |
| Yang et al. [55] | retrospective study | China | 11 October 2023 | 899 | 30.71 ± 3.84 | 141 | ||
| Chillon et al. [56] | observational study | Austria, Germany | 17 March 2023 | 45 | 35.53 (7.00%) | 1 (2.2%) | 6 (13%) | |
| Zhang et al. [57] | case-control study | China | 8 August 2023 | 1084 | 32.00 (30.00–35.00) | 248/1084 (22.88) | 180/1084 (16.61) | 70/504 (13.89) |
| TOTAL | 2362 4920 couples | 1159 | 186 | 74 |
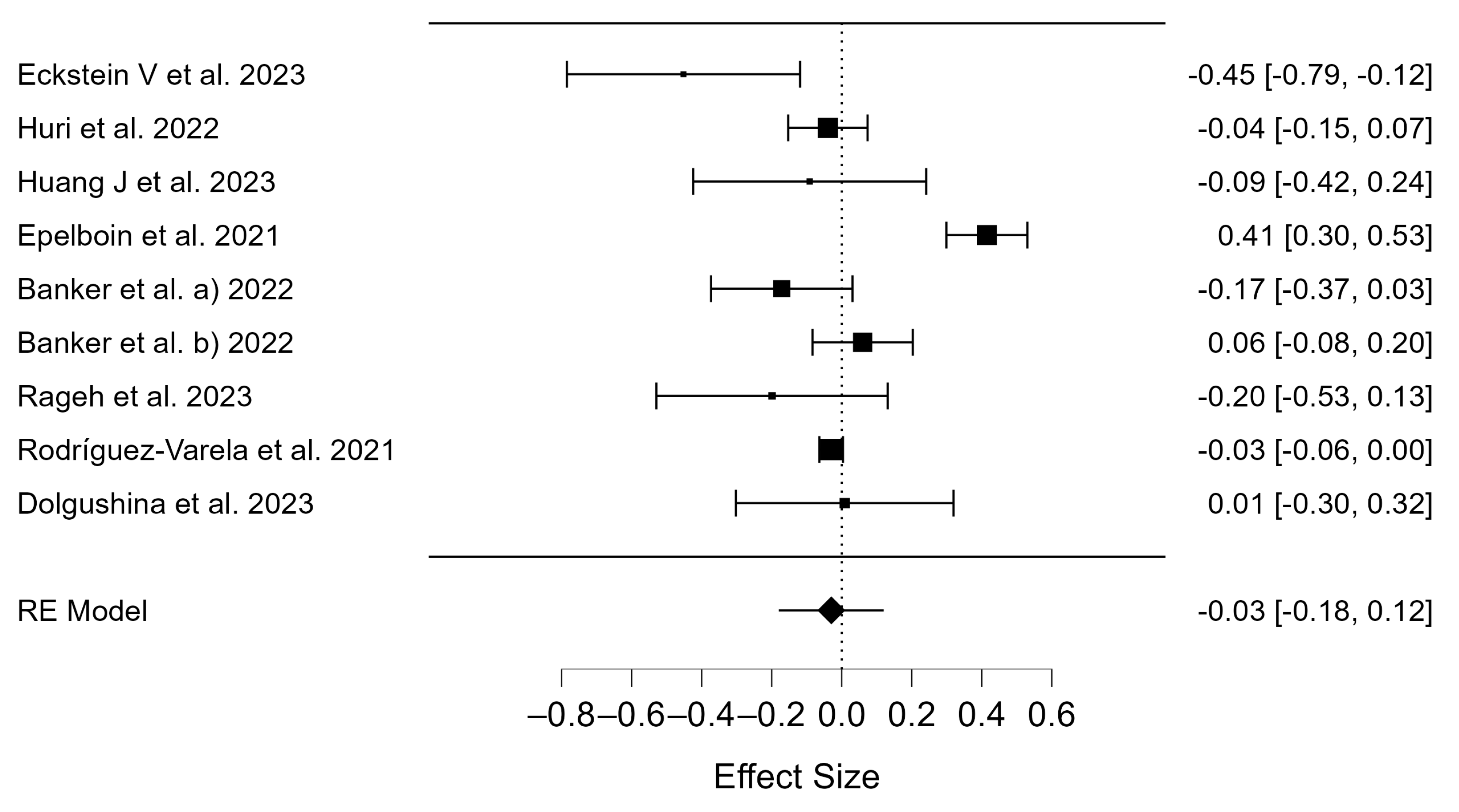
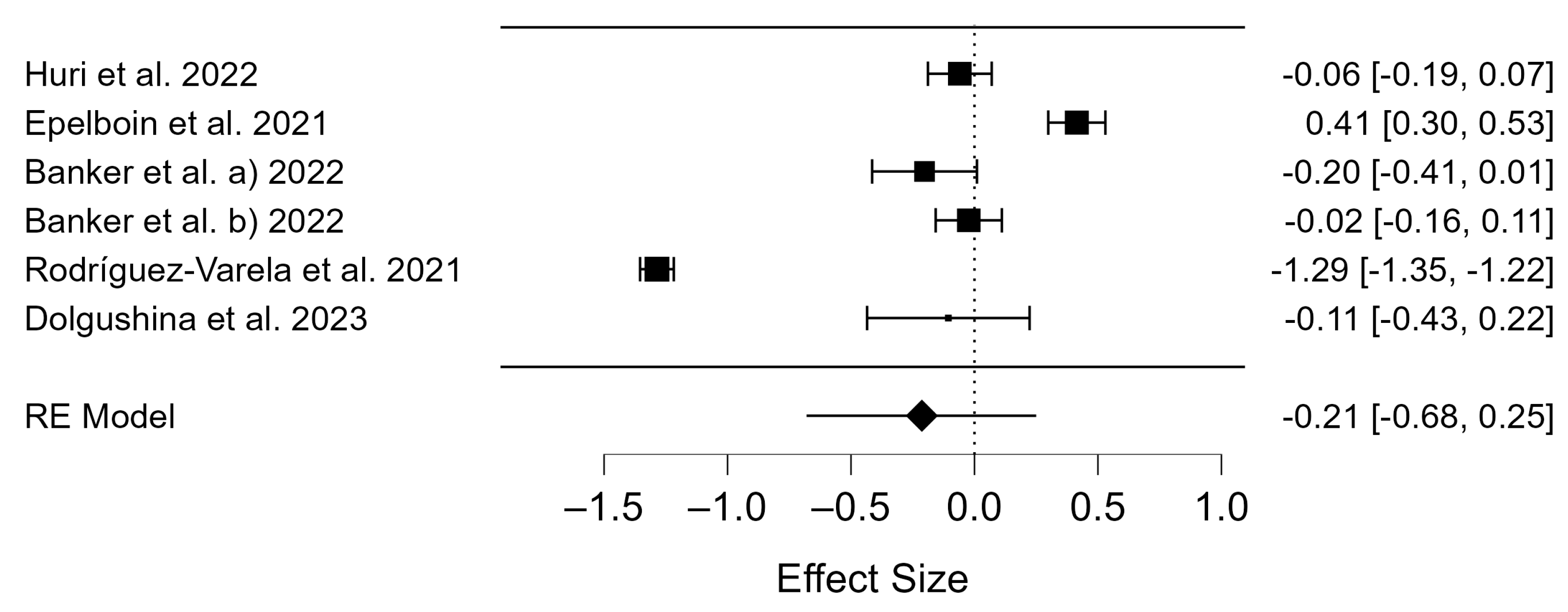
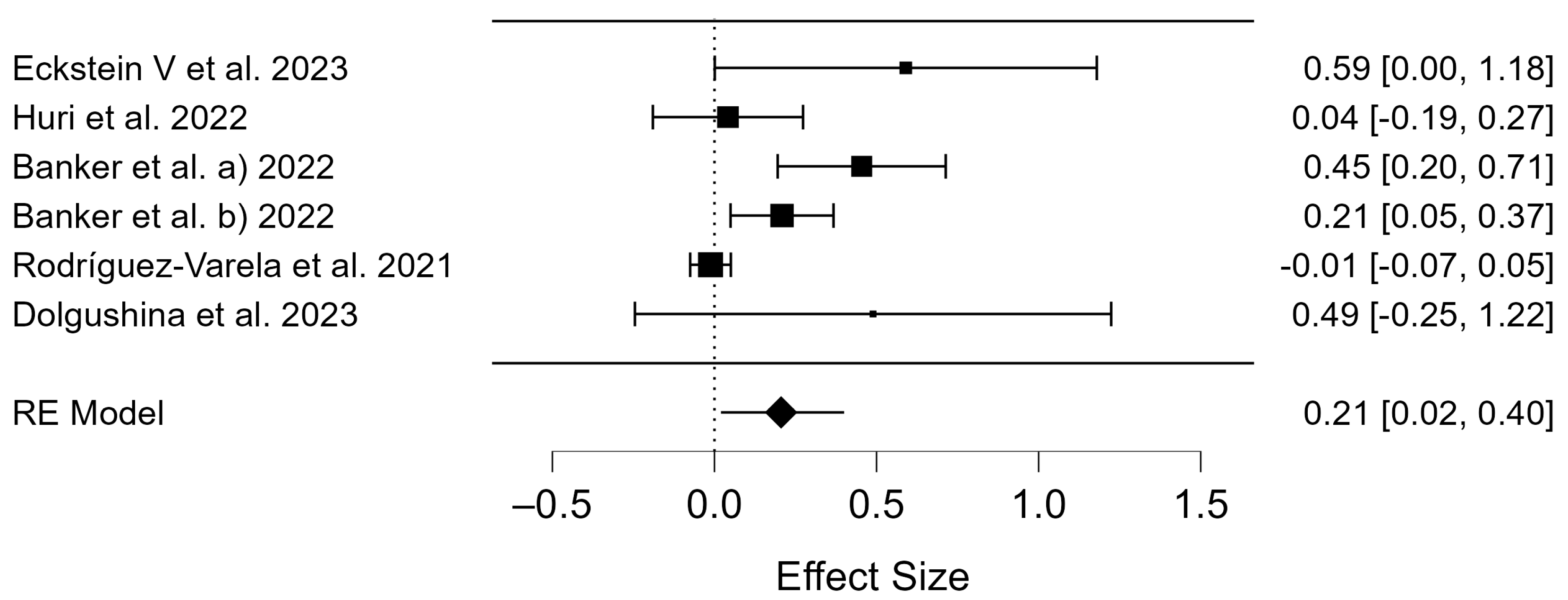
3.2. Meta-Analysis
3.3. Publication Bias
4. Discussion
4.1. Clinical Pregnancy Rate of COVID-19 Patients
4.2. Birth Rates of COVID-19 Patients
4.3. Miscarriage Rates of COVID-19 Patients
4.4. Clinical Pregnancy Rate of COVID-19 Vaccinated Patients
4.5. Birth Rates of COVID-19 Vaccinated Patients
4.6. Miscarriage Rates of COVID-19 Vaccinated Patients
4.7. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.E.K.; Daly, M.J.; Ganna, A. The Human Genetic Epidemiology of COVID-19. Nat. Rev. Genet. 2022, 23, 533–546. [Google Scholar] [CrossRef]
- Xue, Y.; Xiong, Y.; Cheng, X.; Li, K. Impact of SARS-CoV-2 Infection on Clinical Outcomes of in Vitro Fertilization Treatments: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1233986. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-nCoV Infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Kasuga, Y.; Zhu, B.; Jang, K.-J.; Yoo, J.-S. Innate Immune Sensing of Coronavirus and Viral Evasion Strategies. Exp. Mol. Med. 2021, 53, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 Distribution and Extrapulmonary Organ Injury in Patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef]
- Herrero, Y.; Pascuali, N.; Velázquez, C.; Oubiña, G.; Hauk, V.; de Zúñiga, I.; Peña, M.G.; Martínez, G.; Lavolpe, M.; Veiga, F.; et al. SARS-CoV-2 Infection Negatively Affects Ovarian Function in ART Patients. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166295. [Google Scholar] [CrossRef]
- Rajak, P.; Roy, S.; Dutta, M.; Podder, S.; Sarkar, S.; Ganguly, A.; Mandi, M.; Khatun, S. Understanding the Cross-Talk between Mediators of Infertility and COVID-19. Reprod. Biol. 2021, 21, 100559. [Google Scholar] [CrossRef]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and Multiorgan Failure: A Narrative Review on Potential Mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X. scRNA-Seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 2020, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New Understanding of the Damage of SARS-CoV-2 Infection Outside the Respiratory System. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Verrienti, P.; Cito, G.; Di Maida, F.; Tellini, R.; Cocci, A.; Minervini, A.; Natali, A. The Impact of COVID-19 on the Male Genital Tract: A Qualitative Literature Review of Sexual Transmission and Fertility Implications. Clin. Exp. Reprod. Med. 2022, 49, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Saksena, S.; Sadri-Ardekani, H. ACE2 Receptor Expression in Testes: Implications in Coronavirus Disease 2019 Pathogenesis†. Biol. Reprod. 2020, 103, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Mao, Y.; Xiong, Y.; Zhang, Y.; Zhang, M. Effect of SARS-CoV-2 Infection upon Male Gonadal Function: A Single Center-Based Study. MedRxiv 2020, 2020-03. [Google Scholar] [CrossRef]
- Bridwell, R.E.; Merrill, D.R.; Griffith, S.A.; Wray, J.; Oliver, J.J. A Coronavirus Disease 2019 (COVID-19) Patient with Bilateral Orchitis. Am. J. Emerg. Med. 2021, 42, 260.e3–260.e5. [Google Scholar] [CrossRef]
- Xu, J.; Qi, L.; Chi, X.; Yang, J.; Wei, X.; Gong, E.; Peh, S.; Gu, J. Orchitis: A Complication of Severe Acute Respiratory Syndrome (SARS)1. Biol. Reprod. 2006, 74, 410–416. [Google Scholar] [CrossRef]
- Gagliardi, L.; Bertacca, C.; Centenari, C.; Merusi, I.; Parolo, E.; Ragazzo, V.; Tarabella, V. Orchiepididymitis in a Boy with COVID-19. Pediatr. Infect. Dis. J. 2020, 39, e200. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired Spermatogenesis in COVID-19 Patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Larasati, T.; Noda, T.; Fujihara, Y.; Shimada, K.; Tobita, T.; Yu, Z.; Matzuk, M.M.; Ikawa, M. Tmprss12 Is Required for Sperm Motility and Uterotubal Junction Migration in Mice†. Biol. Reprod. 2020, 103, 254–263. [Google Scholar] [CrossRef]
- Batiha, O.; Al-Deeb, T.; Al-zoubi, E.; Alsharu, E. Impact of COVID-19 and Other Viruses on Reproductive Health. Andrologia 2020, 52, e13791. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Scopus Preview—Scopus—Document Details—Single-Cell Transcriptome Analysis of the Novel Coronavirus (SARS-CoV-2) Associated Gene ACE2 Expression in Normal and Non-Obstructive Azoospermia (NOA) Human Male Testes. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85085124021&origin=inward&txGid=c40616db96674ffe52e0079abee92424 (accessed on 4 March 2024).
- Rajput, S.K.; Logsdon, D.M.; Kile, B.; Engelhorn, H.J.; Goheen, B.; Khan, S.; Swain, J.; McCormick, S.; Schoolcraft, W.B.; Yuan, Y.; et al. Human Eggs, Zygotes, and Embryos Express the Receptor Angiotensin 1-Converting Enzyme 2 and Transmembrane Serine Protease 2 Protein Necessary for Severe Acute Respiratory Syndrome Coronavirus 2 Infection. F&S Sci. 2021, 2, 33–42. [Google Scholar] [CrossRef]
- Wu, M.; Ma, L.; Xue, L.; Zhu, Q.; Zhou, S.; Dai, J.; Yan, W.; Zhang, J.; Wang, S. Co-Expression of the SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in Human Ovaries: Identification of Cell Types and Trends with Age. Genomics 2021, 113, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Stanley, K.E.; Thomas, E.; Leaver, M.; Wells, D. Coronavirus Disease-19 and Fertility: Viral Host Entry Protein Expression in Male and Female Reproductive Tissues. Fertil. Steril. 2020, 114, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Bouissou, D.R.; Pereira, V.M.; Camargos, A.F.; dos Reis, A.M.; Santos, R.A. Angiotensin-(1-7), Its Receptor Mas, and the Angiotensin-Converting Enzyme Type 2 Are Expressed in the Human Ovary. Fertil. Steril. 2011, 95, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Tanacan, A.; Yazihan, N.; Erol, S.A.; Anuk, A.T.; Yucel Yetiskin, F.D.; Biriken, D.; Ozgu-Erdinc, A.S.; Keskin, H.L.; Moraloglu Tekin, O.; Sahin, D. The Impact of COVID-19 Infection on the Cytokine Profile of Pregnant Women: A Prospective Case-Control Study. Cytokine 2021, 140, 155431. [Google Scholar] [CrossRef]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical Features and Obstetric and Neonatal Outcomes of Pregnant Patients with COVID-19 in Wuhan, China: A Retrospective, Single-Centre, Descriptive Study. Lancet Infect. Dis. 2020, 20, 559–564. [Google Scholar] [CrossRef]
- Zeng, L.; Xia, S.; Yuan, W.; Yan, K.; Xiao, F.; Shao, J.; Zhou, W. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020, 174, 722–725. [Google Scholar] [CrossRef]
- Matar, R.; Alrahmani, L.; Monzer, N.; Debiane, L.G.; Berbari, E.; Fares, J.; Fitzpatrick, F.; Murad, M.H. Clinical Presentation and Outcomes of Pregnant Women with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2021, 72, 521–533. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.Y.; Ho, L.C.; To, W.W.K.; et al. Pregnancy and Perinatal Outcomes of Women with Severe Acute Respiratory Syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical Analysis of 10 Neonates Born to Mothers with 2019-nCoV Pneumonia. Transl. Pediatr. 2020, 9, 510–560. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, J.; Liu, Y.; Fu, T.; Yu, X.; Zhang, Z.-J. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA 2020, 323, 1313–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, G.; Hou, H.; Liao, Q.; Chen, J.; Bai, H.; Lee, S.; Wang, C.; Li, H.; Cheng, L.; et al. Analysis of Sex Hormones and Menstruation in COVID-19 Women of Childbearing Age. Reprod. Biomed. Online 2021, 42, 260–267. [Google Scholar] [CrossRef] [PubMed]
- 1 in 6 People Globally Affected by Infertility: WHO. Available online: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility (accessed on 23 November 2023).
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing ‘Reviewers’ to ‘Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, V.; Glaß, K.; Leßmann, M.-E.; Schaar, J.; Klimova, A.; Wimberger, P.; Goeckenjan, M. Assisted Reproduction after SARS-CoV-2-Infection: Results of a Single-Center Cohort-Study. Arch. Gynecol. Obstet. 2023, 309, 305–313. [Google Scholar] [CrossRef]
- Huri, M.; Noferi, V.; Renda, I.; Piazzini, F.; Benemei, S.; Coccia, M.E. The COVID-19 Pandemic Impact on the Outcome of Medically Assisted Reproduction Pregnancies. Front. Reprod. Health 2022, 4, 860425. [Google Scholar] [CrossRef]
- Rageh, K.E.A.; Farag, E.A.; Behery, M.A.; Badreldin, M.A.; Ali, E.A. The Impact of Previous Exposure to COVID-19 on the Outcome of ICSI Cycles. JBRA Assist. Reprod. 2023, 27, 367–372. [Google Scholar] [CrossRef]
- Engels Calvo, V.; Cruz Melguizo, S.; Abascal-Saiz, A.; Forcén Acebal, L.; Sánchez-Migallón, A.; Pintado Recarte, P.; Cuenca Marín, C.; Marcos Puig, B.; Del Barrio Fernández, P.G.; Nieto Velasco, O.; et al. Perinatal Outcomes of Pregnancies Resulting from Assisted Reproduction Technology in SARS-CoV-2-Infected Women: A Prospective Observational Study. Fertil. Steril. 2021, 116, 731–740. [Google Scholar] [CrossRef]
- Rodríguez-Varela, C.; Mariani, G.; Dolz, P.; García-Velasco, J.A.; Serra, V.; Pellicer, A.; Labarta, E. Impact of COVID-19 on Infertility Treatments: Not Even a Global Pandemic Was Strong Enough to Hamper Successful Pregnancies. Life 2021, 12, 6. [Google Scholar] [CrossRef]
- Ata, B.; Gianaroli, L.; Lundin, K.; Mcheik, S.; Mocanu, E.; Rautakallio-Hokkanen, S.; Tapanainen, J.S.; Vermeulen, N.; Veiga, A. Outcomes of SARS-CoV-2 Infected Pregnancies after Medically Assisted Reproduction. Hum. Reprod. 2021, 36, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Xia, L.; Zhao, Y.; Tian, L.; Xu, D.; Su, Q.; Hu, Y.; Xie, Q.; Chen, J.; et al. Effect of Prior Female SARS-CoV-2 Infection on IVF Outcomes: A Prospective Cohort Study. Front. Endocrinol. 2023, 14, 1239903. [Google Scholar] [CrossRef]
- Epelboin, S.; Labrosse, J.; De Mouzon, J.; Fauque, P.; Gervoise-Boyer, M.-J.; Levy, R.; Sermondade, N.; Hesters, L.; Bergère, M.; Devienne, C.; et al. Obstetrical Outcomes and Maternal Morbidities Associated with COVID-19 in Pregnant Women in France: A National Retrospective Cohort Study. PLoS Med. 2021, 18, e1003857. [Google Scholar] [CrossRef] [PubMed]
- Ziert, Y.; Abou-Dakn, M.; Backes, C.; Banz-Jansen, C.; Bock, N.; Bohlmann, M.; Engelbrecht, C.; Gruber, T.M.; Iannaccone, A.; Jegen, M.; et al. Maternal and Neonatal Outcomes of Pregnancies with COVID-19 after Medically Assisted Reproduction: Results from the Prospective COVID-19-Related Obstetrical and Neonatal Outcome Study. Am. J. Obstet. Gynecol. 2022, 227, e1–e495. [Google Scholar] [CrossRef]
- Dolgushina, N.V.; Menzhinskaya, I.V.; Ermakova, D.M.; Frankevich, N.A.; Vtorushina, V.V.; Sukhikh, G.T. The Effect of COVID-19 Severity, Associated Serum Autoantibodies and Time Interval after the Disease on the Outcomes of Fresh Oocyte ART Cycles in Non-Vaccinated Patients. J. Clin. Med. 2023, 12, 4370. [Google Scholar] [CrossRef] [PubMed]
- Banker, M.; Arora, P.; Banker, J.; Shah, A.; Gupta, R.; Shah, S. Impact of COVID-19 Pandemic on Clinical and Embryological Outcomes of Assisted Reproductive Techniques. J. Hum. Reprod. Sci. 2022, 15, 150–156. [Google Scholar] [CrossRef]
- Madjunkov, M.; Dviri, M.; Librach, C. A Comprehensive Review of the Impact of COVID-19 on Human Reproductive Biology, Assisted Reproduction Care and Pregnancy: A Canadian Perspective. J. Ovarian Res. 2020, 13, 140. [Google Scholar] [CrossRef]
- Age and Infertility: The Biological Clock: Fact or Fiction? Available online: https://fertilitycenterlv.com/blog/your-biological-clock-fact-or-fiction-the-effects-of-age-on-female-fertility/ (accessed on 22 November 2023).
- Huang, J.; Xia, L.; Lin, J.; Liu, B.; Zhao, Y.; Xin, C.; Ai, X.; Cao, W.; Zhang, X.; Tian, L.; et al. No Effect of Inactivated SARS-CoV-2 Vaccination on in Vitro Fertilization Outcomes: A Propensity Score-Matched Study. J. Inflamm. Res. 2022, 15, 839–849. [Google Scholar] [CrossRef]
- Dong, M.; Wu, S.; Zhang, X.; Zhao, N.; Qi, J.; Zhao, D.; Sang, Y.; Tan, J. Effects of COVID-19 Vaccination Status, Vaccine Type, and Vaccination Interval on IVF Pregnancy Outcomes in Infertile Couples. J. Assist. Reprod. Genet. 2022, 39, 1849–1859. [Google Scholar] [CrossRef]
- Wang, C.; Tang, D.; Liu, J.; Zhang, S.; Xu, Y.; Qiao, J.; Cao, Y. Association Between COVID-19 Vaccination and Artificial Insemination Outcomes for Couples Experiencing Infertility. JAMA Netw. Open 2022, 5, e2247216. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Lin, G.; Gong, F.; Hocher, B. Safety of COVID-19 Vaccination in Women Undergoing IVF/ICSI Treatment—Clinical Study and Systematic Review. Front. Immunol. 2023, 13, 1054273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, Y.; Li, G.; Yin, B.; Tang, X.; Jia, L.; Zhang, X.; Yang, W.; Wang, C.; Peng, X.; et al. Pregnancy Outcomes Following Natural Conception and Assisted Reproduction Treatment in Women Who Received COVID-19 Vaccination Prior to Conception: A Population-Based Cohort Study in China. Front. Med. 2023, 10, 1250165. [Google Scholar] [CrossRef] [PubMed]
- Chillon, T.S.; Weiss, G.; Demircan, K.; Minich, W.B.; Schenk, M.; Schomburg, L. Antibodies to SARS-CoV-2 in Follicular Fluids and Their Association with Assisted Reproduction. Front. Immunol. 2023, 14, 1120328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Chen, Y.-H.; Zhang, S.-P.; Wu, X.-Q.; Wang, X.-P. Effects of the Severe Acute Respiratory Syndrome Coronavirus 2 Inactivated Vaccine on the Outcome of Frozen Embryo Transfers: A Large Scale Clinical Study. Int. J. Womens Health 2023, 15, 1305–1316. [Google Scholar] [CrossRef]
- European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE); Wyns, C.; Geyter, C.D.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; et al. ART in Europe, 2018: Results Generated from European Registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar] [CrossRef]
- European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Cuevas Saiz, I.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; et al. ART in Europe, 2019: Results Generated from European Registries by ESHRE. Hum. Reprod. 2023, 38, dead197. [Google Scholar] [CrossRef]
- Smeenk, J.; Wyns, C.; De Geyter, C.; Bergh, C.; Cuevas, I.; de Neubourg, D.; Kupka, M.S.; Rezabek, K.; Rugescu, I.; Tandler-Schneider, A.; et al. O-153 Assisted Reproductive Technology (ART) in Europe 2020 and Development of a Strategy of Vigilance: Preliminary Results Generated from European Registers by the ESHRE EIM Consortium. Hum. Reprod. 2023, 38, dead093.014. [Google Scholar] [CrossRef]
- Qiao, J.; National Expert Group for Quality Management on Assisted Reproductive Technology. Impacts of COVID-19 pandemics on the services of assisted reproductive technology in Chinese mainland: A national cross-sectional survey. Chin. J. Reprod. Contracept. 2021, 41, 7–11. [Google Scholar]
- Wei, L.; Zhang, J.; Deng, X.; Luo, C.; Bo, L.; Gao, S.; Qian, F.; Lu, S.; Mao, C. Impacts of the COVID-19 Pandemic on Chinese Assisted Reproductive Technology Institutions and Human Sperm Banks: Reflections in the Post-Pandemic Era. J. Health Popul. Nutr. 2023, 42, 82. [Google Scholar] [CrossRef]
- Allotey, J.; Fernandez, S.; Bonet, M.; Stallings, E.; Yap, M.; Kew, T.; Zhou, D.; Coomar, D.; Sheikh, J.; Lawson, H.; et al. Clinical Manifestations, Risk Factors, and Maternal and Perinatal Outcomes of Coronavirus Disease 2019 in Pregnancy: Living Systematic Review and Meta-Analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Adamson, G.D.; de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S. International Committee for Monitoring Assisted Reproductive Technology: World Report on Assisted Reproductive Technology, 2011. Fertil. Steril. 2018, 110, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Balachandren, N.; Davies, M.C.; Hall, J.A.; Stephenson, J.M.; David, A.L.; Barrett, G.; O’Neill, H.C.; Ploubidis, G.B.; Yasmin, E.; Mavrelos, D. SARS-CoV-2 Infection in the First Trimester and the Risk of Early Miscarriage: A UK Population-Based Prospective Cohort Study of 3041 Pregnancies Conceived during the Pandemic. Hum. Reprod. 2022, 37, 1126–1133. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical Transmission of Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2021, 224, 35–53.e3. [Google Scholar] [CrossRef] [PubMed]
- Setti, P.E.L.-; Cirillo, F.; Immediata, V.; Morenghi, E.; Canevisio, V.; Ronchetti, C.; Baggiani, A.; Albani, E.; Patrizio, P. First Trimester Pregnancy Outcomes in a Large IVF Center from the Lombardy County (Italy) during the Peak COVID-19 Pandemic. Sci. Rep. 2021, 11, 16529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zou, Y.; Guo, Y.; Lv, X.; Chen, J.; Guo, X.; Liu, Q. Effect of COVID-19 Inactivated Vaccine on Peripheral Blood Anti-Β2-GPI Antibody and Outcomes in Vitro Fertilization-Embryo Transplantation. Int. Immunopharmacol. 2023, 122, 110596. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, M.; Xue, X.; Li, N.; Chen, L.; Shi, J. Association between Time Interval from COVID-19 Vaccination to In Vitro Fertilization and Pregnancy Rate after Fresh Embryo Transfer. JAMA Netw. Open 2022, 5, e2236609. [Google Scholar] [CrossRef]
- Cao, M.; Wu, Y.; Lin, Y.; Xu, Z.; Liang, Z.; Huang, Q.; Li, S.; Liu, H.; An, C.; Luo, Y.; et al. Inactivated Covid-19 Vaccine Did Not Undermine Live Birth and Neonatal Outcomes of Women with Frozen-Thawed Embryo Transfer. Hum. Reprod. 2022, 37, 2942–2951. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, M.; Lin, Y.; Xu, Z.; Liang, Z.; Huang, Q.; Li, S.; Li, L.; Meng, Y.; An, C.; et al. Inactivated COVID-19 Vaccination Does Not Affect In Vitro Fertilization Outcomes in Women. Hum. Reprod. 2022, 37, 2054–2062. [Google Scholar] [CrossRef]
- Allahbadia, G. Will Procreation Ever Be The Same After COVID-19? J. Obstet. Gynaecol. India 2021, 71, 1–6. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]




| Study | Study Type | City, Country | Publication Date | No. COVID-19 Positive Participants | Mean Age ± SD | Pregnancy | Birth | Miscarriage |
|---|---|---|---|---|---|---|---|---|
| Eckstein V et al. [38] | cohort-study | Germany | 10 October 2023 | 1581 treatment cycles | 35.8 ± 4.1 (23.1–49.5) | 19/87 (21.84%) | not published yet | 10/19 (52.63%) |
| Huri et al. [39] | retro-prospective cohort study | Florence, Italy | 25 April 2022 | 749 | 219 (100%) | 165 (22.02%) | 98 | |
| Rageh et al. [40] | observational study | Manama, Bahrain; Kingdom of Saudi Arabia | 12 September 2023 | 88 | 32.14 ± (4.773) | 32 (36.4%) | ||
| Engels Calvo et al. [41] | multicenter, prospective, observational study | 78 Spanish centers | 12 April 2021 | 74 | 39.6 | 74 (5.5%) | ||
| Rodríguez-Varela et al. [42] | retrospective, multicentric, and double-arm study | Spain, Lisbon and Rome | 21 December 2021 | 6 | 38.6 | 6 | 39 (4.5%)-overall miscarriage rate | |
| ESHRE COVID-19 Working Group et al. [43] | retrospective, multicentric studies | 32 countries worldwide | 13 September 2021 | 105 | 33.7 ± 6.1 | 25 | 67 | 10 (12.5%) |
| Huang J et al. [44] | prospective cohort study | China | 4 October 2023 | 252 | 32.3 ± 5.0 | 83 (70.3%) | ||
| Epelboin et al. [45] | prospective clinical study | France | 30 November 2021. | 16 | 31.1 (±5.9) | 16 (1.8%) | No data | |
| Ziert et al. [46] | multicentric, prospective, observational study | Germany, Austria | 19 April 2022 | 65 | 34.09 ± 5.12 | 65 | 57/65 (87,69%) | 0/57 (0.0) |
| Dolgushina et al. [47] | observational prospective study | Russia | 29 June 2023 | 135 | 34 (31–37) | 39 (28.9%) | 30 (22.2%) | 31 (23.1%) |
| Banker et al. [48] | retrospective cohort study | Ahmedabad, India | 30 June 2022 | 606 | 30.7 | 47.6% Fresh ET 68.7% Frozen ET | 32.4% Fresh ET 46.3% Frozen ET | 10% Fresh ET 6.73% Frozen ET |
| TOTAL | 2096 | 562 | 335 | 188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milostić-Srb, A.; Srb, N.; Talapko, J.; Meštrović, T.; Žiger, T.; Pačarić, S.; Fureš, R.; Makarović, V.; Škrlec, I. The Effect of COVID-19 and COVID-19 Vaccination on Assisted Human Reproduction Outcomes: A Systematic Review and Meta-Analysis. Diseases 2024, 12, 201. https://doi.org/10.3390/diseases12090201
Milostić-Srb A, Srb N, Talapko J, Meštrović T, Žiger T, Pačarić S, Fureš R, Makarović V, Škrlec I. The Effect of COVID-19 and COVID-19 Vaccination on Assisted Human Reproduction Outcomes: A Systematic Review and Meta-Analysis. Diseases. 2024; 12(9):201. https://doi.org/10.3390/diseases12090201
Chicago/Turabian StyleMilostić-Srb, Andrea, Nika Srb, Jasminka Talapko, Tomislav Meštrović, Tihomil Žiger, Stana Pačarić, Rajko Fureš, Vedrana Makarović, and Ivana Škrlec. 2024. "The Effect of COVID-19 and COVID-19 Vaccination on Assisted Human Reproduction Outcomes: A Systematic Review and Meta-Analysis" Diseases 12, no. 9: 201. https://doi.org/10.3390/diseases12090201
APA StyleMilostić-Srb, A., Srb, N., Talapko, J., Meštrović, T., Žiger, T., Pačarić, S., Fureš, R., Makarović, V., & Škrlec, I. (2024). The Effect of COVID-19 and COVID-19 Vaccination on Assisted Human Reproduction Outcomes: A Systematic Review and Meta-Analysis. Diseases, 12(9), 201. https://doi.org/10.3390/diseases12090201







