Abstract
Background: Aging is associated with a decrease in muscle mass. Insulin resistance and hyperglycemia accelerate muscle loss, leading to a deterioration in strength, muscle mass, and physical capacity in older adults. This study was conducted to determine the association between sarcopenia and poor glycemic control in older adults with type 2 diabetes mellitus (T2D). Methods: A cross-sectional study was carried out in older adults with T2D in geriatric outpatient clinics. Sarcopenia was diagnosed as per the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) criteria. According to glycosylated hemoglobin (HbA1c) levels, participants were classified into glycemic control (HbA1c ≤ 7.5%) and poor glycemic control (HbA1c ≥ 7.5%) groups. Results: Older adults with sarcopenia were found to have poor glycemic control compared to adults without sarcopenia (62.3% vs. 47.9%, p = 0.007). Logistic regression analysis showed an association between poor glycemic control and the presence of sarcopenia (odds ratio (OR): 1.79, 95% confidence interval (CI): 1.17–2.75) and low muscle mass (OR: 1.73, 95% CI: 1.07–2.73). Conclusions: Poor glycemic control is associated with the presence of sarcopenia and low muscle mass, which highlights the need to implement better treatment strategies in order to reduce the loss of muscle mass.
1. Introduction
Sarcopenia is defined as the loss of muscle mass and strength related to the aging process [1]. The worldwide prevalence rate of sarcopenia is estimated to be 10% [2]; in Mexico, it is estimated that 33.6% of the population, mainly women, suffer from it, increasing from the age of 80 years old [3]. It is accompanied by a high burden of comorbidity, with cardiovascular disease, T2D, and neurodegenerative diseases being the most frequent conditions [4,5].
Aging is related to a decrease in glucose tolerance, as well as factors such as an increase in adiposity, particularly in the abdominal region, accompanied by a decrease in muscle mass, causing a deterioration in glucose regulation [6]. It has been determined that between 30% and 40% of a person’s muscle mass can decrease by the time they reach an age of 80 years. It is reasonable to think that this loss is related to the development of glucose intolerance and subsequently to the increased risk of developing T2D [7]. Insulin resistance is associated with an increased loss of appendicular lean mass in both men and women [8,9,10]. People with prediabetes and high HbA1c levels experience a loss of muscle mass, muscle strength, and physical performance, particularly in the lower extremities among older ages, and worsening when untreated [11,12,13,14,15].
Sarcopenia is more frequent in patients with diabetes than in normoglycemic patients. Both type 1 diabetes mellitus (T1D) and T2D patients show lower handgrip strength and muscle mass [16], suggesting an inverse correlation between appendicular muscle mass with diabetes duration and fat mass, as well as a positive correlation with appendicular muscle mass based on body mass index (BMI), physical activity level, and muscle strength [17], with T2D being a risk factor for developing sarcopenia (37%) and pre-sarcopenia (73%) compared to individuals without T2D [18]. Other factors, such as female gender [19,20], T1D [16,17], age > 65 years old [18,19], a high BMI [17,21,22,23], hypoalbuminemia [24,25], poor nutritional status [19,20,21,22,23,24], low levels of physical activity [19,22,24,25,26], and high insulin requirements [27], are risk factors for the development of sarcopenia, which was independently associated with short-term mortality after hospital discharge [28].
A progressive increase in HbA1c levels was inversely associated with a low percentage of total, appendicular, and trunk lean muscle mass [13,29]. The same findings were observed in subjects with no previous diagnosis of T2D but who had hyperglycemia and T2D without seeking treatment [29]. In addition, low muscle mass, low handgrip strength, and insulin resistance were independent factors determining poor glycemic control [30] and higher glucose fluctuations [31]. A >1% decrease in HbA1c levels was found to improve muscle mass and gait speed [32]. Conversely, patients with microangiopathic complications have a significantly increased risk of sarcopenia, especially when diabetic retinopathy, nephropathy, and peripheral neuropathy are present [12,24,33].
Insulin use has been shown to be an independent factor in decreasing muscle mass [27,32]. Lower handgrip strength and gait speed were observed when patients were treated with insulin [25]. However, insulin sensitizers and dipeptidyl-peptidase-4 inhibitors (iDPP-4s) minimize the loss of strength and muscle mass [26,34,35]. No association has been found between the duration of T2D and the development of sarcopenia in those within the age range of 6 to 15 years [16] or with the use of other oral antidiabetics [36]. This study was conducted to determine the association between sarcopenia and poor glycemic control in older adults with T2D.
2. Materials and Methods
2.1. Study Participants
A cross-sectional study was conducted in older adults with T2D, recruited from the Geriatrics outpatient clinic of Hospital 24 del Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE, for its acronym in Spanish), Ciudad Guzmán, Mexico. The participants were 60 years old or older with a confirmed diagnosis of T2D, consecutively assessed from July 2022 to June 2023. The inclusion criteria were as follows: (1) an age of ≥60 years and (2) a diagnosis of T2D (according to the American Diabetes Association criteria) [37]. The exclusion criteria were as follows: (1) the presence of severe physical or cognitive limitations, (2) the presence of an acute process that warrants emergency care or hospital admission, (3) the diagnosis of terminal illness or being in palliative care, and (4) an inconclusive diagnosis of T2D or the absence of antidiabetic treatment at the time of assessment.
Our study procedures were approved by our institution’s Ethics and Research Committees under the registration number DJSMEI-13149. All participants agreed and signed a written informed consent form.
2.2. Clinical Features
Each participant was questioned directly or through the main caregiver to acquire demographic variables, as well as their age, medical history, and medication records. Comorbidity was defined through the Charlson Comorbidity Index as ≥3 diseases [38] and the presence of polypharmacy, reporting the consumption of ≥5 medications simultaneously in the last month. The duration of T2D was dichotomously defined as <20 years and >20 years. According to the medical record, the presence of complications related to T2D was recorded: retinopathy, neuropathy, cardiopathy (heart failure, myocardial infarction, angina pectoris, atrial fibrillation); cerebrovascular complications (transient ischemia, cerebrovascular event); angiopathy (peripheral arterial disease in upper or lower limbs, carotid stenosis); nephropathy (estimated GFR via CKD-EPI of creatinine ≤ 60 mL/min/1.73 m2 without dialysis). Weight and height were measured, and BMI was calculated as weight (kg)/height2 (m). The nutritional status of older adults was assessed through the Mini Nutrition Assessment (MNA); a score of ≥18 was defined as adequate nutritional status, and a score of ≤17 was defined as malnutrition [39]. Frailty was defined through the FRAIL (Fatigue, Resistance, Ambulation, Illness, and Loss of weight) scale; a score of ≥3 points was categorized as frailty [40]. Upon direct questioning at the time of care, physical activity was assessed by calculating metabolic equivalents (METs) through the Duke Activity Status Index (DASI). The calculation was as follows: METs = total DASI score × 0.43 + 9.6/3.5, low physical activity was categorized if the METs were ≤5 [41].
The biochemical parameters of interest, such as HbA1c, total cholesterol, high-density cholesterol (HDL-c), low-density cholesterol (LDL-c), triglycerides, uric acid, and albumin, were obtained from the clinical record less than 3 months from the date they were taken. Insulin resistance was measured through the triglyceride/glucose (TyG) index, according to the following formula: Ln (TG [mg/dL] x glucose [mg/dL]/2), with a value of ≥8.80 defined as insulin resistance [42]. Glycemic control was determined through HbA1c levels ≤ 7.5%, and poor glycemic control as an HbA1c ≥ 7.5%.
2.3. Definition of Sarcopenia
According to the EWGSOP2 criteria [1], older adults with low muscle strength and low muscle mass were defined as having sarcopenia. Muscle strength was defined through the handgrip strength of the dominant hand, using a JAMAR® dynamometer. A maximum of 3 attempts were performed, recording the highest value for our subsequent analyses. The cut-off value for low muscle strength was ≤16 kg for women and ≤27 kg for men. The appendicular skeletal muscle mass (ASM) was determined through the formula ASM kg = 0.215 × calf circumference (cm) + 0.093 × handgrip strength (kg) + 0.061 × weight (kg) + 3.637 × sex + 0.112 × height (cm) − 16.449; where sex: male = 1; female = 0. We defined low muscle mass as men with ≤20 kg and women with ≤15 kg [43].
Physical performance was measured by using walking speed in meters/second (m/s), timed over four linear meters. Each older adult was instructed and assessed on 2 occasions; the best-timed record was used to define poor physical performance. The cutoff value was set at ≤0.8 m/s.
2.4. Statistical Analysis
The Kolmogorov–Smirnov test was performed to assess the distribution of the variables. The calculated values were presented as frequencies and percentages or means and standard deviations (SDs). Student’s t-test and Chi-square were used for the comparison of numerical and categorical variables, respectively. The analysis of association was performed through logistic regression models with response variables: sarcopenia and its components. The odds ratios (OR) and 95% confidence intervals (CI) were estimated as a measure of the effect size of the poor glycemic control in the logistic regression. We adjusted the estimates of the OR of poor glycemic control using age, sex, and variables related to T2D. We performed a sensitivity analysis of the estimate of the OR of poor glycemic control adjusting to a different variable related to T2D. The Hosmer–Lemeshow test was used to assess the goodness of fit of the logistic regression models. All statistical analyses were performed by using the IBM SPSS Statistics 29.0.1 software version. Any p-value < 0.05 was considered significant.
3. Results
3.1. Characteristics of the Participants
Figure 1 shows that 443 patients diagnosed with T2D were initially recruited for the study, of whom 87 were eliminated and 356 participants were enrolled.
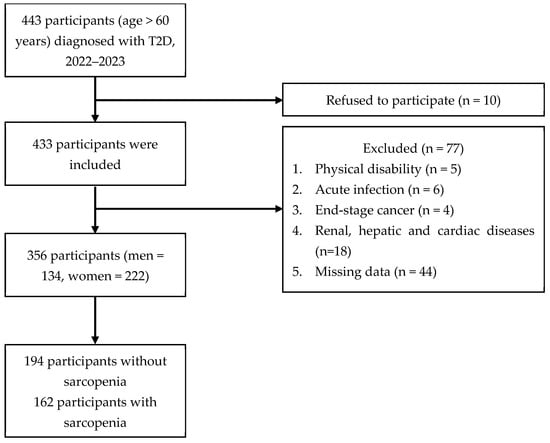
Figure 1.
Flow diagram for study participants.
The general characteristics of the patients according to the diagnosis of sarcopenia are presented in Table 1. The frequency of sarcopenia in our study was 45.5%, of which 97 cases were women (59.9%) and 65 cases were men (40.1%). The overall poor glycemic control in patients was 54.5%.

Table 1.
Clinical characteristics of older adults with or without sarcopenia.
When comparing the groups with or without sarcopenia, it was observed that patients with the presence of sarcopenia were older (p < 0.001), had poor glycemic control (62.3% vs. 47.9%, p = 0.007), burden of comorbidity (p = 0.014), malnutrition (p < 0.001), and frailty (p < 0.001), as well as a longer duration of T2D (p < 0.001). They also presented a higher frequency of complications associated with T2D in general (81.5% vs. 64.4%, p < 0.001), of which heart disease (26.5% vs. 11.9%, p < 0.001) and nephropathy (21.0% vs. 12.9%, p < 0.001) were the most frequent. Older adults with sarcopenia registered lower physical activity measured through METs (4.2 vs. 5.6, p < 0.001).
Conversely, body composition in sarcopenia patients revealed that BMI (p < 0.001), waist circumference (p < 0.001), hip circumference (p < 0.001), and body fat percentage (p < 0.001) were lower. For sarcopenia-defining components, the means for handgrip strength (13.9 kg vs. 23.4 kg, p < 0.001), appendicular skeletal muscle mass (14.2 kg vs. 16.9 kg, p < 0.001), and gait speed (0.60 m/s vs. 0.82 m/s, p < 0.001) were lower than in those older adults without sarcopenia.
HbA1c levels were higher in patients with sarcopenia (8.2% vs. 7.8%, p = 0.027). However, their albumin, total cholesterol, and triglyceride levels were lower than those without sarcopenia. The use of antidiabetic drugs showed that the frequency of insulin use was higher in patients with sarcopenia (68.5% vs. 44.3%, p < 0.001) and a lower use of sulfonylureas and biguanides.
3.2. Components of Sarcopenia According to Glycemic Control
We compared the components that define the diagnosis of sarcopenia with glycemic control. In patients with poor glycemic control, handgrip strength (13.5 kg vs. 23.5 kg, p < 0.001), appendicular skeletal muscle mass (14.0 kg vs. 16.9 kg, p < 0.001), and gait speed (0.62 m/s vs. 0.80 m/s, p < 0.001) were significantly lower for subjects with sarcopenia. In patients with glycemic control, there were no significant differences between subjects with and without sarcopenia for handgrip strength (13.5 kg vs. 14.7 kg, p = 0.091), for appendicular skeletal muscle mass (14.0 kg vs. 14.5 kg, p = 0.486), and for gait speed (0.62 m/s vs. 0.63 m/s, p = 0.701).
3.3. Association of Sarcopenia Risk and Its Components with Glycemic Control
Logistic regression analysis for sarcopenia and its components focused on poor glycemic control (HbA1c ≥ 7.5%), the associations are shown in Table 2. Table A1 of the Appendix A shows the results of the Hosmer–Lemeshow test. Poor glycemic control was significantly associated with the presence of sarcopenia and with low muscle mass, without being significant for low muscle strength and low gait speed. The same associations held for both sarcopenia and low muscle mass, when adjusted for age and sex, and with variables such as comorbidity, T2D-related complication, presence of heart disease, nephropathy, and duration of T2D ≥ 20 years old.

Table 2.
Association between poor glycemic control and sarcopenia and its components.
4. Discussion
In our study, a high frequency of sarcopenia was observed in older adults with T2D, having a significant association with some clinical characteristics such as advanced age, higher comorbidity burden, malnutrition status, presence of frailty, low physical activity, presence of complications associated with T2D, and a disease duration of more than 20 years. More than half of the patients presented poor glycemic control, represented by an HbA1c level ≥ 7.5%, this being more frequent in those older adults who were categorized with sarcopenia.
Poor glycemic control was significantly associated with sarcopenia and low muscle mass (OR 1.79 and OR 1.73, respectively), but not with low muscle strength or low gait speed; these associations were maintained when adjusted for variables related to T2D itself.
The frequency of sarcopenia in our study was 45.5%, which is higher than the 11% reported worldwide [2] and 33.6% in our country [3]. This variability in prevalence arises from the heterogeneity of the criteria used to define sarcopenia. Increasing age and female sex are the non-modifiable factors most frequently associated with sarcopenia found in our study and are similarly referred to in other cross-sectional studies [18,19,20]. In Mexico, Perez-Zepeda et al. found that the prevalence of sarcopenia increases with age, with increasing values between 60 and 69 years old (16.06%), 70 and 79 years old (32.85%), and over 80 years old (51.01%) [44]. Body composition and muscle mass vary according to cultural, regional, and geographical location in our country, both for men and women, and muscle mass was higher for adults living in the center of the country [45].
In the present study, the presence of sarcopenia was further associated with a higher burden of comorbidity (Charlson index ≥ 3 diseases), malnutrition status (MNA ≤ 17 points), presence of frailty (FRAIL ≥ 3 points), clinical conditions related to the natural history of T2D (prolonged duration of the disease, presence of chronic complications), and low physical activity. These same findings have been demonstrated in several studies, where, when evaluating subjects with T2D, the risk of developing sarcopenia was OR 1.55; 95% CI 1.25–1.91, p < 0.001; it is noteworthy that subjects with T2D presented lower physical performance and muscle strength than those with euglycemia, without observing a difference in the amount of muscle mass [25].
Prolonged periods of sedentary lifestyle, poor nutritional status, and lower levels of physical activity have been identified as common risk factors for the presence of sarcopenia, which is exacerbated in the presence of T2D [19]. In a study with similar findings, subjects with sarcopenia have a higher risk of malnutrition than those with normal nutritional status; similarly, in the subgroup of women with malnutrition (OR 4.97; p = 0.003) and women with T2D (OR 5.52; p = 0.019), they were more likely to have sarcopenia [20]. Nutritional status is perhaps the most important determinant associated with sarcopenia. In our study, older adults with T2D who presented sarcopenia were associated with a state of malnutrition; this association is dependent on BMI, since obesity has bimodal behavior, it increases the risk of presenting sarcopenia (OR 3.2; 95% CI 1.24–8.26) [21]. However, high BMI also had a negative association with the development of sarcopenia (men, OR 0.57, 95% CI 0.44–0.73; women, OR 0.48, 95% CI 0.33–0.70) [46]; in addition, lower BMIs, are associated with the development of sarcopenia [22], as nutritional status itself has shown a positive correlation with muscle mass and handgrip strength [20,23,24]; the same relationship holds with low protein intake and lower physical activity [20,47,48]. We defined low physical activity as ≤5 METs by the Duke index, with the mean being lower in subjects with sarcopenia; however, in the multivariate analysis, it was not associated with the presence of sarcopenia, low muscle strength, low muscle mass, or low gait speed. Nevertheless, several studies have reported that a state of pre-frailty (OR 4.75; 95% CI 1.90–11.89; p = 0.001) [49] and low physical activity (OR 15.35; 95% CI 1.69–139.47; p = 0.015) increases the risk of sarcopenia [19,22,24,25,26].
Insulin resistance determines the onset of accelerated skeletal muscle loss [8,9,10]. This loss is more pronounced in the appendicular area, especially in the lower extremities, and this deterioration is substantially more aggressive in patients without antidiabetic treatment. Park SW et al., in their study, showed in a 6-year follow-up that loss of total muscle mass was more pronounced in older adults with T2D but without treatment than in those with T2D receiving treatment and those without T2D (−435 ± 79 vs. −293 ± 72 vs. −193 ± 22 gr/year, respectively, p < 0.01) [13]. In our study, insulin resistance was evaluated via the TyG index; the mean values found for both groups determine the presence of insulin resistance. However, it was slightly higher in patients without sarcopenia (9.1 vs. 9.3; p = 0.046). Another observation was made for body fat index, where patients with sarcopenia registered a lower percentage for calculated body fat (38.4% vs. 42.2%, p < 0. 001), a finding that contrasts with what was found in a recent study, which evaluated the association between body fat percentage and sarcopenia; logistic regression analyses demonstrated that a high body fat percentage was associated with an increased risk of sarcopenia for both sexes (male, OR 1.38, 95% CI 1.15–1.65; female, OR 1.30, 95% CI: 1.07–1.56) [46].
In our study, poor glycemic control was associated with the presence of sarcopenia and low muscle mass among patients. Studies have highlighted the importance of quantitative muscle importance with the risk of developing T2D. Son JW et al. demonstrated in a follow-up of more than 9 years in middle-aged adults without T2D that the presence of low muscle mass increases the risk of developing T2D by 11.9% in non-obese patients and 19.7% in patients with obesity [50]. Cross-sectional studies have also shown this association [13,14,15], as well as an association with low muscle strength [51]. Persistent hyperglycemia and poor glycemic control are determinants for the development of sarcopenia in the elderly; several studies show that higher glucose and HbA1c levels are associated with poorer muscle quality in quantitative aspects, muscle strength, and physical performance [15,16,25,29,32,33]. Low muscle strength was associated with a higher total insulin dose requirement [30], and low muscle mass was associated with fluctuations in glucose levels and greater variability in fasting glucose ranges [31].
Several studies have shown that the class of drugs including iDPP-4 has a neutral and/or attenuating effect on the loss of muscle mass [34,35]; however, in the case of iSGLT-2, a reduction in muscle mass has been reported [52]. The effect of insulin treatment remains controversial; in cross-sectional designs, the use of insulin shows an association with the development of sarcopenia [32]. Nonetheless, in longitudinal designs, insulin treatment and reduction of HbA1c levels have been shown to attenuate the progression of sarcopenia in older adults with T2D [53,54].
Our study was conducted in an older Mexican population and sought to determine the association between sarcopenia and T2D in a context that may differ from the settings of many previous studies. Despite the existing body of evidence, verifying the reproducibility of findings in various populations is paramount to ensure the external validity of research, providing a stronger foundation for translating findings into clinical practice. This is crucial for clinicians and policymakers seeking evidence applicable to diverse demographic and ethnic groups.
Our study has the following limitations: the number of patients assessed is relatively small in terms of giving external validity to the findings in older Mexican adults with T2D. Due to the type of study, only glycemic control was determined at the time of assessment; a longitudinal study could be necessary to assess changes in HbA1c levels, time of glycemic control, adherence to treatment, and the combinations of the different antidiabetic drugs and their doses. As insulin was found to be a deleterious factor in muscle mass and sarcopenia, it was necessary to determine the influence of the type of insulin used, the doses, and the scheme employed. In determining malnutrition as an independent implication of glycemic control, it was necessary to describe the caloric quantity and protein intake per day since it directly influences glucose control and the development of sarcopenia. In contrast, we evaluated physical activity through the estimation of METs performed for certain activities of daily living. However, we did not evaluate the intensity, frequency, and duration nor the history of physical exercise since the level of physical activity can modify changes in muscle mass and strength. Finally, our study did not use any sophisticated method in determining body composition (dual-energy X-ray absorptiometry, bioelectrical impedance) or other imaging methods.
5. Conclusions
In summary, the frequency of poor glycemic control in older adults with T2D was higher when they presented sarcopenia and low muscle mass. Our findings indicate that poor glycemic control is associated with sarcopenia and low muscle mass, which determines the need to implement better treatment strategies to reduce the loss of muscle mass.
Author Contributions
Conceptualization, F.A.A.-A. and C.V.-D.-L.; methodology, J.V.R.-B., M.E.O.-H., D.D.-C. and C.V.-D.-L.; software, F.A.A.-A. and C.V.-D.-L.; validation, J.V.R.-B. and C.V.-D.-L.; formal analysis, F.A.A.-A., J.V.R.-B. and C.V.-D.-L.; investigation, F.A.A.-A. and C.V.-D.-L.; data curation, M.E.O.-H., D.D.-C. and C.V-D-L.; writing—original draft preparation, F.A.A.-A., J.V.R.-B., M.E.O.-H. and D.D.-C.; writing—review and editing, F.A.A.-A. and C.V.-D.-L.; visualization, M.E.O.-H. and D.D.-C.; supervision, J.V.R.-B. and C.V.-D.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the Declaration of Helsinki and approved by our institution’s Ethics and Research Committees under the registration number DJSMEI-13149.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data sets used to support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the research participants of the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE, for its acronym in Spanish) for their participation.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1 shows the Chi-square statistics and the p-value of the Hosmer–Lemeshow goodness-of-fit test of the regression models in Table 1. The results show that all models had a good fit, except for some logistic models to the response variable of low gait speed. The models on which we base our conclusions had a good fit.

Table A1.
Hosmer–Lemeshow test for goodness of fit for logistic regression models.
Table A1.
Hosmer–Lemeshow test for goodness of fit for logistic regression models.
| Sarcopenia | Low Muscle Strength | Low Muscle Mass | Low Gait Speed | |||||
|---|---|---|---|---|---|---|---|---|
| χ2 (df) | p-Value | χ2 (df) | p-Value | χ2 (df) | p-Value | χ2 (df) | p-Value | |
| PC, age, sex | 15.25 (8) | 0.054 | 8.29 (8) | 0.405 | 1.98 (8) | 0.982 | 11.53 (8) | 0.173 |
| PC, age, sex, comorbidity | 5.29 (8) | 0.725 | 5.05 (8) | 0.752 | 9.33 (8) | 0.315 | 15.87 (8) | 0.044 |
| PC, age, sex, malnutrition | 9.26 (8) | 0.320 | 6.12 (8) | 0.634 | 5.51 (8) | 0.702 | 13.39 (8) | 0.099 |
| PC, age, sex, frailty | 11.72 (8) | 0.165 | 4.79 (8) | 0.780 | 5.93 (8) | 0.655 | 2.95 (8) | 0.937 |
| PC, age, sex, DM complication | 12.11 (8) | 0.146 | 6.82 (8) | 0.555 | 4.88 (8) | 0.770 | 17.79 (8) | 0.023 |
| PC, age, sex, activity ≤ 5 METs | 4.86 (8) | 0.772 | 3.50 (8) | 0.899 | 2.68 (8) | 0.953 | 3.92 (8) | 0.915 |
| PC, age, sex, insulin use | 12.13 (8) | 0.146 | 5.79 (8) | 0.671 | 4.57 (8) | 0.802 | 7.77 (8) | 0.456 |
| PC, age, sex, heart disease | 10.04 (8) | 0.262 | 8.94 (8) | 0.348 | 3.83 (8) | 0.872 | 18.18 (8) | 0.020 |
| PC, age, sex, nephropathy | 7.66 (8) | 0.467 | 8.65 (8) | 0.373 | 13.1 (8) | 0.108 | 11.8 (8) | 0.168 |
| PC, age, sex, DM ≥ 20 years | 12.51 (8) | 0.130 | 9.27 (8) | 0.320 | 5.95 (8) | 0.653 | 14.45 (8) | 0.071 |
χ2: Chi-square statistics; df: degrees of freedom; PC: poor glycemic control; DM: diabetes mellitus; METs: metabolic equivalents.
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Arango-Lopera, V.E.; Arroyo, P.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Prevalence of sarcopenia in Mexico City. Eur. Geriatr. Med. 2012, 3, 157–160. [Google Scholar] [CrossRef]
- Pacifico, J.; Geerlings, M.A.J.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef]
- Khadra, D.; Itani, L.; Chebaro, Y.; Obeid, M.; Jaber, M.; Ghanem, R.; Ayton, A.; Masri, D.; Kilmura, A.; Tannir, H. Association Between Sarcopenic Obesity and Metabolic Syndrome in Adults: A Systematic Review and Meta-Analysis. Curr. Cardiol. Rev. 2020, 16, 153–162. [Google Scholar] [CrossRef]
- Koster, A.; Visser, M.; Simonsick, E.M.; Yu, B.; Allison, D.; Newman, A.; Van Eijk, J.T.M.; Satterfield, S.; Harris, T.B. Association between fitness and changes in body composition and muscle strength. J. Am. Geriatr. Soc. 2010, 58, 219–226. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Holloszy, J.O. Loss of skeletal muscle mass with aging: Effect on glucose tolerance. J. Gerontol. Biol. Sci. Med. Sci. 1995, 50, 68–72. [Google Scholar] [CrossRef]
- Lee, C.G.; Boyko, E.J.; Strotmeyer, E.S.; Lewis, C.E.; Cawthon, P.M.; Hoffman, A.R.; Everson-Rose, S.A.; Orwoll, E.S. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J. Am. Geriatr. Soc. 2011, 59, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Mateo, H.; López-Teros, M.T.; Ramírez, F.A.; Astiazarán-García, H. Association between insulin resistance and low relative appendicular skeletal muscle mass: Evidence from a cohort study in community-dwelling older men and women participants. J. Gerontol. Biol. Sci. Med. Sci. 2014, 69, 871–877. [Google Scholar] [CrossRef] [PubMed]
- López-Teros, M.T.; Ramírez, F.A.; Alemán-Mateo, H. Hyperinsulinemia is associated with the loss of appendicular skeletal muscle mass at 4.6 year follow-up in older men and women. Clin. Nutr. 2015, 34, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liu, Y.; Li, X.; Huo, C.; Jia, X.; Yang, J.; Lei, Y.; Xu, R.; Sun, C.; Wang, X.; et al. Changes and Risk Factors of Skeletal Muscle Mass and Strength in Patients with Type 2 Diabetes over 60 Years Old: A Cross-Sectional Study from China. J. Diabetes Res. 2020, 2020, 9815485. [Google Scholar] [CrossRef]
- Qiao, Y.S.; Chai, Y.H.; Gong, H.J.; Zhuldyz, Z.; Stehouwer, C.D.; Zhou, J.B.; Simo, R. The Association between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences from Observational Studies. Front. Endocrinol. 2021, 12, 782391. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; Harris, T.B.; Nevitt, M.; Cho, Y.W. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, K.S.; Kim, M.J.; Kim, S.K.; Cho, Y.W.; Park, S.W. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 115–121. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.; Kranenburg, J.; Nilwik, R.; Loon, L.J. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Yoshida, S.; Yasuda, T.; Umayahara, Y.; Shimizu, S.; Ryomoto, K.; Yamamoto, T.; Shimomura, I. High prevalence and clinical impact of dynapenia and sarcopenia in Japanese patients with type 1 and type 2 diabetes: Findings from the Impact of Diabetes Mellitus on Dynapenia study. J. Diabetes Investig. 2021, 12, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Pollakova, D.; Tubili, C.; Di Folco, U.; De Giuseppe, R.; Battino, M.; Giampieri, F. Muscular involvement in long-term type 1 diabetes: Does it represent an underestimated complication? Nutrition 2023, 112, 112060. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef]
- Yen, H.Y.; Lee, S.C.; Lin, C.F.; Lai, H.R.; Yamaguchi, Y.; Lee, P.H. Prevalence of sarcopenia and its association with diet and physical activity in older adults with type 2 diabetes: A cross-sectional study. Nurs. Health Sci. 2023. [Google Scholar] [CrossRef]
- Velázquez-Alva, M.C.; Irigoyen-Camacho, M.E.; Zepeda-Zepeda, M.A.; Lazarevich, I.; Arrieta-Cruz, I.; D’Hyver, C. Sarcopenia, nutritional status and type 2 diabetes mellitus: A cross-sectional study in a group of Mexican women residing in a nursing home. Nutr. Diet. 2020, 77, 515–522. [Google Scholar] [CrossRef]
- Mattassi, M.; Henríquez Mella, C.; Pérez Bocaz, L. Association between Sarcopenia and Nutritional Status in Chilean Older People Aged 65 Years and Older. Nutrients 2022, 14, 5228. [Google Scholar] [CrossRef] [PubMed]
- Sravya, S.L.; Swain, J.; Sahoo, A.K.; Mangaraj, S.; Kanwar, J.; Jadhao, P.; Das, S. Sarcopenia in Type 2 Diabetes Mellitus: Study of the Modifiable Risk Factors Involved. J. Clin. Med. 2023, 12, 5499. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Shibazaki, K.; Uchida, R.; Imai, Y.; Mukoyama, T.; Shibata, S.; Morita, H. Sarcopenia is associated with the Geriatric Nutritional Risk Index in elderly patients with poorly controlled type 2 diabetes mellitus. J. Diabetes Investig. 2022, 13, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Massimino, E.; Izzo, A.; Castaldo, C.; Ferretti, E.; Rivellese, A.A.; Della Pepa, G. Risk of Sarcopenia and Associated Factors in Older Adults with Type 2 Diabetes: An Exploratory Cross-Sectional Study. Healthcare 2023, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Hiromine, Y.; Noso, S.; Rakugi, H.; Sugimoto, K.; Takata, Y.; Fukuda, M.; Akasaka, H.; Osawa, H.; Tabara, Y.; Ikegami, H. Poor glycemic control rather than types of diabetes is a risk factor for sarcopenia in diabetes mellitus: The MUSCLES-DM study. J. Diabetes Investig. 2022, 13, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Tsiridis, E.; Goulis, G. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef]
- Chen, D.S.; Zhu, Y.Q.; Ni, W.J.; Li, Y.; Yin, G.; Shao, Z.; Zhu, J. Hand grip strength is inversely associated with total daily insulin dose requirement in patients with type 2 diabetes mellitus: A cross-sectional study. PeerJ 2023, 11, e15761. [Google Scholar] [CrossRef]
- Beretta, M.V.; Dantas Filho, F.F.; Freiberg, R.E.; Feldman, J.V.; Nery, C.; Rodrigues, T.C. Sarcopenia and Type 2 diabetes mellitus as predictors of 2-year mortality after hospital discharge in a cohort of hospitalized older adults. Diabetes Res. Clin. Pract. 2020, 159, 107969. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Tra, Y.; Egan, J.M.; Ferrucci, L.; Brancati, F. Hyperglycemia is associated with relatively lower lean body mass in older adults. J. Nutr. Health Aging 2014, 18, 737–743. [Google Scholar] [CrossRef]
- Koo, B.K.; Moon, S.; Moon, M.K. Muscle strength, an independent determinant of glycemic control in older adults with long-standing type 2 diabetes: A prospective cohort study. BMC Geriatr. 2021, 21, 684. [Google Scholar] [CrossRef]
- Shi, X.; Liu, W.; Zhang, L.; Xiao, F.; Huang, P.; Yan, B.; Zhang, Y.; Su, W.; Jiang, Q.; Lin, M.; et al. Sex-Specific Associations Between Low Muscle Mass and Glucose Fluctuations in Patients with Type 2 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 913207. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ikegami, H.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Tabara, Y.; Osawa, H.; Hiromine, Y.; Rakugi, H. Glycemic Control and Insulin Improve Muscle Mass and Gait Speed in Type 2 Diabetes: The MUSCLES-DM Study. J. Am. Med. Dir. Assoc. 2021, 22, 834–838.e1. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Tabara, Y.; Ikegami, H.; Takata, Y.; Kamide, K.; Ikezoe, T.; Kiyoshige, E.; Makutani, Y.; Onuma, H.; Gondo, Y.; et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The Multicenter Study for Clarifying Evidence for Sarcopenia in Patients with Diabetes Mellitus. J. Diabetes Investig. 2019, 10, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Barbieri, M.; Fava, I.; Desiderio, M.; Coppola, C.; Marfella, R.; Paolisso, G. Sarcopenia in Elderly Diabetic Patients: Role of Dipeptidyl Peptidase 4 Inhibitors. J. Am. Med. Dir. Assoc. 2016, 17, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izuyama, H.; Yoshimoto, T.; Ogawa, Y. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 34, e2957. [Google Scholar] [CrossRef]
- Lee, C.G.; Boyko, E.J.; Barrett-Connor, E.; Hoffman, A.R.; Rose, S.A.; Lewis, C.E.; Cawthon, P.M.; Orwoll, E.S. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011, 34, 2381–2386. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Khan, S.A.; Shields, S.; Abusamaan, M.S.; Mathioudakis, N. Association between dysglycemia and the Charlson Comorbidity Index among hospitalized patients with diabetes. J. Diabetes Complicat. 2022, 36, 108305. [Google Scholar] [CrossRef]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef]
- Somagutta, M.R.; Uday, U.; Bathula, N.R.; Pendyala, S.; Jain, M.; Mahmutaj, G.; Gad, M.; Batula, N.; Pendyala, S. Diagnosing Frailty in Primary Care Practice. Cureus 2022, 14, e23329. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Boineau, R.E.; Higginbotham, M.B.; Lee, K.; Mark, D.; Califf, R.; Cobb, F.; Pryor, D. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 1989, 64, 651–654. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, H.S.; Lee, Y.J.; Lee, J.H. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res. Clin. Pract. 2021, 180, 109042. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Enríquez-Reyna, M.C.; Garza-Sepúlveda, G.; Tijerina-Sáenz, A.; Ramos-Peña, E.; Gómez de la Garza, M. Puntos de corte y validación de una ecuación antropométrica para estimar la masa muscular, en el estudio de la sarcopenia en población mexicana. Salud Publica Mex. 2015, 57, 485–486. [Google Scholar] [PubMed]
- Pérez-Zepeda, M.U.; Sánchez-Garrido, N.; González-Lara, M.; Gutiérrez-Robledo, L.M. Sarcopenia prevalence using simple measurements and population-based cutoff values. J. Lat. Am. Geriatr. Med. 2016, 2, 8–13. [Google Scholar] [PubMed]
- Rangel Peniche, D.B.; Alemán Mateo, H.; Barreiro, M.L.A.A.; Ruiz Valenzuela, R.E.; Ramírez-Torres, M.; Urquidez-Romero, R. Differences in Body Composition in Older People from Two Regions of Mexico: Implications for Diagnoses of Sarcopenia and Sarcopenic Obesity. Biomed. Res. Int. 2018, 2018, 7538625. [Google Scholar] [CrossRef] [PubMed]
- Therakomen, V.; Petchlorlian, A.; Lakananurak, N. Prevalence and risk factors of primary sarcopenia in community-dwelling outpatient elderly: A cross-sectional study. Sci. Rep. 2020, 10, 19551. [Google Scholar] [CrossRef]
- Liguori, I.; Curcio, F.; Russo, G.; Cellurale, M.; Aran, L.; Bulli, G.; Morte, D.; Gargiulo, G.; Testa, G. Risk of Malnutrition Evaluated by Mini Nutritional Assessment and Sarcopenia in Noninstitutionalized Elderly People. Nutr. Clin. Pract. 2018, 33, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, I.J.M.; den Braber, N.; Oosterwijk, M.M.; Gant, C.M.; Navis, G.; Hutten, M.; Bejinum, B.; Bakker, S.J.; Laverman, G.D. Low Physical Activity in Patients with Complicated Type 2 Diabetes Mellitus Is Associated with Low Muscle Mass and Low Protein Intake. J. Clin. Med. 2020, 9, 3104. [Google Scholar] [CrossRef]
- Sun, L.; Fu, J.; Mu, Z.; Duan, X.; Chan, P.; Xiu, S. Association between body fat and sarcopenia in older adults with type 2 diabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1094075. [Google Scholar] [CrossRef]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef]
- Li, J.J.; Wittert, G.A.; Vincent, A.; Atlantis, E.; Shi, Z.; Appleton, S.L.; Hill, C.L.; Jenkins, A.J. Muscle grip strength predicts incident type 2 diabetes: Population-based cohort study. Metabolism 2016, 65, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Ogawa, Y. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif. Tissue Int. 2017, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, U.; Then, C.; Rottenkolber, M.; Selte, C.; Seissler, J.; Conzade, R.; Linkohr, B.; Peters, A.; Drey, M.; Thorand, B. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-age study. Acta Diabetol. 2020, 57, 1057–1063. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).