Abstract
Background: Androgenetic alopecia (AGA) is a prevalent condition characterized by progressive follicular miniaturization. Minoxidil topical treatment and finasteride oral treatment are the golden standard, but they are limited by local and systemic adverse effects. Combination therapies targeting both follicular stimulation and nutritional support may enhance clinical outcomes. Objective: To evaluate the efficacy of a combined topical and oral therapy compared to topical monotherapy in patients with AGA using trichoscopic and clinical parameters. Methods: In this open-label, prospective trial, 48 patients were assigned to receive either a topical spray alone (Group A) or in combination with oral capsules (Group B) for 3 months. Trichoscopic parameters were assessed at baseline and post-treatment. Paired and independent t-tests, along with Cohen’s d effect sizes, were used to evaluate intra- and inter-group changes. Results: Both groups demonstrated improvements in hair density, thickness, and anagen/telogen ratio. Group B exhibited significantly greater increases in total hair count and anagen conversion (p < 0.05). The effect sizes ranged from small to large, with the most pronounced changes observed in anagen/telogen ratio (Cohen’s d = 0.841) in males. Conclusions: The combination of topical and oral treatment led to greater trichologic improvements than topical therapy alone. While extrapolated projections at 6 and 12 months suggest continued benefit, future studies with longer duration and placebo controls are required to validate these findings.
1. Introduction
Androgenetic alopecia (AGA), or pattern hair loss, is the most prevalent form of hair loss worldwide, with an estimated 80% of men and nearly 50% of women affected by the age of 50 [1]. AGA is characterized by the progressive miniaturization of hair follicles, a shortened anagen phase, and an increased proportion of hairs in the telogen phase [2]. The condition is hormonally driven, with androgens such as dihydrotestosterone (DHT) playing a central role by binding to androgen receptors in the dermal papilla and promoting the expression of inhibitory cytokines, including transforming growth factor-beta 1 (TGF-β1) and Dickkopf-1 (DKK-1) [3].
The pathogenesis of AGA is multifactorial, encompassing hormonal, genetic, inflammatory, and vascular factors. In men, increased sensitivity to DHT mediated by 5α-reductase types I and II accelerates follicular miniaturization. In women, the etiopathogenesis is more complex, involving estrogen-to-androgen imbalances, perifollicular microinflammation, and age-related hormonal decline [4]. Furthermore, perifollicular fibrosis and oxidative stress are increasingly recognized as contributors to disease progression [4].
Conventional pharmacologic treatments for AGA—such as oral finasteride and topical minoxidil—can promote partial regrowth and are often limited by side effects [5]. These include sexual dysfunction, local dermatitis, orthostatic hypotension, mastodynia (in women), potential proliferative processes in long-term finasteride users (also more prevalent in women due to estrogen excess), and poor compliance due to cosmetic or sensory dissatisfaction [6]. Consequently, novel therapeutics with improved tolerability and multimodal mechanisms are under active investigation.
Minoxicapil is a novel topical formulation that incorporates a patented complex based on pyrrolidinyl diaminopyrimidine oxide and several other bioactive ingredients known to act synergistically on key pathogenic targets in AGA. Oleanolic acid, a pentacyclic triterpenoid found in numerous botanical sources, inhibits both type I and II 5α-reductase enzymes and downregulates DHT while promoting Insulin-like Growth Factor 1 (IGF-1) and Vascular Endothelial Growth Factor (VEGF) expression via activation of the Wnt/β-catenin pathway [3,7]. Apigenin, a plant-derived flavonoid, improves perifollicular microcirculation, inhibits TGF-β1 expression, and directly promotes dermal papilla and hair follicle cell proliferation [8]. Biotinyl tripeptide-1, a matrikine peptide, not only inhibits 5α-reductase but also supports extracellular matrix remodeling and hair follicle anchoring through its interaction with fibronectin and collagen pathways [3,6,7,8]. Oral supplementation with sulphated amino acids methionine and cysteine, specific minerals (zinc, copper, selenium), and vitamins (B-complex, vitamin D3, vitamin C) was also found to be beneficial for hair health [7,8].
Additional actives such as adenosine stimulate the expression of fibroblast growth factor-7 (FGF-7), VEGF, and keratinocyte growth factor (KGF), further enhancing follicular metabolism and extending anagen phase duration [9]. Ginkgo biloba and biotin provide antioxidant, anti-inflammatory, and metabolic support essential for a favorable scalp environment [6,9]. The combination of these actives addresses the androgenic, vascular, and inflammatory dimensions of AGA, aiming for a more sustained and comprehensive therapeutic effect. This aligns with emerging cosmeceutical frameworks for AGA management [10].
The primary objective of this study was to assess the short-term safety and efficacy of Minoxicapil spray, with and without oral supplementation, in patients with AGA. We further aimed to extrapolate treatment outcomes through 6 and 12 months using statistical modeling, to better understand the long-term potential of this therapy.
2. Materials and Methods
2.1. Study Design and Population
This was a prospective, single-center, open-label clinical study conducted over a period of three months. The study protocol has been reviewed and approved by an Institutional Review Board (document no. 12808-1/28.08.2023). A total of 60 patients (36 females and 24 males), aged between 19 and 79 years, with clinically diagnosed androgenetic alopecia (AGA) were initially enrolled. However, 12 participants (9 females and 3 males) were lost to follow-up, resulting in a final evaluable cohort of 48 patients (27 females and 21 males).
All participants were clinically diagnosed with androgenetic alopecia by a board-certified dermatologist (V.M.V.) based on trichoscopic findings and pattern recognition consistent with Norwood–Hamilton and Ludwig classification systems.
The sample size of 60 participants was chosen based on feasibility constraints and aligns with previous exploratory trichoscopic studies of androgenetic alopecia treatments, which typically involve sample sizes ranging from 30 to 60 participants in early-phase trials [6,11,12]. While no a priori power calculation was performed, the moderate-to-large observed effect sizes (Cohen’s d) suggest adequate sensitivity to detect clinically meaningful changes.
2.2. Inclusion Criteria
Participants eligible for enrollment were adults over the age of 18 diagnosed with androgenetic alopecia. Male participants were required to present with stage II–III alopecia on the Hamilton-Norwood scale, while female participants had to present with stage I–III on the Ludwig scale. All participants were required to provide written informed consent prior to inclusion in the study. Additionally, participants agreed to maintain consistent personal hygiene practices and to continue using the same hygiene products throughout the 3-month duration of the study. They also committed to maintaining the same hair color and haircut for the entirety of the study period. Individuals with any degree of severe physical or cognitive disability were not eligible for inclusion.
2.3. Exclusion Criteria
Subjects were excluded if they had a known hypersensitivity to any of the ingredients in the investigational products. Additional exclusion criteria included the presence of infectious or inflammatory conditions affecting the scalp, or any other form of alopecia besides androgenetic alopecia (e.g., lichen plano-pilaris). Patients with chronic systemic illnesses likely to compromise general health or nutritional status—such as active cancer, hepatic or renal disease, or anemia—were also excluded. Furthermore, individuals with comorbidities affecting the gastrointestinal absorption of vitamins and minerals were not eligible. Patients who had undergone a hair transplant procedure within the past 12 months, or who had used any topical anti-hair loss products within the past month, were also excluded from participation.
2.4. Treatment Arms
Participants were randomized using a computer-generated sequence in a 1:1 ratio without stratification and allocated to one of two groups. Group A received Minoxicapil topical spray alone. Group B received Minoxicapil topical spray in combination with oral Minoxicapil capsules.
The investigational treatment consisted of two complementary formulations: a topical solution (Minoxicapil spray) and an oral supplement (Minoxicapil capsules). The spray contained a minoxidil derivative (DPOO complex: diethylene glycol monoethyl ether and purified water) combined with an absorption enhancer and a patented Procapil complex, which includes biotinyl tripeptide-1, oleanolic acid (a 5α-reductase inhibitor), and apigenin, known for its vasodilatory and anti-inflammatory properties. The recommended application was 1–2 sprays (0.2 mL per spray) per 2 × 2 cm area of scalp (for a total of 10–12 sprays for the entire scalp), not exceeding 25 sprays (5 mL) per day. Application involves gentle massage of the treated area with fingertips for 2–3 min. The oral formulation contains a synergistic blend of amino acids (methionine and cysteine), trace elements (zinc, copper, selenium), and a comprehensive panel of vitamins including high-dose biotin (2000 µg), vitamin D3, vitamin C, and B-complex vitamins. A detailed list of ingredients and excipient concentrations for both the spray and oral formulation is provided in Appendix A, to ensure full transparency and reproducibility of the intervention.
The topical spray was applied twice daily to the affected scalp area. The capsules were taken orally, once per day.
A third arm consisting of capsules-only was not included, as oral supplementation alone was not considered sufficient for monotherapy evaluation based on the existing literature [13].
The decision to not include a placebo or oral-only group was based on ethical and practical considerations. Similar combination studies have employed active-controlled, open-label designs, especially in early-stage trials aiming to assess clinical feasibility and tolerability [6,9].
2.5. Outcome Measures
Each subject underwent trichoscopic evaluation at baseline (T0) and after 3 months of treatment (T3). The assessed parameters included: (1) hair density (number of hairs per cm2), (2) hair shaft thickness (mm), (3) proportion of hairs in anagen and (4) telogen phases, (5) anagen/telogen ratio, (6) total number of hairs, (7) number of empty follicles (Figure 1), (8) presence of peripilar signs (perifollicular hyperpigmentation) (Figure 2).
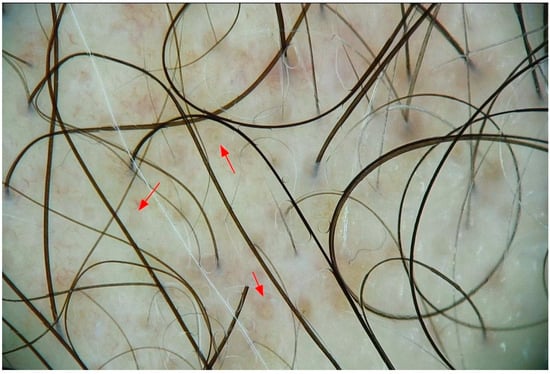
Figure 1.
Empty hair follicles in androgenic alopecia. Trichoscopic image showing multiple empty follicular openings (red arrows), a characteristic finding in androgenetic alopecia, representing sites of miniaturized or regressed hair units.
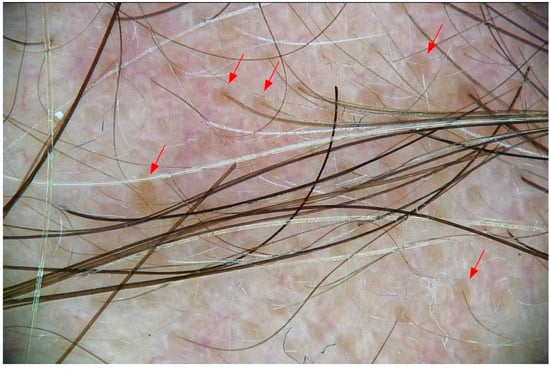
Figure 2.
Peripilar signs in androgenic alopecia. Trichoscopic aspect of peripilar signs (red arrows), seen as brownish halos around hair follicle openings.
These measures were obtained using a digital dermatoscope (Fotofinder Systems, Bad Birnbach, Germany, Fotofinder ATBM II) under standardized lighting and magnification conditions. After each performed investigation, a trichologic report containing several measured parameters was generated (Figure 3).
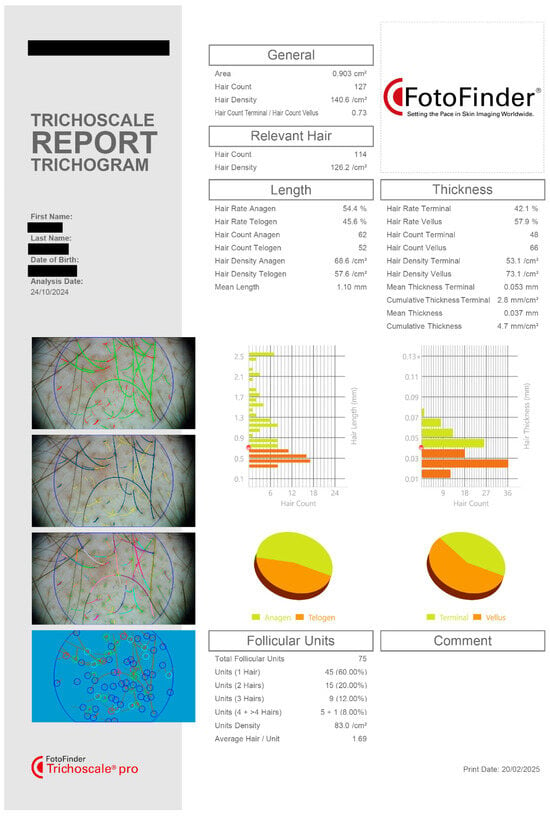
Figure 3.
Trichoscopic report. Trichogram output displaying detailed scalp analysis based on digital trichoscopy. The report includes multiple quantitative and morphological hair parameters used for diagnosis and monitoring in androgenetic alopecia. All key parameters assessed in this study are captured in the analysis, allowing for precise tracking of treatment-related changes in hair growth patterns and follicular health. Data shown for a single patient in the Spray and Capsule group.
To minimize observer bias, all trichoscopic images were anonymized and assigned random codes before evaluation. The evaluator (V.M.V), a board-certified dermatologist with expertise in digital trichoscopy, was blinded to both group allocation and temporal sequence (baseline vs. post-treatment).
2.6. Data Analysis and Extrapolation
Quantitative data were entered into SPSS v24.0 software package for statistical analysis. Descriptive statistics (means, standard deviations) were calculated for each trichologic parameter. Paired t-tests were used to assess intra-group changes between T0 and T3. Independent t-tests were conducted to compare outcomes between Group A and Group B. Cohen’s d was used to estimate effect sizes.
Prior to extrapolating the observed values beyond the 3-month endpoint, a preliminary linearity assessment was conducted to confirm that the progression of trichologic parameters followed a consistent linear trend. This included regression analysis, slope inspection, and residual distribution checks to validate the assumptions required for temporal extrapolation. To predict long-term outcomes, monthly rates of change were calculated for each parameter between T0 and T3. These rates were then applied to extrapolate values at 6 months (T6) and 12 months (T12).
Linear regression models were constructed to identify baseline predictors of treatment response, with changes in hair density and anagen ratio as dependent variables. It is important to note that extrapolations beyond T3 were based on linear modeling of short-term changes and do not account for potential biological plateaus or nonlinear dynamics of hair growth cycles. These projections are hypothetical and should be interpreted cautiously pending long-term empirical validation.
Statistical significance was set at p < 0.05. Results were stratified by sex to evaluate gender-specific responses.
3. Results
3.1. Baseline Characteristics
The study population included 27 females (56.3%) and 21 males (43.8%) with a mean age of 39.4 years (±13.62).
In the male population in the study, severity of androgenetic alopecia was assessed using the Hamilton–Norwood scale. The most frequently observed stage was III (38.10%), followed by stage IIIA (28.57%). Stages III vertex and IV were each present in 14.29% of male patients, while stage Va was the least common, recorded in 4.76% of cases (Figure 4). These findings suggest that most male participants presented with moderate forms of AGA at baseline.
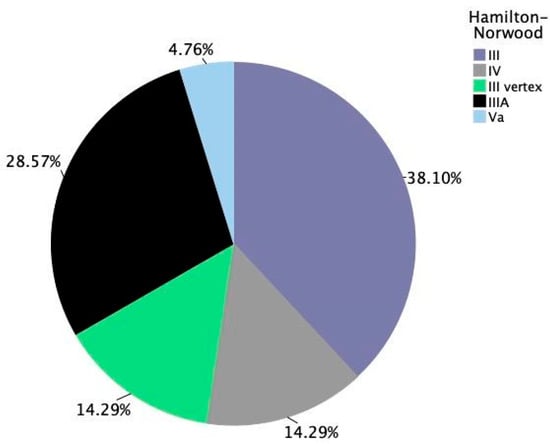
Figure 4.
Distribution of androgenetic alopecia severity among male participants. Hamilton–Norwood staging across all male subjects at baseline (n = 21).
The distribution of androgenetic alopecia stages was relatively balanced between the two treatment arms at baseline, with some notable differences. In Group B (spray and capsules), the majority of participants (45.5%) were classified as Hamilton–Norwood stage III, followed by stage IIIA (27.3%), stage Va (18.2%), and lower proportions in stage IV (9.1%). This suggests a predominance of moderate alopecia with limited vertex involvement (Figure 5B).
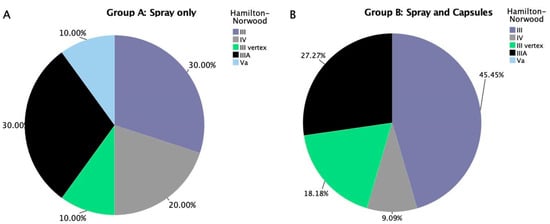
Figure 5.
(A) Stratification of androgenic alopecia severity for male participants in treatment group A (spray only). (B) Stratification of androgenic alopecia severity for male participants in treatment group B (spray and capsules).
In contrast, the spray-only group (Group A) displayed a more even distribution across stages, with stage III and IIIA each comprising 30% of participants, and stages IV, III vertex, and Va each representing 10–20% of the group. Overall, the Spray group included slightly more severe cases with vertex involvement (e.g., stage III vertex), while the Spray + Capsules group skewed more toward frontal and early mid-frontal hairline recession (e.g., stage III and IIIA) (Figure 5A).
For female participants, severity was categorized according to the Ludwig classification system. The most common grade was stage I-3, accounting for 33.33% of cases, followed by I-2 at 25.93% and I-4 at 14.81%. More advanced patterns, such as stages II-1 and II-2, each represented 11.11%, while stage III was seen in only 3.70% of female patients. This distribution reflects a predominance of early to moderate-stage hair loss among women enrolled in the study, with a relatively wide variation in presentation (Figure 6).
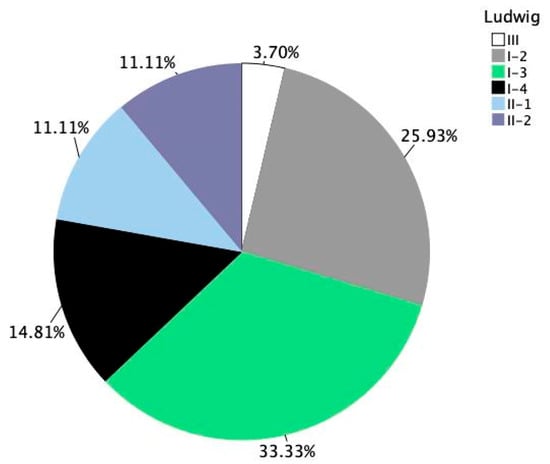
Figure 6.
Distribution of androgenetic alopecia severity among female participants. Ludwig staging across all female subjects at baseline (n = 27).
The Ludwig classification at baseline showed a diverse distribution of alopecia severity among female participants in both treatment groups. In the spray-only group (Group A), the most common stages were Ludwig I-2 and I-3, each representing 30.77% of participants. As for the remaining stages, II-1 and II-2 were equally distributed with 15.38% each, I-4 accounted for 7.69% of subjects, while stage III was not represented (Figure 7A).
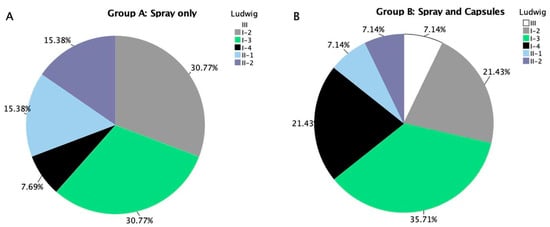
Figure 7.
(A) Stratification of androgenic alopecia severity for female participants in treatment group A (spray only). (B) Stratification of androgenic alopecia severity for female participants in treatment group B (spray and capsules).
In comparison, the Spray + Capsules group (Group B) showed a slightly wider spread. Ludwig I-3 remained the most prevalent (35.71%), followed by I-2 and I-4 (both at 21.43%). Lower severity stages like II-1, II-2, and stage III each represented 7.14% of the group (Figure 7B).
This baseline distribution indicates a slightly broader spectrum of alopecia severity in the combination treatment group, with more cases of early-stage (I-4) and advanced (III) involvement compared to the spray-only group.
Both treatment arms were balanced in terms of baseline demographics and alopecia grading.
3.2. Trichologic Parameters in the Overall Study Population After 3 Months
Significant improvements (p < 0.01) were observed in several parameters in both treatment groups. Hair density increased by an average of 7.8 hairs/cm2. Hair shaft thickness increased by 0.01 mm. Anagen hair count increased by 5.29 in average, while telogen hair count decreased by 5.43, in average. The anagen/telogen ratio improved by 0.75. Empty follicles reduced by 1.5, and peripilar signs diminished by 0.39 (Table 1).

Table 1.
Changes in trichologic parameters between baseline (T0) and 3 months (T3) in the overall study population. Positive values indicate an increase from baseline, while negative values indicate a decrease. For example, a negative change in telogen count or empty follicles reflects a clinical improvement.
Although there was a numerical increase in the total number of hairs, the change did not reach statistical significance (p = 0.602).
Although several parameters in Table 1 exhibit large standard deviations, the corresponding p-values remain statistically significant due to the consistent directionality of intra-subject changes. The paired t-test model evaluates within-subject differences, which can yield strong statistical power even in the presence of inter-individual variability [14,15]. This is particularly important in dermatologic studies, where baseline variability is common and intra-subject designs help control for it [16].
Effect size analysis using Cohen’s d revealed variable magnitudes of change across the eight trichologic parameters following 3 months of treatment (Figure 8). The most substantial improvement was observed in the reduction inempty follicles (d = −0.91), indicating a large effect and suggesting a meaningful increase in follicular activity. Medium-to-large effects were also seen in the decrease intelogen-phase hairs (d = 0.60) and the increase in the anagen/telogen ratio (d = 0.59), both consistent with improved follicular cycling. Moderate improvements were found in anagen hair count (d = 0.48), hair density (d = 0.41), and peripilar signs (d = 0.39). Hair shaft thickness showed a small-to-moderate effect (d = 0.30), while total hair count exhibited only a negligible change (d = 0.08).
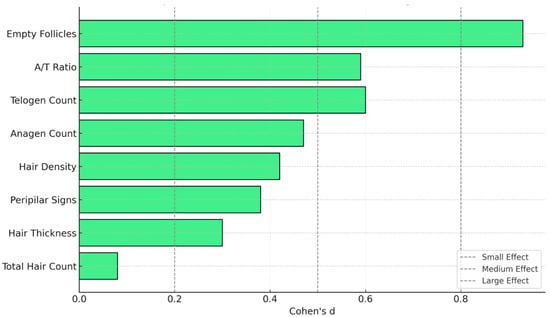
Figure 8.
Effect sizes (Cohen’s d) for trichologic parameters after 3 months of treatment. This chart illustrates the magnitude of change across trichologic parameters using Cohen’s d. Grey vertical lines indicate conventional thresholds for small (d = 0.2), medium (d = 0.5), and large (d = 0.8) effects. A/T = anagen/telogen.
3.2.1. Within-Group Analysis
Paired-sample t-tests were conducted in each of the two groups to assess the changes in trichologic parameters from baseline (T0) to three months (T3). Note that negative values in T3–T0 differences reflect a reduction in parameters such as telogen count and empty follicles—which corresponds to a therapeutic improvement in hair cycling and follicular density.
In Group A (topical spray only), statistically significant improvements were observed in telogen hair count (p = 0.001), anagen/telogen ratio (p = 0.001), empty follicles (p < 0.001), and peripilar signs (p = 0.001). However, no significant changes were detected in total hair count (p = 0.323), hair density (p = 0.513), hair shaft thickness (p = 0.096), or anagen hair count (p = 0.604) (Table 2).

Table 2.
Changes in trichoscopic parameters in Group A (spray only) between baseline (T0) and 3 months (T3). Positive values indicate an increase from baseline, while negative values indicate a decrease. For example, a negative change in telogen count or empty follicles reflects a clinical improvement.
In Group B (topical spray plus oral Minoxicapil), paired-sample t-tests revealed statistically significant improvements in total hair count (p = 0.026), hair density (p < 0.001), anagen hair count (p < 0.001), telogen hair count (p = 0.043), anagen/telogen ratio (p = 0.036), and empty follicle count (p < 0.001). While hair shaft thickness showed a nonsignificant increase (p = 0.148), changes in peripilar signs were not statistically significant (p = 0.341) (Table 3).

Table 3.
Trichoscopic parameter change in Group B (Minoxicapil spray and capsules) between baseline (T0) and 3 months (T3). Positive values indicate an increase from baseline, while negative values indicate a decrease. For example, a negative change in telogen count or empty follicles reflects a clinical improvement.
Effect size analysis using Cohen’s d revealed notable differences in treatment impact between the two groups (Table 4). Group B (spray + capsules) demonstrated consistently larger effect sizes across most parameters, with particularly strong improvements in anagen count (d = 0.96), hair density (d = 0.92), and total hair count (d = 0.48). In contrast, Group A (spray only) showed moderate effects in reducing telogen count (d = −0.77), improving the anagen/telogen ratio (d = 0.81), and decreasing empty follicles (d = −0.95), but changes in growth-related parameters were comparatively limited.

Table 4.
Within-group effect sizes (Cohen’s d) by treatment arm.
3.2.2. Between-Group Comparison
Participants who received the combined treatment of topical spray and oral Minoxicapil experienced statistically significantly greater increases in total hair count after 3 months of treatment (7.13 ± 15.00 hairs) compared to those who received topical spray alone (−4.67 ± 22.14 hairs), t(38.2) = 2.14, p = 0.039. Similarly, the combined treatment group showed a significantly larger increase in anagen hair count (8.99 ± 9.41 hairs) than the spray-only group (1.28 ± 11.70 hairs), t(42.3) = 2.50, p = 0.016 (Table 4).
While the increase in hair density was numerically greater in the combination group (12.15 ± 13.27 hairs/cm2) compared to the spray-only group (3.24 ± 23.36 hairs/cm2), the difference did not reach statistical significance, t(34.2) = 1.61, p = 0.118. Changes in hair shaft thickness also did not differ significantly between groups, with the spray-only group showing a mean increase of 0.017 ± 0.0457 mm and the combination group showing a smaller increase of 0.004 ± 0.0135 mm, t(25.5) = −1.26, p = 0.218 (Table 4).
The spray-only group demonstrated a greater reduction in telogen count (−7.57 ± 9.86 hairs) compared to the combination group (−3.46 ± 8.10 hairs), although this difference was not statistically significant, t(42.7) = −1.57, p = 0.124. No significant between-group differences were observed in the anagen/telogen ratio (spray-only: 0.88 ± 1.09; combination: 0.63 ± 1.42), t(40.6) = 0.65, p = 0.521; in empty follicle count (spray-only: −1.39 ± 1.47; combination: −1.60 ± 1.83), t(41.3) = −0.39, p = 0.698; or in peripilar signs (spray-only: −0.57 ± 0.73; combination: −0.24 ± 1.23), t(32.6) = 1.13, p = 0.266 (Table 5).

Table 5.
Intergroup comparison of trichologic parameter changes in the two treatment arms after 3 months.
Between-group effect sizes were calculated using Cohen’s d to evaluate the magnitude of differences in trichologic parameter changes between Group A (spray only) and Group B (spray + capsules). The most prominent effects were observed for anagen count (d = 0.73), total hair count (d = 0.63), and hair density (d = 0.47), indicating a moderate advantage in favor of the combined treatment. Smaller effects were seen in telogen count (d = 0.46) and peripilar signs (d =0.32), while negligible or slightly negative effects were found for hair thickness (d = −0.38), anagen/telogen ratio (d = −0.20), and empty follicle count (d = −0.13), favoring Group A in these parameters. (Figure 9) These findings support the superior efficacy of the combined intervention in stimulating hair growth.
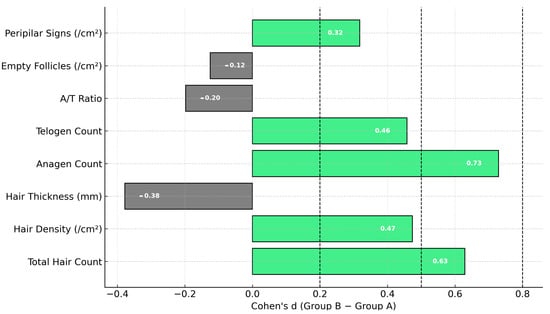
Figure 9.
Cohen’s d effect sizes comparing treatment groups for trichologic parameters. Light green bars represent positive effects favoring Group B, while gray bars indicate effects favoring Group A. Vertical dashed lines denote thresholds for small (0.2), medium (0.5), and large (0.8) effects.
3.3. Trends
Extrapolation models were used to estimate the potential progression of trichologic outcomes beyond the 3-month observation period. These were based on observed monthly rates of change and assumed linear continuity. However, given the cyclic and adaptive nature of hair follicle biology, these projections should be interpreted with caution and are presented for illustrative purposes only.
3.3.1. Treatment-Specific Trends
Model-based extrapolation of trichoscopic parameter evolution over 12 months demonstrated distinct response profiles between the two treatment groups. Group B (spray plus capsules) showed steeper and sustained improvements in hair density, hair thickness, anagen count, and anagen/telogen ratio, particularly evident from 6 to 12 months (extrapolated data). Group A (spray only) exhibited more modest gains across these domains but showed comparable reductions in telogen count, empty follicle presence, and peripilar signs. These trends suggest that while both treatments improved follicular health, the combined regimen elicited a more robust and multidimensional response over time (Figure 10).
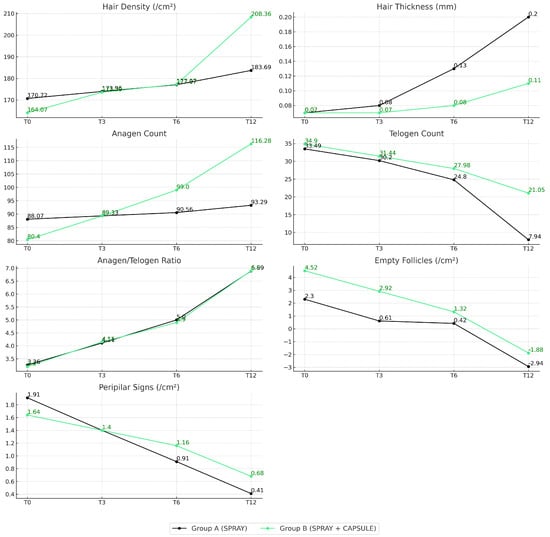
Figure 10.
Evolution of trichologic parameters over 12 months by treatment group. Each panel shows mean values of a specific trichologic parameter at T0, T3, T6, and T12. Group A (black) received topical Minoxicapil spray only, while Group B (green) received both spray and oral Minoxicapil.
3.3.2. Sex-Specific Trends
Sex-stratified analysis (Figure 11) revealed distinct trends in trichologic parameter evolution between male and female participants. Across nearly all domains, females demonstrated more stable and progressive improvements, particularly in hair density (T0: 177.95 to T12: 210.22), anagen count (88.15 to 107.29), and reduction in telogen count (36.25 to 14.26). Males also showed gains in hair growth parameters, but with more pronounced increases in shaft thickness (0.0589 mm to 0.2265 mm) and greater reductions in empty follicle count (6.00 to 2.95). Both groups improved their anagen/telogen ratio over time, though females reached slightly higher values at T12. The reduction in peripilar signs was consistent across sexes, with males reaching negative values by T12, suggesting potential resolution of perifollicular inflammation.
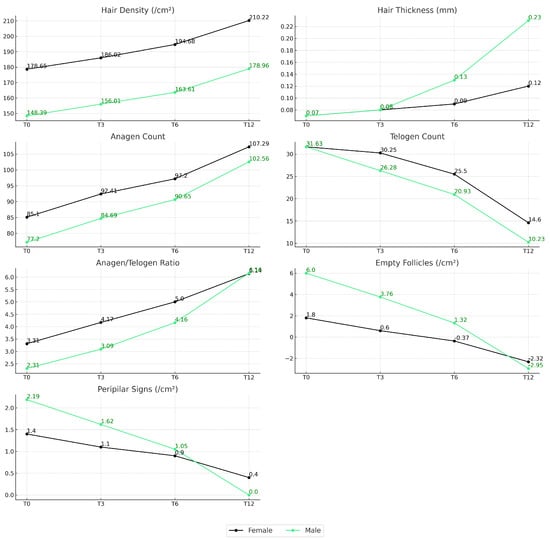
Figure 11.
Evolution of trichologic parameters over 12 months by subject sex. Line graphs depict the mean values of seven trichologic parameters at baseline (T0), 3 months (T3), 6 months (T6), and 12 months (T12), stratified by sex. Female participants are represented by black lines, and male participants by light green lines.
The observed sex-specific response patterns may reflect fundamental differences in AGA pathophysiology between men and women. Female participants demonstrated greater improvements in hair density and anagen conversion, while male subjects exhibited more pronounced reductions in empty follicles and thicker hair shafts. These findings are consistent with the known hormonal dynamics in AGA: in men, androgen sensitivity and DHT-driven miniaturization predominate, often resulting in complete follicular regression; in women, hormonal imbalances, perifollicular inflammation, and microvascular dysfunction play a greater role [4]. The more favorable density gains in women may therefore reflect improved perifollicular circulation and anti-inflammatory effects, while the reduction in empty follicles among men suggests partial reversal of follicular dormancy.
3.4. Patient-Reported Tolerability and Subjective Observations
Not all participants provided complete responses, and some did not answer the self-evaluation or tolerability questions.
Among those who completed the study and answered the final survey, most participants in both the male and female groups rated the overall tolerability of the treatment as good (20F and 17M) to very good (7F and 2M), with common positive remarks highlighting pleasant fragrance (17F and 9M), ease of application (3F and 3M), reduction in hair loss (17F and 10M), and perceived hair thickening or strengthening (10F and 5M). Notably, two participants reported visible nail improvement (1F and 1M) and increased hair resistance. Male participants frequently described a reduction in shedding and a refreshing sensation on the scalp (4M). Some also mentioned indirect benefits such as improved sleep quality.
Minor adverse effects were reported by a subset of subjects and were generally mild and transient. These included scalp dryness (4F and 1M), itching (2F and 3M), mild dandruff (2M), increased sebum production (4F), or mild gastrointestinal effects like increased appetite (3F) or accelerated transit. A few subjects noted capsule size and smell as unpleasant (4F and 2M), while three male users reported initial irritation or mild folliculitis. One participant reported persistent scalp itching, albeit it was proven as neuropathy. Despite these issues, no serious adverse reactions were reported, and several users continued treatment independently beyond the study period (2F). Of note, no minoxidil class effects such as orthostatic hypotension or mastodynia were recorded.
Across 57 participants who completed treatment satisfaction self-evaluations, the mean score was 1.97 (on a scale from 0 to 3), with a standard deviation of 0.63, indicating moderate to high satisfaction overall. The median and mode were both 2, reflecting a generally positive perception of the treatment. Most frequently, participants rated their experience as “2” (37 responses), followed by “1” and “3” (each with 9 responses). One participant rated the experience as “0”, and another gave a nuanced rating of “2.5”. These findings suggest that the majority of users found the treatment effective and tolerable, with relatively few expressing dissatisfaction.
4. Discussion
This open-label study demonstrated significant improvements in trichologic parameters after three months of Minoxicapil treatment, including increased hair density, hair shaft thickness, and a favorable shift in the anagen/telogen ratio. These findings are in line with previous reports evaluating similar formulations containing pyrrolidinyl diaminopyrimidine oxide derivate, oleanolic acid, apigenin, and biotinyl tripeptide-1 [6].
The trichoscopic parameters selected for this study reflect core biological dimensions of follicular health in AGA. Hair density (hairs/cm2) is a direct metric for follicular activity and is commonly used as a primary endpoint in AGA trials. Hair shaft thickness reflects the degree of miniaturization, a hallmark of androgen-sensitive follicular regression. The anagen/telogen ratio is a global marker of follicular cycling; lower values suggest chronic telogen dominance or effluvium overlap, while improvements in this ratio indicate a therapeutic response [11]. The number of empty follicles corresponds to irreversible follicular dropout, particularly in advanced stages, and is associated with permanent hair loss [9]. Peripilar signs—seen as brownish perifollicular halos—have been linked to subclinical inflammation and perifollicular fibrosis, which are increasingly recognized as contributors to AGA progression [9]. Together, these parameters offer a multidimensional framework for evaluating therapeutic efficacy in both early- and late-stage disease. This approach is further supported by recent systematic reviews of trichoscopy in hair disorders [17].
The enhancement in hair density and follicular activity is likely attributable to the synergistic effect of the formulation’s core ingredients. Minoxicapil is a proprietary complex that includes pyrrolidinyl diaminopyrimidine oxide, a compound with structural similarity to minoxidil. It is currently marketed as a cosmetic ingredient, and this study represents one of the first clinical evaluations of its use in androgenetic alopecia.
Oleanolic acid’s inhibition of 5α-reductase and its ability to stimulate Wnt/β-catenin signaling has been shown to induce follicular proliferation and prolong the anagen phase in both in vitro and in vivo models [7]. Apigenin’s vasodilatory and anti-inflammatory properties support angiogenesis and reduce perifollicular fibrosis, while also downregulating TGF-β1—a key catagen-inducing cytokine—thereby helping maintain the follicles in an active growth phase [8].
Biotinyl tripeptide-1 contributes further by inhibiting 5α-reductase and enhancing dermal matrix synthesis, fostering healthier follicular anchoring and cycling. Clinical data suggest that its combination with apigenin and oleanolic acid leads to significant increases in both hair count and thickness in patients with AGA and telogen effluvium [6].
Interestingly, our sex-specific analysis revealed differing response patterns: female participants demonstrated greater gains in density and anagen conversion, while male participants showed more substantial reduction in empty follicles and shaft thickening. This divergence may reflect underlying hormonal differences and follicular DHT sensitivity, as supported by histopathologic and transcriptomic evidence [4].
The extrapolated projections to 6 and 12 months suggest continued improvement, although at a diminishing rate, which may reflect a therapeutic plateau commonly seen in chronic hair growth treatments. These results align with prior longitudinal studies evaluating botanical-based anti-alopecic interventions and highlight the need for long-term maintenance strategies.
While extrapolated data suggest continued improvement in hair density and follicular activity through 12 months, such linear trends are unlikely to persist indefinitely due to the cyclical biology of hair growth and potential treatment plateau effects. These findings serve as a hypothetical model rather than definitive long-term efficacy data.
Patient-reported outcomes supported the favorable tolerability profile of the treatment. Among participants who completed the study, 27 women and 19 men rated tolerability as good or very good. Commonly reported benefits included pleasant fragrance, ease of application, reduced hair shedding, and perceived hair strengthening. Mild adverse effects such as scalp dryness, itching, increased sebum, and gastrointestinal discomfort were reported by a minority, with no serious adverse events observed. The mean self-evaluation score was 1.97 (on a 0–3 scale). These findings are consistent with evidence showing that treatment tolerability and user satisfaction are key predictors of adherence in AGA management [18].
Despite the study’s strengths—including objective trichoscopic measurements and gender-specific subgroup analysis—certain limitations should be acknowledged.
A key limitation of this study is the absence of a placebo or active comparator arm. As an open-label trial, the design is inherently more susceptible to expectation bias, and spontaneous hair regrowth cannot be excluded. While the absence of a placebo or monotherapy capsule group may be viewed as a limitation, similar study designs are common in early-phase AGA research. Garre et al. evaluated a multi-agent topical formulation without a placebo comparator [6], and Oura et al.reported positive outcomes in a short-duration (4-month) combination therapy trial [9]. Moreover, in the context of ethical and adherence concerns related to withholding treatment in a progressive condition like AGA, pragmatic designs with active comparator arms have been increasingly employed [19].
Additionally, a treatment arm consisting solely of oral Minoxicapil capsules was not included in this study. This decision was based on the known limited efficacy of nutritional supplementation as a monotherapy in androgenetic alopecia, which has historically demonstrated inconsistent or minimal impact when used in isolation [20,21]. The primary goal of this study was to assess whether the addition of systemic support to a topical formulation yields enhanced outcomes. Including a capsule-only group would have increased study complexity and required a larger sample size without directly answering the core hypothesis. Furthermore, most clinical protocols involving nutraceuticals in AGA use them adjunctively rather than as standalone interventions [20].
While Minoxicapil demonstrated favorable results, it was not directly compared to conventional therapies such as 5% topical minoxidil or oral finasteride which have demonstrated synergistic efficacy when used in combination [22]. Previous trials have shown that these agents yield an average increase of 10–20 hairs/cm2 over 4–6 months [1].
Our findings are consistent with those of Garre et al., who reported significant improvements in hair count and thickness using a topical botanical complex similar to Minoxicapil [6]. However, unlike their study, our formulation incorporated both topical and oral components, targeting follicular health through both direct follicular stimulation and systemic nutritional support. Similar synergistic effects have been observed in studies combining topical minoxidil with oral finasteride, which yielded superior outcomes compared to monotherapy [22]. Similar synergistic effects were observed in a randomized trial combining platelet-rich plasma (PRP) with topical minoxidil [23]. Furthermore, recent work by Legiawati et al. highlights the relevance of integrating metabolic and vascular support into AGA treatment regimens, particularly in early-onset cases [12]. These comparisons support the rationale for multi-pathway combination therapies in androgenetic alopecia.
Mechanistically, the observed trichologic improvements can be attributed to the dual targeting of hormonal and vascular pathways. Oleanolic acid and biotinyl tripeptide-1 inhibit 5α-reductase, thereby reducing DHT-mediated miniaturization. Apigenin promotes perifollicular vasodilation and angiogenesis through upregulation of VEGF and nitric oxide synthesis. Simultaneously, Wnt/β-catenin signaling—enhanced by oleanolic acid—plays a central role in activating follicular stem cells and sustaining anagen phase duration. The inclusion of oral micronutrients such as zinc, selenium, and methionine support keratin production and scalp homeostasis, complementing topical mechanisms with systemic bioavailability [21]. Recent clinical trials have demonstrated similar systemic benefits from oral probiotic supplementation, further supporting the concept of multimodal therapy in AGA [24].
In our study, the combined therapy (spray and capsules) led to an increase of 12.14 hairs/cm2 over 3 months. Direct head-to-head studies are necessary to establish whether Minoxicapil provides comparable efficacy.
Other identified limitations include the relatively small sample size, and a short primary endpoint. Despite the relatively short 3-month observation period, previous studies investigating combination treatments for AGA have employed similarly brief durations. For example, Garre et al. evaluated a similar botanical complex over a 6-month period with interim analysis at 3 months, demonstrating significant improvements in hair density and thickness at that early point [6]. Some autologous micrograft therapy trials have reported meaningful results in under 3 months [25]. Oura et al. also reported meaningful changes in hair parameters after just 4 months of adenosine-based treatment [9]. Additionally, recent trichoscopic protocols, such as Hoffmann et al. and Legiawati et al., used short-term data for predictive modeling and extrapolation of long-term follicular outcomes [11,12]. These precedents support the validity of short-duration endpoints and the utility of cautious extrapolation in AGA research.
Nonetheless, the consistency of our results with prior mechanistic and clinical research—including the effects of oleanolic acid on follicular proliferation via Wnt/β-catenin [7], apigenin’s role in vascular stimulation and anti-fibrotic signaling [8], and clinical improvements seen with the combined use of biotinyl tripeptide-1, apigenin, and oleanolic acid [6]—reinforces the potential clinical utility of Minoxicapil in the treatment of AGA.
To the best of our knowledge, this is the first consistent confirmation of pyrrolidinyl diaminopyrimidine oxide efficacy in female pattern hair loss.
Future research should explore long-term safety, optimal dosing schedules, and molecular biomarkers predictive of treatment response. To substantiate these preliminary findings, future randomized, double-blind, placebo-controlled trials with longer follow-up durations are essential. Such studies would clarify long-term efficacy and safety, and better define Minoxicapil’s role within the evolving framework of personalized alopecia management.
5. Conclusions
Minoxicapil demonstrated statistically and clinically significant improvements in key trichologic parameters in patients with androgenetic alopecia over 3 months. The combined action of oleanolic acid, apigenin, and biotinyl tripeptide-1 supports a multimodal therapeutic model, capable of addressing the hormonal, inflammatory, and vascular components of AGA. While individual ingredient mechanisms suggest synergistic effects, the present study does not allow for isolated attribution of efficacy.
Extrapolated projections suggest continued improvement up to 12 months, though the rate of change decreases after 6 months, indicating a potential therapeutic plateau.
These findings underscore the potential of Minoxicapil as a viable treatment alternative or adjunct in both male and female AGA and warrant further investigation through randomized controlled trials with longer follow-up periods.
Author Contributions
Conceptualization and study design, V.-M.V. and M.L.; literature research, V.-M.V.; images acquisition V.-M.V.; data and statistical analysis, M.L.; writing—original draft preparation, M.L.; writing—review and editing, V.-M.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of Fiterman Pharma, Iasi, in facilitating the conduct of this study and supporting its publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Elias Emergency University Hospital, Bucharest, Romania (protocol code 12808-1/28.08.2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Composition of oral Minoxicapil capsules.
Table A1.
Composition of oral Minoxicapil capsules.
| Active Ingredient | Amount per Daily Dose | % Daily Value (DV) |
|---|---|---|
| Methionine | 200 mg | – |
| Cysteine | 100 mg | – |
| Zinc | 20 mg | 200% |
| Copper | 1 mg | 100% |
| Selenium | 63.25 µg | 115% |
| Vitamin D3 | 17.8 µg | 356% |
| Vitamin C | 196 mg | 245% |
| Vitamin A | 96 µg | 12% |
| Vitamin B1 (Thiamine) | 0.12 mg | 11% |
| Vitamin B2 (Riboflavin) | 0.16 mg | 11.4% |
| Vitamin B3 (Niacin) | 17.8 mg | 111.2% |
| Vitamin B5 (Pantothenic Acid) | 0.8 mg | 13.5% |
| Vitamin B6 | 0.14 mg | 10% |
| Biotin | 2000 µg | 4000% |
| Vitamin B9 (Folic Acid) | 20 µg | 10% |
| Vitamin B12 | 0.2 µg | 8% |
References
- Whitting, D.A. Hair Growth and Disorders; Blume-Peytavi, U., Whiting, D.A., Trüeb, R.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; p. 564. [Google Scholar] [CrossRef][Green Version]
- Messenger, A.G.; Sinclair, R. Follicular miniaturization in female pattern hair loss: Clinicopathological correlations. Br. J. Dermatol. 2006, 155, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, W.; Luo, J.; He, J.; Zheng, X.; Zhu, S.; Rong, B.; Ai, Y.; Zhang, L.; He, T. Effects of oleanolic acid on hair growth in mouse dorsal skin mediated via regulation of inflammatory cytokines. J. Appl. Biomed. 2023, 21, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan Orhan, İ.; Abacı, N. A Narrowed Look Into Plant-Derived Testosterone 5α-Reductase Inhibitors for Androgenetic Alopecia. Curr. Perspect. Med. Aromat. Plants 2024, 7, 58–72. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Blumeyer, A.; Tosti, A.; Finner, A.; Marmol, V.; Trakatelli, M.; Reygagne, P.; Messenger, A. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br. J. Dermatol. 2011, 164, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Garre Contreras, A.; Piquero-Casals, J.; Trullas, C.; Martinez, G. Efficacy and Safety of a New Topical Hair Loss-Lotion Containing Oleanolic Acid, Apigenin, Biotinyl Tripeptide-1, Diaminopyrimidine Oxide, Adenosine, Biotin and Ginkgo biloba in Patients with Androgenetic Alopecia and Telogen effluvium: A Six-month Open-Label Prospective Clinical Study. J. Cosmetol. Trichol. 2018, 04, 1000132. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Yi, H.; Han, L.; Ji, B.; Chen, H.; Deng, W.; Wan, M. β-Catenin is involved in oleanolic acid-dependent promotion of proliferation in human hair matrix cells in an in vitro organ culture model. Fitoterapia 2017, 121, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Lee, J.; Jung, E.; Kim, S.-C.; Kang, J.-I.; Lee, J.; Kim, Y.-W.; Sung, Y.K.; Kang, H.-K.; Park, D. A cell-based system for screening hair growth-promoting agents. Arch. Dermatol. Res. 2009, 301, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Oura, H.; Iino, M.; Nakazawa, Y.; Tajima, M.; Ideta, R.; Nakaya, Y.; Arase, S.; Kishimoto, J. Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: A pilot, double-blind, randomized, placebo-controlled trial. J. Dermatol. 2008, 35, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.; Batra, S.; Barreto, D.; Rapaport, J. An Updated Etiology of Hair Loss and the New Cosmeceutical Paradigm in Therapy: Clearing ‘the Big Eight Strikes’. Cosmetics 2023, 10, 106. [Google Scholar] [CrossRef]
- Hoffmann, R. TrichoScan: A Novel Tool For The Analysis of Hair Growth In Vivo. J. Investig. Dermatol. Symp. Proc. 2003, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Legiawati, L.; Sitohang, I.B.S.; Yusharyahya, S.N.; Sirait, S.P.; Novianto, E.; Yunir, E.; Lauren, B.C.; Hakiki, N.P.; Rahmadika, F.D. The comparison of metabolic syndrome parameters, trichoscopic and trichoscan characteristics in androgenetic alopecia (AGA) and early-onset androgenetic alopecia (early-onset AGA). Arch. Dermatol. Res. 2024, 316, 581. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. “Let Food be Thy Medicine”: Value of Nutritional Treatment for Hair Loss. Int. J. Trichol. 2021, 13, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet 1995, 346, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, L.; Cottle, P.; Grimalt, R.; Kasprzak, M.; Sicińska, J.; Sinclair, R.; Tosti, A. Efficiency of Hair Detection in Hair-to-Hair Matched Trichoscopy. Skin. Appendage Disord. 2022, 8, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kuczara, A.; Waśkiel-Burnat, A.; Rakowska, A.; Olszewska, M.; Rudnicka, L. Trichoscopy of Androgenetic Alopecia: A Systematic Review. J. Clin. Med. 2024, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Van Zuuren, E.J.; Fedorowicz, Z.; Carter, B.; Schoones, J. Interventions for female pattern hair loss. Cochrane Database Syst. Rev. 2016, 2016, CD007628. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Finner, A.M. Nutrition and hair: Deficiencies and supplements. Dermatol. Clin. 2013, 31, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The role of vitamins and minerals in hair loss: A review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Caro, G. Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: A prospective, randomized, controlled, assessor blinded, 3-arm, pilot trial. J. Cosmet. Dermatol. 2024, 23, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, E.E.; Smirnova, I.O. Comparative Evaluation of the Clinical Efficacy of PRP-Therapy, Minoxidil, and Their Combination with Immunohistochemical Study of the Dynamics of Cell Proliferation in the Treatment of Men with Androgenetic Alopecia. Int. J. Mol. Sci. 2020, 21, 6516. [Google Scholar] [CrossRef] [PubMed]
- García-Navarro, A.; Vasallo-Morillas, M.I.; Navarro-Belmonte, R.; Vilanova, C.; Torrent, D.; Kilasoniya, A.; Moles-Ugeda, I.; Gallego-Herrera, E.; Ramírez-Boscá, A. Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia. Nutrients 2024, 16, 2900. [Google Scholar] [CrossRef] [PubMed]
- Krefft-Trzciniecka, K.; Piętowska, Z.; Pakiet, A.; Nowicka, D.; Szepietowski, J.C. Short-Term Clinical Assessment of Treating Female Androgenetic Alopecia with Autologous Stem Cells Derived from Human Hair Follicles. Biomedicines 2024, 12, 153. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).