Isolation and Characterization of Cultivable Microbes from the Gut of Zophobas atratus (Coleoptera: Tenebrionidae) Larvae Reared on Two Types of Artificial Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zophobas atratus Origin and Rearing on Experimental Diets
2.2. Obtaining Intestinal Tract Tissues from Zophobas atratus Larvae
2.3. Analysis of Culturable Aerobic/Facultative Anaerobic Microbial Groups from Zophobas atratus Larval Gut
2.4. Analysis of Cultured Aerobic/Facultative Anaerobic Bacterial Isolates from Zophobas atratus Larvae Gut
2.5. Statistical Analysis
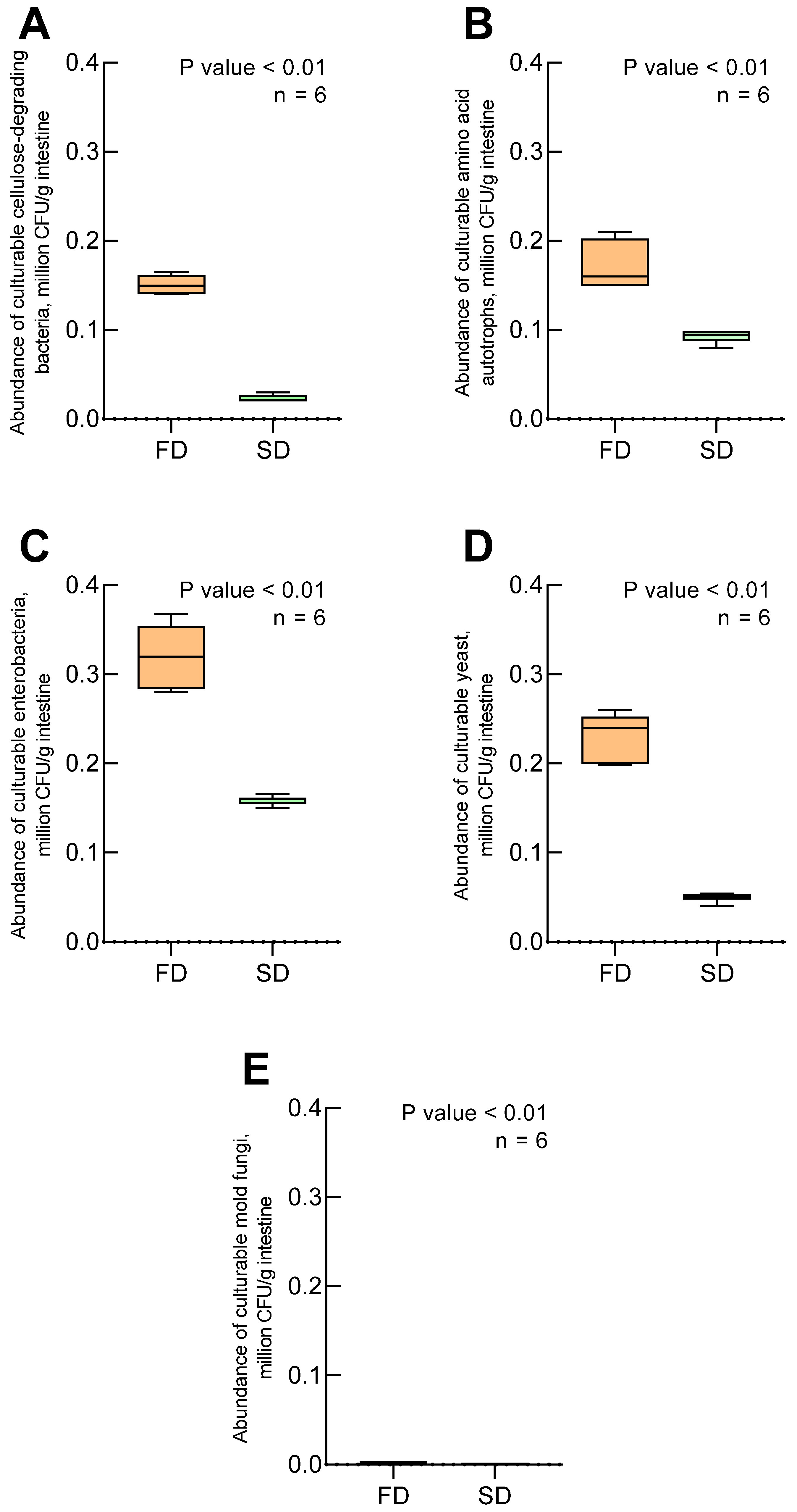
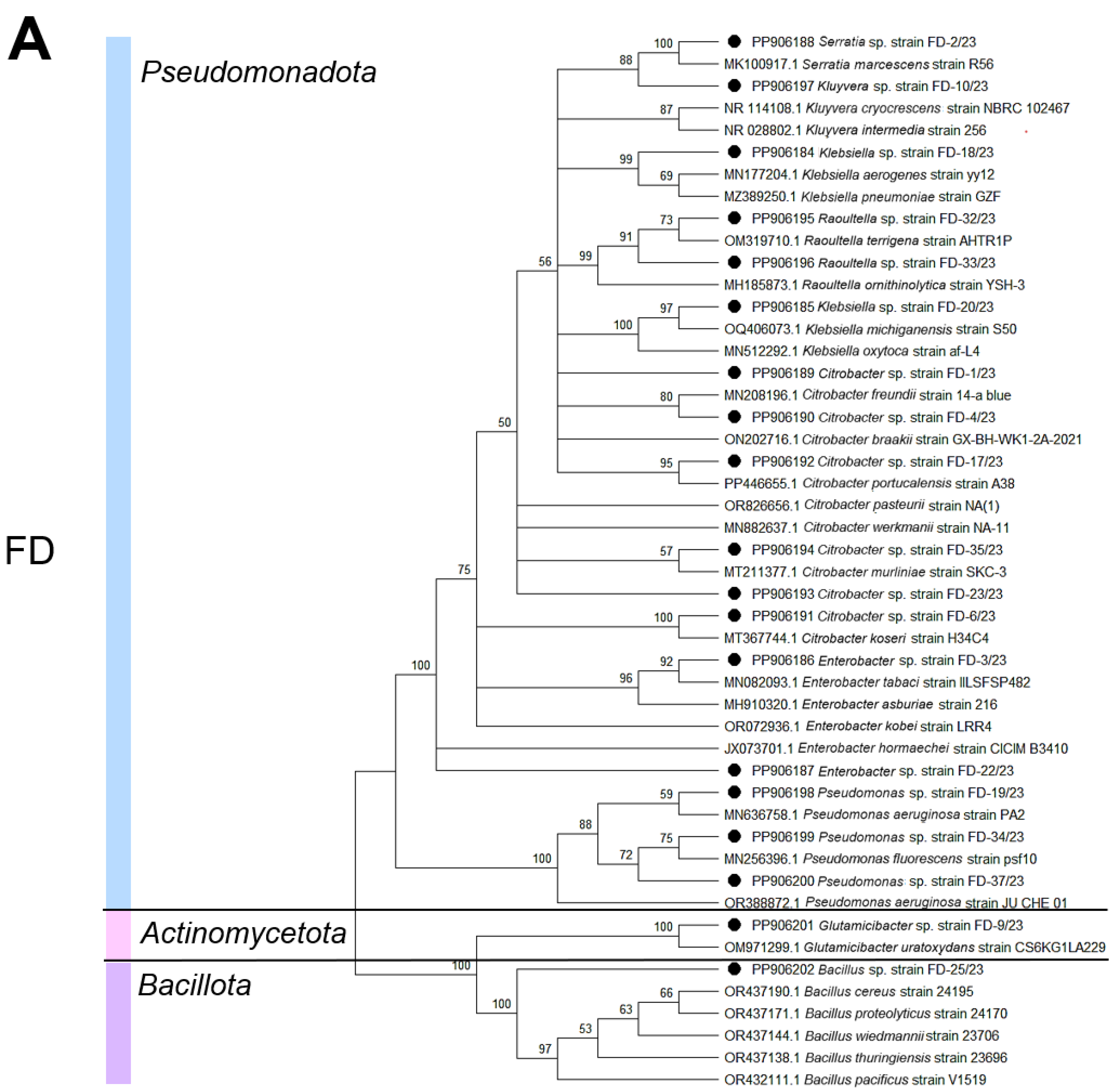
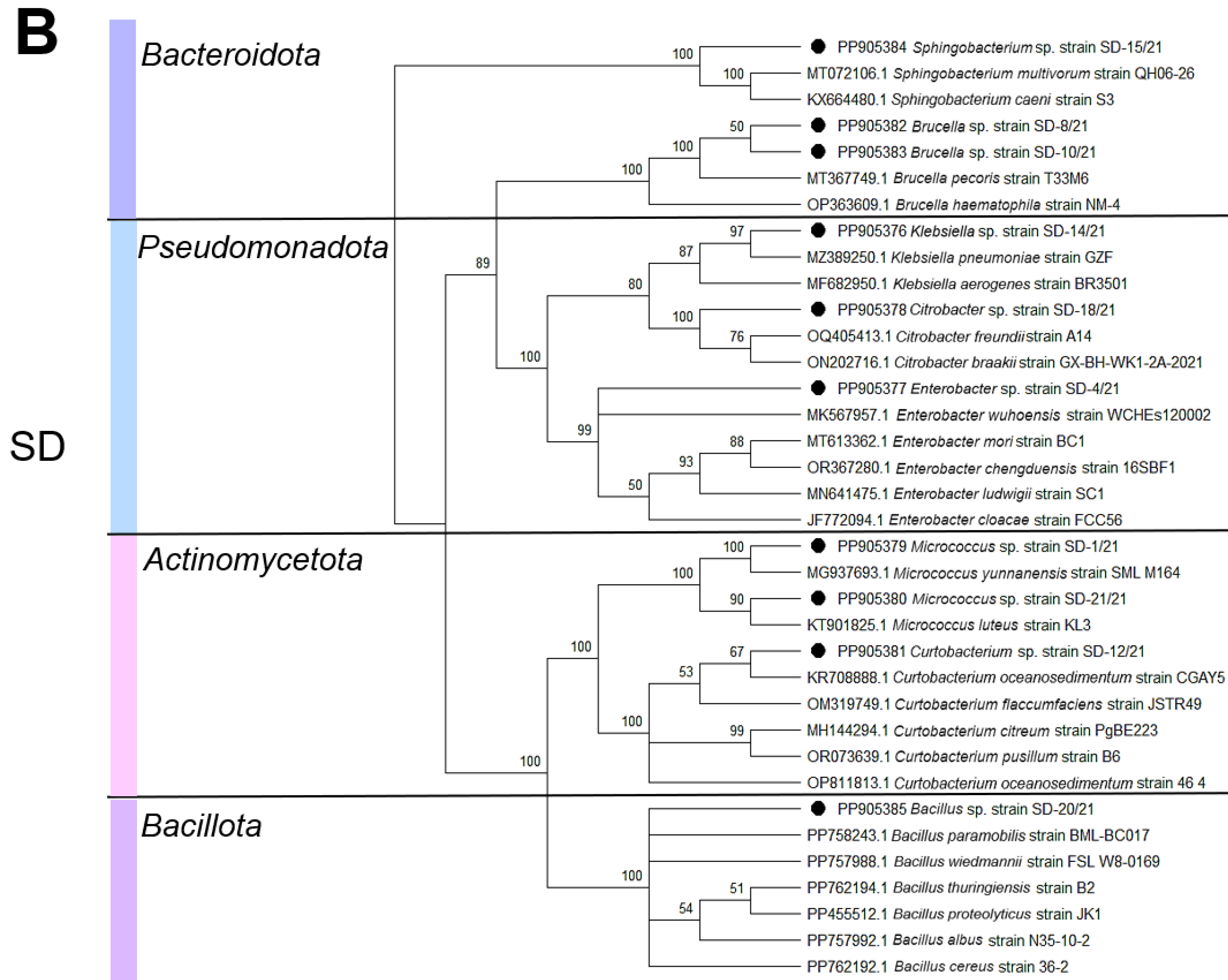
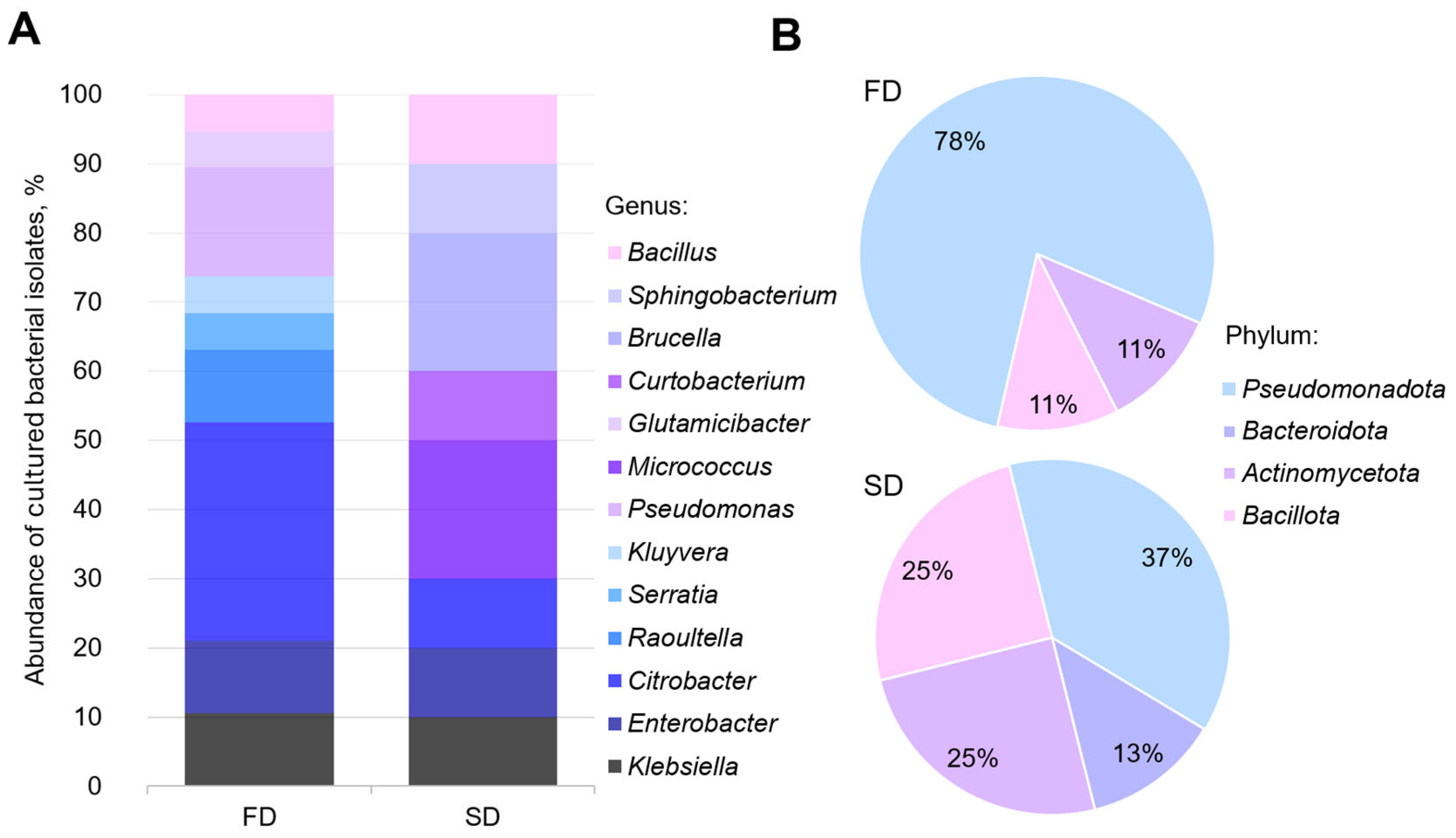
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagarajan, V.M.; Yuvan, M.; Srinivasan, R.; Satagopan, N.R.; Asokan, A.; A., A. Status of Important Coastal Habitats of North Tamil Nadu: Diversity, Current Threats and Approaches for Conservation. Reg. Stud. Mar. Sci. 2022, 49, 102106. [Google Scholar] [CrossRef]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-Mediated Insecticide Resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Suárez-Moo, P.; Cruz-Rosales, M.; Ibarra-Laclette, E.; Desgarennes, D.; Huerta, C.; Lamelas, A. Diversity and Composition of the Gut Microbiota in the Developmental Stages of the Dung Beetle Copris incertus Say (Coleoptera, Scarabaeidae). Front. Microbiol. 2020, 11, 1698. [Google Scholar] [CrossRef]
- Salem, H.; Kaltenpoth, M. Beetle–Bacterial Symbioses: Endless Forms Most Functional. Annu. Rev. Entomol. 2022, 67, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. The Microbial Dimension in Insect Nutritional Ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Sudakaran, S.; Retz, F.; Kikuchi, Y.; Kost, C.; Kaltenpoth, M. Evolutionary Transition in Symbiotic Syndromes Enabled Diversification of Phytophagous Insects on an Imbalanced Diet. ISME J. 2015, 9, 2587–2604. [Google Scholar] [CrossRef] [PubMed]
- Funaro, C.F.; Kronauer, D.J.C.; Moreau, C.S.; Goldman-Huertas, B.; Pierce, N.E.; Russell, J.A. Army Ants Harbor a Host-Specific Clade of Entomoplasmatales Bacteria. Appl. Environ. Microbiol. 2011, 77, 346–350. [Google Scholar] [CrossRef]
- Dang, K.; Doggett, S.L.; Veera Singham, G.; Lee, C.-Y. Insecticide Resistance and Resistance Mechanisms in Bed Bugs, Cimex spp. (Hemiptera: Cimicidae). Parasit. Vectors 2017, 10, 318. [Google Scholar] [CrossRef]
- Nicoletti, R.; Becchimanzi, A. Ecological and Molecular Interactions between Insects and Fungi. Microorganisms 2022, 10, 96. [Google Scholar] [CrossRef]
- Zhou, L.-F.; Wu, J.; Li, S.; Li, Q.; Jin, L.-P.; Yin, C.-P.; Zhang, Y.-L. Antibacterial Potential of Termite-Associated Streptomyces spp. ACS Omega 2021, 6, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M. Gut Microbiotas and Host Evolution: Scaling Up Symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut Microbiota Mediate Caffeine Detoxification in the Primary Insect Pest of Coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Dietrich, C.; Köhler, T.; Poulsen, M.; Sillam-Dussès, D.; Brune, A. Diet Is the Primary Determinant of Bacterial Community Structure in the Guts of Higher Termites. Mol. Ecol. 2015, 24, 5284–5295. [Google Scholar] [CrossRef]
- Warnecke, F.; Luginbühl, P.; Ivanova, N.; Ghassemian, M.; Richardson, T.H.; Stege, J.T.; Cayouette, M.; McHardy, A.C.; Djordjevic, G.; Aboushadi, N.; et al. Metagenomic and Functional Analysis of Hindgut Microbiota of a Wood-Feeding Higher Termite. Nature 2007, 450, 560–565. [Google Scholar] [CrossRef]
- Aylward, F.O.; Suen, G.; Biedermann, P.H.W.; Adams, A.S.; Scott, J.J.; Malfatti, S.A.; Glavina del Rio, T.; Tringe, S.G.; Poulsen, M.; Raffa, K.F.; et al. Convergent Bacterial Microbiotas in the Fungal Agricultural Systems of Insects. mBio 2014, 5, e02077. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, P.H.W.; Vega, F.E. Ecology and Evolution of Insect–Fungus Mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Hoover, K.; Tien, M.; Tringe, S.G.; Barry, K.W.; del Rio, T.G.; Chovatia, M.; Herr, J.R.; Carlson, J.E. Metagenomic Profiling Reveals Lignocellulose Degrading System in a Microbial Community Associated with a Wood-Feeding Beetle. PLoS ONE 2013, 8, e73827. [Google Scholar] [CrossRef]
- Yi, D.-A.; Kuprin, A.V.; Lee, Y.H.; Bae, Y.J. Newly Developed Fungal Diet for Artificial Rearing of the Endangered Long-horned Beetle Callipogon relictus (Coleoptera: Cerambycidae). Entomol. Res. 2017, 47, 373–379. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Wood Decomposition as Influenced by Invertebrates. Biol. Rev. 2014, 91, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, M. Nutrient Dynamics in Decomposing Dead Wood in the Context of Wood Eater Requirements: The Ecological Stoichiometry of Saproxylophagous Insects. In Saproxylic Insects: Diversity, Ecology and Conservation; Ulyshen, M.D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 429–469. ISBN 978-3-319-75937-1. [Google Scholar]
- Ulyshen, M.D.; Šobotník, J. An Introduction to the Diversity, Ecology, and Conservation of Saproxylic Insects. In Saproxylic Insects: Diversity, Ecology and Conservation; Ulyshen, M.D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–47. ISBN 978-3-319-75937-1. [Google Scholar]
- Yi, D.-A.; Kuprin, A.V.; Bae, Y.J. Effects of Temperature on Instar Number and Larval Development in the Endangered Longhorn Beetle Callipogon relictus (Coleoptera: Cerambycidae) Raised on an Artificial Diet. Can. Entomol. 2019, 151, 537–544. [Google Scholar] [CrossRef]
- Yi, D.-A.; Kuprin, A.V.; Bae, Y.J. Distribution of the Longhorned Beetle Callipogon relictus (Coleoptera: Cerambycidae) in Northeast Asia. Zootaxa 2018, 4369, 101–108. [Google Scholar] [CrossRef]
- Kang, J.H.; Yi, D.-A.; Kuprin, A.V.; Han, C.; Bae, Y.J. Phylogeographic Investigation of an Endangered Longhorn Beetle, Callipogon Relictus (Coleoptera: Cerambycidae), in Northeast Asia: Implications for Future Restoration in Korea. Insects 2021, 12, 555. [Google Scholar] [CrossRef]
- Veremenko, V.S.; Khandy, M.T.; Shevchenko, E.A.; Grinchenko, A.V.; Kumeiko, V.V.; Kuprin, A.V. Effects of Diet and Feed Composition on Antibacterial Activity of Hemolymph of Saproxylic Beetles: A Case Study of Zophobas atratus (Coleoptera: Tenebrionidae). Far East. Entomol. 2022, 458, 1–12. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Ding, M.-Q.; Li, M.-X.; Ding, J.; Bai, S.-W.; Wu, Q.-L.; Zhao, L.; Cao, G.-L.; Ren, N.-Q.; et al. Sustainable Strategy for Lignocellulosic Crop Wastes Reduction by Tenebrio molitor Linnaeus (Mealworm) and Potential Use of Mealworm Frass as a Fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Tschinkel, W.R. Zophobas atratus (Fab.) and Z. rugipes Kirsch (Coleoptera: Tenebrionidae) Are the Same Species. Coleopt. Bull. 1984, 38, 325–333. [Google Scholar]
- Rumbos, C.I.; Athanassiou, C.G. The Superworm, Zophobas Morio (Coleoptera: Tenebrionidae): A ‘Sleeping Giant’ in Nutrient Sources. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef]
- Bulet, P.; Cociancich, S.; Dimarcq, J.L.; Lambert, J.; Reichhart, J.M.; Hoffmann, D.; Hetru, C.; Hoffmann, J.A. Insect Immunity. Isolation from a Coleopteran Insect of a Novel Inducible Antibacterial Peptide and of New Members of the Insect Defensin Family. J. Biol. Chem. 1991, 266, 24520–24525. [Google Scholar] [CrossRef]
- VandenBrooks, J.M.; Ford, C.F.; Harrison, J.F. Responses to Alteration of Atmospheric Oxygen and Social Environment Suggest Trade-Offs among Growth Rate, Life Span, and Stress Susceptibility in Giant Mealworms (Zophobas morio). Physiol. Biochem. Zool. 2020, 93, 358–368. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, H.; Shin, Y.P.; Kim, M.-A.; Natarajan, S.; Veerappan, K.; Kim, S.H.; Park, J.; Hwang, J.S. Uncovering Antimicrobial Peptide from Zophobas atratus Using Transcriptome Analysis. Int. J. Pept. Res. Ther. 2021, 27, 1827–1835. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, S.Y.; Yoon, H.J. Development of Optimized Artificial Diets for Enhanced Growth of Zophobas atratus Larvae. Entomol. Res. 2024, 54, e70002. [Google Scholar] [CrossRef]
- Yang, S.-S.; Brandon, A.M.; Andrew Flanagan, J.C.; Yang, J.; Ning, D.; Cai, S.-Y.; Fan, H.-Q.; Wang, Z.-Y.; Ren, J.; Benbow, E.; et al. Biodegradation of Polystyrene Wastes in Yellow Mealworms (Larvae of Tenebrio molitor Linnaeus): Factors Affecting Biodegradation Rates and the Ability of Polystyrene-Fed Larvae to Complete Their Life Cycle. Chemosphere 2018, 191, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Arunrattiyakorn, P.; Ponprateep, S.; Kaennonsang, N.; Charapok, Y.; Punphuet, Y.; Krajangsang, S.; Tangteerawatana, P.; Limtrakul, A. Biodegradation of Polystyrene by Three Bacterial Strains Isolated from the Gut of Superworms (Zophobas atratus Larvae). J. Appl. Microbiol. 2022, 132, 2823–2831. [Google Scholar] [CrossRef]
- Vital-Vilchis, I.; Karunakaran, E. Using Insect Larvae and Their Microbiota for Plastic Degradation. Insects 2025, 16, 165. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects–Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Hiergeist, A.; Gläsner, J.; Reischl, U.; Gessner, A. Analyses of Intestinal Microbiota: Culture versus Sequencing. ILAR J. 2015, 56, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.G.; Steen, A.D.; Ladau, J.; Yin, J.; Crosby, L. Phylogenetically Novel Uncultured Microbial Cells Dominate Earth Microbiomes. mSystems 2018, 3, e00055-18. [Google Scholar] [CrossRef]
- Kuprin, A.; Baklanova, V.; Khandy, M.; Grinchenko, A.; Kumeiko, V. Newly Woody Artificial Diet Reveals Antibacterial Activity of Hemolymph in Larvae of Zophobas atratus (Fabricius, 1775) (Coleoptera: Tenebrionidae). Insects 2024, 15, 435. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best Practices for Analysing Microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I. Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Academic Press: Cambridge, MA, USA, 2022; ISBN 978-0-12-822148-8. [Google Scholar]
- Koch, R. Zur Untersuchung von pathogenen Organismen; Norddeutschen Buchdruckerei und Verlagsanstalt: Berlin, Germany, 1881. [Google Scholar]
- Atlas, R.M.; Bartha, R. Microbial Ecology: Fundamentals and Applications; Addison Wesley Publishing Company: London, UK, 1981; ISBN 978-0-201-00051-1. [Google Scholar]
- Krieg, N.R.; Staley, J.T.; Brown, D.R.; Hedlund, B.P.; Paster, B.J.; Ward, N.L.; Ludwig, W.; Whitman, W.B. (Eds.) Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2010; ISBN 978-0-387-95042-6. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, J.K.; Smith, K.F.; Staden, R. A New DNA Sequence Assembly Program. Nucleic Acids Res. 1995, 23, 4992–4999. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evol. Int. J. Org. Evol. 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000; ISBN 978-0-19-535051-7. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic Digestion of Lignocellulose in Termite Guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional Genomics and Microbiome Profiling of the Asian Longhorned Beetle (Anoplophora glabripennis) Reveal Insights into the Digestive Physiology and Nutritional Ecology of Wood Feeding Beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.-S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef]
- Li, C.; Han, G.; Sun, J.; Huang, L.; Lu, Y.; Xia, Y.; Liu, Q.; Xu, J. The Gut Microbiota Composition of Cnaphalocrocis medinalis and Their Predicted Contribution to Larval Nutrition. Front. Microbiol. 2022, 13, 909863. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xu, H.; Liu, Y.; Lu, Z. Effects of Host Plant and Insect Generation on Shaping of the Gut Microbiota in the Rice Leaffolder, Cnaphalocrocis medinalis. Front. Microbiol. 2022, 13, 824224. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Fundamentals of Microbiome Science: How Microbes Shape Animal Biology; Princeton University Press: Princeton, NJ, USA, 2018; ISBN 978-0-691-16034-4. [Google Scholar]
- Hammer, T.J.; Sanders, J.G.; Fierer, N. Not All Animals Need a Microbiome. FEMS Microbiol. Lett. 2019, 366, fnz117. [Google Scholar] [CrossRef]
- Leite-Mondin, M.; DiLegge, M.J.; Manter, D.K.; Weir, T.L.; Silva-Filho, M.C.; Vivanco, J.M. The Gut Microbiota Composition of Trichoplusia Ni Is Altered by Diet and May Influence Its Polyphagous Behavior. Sci. Rep. 2021, 11, 5786. [Google Scholar] [CrossRef] [PubMed]
- Mugo-Kamiri, L.; Querejeta, M.; Raymond, B.; Herniou, E.A. The Effect of Diet Composition on the Diversity of Active Gut Bacteria and on the Growth of Spodoptera exigua (Lepidoptera: Noctuidae). J. Insect Sci. 2024, 24, 13. [Google Scholar] [CrossRef]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Tinker, K.A.; Ottesen, E.A. The Core Gut Microbiome of the American Cockroach, Periplaneta americana, Is Stable and Resilient to Dietary Shifts. Appl. Environ. Microbiol. 2016, 82, 6603–6610. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.E.; Leonard, S.P.; Kwong, W.K.; Engel, P.; Moran, N.A. Genome-Wide Screen Identifies Host Colonization Determinants in a Bacterial Gut Symbiont. Proc. Natl. Acad. Sci. USA 2016, 113, 13887–13892. [Google Scholar] [CrossRef]
- Grünwald, S.; Pilhofer, M.; Höll, W. Microbial Associations in Gut Systems of Wood- and Bark-Inhabiting Longhorned beetles [Coleoptera: Cerambycidae]. Syst. Appl. Microbiol. 2010, 33, 25–34. [Google Scholar] [CrossRef]
- Rizzi, A.; Crotti, E.; Borruso, L.; Jucker, C.; Lupi, D.; Colombo, M.; Daffonchio, D. Characterization of the Bacterial Community Associated with Larvae and Adults of Anoplophora chinensis Collected in Italy by Culture and Culture-Independent Methods. BioMed Res. Int. 2013, 2013, 420287. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, M.-Y.; Kim, J.-W.; Lee, S.A.; Ahn, J.-H.; Song, J.; Kim, S.-H.; Weon, H.-Y. Effects of Diet Type, Developmental Stage, and Gut Compartment in the Gut Bacterial Communities of Two Cerambycidae Species (Coleoptera). J. Microbiol. 2017, 55, 21–30. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Mohammed, W.S.; Shagimardanova, E.I.; Vankov, P.Y.; Gogoleva, N.E.; Ziganshin, A.M. Fungal, Bacterial, and Archaeal Diversity in the Digestive Tract of Several Beetle Larvae (Coleoptera). BioMed Res. Int. 2018, 2018, 6765438. [Google Scholar] [CrossRef]
- Kolasa, M.; Ścibior, R.; Mazur, M.A.; Kubisz, D.; Dudek, K.; Kajtoch, Ł. How Hosts Taxonomy, Trophy, and Endosymbionts Shape Microbiome Diversity in Beetles. Microb. Ecol. 2019, 78, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.; Boddy, L.; Weightman, A.J. Bacteria in Decomposing Wood and Their Interactions with Wood-Decay Fungi. FEMS Microbiol. Ecol. 2016, 92, fiw179. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.A.; Lang, J.M.; Bhatnagar, S.; Eisen, J.A.; Kopp, A. Bacterial Communities of Diverse Drosophila Species: Ecological Context of a Host–Microbe Model System. PLOS Genet. 2011, 7, e1002272. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Moran, N.A. Links between Metamorphosis and Symbiosis in Holometabolous Insects. Philos. Trans. R. Soc. B 2019, 374, 20190068. [Google Scholar] [CrossRef]
- Paniagua Voirol, L.R.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial Symbionts in Lepidoptera: Their Diversity, Transmission, and Impact on the Host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut Microbiota of the Pine Weevil Degrades Conifer Diterpenes and Increases Insect Fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef]
- Adams, A.S.; Aylward, F.O.; Adams, S.M.; Erbilgin, N.; Aukema, B.H.; Currie, C.R.; Suen, G.; Raffa, K.F. Mountain Pine Beetles Colonizing Historical and Naïve Host Trees Are Associated with a Bacterial Community Highly Enriched in Genes Contributing to Terpene Metabolism. Appl. Environ. Microbiol. 2013, 79, 3468–3475. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Jones, R.T.; Sanchez, L.G.; Fierer, N. A Cross-Taxon Analysis of Insect-Associated Bacterial Diversity. PLoS ONE 2013, 8, e61218. [Google Scholar] [CrossRef]
- Ellegaard, K.M.; Engel, P. Genomic Diversity Landscape of the Honey Bee Gut Microbiota. Nat. Commun. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.-X.; Niu, Y.-M.; Ren, L.-L.; Zong, S.-X. Inheritance or Recruitment? The Assembly Mechanisms and Functional Dynamics of Microbial Communities in the Life Cycle of a Wood-Feeding Beetle. Mol. Ecol. 2025, 34, e17751. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, A.; Hortala, M.; Poole, P.S. Absolute Quantitation of Microbiota Abundance in Environmental Samples. Microbiome 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.K.; Chatterjee, S.; Sharma, S.; Mazumder, P.B.; Vairale, M.G.; Raju, P.S. Insect Gut Bacteria and Their Potential Application in Degradation of Lignocellulosic Biomass: A Review. In Bioremediation: Applications for Environmental Protection and Management; Varjani, S.J., Agarwal, A.K., Gnansounou, E., Gurunathan, B., Eds.; Springer: Singapore, 2018; pp. 277–299. ISBN 978-981-10-7485-1. [Google Scholar]
| Name of Component | Fungal-Based Diet, % | Standard Diet, % | Control Diet, % |
|---|---|---|---|
| Sawdust of Ulmus japonica | 24.0 | - | 90 |
| Distilled water | 62.9 | 10 | 10 |
| Wheat flakes and bran | - | 90 | - |
| Mycelium of Pleurotus citrinopileatus | 5.0 | - | - |
| Feed yeast | 2.0 | - | - |
| Ascorbic acid | 0.9 | - | - |
| Sucrose | 4.0 | - | - |
| Agar | 1.2 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baklanova, V.; Kuprin, A.; Baklanov, I.; Kumeiko, V. Isolation and Characterization of Cultivable Microbes from the Gut of Zophobas atratus (Coleoptera: Tenebrionidae) Larvae Reared on Two Types of Artificial Diets. Biology 2025, 14, 824. https://doi.org/10.3390/biology14070824
Baklanova V, Kuprin A, Baklanov I, Kumeiko V. Isolation and Characterization of Cultivable Microbes from the Gut of Zophobas atratus (Coleoptera: Tenebrionidae) Larvae Reared on Two Types of Artificial Diets. Biology. 2025; 14(7):824. https://doi.org/10.3390/biology14070824
Chicago/Turabian StyleBaklanova, Vladislava, Alexander Kuprin, Ivan Baklanov, and Vadim Kumeiko. 2025. "Isolation and Characterization of Cultivable Microbes from the Gut of Zophobas atratus (Coleoptera: Tenebrionidae) Larvae Reared on Two Types of Artificial Diets" Biology 14, no. 7: 824. https://doi.org/10.3390/biology14070824
APA StyleBaklanova, V., Kuprin, A., Baklanov, I., & Kumeiko, V. (2025). Isolation and Characterization of Cultivable Microbes from the Gut of Zophobas atratus (Coleoptera: Tenebrionidae) Larvae Reared on Two Types of Artificial Diets. Biology, 14(7), 824. https://doi.org/10.3390/biology14070824






