Abstract
Phallus indusiatus, or bamboo mushroom, has been reported for its nutraceutical properties, while its cosmeceutical properties remain unclear. In this study, we conducted extractions of whole, fresh P. indusiatus using both aqueous and ethanolic methods. Among the extracts, ultrasonic-assisted extraction method with DI showed the highest antioxidant activity compared to the others. For cosmeceutical assessment, we evaluated the extracts’ inhibitory effects against ECM-degrading enzymes and found that they exhibited a modest inhibitory effect of approximately 50%. Remarkably, ultrasonic-assisted extraction with DI demonstrated promising cosmeceutical properties. Additionally, pressure-assisted extraction with DI showed a potentially protective effect against H2O2-induced DNA damage. To investigate the anti-melanogenic effect on MNT-1 cells, we treated them with the extracts and observed a significant decrease in cellular tyrosinase activity under α-MSH stimulation. This resulted in a relative reduction in melanin content. Notably, autoclaving extraction exhibited a significantly greater anti-melanogenic effect than the other extracts at the lowest concentration tested. Furthermore, the extracts demonstrated a reduction in NO production under LPS-induced inflammation. Hot water extraction with DI and ethanol exhibited a stronger anti-inflammatory effect compared to diclofenac, without any cytotoxicity. These findings highlight the hidden cosmeceutical properties of P. indusiatus and suggest its potential use as a bioactive ingredient in cosmetic formulations.
1. Introduction
The trend of mushroom-based products has been driven by growing consumer interest in natural and plant-based ingredients. Mushroom extracts are used as functional ingredients in various dietary supplements as nutraceutical sources, leading to overall well-being. The term cosmeceutical was proposed as a combination of pharmaceuticals and cosmetics [1]. Cosmetic ingredients with cosmeceutical properties offer skin-enhancing benefits and exhibit therapeutic actions against skin pathologies, including skin inflammation [2]. Mushrooms have traditionally been consumed as a nutrition source and used in alternative medicine. Well-known studies of bioactive compounds from mushroom have become interesting in cosmeceutical fields. Mushroom cosmetic formulations have been used in several cosmetics industries. Mushroom extracts were functionally claimed to have cosmeceutical properties, such as moisturizing, anti-aging, anti-acne, and skin lightening effects [3]. Seeking a new species to explore with regard to cosmetic efficacy would shed light a promising ingredient.
Wrinkles, dryness, freckles, melasma, and solar lentigines have emerged as significant beauty concerns, impacting individual self-confidence and overall appearance. Photo-aging is one of the daily risks that impacts skin health. UV-induced melanin biosynthesis upregulated tyrosinase gene expression, resulting in melanin biosynthesis acceleration [4,5]. Tyrosinase is a key enzyme in melanin biosynthesis. The hydroxylation of tyrosine to 3,4 dihydroxyphenylalanine (DOPA) and the oxidation of DOPA to DOPA quinone leads to melanin accumulation [6]. Moreover, ECM-degrading enzymes also play a critical role in skin aging. Proteomic analysis of UV-induced skin biopsies revealed collagen fibril fragmentation through increasing pro-inflammatory proteases expression [7]. Matrix metalloprotease was reportedly increased upon exposure to UV radiation, leading to ECM protein degradation [8]. UV radiation and other environmental factors contribute to the generation of reactive oxygen species [2], which play a pivotal role in the skin changes associated with skin aging [9,10,11]. Attenuation of these key enzymes, or inflammatory attenuation, would be a promising strategy for cosmeceutical ingredient development.
Hydroquinone, azelaic, phenol, corticosteroid, retinoids, kojic acid, and arbutin were developed as anti-tyrosinase inhibitors for hyperpigmentation treatment [12,13,14,15,16,17]. However, penetration ability, cytotoxicity, irritation, and stability are still challenges. Nowadays, numerous of edible mushrooms have been well-studied for their cosmeceutical properties. Polysaccharide of straw mushroom (Volaeriella volvacea) was formulated as a cosmetic product that provided significant skin benefits, such as increasing moisture, elasticity, net, and skin firmness [18]. High polyphenol contents of Ganoderma lucidum and its varieties were identified as having 16 bioactive compounds containing anti-melanogenic activities [19]. Dictyophora indusiate or Phallus indusiatus (bamboo mushroom) is an edible mushroom that belongs to Phallaceae family. The pharmaceutical properties of P. indusiatus have been extensively reviewed elsewhere [20]. However, their cosmeceutical potential is still limited. Antioxidant activity is a common activity that has been investigated through various methods. The majority of water-soluble and crude extracts derived from P. indusiatus demonstrated moderate antioxidant activity within the concentration range of less than 1 mg/mL up to 2 mg/mL [21,22]. 5-(hydroxymethyl)-2-furfural (HMF) was identified as an anti-tyrosinase inhibitor from the methanolic extract of P. indusiatus [23]. Aqueous extraction of P. indusiatus showed potential in wound healing through reducing pro-inflammatory cytokines and stimulating collagen production [24]. However, the cosmeceutical potential of P. indusiatus, in term of anti-melanogenic activity and ECM-degrading enzyme inhibition, remains unclear.
In this study, fresh P. indusiatus was subjected to extraction using mild solvents and various extraction methods in order to investigate its cosmeceutical properties. Phytochemical analysis and antioxidant activity assessment were performed. The focus of the cosmeceutical property evaluation was on the inhibition of ECM-degrading enzymes and anti-melanogenic activity. The extracts exhibited inhibitory effects on collagenase and elastase. To examine the effects on melanin synthesis, MNT-1 melanoma cells were treated with the extracts, and cellular tyrosinase activity and melanin content were assessed. The anti-inflammatory effect and cell viability of the extracts were also evaluated using RAW264.7 cells. Overall, the findings highlight the cosmeceutical potential of P. indusiatus, suggesting its suitability as a bioactive ingredient in the cosmetic industry.
2. Materials and Methods
2.1. Phallus indusiatus Extraction
A total of 50 g of fresh P. indusiatus was extracted using different methods, as shown in Figure 1. Distilled water and ethanol were used as an extraction solvent basis. Boiling water (50 °C), autoclaving (121 °C), and sonication (50 °C) were applied to accelerate the extraction process following the indicated time. The ethanolic extracts (S2 and S5) were evaporated by a SpeedVac vacuum concentrator(Eppendrof, Switzerland). The distilled water extracts (S1, S3, and S4) were lyophilized. All sample were reconstituted in dimethyl sulfoxide and kept at −20 °C before the further analysis.
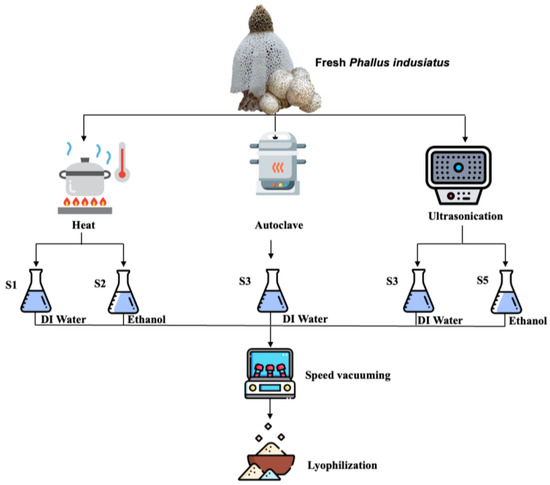
Figure 1.
Schematic of P. indusiatus extraction procedure using various physical methods and solvents.
2.2. Phytochemical Analysis of Crude Extracts
2.2.1. Total Flavonoids Content Determination
Total flavonoid content was determined using the aluminum chloride method, following the previous, with minor modification [25]. A total of 10 µL of each extract was mixed with 190 µL of 2% AlCl3 solution and incubated at room temperature for 1 h. Various concentrations (0–100 µg/mL) of quercetin were used to generate a standard curve following the procedure described above. The absorbance was measured at 420 nm. Total flavonoids content of the extracts was expressed in quercetin equivalent per gram extract (µg QAE/g extract) in regard to the quercetin calibration curve.
2.2.2. Total Phenolics and Tannin Content
Total phenolic content was determined as referenced in the previous study, with some minor modifications [26]. A total of 10 µL of the sample was mixed with 40 µL of Folin–Ciocalteu reagent before adding 200 µL of 75 mg/mL sodium carbonate solution. Gallic acid and tannic acid were used as polyphenol standard compounds for generating a calibrating curve. After 90 min of incubation time, the reactions were measured using the absorbance at 760 nm. All experiments were performed in at least triplicate for each test. Data are expressed as means ± standard deviation (SD). Total phenolics contents are expressed as micrograms of gallic acid equivalents per gram extracts (µg GAE/g extract). Total tannin contents are expressed as micrograms of tannic acid equivalents per gram extracts (µg TAE/g extract).
2.2.3. Total Sugar Analysis
Phenol-sulfuric colorimetric assay was performed to determine the total sugar of each extract. Mixing of the extract with 5% phenol solution was conducted by 1:1 ratio in a glass test tube. A total of 2 volume of absolute sulfuric acid was added to the mixture and left at room temperature in a fume hood for 30 min. Various concentrations of D-glucose were used to generate a calibration curve, as described above. Each reaction and the D-glucose standard mixture were measured at 490 nm by a microplate reader. Total sugar was calculated regrading the D-glucose calibration curve.
2.3. Antioxidant Analysis
2.3.1. DPPH Assay
2,2-diphenyl-1-picrylhydrazyl (DPPH) was used as a colorimetric free radical for determining the scavenging activity of the extracts. DPPH solution (2 µg/mL) was freshly prepared in absolute ethanol and kept in a dark place. A total of 10 µL of the extracts was mixed with 190 µL of the DPPH solution into a 96-well plate. The mixture was further incubated at room temperature in a dark place for 30 min. The absorbance was measured at 517 nm wavelength using a microplate reader. The absorbance was calculated as %scavenging of control and represented as IC50. All experiments were performed in triplicate.
2.3.2. ABTS Assay
Oxidation of ABTS was performed by mixing 7 mM ABTS with 2.45 mM potassium persulfate in DI water and incubating in the dark at 25 °C overnight. The solution was diluted to 1:10 (v/v), mixed with the sample, and the absorbance was measured at 734 nm. The absorbance was calculated as %scavenging of control and represented as IC50. All experiments were performed in triplicate.
2.3.3. ORAC Assay
A total of 25 µL of each sample was mixed with 150 µL of 40 µM Fluorescein solution and further incubated at 37 °C for 30 min. A 200 mM AAPH solution was used as a radial initiator by adding 25 µL of AAPH into the mixture. The decay of fluorescence intensity was measured by a fluorescence microplate reader at Ex485/Em528 at 1 min intervals for 1 h. Ascorbic acid was used to generate AUC for the calibration curve.
2.3.4. DNA Damage Assay
A total of 0.5 mM iron (III) sulphate was combined with an aliquot of 300 ng pET28a (+) plasmid and incubated at 37 °C for 20 min. A total of 3 µL of 15% hydrogen peroxide was then added into the mixture and continually incubated at 37 °C for 25 min. The integrity of the DNA was then monitored using agarose gel electrophoresis.
2.4. Biological Function Analysis
2.4.1. Collagenase Inhibition Assay
The extracts were assessed for their inhibitory effect on collagenase activity. The extracts were mixed with collagenase in 50 mM tricine buffer, pH 7.5, 400 mM, in the presence of Ca2+ and incubated at 37 °C for 15 min. At the indicated time, FALGPA substrate solution was added to initiate the reaction. Epigallocatechin gallate (EGCG) was used as a positive control. The absorbance was then measured by a spectrophotometer at 340 nm, and the %collagenase inhibition was calculated compared to the control.
2.4.2. Elastase Inhibition Assay
The extracts were pre-incubated with elastase (0.05 mg/mL) at room temperature for 15 min. The reaction mixture was initiated with 0.8 mM N-Succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN) in 200 mM Tris-HCl pH 8.0. Epigallocatechin gallate (EGCG) was used as a positive control. The reaction was immediately measured at an absorbance value between 381 and 402 nm. The %elastase inhibition was calculated compared to the control.
2.4.3. Tyrosinase Inhibition Assay
Mushroom tyrosinase was reacted with the desired concentrations of the extracts in 50 mM potassium phosphate buffer pH 6.8. Kojic acid was used as an anti-tyrosinase inhibitor. After 5 min incubation, 10 mM L-DOPA in 50 mM potassium phosphate buffer pH 6.8 was added and continually incubated at room temperature for 25 min. Oxidation of L-DOPA was measured at a wavelength of 470 nm. The data were analyzed as %tyrosinase inhibition compared to control.
2.4.4. Cellular Tyrosinase Inhibition Assay
MNT-1 (5 × 104 cells) was seeded into a 12-well plate. After cell attachment, the cells were replenished with fresh culture medium containing various concentrations of the extracts. α-MSH was added to stimulate melanogenesis as a negative control. Kojic acid was used as positive control. After 48 h, the treated cells were washed, fixed, and staining with L-DOPA for determining tyrosinase activity. The stained cells were photographed under an inverted microscopy.
2.4.5. Melanin Quantitation
To quantify melanin production in melanocytes, the procedures was performed as described earlier. The treated cells were trypsinized and collected by centrifugation. The cell pellets were thrice washed and solubilized with 1N NaOH/10%DMSO solution. The mixture was completely solubilized at 80 °C for 2 h. At the indicated time, the mixture was transferred into a 96-well plate and the absorbance was measured at 405 nm.
2.4.6. Anti-Inflammatory Assay
RAW 264.7 cells (1 × 105 cells/well) were seeded into a 96-well plate. After 18 h, the cells were treated with the extracts for 1 h before LPS stimulation. Diclofenac and untreated were used as positive and negative controls. After 24 h LPS-stimulated inflammation, the treated medium as collected and determined released NO through Griess’s reagent, following the instructions. The remaining cells were investigated for cell viability by MTT assay. Briefly, fresh medium containing MTT solution was replenished and incubated for 3 h. The forming formazan crystal was dissolved with DMSO. The absorbance was measured at 570 nm with a reference wavelength of 630 nm.
2.5. Statistical Analysis
All results were performed in triplicate and analyzed using statistical analysis. Graphs were plotted with GraphPad Prism 9.0 software. For melanin biosynthesis results, color intensity was measured using Image J software version 1.53t. Data were expressed as means ± standard deviation (SD). Significant differences between groups were determined by one-way ANOVA following desired post-Hoc; p < 0.05 was considered a significant difference.
3. Results
3.1. Study of Phytochemical Contents of Fresh P. indusiatus Extraction by Different Methods
Various extraction processes have been proposed to achieve the highest yield and still retain the biological functions of edible mushrooms. Hot water, autoclaving, and ultrasonication are the extraction methods that retain high bioactive compound recovery. Distilled water and ethanol were less-toxic solvents used as the basic solvent for fresh P. indusiatus extraction. All extracts were denoted as “S”. With regard to the results, the highest to lowest % extraction yield was S3 > S2 > S4 > S1 > S5. The fruiting body of P. indusiatus was completely destroyed by autoclaving (S3). Total phenolics, flavonoids, total tannin, and total sugar were seemingly elevated in S3 compared to the others (Table 1). Distilled water extraction in both hot water (S1) and ultrasonication (S4) did not differ in the percentage of extraction yield. Interestingly, total tannin contents were insignificantly the highest in hot water extraction, regarding its hydrophobic property, while the lowest was obtained by ultrasonication. Interestingly, S2 dramatically enriched total flavonoid content in ethanol–hot water extraction. Notably, ultrasonication (S4) with hot water extraction could not enrich the phytochemical contents from P. indusiatus. Ethanol-based extraction in both hot water and ultrasonication extraction elicited a lower % extraction yield than water-based extraction. For biological functions, antioxidant activity was evaluated by several methods. Ethanol-based reaction of artificial colored-free radical DPPH demonstrated the antioxidant activity of lipophilic molecules. As anticipated, S2, S3, and S4 demonstrated IC50 ~80 µg/mL by DPPH, which was greater than S1 and S5 regarding their total flavonoid contents. Interestingly, water-based reaction or oxidized ABTS scavenging revealed antioxidant activity in all fractions. Hot water extraction in both water (S1) and ethanol (S2) significantly demonstrated ABTS•+ reduction capacity regarding their total phenolic contents. However, both methods might not reflect their authentic antioxidant activity. Protection of florescence molecules with antioxidant molecules under an oxidation environment was considered in order to examine antioxidant activity. ORAC was performed and expressed as mM ascorbic equivalent. Notably, S4 elicited the highest netAUC value following S1, S2, and S5, respectively, at identical concentrations. NetAUC determination of S3 could not be determined. Interestingly, S3 dramatically demonstrated a protection effect on DNA damage under H2O2-induced DNA damage, indicating that the protective effect against DNA damage was similar to the antioxidant value (Figure 2). S1 slightly exhibited a protective effect, while the others did not. Taken together, various phytochemical contents were assessed, based on the extraction methods and the solvent polarity, that reflect their antioxidant activity.

Table 1.
Phytochemical composition analysis and antioxidant capacity of the mushroom extracts. Mean ± SD; FW fresh weight; N.D. (not determined); n = 3 (Independent experiments were done in triplicate), abc in the same column indicated significant differences (p < 0.05).
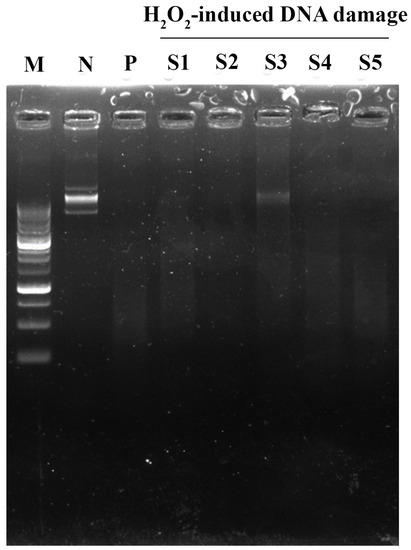
Figure 2.
DNA protective effect of P. indusiatus extracts under H2O2 induction, M; DNA ladder 12,000–200 bp, N; pET26b + P; H2O2-induced damage, S1–S5 (100 µg/mL); H2O2-induced damage in the presence of P. indusiatus extracts.
3.2. Inhibitory Effect on ECM-Degrading Enzymes of the P. indusiatus Extracts
Skin integrity experiences aging from the surrounding environment. Collagen and elastin play critical roles in the outermost part of the skin in retaining skin integrity. Herein, the anti-ECM-degrading enzyme effect of the extracts was evaluated on collagenase and elastase enzymatic activities. At 1 mg/mL of each extract, EGCG was used as a positive control that exhibited 77.79 ± 2.68% inhibition against collagenase (Figure 3A). S1, S3, S4, and S5 demonstrated a modest effect on collagenase activity compared to the control. Only 24.66 ± 7.65% inhibition was observed in S2. For elastase activity, the inhibitory effect was slightly observed for each fraction following S1 (28.41 ± 1.75%), S2 (23.41 ± 4.36%), S3 (21.11 ± 6.40%), S4 (32.73 ± 3.09%), and S5 (24.61 ± 1.90%) (Figure 3B). The inhibitory effect was larger on collagenase than elastase. The P. indusiatus extracts have a promising inhibitory effect on ECM-degrading enzymes (Figure 3A,B).
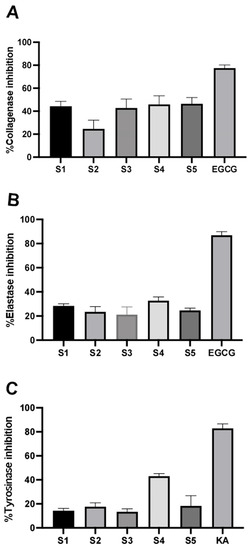
Figure 3.
Inhibition of ECM-degrading enzymes and anti-tyrosinase activity of P. indusiatus; 100 µg/mL of the extracts was used to determine their inhibitory effect against collagenase (A), elastase (B), and tyrosinase (C).
3.3. Assessment of Anti-Melanogenesis of the P. indusiatus Extracts in Both Enzymatic and Cellular Functions
Melanin biosynthesis was determined based on L-DOPA oxidation by tyrosinase enzymatic activity. Tyrosinase is a rate-limiting enzyme for melanin synthesis that directly catalyzes L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). Edible mushrooms reportedly have an anti-melanogenic effect for cosmeceutical ingredient formulations [27]. Notably, S4 exhibited the highest %inhibition by 40.01 ± 2.18%, while the others demonstrated a modest %inhibition at the identical concentration compared to the positive control (Figure 3C). Cellular tyrosinase activity on melanin biosynthesis was evaluated in MNT-1 cells. Under α-melanocyte-stimulating hormone (α-MSH) stimulation, the relative dark area was dramatically increased compared to the unstimulated condition, which could reflect cellular tyrosinase activity. In the presence of the extracts, the relative dark area gradually decreased in a dose-dependent manner that was similar to KA treatment (Figure 4). As mentioned earlier, another role of tyrosinase is its ability to rapidly convert L-DOPA into L-dopaquinone. To further ensure the effect of the extract, L-DOPA staining was performed to determine the cellular tyrosinase-associated melanin content. According to the results, the extracts could attenuate melanin content in a dose-dependent manner. At the highest concentration of the extract, melanin content was similar to KA treatment. Interestingly, S3 significantly reduced melanin content at low concentrations (Figure 5). These findings demonstrated that P. indusiatus extracts strongly exhibited anti-melanogenesis activity.
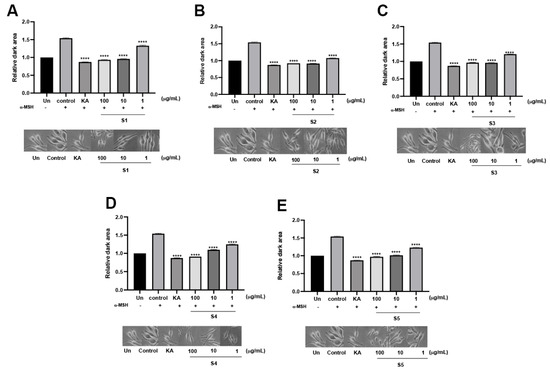
Figure 4.
Determination of cellular tyrosinase activity by L-DOPA staining in MNT-1 cells in the presence of P. indusiatus extracts (S1–S5) (A–E). α-MSH was used as a melanogenic inducer. KA; kojic acid was used positive control. (n = 3, p < 0.05; **** p < 0.0001).
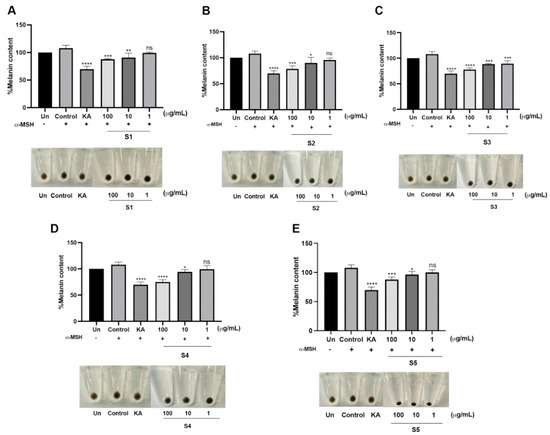
Figure 5.
Quantitative analysis of melanin content in MNT-1 cells in the presence of P. indusiatus extracts (S1–S5) (A–E). α-MSH was used as a melanogenic inducer. KA; kojic acid was used positive control. (n = 3, p < 0.05, ns; p < 0.1234; * p < 0.0322; ** p < 0.0021; *** p < 0.0002; **** p < 0.0001).
3.4. Anti-Inflammatory Activity of the P. indusiatus Extracts by Decreasing NO Production
Murine macrophages have been widely used to study the anti-inflammatory effects of LPS stimulation. LPS-induced inflammation increased NO production, leading to an increase in cellular stress. Based on their antioxidant activity, the extracts were further investigated to determine their cellular antioxidant activity and whether they exert anti-inflammatory effects. According to the results, the level of released NO was significantly elevated upon LPS stimulation. Diclofenac, known for its positive effects on macrophages, attenuated the level of released NO. Interestingly, in the presence of the extracts, the level of released NO was reduced compared to the LPS-stimulated condition. S1 and S2 demonstrated anti-inflammatory effects greater than Diclofenac by lowering the released NO level under LPS stimulation. S3, S4, and S5 insignificantly decreased the released NO level compared to Diclofenac (Figure 6). Interestingly, the extracts seemingly improved cell survival under LPS stimulation. These findings indicated that the extracts have an anti-inflammatory effect by decreasing NO production and improving the survival rate of murine macrophages.
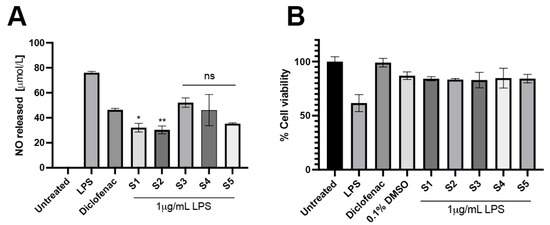
Figure 6.
Anti-inflammatory activity of the P. indusiatus extracts. Released NO level was determined in the presence of the extracts under LPS stimulation (A). Diclofenac was used as positive control. MTT was performed to assess cell viability of the remaining cells (B). (Compared to diclofenac, n = 3, p < 0.01, ns; p < 0.0332, * p < 0.0021; ** p < 0.0002).
4. Discussion
Mushroom-based products have gained significant interest in the formulation of bioactive ingredients. Mushroom extracts are known for possessing health-promoting benefits [27]. The cosmeceutical properties of mushrooms have attracted attention for their potential application in cosmetic products, thanks to their multifunctional properties [28]. The investigation of antioxidants, antibacterial agents, anti-tyrosinase compounds, and anti-ECM-degrading enzymes has been extensive, focusing on the phytochemical components of mushrooms. Ethanol extracts of Calocybe indica, Ganoderma lucidum, and Ganoderma tropicum have shown significant antioxidant and antibacterial activity, which is correlated with their phenolic content [29]. In our study, aqueous extraction of dried P. indusiatus (D. indusiata) resulted in a high total phenolic content, while the total flavonoid content was relatively low [24]. The presence of flavonoids in mushrooms has been a topic of debate. Our results indicated an enrichment of flavonoid content in fresh P. indusiatus, particularly with ethanolic extraction. Flavonoids are plant secondary metabolites known for their protective effects against UV exposure and oxidative stress [30]. Although chalcone synthase or chalcone isomerase, key enzymes in flavonoid biosynthesis, are not found in mushroom genomes [31], alternative flavonoid biosynthesis enzymes have been proposed. Phenylalanine ammonia lyase, identified in Sanghuangporus baumii, plays a role in the flavonoid synthesis pathway by converting L-phenylalanine to trans-cinnamic acid [32]. It is possible that mushrooms absorb flavonoids from their surroundings or from mycorrhizal plants [31] In conclusion, further in-depth investigation of the phytochemical analysis of P. indusiatus is needed to better understand its bioactive compounds.
For biological activity, antioxidant activity of aqueous P. indusiatus extracts were assessed using several methods. Aqueous extraction of P. indusiatus reportedly elicited radical scavenging activity up to 2 mg/mL [21,33]. According to the results, the antioxidant activity of P. indusiatus was promising in the sub-microgram range IC50 (~20–100 µg/mL). DPPH insignificantly exhibited antioxidant activity at a similar level. Among the different extraction methods employed, the ABTS and ORAC assays showed that P. indusiatus extracts exhibited significant antioxidant activity. However, the antioxidant capacities measured by ABTS and ORAC strongly confirmed the effect better than DPPH [34]. The highest level of phenolic content that was found in S3, which exhibited a protective effect on H2O2-induced DNA damage. Unbalancing of the oxidant/antioxidant ratio occurs upon cellular damage through an increase in ROS, leading to DNA fragmentation [35]. UV-induced pyrimidine dimer formation and its oxidation product lead to DNA damage, which leads to photoaging and skin cancer [36]. The enrichment of phenolic and flavonoid contents in Russula virescens was found to be associated with DNA protection, which correlated with its antioxidant activity [37]. ECM degradation is a major concern when it comes to skin aging. Collagenase and elastase play critical roles in ECM modelling. ECM consist of laminin, elastin, fibroblast, and glycosaminoglycans, including collagen, that are responsible for maintaining the integrity and strength of organs and skin [38]. A combination of UVA and polyphenol increased tropoelastin, an elastin monomer, resulting in elastin and collagen deposition in human dermal fibroblasts that improved skin properties [39]. Aqueous extraction of P. indusiatus stimulated collagen deposition by inhibiting MMP-2 activity, leading to enhanced wound healing [24]. Our results seemingly reveled a promising cosmeceutical property by moderately inhibiting ECM-degrading enzymes. Melanogenesis is a homeostatic process in the skin that serves to protect our body against external stimuli, particularly UV radiation, through skin pigmentation. This complex process is regulated by secreted factors such as αMSH, SCF, KGF, and bFGF, which are involved in the intrinsic pathway [40]. Tyrosinase is a key enzyme involved in the process of melanogenesis. It plays a crucial role in two different reactions: the hydroxylation of tyrosine to L-DOPA, followed by the oxidation of L-dopa to form dopaquinone through its catecholase activity [41]. 5-(hydroxymethyl)-2-furfural is a natural compound that rises during heat treatment due to the Maillard reaction. We identified 5-(hydroxymethyl)-2-furfural from P. indusiatus, which has a demonstrated inhibitory effect on tyrosinase activity [23]. Aqueous extract of P. indusiatus with an ultrasonic method (S4) enhanced the inhibitory effect on tyrosinase activity. Ultrasonic-assisted extraction increased the mass transfer rate during extraction, resulting in bioactive compound enrichment [42]. Flavonoids seemingly exerted their inhibitory effect on tyrosinase activity [43]. Some flavonoids, such as chalcone, resveratrol, and coumarin, strongly inhibited tyrosinase activity [44]. P. indusiatus extracts decreased cellular tyrosinase activity and melanin synthesis in MNT-1 cells. A correlation between flavonoid content and anti-melanogenesis would be a promising form of cosmetic efficacy. However, the characterization of atopic dermatitis simultaneously appeared in various symptoms [45]. P. indusiatus extracts dramatically decreased NO production by 10-fold compared to Diclofinac [24]. In this study, P. indusiatus demonstrated a greater anti-inflammatory effect compared to Diclofenac by reducing NO production. The magnitude of the anti-inflammatory effect was found to be dependent on the extraction method used. The amount of released NO was dramatically different, which could be explained in terms of cell passaging [46]. These findings shed light on the cosmeceutical properties of P. indusiatus for mushroom-based cosmetic product development.
5. Conclusions
In this study, different P. indusiatus extraction methods were performed to evaluate cosmetic efficacy on their biological functions. The extracts were analyzed in term of phytochemical components, antioxidant activity, inhibitory effect against ECM-degrading enzymes, and anti-melanogenic properties in both enzymatic and cell-based experiments. Ultrasonic-assisted extraction with DI revealed the highest antioxidant activity of the P. indusiatus extracts. Interestingly, high pressure-assisted extraction with DI elicited a protective effect on DNA integrity. All of the extracts significantly decreased melanin contents that related to cellular tyrosinase activity in MNT-1. Moreover, an anti-inflammatory effect was attenuated by the extracts without any cytotoxicity in the presence of the extracts. Taken together, the P. indusiatus extracts revealed various biological functions that could be promising in terms of cosmetic efficacy for cosmetic ingredient development.
Author Contributions
K.C. and L.T.: conceptualization and experimental design; K.T. and L.T.: methodology; K.J.: phytochemical analysis; K.T. and L.T.: data gathering and analysis; K.T.: first draft manuscript; L.T. and K.C.: editing and discussion. All authors have read and agreed to the published version of the manuscript.
Funding
The Thailand Science Research and Innovation through the Kasetsart University Reinventing University Program 2021; and the Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand (FF (KU) 25.64).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon requested.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; Gonzalez-Paramas, A.M.; Ferreira, I.C. Development of Mushroom-Based Cosmeceutical Formulations with Anti-Inflammatory, Anti-Tyrosinase, Antioxidant, and Antibacterial Properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Visvanathan, S.; Krishnamoorthy, R.; Sabesan, G.S. Fungal Cosmetics: Mushrooms in Beauty Care and the New Age of Natural Cosmetics. In Fungal Biology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–37. [Google Scholar]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121 Pt 24, 3995–3999. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187. [Google Scholar] [CrossRef]
- Kameyama, K.; Takemura, T.; Hamada, Y.; Sakai, C.; Kondoh, S.; Nishiyama, S.; Urabe, K.; Hearing, V.J. Pigment production in murine melanoma cells is regulated by tyrosinase, tyrosinase-related protein 1 (TRP1), DOPAchrome tautomerase (TRP2), and a melanogenic inhibitor. J. Investig. Dermatol. 1993, 100, 126–131. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Fussell, J.C.; Kelly, F.J. Oxidative contribution of air pollution to extrinsic skin ageing. Free Radic. Biol. Med. 2020, 151, 111–122. [Google Scholar] [CrossRef]
- Garcia-Gavin, J.; Gonzalez-Vilas, D.; Fernandez-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermat. 2010, 62, 63–64. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Xin, X.; Mo, L.; Zou, Y.; Zhao, G.; Yu, Y.; Chen, K. Antityrosinase Mechanism and Antimelanogenic Effect of Arbutin Esters Synthesis Catalyzed by Whole-Cell Biocatalyst. J. Agric. Food Chem. 2021, 69, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.A.; Fitzpatrick, T.B. Topical use of hydroquinone as a depigmenting agent. JAMA 1965, 194, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.C.; Nazzaro-Porro, M.; Passi, S.; Zina, G. Azelaic acid therapy in disorders of pigmentation. Clin. Dermatol. 1989, 7, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Neering, H. Treatment of melasma (chloasma) by local application of a steroid cream. Dermatologica 1975, 151, 349–353. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy. J. Fungi 2022, 8, 572. [Google Scholar] [CrossRef]
- Ha, H.T.; Tran-Van, H.; Tran-Van, H.; Tran, T.V.; Ha, H.T.; Tran-Van, H.; Tran, T.V.; Nguyen, H.T.N.; Phan, D.T.A. Study on chemical compositions, antioxidants and intracellular anti-melanogenic activities of varieties of Ganoderma lucidum in Vietnam. Int. J. Food Sci. Technol. 2023, 58, 4127–4135. [Google Scholar] [CrossRef]
- Habtemariam, S. The Chemistry, Pharmacology and Therapeutic Potential of the Edible Mushroom Dictyophora indusiata (Vent ex. Pers.) Fischer (Synn. Phallus indusiatus). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef]
- Oyetayo, V.; Dong, C.; Yao, Y. Antioxidant and antimicrobial properties of aqueous extract from Dictyophora indusiata. Open Mycol. J. 2009, 3, 20–26. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Wu, L.; Wu, X.; Huang, Y.; Liu, B. Optimization of polysaccharides extraction from Dictyophora indusiata and determination of its antioxidant activity. Int. J. Biol. Macromol. 2017, 103, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Choi, J.; Sharma, N.; Choi, M.; Seo, S.Y. In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata. Phytother. Res. 2004, 18, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; et al. High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms. J. Fungi 2021, 7, 1100. [Google Scholar] [CrossRef]
- Giordano, A.; Morales-Tapia, P.; Moncada-Basualto, M.; Pozo-Martinez, J.; Olea-Azar, C.; Nesic, A.; Cabrera-Barjas, G. Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules 2022, 27, 610. [Google Scholar] [CrossRef] [PubMed]
- Phonphoem, W.; Sinthuvanich, C.; Aramrak, A.; Sirichiewsakul, S.; Arikit, S.; Yokthongwattana, C. Nutritional Profiles, Phytochemical Analysis, Antioxidant Activity and DNA Damage Protection of Makapuno Derived from Thai Aromatic Coconut. Foods 2022, 11, 3912. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Mago, P.; Sharma, R.; Hafeez, I.; Nawaz, I.; Joshi, M.; Mehrotra, R. Mushroom based Cosmeceuticals: An Upcoming Biotechnology Sector. Biosci. Biotechnol. Res. Asia 2023, 20, 2. [Google Scholar] [CrossRef]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of Total Phenolic Content, HPLC Analysis, and Antioxidant Potential of Three Local Varieties of Mushroom: A Comparative Study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar] [CrossRef]
- Rensing, S.A. Great moments in evolution: The conquest of land by plants. Curr. Opin. Plant Biol. 2018, 42, 49–54. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F.R.; Soler-Rivas, C. Mushrooms do not contain flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, X.; Liu, R.; Zou, L. Mushrooms Do Produce Flavonoids: Metabolite Profiling and Transcriptome Analysis of Flavonoid Synthesis in the Medicinal Mushroom Sanghuangporus baumii. J. Fungi 2022, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Hu, Z.; Fu, H.; Hu, M.; Xu, X.; Chen, J. Chemical analysis and antioxidant activity in vitro of a beta-D-glucan isolated from Dictyophora indusiata. Int. J. Biol. Macromol. 2012, 51, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Debnath, T.; Lim, B.O. DNA Protection, Total Phenolics and Antioxidant Potential of the Mushroom Russula Virescens. J. Food Biochem. 2014, 38, 6–17. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Fabi, S.G.; Palestine, R.F.; Rivkin, A.; Ortiz, A.; Bucay, V.W.; Chiu, A.; Naga, L.; Emer, J.; Chasan, P.E. Extracellular Matrix Modulation: Optimizing Skin Care and Rejuvenation Procedures. J. Drugs Dermatol. 2016, 15, s63–s71. [Google Scholar]
- Chowdhury, A.; Nosoudi, N.; Karamched, S.; Parasaram, V.; Vyavahare, N. Polyphenol treatments increase elastin and collagen deposition by human dermal fibroblasts; Implications to improve skin health. J. Dermatol. Sci. 2021, 102, 94–100. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Varghese, P.K.; Abu-Asab, M.; Dimitriadis, E.K.; Dolinska, M.B.; Morcos, G.P.; Sergeev, Y.V. Tyrosinase Nanoparticles: Understanding the Melanogenesis Pathway by Isolating the Products of Tyrosinase Enzymatic Reaction. Int. J. Mol. Sci. 2021, 22, 734. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Sandby-Moller, J.; Kobayasi, T.; Gniadecki, R. Skin aging and natural photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lucy, T.T.; Mamun-Or-Rashid, A.N.M.; Yagi, M.; Yonei, Y. Serial Passaging of RAW 264.7 Cells Modulates Intracellular AGE Formation and Downregulates RANKL-Induced In Vitro Osteoclastogenesis. Int. J. Mol. Sci. 2022, 23, 2371. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).