Abstract
Electrochemical sensing arrays enable the spatial study of dopamine levels throughout brain slices, the diffusion of electroactive molecules, as well as neurotransmitter secretion from single cells. The integration of complementary metal-oxide semiconductor (CMOS) devices in the development of electrochemical sensing devices enables large-scale parallel recordings, providing beneficial high-throughput for drug screening studies, brain–machine interfaces, and single-cell electrophysiology. In this paper, an electrochemical sensor capable of recording at 40,000 frames per second using a CMOS sensor array with 1024 electrochemical detectors and a custom field-programmable gate array data acquisition system is detailed. A total of 1024 on-chip electrodes are monolithically integrated onto the designed CMOS chip through post-CMOS fabrication. Each electrode is paired with a dedicated transimpedance amplifier, providing 1024 parallel electrochemical sensors for high-throughput studies. To support the level of data generated by the electrochemical device, a powerful data acquisition system is designed to operate the sensor array as well as digitize and transmit the output of the CMOS chip. Using the presented electrochemical sensing system, both dopamine and hydrogen peroxide diffusions across the sensor array are successfully recorded at 40,000 frames per second across the 32 × 32 electrochemical detector array.
1. Introduction
Recent advancements have provided enhanced spatiotemporal resolution for electrochemical studies such as the imaging of diffusing electroactive molecules [1,2], detection of toxic metabolites such as pyocyanin [3,4], and mapping of hydrogen peroxide (H2O2) levels, in association with neurological diseases, throughout a brain slice [5] using electrochemical sensor arrays. Using electrochemical techniques, the redox molecules present in electrochemical studies will either release electrons, oxidization, producing a negative current as the electrons are accepted by the electrode, or will take electrons from the electrode, reduction, producing a positive current. For example, the presence of neurotransmitters, such as dopamine, can be monitored by direct measurement of the released electrons during an oxidation reaction at an electrode. The dynamics of redox molecule diffusion may only last milliseconds to microseconds; as such, the high temporal resolution provided by electrochemical recordings provides a unique advantage in capturing details of these reactions. Additionally, it is possible to be highly specific to, and highly sensitive of, the electroactive molecules using electrochemical detection techniques. Dopamine mapping at the microscale can help reveal the real-time neurological degeneration, particularly associated with Parkinson’s disease, and help better understand the early-stage progress of the disorders. Oxidative stress is a widespread symptom of ADHD, Parkinson’s disease, and Alzheimer’s disease, which produces peroxides and free radicals as a byproduct, and therefore, the mapping of peroxide can probe the oxidative stress from tissues at a cellular level. To further enhance the high specificity and temporal resolution of electrochemical recordings, the miniaturization and parallelization of the electronics and sensors are prevalent in emerging devices that explore the integration of microelectrode arrays (MEA) and complementary metal-oxide semiconductor (CMOS) technology. The parallelization of the sensor array enables the construction of spatial data sets based on the sensor array’s geometric arrangement. However, as the electrode count of MEAs increases, the resulting expansion of parallel electrochemical recordings introduces an instrumentation challenge. Direct wiring of electrodes to their corresponding amplifiers becomes impractical, and using commercially available amplifiers is costly. To support the increasing throughput of electrochemical recordings, CMOS technology is being explored as an interface, as it can provide arrays of readout circuitry that amplify and process the electrochemical signals detected by MEAs [6]. Through on-chip integration of MEAs, interconnections are simplified, and readout circuitry is scalable, allowing large-scale electrochemical sensor arrays on small silicon chips. Recently developed devices include an array of 59,760 electrodes capable of up to 2048 simultaneous recordings [7,8] and an array of 10 × 20 electrodes with the capability of performing voltammetry as well as amperometry [9]. Recent state-of-the-art electrochemical imaging sensors include a 32 × 32 array that provides electrochemical images at 90 frames per second [1] and an array of 100 electrodes for up to 100 parallel recordings of neurotransmitter release from chromaffin cells [10,11]. A 64 × 64 potentiostat array was developed for electrochemical impedance spectroscopy (EIS) that was able to achieve 0.025 frames per second [12]. Another EIS sensor integrated 512 × 256 on-chip electrodes with 8 potentiostats which achieved a frame rate of ~0.14 frames per second [13].
The monolithic integration of the electrochemical sensors simplifies the connections between the electrodes and the readout circuitry, but an external data acquisition system remains necessary to support operation of the complex functionality of the sensor arrays and transfer of the recorded electrochemical signals to a computer for storage and analysis. The external system can be simplified through the integration of analog-to-digital converters (ADCs) in the CMOS chip. However, the integration of ADCs in the CMOS chip requires a large area; as such, it can be preferable to instead use external ADCs. External ADCs have additional benefits, such as cost effectiveness, as sensors are generally being treated as single-use devices, due to their need to make direct contact with the biological or chemical substances being investigated. Future advancements in biotechnology will require high-throughput data acquisition to enable high-throughput gene sequencing [14,15], high-throughput drug screening studies [16,17], high-density brain–machine interfacing [18,19,20], and high-throughput single-cell electrophysiology [10,11,21,22]. To support high-throughput applications, the external acquisition system must support fast analog-to-digital conversion, extensive data transmission, and highly parallelized signal processing. For an electrochemical imaging device with 1000 sensors operating at 40,000 frames per second, a minimum cumulative sampling rate of 40 million samples per second (S/s) is required. If 16-bit ADCs are used for digitization, 2 bytes are created per sample, and the minimum data transmission rate is 80 Mbytes per second (MB/s). A high-performance acquisition system was designed using commercially available data acquisition modules and is capable of digitizing 16 analog inputs at a maximum sampling rate of 120 MS/s at a 16-bit resolution [23,24]. However, this system is very costly and lacks a sufficient number of analog inputs for a high-throughput CMOS device used for parallel recordings of neurotransmitter release from single cells [2,22,25]. As an alternative to commercially available systems, low-cost data acquisition systems have been presented using Arduinos, one 16-bit analog input with a sampling rate of up to 860 S/s [26], and custom acquisition systems controlled by microcontrollers, eight 16-bit analog inputs and a maximum sampling rate of 20 kS/s [27], or field-programmable gate array (FPGAs), 1024 16-bit analog inputs at a sampling rate of 30 kS/s [28] or 64 16-bit analog inputs with a sampling rate up to 200 kS/s/ch [29]. Although the FPGA-based custom acquisition systems may have the adequate number of analog channels for the custom CMOS electrochemical imaging device discussed in Section 2.1 (32 parallel outputs), these FPGA systems do not provide sampling rates sufficient to achieve a 40 kHz frame rate as a minimum sampling rate of 1.28 MS/s/ch is required, as discussed in Section 2.3. To support the data transmission demands for high-throughput applications, the selected interface must be considered. Common interfaces used for data acquisition systems are universal serial bus (USB), peripheral component interconnect (PCI), and PCI Express (PCIe). Among these interfaces, USB is the most accessible due to its availability on virtually all desktop computers as well as laptops, whereas PCI and PCIe interfaces are limited on desktop computers and require external adapters for use on laptops. For high-throughput electrochemical imaging, the growing prevalence of the USB 3.0 interface provides sufficient data transmission at up to 625 MB/s and broadens the applicability of data acquisition systems outside of well-equipped research laboratories. By using the power delivered through the USB interface, ranging from 4.5 watts (W) for USB 3.0 and up to 15 W for type-C, the data acquisition systems can be designed for applications requiring portable high-performance devices.
In this paper, we present the design of an electrochemical sensing platform that is capable of 1024 simultaneous electrochemical recordings at a frame rate of 40,000 per second. A recent state-of-the-art electrochemical imaging system using a CMOS device with a 16,064 electrode MEA for monitoring oxygen gradients across bovine cumulus-oocytes-complexes cells [30] uses a custom data acquisition system to provide a frame rate up to 4 Hz [31]. Another system with a 128 × 128 array achieves real-time ion imaging with a custom acquisition system to provide a maximum frame rate of 6100 frames per second [32]. A third CMOS-based electrochemical imaging system with 4096 pixels was post-processed to have a 256-electrochemical-cell array for pH spatiotemporal mapping [33], and the developed setup for this CMOS device can use up to three commercial data acquisition cards to acquire data from the entire 4096 pixel array at a frame rate up to 9.4 kHz [34]. Thus, the new electrochemical imaging system presented in this manuscript, which is capable of a frame rate of 40,000 frames per second, is one of the fastest devices reported to date with high spatial resolution (Figure 1). This is achieved by collective innovations in the system design, including CMOS design, packaging, a FPGA-based data acquisition system, and a signal processing and analysis program. The designed system provides significant benefits for studies that require high-throughput and high spatial and/or temporal resolution, such as high-throughput single-cell amperometry to study the mechanisms of neurological diseases, the mechanisms of vesicle secretion, and the dynamics of neurotransmitter release. In the following sections, the design of the 1024-pixel electrochemical imaging sensor will be presented alongside the custom data acquisition system that enables a frame rate of 40,000 frames per second. In Section 2.1, the design of the CMOS electrochemical detector is discussed, and in Section 2.2, the post-CMOS processing of the CMOS chip is discussed. Section 2.3 discusses the packaging of the CMOS chip to ensure the device is biocompatible and waterproof. In Section 2.4, the data acquisition system and its parts are discussed, while in Section 2.5, the design of the FPGA architecture is discussed. Section 2.6 presents the experimental procedures for the electrochemical imaging of dopamine and H2O2 diffusion using the designed electrochemical imaging sensor. Section 3.1 discusses the noise characterization and performance of the data acquisition system, and Section 3.2 and Section 3.3 discuss the electrochemical imaging of dopamine and hydrogen peroxide, respectively, diffusing over the electrochemical sensor array.
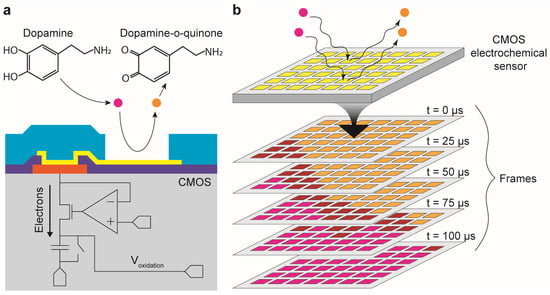
Figure 1.
Rapid electrochemical imaging sensor array with 1024 pixels and a frame rate of 40,000 frames per second. (a) Electroactive molecules such as dopamine release electrons into individual electrochemical sensors through electrochemical reactions. Individual sensors consist of on-chip platinum electrodes and integrated transimpedance amplifiers for immediate amplification of pA-nA signals. (b) The CMOS electrochemical sensor array integrates 1024 electrochemical sensors in a single silicon chip which can operate parallel electrochemical sensing. Therefore, the electrochemical imaging can be done at the full sampling rate which is 40,000 frames per second.
2. Materials and Methods
2.1. CMOS Electrochemical Detector Design
For two-dimensional imaging of dopamine diffusion, a CMOS device with an electrochemical detector array is designed to measure the electrochemical signals from molecules diffusing on the surface of the chip in parallel. The CMOS electrochemical detector array is designed using Cadence Virtuoso Analog Design Environment (Cadence Design Systems Inc., San Jose, CA, USA) and fabricated using a standard 2-poly 4-metal 0.35 µm CMOS process [22,25]. The flow of electrons into the electrode results in small currents that range from pA to nA. To record these small currents, an electrochemical detector is designed which consists of an integration capacitor-based transimpedance amplifier (TIA) (Figure 2). Using the feedback of the operational amplifier, the potential of the electrode is regulated to the input Vref, thus enabling redox reactions at the electrode for electrochemical sensing. As electrons flow through the electrode due to oxidation or reduction current, the electrochemical current is integrated into a capacitor. A regulated cascode topology is used to maximize the injection efficiency (Figure 2) [35]. This regulated cascode topology reduces the input impedance, and since the input current splits between the input impedance of the amplifier and the impedance from the double layer created at the electrode/electrolyte interface, the lower input impedance achieves higher injection efficiency of the input current into the amplifier. The integration of current on the capacitor results in a change in voltage at the TIA’s readout node. The voltage at the readout node is read periodically, and the voltage of the integration capacitor is reset during the readout phase to start the next integration cycle. The length of the periodic readout and subsequent reset of the integration capacitor by switch (Figure 2), determines the sampling rate of the TIA. To achieve a 40 kS/s sampling rate, the periodic readout must occur every 25 µs. The designed TIA occupies an area of 30 × 30 µm2 and includes a 1-bit static random-access memory (SRAM) to enable testing circuitry and a dedicated switch to alter the total integration capacitance, thus changing the current to voltage gain of the TIA.
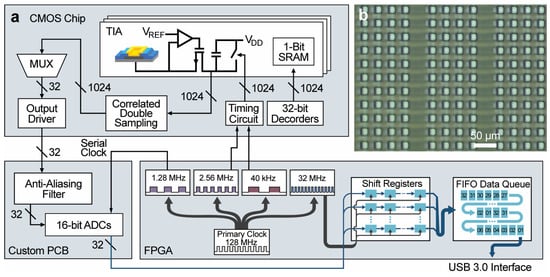
Figure 2.
Overview of the custom electrochemical imaging system’s architecture. (a) An array of 1024 amplifiers, with an array of 1024 monolithically integrated electrodes, detects electroactive molecules at the electrode through electron transactions by redox reactions. The 32 parallel outputs from the CMOS chip are passed to the custom PCB containing 32 ADCs. (b) Microphotograph of a section of the integrated microelectrode array. Each platinum electrode is insulated by SU-8 and has an active area of 15 μm × 15 μm. The pitch between electrodes is 30 μm.
To enable large-scale electrochemical recordings, the CMOS chip has an array of 32 × 32 electrochemical detectors. The recordings from the 1024 individual detectors are multiplexed to 32 parallel outputs on a column basis. Each multiplexer selects one electrochemical sensor through a column of the CMOS array using time-division multiplexing. This time-division multiplexing scheme sequentially staggers the readout period of the rows such that one detector is being read while the rest are integrating, eliminating dead time due to readout. To operate the integrating TIAs and multiplex the 1024 electrochemical detectors, the timing circuits are designed to generate all necessary signals using two external clock inputs. To send the electrochemical recordings to a computer for analysis, the CMOS chip relies on external ADCs in the FPGA-based data acquisition system described in Section 2.4. The multiplexed outputs of the electrochemical detector array are fed into output buffers in order to drive the capacitive load from the output lines, bonding pads, and the input capacitance of the ADCs.
2.2. Post-CMOS Processing
A common material used for the top metal layer for CMOS fabrication is an aluminum/copper alloy, which is reactive to solutions used for electrochemical experiments and is inadequate for applications requiring high signal-to-noise performance. To achieve a high signal-to-noise ratio, polarizable electrode materials, such as platinum and gold, are used because of their low reactivity to electrolytic solutions. Through post-CMOS processing, a MEA using a polarizable material, platinum, is integrated onto the surface of the CMOS chip. To integrate the MEA onto the device, a lift-off process is accomplished through photolithography using negative photoresist. The lift-off process consists of creating a sacrificial layer of photoresist, patterning the desired electrode patterns into the sacrificial layer, permitting access to the surface of the CMOS chip, deposition of metal materials, and removal of the sacrificial layer, leaving only the desired electrode patterns on the surface of the device. Prior to the photolithography process, each CMOS die (5 mm × 5 mm) is bonded to a coverslip (25 mm × 25 mm) using epoxy in order to reduce the complications of handling small samples for post-CMOS processing. The bonded die is then cleaned by bathing it in acetone, isopropanol, and then DI water. The bonded die is spin-coated with a negative photoresist, NR9-1500PY (Futurrex Inc., Franklin, NJ, USA), to cover the entire surface of the device. The device is then put into a contact aligner (EVG 620, EV Group, St. Florian am Inn, Austria) with a custom-designed photomask to correctly position the desired MEA pattern on the CMOS chip. The chip is exposed to UV light in the aligner and then developed to remove photoresist from the surface of the device where metal deposition is desired. To integrate the platinum MEA, 20 nm of Ti is first deposited to act as an adhesion layer between the Al/Cu and Pt, then 200 nm of Pt is deposited using a sputtering machine (AJA Six-Gun Sputtering System, AJA International Inc., Hingham, MA, USA). After the lift-off process, the dies are cleaned by bathing the chips in acetone, isopropanol, and then DI water. The chip is spin-coated to cover the CMOS chip with SU-8 photoresist (Kayaku Advanced Materials Inc., Westborough, MA, USA), exposed to UV light using the contact aligner, and a second image on the designed photomask containing an array of well structures that are 15 μm × 15 μm in size. Finally, the exposed SU-8 is developed to remove undesired SU-8 from the surface of the CMOS chip and provide access to the platinum electrodes throughout the MEA [22]. After all post-CMOS fabrication is complete, the integrated electrode array consists of 1024 SU-8 insulated platinum electrodes, providing an active electrode area of 15 μm × 15 μm, with a pitch of 30 μm. In addition to electrode passivation, the SU-8 provides a trap feature that enables single-cell analysis as previously shown [22]. The biocompatible nature of platinum and SU-8 has been previously shown with cell viability [36,37].
2.3. Biocompatible Packaging
After the MEA is integrated onto the CMOS chip, it is bonded onto a custom-designed PCB die holder using epoxy. After the epoxy has cured, the pads on the CMOS chip are wire bonded to the PCB pads to provide an interface between the CMOS electrochemical detector array and the external data acquisition system. To protect the wire bonds when handling the device, as well as from liquids during electrochemical experiments, a 3D-printed well made of acrylonitrile butadiene styrene (ABS) plastic is coated with polydimethylsiloxane (PDMS), which is commonly used for its biocompatible properties [38], and then bonded to the surface of the CMOS chip using additional PDMS. The custom-designed ABS well provides an opening to access the surface of the CMOS device and accommodates 2 mL of liquid for electrochemical experiments. This packaging approach is based on our previous work [21] that has reliably demonstrated stability in cell culture conditions for up to 24 h.
2.4. Design of FPGA-Based Data Acquisition System
The integration and readout of the electrochemical detector array during electrochemical recording is controlled using an FPGA-based data acquisition system that interfaces with the CMOS chip. The acquisition system includes 32 ADCs, each with a 16-bit resolution, one adjustable regulator to set the redox potential Vref (Figure 2), 16 digital inputs and outputs (DIO), and a USB 3.0 SuperSpeed interface. The acquisition system consists of an Opal Kelly (Opal Kelly Inc., Portland, OR, USA) FPGA board and a custom-designed PCB. The FPGA board has a Cypress FX3 ( Infineon Technologies AG, Neubiberg, Germany) chip that provides the SuperSpeed USB 3.0 interface through which commands can be received to control the I/O of the Kintex-7 (Xilinx Inc., San Jose, CA, USA) FPGA chip. The FPGA chip generates the DIO that operates the electrochemical detector array, which includes the generated clocks used in the timing circuitry, and receives the parallel streams of data from the ADCs. To achieve synchronous operation of the electrochemical detector array and the ADCs, the FPGA generates all clock signals used throughout the electrochemical sensing system (Figure 2).
The custom-designed PCB contains 16 ADC chips (LTC2323-16, Analog Devices Inc., Wilmington, MA, USA) that each have two embedded ADCs, each of which can individually sample their input up to 5 MS/s, providing 32 high-speed 16-bit ADCs. Two stages of filters using the Sallen–Key filter topology are used for anti-aliasing. The ADCs convert the 32 parallel outputs from the electrochemical detector array to 16-bit digital representations which are sent serially to the DIO pins of the FPGA. The received data is then processed and packaged by the FPGA to be transferred to a PC through the USB 3.0 interface chip.
In summary (Table 1), the sampling rate from each electrode (potentiostat) is 40 kS/s. Because this CMOS chip consists of 1024 potentiostats, the total sampling rate of the entire array is 40.96 MS/s. The outputs are grouped in columns which consist of 32 potentiostats. Each output is sampled with an external 16-bit ADC at 1.28 MS/s. The chip has 32 columns of outputs, and therefore, the external board includes 32 ADCs. The total data rate output from all ADCs is 40.96 MS/s, which translates to 81.92 MB/s (2 bytes per sample).

Table 1.
Specifications of presented electrochemical imaging system.
2.5. FPGA Architecture
The FPGA’s architecture consists of clock generation, shift registers for parallel serial streams of data, a first-in, first-out (FIFO) data manager, and a USB 3.0 interface to control the functionality of the electrochemical sensing system and send data to a computer (Figure 2). Using a 200 MHz oscillator as a master source, the FPGA synchronously generates all the clocks required to initiate the sampling and readout of the ADCs as well as the integration and readout of the electrochemical detector array. The generated clocks are subdivided from a master clock of 128 MHz into frequencies of 32 MHz, 2.56 MHz, 1.28 MHz, and 40 kHz. By producing the clocks in this manner, the system is able to run synchronously across all of its operations. In addition to synchronously running the CMOS chip and the ADCs, the generated clocks are also repurposed inside the FPGA to operate the input shift registers and the FIFO data manager. The 40 kHz clock initiates the readout of the first row in the electrochemical detector array, the 2.56 MHz clock propagates the readout period over two clock pulses across the 32 columns to the next row in the array while returning the previous row to an integration period, producing an output frequency of 1.28 MHz for each column’s multiplexer. This results in an effective frame rate of 40,000 frames per second. Each of the 32 ADCs sample the 32 parallel CMOS outputs simultaneously at 1.28 MS/s and the 16-bit digital conversions are output from the ADCs in serial data streams at a rate of 32 MHz. The data from the ADCs are input to shift registers in the FPGA to allow parallel processing of the data. The data in the shift registers is then packaged by column such that the first data package contains the data from column 1, the next package contains data from column 2, and so on. The packaged data is then passed into a FIFO data queue that continuously fills until the computer requests data from the FPGA via a USB 3.0 interface. A custom-designed C++ program running on the connected computer is used to send the system reset signals and initialize the system prior to data collection, as well as receive data from the FIFO data queue using the FPGA’s application programming interface (API). The program allows a desired data amount to be specified and multiple sets of data to be easily collected back-to-back, useful for multiple successive experiments, with limitations on request size based only on the hardware present within the computer. The FPGA-based acquisition system can acquire 32 parallel streams of data at 1.28 MS/s/ch, which produces 40.96 MS/s, accumulatively (Figure 3). With each sample being at 16-bit resolution, the data produced is 655.36 Mb/s. The FPGA-based data acquisition system is tested by directly feeding a 50-kHz square and sinusoidal signal to all 32 channels (Figure 3c).
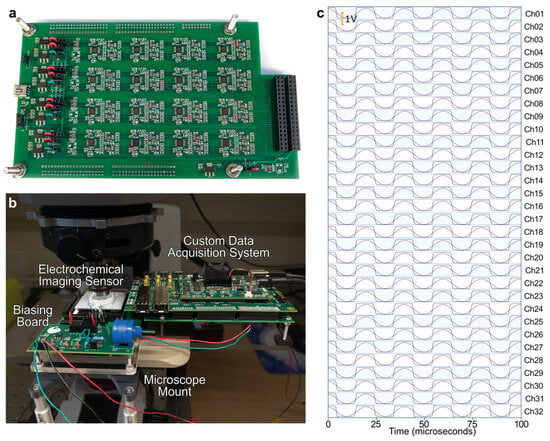
Figure 3.
High-throughput FPGA-based electrochemical imaging system. (a) Custom PCB with 32 high-speed high-accuracy ADCs. (b) Complete electrochemical imaging system consisting of the custom acquisition system and the CMOS electrochemical imaging sensor. (c) Demonstration of 32 parallel data streams input to the acquisition system at 1.28 MS/s/ch (40.96 MS/s, accumulatively).
2.6. Experimental Procedure for Electrochemical Imaging
To demonstrate rapid electrochemical imaging at 40,000 frames per second, the diffusion of both dopamine (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) and hydrogen peroxide (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) is recorded. For the dopamine diffusion experiment, 1mL of a phosphate-buffered saline (PBS) (Gibco, Waltham, MA, USA) solution is first added to the CMOS electrochemical sensor’s well, and a reference Ag|AgCl electrode is connected to ground and put in the well. To ensure that no bubbles are blocking access to the electrodes throughout the array, preventing an interface between the electrode and the electrolytic solution used for electrochemical experiments, the sensor and solution are agitated until no bubbles remain. When performing consecutive experiments, the electrolytic solutions used are never fully removed from the well to avoid drying of the electrodes, thus preventing bubbles from reappearing and blocking the electrode/electrolyte interface. To obtain a baseline recording of the MEA, a recording with only 1 mL of PBS solution on the electrochemical sensor is taken to ensure functionality of the array.
Then, a control experiment is performed by injecting 1 mL of PBS into the well. The control experiment is performed to ensure that the signals recorded from the later injected redox molecules are not a result of contamination in the PBS or changing influence of the reference electrode’s interface with the increasing electrolytic solution volume. When injecting into the well, for all experiments, the pipette is pointed into the corner of the well to avoid directly injecting onto the electrodes. This prevents signal saturation throughout the array immediately after injection, allowing the imaging of redox molecules diffusing across the sensor array. Following the control experiment, we record the injection of 1 mL of an 8 mM dopamine solution into the well, resulting in a final concentration of 4 mM. For these recordings, amperometry is the electrochemical technique used to detect the presence of dopamine throughout the array, and the electrode is held at a potential of 920 mV, enabling the oxidation of the dopamine molecules. The oxidation of dopamine molecules results in an increase in the current, ending in a higher steady-state level of the electrochemical sensor (Figure 4). The H2O2 experiment follows the same procedure outlined for the dopamine experiments; an 8 mM H2O2 injection is performed, resulting in 4 mM final concentration, for the electrochemical imaging of H2O2 diffusion.
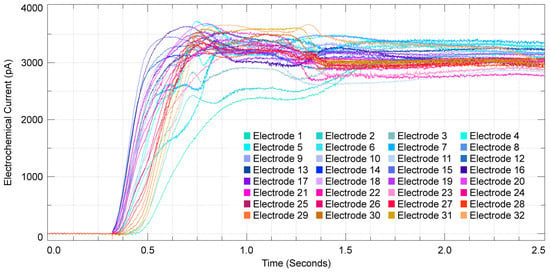
Figure 4.
Dopamine diffusion across a single column of the electrochemical array, each trace represents the output of an individual electrode over a period of 2.5 s.
3. Results and Discussion
3.1. Noise Characteristics and Performance
For the noise characterization of the ADCs in the data acquisition system, the filter setup on the custom-designed PCB consists of fourth-order low-pass filters with cutoff frequencies at 1 MHz. The noise is characterized at a 1.28 MS/s sampling rate, the minimum rate required to operate the CMOS imaging sensor at a frame rate of 40,000 frames per second. With the battery powered voltage input, the recorded noise floor is ~3 × 10−12 V2/Hz up until 3 kHz and starts to taper down to ~5 × 10−14 V2/Hz. The noise of the ADCs is 255 µVRMS when sampling at 1.28 MS/s. Based on the transimpedance gain of 84.6 µV/pA, the input-referred noise translates to 3.01 pARMS at 1.28 MS/s. The noise is also characterized by electrolytic solution on the electrode array. The total noise with the electrolytic solution is measured at 4.71 pARMS at 40 kS/s on each electrode/potentiostat after demultiplexing. The total noise of a single electrochemical sensor is dominated by the potentiostat and the electrode-electrolytic interface.
3.2. Fast Frame Rate Dopamine Electrochemical Imaging
Using the electrochemical imaging array, the diffusion of dopamine over time can be observed through the changes in the oxidation currents of the electrodes. The electrochemical measurements from a single column in the time domain have a rapid response from the dopamine diffusion across the array (Figure 4). Interestingly, the oxidation current increases in the middle channels of the selected column first (channels 14–19). Then, as dopamine diffuses across the array, we begin to see oxidation in the bottom channels (channels 28–32) and then finally the upper channels (channels 0–4). Therefore, the temporal resolution of the electrochemical electrode array is sufficiently fast to resolve the varying arrival time of electroactive molecules on each electrode. Using all column outputs, we can plot the complete electrochemical imaging for the diffusion of 4 mM of dopamine. Dopamine diffusion is recorded with a full framerate of 40,000 frames per second, and Figure 5a is created by selecting nine representative frames that span a 160-millisecond duration. In order to visualize the diffusion characteristics of dopamine, the electrochemical levels have been normalized for each electrochemical sensor and are represented by the yellow to black gradient, with yellow denoting the greatest recorded current levels, which directly relate to the amount of dopamine detected by a sensor until its steady-state point is reached. In the presented electrochemical imaging, the dopamine traverses from the right side to the left side of the sensor array (Figure 5a). The transient data shown in Figure 4 corresponds to a column of sensors on the left side of the array shown in Figure 5a, and the diffusion imaging shown in Figure 5a corroborates the diffusion direction that was captured in Figure 4. The diffusion time of dopamine from the bottom right corner of the array (the location of the pipette injection) to the top left corner (furthest from the injection) is measured to be 375.850 milliseconds. This was measured by comparing the time between the half-max value of their respective oxidation signals. Beyond the time presented in Figure 5a, the dopamine continues to diffuse across the entire array until nearly all of the sensors reach their own steady-state levels of oxidation current. To confirm that the signal we are recording is a result of the oxidation of the injected dopamine, control experiments are performed where PBS is injected into the PBS that is on the electrochemical sensor array. As shown in Figure 5c, there is no electrochemical signal recorded from injected PBS, and any fluctuations seen are only noise (Figure 5c,d). The temporal resolution of the presented device enables electrochemical imaging data frames at 25 microsecond intervals, providing the opportunity to observe events with very limited lifetimes.
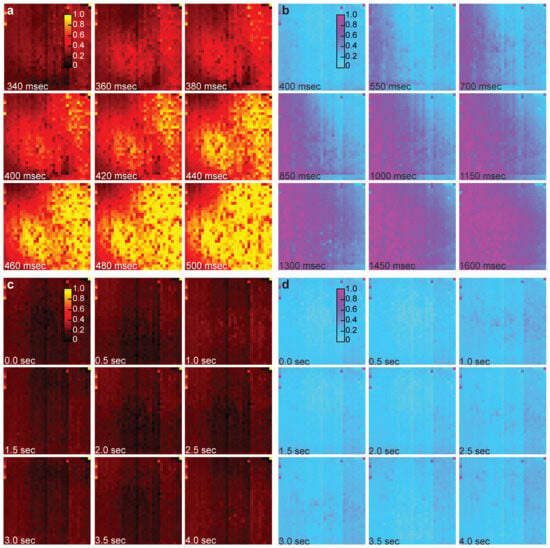
Figure 5.
Rapid electrochemical imaging of dopamine and hydrogen peroxide at 40,000 frames per second. (a) Electrochemical imaging of dopamine diffusion across the sensor array. Dopamine diffusion is recorded with a full framerate of 40,000 frames per second, and the image is created by selecting nine representative frames that span a 160-millisecond duration. (b) Electrochemical imaging of hydrogen peroxide diffusion across the sensor array. Hydrogen peroxide diffusion is also recorded with a full framerate of 40,000 frames per second, and the image is created by selecting nine representative frames that span a 1200-millisecond duration. (c) Control experiment for the dopamine imaging data set. (d) Control experiment for the peroxide imaging data set.
For the dopamine experiment shown in Figure 5a, the oxidation current recorded across the array is 2182.8 ± 832.6 pARMS (mean ± SD). Another experiment was conducted wherein the same concentration of dopamine was injected into the electrochemical array. For this second experiment, the oxidative signal that was recorded across the array is 2239.0 ± 1057.5 pARMS (mean ± SD). The repeated experiments showed consistent results. The signal-to-noise level is greater than 460 in both experiments. Based on the noise level of the electrochemical sensors, 8–9 µM of dopamine concentration will result in a signal above the noise floor.
3.3. Dynamic Hydrogen Peroxide Imaging
The diffusion of H2O2 (4 mM) across the sensor array is also imaged using the CMOS electrochemical sensor. Opposite to the dopamine experiment, the H2O2 spreads from the left to the right of the sensor array. The hydrogen peroxide imaging is recorded with a full frame rate of 40,000 frames per second, and the imaging shown in Figure 5b is generated by selecting nine representative frames across a 1200 millisecond span. The levels have been normalized for each electrochemical sensor and are represented by the purple to blue gradient, with purple denoting the greatest recorded current levels, which directly relate to the amount of hydrogen peroxide detected by a sensor until its steady-state point is reached. For this experiment, the diffusion time of hydrogen peroxide from the bottom right corner of the array (the location of the pipette injection) to the top left corner (furthest from the injection) is measured to be 1895.075 milliseconds. This was measured by comparing the time between the half-max value of their respective oxidation signals. Additionally, the recorded signals for the experiment shown in Figure 5b are 2386.0 ± 820.1 pARMS (mean ± SD). Another peroxide experiment was performed using the same concentration for injection, and the recorded signals are 2251.2 ± 889.9 pARMS (mean ± SD), nearly identical results to the experiment shown in Figure 5b. To ensure the recorded diffusion in Figure 5b was a result of peroxide oxidation, PBS was injected onto the array. As shown in Figure 5d, there is no significant signal recorded by the electrochemical sensors over 4 s. From these results, we see, similar to the dopamine experiment, that the sensor array is capable of providing spatiotemporal data that can be used to visualize the diffusion of the oxidizing agent through the PBS solution. The signal-to-noise level is greater than 470 in both experiments. Based on the noise level of the electrochemical sensors, 8–9 µM of hydrogen peroxide concentration will result in a signal above the noise floor.
4. Conclusions
In this paper, we present an electrochemical imaging platform, which consists of an electrochemical sensor and an FPGA-based data acquisition system, capable of recording a 32 × 32 pixel array of sensors at 40,000 frames per second, one of the fastest reported to date. The presented electrochemical detector, an integration-capacitor-based TIA with an integrated electrode, is used throughout a 32 × 32 array to enable large-scale electrochemical recordings. To prepare the device for electrochemical experiments, post-CMOS processing is used to integrate a platinum MEA onto the CMOS. To enable a fast 40,000 frames per second frame rate, an FPGA-based data acquisition system is designed to operate the electrochemical sensor and digitize the 32 parallel outputs of the CMOS chip. Compared to recent state-of-the-art electrochemical imaging sensors (Table 2) [39,40], the presented work demonstrates the highest frame rate at 40,000 frames per second, which is achieved by the large potentiostat array integrated into the custom CMOS chip.

Table 2.
Survey of recent electrochemical imaging systems.
The device is used to successfully image the diffusion of dopamine and hydrogen peroxide over the 32 × 32 pixel array of sensors at a frame rate of 40,000 frames per second. The recordings are done in real-time, and every sample from all channels (40,000 samples per second per channel, ~41 MSamples per second collectively) is collected with an external FPGA-based data acquisition system. This is possible because every clock in all steps of processing is synchronized (current integration of amplifier to digitization).
At this time, the data analysis and display are performed offline; however, we are currently investigating a method to integrate the digital signal processing, analysis, and display into the FPGA programming which can then selectively transmit the user-requested data for real-time display and quantifications. The integrated FPGA can also enable the integration of neural networks to further process the data in real-time and reduce the computational resources required by the computer to visualize and analyze the data. These solutions can potentially relax the USB 3.0 data rate and CPU usage.
The presented system provides significant benefits for studies that require high-throughput and high spatiotemporal resolution, such as high-throughput single-cell amperometry to study vesicle secretion, mechanisms of neurological diseases, and the dynamics of neurotransmitter release.
Author Contributions
Conceptualization, K.A.W. and B.N.K.; methodology, K.A.W. and B.N.K.; software, K.A.W., M.A.C. and B.N.K.; validation, K.A.W., M.A.C. and B.N.K.; formal analysis, K.A.W., M.A.C. and B.N.K.; investigation, K.A.W., M.A.C. and B.N.K.; resources, B.N.K.; data curation, K.A.W. and B.N.K.; writing—original draft preparation, K.A.W., M.A.C. and B.N.K.; writing—review and editing, K.A.W. and B.N.K.; visualization, K.A.W., M.A.C. and B.N.K.; supervision, B.N.K.; project administration, B.N.K.; funding acquisition, B.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation, grant numbers #2411567 and #2411566, and the United States Air Force Office of Scientific Research, grant number FA9550-21-1-0117.
Data Availability Statement
The data presented in this manuscript are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rothe, J.; Frey, O.; Stettler, A.; Chen, Y.; Hierlemann, A. Fully Integrated CMOS Microsystem for Electrochemical Measurements on 32 × 32 Working Electrodes at 90 Frames Per Second. Anal. Chem. 2014, 86, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Mulberry, G.; Kim, B.N. Rapid 1024-Pixel Electrochemical Imaging at 10,000 Frames Per Second Using Monolithic CMOS Sensor and Multifunctional Data Acquisition System. IEEE Sens. J. 2018, 18, 5507–5514. [Google Scholar] [CrossRef]
- Elliott, J.; Simoska, O.; Karasik, S.; Shear, J.B.; Stevenson, K.J. Transparent Carbon Ultramicroelectrode Arrays for the Electrochemical Detection of a Bacterial Warfare Toxin, Pyocyanin. Anal. Chem. 2017, 89, 6285–6289. [Google Scholar] [CrossRef] [PubMed]
- Simoska, O.; Sans, M.; Fitzpatrick, M.D.; Crittenden, C.M.; Eberlin, L.S.; Shear, J.B.; Stevenson, K.J. Real-Time Electrochemical Detection of Pseudomonas aeruginosa Phenazine Metabolites Using Transparent Carbon Ultramicroelectrode Arrays. ACS Sens. 2019, 4, 170–179. [Google Scholar] [CrossRef]
- Kasai, N.; Shimada, A.; Nyberg, T.; Torimitsu, K. An electrochemical sensor array and its application to real-time brain slice imaging. Electron. Commun. Jpn. 2009, 92, 1–6. [Google Scholar] [CrossRef]
- White, K.A.; Darroudi, M.; Park, J.; Kim, B.N. A 128-ch Area-Efficient Neurochemical-Sensing Front-End for FSCV Recordings of Dopamine. IEEE Sens. J. 2024, 24, 8788–8797. [Google Scholar] [CrossRef]
- Dragas, J.; Viswam, V.; Shadmani, A.; Chen, Y.; Bounik, R.; Stettler, A.; Radivojevic, M.; Geissler, S.; Obien, M.E.J.; Müller, J.; et al. In Vitro Multi-Functional Microelectrode Array Featuring 59 760 Electrodes, 2048 Electrophysiology Channels, Stimulation, Impedance Measurement, and Neurotransmitter Detection Channels. IEEE J. Solid-State Circuits 2017, 52, 1576–1590. [Google Scholar] [CrossRef]
- Viswam, V.; Dragas, J.; Shadmani, A.; Chen, Y.; Stettler, A.; Müller, J.; Hierlemann, A. 22.8 Multi-functional microelectrode array system featuring 59,760 electrodes, 2048 electrophysiology channels, impedance and neurotransmitter measurement units. In Proceedings of the 2016 IEEE International Solid-State Circuits Conference (ISSCC), San Francisco, CA, USA, 31 January–4 February 2016; pp. 394–396. [Google Scholar]
- Guo, J.; Ng, W.; Yuan, J.; Li, S.; Chan, M. A 200-Channel Area-Power-Efficient Chemical and Electrical Dual-Mode Acquisition IC for the Study of Neurodegenerative Diseases. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 567–578. [Google Scholar] [CrossRef]
- Huang, M.; Delacruz, J.B.; Ruelas, J.C.; Rathore, S.S.; Lindau, M. Surface-modified CMOS IC electrochemical sensor array targeting single chromaffin cells for highly parallel amperometry measurements. Pflügers Arch. Eur. J. Physiol. 2018, 470, 113–123. [Google Scholar] [CrossRef]
- Kim, B.N.; Herbst, A.D.; Kim, S.J.; Minch, B.A.; Lindau, M. Parallel recording of neurotransmitters release from chromaffin cells using a 10×10 CMOS IC potentiostat array with on-chip working electrodes. Biosens. Bioelectron. 2013, 41, 736–744. [Google Scholar] [CrossRef]
- Abbott, J.; Mukherjee, A.; Wu, W.; Ye, T.; Jung, H.S.; Cheung, K.M.; Gertner, R.S.; Basan, M.; Ham, D.; Park, H. Multi-parametric functional imaging of cell cultures and tissues with a CMOS microelectrode array. Lab Chip 2022, 22, 1286–1296. [Google Scholar] [CrossRef]
- Hu, K.; Ho, J.; Rosenstein, J.K. Super-Resolution Electrochemical Impedance Imaging With a 512 × 256 CMOS Sensor Array. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 502–510. [Google Scholar] [CrossRef]
- Rosenstein, J.K.; Wanunu, M.; Merchant, C.A.; Drndic, M.; Shepard, K.L. Integrated nanopore sensing platform with sub-microsecond temporal resolution. Nat. Methods 2012, 9, 487–492. [Google Scholar] [CrossRef]
- Shekar, S.; Niedzwiecki, D.J.; Chien, C.-C.; Ong, P.; Fleischer, D.A.; Lin, J.; Rosenstein, J.K.; Drndić, M.; Shepard, K.L. Measurement of DNA Translocation Dynamics in a Solid-State Nanopore at 100 ns Temporal Resolution. Nano Lett. 2016, 16, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.; Bowlby, M.; Peri, R.; Vasilyev, D.; Arias, R. High-throughput electrophysiology: An emerging paradigm for ion-channel screening and physiology. Nat. Rev. Drug Discov. 2008, 7, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Aziz, M.K.; Li, S.; Chi, T.; Grijalva, S.I.; Sung, J.H.; Cho, H.C.; Wang, H. 1024-Pixel CMOS Multimodality Joint Cellular Sensor/Stimulator Array for Real-Time Holistic Cellular Characterization and Cell-Based Drug Screening. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 80–94. [Google Scholar] [CrossRef]
- Borton, D.A.; Yin, M.; Aceros, J.; Nurmikko, A. An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 2013, 10, 026010. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, E.; Basu, A. A 128-Channel Extreme Learning Machine-Based Neural Decoder for Brain Machine Interfaces. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.W.; Lee, J.; Li, S.; Yu, S.; Kilfovle, C.; Larson, L.; Nurmikko, A.; Laiwalla, F. A CMOS Distributed Sensor System for High-Density Wireless Neural Implants for Brain-Machine Interfaces. In Proceedings of the ESSCIRC 2018—IEEE 44th European Solid State Circuits Conference (ESSCIRC), Dresden, Germany, 3–6 September 2018; pp. 230–233. [Google Scholar]
- White, K.A.; Kim, B.N. Quantifying neurotransmitter secretion at single-vesicle resolution using high-density complementary metal–oxide–semiconductor electrode array. Nat. Commun. 2021, 12, 431. [Google Scholar] [CrossRef]
- White, K.A.; Mulberry, G.; Smith, J.; Lindau, M.; Minch, B.A.; Sugaya, K.; Kim, B.N. Single-Cell Recording of Vesicle Release From Human Neuroblastoma Cells Using 1024-ch Monolithic CMOS Bioelectronics. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zheng, W.; Zhang, M.; Yuan, T.; Zhuang, G.; Pan, Y. A Real-Time Data Acquisition and Processing Framework Based on FlexRIO FPGA and ITER Fast Plant System Controller. IEEE Trans. Nucl. Sci. 2016, 63, 1715–1719. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, R.; Zhang, M.; Zhuang, G.; Yuan, T. Design of FPGA based high-speed data acquisition and real-time data processing system on J-TEXT tokamak. Fusion Eng. Des. 2014, 89, 698–701. [Google Scholar] [CrossRef]
- Mulberry, G.; White, K.A.; Kim, B.N. Analysis of Simple Half-Shared Transimpedance Amplifier for Picoampere Biosensor Measurements. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Grinias, J.P.; Whitfield, J.T.; Guetschow, E.D.; Kennedy, R.T. An Inexpensive, Open-Source USB Arduino Data Acquisition Device for Chemical Instrumentation. J. Chem. Educ. 2016, 93, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Ferrero Martín, F.J.; Valledor Llopis, M.; Campo Rodríguez, J.C.; Blanco González, J.R.; Menéndez Blanco, J. Low-cost open-source multifunction data acquisition system for accurate measurements. Measurement 2014, 55, 265–271. [Google Scholar] [CrossRef]
- Kinney, J.P.; Bernstein, J.G.; Meyer, A.J.; Barber, J.B.; Bolivar, M.; Newbold, B.; Scholvin, J.; Moore-Kochlacs, C.; Wentz, C.T.; Kopell, N.J.; et al. A direct-to-drive neural data acquisition system. Front. Neural Circuits 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, R.; Mandaliya, H.; Patel, J.; Kumari, P.; Gautam, P.; Raulji, V.; Edappala, P.; Pujara, H.D.; Jha, R. Embedded multi-channel data acquisition system on FPGA for Aditya Tokamak. Fusion Eng. Des. 2016, 112, 964–968. [Google Scholar] [CrossRef]
- Tedjo, W.; Obeidat, Y.; Catandi, G.; Carnevale, E.; Chen, T. Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array. Biosensors 2021, 11, 256. [Google Scholar] [CrossRef]
- Tedjo, W.; Chen, T. An Integrated Biosensor System With a High-Density Microelectrode Array for Real-Time Electrochemical Imaging. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 20–35. [Google Scholar] [CrossRef]
- Zeng, J.; Kuang, L.; Cacho-Soblechero, M.; Georgiou, P. An Ultra-High Frame Rate Ion Imaging Platform Using ISFET Arrays With Real-Time Compression. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 820–833. [Google Scholar] [CrossRef]
- Jung, H.S.; Jung, W.-B.; Wang, J.; Abbott, J.; Horgan, A.; Fournier, M.; Hinton, H.; Hwang, Y.-H.; Godron, X.; Nicol, R.; et al. CMOS electrochemical pH localizer-imager. Sci. Adv. 2022, 8, eabm6815. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.; Ye, T.; Krenek, K.; Qin, L.; Kim, Y.; Wu, W.; Gertner, R.S.; Park, H.; Ham, D. The Design of a CMOS Nanoelectrode Array With 4096 Current-Clamp/Voltage-Clamp Amplifiers for Intracellular Recording/Stimulation of Mammalian Neurons. IEEE J. Solid-State Circuits 2020, 55, 2567–2582. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Gillis, K.D.; Lindau, M.; Minch, B.A. Design of a CMOS Potentiostat Circuit for Electrochemical Detector Arrays. IEEE Trans. Circuits Syst. I Regul. Pap. 2007, 54, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Yoshida, K.; Koch, K.P.; Navarro, X. Assessment of Biocompatibility of Chronically Implanted Polyimide and Platinum Intrafascicular Electrodes. IEEE Trans. Biomed. Eng. 2007, 54, 281–290. [Google Scholar] [CrossRef]
- Nemani, K.V.; Moodie, K.L.; Brennick, J.B.; Su, A.; Gimi, B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater. Sci. Eng. C 2013, 33, 4453–4459. [Google Scholar] [CrossRef]
- Bélanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef]
- Huang, M.; Dorta-Quiñones, C.I.; Minch, B.A.; Lindau, M. On-Chip Cyclic Voltammetry Measurements Using a Compact 1024-Electrode CMOS IC. Anal. Chem. 2021, 93, 8027–8034. [Google Scholar] [CrossRef]
- White, K.A.; Mulberry, G.; Kim, B.N. Parallel 1024-ch Cyclic Voltammetry on Monolithic CMOS Electrochemical Detector Array. IEEE Sens. J. 2020, 20, 4395–4402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).