Fractional CO2 Laser 2-Mercaptonicotinoyl Glycine Drug Delivery for Melasma and Facial Hyperpigmentation: A Real-Observational World Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Informed Consent and Data Collection
Treatment Groups and Clinical Procedures
2.4. Outcome Measures
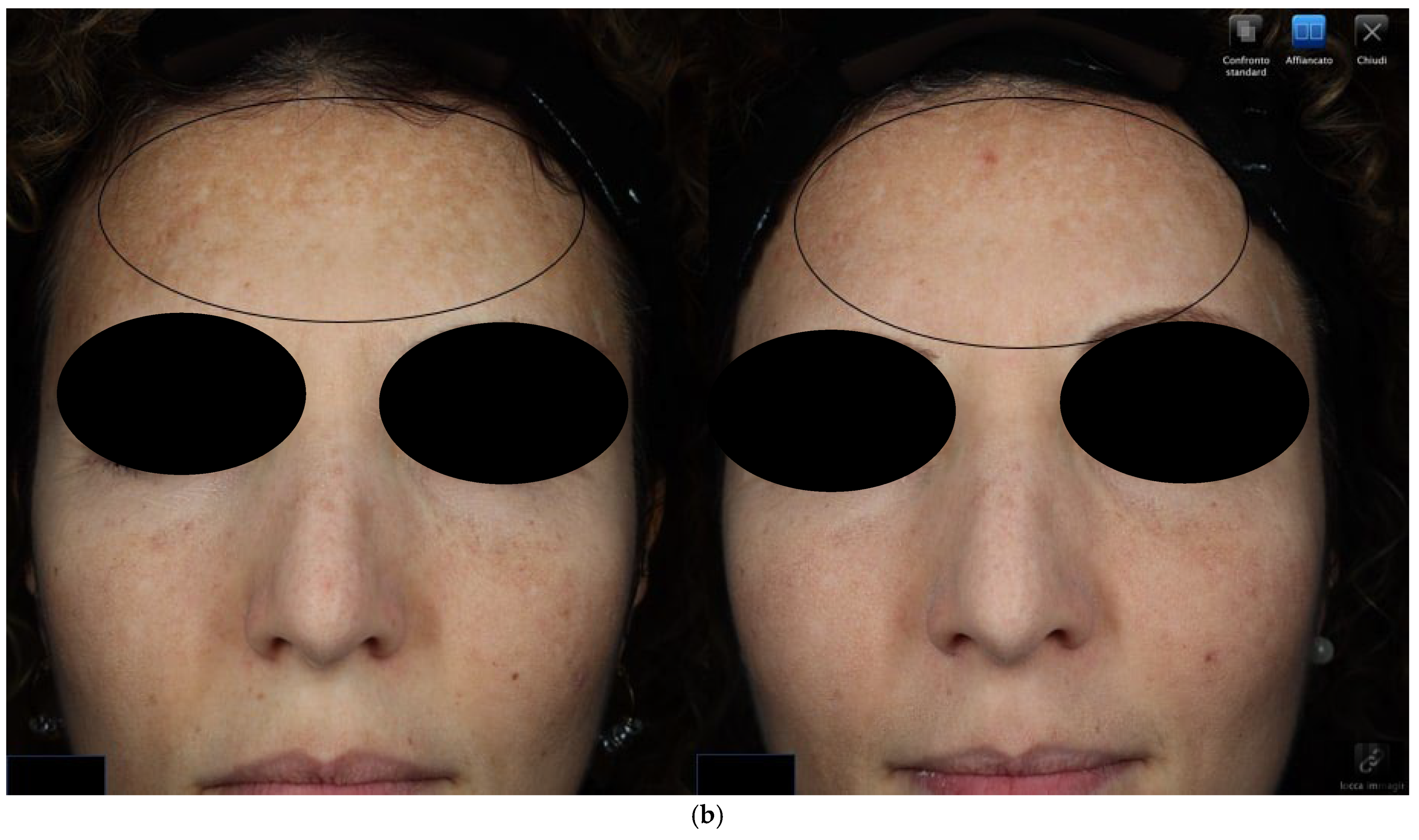
2.4.1. Objective Hyperpigmentation Assessment
2.4.2. Modified Melasma Area and Severity Index (mMASI)
2.4.3. Dermatology Life Quality Index (DLQI)
2.5. Statistical Analysis
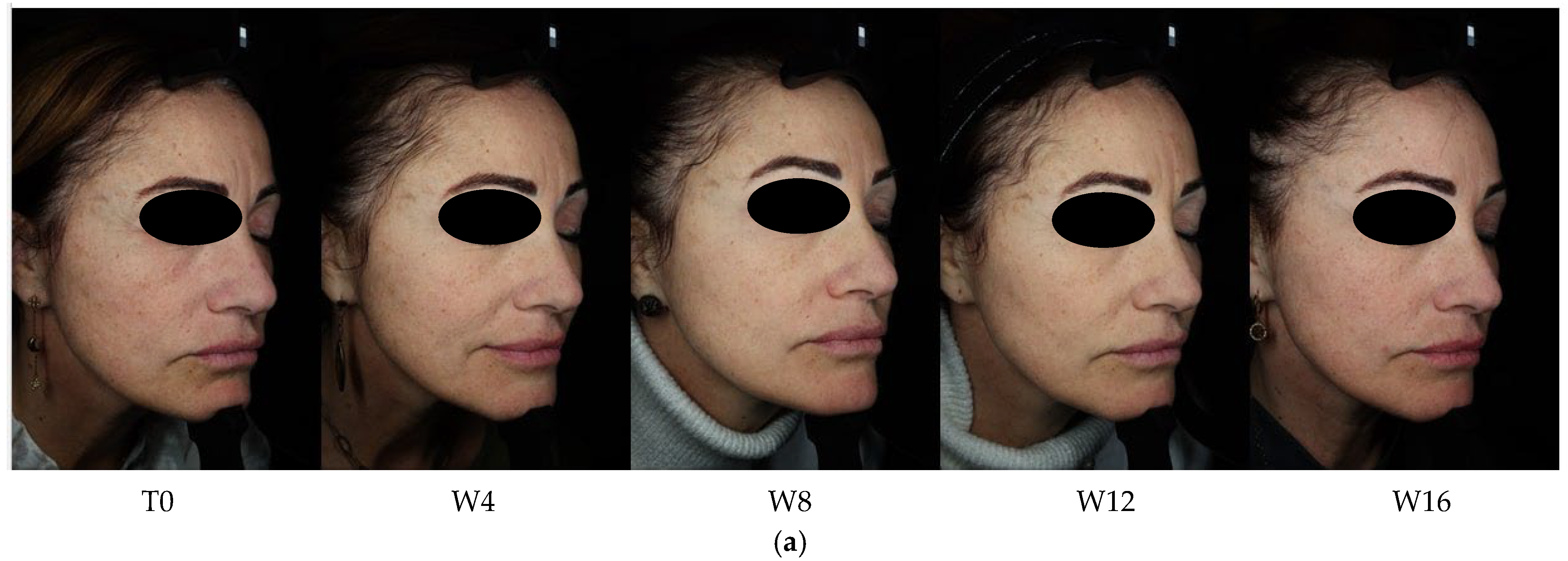
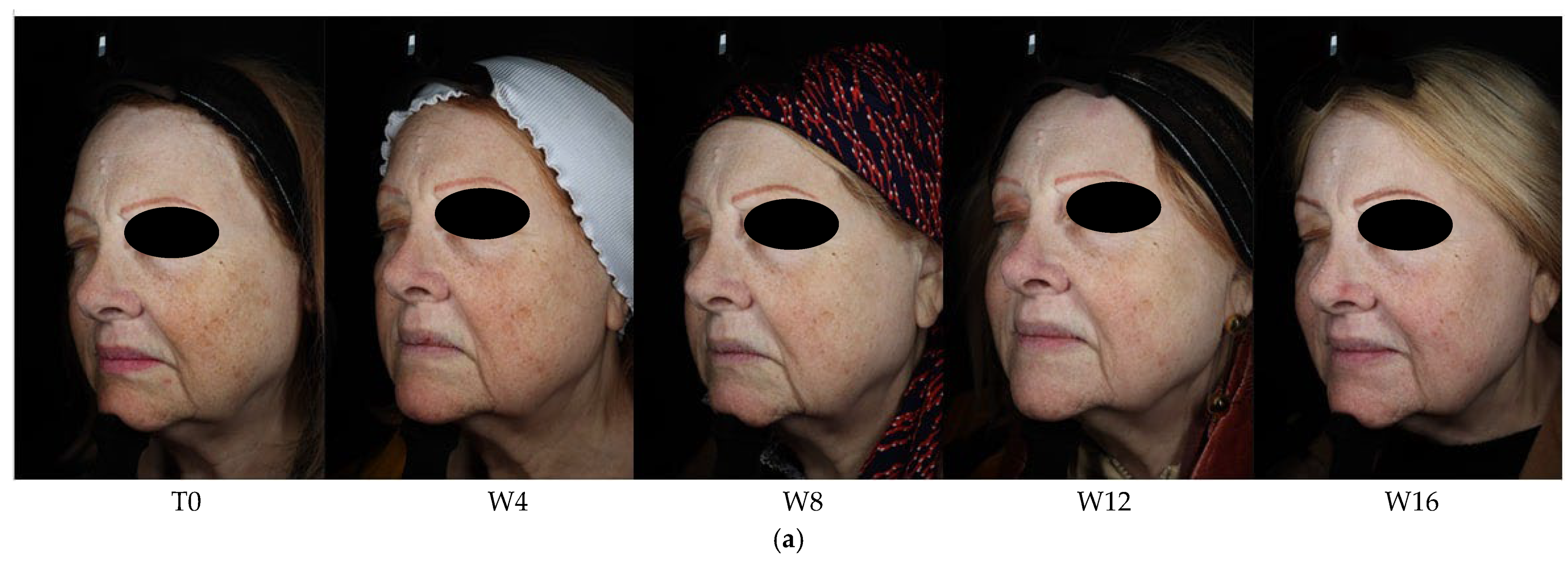
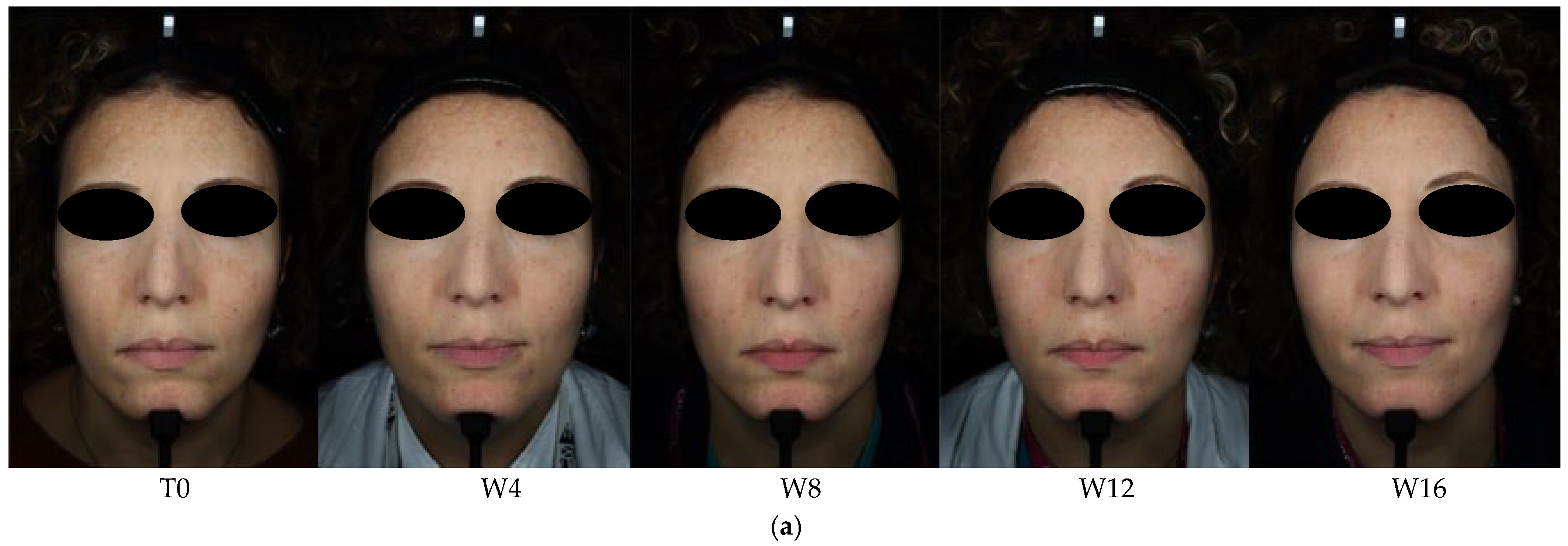
3. Results
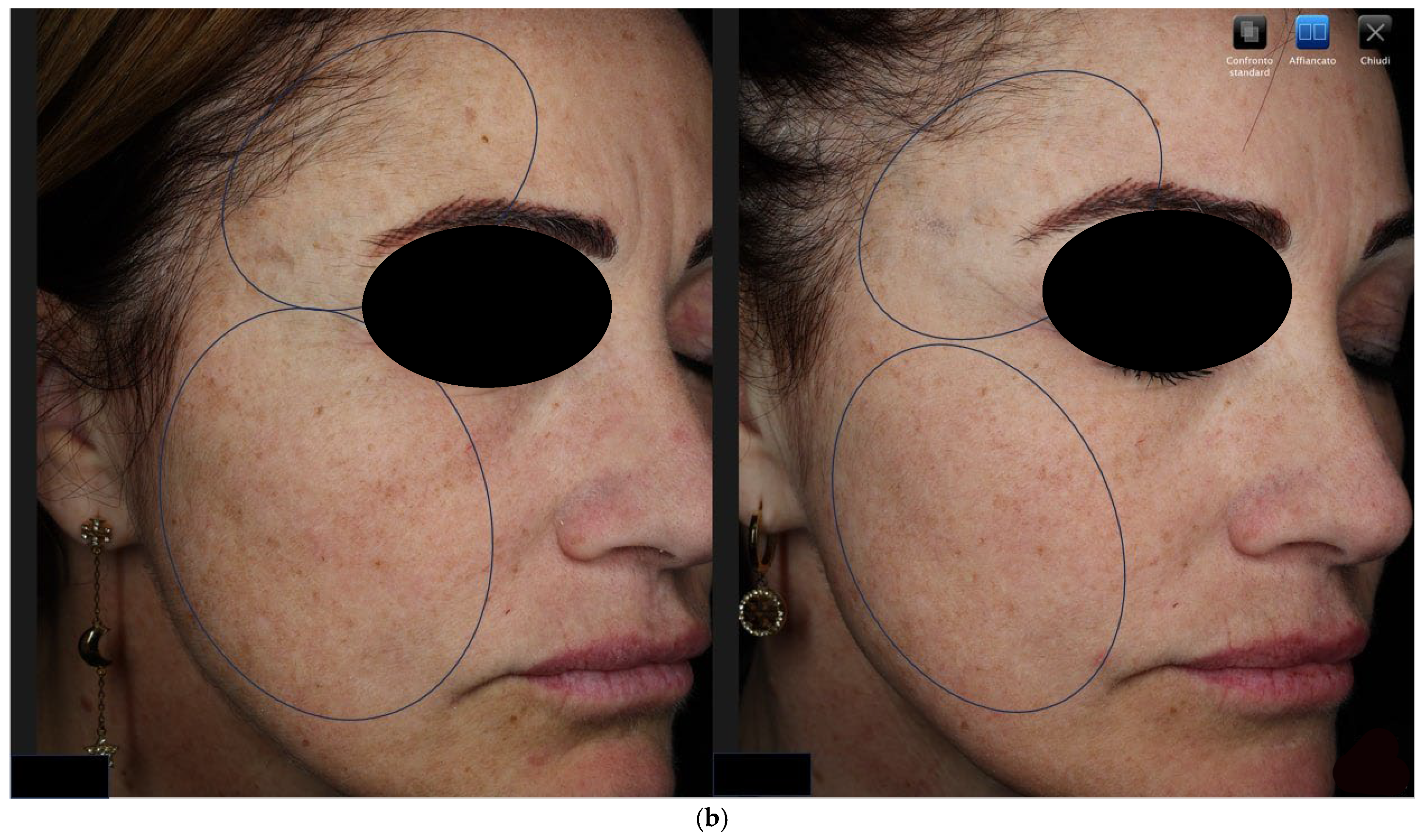
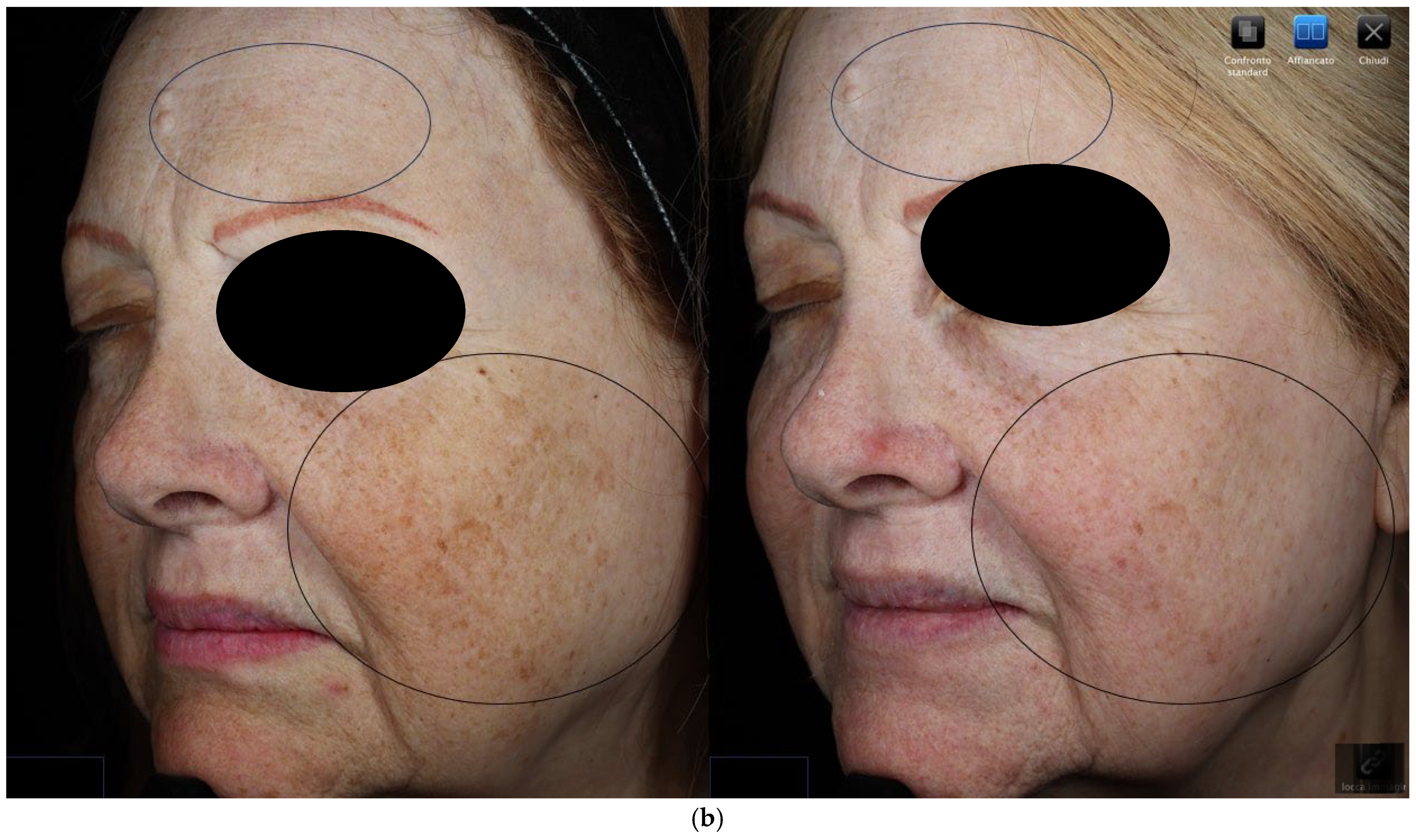
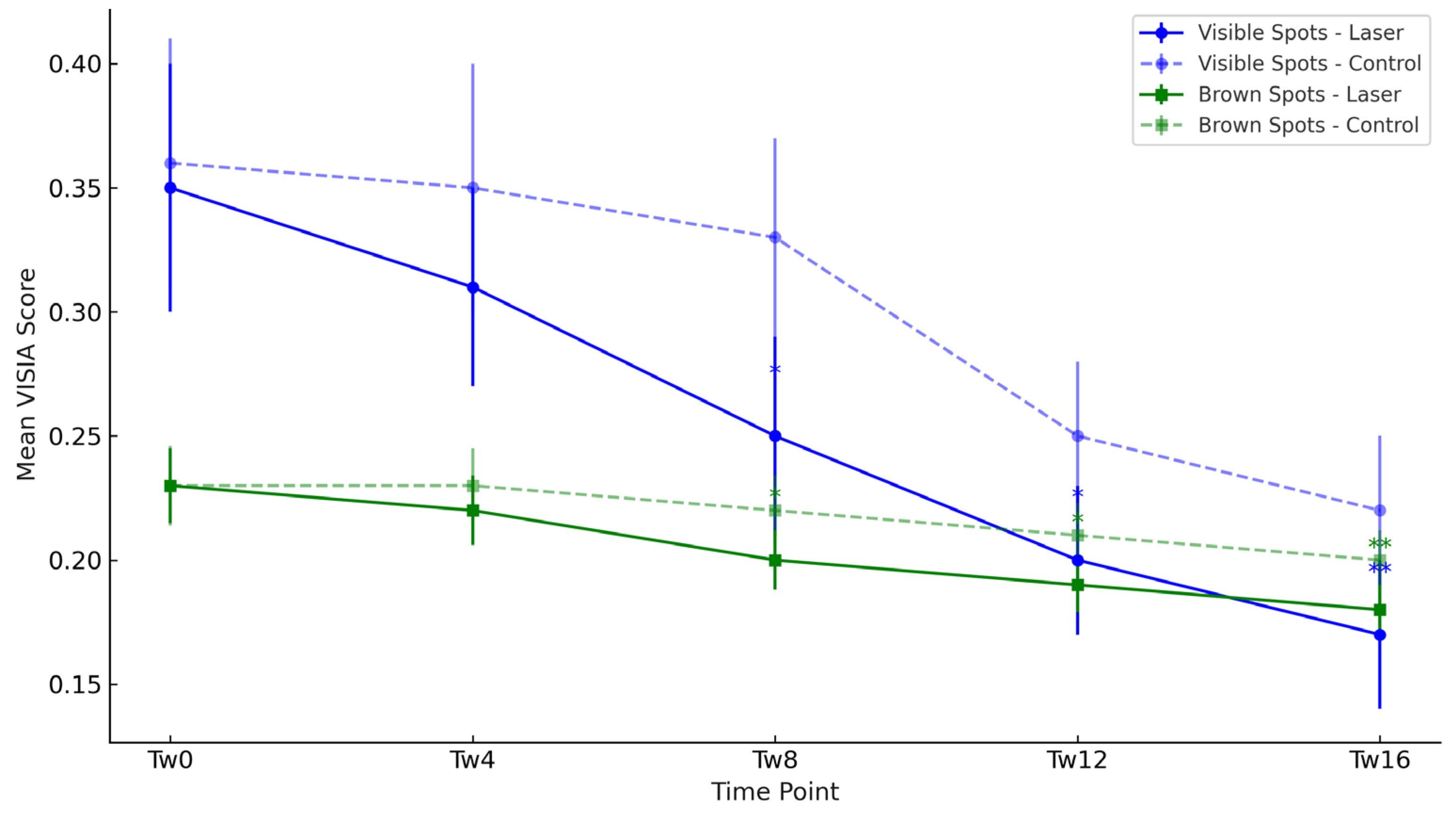
3.1. Objective Hyperpigmentation Assessment (VISIA-CR® Analysis)
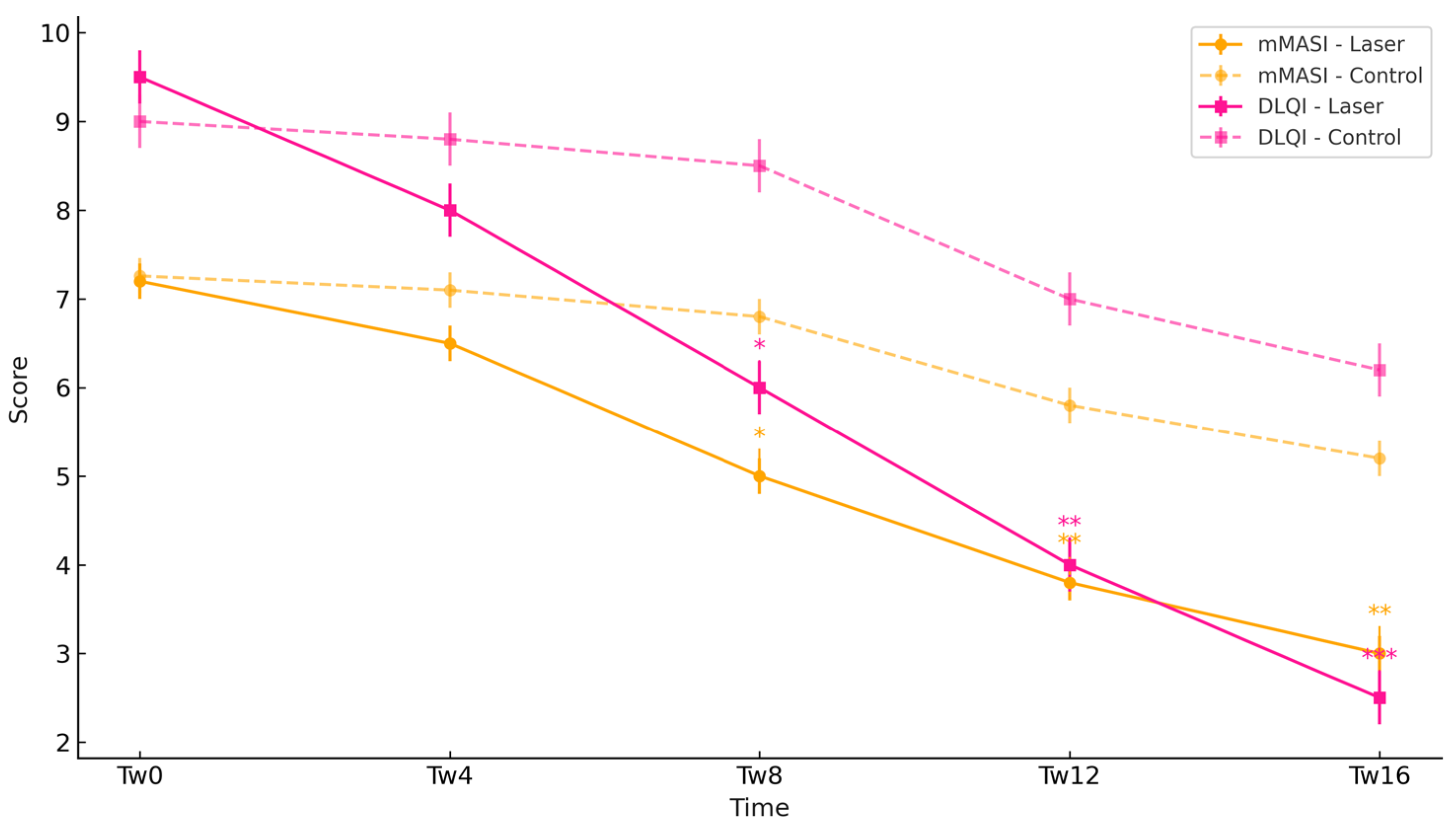
3.2. Clinical Severity Assessment (mMASI Scores) and Patient-Reported Outcomes (DLQI Scores)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syder, N.C.; Quarshie, C.; Elbuluk, N. Disorders of Facial Hyperpigmentation. Dermatol. Clin. 2023, 41, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Ko, D.; Friedman, B.J.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part I. Pathogenesis and clinical features of common pigmentary disorders. J. Am. Acad. Dermatol. 2023, 88, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Elbuluk, N.; Grimes, P.; Chien, A.; Hamzavi, I.; Alexis, A.; Taylor, S.; Gonzalez, N.; Weiss, J.; Desai, S.R.; Kang, S. The Pathogenesis and Management of Acne-Induced Post-inflammatory Hyperpigmentation. Am. J. Clin. Dermatol. 2021, 22, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, X.; Cheng, H. Prevention of Melasma During Pregnancy: Risk Factors and Photoprotection-Focused Strategies. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2301–2310. [Google Scholar] [CrossRef]
- Bostan, E.; Cakir, A. The dermoscopic characteristics of melasma in relation to different skin phototypes, distribution patterns and wood lamp findings: A cross-sectional study of 236 melasma lesions. Arch. Dermatol. Res. 2023, 315, 1927–1938. [Google Scholar] [CrossRef]
- Ozbagcivan, O.; Akarsu, S.; Ikiz, N.; Semiz, F.; Fetil, E. Dermoscopic Differentiation of Facial Lentigo Maligna from Pigmented Actinic Keratosis and Solar Lentigines. Acta Dermatovenerol. Croat. 2019, 27, 146–152. [Google Scholar]
- Woolery-Lloyd, H.; Kammer, J.N. Treatment of hyperpigmentation. Semin. Cutan. Med. Surg. 2011, 30, 171–175. [Google Scholar] [CrossRef]
- Charoo, N.A. Hyperpigmentation: Looking beyond hydroquinone. J. Cosmet. Dermatol. 2022, 21, 4133–4145. [Google Scholar] [CrossRef]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef]
- Borelli, C.; Fischer, S. Chemical Peelings zur Behandlung von Melasma, Pigmentstörungen und Hyperpigmentierungen: Indikationen, Effektivität und Risiken. Hautarzt 2020, 71, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Zhou, S.; Cheng, S.; Liu, H.; Cui, Y. Laser therapy in the treatment of melasma: A systematic review and meta-analysis. Lasers Med. Sci. 2022, 37, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.E.; Goldman, M.P.; Fitzpatrick, R.E. Ablative CO2 lasers for skin tightening: Traditional versus fractional. Dermatol. Surg. 2014, 40 (Suppl. S12), S147–S151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, M.; Lai, X.; Yan, Y. Efficacy and Safety of Ablative Fractional Laser in Melasma: A Meta-analysis and Systematic Review. Lasers Med. Sci. 2024, 39, 71. [Google Scholar] [CrossRef]
- Sextius, P.; Warrick, E.; Prévot-Guéguiniat, A.; Lereaux, G.; Boirre, F.; Baux, L.; Hassine, S.B.; Qiu, J.; Huang, X.; Xu, J.; et al. 2-Mercaptonicotinoyl glycine, a new potent melanogenesis inhibitor, exhibits a unique mode of action while preserving melanocyte integrity. Pigment Cell Melanoma Res. 2024, 37, 462–479. [Google Scholar] [CrossRef]
- de Dormael, R.; Sextius, P.; Bourokba, N.; Mainguene, E.; Tachon, R.; Gaurav, K.; Jouni, H.; Bastien, P.; Diridollou, S. 2-Mercaptonicotinoyl glycine prevents UV-induced skin darkening and delayed tanning in healthy subjects: A randomized controlled clinical study. J. Cosmet. Dermatol. 2024, 23, 1745–1752. [Google Scholar] [CrossRef]
- Muller, B.; Flament, F.; Jouni, H.; Sextius, P.; Tachon, R.; Wang, Y.; Wang, H.; Qiu, H.; Qiu, J.; Amar, D.; et al. A Bayesian network meta-analysis of 14 molecules inhibiting UV daylight-induced pigmentation. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1566–1574. [Google Scholar] [CrossRef]
- Demessant-Flavigny, A.L.; Petkar, G.; Jodun, D.; Le Dantec, G.; Le Floc’h, C.; Kerob, D. Efficacy of a 2-MNG-Containing Depigmenting Serum in the Treatment of Post-Inflammatory Hyperpigmentation. J. Cosmet. Dermatol. 2025, 24, e16735. [Google Scholar] [CrossRef]
- Passeron, T.; Kerob, D.; Le Dantec, G.; Demessant-Flavigny, A.L.; do Nascimer, A.R.; Moura, R.; Salah, S.; Feiges, M.; Fernandez, E.; Alexis, A. Efficacy and Tolerability of a New Facial 2-Mercaptonicotinoyl Glycine-Containing Depigmenting Serum versus Hydroquinone 4% over 3-Month Treatment of Facial Melasma. Dermatol. Ther. 2025; in press. [Google Scholar] [CrossRef]
- Wu, W.; Su, Q.; Zhang, Y.; Du, Y.; Hu, Y.; Wang, F. Novel 532-nm Q-switched Nd:YAG laser for the treatment of melasma and rejuvenation: A prospective, randomized controlled comparison with 1064-nm Q-switched Nd:YAG laser. Int. J. Dermatol. 2024, 63, 1242–1251. [Google Scholar] [CrossRef]
- Duteil, L.; Queille-Roussel, C.; Lacour, M.; Zastrow, L. Clinical proof of concept for Thiamidol, a new powerful inhibitor of human tyrosinase. J. Investig. Dermatol. 2014, 134, S62. [Google Scholar]
- Khemis, A.; Kaiafa, A.; Queille-Roussel, C.; Duteil, L. A randomized, double-blind, vehicle-controlled study to evaluate the efficacy of a topical formulation containing Thiamidol in the treatment of melasma. J. Cosmet. Dermatol. 2020, 19, 1106–1112. [Google Scholar]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2010, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- El-Sinbawy, Z.G.; Abdelnabi, N.M.; Sarhan, N.E.; Elgarhy, L.H. Clinical & ultrastructural evaluation of the effect of fractional CO2 laser on facial melasma. Ultrastruct. Pathol. 2019, 43, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gao, J.C.; Moy, J.; Lee, H.S. Fractional CO2 laser and adjunctive therapies in skin of color melasma patients. JAAD Int. 2022, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, J.F.; Piérard, G.E. Skin Colour and Ethnic Variations. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2006; pp. 303–314. [Google Scholar]
| Component. | Score | Description |
|---|---|---|
| Area (A) | 0–6 | 0 = No involvement; 6 = 90–100% of the region affected |
| Darkness (D) | 0–4 | 0 = Normal; 4 = Very markedly darker |
| Homogeneity (H) | 0–4 | 0 = Uniform; 4 = Very mottled/confetti-like |
| Total DLQI Score | Interpretation |
|---|---|
| 0–1 | No effect at all |
| 2–5 | Small effect |
| 6–10 | Moderate effect |
| 11–20 | Very large effect |
| 21–30 | Extremely large effect |
| Parameter | Group | Tw0 | Tw4 | Tw8 | Tw12 | Tw16 |
|---|---|---|---|---|---|---|
| Visible Spots | Laser | 0.35 | 0.31 | 0.25 # | 0.20 #* | 0.17 #** |
| Visible Spots | Control | 0.36 | 0.35 | 0.33 | 0.25 # | 0.22 # |
| UV Spots | Laser | 0.40 | 0.41 | 0.39 | 0.40 | 0.39 |
| UV Spots | Control | 0.41 | 0.41 | 0.42 | 0.40 | 0.41 |
| Brown Spots | Laser | 0.23 | 0.22 | 0.20 # | 0.19 #* | 0.18 #** |
| Brown Spots | Control | 0.23 | 0.23 | 0.22 | 0.21 # | 0.20 # |
| Parameter | Group | Tw0 | Tw4 | Tw8 | Tw12 | Tw16 |
|---|---|---|---|---|---|---|
| mMASI | Laser | 7.20 ± 0.82 | 6.50 ± 0.75 | 5.00 ± 0.70 #* | 3.80 ± 0.60 #** | 3.00 ± 0.50 #** |
| mMASI | Control | 7.26 ± 0.90 | 7.10 ± 0.88 | 6.80 ± 0.85 | 5.80 ± 0.80 # | 5.20 ± 0.75 # |
| DLQI | Laser | 9.5 ± 1.9 | 8.0 ± 1.7 | 6.0 ± 1.5 #* | 4.0 ± 1.2 #** | 2.5 ± 1.0 #*** |
| DLQI | Control | 9.0 ± 1.6 | 8.8 ± 1.5 | 8.5 ± 1.4 | 7.0 ± 1.2 # | 6.2 ± 1.1 # |
| Topical 2-MNG-Serum | Fractional CO2 Laser + 2-MNG-Serum | Q-Switched Laser Alone | |
|---|---|---|---|
| Mechanism | Inhibits melanin synthesis, strengthens skin barrier, anti-inflammatory, antioxidant | Enhances skin penetration + binds melanin precursors | Direct pigment fragmentation (photoacoustic) |
| Target Depth | Mainly epidermal | Epidermal + superficial dermal | Dermal and epidermal (depending on settings) |
| Efficacy | High for superficial pigmentation | Slightly superior for deep pigmentation | High for discrete pigmented lesions |
| Effectiveness on Deep Spots (Visia CR) | Moderate | Superior | Variable (effective on localized pigment) |
| Risk of PIH (Post-Inflammatory Hyperpigmentation) | Very low | Low | Higher (especially in darker phototypes) |
| Healing Time | None | 3–7 days downtime (mild redness, dryness) | 7–14 days downtime (possible crusting) |
| Patient Comfort | Excellent | Good (mild discomfort post-laser) | Moderate (risk of pain, more visible downtime) |
| Cost | Low | Higher (laser + topical product) | High (multiple sessions often needed) |
| Best Indicated For | Mild to moderate superficial hyperpigmentation | Deep melasma, resistant PIH, dermal pigmentation | Tattoos, lentigines, nevus of Ota |
| Overall Safety | Excellent | Very good (if properly performed) | Good but with higher caution needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattola, A.; Amore, E.; Amato, S.; Nisticò, S.P.; Pellacani, G. Fractional CO2 Laser 2-Mercaptonicotinoyl Glycine Drug Delivery for Melasma and Facial Hyperpigmentation: A Real-Observational World Experience. Cosmetics 2025, 12, 251. https://doi.org/10.3390/cosmetics12060251
Dattola A, Amore E, Amato S, Nisticò SP, Pellacani G. Fractional CO2 Laser 2-Mercaptonicotinoyl Glycine Drug Delivery for Melasma and Facial Hyperpigmentation: A Real-Observational World Experience. Cosmetics. 2025; 12(6):251. https://doi.org/10.3390/cosmetics12060251
Chicago/Turabian StyleDattola, Annunziata, Emanuele Amore, Simone Amato, Steven Paul Nisticò, and Giovanni Pellacani. 2025. "Fractional CO2 Laser 2-Mercaptonicotinoyl Glycine Drug Delivery for Melasma and Facial Hyperpigmentation: A Real-Observational World Experience" Cosmetics 12, no. 6: 251. https://doi.org/10.3390/cosmetics12060251
APA StyleDattola, A., Amore, E., Amato, S., Nisticò, S. P., & Pellacani, G. (2025). Fractional CO2 Laser 2-Mercaptonicotinoyl Glycine Drug Delivery for Melasma and Facial Hyperpigmentation: A Real-Observational World Experience. Cosmetics, 12(6), 251. https://doi.org/10.3390/cosmetics12060251








