Abstract
Centella asiatica (CICA)-derived exosomes have emerged as bioactive agents for skin rejuvenation due to their regenerative and anti-inflammatory properties. This study evaluated the safety and efficacy of a topical ampoule containing CICA-derived extracellular vesicles (EVs) in healthy Korean adults. This human application study was conducted over a 15-day period, during which the test formulation was topically applied to the skin following a controlled regimen. A 24-h patch test with 30 participants confirmed non-irritation (irritation index: 0.00). In a separate two-week trial (n = 20; mean age 50.7 years), 3D imaging and ultrasound assessed five-dimensional pore improvement (area, density, volume, filling, texture), wrinkle depth reduction in five facial regions, dermal hydration at 0.5, 1.5, and 2.5 mm depths, and skin density. Significant reductions were observed in mean pore area (−17.9%) and pore density (−26.9%), with a 9.0% decrease in surface roughness. Wrinkle depths decreased by 7.8–18.8% across the forehead, glabella, crow’s feet, nasolabial folds, and neck. Hydration increased by 7.9% at 0.5 mm, and dermal density improved by 12.7% (p < 0.05). These findings highlight the excellent skin compatibility and multifaceted cosmetic benefits of the formulation containing CICA-derived exosomes and other active ingredients, underscoring its potential as a safe, effective, and innovative anti-aging cosmetic agent.
1. Introduction
The skin, as the largest organ of the human body, acts as the primary defense barrier against environmental aggressors such as ultraviolet (UV) radiation, airborne pollutants, and microbial pathogens. In addition to its protective function, the skin plays vital roles in thermoregulation, immune surveillance, and maintaining homeostasis [1]. Anatomically, the skin comprises three layers: the epidermis, which contains keratinocytes, melanocytes, and Langerhans cells; the dermis, composed mainly of fibroblasts and extracellular matrix (ECM) proteins such as collagen and elastin; and the subcutaneous layer, predominantly made up of adipocytes and vascular stromal cells [2].
Skin aging is a multifactorial process influenced by both intrinsic and extrinsic factors. Intrinsic aging is primarily driven by genetic and metabolic changes that reduce cellular turnover and collagen synthesis. Extrinsic aging, in contrast, is induced by chronic exposure to UV radiation, oxidative stress, and environmental toxins, leading to dryness, pigmentation, loss of elasticity, and wrinkle formation [3,4]. In response to this, the global skincare market has shifted toward bioactive formulations aimed at restoring skin health and appearance through hydration, antioxidant protection, and ECM support [5].
Among natural-origin actives, Centella asiatica (Apiaceae; commonly known as CICA) has gained prominence in both traditional medicine and modern skincare for its broad-spectrum therapeutic properties. Rich in triterpenoids, flavonoids, and polyphenols, CICA has demonstrated potent anti-inflammatory, antioxidant, and wound-healing activities in preclinical and clinical settings [6,7,8]. These properties have led to its widespread incorporation into skincare products targeting sensitive, aging, or damaged skin [9].
More recently, extracellular vesicles (EVs) derived from plants have emerged as a next-generation bioactive class. These nanosized, lipid bilayer-bound particles transport signaling molecules such as proteins, miRNAs, and lipids, and have been shown to mediate cross-kingdom biological communication [10]. EVs isolated from CICA in particular have demonstrated enhanced efficacy in skin regeneration and inflammation reduction compared to crude extracts [11].
In this context, the CICA Exosome Ampoule, ExoGlow (JUVEV®, Microgentas, Seoul, Republic of Korea), a cosmetic formulation enriched with CICA-derived extracellular vesicles, was developed to leverage these advantages for topical skin care. This study aimed to evaluate the clinical safety and efficacy of the CICA Exosome Ampoule (hereinafter “CICA-EV ampoule”) through two separate trials: (1) a 24-h human patch test involving 30 participants to assess primary skin irritation potential; and (2) a 2-week clinical evaluation in 20 adult volunteers to assess effects on pore characteristics, wrinkle depth, dermal hydration, and skin density using high-resolution imaging and biophysical instruments. The goal of this investigation is to validate the application of CICA-derived EVs as a safe and multifunctional ingredient in advanced anti-aging skincare.
2. Materials and Methods
2.1. Isolation and Characterization of CICA-Derived Exosomes
Centella asiatica extract solution (1 L) was purchased from Freshfarm Co. (Seoul, Republic of Korea; Cat. No. 4345825554) and used directly without further modification. CICA-derived extracts (400 mL) were applied to an electrokinetic-assisted mesh filtration system [12], ExoFilter® (Microgentas, Seoul, Republic of Korea), as shown in Figure A1a,b. The filtration unit comprised a multilayer mesh coated with a positively charged salt to selectively bind negatively charged extracellular vesicles (EVs) while allowing contaminants to pass through. Samples were loaded onto the filter under vacuum pressure flow at ∼400 mL/min (Figure A1c). After loading, an optional 100 mL wash with a washing buffer removed residual contaminants. Bound EVs were then eluted by passing 100 mL of elution buffer through the filter under vacuum (Figure A1c, center), and the eluate containing purified EVs was collected in sterile tubes (Figure A1c, right). SEM and TEM imaging confirmed that the CICA-derived nanoparticles exhibit characteristic EV morphology and size, appearing as ~100–150 nm cup-shaped vesicles with a double membrane. (Figure A1d,e). Nanoparticle tracking analysis was performed using a ZetaView system (Particle Metrix, Ammersee, Germany). The isolated particles displayed a size distribution peaking at approximately 130 nm, with a total concentration of 2.3 × 109 particles/mL (Figure A1f).
2.2. Test Product and Full Ingredient Disclosure
The study product, CICA-EV ampoule, is a clear, water-based serum formulated to deliver high-purity CICA-derived exosomes (20,000 ppm) together with moisturizing and barrier-enhancing actives. The full ingredient list (INCI names) is shown in Table 1.

Table 1.
Full ingredient list for CICA-EV ampoule.
2.3. Participants
Two independent studies were conducted to assess the safety and efficacy of CICA-EV ampoule. The first was a 24-h primary skin irritation test involving 30 healthy adult volunteers (age range, 35–69 years; mean and median age, 54 years). The second was a two-week efficacy evaluation involving 20 healthy female participants (age range, 34–63 years; mean and median age, 51 years). All subjects were screened to exclude those with chronic illnesses, dermatological disorders, known allergies, or use of systemic medications or topical treatments during the study period.
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and followed relevant domestic regulations, including the Bioethics and Safety Act of Korea. The trial was performed in compliance with the Standard Operating Procedures (SOPs) of ACE Clinical Research Center, the ICH-GCP guidelines (Integrated Addendum to ICH E6: Guideline for Good Clinical Practice E6), and the guidelines for Human Application Tests of Cosmetics issued by the Ministry of Food and Drug Safety (MFDS), Republic of Korea. The reliability of the study was confirmed by the quality assurance officer. The protocol was approved by the Institutional Review Board (IRB) of ACE Clinical Research Center (Approval No. ASC-IRB-2411-150, 29/10/2024).
2.3.1. Inclusion Criteria
- Male or female adults aged 30 years or older.
- Individuals who voluntarily agreed to participate after receiving a full explanation of the study protocol and restrictions from the investigator or delegated personnel.
- Individuals with no acute or chronic systemic diseases, including dermatological conditions.
- Individuals who fully understood the potential risks and signed a written informed consent form.
- Individuals who were available for follow-up during the study period.
2.3.2. Exclusion Criteria
- Pregnant or breastfeeding women, or those planning to become pregnant.
- Individuals with psychiatric disorders or infectious skin diseases.
- Individuals who have used topical corticosteroids for more than one month to treat skin diseases.
- Individuals who participated in a similar clinical study within the past 6 months.
- Individuals with dermatological abnormalities at the test site (e.g., moles, acne, erythema, telangiectasia).
- Individuals who used cosmetics or pharmaceutical products with similar efficacy at the test site within the past 3 months.
- Individuals participating in multiple concurrent studies.
- Individuals deemed unsuitable for the study by the principal investigator.
2.3.3. Drop-Out Criteria
- Participants were excluded from the final analysis if any of the following occurred:
- Voluntary withdrawal of consent at any point during the study.
- Subjective decision by the participant to discontinue due to personal reasons or inability to attend follow-up visits.
- Occurrence of a serious adverse event or a reaction deemed related to the product.
- Non-compliance with the scheduled study visits or protocol violations.
Participants were instructed to use only the provided CICA-EV ampoule and the standardized, fragrance-free cleanser supplied by the study team, and to refrain from applying any other topical products (e.g., serums, masks, lotions) to the test area throughout the trial. Before each measurement, subjects washed their faces with the provided cleanser and rested for 30 min in a controlled environment (22 ± 2 °C, 50 ± 10% RH). No specific dietary restrictions were imposed; participants maintained their habitual diet and lifestyle throughout the study.
2.4. Study Design and Ethical Approval
The clinical study was conducted at Ace Clinical Research Center (Seoul, Republic of Korea) under IRB approval no. ASC-IRB-2411-150 and in compliance with the Korean Bioethics and Safety Act, the Declaration of Helsinki, ICH-GCP E6(R2), and MFDS guidelines for human application testing of cosmetics. Written informed consent was obtained from every participant, and the quality assurance manager confirmed that all procedures adhered fully to the approved protocol with no critical deviations. This investigation included both a skin irritation test and a clinical efficacy evaluation under controlled conditions.
2.5. Irritation Test
A 24-h closed patch application was performed on a non-lesional, flat area of the upper back (excluding the spine) in healthy Korean adults aged ≥ 19 years. Approximately 20 μL of the test product (liquid or diluted) was applied via a 10 mm × 10 mm cut sample of the patch-type formulation. A board-certified dermatologist visually assessed skin reactions at 30 min and 24 h after patch removal using a standardized 0–4 grading scale (0 = no erythema).
2.6. Efficacy Evaluation
The clinical efficacy trial was conducted at Ace Clinical Research Center (Seoul, Republic of Korea) under controlled temperature and humidity (22 ± 2 °C, 50 ± 10% RH). Prior to any assessments, participants washed their faces with a standardized cleanser and then rested for 30 min to acclimatize. The CICA-EV ampoule was applied twice daily (morning and evening) for 14 consecutive days. Instrumental assessments—including pore parameters, layered hydration, wrinkle depth, and dermal density—were performed at baseline (Day 0) and after the final application (Day 14). All measurements took place in a controlled environment (20 ± 1 °C, 50 ± 5% RH), with participants resting for at least 15 min before each session.
2.7. Instrumentation and Measurements
2.7.1. Pore Analysis
Five pore characteristics—mean pore area (mm2), pore density (ea/cm2), mean pore volume (10−3 mm3), total pore volume (mm3), and maximum pore depth (mm)—were measured at baseline and after treatment using Antera 3D® imaging (Miravex Ltd., Dublin, Ireland) under standardized lighting conditions. Images were captured at identical facial regions, and proprietary software (version X.Y) provided numerical outputs for each parameter.
2.7.2. Layered Hydration
Deep skin moisture content, expressed as tissue dielectric constant (TDC), was assessed at 0.5, 1.5, and 2.5 mm depths using MoistureMeter D® (Delfin Technologies Ltd., Kuopio, Finland). Prior to each measurement session, the probe was calibrated according to the manufacturer’s protocol. Three consecutive readings were obtained at each depth per subject, and the average value was recorded.
2.7.3. Wrinkle Depth
Wrinkle depth (mm) for five anatomical sites—forehead, glabella, periorbital (crow’s feet), nasolabial fold, and neck—was quantified using Antera 3D® at baseline (Day 0) and after two weeks of treatment (Day 14). The same facial landmarks and imaging settings were maintained to ensure consistency across both time points.
2.7.4. Dermal Density and Elasticity
Dermal density (g/cm3) and elasticity (E %) at predefined facial landmarks were optionally measured using Ultrascan UC22® (Courage+Khazaka, Köln, Germany). Measurements were performed in triplicate, and median values were recorded for analysis.
2.8. Statistical Analysis
Data were analyzed using IBM SPSS Statistics 23 (SPSS Inc., Chicago, IL, USA). The normality of continuous variables was assessed by the Shapiro–Wilk test. For outcomes measured at two time points, normally distributed data were evaluated with a paired t-test, whereas non-normally distributed data were assessed with the Wilcoxon signed-rank test. Statistical significance was defined as p < 0.05 (two-tailed). Improvement (%) was calculated as follows:
3. Results
A total of 30 healthy Korean adults participated in the 24-h patch test, and 20 were subsequently enrolled in a two-week efficacy trial to assess improvements in skin condition following daily application of the CICA-EV ampoule over 14 days.
3.1. Subject Demographics
A total of 21 healthy adults were initially enrolled in the two-week efficacy study; one subject (S15) withdrew at week 2 for noncompliance, leaving 20 subjects (all female) for final analysis. The mean age of the efficacy cohort was 50.7 ± 8.9 years (range 34–63 years). Age distribution was as follows: 5% in their 30 s, 35% in their 40 s, 50% in their 50 s, and 10% in their 60 s (Figure 1a). Skin types were classified by a board-certified dermatologist at baseline: 20% of subjects had normal skin, 5% oily, 50% dry, and 25% combination (Figure 1b).
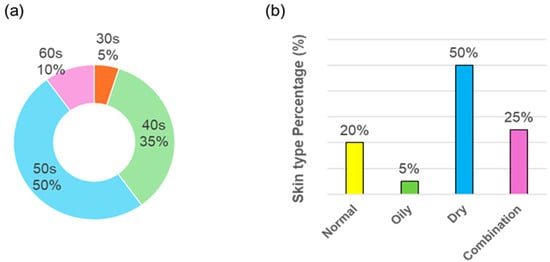
Figure 1.
Demographics of study participants (n = 20). (a) Age distribution, (b) Skin type distribution.
3.2. Primary Irritation Evaluation
A 24-h closed patch test was conducted on the upper backs of 30 healthy adults (all female; age range, 35–69 years; mean and median age, 54 years) to assess the primary irritation potential of the CICA-EV ampoule. A board-certified dermatologist graded skin responses at 30 min and 24 h after patch removal using a standard 0–4 scale (0 = no erythema). As shown in Table 2, no participant exhibited any signs of erythema, edema, or other irritant reactions at either time point (mean irritation score = 0.00), classifying the product as “Excellent” (non-irritant).

Table 2.
Primary irritation scores for the CICA-EV ampoule (n = 30).
During the two-week efficacy trial (n = 20; all female; mean and median age, 51 years), subjects applied the CICA-EV ampoule twice daily to the face. No adverse skin reactions—such as pruritus, dryness, or contact dermatitis—were reported or observed, and no concomitant topical or systemic medications were used. Taken together, these findings demonstrate that the CICA-EV ampoule is non-irritating and well tolerated under both patch-test and extended-use conditions.
3.3. Visual Assessment of Pore and Wrinkle Improvements
Figure 2a shows enlarged 3D pore images of the right cheek captured with Antera 3D (Miravex Ltd., Dublin, Ireland) before treatment (left) and two weeks after daily application of the CICA-EV ampoule (right). Blue dots mark the automatically detected pore openings.

Figure 2.
Representative Antera 3D images illustrating the effects of daily CICA-EV ampoule application on (a) cheek pore distribution and (b) forehead wrinkle mapping. Left panels show baseline (“Before use”) and right panels show results after two weeks of treatment (“2 weeks after”). In (a), blue dots mark automatically detected pore openings; in (b), yellow-to-red color overlays indicate increasing wrinkle depth and length.
Also, Figure 2b shows the typical wrinkle maps of the forehead region acquired with Antera 3D before treatment (left) and two weeks post-treatment (right). Yellow-to-red color overlays indicate increasing wrinkle depth and length.
While these images clearly illustrate the qualitative reduction in pore size and wrinkle depth following CICA-EV ampoule treatment, a more rigorous evaluation requires quantitative analysis. Accordingly, we employed the Antera 3D system to measure changes in pore density and wrinkle parameters, enabling an objective assessment of the ampoule’s efficacy.
3.4. Pore Improvement Outcomes
Figure 3 presents the changes in five key parameters of skin pores measured by Antera 3D before and after a single use of the CICA-EV ampoule (Day 1). All five parameters—mean pore area, pore density, mean pore volume, total pore volume, and maximum pore depth—showed statistically significant reductions (p < 0.05, Table A1).
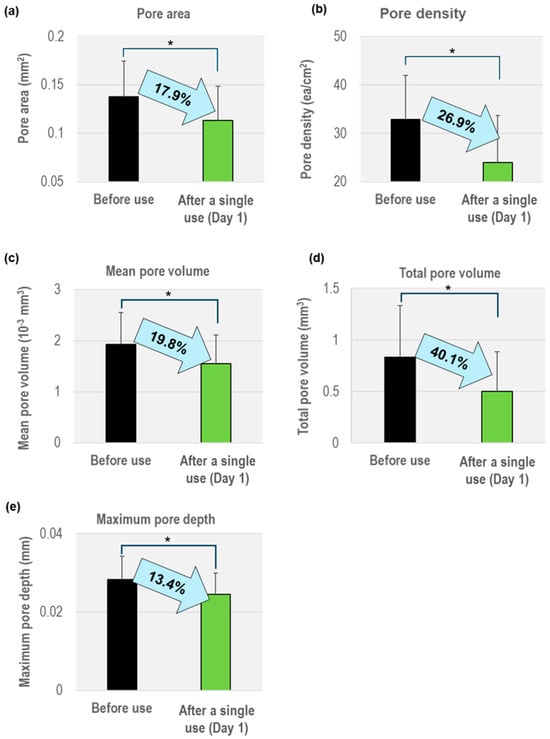
Figure 3.
Percentage reductions in pore parameters after a single use (Day 1) of the CICA-EV ampoule (n = 20). (a) Mean pore area, (b) Pore density, (c) Mean pore volume, (d) Total pore volume, (e) Maximum pore depth. All reductions were statistically significant (* p < 0.05; paired t-test).
As shown in Figure 3a, the mean pore area decreased from 0.1378 ± 0.0366 mm2 at baseline to 0.1131 ± 0.0352 mm2 after a single use (−17.9%). Pore density fell from 32.83 ± 9.20 ea/cm2 to 24.00 ± 9.74 ea/cm2 (−26.9%, Figure 3b). Mean pore volume was reduced from 0.001934 ± 0.000614 mm3 to 0.001551 ± 0.000566 mm3 (−19.8%, Figure 3c), while total pore volume dropped from 0.8350 ± 0.4984 mm3 to 0.5005 ± 0.3844 mm3 (−40.1%, Figure 3d). Maximum pore depth decreased from 0.0283 ± 0.0059 mm to 0.0245 ± 0.0054 mm (−13.4%, Figure 3e). These results demonstrate that a single application produces rapid, statistically significant refinements in pore structure. Each parameter’s before-and-after comparison was analyzed using the paired t-test (or Wilcoxon signed-rank test when normality was not met), and all changes reached statistical significance (p < 0.05). These results demonstrate that the CICA-EV ampoule produces rapid and robust pore-refining effects, with a pronounced impact on total pore volume.
3.5. Hydration Outcomes
Table 3 summarizes the effects of a single application of the CICA-EV ampoule on deep skin moisture content at three skin depths (0.5, 1.5, and 2.5 mm; n = 20). All depths showed statistically significant increases in moisture, as measured by the tissue dielectric constant (TDC). At the 0.5 mm measurement depth, a single application of the CICA-EV ampoule increased TDC from 40.4 ± 6.3 at baseline to 43.5 ± 5.9 after use, representing a 7.9% improvement (p < 0.05, Wilcoxon signed-rank test).

Table 3.
Effects of skin moisture content at three different depths (n = 20).
Similar, though slightly attenuated, gains were observed at deeper layers: at 1.5 mm depth, TDC rose by 6.5% (from 37.3 ± 6.2 to 39.8 ± 5.7; p < 0.05), and at 2.5 mm depth by 4.5% (from 32.3 ± 3.7 to 33.77 ± 3.8; p < 0.05). These findings indicate that the CICA-EV ampoule delivers rapid and significant hydration enhancements throughout the skin strata, with the most substantial effect at the superficial 0.5 mm layer.
3.6. Wrinkle Reduction
As illustrated in Figure 4, the application of the CICA-EV ampoule over a 2-week period resulted in statistically significant improvements in wrinkle depth across all five evaluated facial and neck regions (n = 20; p < 0.05, see Table A2). These results highlight both the speed and breadth of efficacy offered by the test formulation.

Figure 4.
Reduction in average wrinkle depth at five anatomical sites following two weeks of the CICA-EV ampoule use (n = 20) (a) Forehead wrinkles decreased by 7.8%, (b) Glabellar lines decreased by 11.3%, (c) Periorbital (crow’s-feet) wrinkles decreased by 11.7%, (d) Nasolabial fold wrinkles decreased by 18.8%, (e) Neck wrinkles decreased by 9.5%. All reductions were statistically significant (* p < 0.05; paired t-test).
In particular, forehead wrinkles, often associated with early signs of aging due to repetitive facial expressions, were reduced by 7.8%, decreasing from 0.0603 ± 0.0043 mm at baseline to 0.0556 ± 0.0034 mm after treatment. Similarly, glabellar lines, which tend to be deeper and more persistent, showed an even greater reduction of 11.3%, from 0.0743 ± 0.0102 mm to 0.0659 ± 0.0066 mm, indicating notable improvement in dynamic wrinkle regions.
The periorbital (crow’s feet) area, which is characterized by fine lines due to thin skin and high mobility, demonstrated an 11.7% decrease in wrinkle depth, from 0.1173 ± 0.0266 mm to 0.1036 ± 0.0228 mm. Impressively, the nasolabial folds—a key marker of facial aging—exhibited the most substantial reduction of 18.8%, suggesting volumizing or smoothing effects in this relatively deep static wrinkle region (from 0.0860 ± 0.0191 mm to 0.0698 ± 0.0160 mm). In the neck, where the skin is thinner and more prone to creasing, a 9.5% improvement was observed, with wrinkle depth decreasing from 0.0781 ± 0.0101 mm to 0.0707 ± 0.0082 mm.
Additionally, a 12.67% increase in dermal density, measured by ultrasound, further supports the underlying skin-reinforcing effects of the CICA-EV formulation.
Taken together, these outcomes provide compelling evidence that the test formulation not only improves superficial wrinkle appearance but also promotes measurable skin structural enhancement, offering rapid and visible anti-aging benefits across both dynamic (expression-driven) and static (gravity-related) facial regions.
3.7. Dermal Density Improvement
Dermal density was assessed using DUB® SkinScanner (Courage+Khazaka, Köln, Germany) at baseline and after two weeks of twice-daily CICA-EV ampoule application (n = 20). As shown in Table 4, mean dermal density increased from 21.31 ± 3.42 at baseline to 24.01 ± 3.96 after two weeks, representing a 12.67% improvement. This change was statistically significant (p < 0.05, paired t-test), indicating that the ampoule promotes a measurable increase in dermal density under standard measurement conditions.

Table 4.
Effects of dermal density after two-week application of the CICA-EV ampoule (n = 20).
3.8. In-Vitro Test for CICA-Derived Exosomes
3.8.1. Anti-Wrinkle Efficacy
The anti-wrinkle potential of CICA-derived EVs was evaluated through multiple mechanisms. In a collagen synthesis assay using HDF cells, treatment with 25 µg/mL of EVs significantly upregulated type I collagen (COL1A1) expression by 136.6% compared to the control. Additionally, an elastase inhibition assay showed 21.6% suppression of enzyme activity at 25 µg/mL, indicating the formulation’s capability to reduce the degradation of dermal matrix components. Matrix metalloproteinase-1 (MMP-1), a key enzyme in collagen breakdown, was also significantly downregulated—by up to 49.3% at 25 µg/mL—further supporting the anti-aging effects. In vitro assays for CICA-derived exosomes was summarized in Table 5.

Table 5.
Summary of in vitro assays for CICA-derived exosomes.
3.8.2. Anti-Inflammatory Effects
The anti-inflammatory activity of the EVs was confirmed in LPS-stimulated HaCaT keratinocytes. Treatment with CICA-derived EVs reduced mRNA expression of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α in a dose-dependent manner. At 25 µg/mL, IL-6 and IL-1β were reduced by 47.1% and 48.6%, respectively. TNF-α expression decreased by 30.1%, suggesting that these EVs may modulate inflammation associated with skin aging and irritation.
3.8.3. Wound Healing and Cell Proliferation
A scratch wound healing assay and WST-1 cell viability assay in HaCaT cells demonstrated that CICA-EVs enhance cellular regeneration. At concentrations of 5, 10, and 25 µg/mL, significant increases in wound closure were observed at both 24 and 48 h, with the highest closure rate reaching 98.7% at 25 µg/mL. This indicates robust proliferative and migratory effects, supporting the regenerative capacity of the EVs.
3.8.4. Cytotoxicity Assessment
To evaluate safety, cytotoxicity tests were conducted on Hs68 fibroblasts, HaCaT keratinocytes, and HDF cells. At concentrations up to 100 µg/mL, the EVs exhibited no cytotoxicity, maintaining >90% cell viability in all tested lines. These findings confirm the formulation’s biocompatibility and support its safe use in cosmetic applications.
4. Discussion
In this pilot study, a two-week regimen of twice-daily CICA-EV ampoule produced robust, multidimensional improvements: pore volume decreased by 40.1%, pore area by 17.9%, and pore density by 26.9%; skin hydration increased by 7.9% at 0.5 mm, 6.5% at 1.5 mm, and 4.5% at 2.5 mm; wrinkle depths diminished by 7.8–18.8% across five facial regions; and dermal density rose by 12.7%. These changes were all statistically significant (p < 0.05).
Mechanistically, we attribute these pronounced effects to the exceptional penetration capacity of CICA-derived exosomes and their bioactive cargo [6,7]. In vitro assays using pure CICA-derived exosome water (absent additional cosmetic ingredients) have demonstrated upregulation of collagen I/III gene expression in dermal fibroblasts, enhanced hyaluronic acid synthesis in keratinocytes, and suppression of MMP-1 activity. Such findings support a model wherein exosome-delivered lipids, growth factors, and miRNAs synergistically stimulate extracellular matrix remodeling and epidermal barrier restoration. The consequent reinforcement of the collagen–elastin network likely underlies the observed pore-refining and wrinkle-smoothing effects, while upregulation of aquaporin channels and enhanced lipid lamellae formation account for the full-thickness hydration gains evidenced by TDC measurements at 0.5, 1.5, and 2.5 mm.
Our clinical outcomes align with prior reports of exosome-enriched topicals enhancing matrix protein synthesis and barrier recovery [13,14]. Notably, the 40% reduction in total pore volume surpasses improvements typically reported with peptide or retinoid creams (often 10–20% pore reductions) [14], highlighting the potency of CICA-derived exosomes. The graded hydration increases at progressive depths further distinguish this formulation from conventional humectants, which rarely achieve statistically significant moisture gains below the superficial epidermis.
Nevertheless, this pilot study has several limitations that should be acknowledged. First, our cohort—20 participants aged 34–63 years (mean and median, 51 years)—represents an age group in which skin elasticity declines and pore size increases are most pronounced, making it suitable for assessing anti-aging efficacy; however, the relatively narrow demographic and single-arm, before-and-after design limit the generalizability of our findings. Second, although the Antera 3D system’s inherent variability cannot be fully eliminated, our within-subject paired design—and concurrent reporting of median alongside mean values—helped mitigate inter-individual differences, with median-based analyses yielding trends consistent with those observed using mean values. Third, while the two-week application demonstrated initial efficacy, longer-term studies are required to evaluate the durability and cumulative benefits of CICA-EV treatment; future trials should include extended follow-up periods (e.g., 8–12 weeks). Finally, because our formulation contains both CICA-derived extracellular vesicles and complementary actives (e.g., peptides, humectants), the observed efficacy may reflect combined or synergistic mechanisms. To isolate the specific contribution of exosomes, future placebo-controlled, randomized studies should compare the CICA-EV ampoule against an identical base formulation lacking CICA-EVs.
One important consideration in exosome-based cosmetic development is the stability and shelf-life of EVs within complex formulations. While trehalose or mannitol may help preserve exosome integrity, even refrigerated storage (4 °C) cannot fully prevent degradation over time. Moreover, verifying stability is technically challenging due to the interference of formulation components—such as humectants and surfactants—with nanoparticle tracking analysis (NTA). As such, alternative methods like fluorescence-labeled EV marker detection, ELISA, or Western blotting may be more suitable for assessing exosome presence and integrity in cosmetic products.
Future research should include randomized, double-blind, placebo-controlled trials with larger, more diverse populations to confirm these findings. Extended follow-up will clarify the persistence of effects, while dose–response studies can identify optimal exosome concentrations. Mechanistic investigations—such as transcriptomic or proteomic profiling of skin biopsies—will be critical for validating exosome-mediated biomarker changes. Finally, head-to-head comparisons with established anti-aging actives (e.g., retinoids, peptides) will help position CICA exosome ampoules within the broader cosmetic landscape.
5. Conclusions
In summary, the CICA-EV ampoule delivers rapid and significant improvements in multiple aspects of skin quality. A single application reduced pore area, density, volume, and depth, while two weeks of twice-daily use increased full-thickness hydration, decreased wrinkle depth across five facial regions, and enhanced dermal density by 12.7%. No irritation or adverse reactions were observed, confirming excellent skin compatibility. These findings support the ampoule’s potential as a safe, effective cosmetic for anti-aging and barrier-repair applications. Future randomized, placebo-controlled trials with larger, more diverse cohorts and longer follow-up periods are warranted to confirm the durability of these effects, optimize dosing, and further elucidate underlying molecular mechanisms.
Author Contributions
H.S.P. contributed formal analysis, investigation, methodology, writing—original draft; S.S. contributed conceptualization; formal analysis; methodology; supervision; visualization; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This project was conducted with the support of the ERC Project of the National Research Foundation of Korea (NRF) Grant funded by the Korean Government, MSIP (RS-2023-00207833).
Institutional Review Board Statement
The study protocol was approved by the Ace Clinical Research Center Institutional Review Board (IRB No. ASC-IRB-2411-150, 29/10/2024).
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study prior to any procedures.
Data Availability Statement
The main data supporting the results of this study are available within the manuscript and Appendix A. The raw data files are available for research purposes from the corresponding author upon reasonable request. Source data are provided in this paper.
Acknowledgments
We thank Ace Clinical Research Center for conducting the clinical study.
Conflicts of Interest
The author, Sehyun Shin, is a shareholder of Microgentas. However, the research presented in this paper was conducted objectively, and the conclusions were drawn independently of any influence from Microgentas.
Abbreviations
| EV | Extracellular vesicle |
| CICA | Centella asiatica |
| TDC | Tissue dielectric constant |
Appendix A

Table A1.
Analysis results of improvement in five pore parameters using Antera 3D (n = 20).
Table A1.
Analysis results of improvement in five pore parameters using Antera 3D (n = 20).
| Parameter | Time Point | Mean ± SD | p-Value | Improvement Rate (%) |
|---|---|---|---|---|
| Mean pore area (mm2) | Before use | 0.1378 ± 0.0366 | <0.05 | 17.9 |
| After a single use | 0.1131 ± 0.0352 | |||
| Pore density (ea/cm2) | Before use | 32.83 ± 9.20 | <0.05 | 26.9 |
| After a single use | 24.00 ± 9.74 | |||
| Mean pore volume (10−3 mm3) | Before use | 1.934 ± 0.614 | <0.05 | 19.8 |
| After a single use | 1.551 ± 0.566 | |||
| Total pore volume (mm3) | Before use | 0.8350 ± 0.4984 | <0.05 | 40.1 |
| After a single use | 0.5005 ± 0.3844 | |||
| Maximum pore depth (mm) | Before use | 0.0283 ± 0.0059 | <0.05 | 13.4 |
| After a single use | 0.0245 ± 0.0054 |

Table A2.
Changes in average wrinkle depth at five measurement sites (n = 20).
Table A2.
Changes in average wrinkle depth at five measurement sites (n = 20).
| Site | Time Point | Average Depth (mm) | p-Value | Improvement Rate (%) |
|---|---|---|---|---|
| Forehead wrinkle | Before use | 0.0603 ± 0.0043 | <0.05 | 7.8 |
| 2 weeks after | 0.0556 ± 0.0034 | |||
| Glabellar wrinkle | Before use | 0.0743 ± 0.0102 | <0.05 | 11.3 |
| 2 weeks after | 0.0659 ± 0.0066 | |||
| Periorbital (crow’s feet) wrinkle | Before use | 0.1173 ± 0.0266 | <0.05 | 11.7 |
| 2 weeks after | 0.1036 ± 0.0228 | |||
| Nasolabial fold wrinkle | Before use | 0.0860 ± 0.0191 | <0.05 | 18.8 |
| 2 weeks after | 0.0698 ± 0.0160 | |||
| Neck wrinkle | Before use | 0.0781 ± 0.0101 | <0.05 | 9.5 |
| 2 weeks after | 0.0707 ± 0.0082 |
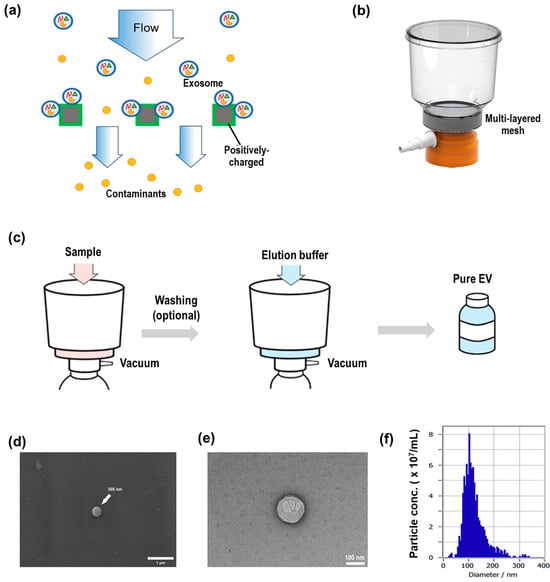
Figure A1.
Isolation and characterization of CICA-derived exosomes. (a) Schematic of the electrokinetic filtration principle, (b) Photograph of the ExoFilter, (c) Workflow for EV isolation: sample loading under vacuum or gravity, optional wash, and elution of bound EVs with buffer to yield pure vesicles, (d) Scanning electron microscopy (SEM) image showing a representative EV, (e) Transmission electron microscopy (TEM) image confirming the double-membrane morphology of EV, (f) Size distribution of EVs.
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Bressler, R.S.; Bressler, C.H. Functional anatomy of the skin. Clin. Podiatr. Med. Surg. 1989, 6, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.K.; Masub, N.; Jagdeo, J. Bioactive ingredients in Korean cosmeceuticals: Trends and research evidence. J. Cosmet. Dermatol. 2020, 19, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, K.; Paczkowska-Walendowska, M.; Garbiec, E.; Cielecka-Piontek, J. Topical Application of Centella asiatica in Wound Healing: Recent Insights into Mechanisms and Clinical Efficacy. Pharmaceutics 2024, 16, 1252. [Google Scholar] [CrossRef]
- Witkowska, K.; Paczkowska-Walendowska, M.; Plech, T.; Szymanowska, D.; Michniak-Kohn, B.; Cielecka-Piontek, J. Chitosan-Based Hydrogels for Controlled Delivery of Asiaticoside-Rich Centella asiatica Extracts with Wound Healing Potential. Int. J. Mol. Sci. 2023, 24, 17229. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Centella asiatica-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2023, 42, 5S–22S. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ren, X.; Yi, F.; Zhang, X.; Hou, J.; Zhang, Z.; Yuan, L.; Li, L.; Gao, Q. Innovative Plant Exosome Delivery System for Enhancing Antiaging Potency on Skin. ACS Appl. Bio. Mater. 2025, 8, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Bae, M.; Kim, Y.; Jeon, S.; Kang, S.; Rhee, W.; Shin, S. Scalable, High-Throughput Isolation of Extracellular Vesicles Using Electrokinetic-Assisted Mesh Filtration: ExoFilter. J. Extracell. Biol. 2025, 4, e70054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, T.; Li, M.; Liu, Q.; Yu, A.; Cheng, K.; Ma, J.; Murphy, S.; McNutt, P.M.; Zhang, Y. Insights into optimizing exosome therapies for acute skin wound healing and other tissue repair. Front. Med. 2024, 18, 258–284. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Krzyżanowska, M.; Polak, A.; Puzianowska-Kuźnicka, M. Effectiveness of Extracellular Vesicle Application in Skin Aging Treatment and Regeneration: Do We Have Enough Evidence from Clinical Trials? Int. J. Mol. Sci. 2025, 26, 2354. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Jones, C.D. Comparative efficacy of a dual-action retinoid/AHA cream on pore size and skin texture: An 8-week, double-blind trial. Int. J. Cosmet. Sci. 2016, 38, 321–328. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).