Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Diet
2.2. In Vivo Metabolic Tests
2.3. Ex Vivo Lipolysis Assay
2.4. Other Metabolic Variables
2.5. Liver Lipid Analysis
2.6. Real-Time PCR Analysis
2.7. Histology
2.8. Assessment of Hepatic Oxidative Stress
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
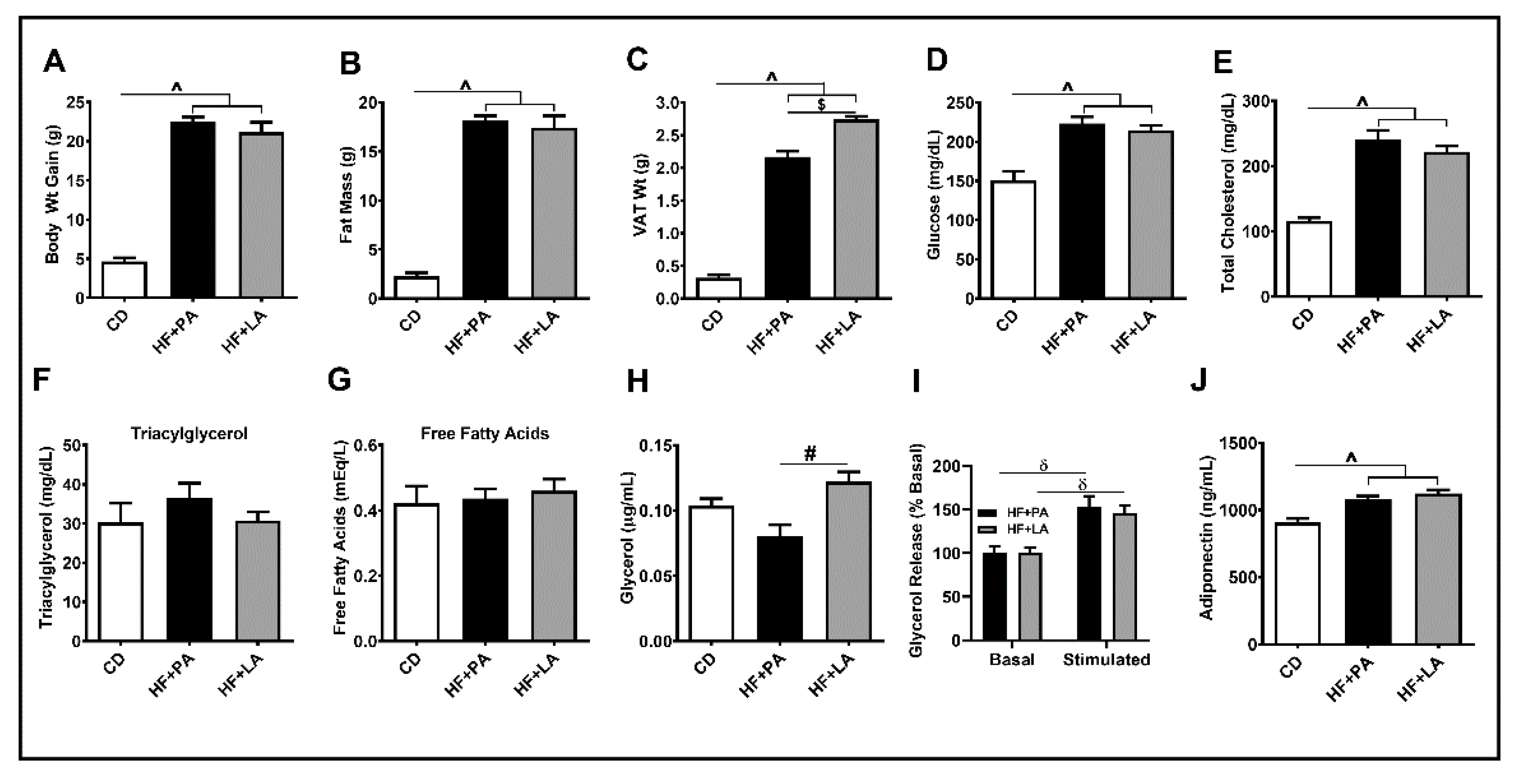
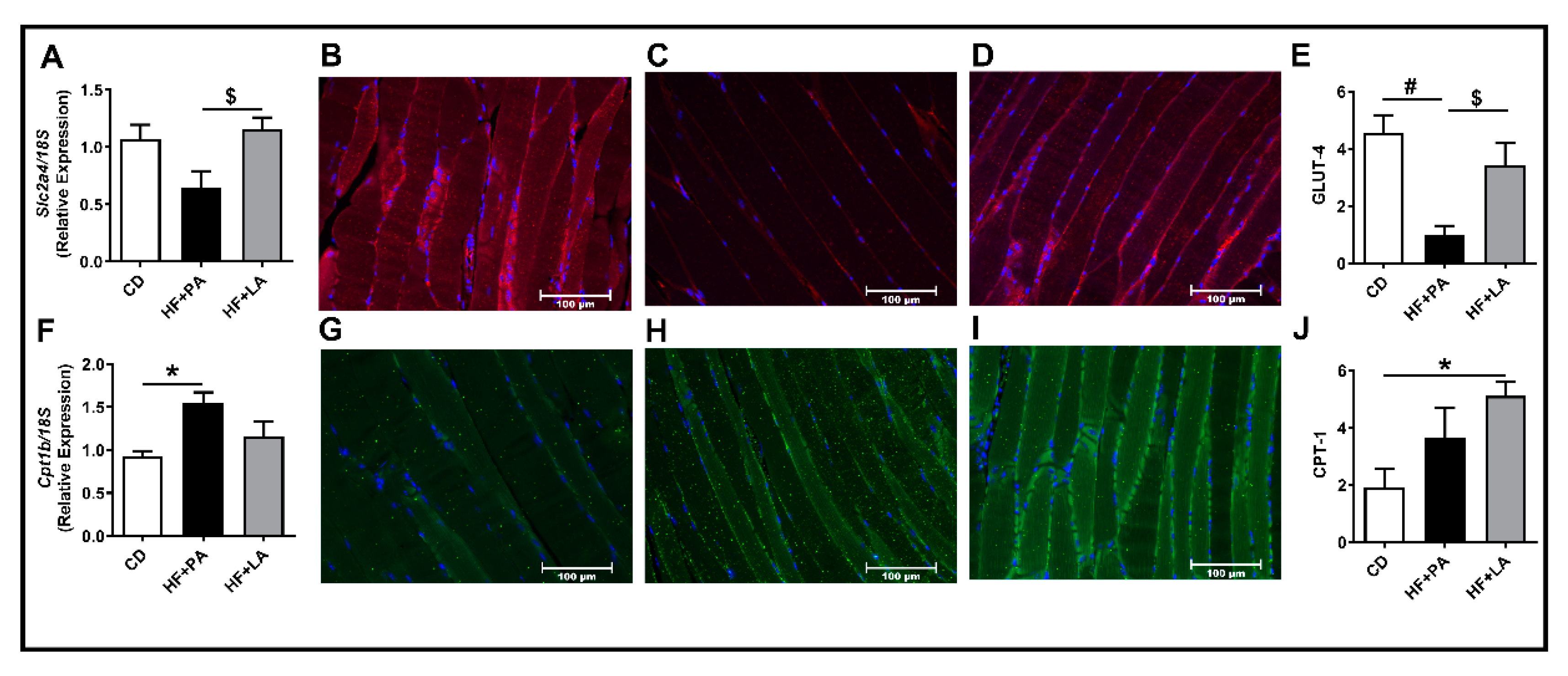
3.1. LA Increased VAT Mass Compared to PA without Changes in Total Fat Mass
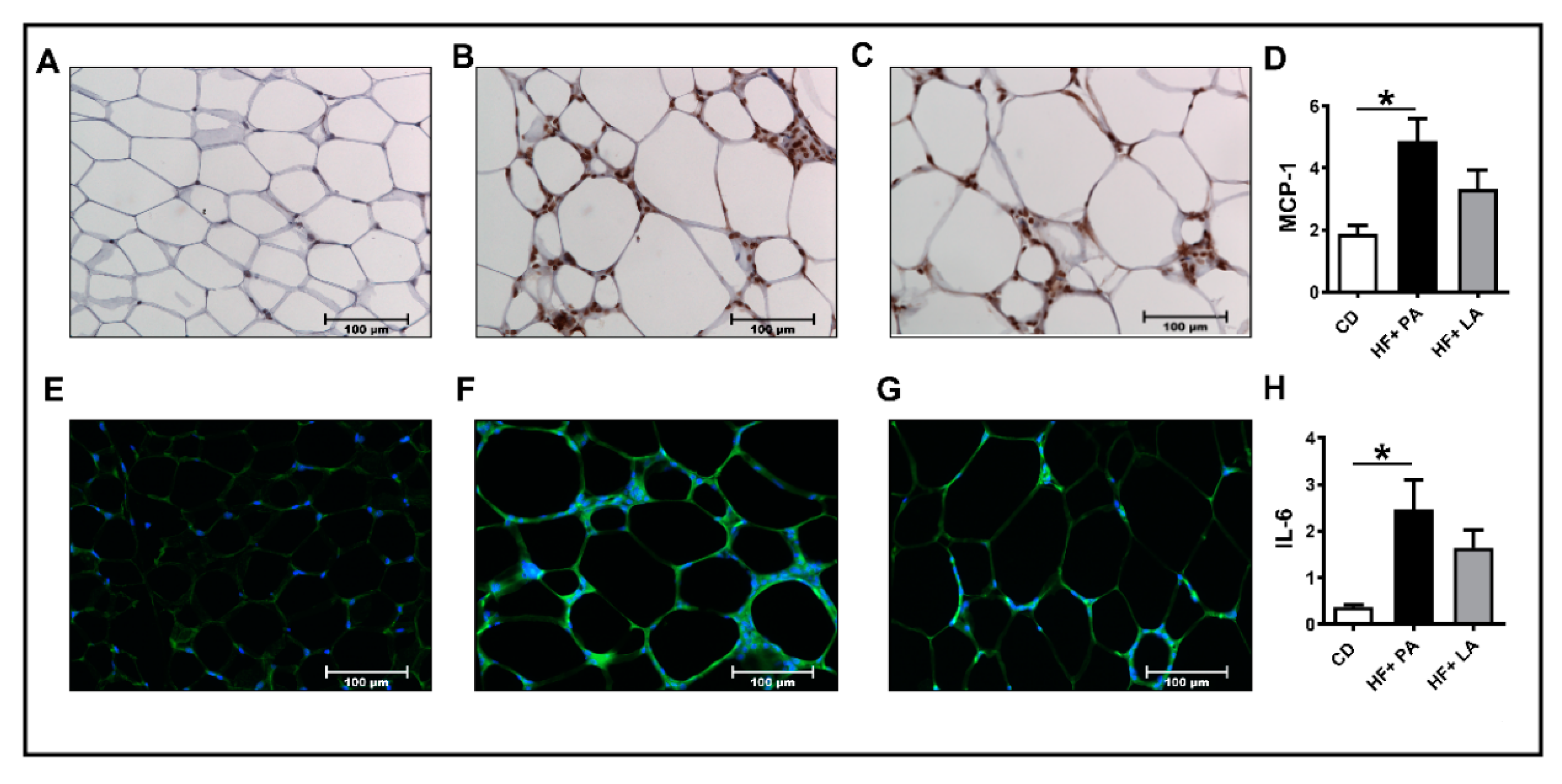
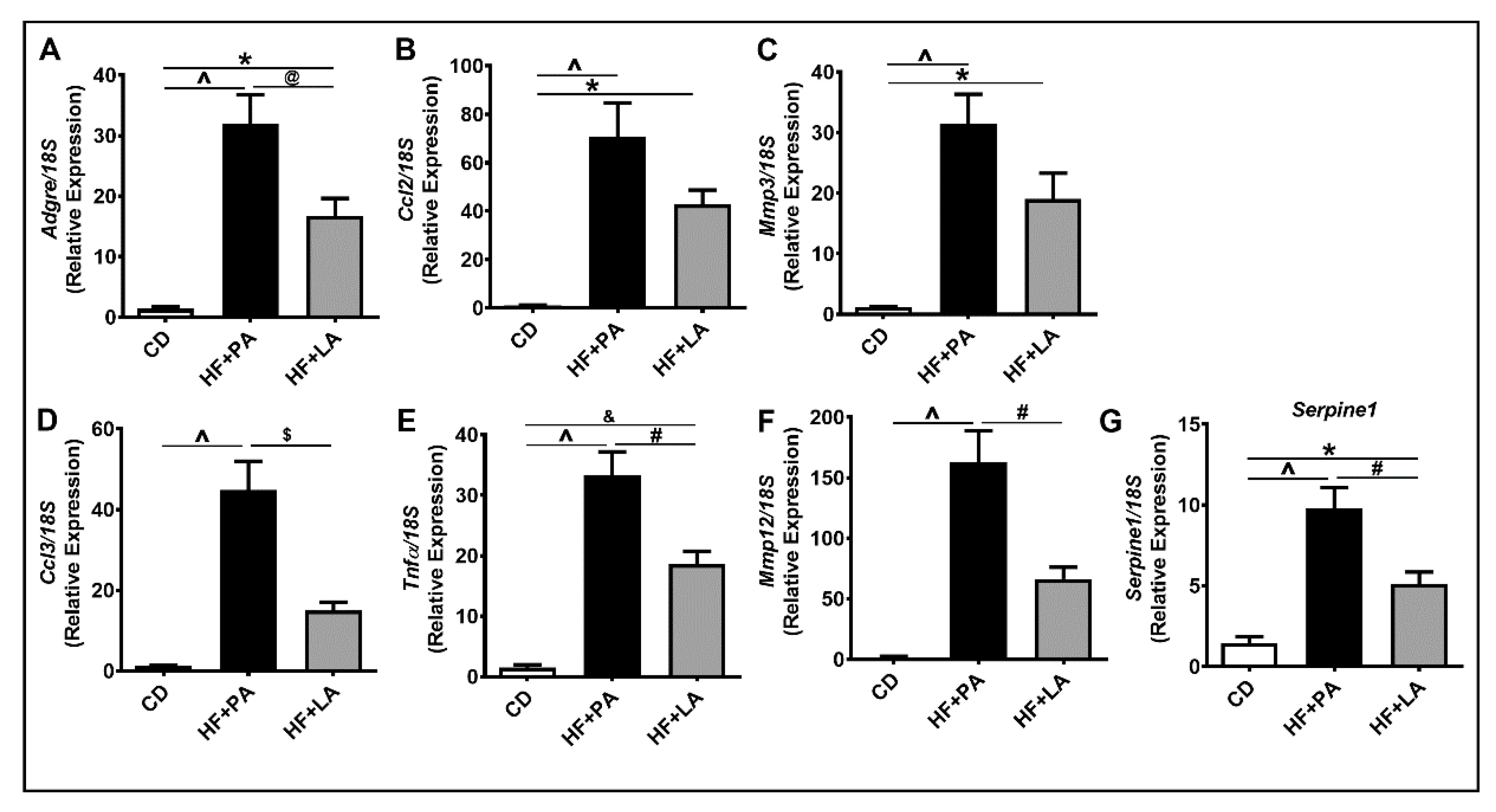
3.2. A High Fat Diet Supplemented with LA Promoted Less AT Inflammation Compared to PA
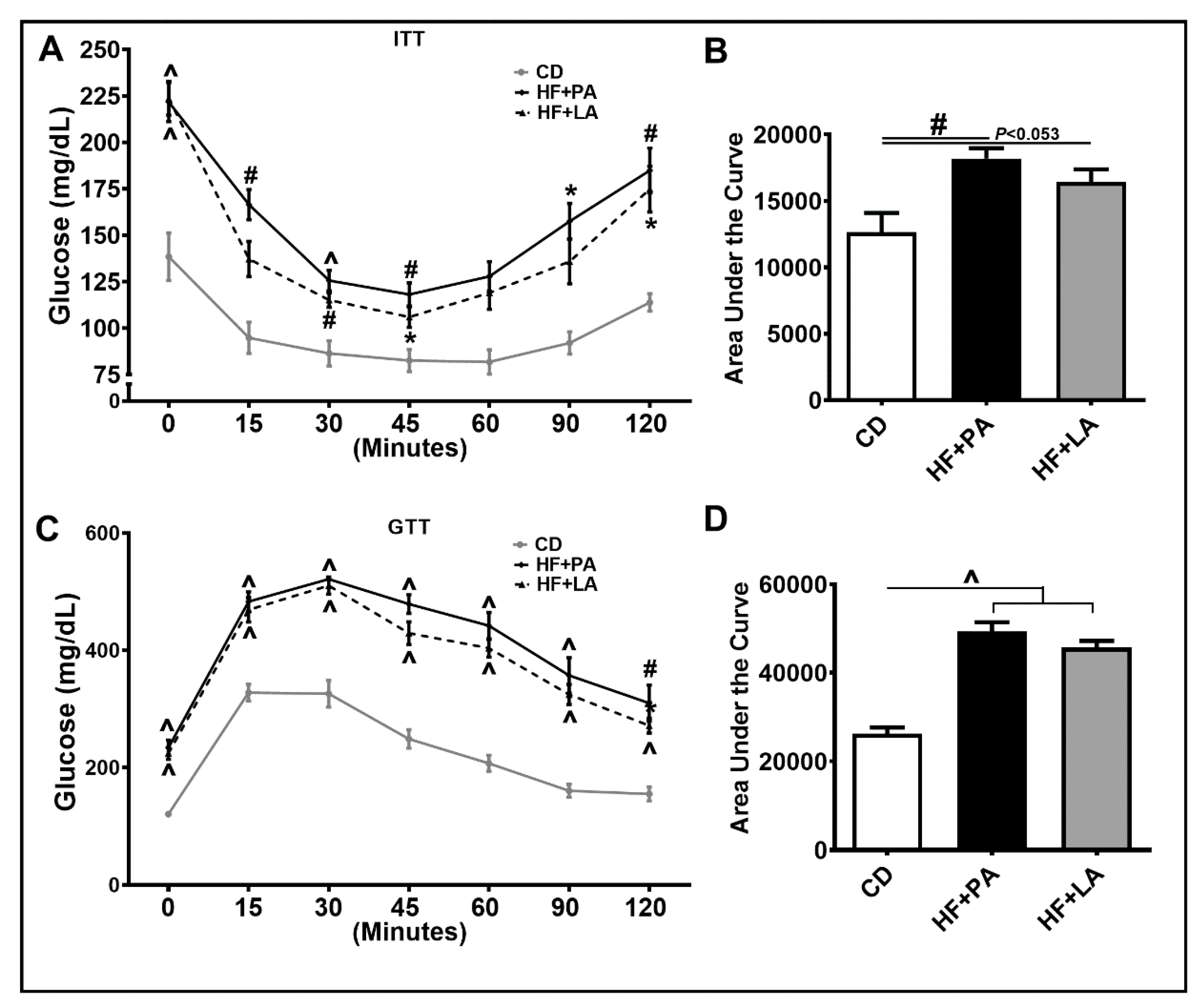
3.3. Both PA and LA Supplementation Induced IR, But LA Was Less Potent Than PA in Causing IR Compared to CD Controls
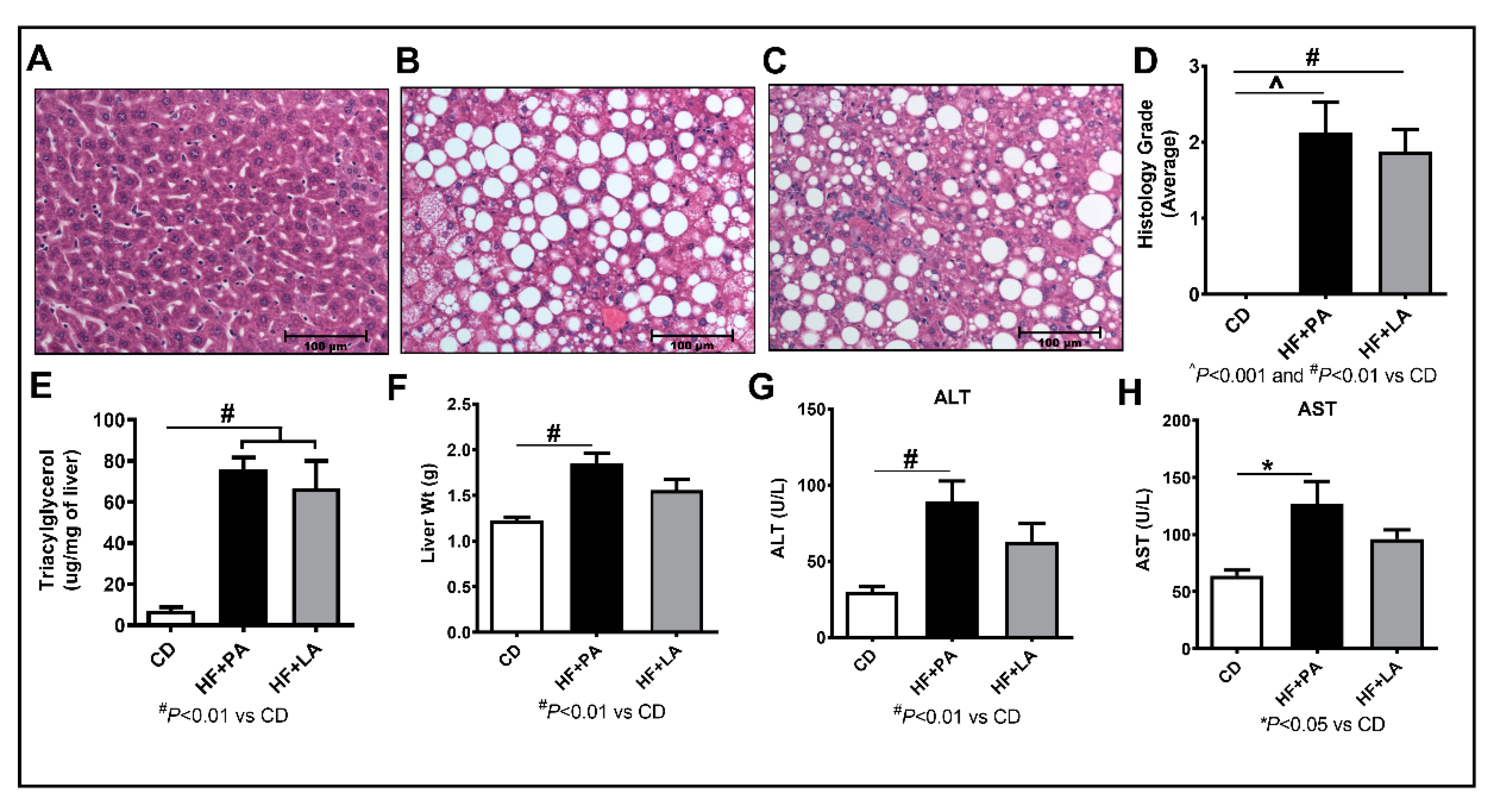
3.4. LA Supplementation Caused Less Liver Injury Compared to PA
3.5. A High Fat Diet Supplemented with LA or PA Exerted Similar Effects on Fatty Acid Profile in Liver Triglycerides
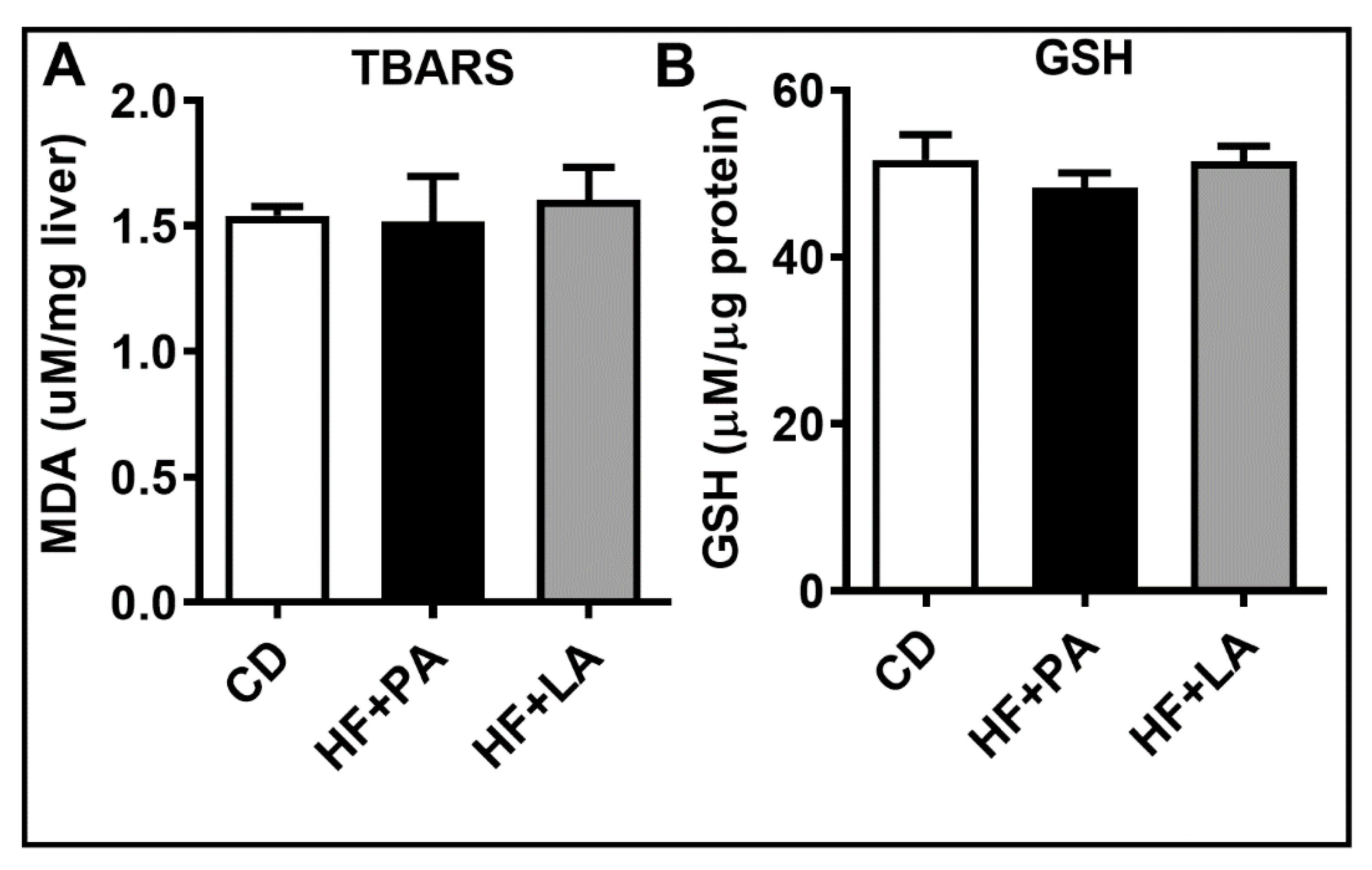
3.6. Hepatic Oxidative Stress Was Not Altered by a HF Diet Supplemented with LA or PA
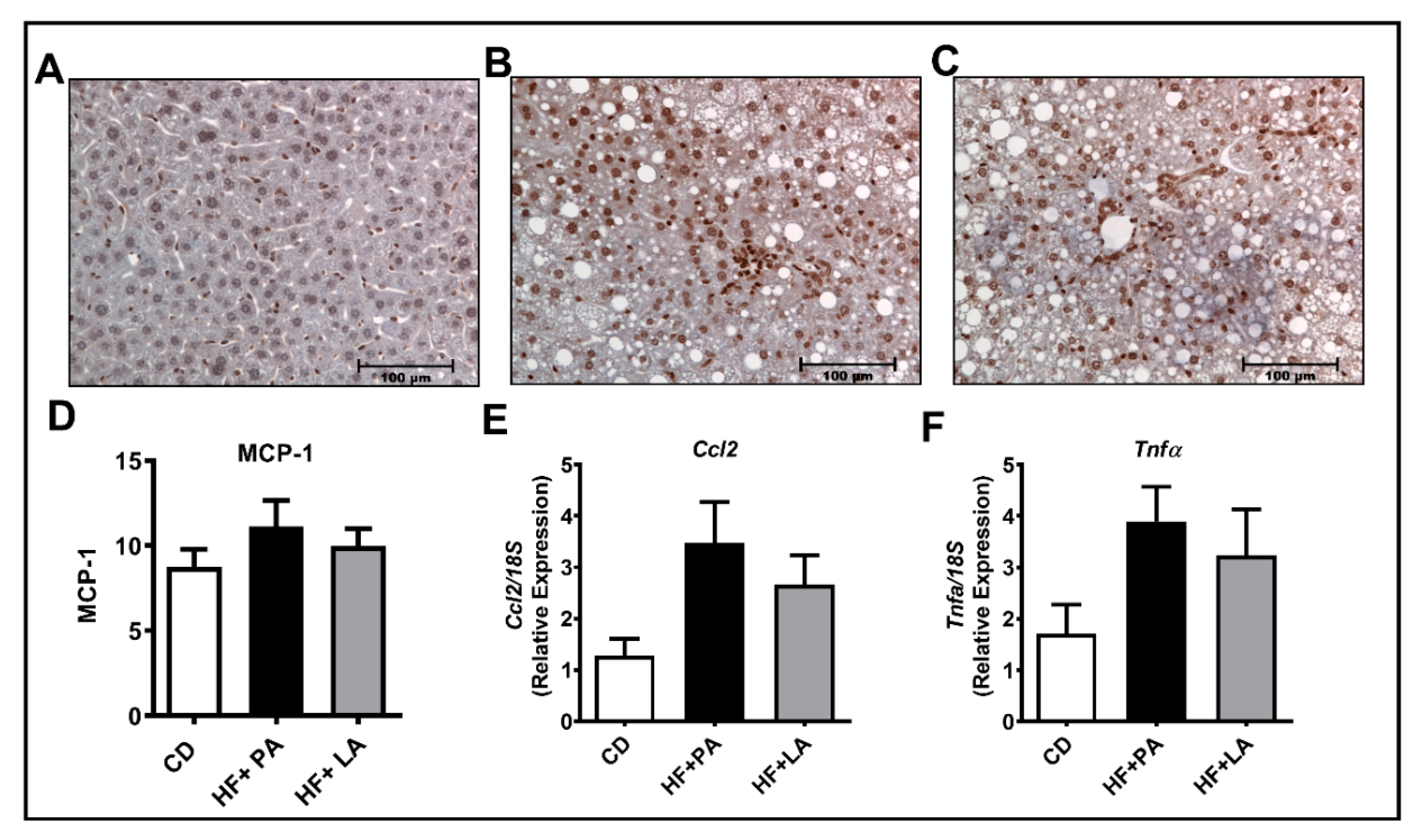
3.7. A High Fat Diet Supplemented with LA or PA Exerted Similar Effects on Markers of Hepatic Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orru, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Muller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Physiol. Gastrointest. Liver. Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [PubMed]
- Sales, R.C.; Medeiros, P.C.; Spreafico, F.; de Velasco, P.C.; Goncalves, F.K.A.; Martin-Hernandez, R.; Mantilla-Escalante, D.C.; Gil-Zamorano, J.; Peres, W.A.F.; de Souza, S.A.L.; et al. Olive Oil, Palm Oil, and Hybrid Palm Oil Distinctly Modulate Liver Transcriptome and Induce NAFLD in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2018, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlstrom, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Roden, M. Mechanisms of Disease: Hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat. Clin. Pr. Endocrinol. Metab. 2006, 2, 335–348. [Google Scholar] [CrossRef]
- Milic, S.; Lulic, D.; Stimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef]
- Laugerette, F.; Furet, J.P.; Debard, C.; Daira, P.; Loizon, E.; Geloen, A.; Soulage, C.O.; Simonet, C.; Lefils-Lacourtablaise, J.; Bernoud-Hubac, N.; et al. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E374–E386. [Google Scholar] [CrossRef]
- Reynolds, C.M.; McGillicuddy, F.C.; Harford, K.A.; Finucane, O.M.; Mills, K.H.; Roche, H.M. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol. Nutr. Food Res. 2012, 56, 1212–1222. [Google Scholar] [CrossRef]
- Rial, S.A.; Jutras-Carignan, A.; Bergeron, K.F.; Mounier, C. A high-fat diet enriched in medium chain triglycerides triggers hepatic thermogenesis and improves metabolic health in lean and obese mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158582. [Google Scholar] [CrossRef]
- Zacek, P.; Bukowski, M.; Mehus, A.; Johnson, L.; Zeng, H.; Raatz, S.; Idso, J.P.; Picklo, M. Dietary saturated fatty acid type impacts obesity-induced metabolic dysfunction and plasma lipidomic signatures in mice. J. Nutr. Biochem. 2019, 64, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zicker, M.C.; Silveira, A.L.M.; Lacerda, D.R.; Rodrigues, D.F.; Oliveira, C.T.; de Souza Cordeiro, L.M.; Lima, L.C.F.; Santos, S.H.S.; Teixeira, M.M.; Ferreira, A.V.M. Virgin coconut oil is effective to treat metabolic and inflammatory dysfunction induced by high refined carbohydrate-containing diet in mice. J. Nutr. Biochem. 2019, 63, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet 2015, 115, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Han, Y.; Xu, J.; Huang, W.; Li, Z. Medium Chain Triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism. PLoS ONE 2018, 13, e0191182. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Lee, Y.Y. Virgin Coconut Oil and its Cardiovascular Health Benefits. Nat. Prod. Commun. 2016, 11, 1151–1152. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Sharp, S.J.; Finikarides, L.; Afzal, I.; Lentjes, M.; Luben, R.; Forouhi, N.G. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 2018, 8, e020167. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Palliyil, D.M.; Kuruvilla, K.; Raghavamenon, A.C. Virgin coconut oil reverses hepatic steatosis by restoring redox homeostasis and lipid metabolism in male Wistar rats. J. Sci. Food Agric. 2018, 98, 1757–1764. [Google Scholar] [CrossRef]
- Wang, M.E.; Singh, B.K.; Hsu, M.C.; Huang, C.; Yen, P.M.; Wu, L.S.; Jong, D.S.; Chiu, C.H. Increasing Dietary Medium-Chain Fatty Acid Ratio Mitigates High-fat Diet-Induced Non-Alcoholic Steatohepatitis by Regulating Autophagy. Sci. Rep. 2017, 7, 13999. [Google Scholar] [CrossRef]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Saraswathi, V.; Hasty, A.H. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J. Lipid Res. 2006, 47, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.S.; Niskanen, L.K.; Maliranta, H.M.; Savolainen, M.J.; Kesaniemi, Y.A.; Uusitupa, M.I. Lauric and palmitic acid-enriched diets have minimal impact on serum lipid and lipoprotein concentrations and glucose metabolism in healthy young women. J. Nutr. 1995, 125, 466–473. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ross, R.; Parsons, W.D.; Jones, P.J. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes. Res. 2003, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Fragua, V.; Barroeta, A.C.; Manzanilla, E.G.; Codony, R.; Villaverde, C. Evaluation of the use of esterified fatty acid oils enriched in medium-chain fatty acids in weight loss diets for dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2015, 99 (Suppl. S1), 48–59. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, E.; Browne, L.; Irving-Rodgers, H.; Massa, H.M.; Fozzard, N.; Jennings, M.P.; Peak, I.R. Effect of GPR84 deletion on obesity and diabetes development in mice fed long chain or medium chain fatty acid rich diets. Eur. J. Nutr. 2018, 57, 1737–1746. [Google Scholar] [CrossRef]
- Wang, L.; Luo, L.; Zhao, W.; Yang, K.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; Xi, Q.; Zhang, Y.; et al. Lauric Acid Accelerates Glycolytic Muscle Fiber Formation through TLR4 Signaling. J. Agric. Food Chem. 2018, 66, 6308–6316. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Q.; Li, F.; Yi, W.; Liu, F.; Wang, S.; Jiang, Q. Effects of Dietary Supplementation of Lauric Acid on Lactation Function, Mammary Gland Development, and Serum Lipid Metabolites in Lactating Mice. Animals 2020, 10, 529. [Google Scholar] [CrossRef]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Haversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pacheco, F.; Gutierrez-Repiso, C.; Garcia-Serrano, S.; Alaminos-Castillo, M.A.; Ho-Plagaro, A.; Valdes, S.; Garcia-Arnes, J.; Gonzalo, M.; Andrade, R.J.; Moreno-Ruiz, F.J.; et al. The pro-/anti-inflammatory effects of different fatty acids on visceral adipocytes are partially mediated by GPR120. Eur. J. Nutr. 2017, 56, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, V.; Gao, L.; Morrow, J.D.; Chait, A.; Niswender, K.D.; Hasty, A.H. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J. Nutr. 2007, 137, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.A.; Oyama, L.M.; de Macedo Motoyama, C.S.; da Silva Biz, C.R.; Silveira, V.L.; Ribeiro, E.B.; Oller do Nascimento, C.M. Long chain saturated fatty acids increase haptoglobin gene expression in C57BL/6J mice adipose tissue and 3T3-L1 cells. Eur. J. Nutr. 2010, 49, 235–241. [Google Scholar] [CrossRef]
- Stroher, D.J.; de Oliveira, M.F.; Martinez-Oliveira, P.; Pilar, B.C.; Cattelan, M.D.P.; Rodrigues, E.; Bertolin, K.; Goncalves, P.B.D.; Piccoli, J.; Manfredini, V. Virgin Coconut Oil Associated with High-Fat Diet Induces Metabolic Dysfunctions, Adipose Inflammation, and Hepatic Lipid Accumulation. J. Med. Food. 2019. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Good Fats versus Bad Fats: A Comparison of Fatty Acids in the Promotion of Insulin Resistance, Inflammation, and Obesity. Miss. Med. 2017, 114, 303–307. [Google Scholar]
- Deol, P.; Evans, J.R.; Dhahbi, J.; Chellappa, K.; Han, D.S.; Spindler, S.; Sladek, F.M. Soybean Oil Is More Obesogenic and Diabetogenic than Coconut Oil and Fructose in Mouse: Potential Role for the Liver. PLoS ONE 2015, 10, e0132672. [Google Scholar] [CrossRef]
- Buettner, R.; Ascher, M.; Gabele, E.; Hellerbrand, C.; Kob, R.; Bertsch, T.; Bollheimer, L.C. Olive oil attenuates the cholesterol-induced development of nonalcoholic steatohepatitis despite increased insulin resistance in a rodent model. Horm. Metab. Res. 2013, 45, 795–801. [Google Scholar] [CrossRef]
- Valente, F.X.; Candido, F.G.; Lopes, L.L.; Dias, D.M.; Carvalho, S.D.L.; Pereira, P.F.; Bressan, J. Effects of coconut oil consumption on energy metabolism, cardiometabolic risk markers, and appetitive responses in women with excess body fat. Eur. J. Nutr. 2018, 57, 1627–1637. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brown, S.H.; Small, L.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J. Lipid. Res. 2013, 54, 3322–3333. [Google Scholar] [CrossRef]
- Stanton, M.C.; Chen, S.C.; Jackson, J.V.; Rojas-Triana, A.; Kinsley, D.; Cui, L.; Fine, J.S.; Greenfeder, S.; Bober, L.A.; Jenh, C.H. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. (Lond.) 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Derm. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, E.; Sauer, G. Tumor necrosis factor induces necrosis of human carcinoma xenografts in the presence of tricyclodecan-9-yl-xanthogenate and lauric acid. Int. J. Cancer 1990, 45, 1113–1118. [Google Scholar] [CrossRef]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef]
| Gene (Abbr) | Description | Catalog Number |
|---|---|---|
| 18s | 18S ribosomal RNA | 4352930E |
| Adgre1 (EMR-1; F4/80) | EGF-like module containing, mucin-like, hormone receptor-like 1 | Mm00802529_m1 |
| Ccl2 (MCP-1) | Chemokine ligand 2/monocyte chemotactic protein 1 | Mm00441242_m1 |
| Ccl3 (MIP-1a) | Chemokine ligand 3/macrophage inflammatory protein 1alpha | Mm00441258_m1 |
| Cpt1b | Carnitine palmitoyl transferase 1 (A = Liver; B = Muscle) | Mm00487191_g1 |
| Cpt2 | Carnitine palmitoyl transferase 2 | Mm00487205_m1 |
| Mmp12 | Matrix metallopeptidase 12 (macrophage elastase) | Mm00500554_m1 |
| Mmp3 | Matrix metallopeptidase 3 | Mm00440295_m1 |
| Serpine/PAI-1 | Serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1) | Mm00435860_m1 |
| Slc2a4 (Glut-4) | Solute carrier family 2 (facilitated glucose transporter), member 4 | Mm01245502_m1 |
| Tnfa | Tumor necrosis factor alpha | Mm00443258_m1 |
| Fatty Acid | CD | HF + PA | HF + LA |
|---|---|---|---|
| Myristic acid (14:0) | 0.02 ± 0.02 | 0.59 ± 0.04 ^ | 0.61 ± 0.11 ^ |
| Palmitic acid (16:0) | 2.66 ± 0.50 | 24.7 ± 1.9 ^ | 21.0 ± 4.3 # |
| Palmitoleic acid (16:1) | 0.26 ± 0.06 | 3.28 ± 0.24 ^ | 3.05 ± 0.64 ^ |
| Stearic acid (18:0) | 0.28 ± 0.05 | 1.38 ± 0.11 ^ | 1.18 ± 0.18 ^ |
| Oleic acid (18:1) | 2.86 ± 0.56 | 29.2 ± 2.6 ^ | 26.7 ± 5.5 # |
| Linoleic acid (18:2) | 2.10 ± 0.56 | 10.7 ± 0.7 ^ | 9.54 ± 1.50 ^ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology 2020, 9, 346. https://doi.org/10.3390/biology9110346
Saraswathi V, Kumar N, Gopal T, Bhatt S, Ai W, Ma C, Talmon GA, Desouza C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology. 2020; 9(11):346. https://doi.org/10.3390/biology9110346
Chicago/Turabian StyleSaraswathi, Viswanathan, Narendra Kumar, Thiyagarajan Gopal, Saumya Bhatt, Weilun Ai, Carmen Ma, Geoffrey A. Talmon, and Cyrus Desouza. 2020. "Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity" Biology 9, no. 11: 346. https://doi.org/10.3390/biology9110346
APA StyleSaraswathi, V., Kumar, N., Gopal, T., Bhatt, S., Ai, W., Ma, C., Talmon, G. A., & Desouza, C. (2020). Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology, 9(11), 346. https://doi.org/10.3390/biology9110346






