pH-Sensitive TRPC5 Is Differentially Expressed in Various Common Skin Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
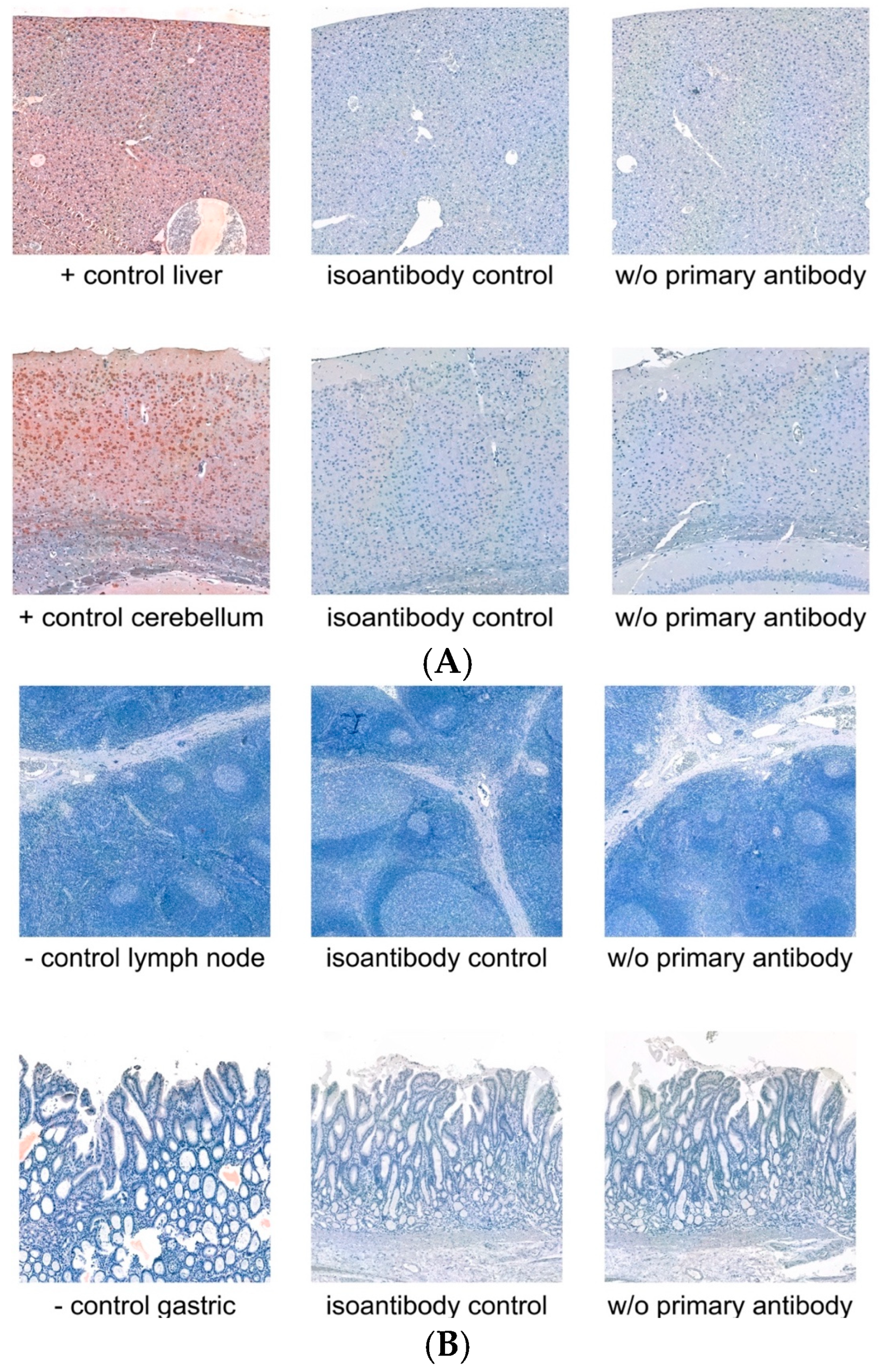
2.1. Immunohistochemistry
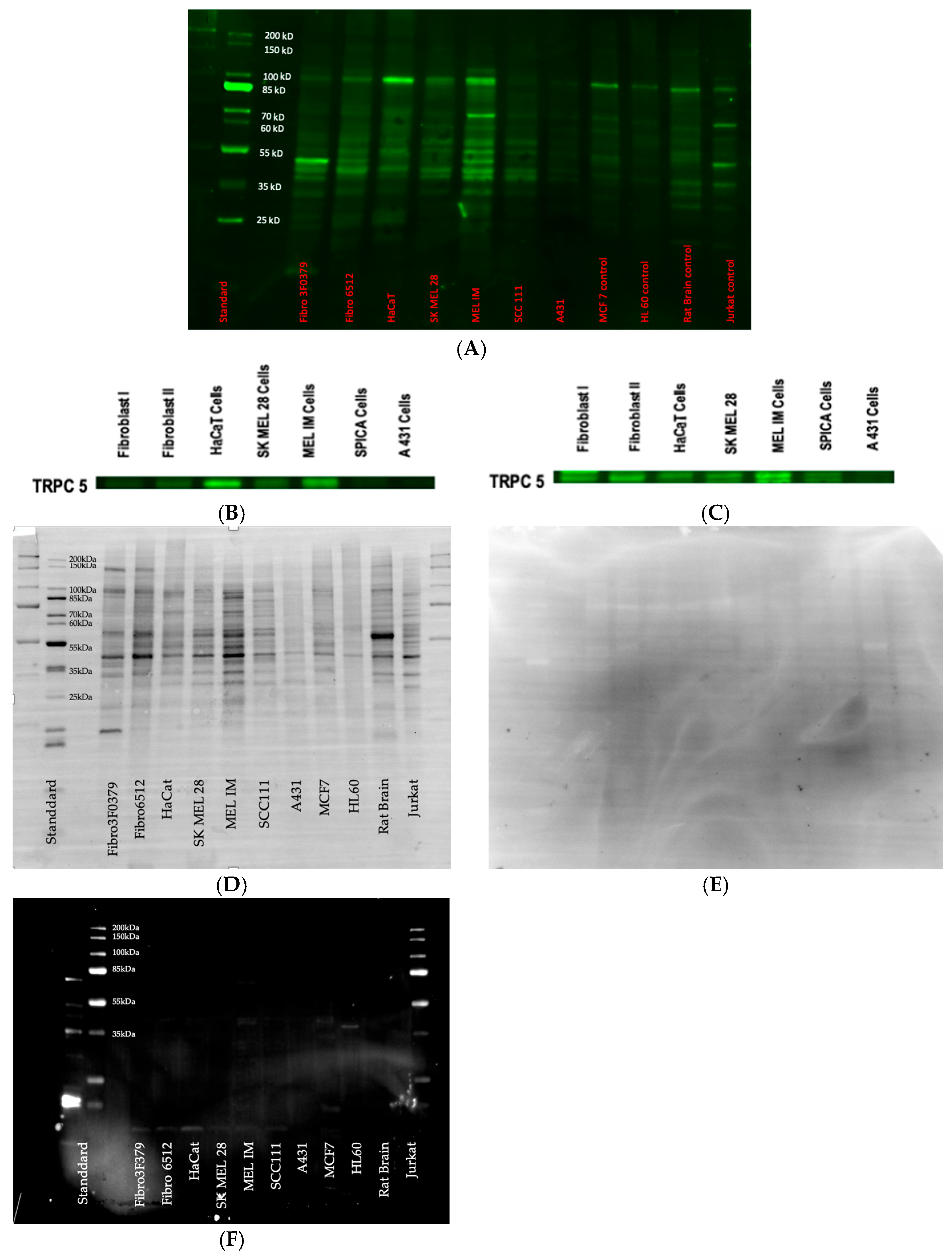
2.2. Western Blot
2.3. Immunfluorescence
2.4. Scoring
2.5. Statistics
3. Results
3.1. Controls
3.2. Immunfluorescence
3.3. Western Blot
3.4. Samples
3.5. BCC
3.6. SCC
3.7. NCN
3.8. MM
3.9. Statistical Analysis of TRPC5—Comparison of All Entities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7 (Suppl. S1), 5–19. (In English) [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. (In English) [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [CrossRef]
- Bolick, N.L.; Geller, A.C. Epidemiology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 57–72. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Kristiansen, I.S.; Thapa, S. Increasing melanoma incidence with unchanged mortality: More sunshine, better treatment, increased diagnostic activity, overdiagnosis or lowered diagnostic threshold? Br. J. Dermatol. 2024, 191, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. Morb. Mortal. Wkly. Rep.-NLM Cat. 2015, 64, 591–596. (In English) [Google Scholar]
- Damsky, W.E.; Bosenberg, M. Melanocytic nevi and melanoma: Unraveling a complex relationship. Oncogene 2017, 36, 5771–5792. (In English) [Google Scholar] [CrossRef]
- Viana, A.C.; Gontijo, B.; Bittencourt, F.V. Giant congenital melanocytic nevus. Bras. Dermatol. 2013, 88, 863–878. (In English) [Google Scholar] [CrossRef]
- Ackermann, K.; Wallner, S.; Brochhausen, C.; Schreml, S. Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors. Int. J. Mol. Sci. 2021, 22, 6024. (In English) [Google Scholar] [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. (In English) [Google Scholar] [CrossRef]
- Nassios, A.; Wallner, S.; Haferkamp, S.; Klingelhöffer, C.; Brochhausen, C.; Schreml, S. Expression of proton-sensing G-protein-coupled receptors in selected skin tumors. Exp. Dermatol. 2019, 28, 66–71. (In English) [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. (In English) [Google Scholar] [CrossRef]
- Weiß, K.T.; Fante, M.; Köhl, G.; Schreml, J.; Haubner, F.; Kreutz, M.; Haverkampf, S.; Berneburg, M.; Schreml, S. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 2017, 26, 127–132. (In English) [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. (In English) [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. (In English) [Google Scholar] [CrossRef]
- Martínez-Zaguilán, R.; Seftor, E.A.; Seftor, R.E.; Chu, Y.W.; Gillies, R.J.; Hendrix, M.J. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. (In English) [Google Scholar] [CrossRef]
- Kurz, B.; Michael, H.P.; Förch, A.; Wallner, S.; Zeman, F.; Decking, S.-M.; Ugele, I.; Hintschich, C.; Haubner, F.; Ettl, T.; et al. Expression of pH-Sensitive TRPC4 in Common Skin Tumors. Int. J. Mol. Sci. 2023, 24, 1037. (In English) [Google Scholar] [CrossRef]
- Stolwijk, J.A.; Sauer, L.; Ackermann, K.; Nassios, A.; Aung, T.; Haerteis, S.; Bäumner, A.J.; Wegener, J.; Schreml, S. pH sensing in skin tumors: Methods to study the involvement of GPCRs, acid-sensing ion channels and transient receptor potential vanilloid channels. Exp. Dermatol. 2020, 29, 1055–1061. (In English) [Google Scholar] [CrossRef]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. Biol. Sci. 2020, 287, 20201309. (In English) [Google Scholar] [CrossRef] [PubMed]
- Pattison, L.A.; Callejo, G.; Smith, E.S.J. Evolution of acid nociception: Ion channels and receptors for detecting acid. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190291. (In English) [Google Scholar] [CrossRef]
- Forch, A.; Wallner, S.; Zeman, F.; Ettl, T.; Brochhausen, C.; Schreml, S. Expression of Proton-Sensitive GPR31, GPR151, TASK1 and TASK3 in Common Skin Tumors. Cells 2021, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014, 14, 457–480. (In English) [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zhang, A.; Gupte, A.A.; Hamilton, D.J. The Role of Calcium Signaling in Melanoma. Int. J. Mol. Sci. 2022, 23, 1010. (In English) [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Bikle, D.D. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J. Biol. Chem. 2001, 276, 41079–41085. [Google Scholar] [CrossRef]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, W.; Guo, S.; Zuo, Z. Discovery of novel modulators targeting human TRPC5: Docking-based virtual screening, molecular dynamics simulation and binding affinity predication. J. Mol. Graph. Model. 2021, 102, 107795. (In English) [Google Scholar] [CrossRef]
- Mederos, Y.S.M.; Gudermann, T.; Storch, U. Emerging Roles of Diacylglycerol-Sensitive TRPC4/5 Channels. Cells 2018, 7, 218. [Google Scholar] [CrossRef]
- Tóth, B.I.; Oláh, A.; Szöllősi, A.G.; Bíró, T. TRP channels in the skin. Br. J. Pharmacol. 2014, 171, 2568–2581. (In English) [Google Scholar] [CrossRef]
- Hennings, H.; Kruszewski, F.H.; Yuspa, S.H.; Tucker, R.W. Intracellular calcium alterations in response to increased external calcium in normal and neoplastic keratinocytes. Carcinogenesis 1989, 10, 777–780. (In English) [Google Scholar] [CrossRef]
- Wang, T.; Ning, K.; Lu, T.X.; Hua, D. Elevated expression of TrpC5 and GLUT1 is associated with chemoresistance in colorectal cancer. Oncol. Rep. 2017, 37, 1059–1065. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ning, K.; Lu, T.-X.; Sun, X.; Jin, L.; Qi, X.; Jin, J.; Hua, D. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci. 2017, 108, 448–454. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cai, Y.; He, D.; Zou, C.; Zhang, P.; Lo, C.Y.; Xu, Z.; Chan, F.L.; Yu, S.; Chen, Y.; et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16282–16287. (In English) [Google Scholar] [CrossRef] [PubMed]
- He, D.X.; Ma, X. Transient receptor potential channel C5 in cancer chemoresistance. Acta Pharmacol. Sin. 2016, 37, 19–24. (In English) [Google Scholar] [CrossRef]
- Wu, S.; Singh, R.K. Resistance to chemotherapy and molecularly targeted therapies: Rationale for combination therapy in malignant melanoma. Curr. Mol. Med. 2011, 11, 553–563. (In English) [Google Scholar] [CrossRef]
- Piciu, F.; Balas, M.; Badea, M.A.; Cucu, D. TRP Channels in Tumoral Processes Mediated by Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1327. (In English) [Google Scholar] [CrossRef]
- Gaunt, H.J.; Vasudev, N.S.; Beech, D.J. Transient receptor potential canonical 4 and 5 proteins as targets in cancer therapeutics. Eur. Biophys. J. 2016, 45, 611–620. [Google Scholar] [CrossRef]
- Carson, C.; Raman, P.; Tullai, J.; Xu, L.; Henault, M.; Thomas, E.; Yeola, S.; Lao, J.; McPate, M.; Verkuyl, J.M.; et al. Englerin A Agonizes the TRPC4/C5 Cation Channels to Inhibit Tumor Cell Line Proliferation. PLoS ONE 2015, 10, e0127498. [Google Scholar] [CrossRef]
- Akbulut, Y.; Gaunt, H.J.; Muraki, K.; Ludlow, M.J.; Amer, M.S.; Bruns, A.; Vasudev, N.S.; Radtke, L.; Willot, M.; Hahn, S.; et al. (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew. Chem. Int. Ed. Engl. 2015, 54, 3787–3791. [Google Scholar] [CrossRef]
- Rubaiy, H.N.; Ludlow, M.J.; Siems, K.; Norman, K.; Foster, R.; Wolf, D.; A Beutler, J.; Beech, D.J. Tonantzitlolone is a nanomolar potency activator of transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2018, 175, 3361–3368. [Google Scholar] [CrossRef] [PubMed]



| Pairs | p-Value | Adj. p-Value |
|---|---|---|
| BCC-MM dermal | 0.351 | 1.000 |
| BCC-NCN dermal | 0.263 | 1.000 |
| BCC-MM epidermal | 0.039 | 0.585 |
| BCC-NCN epidermal | 0.001 | 0.018 |
| BCC-SCC | <0.001 | <0.001 |
| MM dermal-NCN dermal | 0.727 | 1.000 |
| MM dermal-SCC | <0.001 | 0.002 |
| NCN dermal-SCC | 0.005 | 0.077 |
| MM epidermal-NCN epidermal | 0.125 | 1.000 |
| MM epidermal-SCC | 0.007 | 0.112 |
| NCN epidermal-SCC | 0.487 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopmann, L.; Heider, J.; Niebel, D.; Evert, K.; Zeman, F.; Hammers, C.M.; Ettl, T.; Brochhausen, C.; Schreml, S. pH-Sensitive TRPC5 Is Differentially Expressed in Various Common Skin Tumors. Biology 2025, 14, 823. https://doi.org/10.3390/biology14070823
Hopmann L, Heider J, Niebel D, Evert K, Zeman F, Hammers CM, Ettl T, Brochhausen C, Schreml S. pH-Sensitive TRPC5 Is Differentially Expressed in Various Common Skin Tumors. Biology. 2025; 14(7):823. https://doi.org/10.3390/biology14070823
Chicago/Turabian StyleHopmann, Lara, Judith Heider, Dennis Niebel, Katja Evert, Florian Zeman, Christoph M. Hammers, Tobias Ettl, Christoph Brochhausen, and Stephan Schreml. 2025. "pH-Sensitive TRPC5 Is Differentially Expressed in Various Common Skin Tumors" Biology 14, no. 7: 823. https://doi.org/10.3390/biology14070823
APA StyleHopmann, L., Heider, J., Niebel, D., Evert, K., Zeman, F., Hammers, C. M., Ettl, T., Brochhausen, C., & Schreml, S. (2025). pH-Sensitive TRPC5 Is Differentially Expressed in Various Common Skin Tumors. Biology, 14(7), 823. https://doi.org/10.3390/biology14070823








