Effect of Acrylonitrile Butadiene Styrene (ABS) Secondary Microplastics on the Demography of Moina macrocopa (Cladocera)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Moina macrocopa and Chlorella vulgaris Maintenance
2.2. Preparation of ABS Microplastics
2.3. Ingestion of Moina macrocopa to ABS-MPs
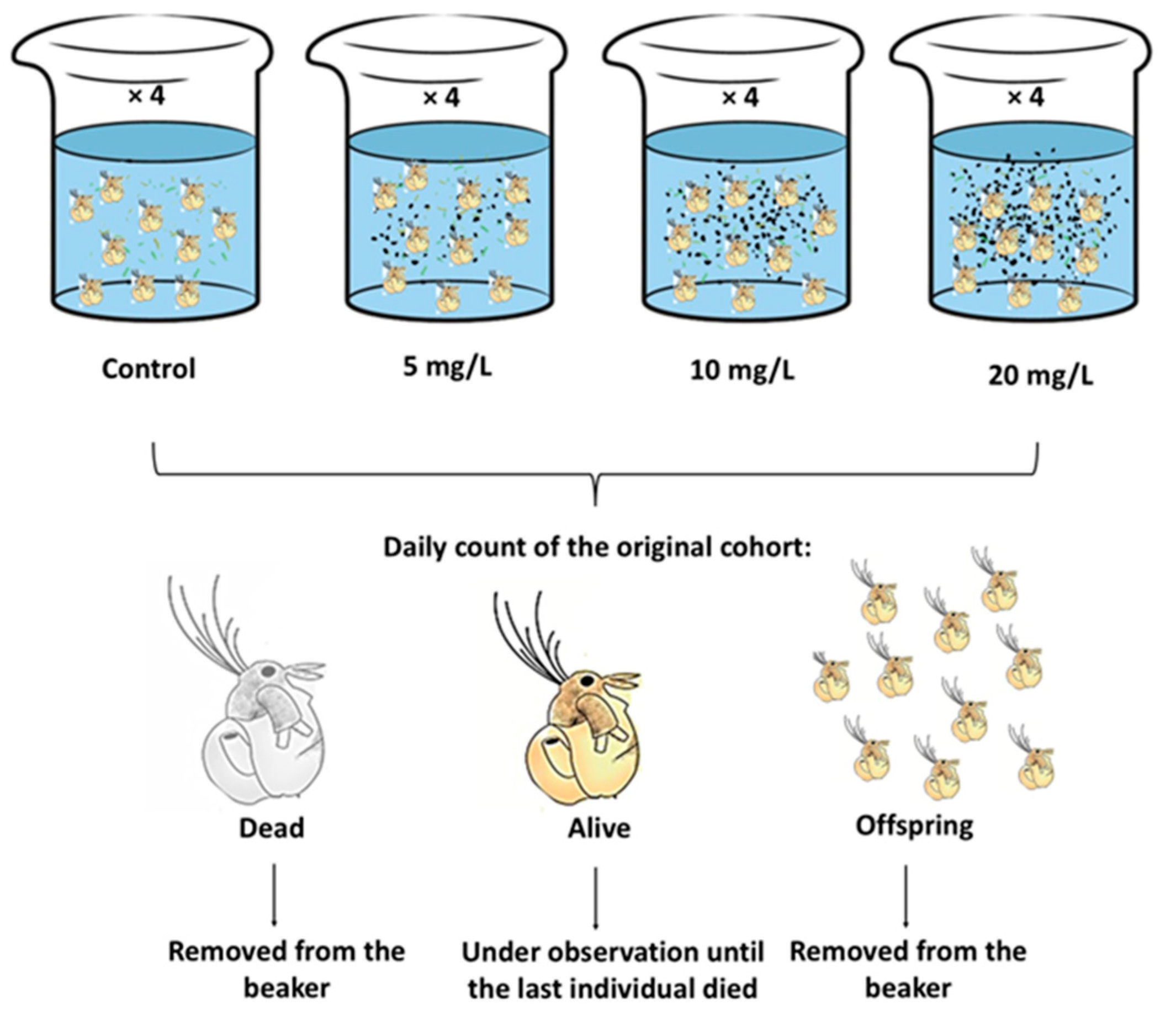
2.4. Exposure of Moina macrocopa to ABS-MPs
2.5. Statistical Analysis
3. Results
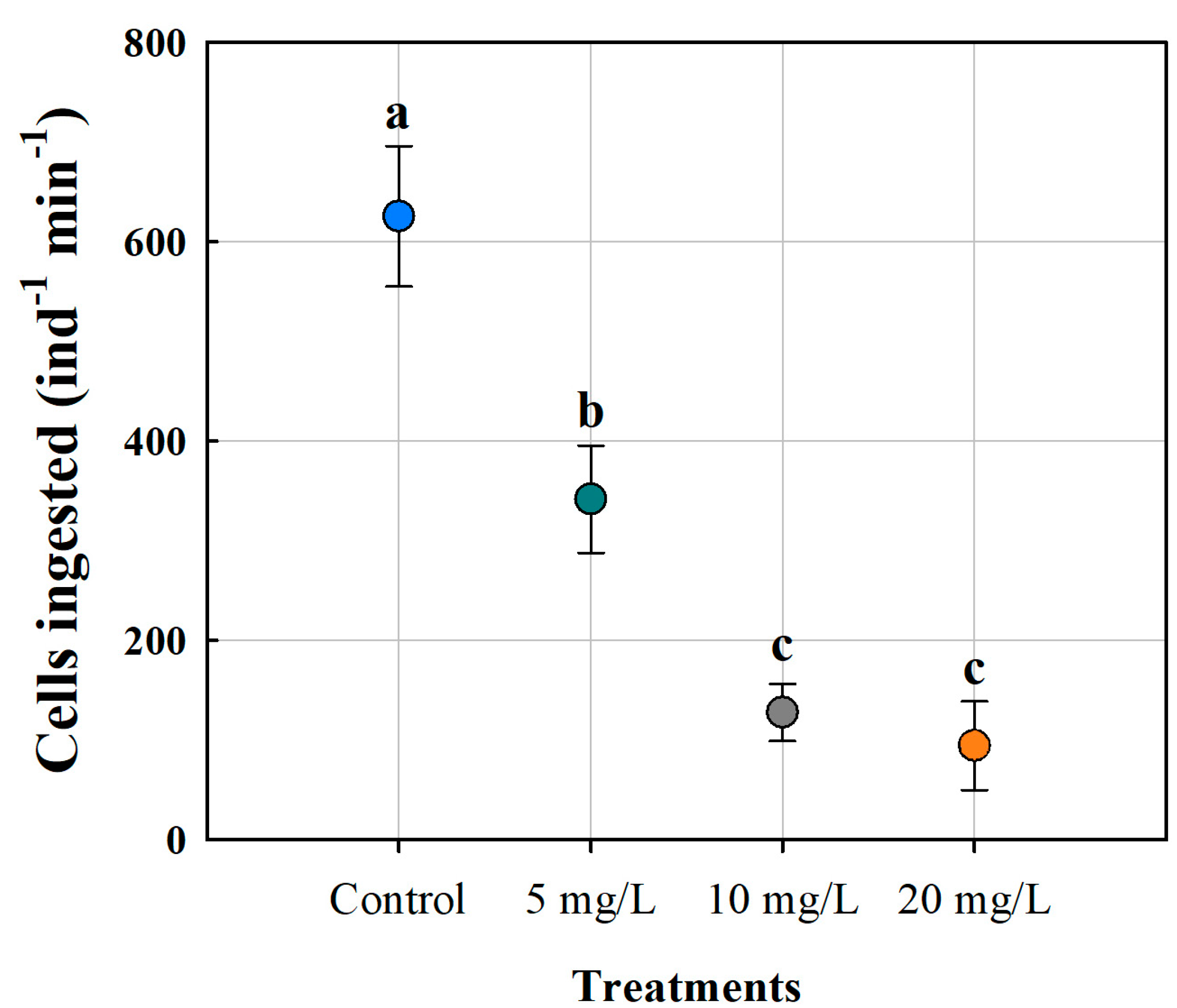
3.1. Cell Ingestion of Moina macrocopa
3.2. Survivorship
3.3. Mortality Rate
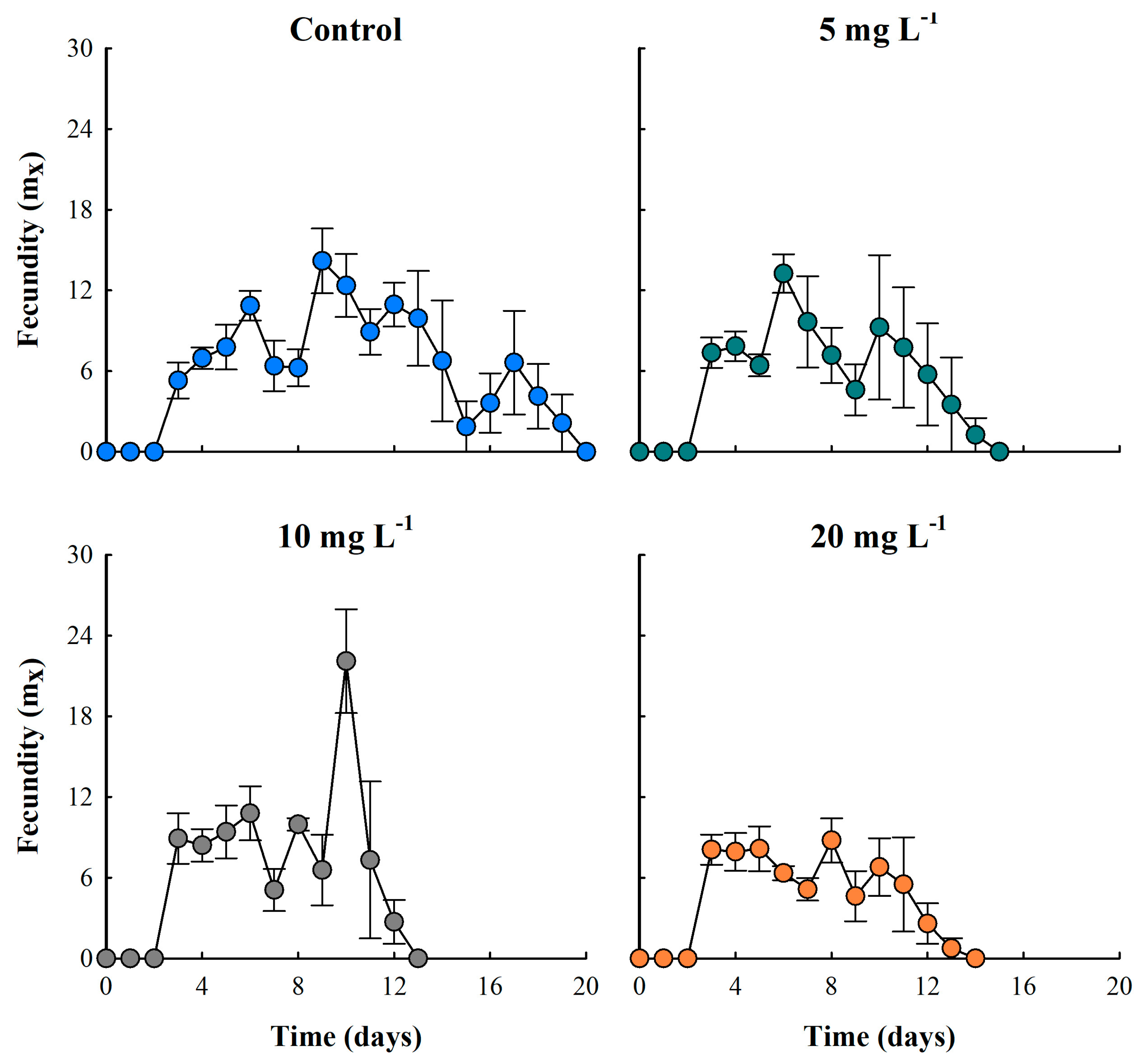
3.4. Fecundity
3.5. Life Table Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| ABS-MPs | Acrylonitrile butadiene styrene microplastics |
| PS | Polystyrene |
| PE | Polyethylene |
| PVC | Polyvinyl chloride |
| PET | Polyethylene terephthalate |
References
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Sakane, F.; Kinoshita, C.; Sato, K.; Mizukawa, K.; Takada, H. COVID-19-derived plastic debris contaminating marine ecosystem: Alert from a sea turtle. Mar. Pollut. Bull. 2022, 175, 113389. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Hamid, A.K.; Krebsbach, S.A.; He, J.; Wang, D. Critical review of microplastics removal from the environment. Chemosphere 2022, 293, 133557. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Hall, N.M.; Berry, K.L.E.; Rintoul, L.; Hoogenboom, M.O. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015, 162, 725–732. [Google Scholar] [CrossRef]
- Cunningham, E.M.; Ehlers, S.M.; Dick, J.T.; Sigwart, J.D.; Linse, K.; Dick, J.J.; Kiriakoulakis, K. High abundances of microplastic pollution in deep-sea sediments: Evidence from Antarctica and the Southern Ocean. Environ. Sci. Technol. 2020, 54, 13661–13671. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in lakes and rivers: An issue of emerging significance to limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Nava, V.; Chandra, S.; Aherne, J.; Alfonso, M.B.; Antão-Geraldes, A.M.; Attermeyer, K.; Leoni, B. Plastic debris in lakes and reservoirs. Nature 2023, 619, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Tassin, B. Sources and fate of microplastics in urban areas: A focus on Paris Megacity. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer: Heidelberg, Germany, 2017; pp. 69–84. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics are contaminants of emerging concern in freshwater environments: An overview. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer: Heidelberg, Germany, 2018; pp. 1–23. [Google Scholar] [CrossRef]
- Hurley, R.R.; Woodward, J.C.; Rothwell, J.J. Ingestion of microplastics by freshwater tubifex worms. Environ. Sci. Technol. 2017, 51, 12844–12851. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 12549. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton fecal pellets, marine snow, phytodetritus, and the ocean’s biological pump. Prog. Oceanogr. 2015, 130, 205–248. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Elliott, M.; Almeida, C.M.R.; Ramos, S. Microplastics and plankton: Knowledge from laboratory and field studies to distinguish contamination from pollution. J. Hazard. Mater. 2021, 417, 126057. [Google Scholar] [CrossRef]
- Cera, A.; Scalici, M. Freshwater wild biota exposure to microplastics: A global perspective. Ecol. Evol. 2021, 11, 9904–9916. [Google Scholar] [CrossRef]
- Lan, R.; Wei, Y.; Xue, R. Uptake of polystyrene microplastics by marine rotifers under different experimental conditions. Environ. Earth Sci. 2021, 687, 012071. [Google Scholar] [CrossRef]
- Huang, C.H.; Chu, T.W.; Kuo, C.H.; Hong, M.C.; Chen, Y.Y.; Chen, B. Effects of microplastics on reproduction and growth of freshwater live feeds Daphnia magna. Fishes 2022, 7, 181. [Google Scholar] [CrossRef]
- Zamora-Barrios, C.A.; Nandini, S.; Sarma, S.S.S. Effect of microplastics on the demography of Brachionus calyciflorus Pallas (Rotifera) over successive generations. Aquat. Toxicol. 2024, 275, 107061. [Google Scholar] [CrossRef] [PubMed]
- Frydkjær, C.K.; Iversen, N.; Roslev, P. Ingestion and egestion of microplastics by the cladoceran Daphnia magna: Effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of potential hazards from microplastic polymers in the marine environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef]
- Vignatti, A.M.; Cabrera, G.C.; Echaniz, S.A. Distribution and biological aspects of the introduced species Moina macrocopa (Straus, 1820) (Crustacea, Cladocera) in the semi-arid central region of Argentina. Biota Neotrop. 2013, 13, 86–92. [Google Scholar] [CrossRef]
- Weber, C.I. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 4th ed.; United States Environmental Protection Agency: Cincinnati, OH, USA, 1993; EPA/600/4-90/027F, xv.
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Uurasjärvi, E.; Hartikainen, S.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake. Water Environ. Res. 2020, 92, 149–156. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Kunej, U.; Skalar, T. Screening study of four environmentally relevant microplastic pollutants: Uptake and effects on Daphnia magna and Artemia franciscana. Chemosphere 2018, 208, 522–529. [Google Scholar] [CrossRef]
- Kumar, R.; Hwang, J.S. Ontogenetic shifts in the ability of the cladoceran, Moina macrocopa Straus and Ceriodaphnia cornuta Sars to utilize ciliated protists as food source. Int. Rev. Hydrobiol. 2008, 93, 284–296. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.K.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.K.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function, and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Reyes-Santillán, M.C.; Nandini, S.; Sarma, S.S.S. The combined effect of temperature and microplastics on Daphnia pulex Leydig, 1860 (Cladocera). Inland Waters 2025, 1. just-accepted. [Google Scholar] [CrossRef]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.A.; Parolini, M. Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilization of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef]
- Castro, D.G.D.; Destro, A.L.F.; Coimbra, E.C.L.; Silva, A.L.L.D.; Mounteer, A.H. Effects of PET microplastics on the freshwater crustacean Daphnia similis Claus, 1976. Acta Limnol. Bras. 2023, 35, e6. [Google Scholar] [CrossRef]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Yin, J.; Han, Y.; Yang, J.; Lu, X.; Xie, T.; Akbar, S.; Lyu, K.; Yang, Z. Molecular characterization of thioredoxin reductase in waterflea Daphnia magna and its expression regulation by polystyrene microplastics. Aquat. Toxicol. 2019, 208, 90–97. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D. Impact of microplastic beads and fibers on waterflea (Ceriodaphnia dubia) survival, growth, and reproduction: Implications of single and mixture exposures. Environ. Sci. Technol. 2017, 51, 13397–13406. [Google Scholar] [CrossRef]
- Enserink, L.; de la Haye, M.; Maas, H. Reproductive strategy of Daphnia magna: Implications for chronic toxicity tests. Aquat. Toxicol. 1993, 25, 111–123. [Google Scholar] [CrossRef]




| Source of Variation | DFs | SS | MS | F | p |
|---|---|---|---|---|---|
| Between Groups | 3 | 179,587.383 | 59,862.461 | 22.398 | <0.001 |
| Residual | 12 | 32,071.468 | 2672.622 | ||
| Total | 15 | 211,658.851 |
| Source of Variation | DFs | SS | MS | F | p |
|---|---|---|---|---|---|
| Average lifespan | |||||
| Between groups | 3 | 25.007 | 8.336 | 10.474 | 0.001 |
| Residual | 12 | 9.550 | 0.796 | ||
| Total | 15 | 34.557 | |||
| Net reproductive rate | |||||
| Between groups | 3 | 2114.262 | 704.754 | 10.429 | 0.001 |
| Residual | 12 | 810.915 | 67.576 | ||
| Total | 15 | 2925.177 | |||
| Generation time | |||||
| Between groups | 3 | 12.974 | 4.325 | 5.826 | 0.011 |
| Residual | 12 | 8.908 | 0.742 | ||
| Total | 15 | 21.882 | |||
| Population growth rate | |||||
| Between groups | 3 | 0.0191 | 0.00638 | 0.872 | 0.483 |
| Residual | 12 | 0.0879 | 0.00732 | ||
| Total | 15 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manríquez-Guzmán, D.L.; Chaparro-Herrera, D.d.J.; Ramírez-García, P.; Zamora-Barrios, C.A. Effect of Acrylonitrile Butadiene Styrene (ABS) Secondary Microplastics on the Demography of Moina macrocopa (Cladocera). Biology 2025, 14, 555. https://doi.org/10.3390/biology14050555
Manríquez-Guzmán DL, Chaparro-Herrera DdJ, Ramírez-García P, Zamora-Barrios CA. Effect of Acrylonitrile Butadiene Styrene (ABS) Secondary Microplastics on the Demography of Moina macrocopa (Cladocera). Biology. 2025; 14(5):555. https://doi.org/10.3390/biology14050555
Chicago/Turabian StyleManríquez-Guzmán, Diana Laura, Diego de Jesús Chaparro-Herrera, Pedro Ramírez-García, and Cesar Alejandro Zamora-Barrios. 2025. "Effect of Acrylonitrile Butadiene Styrene (ABS) Secondary Microplastics on the Demography of Moina macrocopa (Cladocera)" Biology 14, no. 5: 555. https://doi.org/10.3390/biology14050555
APA StyleManríquez-Guzmán, D. L., Chaparro-Herrera, D. d. J., Ramírez-García, P., & Zamora-Barrios, C. A. (2025). Effect of Acrylonitrile Butadiene Styrene (ABS) Secondary Microplastics on the Demography of Moina macrocopa (Cladocera). Biology, 14(5), 555. https://doi.org/10.3390/biology14050555





