Simple Summary
This review explores the role of primary cilia and extracellular vesicles in neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s diseases. We discuss how ciliogenesis can disrupt important signaling pathways, as well as its involvement in the aggregation of neurotoxic proteins and the trafficking and clearance of these proteins. We also highlight the promising and novel diagnostic and therapeutic approaches currently being developed to detect or help slow the progression of these diseases.
Abstract
Neurodegenerative diseases (NDDs), including Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD), share pathologic mechanisms including oxidative stress, mitochondrial dysfunction, and protein aggregation. However, they differ in age of onset and clinical progression. Emerging evidence highlights primary cilia (PC) as a key regulator of neuronal aging and the progression of these diseases. Dysfunctional PC may impair key signaling pathways, such as Sonic Hedgehog (Shh) and Wnt, promote oxidative stress, mitochondrial damage, and epigenetic instability. PC may also influence intercellular communication by regulating the biogenesis of exosomes and modulating tunneling nanotube (TNT) formation, both of which propagate toxic proteins between neurons. Mechanistically, the regulation of ciliary length is disrupted in AD, which leads to ciliary dysfunction that interferes with signaling pathways and promotes the aggregation of amyloid-beta. This amyloid-beta is then propagated through TNTs and exosomes, spreading neuronal damage. In PD, the accumulation of alpha-synuclein (α-syn) also impairs cilia function, thereby compromising the cell’s response to oxidative stress. This results in the formation of abnormal TNTs and defective exosome-mediated clearance, ultimately contributing to neurodegeneration. Similarly, the mutant huntingtin protein aggregates within primary cilia in HD, morphologically disrupting them by obstructing intraflagellar transport. Damaged cilia are also associated with increased TNT formation and the exosomal release of toxic proteins, which leads to mitochondrial and epigenetic instability, ultimately promoting neuronal aging. Together, targeting ciliary function and its downstream regulation of TNTs and exosomes may provide a novel approach for slowing or halting disease progression across neurodegenerative diseases.
1. Introduction
Neurodegenerative diseases (NDDs) constitute some of the most debilitating diseases facing aging populations today, due to both severity and significant public health ramifications. Despite extensive research efforts, the incidence of NDDs has steadily increased, particularly in Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD). For AD, the most prevalent NDD, concern extends beyond its rising incidence to include a substantial increase in mortality, which has escalated by approximately 140% between 2000 and 2021 [1]. This trend stands in stark contrast to the decline observed over the same period in other leading causes of death in the United States, including stroke, heart disease, and human immunodeficiency virus (HIV) [2]. While the severity of these diseases stems in part from their rising incidence, their profound impact on daily life, as well as the substantial economic burden they impose, highlights the urgency of understanding their pathogenesis and potential intersections [3]. The pathogenesis of AD, PD, and HD can conceptually be broken down into three phases: (1) creation of an abnormal protein, (2) aggregation or lack of clearance, and (3) propagation. This triphasic framework is also shared by other NDDs, such as amyotrophic lateral sclerosis [4,5]. Thus, while these three phases are not exclusive to AD, PD, and HD, they provide essential context for the two primary reasons these three diseases are emphasized over other NDDs in this review.
Although different regions of the brain are impacted, there are common and unique characteristics of AD, PD, and HD. While PD and HD both demonstrate motor impairment symptoms that are not typically seen in AD, both AD and PD exhibit memory impairment, which is also observed in HD. However, in these three diseases, the accumulation of neurotoxin proteins is a common feature (Figure 1). In addition, there are distinct linkages between AD, PD, HD, and the dysfunction of primary cilia (PC), as well as two forms of intercellular transport, including tunneling nanotubes (TNTs), and exosomes [6,7,8,9]. PC are microtubule-based, non-motile organelles that extend from the surface of most vertebrate cell types. They act as sensory structures that regulate diverse signaling pathways essential for development and homeostasis [10,11]. This immense structural and functional organelle relies on the coordinated trafficking and temporal localization of specific receptors and their associated signal transduction modules in the cilium [12]. As a center for both sensory and signaling functionality, neuronal pathways like the Sonic Hedgehog (Shh) and Wingless-related integration site (Wnt) are coordinated by PC, allowing for neural tube patterning, synapse formation, neuronal migration, and a host of other functions [13,14,15,16]. To ensure that PC can flexibly conduct the broad array of signaling pathways necessary for proper cellular functioning, cells have evolved diverse mechanisms to monitor and fine-tune cell-type-specific signaling outputs from their cilia in varying developmental and environmental cues [17]. With such a predominant role in cellular signaling, exploring PC’s connection to intercellular transport, and hence communication, is highly warranted. Further, TNTs are thin, F-actin-rich membrane bridges, which are transient and versatile structures that facilitate the bidirectional intercellular transfer of various cargoes, including organelles, plasma membrane components, pathogens, and calcium [18,19]. Exosomes are extracellular vesicles (EVs) derived from endosomes within the cell and whose contents vary depending on their site of origin. Exosomes carry a complex cargo of proteins, lipids, nucleic acids, and other biomolecules that enable them to mediate intercellular communication, modulate signaling pathways, and reflect the molecular signature of their parent cells [20]. Thus, exosomes are produced by almost all cell types [21,22,23,24,25,26]. The functions of exosomes in the brain can be protective or degenerative, depending on the contents and biological signals that they carry [27,28]. This is especially true if the aggregated neurotoxic proteins incur damage to PC, a vital component of neural signaling pathways [29]. Recent proteomic and transcriptomic analyses have revealed disease-specific EV biomarkers across major neurodegenerative disorders. In AD, EVs are enriched in APP fragments, tau, and synaptic proteins; in PD, they carry citrullinated protein signatures, α-syn, SNAP23, and calbindin; and in HD, they contain N-terminal fragments of mutant huntingtin protein with expanded polyglutamine repeats [30,31,32].
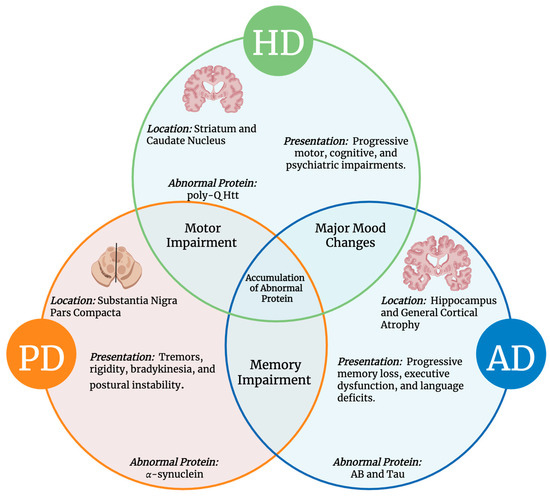
Figure 1.
Common and Unique Features of AD, PD, and HD. The Venn diagram illustrates overlapping and distinct aspects of these neurodegenerative disorders, including disease location, clinical presentation, hallmark symptoms, and associated neurotoxic proteins [33].
Collectively, PC, TNTs, and EVs form an interconnected network of cellular communication that plays a major role in neuronal development. Functionally, PC integrates extracellular cues into intracellular signaling pathways, thereby influencing how cells respond to morphogens and growth factors [9]. TNTs enable rapid and direct exchange of cytoplasmic components such as ions, vesicles, and organelles, providing a structural basis for long-range cell-to-cell transfer [34]. EVs further complement these processes by transporting molecules across the extracellular space, enabling stable intercellular communication and key protein distributions [30,31]. Importantly, emerging evidence suggests that these pathways may not act in isolation; for example, EVs can induce ciliary signaling pathways and ciliogenesis, ciliary signaling can regulate vesicle release, and TNTs have been shown to transfer vesicles between cells [35,36]. Thus, the interplay among PCs, TNTs, and EVs reflects a hierarchical and cooperative system in which localized, direct, and extracellular modes of communication reinforce one another to coordinate complex cellular behaviors.
While the pathways leading to ciliary dysfunction vary across AD, PD, and HD, the downstream effects remain consistent, where impaired primary cilia amplify aging-related damage through three key processes: (1) oxidative stress, (2) mitochondrial dysfunction, and (3) epigenetic alterations [34,35]. However, there is currently a gap in the literature that explores the interplay between PC, TNTs, and EVs in the context of these three diseases. Therefore, in this review, we examine the role of PC as a key regulator of neuronal development and their involvement in intercellular communication via TNTs and EVs. Additionally, this review will discuss both the common and unique elements of epidemiology, pathogenesis, preventive measures, diagnostic strategies, and therapeutic applications related to these three diseases.
2. Methods
For this literature review, a comprehensive search of existing studies on TNTs, exosomes, and primary cilia in neurodegenerative diseases was conducted using the academic databases PubMed and Google Scholar. The search terms included “tunneling nanotubes,” “exosomes,” “primary cilia,” “neurodegenerative diseases,” “Alzheimer’s disease,” “Huntington’s disease,” “Parkinson’s disease,” “ciliopathy,” and “cilia.”
Literature was screened for studies that addressed the intersection of TNTs, exosomes, and primary cilia in the context of neurodegenerative diseases. Exclusion criteria included all other neurodegenerative diseases, such as “Frontotemporal Dementia”, “Amyotrophic Lateral Sclerosis”, and “Lewy Body Dementia”, among others.
Both original research articles and review papers were included to capture important findings and emerging perspectives. Additional studies identified through the reference lists of selected papers were included when relevant. Emphasis was placed on literature addressing protein aggregation, cellular communication, and alterations in ciliary signaling pathways relevant to disease progression.
3. Epidemiology and Clinical Presentations
AD has emerged as one of the leading causes of physical and cognitive disability globally, accounting for almost 80% of all reported dementia diagnoses [36,37,38,39]. With its rising prevalence, AD is quickly becoming one of the most costly and lethal neurodegenerative diseases, placing a significant burden on healthcare systems and long-term care facilities. AD is marked by a slow decline in cognitive abilities, including memory, executive function, and language skills [40,41]. Early in AD progression, damage to neuronal connections occurs primarily in the medial temporal lobe and neocortical areas (Figure 2B) [42,43].
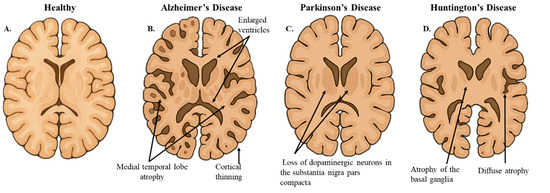
Figure 2.
Hallmarks of Neurodegenerative Brain Morphology. (A). The illustrative image represents a coronal section of healthy brain morphology. (B). In AD, the coronal slice shows cortical thinning and medial temporal lobe atrophy. (C). In PD, the coronal slice highlights the pallor of the substantia nigra, indicative of dopaminergic neuron loss. (D). In HD, the coronal slice demonstrates diffuse cerebral atrophy and atrophy of the basal ganglia. This figure was created using OpenAI GTP-4o.
PD, the second most common neurodegenerative disease, follows a similar pattern to AD, albeit on a smaller scale [44]. A global study analyzing trends in PD reports that the incidence of the disease has increased by an average of 61% per year from 1990 to 2019 [45]. PD is associated with four cardinal motor symptoms, including tremors, rigidity, bradykinesia, and postural instability, alongside the characteristic shuffling and stooped posture of the “Parkinsonian gait” [46]. Progressive neurodegeneration is most pronounced within the nigrostriatal dopaminergic neurons of the substantia nigra pars compacta, a region of the brain associated with movement control and reward behaviors (Figure 2C) [47].
HD, in contrast to AD and PD, is a rarer but highly severe neurodegenerative disorder with a global prevalence of 0.48 per 100,000 person-years (2010–2022), and a U.S. prevalence of 1.76 per 100,000 person-years in the same period [48]. Unlike AD and PD, HD follows an autosomal dominant inheritance pattern and presents earlier in life, typically between 30 and 50 years [49,50]. HD is primarily characterized by progressive motor, cognitive, and psychiatric impairments [51]. Markedly, the frontostriatal circuits are among the earliest and most consistently affected brain regions in HD, followed by more widespread atrophy of the frontal and temporal regions, and eventually deeper subcortical structures, including the limbic system and cerebellum (Figure 2D) [52,53].
Emerging evidence from both mouse models and post-mortem analyses of human brains indicates changes in the morphology of PC, with several studies reporting differences in ciliary length and abundance [54,55]. While these alterations are consistently observed in NDDs, the relationship between clinical manifestations and ciliogenesis remains correlational rather than causative.
4. Mechanisms and Etiology
Aging is widely regarded as the most significant risk factor influencing the NDDs’ progression, severity, and impact [56]. This is due to the brain’s limited regenerative capacity compared to tissues, such as skeletal muscle and skin, which rely on stem cells to repair damage [57,58]. When neurons and oligodendrocytes sustain age-related damage through altered DNA methylation, mitochondrial dysfunction, and reactive oxygen species (ROS), their lack of regenerative capacity renders them more susceptible to irreversible damage compared to other cell types [59,60]. In AD, PD, and HD, aging has also been associated with increased telomere attrition [61,62,63]. Consequently, the absence of telomerase activity, coupled with poor regenerative capacity, creates an environment that is conducive to the progression of neurodegenerative disease. Despite their shared pathological stages, these diseases display striking differences in the average age of onset [64,65,66,67]. As discussed later in this review, age-related cellular stressors not only heighten neuronal vulnerability but also alter PC function and modulate EV and TNT-mediated propagation of pathogenic proteins, further shaping NDDs progression.
4.1. Alzheimer’s Disease Pathogenesis
AD is a multifactorial, progressive neurodegenerative disorder primarily affecting the brain’s memory and cognitive functions. Pathogenesis of AD has been linked to genetic alterations and the accumulation of two proteinaceous lesions in the brain, amyloid-beta plaques (Aβ) and abnormally phosphorylated tau tangles, that disrupt neural communication and impair synaptic transmission. These changes interrupt neuronal processes and extend to their larger networks, impeding communication, metabolism, and repair [68]. Together, Aβ plaques and tau tangles are referred to as the amyloid tau neurodegeneration (ATN) biomarkers [69]. Genetic mutations associated with AD, such as those in the APP gene on chromosome 21, PSEN1 and PSEN2 on chromosomes 14 and 1, respectively, result in altered processing of APP and excessive production of Aβ, which aggregates into plaques [70]. These plaques, in turn, are thought to trigger neuroinflammation and neuronal toxicity, initiating a cascade of events that lead to neuronal death [71]. The other proteinaceous lesion associated with AD, Tau, a protein that normally stabilizes microtubules, is critical to guiding nutrients and molecules from the cell body to the axon and dendrites [72]. In AD, tau becomes hyperphosphorylated, causing it to detach from microtubules and form neurofibrillary tangles with other tau molecules [73]. These tangles disrupt axonal transport, leading to a loss of synapses and further neuronal death [74]. As neuronal damage increases, neuronal networks break down, and susceptible areas of the brain begin to atrophy [75]. The resulting neurodegeneration predominantly affects the hippocampus, the brain region critical for memory formation, contributing to the hallmark cognitive decline seen in AD patients [76,77]. Another study has also linked impaired neurogenesis to AD-related memory and cognitive deficits [78]. As the disease progresses, atrophy spreads to the temporal, parietal, and frontal lobes of the brain, leading to widespread cortical dysfunction [79,80].
Primary Cilia, Exosomes, and TNTs in Alzheimer’s Disease
Degeneration of ciliary signaling pathways reduces cell proliferation and contributes to age-related diseases, such as AD [81]. Microglia, the brain’s phagocytic cells, maintain homeostasis by responding to injury, pruning synapses, and promoting tissue repair [82,83]. In AD mouse models, both the number and morphology of microglial PC are significantly reduced [84,85,86]. Disease-associated microglia (DAM) actively phagocytose dystrophic neurites and Aβ plaques [87,88]. Notably, inhibition of primary cilia in septal microglia leads to larger Aβ plaque accumulation and increased neurite damage [84]. Further, APP has been demonstrated to localize to the PC along with the Shh signaling component Smo [89]. In addition, brain regions with greater Aβ deposition fail to show the normal age-dependent increase in neuronal cilia length [90]. Interestingly, a study found that dentate granule cell primary cilia were shortened by ~50% only in models expressing both Aβ and tau, whereas models expressing Aβ alone or tau alone showed no significant change [9]. Additionally, the most supported mechanism for Aβ-induced cilia shortening involves structural distortion and disruption of Shh signaling, which is essential for ciliary structure and elongation [91]. Moreover, the alterations in cilia length and frequency correlate with Aβ plaque burden and disease severity [91]. AD pathogenesis begins with APP being cleaved abnormally by a β or ɣ-secretase, producing Aβ, which later aggregates to form plaques [92,93,94,95]. Tau, a microtubule-associated protein, subsequently becomes hyperphosphorylated and forms intracellular tangles [73,96]. APP and other AD-related proteins accumulate at the ciliary basal body, contributing to shortening of cilia and subsequent dysfunction of ciliary signaling and neuroprotective pathways [29,89,97]. Ultimately, Aβ and tau propagate to other neurons via TNT and exosomes [98,99,100,101].
Furthermore, PC are key regulators of autophagy-endolysosomal pathways, and when ciliary-autophagic trafficking is disturbed, ciliary cargo can be redirected toward EV secretion [102,103,104]. These pathway alterations enhance the EV-mediated export of pathogenic proteins, including Aβ and tau, facilitating their propagation between cells [98,99]. Concurrently, AD-related cellular stressors, such as Aβ exposure, activate actin remodeling pathways that may drive TNT formation, enabling direct neuron-to-neuron transfer of pathological tau protein [100,101]. These coordinated defects in ciliary signaling and intracellular trafficking may collectively facilitate the EV and TNT-dependent propagation of neurotoxic proteins across neural networks, thereby potentially reinforcing progressive circuit dysfunction (Figure 3A) [98,100,101,105].
Chronic inflammation in the brain may be associated with the buildup of malfunctioning glial cells, which are normally responsible for clearing debris [83,106]. Studies in both animal and human models show that increased Aβ plaque burden correlates with dysregulation of key ciliary signaling pathways, including Shh and Wnt [107,108,109,110]. The Shh pathway displays upregulation of its components, Shh, SMO, GLI1, and GLI2, in the hippocampus of human and mouse AD models, whereas PTCH, GLI3, and Wnt signaling components are downregulated [111,112]. Similar to other neurodegenerative diseases, dysfunction of these cilia-dependent pathways in AD has been associated with disturbances in critical homeostatic processes governed by PC [113,114].
In physiological conditions, exosomes assist neuronal function and intercellular communication, serving as transporters for lipids, proteins, miRNA, and mRNA [115]. However, in damaged environments, like AD, exosomes are involved in the transportation of damaging proteins like Aβ and Tau [116]. Further, an intracranial injection of exosomes from AD mice brains into WT mice caused marked mitochondrial damage and neurotoxicity [117]. The exosomes isolated from the AD mice showed significantly higher levels of tau and Aβ 1–42 proteins [117]. A recent article introduced TNTs as potential contributors to the pathogenesis of AD [118]. As mentioned above, TNTs may play a significant role in the spreading of misfolded proteins like Aβ and Tau, which are central to AD pathology [100]. TNTs allow the direct transfer of these pathogenic aggregates between neurons, bypassing the extracellular space where they might be cleared by immune cells [101,119]. This facilitates the propagation of AD pathology in a predictable, spatiotemporal manner, supporting the progression of the disease. Moreover, TNT formation is often associated with oxidative stress, another key feature of AD [120,121,122]. Given their role in intercellular communication and the spread of protein aggregates, TNTs are emerging as potential therapeutic targets for slowing the disease’s progression [100,123,124].
4.2. Parkinson’s Disease Pathogenesis
The etiology of PD is currently thought to be multifactorial. However, the exact mechanism underlying Parkinsonian symptoms, including bradykinesia, tremor, and postural instability, remains largely unclear. The current understanding of PD pathogenesis suggests that a combination of genetic and environmental factors contributes to the progressive neurodegeneration of nigrostriatal dopaminergic neurons in the pars compacta of the substantia nigra, as well as other affected cortical regions [125,126]. This lack of dopamine is understood to be the primary cause of many of the movement disorders [127,128].
One of the main mediators of this neurodegeneration is the α-syn protein. Derived from 140 amino acids, α-syn is made of three main groups: a highly conserved N-terminal, a hydrophobic non-amyloid β compound, and an unstructured C-terminus [129]. Similar to Aβ and Tau protein in AD, α-syn causes extensive damage when it becomes misfolded [130]. In PD, aggregation of α-syn leads to the formation of Lewy Bodies, a notable characteristic marker of the disease [131]. The aggregation of abnormal Lewy Bodies causes resulting damage and neuroinflammation, leading to deficits and disruption in neurotransmitter signaling [132]. Notably, although α-syn appears early in peripheral tissues, brain damage in PD begins in the brainstem and spreads to the cortex [125,133,134,135]. While the misfolding of α-syn protein is considered to be the main issue in PD, its impact is further exacerbated when it is propagated and not cleared properly [136].
4.2.1. Genetic Relevance in Parkinson’s Disease
Despite most cases of PD resulting from sporadic mutations, inherited PD remains a legitimate concern and is highly correlated with many genes, including Synuclein alpha (SNCA), Leucine-rich Repeat Kinase 2 (LRRK2), Parkin (PRKN), PTEN Induced Kinase 1 (PINK1), VPS35 Retromer Complex Component (VPS35), and Vacuolar Protein Sorting 13 Homolog C (VPS13C) [137]. While α-syn does the most damage, mutation of the gene responsible for its production, the SNCA gene, does not have sole responsibility for the manifestation of PD. In fact, no single gene has complete penetrance in PD, but rather, different combinations of genes have been shown to have an effect in causing PD [138]. Given the three-pronged approach to pathology that α-syn has, this stands to bear reason.
Beyond SNCA mutations, another key gene implicated in PD pathogenesis is PINK1, which encodes a protective mitochondrial Serine/Threonine kinase [139]. Collectively, mutations in these two genes illustrate dual problems in PD pathogenesis: SNCA mutations disrupt protein formation and PNK1 mutations disrupt clearance of these misfolded proteins. Normally, PINK1 serves a protective role by helping to produce the PTEN-induced kinase protein, which shields mitochondria from damage caused by the reactive oxygen species (ROS) [140]. In PD, where there is an overexpression of α-syn, there is an increase in ROS generation, and PINK1 ameliorates the resulting damage. However, when PINK1 is mutated, it loses its function, which means there is no longer any protection against ROS [141]. Thus, this lack of PINK1 leads to the accumulation of α-syn, which further damages the mitochondria [142].
4.2.2. Primary Cilia, Exosomes, and TNTs in Parkinson’s Disease
While α-syn’s role in causing physical damage is a pivotal piece of PD, α-syn has also been shown to further this neurological ailment through faults in cellular signaling and autophagy due to ciliary dysfunction [143]. Emerging evidence has shown that the LRRK2 gene is associated with both α-syn accumulation and PC [144]. The mutation of LRRK2 leads to the hyperactivation of Rab-GTPases, which have been directly shown to decrease ciliogenesis [145].
Furthermore, α-syn aggregates to form complexes called aggresomes that localize adjacent to the centrosome, or the basal body of the cilium, which inhibit ciliary function and ciliogenesis [143]. Next, the loss of cilia function in PD, driven by pathogenic LRRK2 accumulation, disrupts Shh signaling, causing dopaminergic neurons to lose vital growth and survival support, thereby increasing their vulnerability to degeneration [146,147,148]. Similar to AD, disruption of cilia–autophagy crosstalk in PD has been shown to cause neurons to redirect α-syn into EVs, increasing its intercellular transfer, while autophagy inhibition further amplifies α-syn-containing EV release and spread [149,150]. Concurrently, α-syn fibrils damage lysosomes and propagate between neurons via lysosome-associated cargo transported through TNTs, providing a TNT-dependent route for pathology spread [151,152]. Because TNT formation relies on actin-dependent machinery (e.g., M-Sec/RalA/exocyst) and ciliary Shh signaling regulates neuronal actin dynamics, ciliary dysfunction may promote TNT formation, while impaired autophagy increases EV loading, collectively enhancing α-syn dissemination (Figure 3B) [102,149,150,153,154,155].
Currently, there are two main proposed mechanisms for neurodegenerative spread. First, TNTs have long been postulated to play a role in both communication and transportation between distant cells [156]. A recent study has shown that TNTs can indeed transfer cellular components between cells [157]. In PD, a primary cell can get overloaded with α-syn and then use TNTs to dispose of the excess protein, which then gets spread to other cells [118]. Second, the propagation of α-syn has been proposed to be trafficked by EVs. Notably, vesicles transfer a diverse cargo of cellular materials, fuse with the plasma membrane, and are then released to target other cells [156]. While TNTs represent a more localized and direct transfer of α-syn, exosomes have been shown to spread contents of one cell, such as organelles, plasma membrane components, pathogens, and calcium, to another across far distances [20]. Microglia-derived exosomes have also been shown to carry and transfer pathogenic α-syn species, potentially further contributing to this spread [158]. Moreover, strain-specific α-syn conformations exhibit distinct propensities for exosomal packaging, likely driving variability in “prion-like” propagation [159]. However, the exosomes implicated in PD pathogenesis are not limited to transferring only α-syn. Emerging evidence indicates that EVs may also transfer faulty genetic material, such as miR-1, miR-19b, miR-153, and miR-409-3p, to other cells, suggesting an additional mechanism for disease propagation [160].
4.3. Huntington’s Disease Pathogenesis
Huntington’s Disease arises from an expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats in the HTT gene (formerly IT15) of chromosome 4p16.3, leading to the production of a dysfunctional huntingtin protein (polyQ-HTT) [161,162]. The unstable CAG repeats in exon 1 of HTT encode the N-terminal polyglutamine tract of the ~350 kDa, ~3100 amino acid huntingtin protein [163]. The normal huntingtin protein is multifunctional, with roles in transcriptional regulation, vesicular transport, and synaptic transmission [164,165,166]. Elongation of polyglutamine stretches in the abnormal protein is currently regarded as the causal factor for normal huntingtin’s pathogenic transformation, specifically by way of losing its normal solubility in nerve cells and aggregating into intraneuronal inclusions in different compartments of vulnerable brain regions [167,168].
Similar to α-syn in PD and Tau in AD, polyQ-HTT aggregates exert toxic gain-of-function effects, forming β-sheet-rich structures that disrupt essential cellular pathways and cause neurotoxicity [169,170]. Impaired intracellular transport, a hallmark of HD, is closely linked to PC dysfunction, which can alter ciliary structure and disrupt the trafficking of signaling molecules regulating Shh and Wnt pathways [54,171,172]. Further, in HD models, ciliary elongation has been linked to disrupted intraflagellar transport (IFT), a process essential for trafficking signaling molecules along the ciliary axoneme [173]. While these findings establish a connection between ciliary alterations and impaired trafficking, the underlying mechanisms remain poorly understood [174]. Moreover, while both ciliary elongation and IFT disruption have been reported in HD models, supporting evidence from human post-mortem tissue remains limited [69,175]. In contrast to AD and PD, where ciliary involvement is increasingly recognized, the role of cilia in HD pathogenesis remains underexplored [176,177,178].
Primary Cilia, Exosomes, and TNTs in Huntington’s Disease
Pathogenic polyQ-HTT promotes the accumulation of huntingtin-associated protein 1 (HAP1) and pericentriolar material 1 (PCM1) at the centrosome, leading to elongation of PC and accumulation of N-acetylated tubulin in HD neurons [54,179]. Normally, huntingtin transits between the basal body and cilium via phosphorylation within its N17 domain, but polyQ-HTT exhibits reduced N17 phosphorylation, resulting in accumulation along the ciliary stalk [180,181].
Defective autophagy and lysosomal trafficking in HD further redirect neuronal cargo to secretory pathways, enhancing exosome release and facilitating mutant HTT export through late endosomal and lysosomal routes [182,183]. Ciliary signaling regulates actin networks and drives ciliary exocytosis, linking cilium function to EV biogenesis [184,185]. In HD, actin remodeling promotes TNT formation, with Rhes-driven “Rhes tunnels” and TNTs facilitating intercellular transfer of mutant huntingtin and polyglutamine aggregates, potentially complementing EV-mediated export observed in patient biofluids (Figure 3C) [186,187,188].
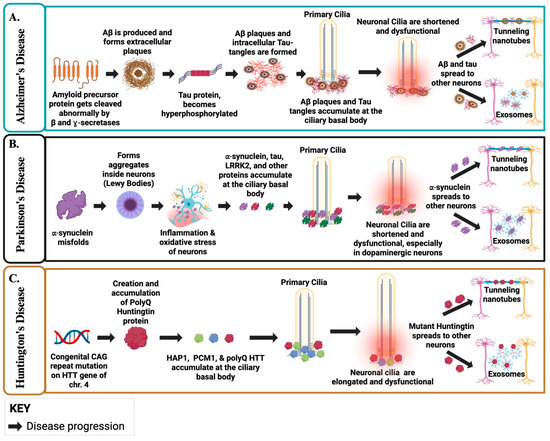
Figure 3.
Ciliary Dysfunction and Protein Propagation in AD, PD, and HD. (A). In AD, Abnormal cleavage of APP generates Aβ, which accumulates at the ciliary basal body, leading to shortened and dysfunctional cilia and impaired signaling. Hyperphosphorylated tau forms tangles, and both Aβ and tau propagate via EVs and TNTs, beginning in the neocortex and entorhinal cortex before spreading more broadly. Disrupted ciliary–autophagy coupling diverts pathogenic cargo toward EV release, amplifying intercellular propagation of toxic proteins. (B). In PD, misfolded α-syn aggregates into Lewy bodies at the basal body, damaging cilia in dopaminergic neurons and impairing Shh signaling. Loss of primary cilia and impaired autophagy enhance EV-mediated α-syn export, while lysosomal damage further drives cargo release. TNT formation, promoted by actin remodeling under ciliary control, provides an additional pathway for α-syn spread from brainstem nuclei upward into cortical circuits. (C). In HD, mutant huntingtin (HTT) accumulates at the centrosome/basal body, producing elongated and dysfunctional cilia and disrupting cilia-dependent signaling. Defects in autophagy and lysosomal trafficking redirect cargo toward secretory pathways, increasing EV release of HTT. Ciliary signaling also directly regulates actin-dependent EV biogenesis. In parallel, actin remodeling promotes TNT formation, including Rhes-driven “tunnels” that transport HTT between neurons, complementing EV-mediated spread observed in patient biofluids. Created in BioRender. Danna, R. (2025) https://BioRender.com/jasqo6c [189].
Beyond aggregation, polyQ-HTT impairs protein clearance pathways, exacerbating cellular dysfunction, and disrupts autophagy by impairing lysosomal degradation and cargo recognition [190]. New emerging evidence shows that mutant HTT sequesters key autophagy regulators, such as p62 and LC3, preventing the clearance of damaged organelles and misfolded proteins [191]. This defect leads to further accumulation of mutant HTT and other toxic components, exacerbating neuronal stress and accelerating death [54]. In addition to direct neuronal toxicity, HD pathogenesis is accompanied by significant neuroinflammation [192]. Brains impacted with HD exhibit increased microglial activation and elevated pro-inflammatory cytokine levels, including IL-6 and TNF-α [193,194]. Further, mutant HTT has been shown to trigger an elevated immune response, leading to chronic neuroinflammation that increases neuronal damage [171,195].
In addition to disrupted intracellular transport, accumulating evidence suggests that mutant HTT propagates between neurons via TNTs and EVs, including exosomes carrying misfolded protein cargo [186,187,196]. Notably, exosomes derived from HD patient neurons contain mutant HTT, providing direct evidence that exosome-mediated transfer contributes to disease spread [197].
5. Preventive Measures, Diagnostics, and Therapeutic Strategies
NDDs remain a major cause of disability and mortality worldwide, with no definitive cure. Increasing evidence suggests that a combination of lifestyle modifications, diagnostic innovations, and emerging therapeutic strategies can significantly influence disease onset, progression, and management. Of particular interest are EVs, especially exosomes, which have emerged as critical mediators of intercellular communication, pathogenic protein propagation, and potential therapeutic delivery. The following section highlights recent advances in preventive strategies, diagnostic modalities, and therapeutic approaches across AD, PD, and HD.
5.1. Preventive Measures
There have been many strategies to delay or prevent the progression of NDDs, including lifestyle changes, physical activities, and high cognitive reserve. For instance, maintaining a healthy lifestyle through balanced caloric intake and adequate sleep supports the structural and functional integrity of PC [198,199,200,201]. In return, this may enhance the activation of ciliary EVs (ciEVs) and neuroprotective signaling pathways like Shh and Wnt, contributing to neuronal resilience and potentially reducing the risk of NDDs. Congruently, recent studies have increasingly focused on lifestyle factors as the mainstay of preventive strategy against the development and progression of NDDs [202,203]. In AD, poor sleep quality and shorter sleep duration are significantly correlated with higher Aβ levels in non-demented populations [204]. Adequate amounts of sleep per night, around 7 h or more, powerfully attenuates oxidative stress and neuroinflammation, key drivers of neurodegeneration, lowering the risk of neurodegenerative disease [205].
While the direct impact of physical activity on TNT formation remains unclear, maintaining neuronal health through exercise may influence TNT dynamics. However, regular exercise has been shown to enhance PC length and ciliogenesis in astrocytes, promoting neuroprotective effects and improving cognitive function in animal models of chronic cerebral hypoperfusion [206]. In addition, physical activity has also been shown to induce the release of EVs carrying neuroprotective factors, which may contribute to the beneficial effects of exercise in preventing NDDs [207]. In particular, higher levels of physical activity have been shown to reduce the risk of AD and PD [208]. In HD, exercise training stabilized patients’ motor deficits and increased their peak oxygen uptake and cardiovascular function [209].
Notably, NDDs, including AD, PD, and HD, tend to exhibit accelerated disease progression and worsening symptoms under stress [210,211,212]. Consistently, it has been shown that stress may significantly influence PC, EVs, and TNTs, modulating neuronal signaling and intercellular communication. In return, PC may alter neuronal excitability and sensitivity, EVs may modify their cargo and release, transmitting stress signals that influence neuroinflammation and synaptic plasticity, and TNTs may increase under oxidative or cellular stress, potentially propagating both protective and pathological effects [213,214,215]. To attenuate this effect, both yoga and mindfulness meditation have been shown to improve health-related quality of life by reducing anxiety and depressive symptoms in neurodegenerative diseases [216,217].
5.1.1. Diagnostics in Alzheimer’s Disease
AD can be confirmed only through a postmortem brain autopsy [218]. In living patients, diagnosis is based on cognitive assessments and cerebral spinal fluid (CSF) biomarkers, such as Aβ, total tau, and phospho-tau-181, using enzyme-linked immunosorbent assay (ELISA) analysis [219,220]. Since CSF collection is invasive, positron emission tomography (PET) and magnetic resonance imaging (MRI) scans are used as noninvasive methods to detect metabolic and structural brain changes associated with AD [221,222,223]. However, alterations in neuronal PC and TNTs may also serve as early mechanistic indicators, but no clinical tools currently exist to evaluate or quantify them in patients. Instead, ongoing clinical trial studies are investigating EVs’ cargo, such as Aβ and tau proteins, for potential AD markers, due to their superior sensitivity, specificity, and accessibility in saliva, blood, and other body fluids [224,225].
Currently, there are two clinical trials aimed at diagnosing AD using EV biomarkers. One actively recruiting clinical trial (NCT06869135) is investigating the potential utilization of Raman Spectroscopy to identify biomarkers of neuronal damage and inflammation present in saliva and salivary extracellular vesicles (sEVs), as a novel approach to early diagnosis of NDDs. By analyzing and tracking the progression of salivary components, focusing on the contents of sEVs, the study aims to facilitate primary early diagnosis, accurately monitor stages of progression, as well as isolate associated biomolecular markers. The other clinical trial (NCT03381482) aims to measure tau quantity in EVs from cerebrospinal fluid (CSF), lumbar puncture, or blood collected from patients at varying AD risk and stages. Collectively, in clinical diagnostic and prognostic studies of AD, EVs have been isolated from plasma, CSF, blood, and saliva, originating from stem cells, neurons, and immune cells. If successful, some of these clinical trials could serve as a noninvasive and early diagnostic tool for AD.
5.1.2. Therapeutics in Alzheimer’s Disease
Currently, there are no clinical studies or approved AD therapies that specifically target PC or TNT. However, mesenchymal stem-cell (MSC)-derived exosomes have been shown to reduce Aβ expression and restore the expression of neuronal memory and synaptic plasticity-related genes in a cell culture model of AD [226,227]. Furthermore, MSC-derived exosomes have been shown to significantly improve brain glucose metabolism and cognitive function compared to controls in AD mice [228]. Neural-stem cell (NSC)-derived exosomes have been shown to alleviate mitochondrial dysfunction, enhance synaptic activity, and decrease inflammation in mice [229]. NSC exosomes have also been shown to effectively repair damage to the blood–brain barrier induced by Aβ [230]. Despite their strong therapeutic promise in AD, the production of stem cell-derived exosomes remains challenging. Their yield from cultured media is extremely low, posing a major challenge for large-scale manufacturing and clinical translation [231]. As a result, strategies such as 3D culture systems, bioreactors, or chemical stimulation are being explored to enhance exosome yield and scalability for therapeutic applications [232,233,234].
Traditionally, AD treatment relied on cholinesterase inhibitors and memantine to manage symptoms [235,236,237]. At present, one clinical trial is assessing MSC-derived EVs as a potential therapy for AD, utilizing intranasal administration of EV-containing drops (HUC-MSC-sEV-00; NCT0660790). The trial is currently in the recruiting phase and will assess therapeutic outcomes using neuropsychological evaluations, without molecular assessment of pharmacological effects.
5.1.3. Diagnostics in Parkinson’s Disease
Currently, PD is primarily diagnosed through the presence of clinical symptoms. Due to PD having multiple variants of disease, accurate and thorough history taking allows clinicians the best opportunity to make the proper diagnosis [238]. Understanding variant differences helps clinicians understand more accurate prognoses, as some variants are more aggressive than others [239]. Typically, bradykinesia, a symptom of a more dominant variant, occurs in concert with either tremor or rigidity, or both [239]. To prevent misdiagnoses in situations where Parkinsonian symptoms are present, but the disease course is aggressive or at an atypical age, dopamine transporter single-photon emission computed tomography is used to confirm PD [240].
Similar to AD, EVs are being actively investigated in clinical trials for the diagnosis and potential treatment of PD. EVs isolated from saliva, blood, urine, and cerebrospinal fluid are evaluated for multiple PD-associated markers. Notably, a recently developed assay identified membrane-associated α-syn in EVs as an early-stage biomarker for PD [241]. Although these assays are not yet standard clinical practice, there are ongoing studies aiming to validate their clinical utility. Currently, different clinical trials are examining EVs isolated from biofluids such as urine, blood, CSF, and saliva for PD diagnostic applications. Of particular interest, several trials have focused on LRRK2-derived EVs (NCT01860118, NCT04603326, NCT03775447) as potential PD biomarkers, which are known to regulate vesicle trafficking and cytoskeletal dynamics associated with PC and TNTs.
5.1.4. Therapeutics in Parkinson’s Disease
Current PD treatments primarily use a combination of carbidopa and levodopa medications to improve motor symptoms [242]. However, side effects, such as dyskinesias, can be managed with dopamine agonists or monoamine-oxidase-B inhibitors, with deep-brain stimulation serving as an option for patients unresponsive to medications [243]. In contrast, EVs may cross the blood–brain barrier and can deliver proteins, RNAs, or drugs directly to target cells, enabling disease-modifying, cell-specific, and multifunctional therapy beyond symptomatic relief [244]. As a result, EVs have been used to transfer PD-specific drugs, specifically targeting dopaminergic neurons [245]. In addition, genetic material such as miR-124-3p can also be transported, which has shown promise in preventing dopaminergic neuron degradation [246]. Similar to AD, the introduction of NSCs has also been shown to increase neuroprotection, especially when enriched with catalase, which is an antioxidant protein [247]. Further, there are currently no clinical studies that specifically target PC or TNT for PD therapy. Instead, a couple of clinical trials (NCT06607900, NCT05152394) are currently evaluating intranasal-derived EVs from human umbilical cord mesenchymal stem cells (hUC-MSCs) as a therapeutic application for PD. The goals for these trials are to modulate neuroinflammation, support neuronal survival, and target disease pathways by delivering bioactive proteins and RNAs directly to the central nervous system (CNS). The trials primarily assess safety, tolerability, and preliminary efficacy using motor and cognitive function tests.
5.1.5. Diagnostics in Huntington’s Disease
HD is usually confirmed diagnostically through the identification of 36 or more CAG repeats on the short arm of chromosome 4p16.3 in the Huntingtin gene [162]. The 36–39 repeat range leads to incomplete penetrance or very late onset of the disease, while definite clinical manifestation is seen at repeat levels of above 40 [248]. One of the most pertinent genetic phenomena in HD is called anticipation, which is when the number of repeats exceeds 55, increasing in each generation [249]. This process can result in Juvenile HD, an aggressive form of the disease that has a significantly earlier onset [250]. To both monitor and predict HD’s manifestation in individuals and across generations, three main categories are assessed: (1) imaging, (2) CSF biomarkers, and (3) bloodborne biomarkers [251,252]. Key biomarkers include polyQ-Htt and Tau, which are detected in the CSF, as well as BDNF, which is measurable in the blood [253,254,255,256]. Imaging is often magnetic resonance imaging, which is useful in understanding cerebral volumes, blood flow, and structural changes [257].
While PC and TNTs have been implicated in HD pathophysiology, they are not yet validated as robust diagnostic tools. Currently, only one clinical trial (NCT06082713) is investigating the HD marker HTT in blood-derived EVs. Moreover, platelet-derived EVs from both pre-manifest and manifest HD patients were shown to carry mutant huntingtin protein; however, EV release levels did not significantly differ from controls, limiting their present diagnostic utility [258].
5.1.6. Therapeutics in Huntington’s Disease
In HD, current therapies revolve around the treatment of symptoms, such as the use of tetrabenazine to treat chorea, involuntary and irregular movements, one of the hallmark symptoms [259]. As for exosomes or TNTs, there is little evidence of these being useful in actual symptom reduction. However, similar to PD, exosomes containing miRNA have shown promise, as their uptake in the brain has been observed in patients with HD [260]. In fact, miR-124, a miRNA shown to be repressed in HD, was utilized in a mouse study via an exosome-based delivery method. While miR-124 delivery was not successful in behavioral symptom reduction, it still demonstrated promise. This is because it could serve as a vehicle for delivering a different miRNA within the exosomes that might have a therapeutic effect. However, there is no current clinical trial investigating either PC, EVs, or TNTs as a potential therapeutic application. For recent clinical trials investigating the diagnostic, prognostic, and therapeutic applications of EVs in AD, PD, and HD, refer to Table 1.

Table 1.
A summary of current Clinical Trials and EVs-Based Diagnostic and therapeutic applications in AD, PD, and HD.
6. Discussion
The increasing prevalence of NDDs underscores the urgent need for improved diagnostics, therapeutics, and care strategies. Early detection combined with emerging therapeutics could slow disease progression and improve quality of life. The shared mechanisms across AD, PD, and HD highlight the critical roles of PC, TNTs, and EVs in NDDs. While each disease has unique etiological factors, the convergence of ciliary dysfunction and protein propagation emphasizes the importance of studying these components in tandem.
PC are essential organelles in neurodevelopment, acting as hubs for major signaling pathways, which regulate brain structure formation and cellular differentiation [259,260,261,262]. During early embryonic development, the disruption of these cilia-associated signaling pathways can result in neurodevelopmental abnormalities [261]. Functionally, these pathways are vital for the proper formation and spatial organization of the brain [262]. Moreover, these signaling pathways also govern cell fate decisions, influencing processes such as differentiation, survival, and programmed cell death [114,263].
In the context of NDDs, PC dysfunction manifests primarily in two ways: (1) a reduction in cilia frequency or (2) alterations in ciliary length. Specifically, neurons and glial cells may either lack cilia altogether or display shortened or elongated cilia. Notably, ciliary shortening has been observed in AD and PD, whereas elongation is characteristic of HD. Although the manifestations of ciliary dysfunction vary across AD, PD, and HD, a unifying mechanism emerges through the role of PC as an integrative signaling hub. In each disease, pathogenic protein accumulation has been associated with cilia dysfunction and impairments in key pathways such as Shh, Wnt-7, and Notch, tipping the balance from neuroprotection to degeneration. The loss of these coordinated signaling diminishes neural patterning, synaptic plasticity, and mitochondrial homeostasis, neuronal resilience, rendering neuronal cells more vulnerable to inflammation and toxicity [147,264,265,266,267,268]. Further, the interplay between EVs, TNTs, and ciliary-associated Shh, Wnt, and Notch pathways represents a convergent mechanism driving the neurodegeneration in NDDs (Figure 4). Precisely, the Shh signaling pathway, supported by EV-associated brain-derived neurotrophic factor (BDNF), has been shown to promote synaptic formation and dopaminergic neuron survival, whereas its disruption exacerbates oxidative stress and neuronal vulnerability [269,270,271]. Wnt signaling pathway has also been shown to maintain synaptic integrity, mitochondrial stability, and blood–brain barrier function; while its dysregulation could upregulate Wnt7a activity to which may induce TNT formation and EV-mediated propagation of neurotoxin proteins, potentially contributing to neuroinflammation and metabolic stress [272,273,274,275,276,277,278,279,280]. Notch signaling pathway may further contribute to EV-driven synaptic remodeling and neuron-glia communication, and its alteration may trigger neuronal remodeling and neuroinflammation [281,282,283]. The crosstalk between Shh, Wnt, and Notch signaling pathways further shows the complexity of neurodegeneration when these pathways are interrupted [267]. Beyond signaling disruption, ciliary dysfunction perturbs cytoskeletal organization and vesicular trafficking, potentially linking it to dysregulated TNT and EV biogenesis [284,285,286]. Thus, while functional PC integrate protective signaling and structural stability, their dysfunction amplifies neurotoxicity by dismantling defense mechanisms and promoting aberrant intercellular protein propagation (Figure 4). While the Shh and Wnt signaling are well-established in supporting neuronal survival, synaptic integrity, and mitochondrial function, the links between Wnt7a, Notch, TNTs, EVs, and ciliary dysfunction in propagating neurotoxicity remain largely speculative and require further validation. Neverthless, all three pathways (Shh, Wnt, and Notch) can simultaneously be disrupted in AD, PD, and HD, with primary cilia loss amplifying pathway dysfunction in disease-relevant neurons. For example, Shh signaling is highly cilia-dependent and is crucial for dopaminergic neuron survival, which could be more relevant to PD [146]. Wnt/Notch signaling may retain partial function without cilia and is strongly associated with AD and HD, which may contribute to synaptic remodeling and neuroinflammation [176,287,288,289].
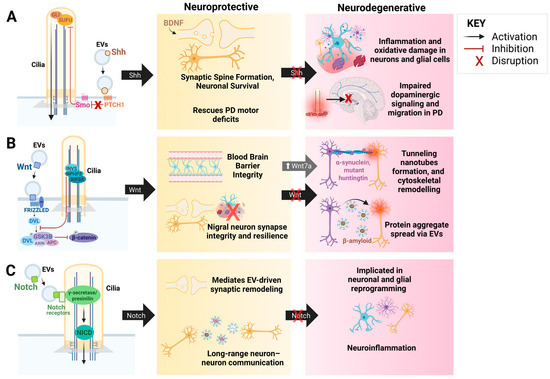
Figure 4.
The Interplay Between EVs, TNTs, and Ciliary Signaling Pathways: Shh, Wnt, and Notch. This figure represents the functional interactions between EVs, TNTs, and ciliary-mediated signaling pathways, demonstrating their dual roles in neuronal maintenance and neurodegenerative disorders. (A). Shh signaling promotes synaptic spine formation and neuronal survival via EV-associated brain-derived neurotrophic factor (BDNF), rescuing dopaminergic neuron loss and motor deficits in PD. Dysregulation of Shh signaling impairs dopaminergic signaling and migration, contributing to inflammation, oxidative stress, and neuronal degeneration. (B). Wnt signaling pathway maintains blood-brain barrier integrity, synaptic resilience, and nigral neuron survival. Abnormal Wnt activity, via the Wnt7a pathway, facilitates tunneling nanotube formation, cytoskeletal remodeling, and EV-mediated propagation of protein aggregates (α-syn, Aβ, mutant huntingtin), linking this pathway to the spread of neurotoxin proteins and neuroinflammation seen in NDDs. (C). Notch signaling mediates EV-driven synaptic remodeling and neuron-neuron communication. Dysregulated Notch signaling may contribute to glial and neuronal reprogramming and neuroinflammation during neurodegeneration. Created in BioRender. Chen, G. (2025) https://BioRender.com/3h40i7a [290].
Epidemiologically, age is one of the major risk factors in NDDs, exacerbating neurodegenerative processes by inducing structural and functional brain changes [280,281]. Key consequences include impaired autophagy and reduced resistance to oxidative stress, both of which contribute to mitochondrial dysfunction and heightened vulnerability to neurodegenerative diseases [291,292,293]. PC and autophagy can work synergistically to regulate protein clearance, with each influencing the other’s function. Autophagy contributes to ciliogenesis by degrading negative regulators (Oral-Facial-Digital Syndrome 1, Centriolar Coiled-Coil Protein 110), modulating ciliary transport (Intraflagellar Transport Protein 20), and controlling ciliary length (Kinesin Family Member 19) [294,295,296]. Conversely, cilia serve as a site for early autophagosome formation, enabling autophagy activation, as evidenced by reduced autophagic functions in PC-depleted cells [102]. Together, autophagy and cilia form a bidirectional regulatory axis that works simultaneously to organize environmental, mechanical, and metabolic signals to maintain homeostasis [297].
Emerging evidence has also demonstrated that mitochondrial stress triggers protective ciliogenesis via protein kinase B (AKT) signaling pathway-mediated autophagy and mitophagy in a human-derived neuroblastoma cell line, a response that declines with aging [268,298]. This further supports the new view that PC may act in a unique role as a cellular timekeeper, regulating the pace of neuronal aging [299]. In line with this, Aβ-mediated circadian dysregulation in AD has been shown to disturb the intrinsic rhythmicity of cilia, thereby compromising their structural integrity and signaling capacity [196,294]. Consequently, loss of proper PC regulation accelerates neuronal aging, marked by increased oxidative stress, mitochondrial disarray, and epigenetically driven senescence that limits DNA repair in vulnerable neurons [289,290,297].
Moreover, aging may also alter intercellular communication by shifting the functions of EVs and TNTs from protective to pathogenic. Senescent cells release EVs enriched in pro-inflammatory and neurotoxic cargo, while aging impairs TNT-mediated mitochondrial transfer, resulting in reduced neuronal resilience and facilitating the spread of protein aggregates in the brain [300,301].
The crosstalk between PC, TNT, and EVs forms an integrated network regulating neuronal homeostasis and intercellular communication. However, the precise mechanism of ciEVs remains poorly defined in NDDs. While studies have shown that ciEVs are selectively released and can be altered when cilia are disrupted, these findings have yet to be validated in NDD models [302]. Similarly, TNT can be formed by distinct processes, and it remains unclear whether their molecular structure or functions are identical across NDDs [303]. Moreover, TNTs are also difficult to visualize in vivo because their nanoscale diameter, fragile structure, and rapid, transient dynamics often necessitate highly skilled imaging approaches and advanced microscopy techniques [304,305,306]. Thus, most evidence on TNT regulation in NDDs comes from in vitro studies, limiting their in vivo applicability. Notably, TNT-like structures have been observed in astrocytic tau propagation (AD models), heart development, and tumors; however, these findings are sparse, context-specific, and require advanced imaging [307,308,309,310]. Further, the reproducibility of these studies is thought to be inconsistent, raising questions about their prevalence, stability, and physiological significance in vivo [311,312,313,314]. However, diagnostic and therapeutic innovation is rapidly advancing through the use of EVs as biomarkers and delivery systems. In AD, PD, and HD, EV-based assays targeting Aβ, α-syn, LRRK2, and mutant huntingtin show promise as noninvasive diagnostic tools. Therapeutically, stem cell-derived EVs exhibit neuroprotective, anti-inflammatory, and mitochondrial-restorative effects, while exosome-mediated miRNA delivery offers experimental potential. However, challenges in yield, scalability, and target specificity remain barriers to clinical translation. Thus, future advancement approaches that incorporate integrated proteomic, transcriptomic, lipidomic, and single-EV sequencing are likely to reveal more selective and disease-specific EV signatures across NDDs. This multi-modal profiling will help differentiate cell-type-specific vesicle populations, identify post-translationally modified cargo, and uncover subtle molecular changes associated with disease progression. In parallel, dissecting the crosstalk among PC, EVs, and TNTs may illuminate how these intercellular communication pathways converge to regulate neuronal homeostasis.
7. Conclusions
The interplay between ciliopathies and EVs suggests a broader framework for understanding neurodegenerative diseases. Regardless of an initial age-related, genetic, and/or proteinaceous insult, a dual-hit model, where ciliary dysfunction both drives and amplifies pathogenic propagation, may explain both the early onset of genetically driven diseases, like in HD, and the progressive deterioration in aging-related diseases, such as in AD and PD. Earlier detection could enable more timely and effective interventions, and the identification of disease-specific biomarkers within exosomes may greatly improve diagnostic accuracy. Therapeutic strategies targeting the cilia, tunneling nanotubes, and exosome pathway, by stabilizing cilia structures, regulating vesicle transport, or inhibiting the spread of toxic proteins, may offer a promising approach to slow or halt the progression of neurodegenerative diseases.
Author Contributions
Conceptualization, A.M.M.; writing—original draft preparation, R.D., S.K., O.A. and M.M.; writing—review and editing, R.D., S.K., O.A., M.M., G.C., W.B.F. and A.M.M.; visualization, R.D., S.K., O.A., G.C. and A.M.M.; supervision, A.M.M.; project administration, R.D., S.K. and A.M.M.; funding acquisition, A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the College of Graduate Studies, California Northstate University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2021. NCHS Data Brief 2022, 456, 1–8. [Google Scholar]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, M.; Borchelt, D.R. Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states. Mol. Neurodegener. 2011, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef]
- Mohieldin, A.M.; Alachkar, A.; Yates, J.; Nauli, S.M. Novel biomarkers of ciliary extracellular vesicles interact with ciliopathy and Alzheimer’s associated proteins. Commun. Integr. Biol. 2021, 14, 264–269. [Google Scholar] [CrossRef]
- Karunakaran, K.B.; Chaparala, S.; Lo, C.W.; Ganapathiraju, M.K. Cilia interactome with predicted protein-protein interactions reveals connections to Alzheimer’s disease, aging and other neuropsychiatric processes. Sci. Rep. 2020, 10, 15629. [Google Scholar] [CrossRef]
- Miyoshi, K.; Kasahara, K.; Murakami, S.; Takeshima, M.; Kumamoto, N.; Sato, A.; Miyazaki, I.; Matsuzaki, S.; Sasaoka, T.; Katayama, T.; et al. Lack of dopaminergic inputs elongates the primary cilia of striatal neurons. PLoS ONE 2014, 9, e97918. [Google Scholar] [CrossRef]
- Chakravarthy, B.; Gaudet, C.; Menard, M.; Brown, L.; Atkinson, T.; Laferla, F.M.; Ito, S.; Armato, U.; Dal Pra, I.; Whitfield, J. Reduction of the immunostainable length of the hippocampal dentate granule cells’ primary cilia in 3xAD-transgenic mice producing human Aβ1-42 and tau. Biochem. Biophys. Res. Commun. 2012, 427, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, L.; Revenkova, E.; Gordon, R.E.; Iomini, C. Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Development 2016, 143, 2160–2171. [Google Scholar] [CrossRef]
- Mill, P.; Christensen, S.T.; Pedersen, L.B. Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat. Rev. Genet. 2023, 24, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, S.; Kornberg, T.B. The C-terminal tail of the Hedgehog receptor Patched regulates both localization and turnover. Genes. Dev. 2006, 20, 2539–2551. [Google Scholar] [CrossRef]
- Zhang, K.; Da Silva, F.; Seidl, C.; Wilsch-Brauninger, M.; Herbst, J.; Huttner, W.B.; Niehrs, C. Primary cilia are WNT-transducing organelles whose biogenesis is controlled by a WNT-PP1 axis. Dev. Cell 2023, 58, 139–154.e8. [Google Scholar] [CrossRef]
- Coschiera, A.; Yoshihara, M.; Lauter, G.; Ezer, S.; Pucci, M.; Li, H.; Kavsek, A.; Riedel, C.G.; Kere, J.; Swoboda, P. Primary cilia promote the differentiation of human neurons through the WNT signaling pathway. BMC Biol. 2024, 22, 48. [Google Scholar] [CrossRef]
- Bangs, F.; Anderson, K.V. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028175. [Google Scholar] [CrossRef]
- Schneider, L.; Clement, C.A.; Teilmann, S.C.; Pazour, G.J.; Hoffmann, E.K.; Satir, P.; Christensen, S.T. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 2005, 15, 1861–1866. [Google Scholar] [CrossRef]
- Hu, H.B.; Song, Z.Q.; Song, G.P.; Li, S.; Tu, H.Q.; Wu, M.; Zhang, Y.C.; Yuan, J.F.; Li, T.T.; Li, P.Y.; et al. LPA signaling acts as a cell-extrinsic mechanism to initiate cilia disassembly and promote neurogenesis. Nat. Commun. 2021, 12, 662. [Google Scholar] [CrossRef]
- Abounit, S.; Zurzolo, C. Wiring through tunneling nanotubes--from electrical signals to organelle transfer. J. Cell Sci. 2012, 125, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Lou, E.; Fujisawa, S.; Morozov, A.; Barlas, A.; Romin, Y.; Dogan, Y.; Gholami, S.; Moreira, A.L.; Manova-Todorova, K.; Moore, M.A. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 2012, 7, e33093. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Faure, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Kesimer, M.; Scull, M.; Brighton, B.; DeMaria, G.; Burns, K.; O’Neal, W.; Pickles, R.J.; Sheehan, J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009, 23, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef] [PubMed]
- Sheller, S.; Papaconstantinou, J.; Urrabaz-Garza, R.; Richardson, L.; Saade, G.; Salomon, C.; Menon, R. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS ONE 2016, 11, e0157614. [Google Scholar] [CrossRef]
- Kunou, S.; Shimada, K.; Takai, M.; Sakamoto, A.; Aoki, T.; Hikita, T.; Kagaya, Y.; Iwamoto, E.; Sanada, M.; Shimada, S.; et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma. Oncogene 2021, 40, 3989–4003. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Ma, T.; Liu, Z.; Gao, L. Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice. Stem Cell Res. Ther. 2023, 14, 278. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Genc, S.; Pennisi, M.; Yeni, Y.; Yildirim, S.; Gattuso, G.; Altinoz, M.A.; Taghizadehghalehjoughi, A.; Bolat, I.; Tsatsakis, A.; Hacimuftuoglu, A.; et al. Potential Neurotoxic Effects of Glioblastoma-Derived Exosomes in Primary Cultures of Cerebellar Neurons via Oxidant Stress and Glutathione Depletion. Antioxidants 2022, 11, 1225. [Google Scholar] [CrossRef]
- Jang, J.; Yeo, S.; Baek, S.; Jung, H.J.; Lee, M.S.; Choi, S.H.; Choe, Y. Abnormal accumulation of extracellular vesicles in hippocampal dystrophic axons and regulation by the primary cilia in Alzheimer’s disease. Acta Neuropathol. Commun. 2023, 11, 142. [Google Scholar] [CrossRef]
- Wang, S.; Kojima, K.; Mobley, J.A.; West, A.B. Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease. EBioMedicine 2019, 45, 351–361. [Google Scholar] [CrossRef]
- Sancandi, M.; Uysal-Onganer, P.; Kraev, I.; Mercer, A.; Lange, S. Protein Deimination Signatures in Plasma and Plasma-EVs and Protein Deimination in the Brain Vasculature in a Rat Model of Pre-Motor Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2743. [Google Scholar] [CrossRef]
- Ciregia, F.; Urbani, A.; Palmisano, G. Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front. Mol. Neurosci. 2017, 10, 276. [Google Scholar] [CrossRef]
- Kondle, S. Figure 1. Common and Unique Features of AD, PD, and HD. 2025, Created in BioRender. Available online: https://BioRender.com/usiaa3q (accessed on 4 December 2025).
- Moruzzi, N.; Valladolid-Acebes, I.; Kannabiran, S.A.; Bulgaro, S.; Burtscher, I.; Leibiger, B.; Leibiger, I.B.; Berggren, P.O.; Brismar, K. Mitochondrial impairment and intracellular reactive oxygen species alter primary cilia morphology. Life Sci. Alliance 2022, 5, 12. [Google Scholar] [CrossRef]
- Ignatenko, O.; Malinen, S.; Rybas, S.; Vihinen, H.; Nikkanen, J.; Kononov, A.; Jokitalo, E.S.; Ince-Dunn, G.; Suomalainen, A. Mitochondrial dysfunction compromises ciliary homeostasis in astrocytes. J. Cell Biol. 2023, 222, e202203019. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025, 21, e70235. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive Functions in Alzheimer Disease: A Systematic Review. Front. Aging Neurosci. 2018, 10, 437. [Google Scholar] [CrossRef]
- Wu, B.S.; Zhang, Y.R.; Li, H.Q.; Kuo, K.; Chen, S.D.; Dong, Q.; Liu, Y.; Yu, J.T. Cortical structure and the risk for Alzheimer’s disease: A bidirectional Mendelian randomization study. Transl. Psychiatry 2021, 11, 476. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Willis, A.W.; Roberts, E.; Beck, J.C.; Fiske, B.; Ross, W.; Savica, R.; Van Den Eeden, S.K.; Tanner, C.M.; Marras, C.; Parkinson’s Foundation, P.G. Incidence of Parkinson disease in North America. npj Park. Dis. 2022, 8, 170. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- Schulz-Schaeffer, W.J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Nopoulos, P.C. Huntington disease: A single-gene degenerative disorder of the striatum. Dialogues Clin. Neurosci. 2016, 18, 91–98. [Google Scholar] [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef]
- Novak, M.J.; Tabrizi, S.J. Huntington’s disease: Clinical presentation and treatment. Int. Rev. Neurobiol. 2011, 98, 297–323. [Google Scholar] [CrossRef] [PubMed]
- Ciarochi, J.A.; Calhoun, V.D.; Lourens, S.; Long, J.D.; Johnson, H.J.; Bockholt, H.J.; Liu, J.; Plis, S.M.; Paulsen, J.S.; Turner, J.A.; et al. Patterns of Co-Occurring Gray Matter Concentration Loss across the Huntington Disease Prodrome. Front. Neurol. 2016, 7, 147. [Google Scholar] [CrossRef]
- Rub, U.; Seidel, K.; Heinsen, H.; Vonsattel, J.P.; den Dunnen, W.F.; Korf, H.W. Huntington’s disease (HD): The neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol. 2016, 26, 726–740. [Google Scholar] [CrossRef]
- Keryer, G.; Pineda, J.R.; Liot, G.; Kim, J.; Dietrich, P.; Benstaali, C.; Smith, K.; Cordelieres, F.P.; Spassky, N.; Ferrante, R.J.; et al. Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. J. Clin. Investig. 2011, 121, 4372–4382. [Google Scholar] [CrossRef]
- Tozser, J.; Earwood, R.; Kato, A.; Brown, J.; Tanaka, K.; Didier, R.; Megraw, T.L.; Blum, M.; Kato, Y. TGF-β Signaling Regulates the Differentiation of Motile Cilia. Cell Rep. 2015, 11, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Elobeid, A.; Libard, S.; Leino, M.; Popova, S.N.; Alafuzoff, I. Altered Proteins in the Aging Brain. J. Neuropathol. Exp. Neurol. 2016, 75, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Ferrer, I.; Aronica, E.; Kovacs, G.G.; Verney, C.; Nardelli, J.; Khung, S.; Delezoide, A.L.; Milenkovic, I.; Rasika, S.; et al. Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cereb. Cortex 2018, 28, 2458–2478. [Google Scholar] [CrossRef]
- Horvathy, D.B.; Nardai, P.P.; Major, T.; Schandl, K.; Cselenyak, A.; Vacz, G.; Kiss, L.; Szendroi, M.; Lacza, Z. Muscle regeneration is undisturbed by repeated polytraumatic injury. Eur. J. Trauma. Emerg. Surg. 2011, 37, 161–167. [Google Scholar] [CrossRef]
- Haxaire, C.; Turpin, F.R.; Potier, B.; Kervern, M.; Sinet, P.M.; Barbanel, G.; Mothet, J.P.; Dutar, P.; Billard, J.M. Reversal of age-related oxidative stress prevents hippocampal synaptic plasticity deficits by protecting D-serine-dependent NMDA receptor activation. Aging Cell 2012, 11, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, R.; Wong, A.; Silva, J.; Li, M.; Itoh, A.; Horiuchi, M.; Itoh, T.; Pleasure, D.; Cortopassi, G. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 2010, 10, 143–150. [Google Scholar] [CrossRef]
- Forero, D.A.; Gonzalez-Giraldo, Y.; Lopez-Quintero, C.; Castro-Vega, L.J.; Barreto, G.E.; Perry, G. Meta-analysis of Telomere Length in Alzheimer’s Disease. J. Gerontol. Ser. A 2016, 71, 1069–1073. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, Y.; Yang, Z.; Li, K.; Lou, X.; Cui, W. Accelerated telomere shortening independent of LRRK2 variants in Chinese patients with Parkinson’s disease. Aging 2020, 12, 20483–20492. [Google Scholar] [CrossRef]
- PerezGrovas-Saltijeral, A.; Ochoa-Morales, A.; Miranda-Duarte, A.; Martinez-Ruano, L.; Jara-Prado, A.; Camacho-Molina, A.; Hidalgo-Bravo, A. Telomere length analysis on leukocytes derived from patients with Huntington Disease. Mech. Ageing Dev. 2020, 185, 111189. [Google Scholar] [CrossRef]
- Ranganathan, M.; Kostyk, S.K.; Allain, D.C.; Race, J.A.; Daley, A.M. Age of onset and behavioral manifestations in Huntington’s disease: An Enroll-HD cohort analysis. Clin. Genet. 2021, 99, 133–142. [Google Scholar] [CrossRef]
- Velez, J.I.; Lopera, F.; Patel, H.R.; Johar, A.S.; Cai, Y.; Rivera, D.; Tobon, C.; Villegas, A.; Sepulveda-Falla, D.; Lehmann, S.G.; et al. Mutations modifying sporadic Alzheimer’s disease age of onset. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Gaare, J.J.; Brugger, K.; Nido, G.S.; Tzoulis, C. DNA Methylation Age Acceleration Is Not Associated with Age of Onset in Parkinson’s Disease. Mov. Disord. 2023, 38, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Raket, L.L.; Oudin Astrom, D.; Norlin, J.M.; Kellerborg, K.; Martinez-Martin, P.; Odin, P. Impact of age at onset on symptom profiles, treatment characteristics and health-related quality of life in Parkinson’s disease. Sci. Rep. 2022, 12, 526. [Google Scholar] [CrossRef]
- Malkov, A.; Popova, I.; Ivanov, A.; Jang, S.S.; Yoon, S.Y.; Osypov, A.; Huang, Y.; Zilberter, Y.; Zilberter, M. Aβ initiates brain hypometabolism, network dysfunction and behavioral abnormalities via NOX2-induced oxidative stress in mice. Commun. Biol. 2021, 4, 1054. [Google Scholar] [CrossRef]
- Ebenau, J.L.; Timmers, T.; Wesselman, L.M.P.; Verberk, I.M.W.; Verfaillie, S.C.J.; Slot, R.E.R.; van Harten, A.C.; Teunissen, C.E.; Barkhof, F.; van den Bosch, K.A.; et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020, 95, e46–e58. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Sergeant, N.; Buee, L. Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front. Physiol. 2012, 3, 229. [Google Scholar] [CrossRef]
- Sondag, C.M.; Dhawan, G.; Combs, C.K. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J. Neuroinflammation 2009, 6, 1. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Grundke-Iqbal, I.; Iqbal, K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Murrell, J.R.; Goedert, M.; Farlow, M.R.; Klug, A.; Ghetti, B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl. Acad. Sci. USA 1998, 95, 7737–7741. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef]
- Josephs, K.A.; Dickson, D.W.; Tosakulwong, N.; Weigand, S.D.; Murray, M.E.; Petrucelli, L.; Liesinger, A.M.; Senjem, M.L.; Spychalla, A.J.; Knopman, D.S.; et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: A longitudinal retrospective study. Lancet Neurol. 2017, 16, 917–924. [Google Scholar] [CrossRef]
- Petersen, R.C.; Jack, C.R., Jr.; Xu, Y.C.; Waring, S.C.; O’Brien, P.C.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Boeve, B.F.; Kokmen, E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 2000, 54, 581–587. [Google Scholar] [CrossRef]
- Hollands, C.; Tobin, M.K.; Hsu, M.; Musaraca, K.; Yu, T.S.; Mishra, R.; Kernie, S.G.; Lazarov, O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017, 12, 64. [Google Scholar] [CrossRef]
- Singh, V.; Chertkow, H.; Lerch, J.P.; Evans, A.C.; Dorr, A.E.; Kabani, N.J. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain 2006, 129, 2885–2893. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Shiung, M.M.; Przybelski, S.A.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Petersen, R.C.; Jack, C.R., Jr. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 2008, 70, 512–520. [Google Scholar] [CrossRef]
- Amador-Arjona, A.; Elliott, J.; Miller, A.; Ginbey, A.; Pazour, G.J.; Enikolopov, G.; Roberts, A.J.; Terskikh, A.V. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: Implications for learning and memory. J. Neurosci. 2011, 31, 9933–9944. [Google Scholar] [CrossRef] [PubMed]
- Stence, N.; Waite, M.; Dailey, M.E. Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia 2001, 33, 256–266. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.; Franklin, R.J. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Yeo, S.; Jang, J.; Jung, H.J.; Lee, H.; Choe, Y. Primary cilia-mediated regulation of microglial secretion in Alzheimer’s disease. Front. Mol. Biosci. 2023, 10, 1250335. [Google Scholar] [CrossRef]
- Guo, A.; Wang, H.; Zhang, Y.; Huang, H. Changes of the Primary Cilia in Alzheimer’s Disease Pathogenesis. Eur. J. Neurosci. 2025, 61, e70125. [Google Scholar] [CrossRef]
- Ding, X.; Wang, J.; Huang, M.; Chen, Z.; Liu, J.; Zhang, Q.; Zhang, C.; Xiang, Y.; Zen, K.; Li, L. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat. Commun. 2021, 12, 2030. [Google Scholar] [CrossRef]
- Ren, X.; Yao, L.; Wang, Y.; Mei, L.; Xiong, W.C. Microglial VPS35 deficiency impairs Aβ phagocytosis and Aβ-induced disease-associated microglia, and enhances Aβ associated pathology. J. Neuroinflammation 2022, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Socodato, R. Beyond Amyloid and Tau: The Critical Role of Microglia in Alzheimer’s Disease Therapeutics. Biomedicines 2025, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Chebli, J.; Rahmati, M.; Lashley, T.; Edeman, B.; Oldfors, A.; Zetterberg, H.; Abramsson, A. The localization of amyloid precursor protein to ependymal cilia in vertebrates and its role in ciliogenesis and brain development in zebrafish. Sci. Rep. 2021, 11, 19115. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kohbuchi, S.; Koganezawa, N.; Sekino, Y.; Shirao, T.; Saido, T.C.; Saito, T.; Saito, Y. Impairment of ciliary dynamics in an APP knock-in mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2022, 610, 85–91. [Google Scholar] [CrossRef]
- Vorobyeva, A.G.; Lee, R.; Miller, S.; Longen, C.; Sharoni, M.; Kandelwal, P.J.; Kim, F.J.; Marenda, D.R.; Saunders, A.J. Cyclopamine modulates γ-secretase-mediated cleavage of amyloid precursor protein by altering its subcellular trafficking and lysosomal degradation. J. Biol. Chem. 2014, 289, 33258–33274. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Zhang, Z.; Nadeau, P.; Song, W.; Donoviel, D.; Yuan, M.; Bernstein, A.; Yankner, B.A. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol. 2000, 2, 463–465. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.G.; Saunders, A.J. Amyloid-β interrupts canonical Sonic hedgehog signaling by distorting primary cilia structure. Cilia 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.; et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef]
- Tardivel, M.; Begard, S.; Bousset, L.; Dujardin, S.; Coens, A.; Melki, R.; Buee, L.; Colin, M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 2016, 4, 117. [Google Scholar] [CrossRef]
- Dilna, A.; Deepak, K.V.; Damodaran, N.; Kielkopf, C.S.; Kagedal, K.; Ollinger, K.; Nath, S. Amyloid-β induced membrane damage instigates tunneling nanotube-like conduits by p21-activated kinase dependent actin remodulation. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166246. [Google Scholar] [CrossRef] [PubMed]
- Pampliega, O.; Orhon, I.; Patel, B.; Sridhar, S.; Diaz-Carretero, A.; Beau, I.; Codogno, P.; Satir, B.H.; Satir, P.; Cuervo, A.M. Functional interaction between autophagy and ciliogenesis. Nature 2013, 502, 194–200. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Barnett, J.L.; Pieroni, C.; Nixon, R.A. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: Neuropathologic evidence for a mechanism of increased β-amyloidogenesis. J. Neurosci. 1997, 17, 6142–6151. [Google Scholar] [CrossRef]
- Kumamoto, N.; Gu, Y.; Wang, J.; Janoschka, S.; Takemaru, K.; Levine, J.; Ge, S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci. 2012, 15, 399–405, S391. [Google Scholar] [CrossRef]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Kruger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef]
- Han, T.; Xu, Y.; Sun, L.; Hashimoto, M.; Wei, J. Microglial response to aging and neuroinflammation in the development of neurodegenerative diseases. Neural Regen. Res. 2024, 19, 1241–1248. [Google Scholar] [CrossRef]
- Pandey, R.S.; Graham, L.; Uyar, A.; Preuss, C.; Howell, G.R.; Carter, G.W. Genetic perturbations of disease risk genes in mice capture transcriptomic signatures of late-onset Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.M.; Soto, I.; Graham, L.C.; Carter, G.W.; Howell, G.R. Clustering of transcriptional profiles identifies changes to insulin signaling as an early event in a mouse model of Alzheimer’s disease. BMC Genom. 2013, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Davoody, S.; Asgari Taei, A.; Khodabakhsh, P.; Dargahi, L. mTOR signaling and Alzheimer’s disease: What we know and where we are? CNS Neurosci. Ther. 2024, 30, e14463. [Google Scholar] [CrossRef]
- Pan, J.; Yao, Q.; Wang, Y.; Chang, S.; Li, C.; Wu, Y.; Shen, J.; Yang, R. The role of PI3K signaling pathway in Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1459025. [Google Scholar] [CrossRef]
- Folke, J.; Pakkenberg, B.; Brudek, T. Impaired Wnt Signaling in the Prefrontal Cortex of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Riise, J.; Plath, N.; Pakkenberg, B.; Parachikova, A. Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer’s disease. J. Neural Transm. 2015, 122, 1303–1318. [Google Scholar] [CrossRef]
- Gazea, M.; Tasouri, E.; Tolve, M.; Bosch, V.; Kabanova, A.; Gojak, C.; Kurtulmus, B.; Novikov, O.; Spatz, J.; Pereira, G.; et al. Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev. Biol. 2016, 409, 55–71. [Google Scholar] [CrossRef]
- Breunig, J.J.; Sarkisian, M.R.; Arellano, J.I.; Morozov, Y.M.; Ayoub, A.E.; Sojitra, S.; Wang, B.; Flavell, R.A.; Rakic, P.; Town, T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 13127–13132. [Google Scholar] [CrossRef]
- Sharma, P.; Schiapparelli, L.; Cline, H.T. Exosomes function in cell-cell communication during brain circuit development. Curr. Opin. Neurobiol. 2013, 23, 997–1004. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjo, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Elsherbini, A.; Kirov, A.S.; Dinkins, M.B.; Wang, G.; Qin, H.; Zhu, Z.; Tripathi, P.; Crivelli, S.M.; Bieberich, E. Association of Aβ with ceramide-enriched astrosomes mediates Aβ neurotoxicity. Acta Neuropathol. Commun. 2020, 8, 60. [Google Scholar] [CrossRef]
- Chakraborty, R.; Nonaka, T.; Hasegawa, M.; Zurzolo, C. Tunnelling nanotubes between neuronal and microglial cells allow bi-directional transfer of α-Synuclein and mitochondria. Cell Death Dis. 2023, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, Z.; Chen, X.; Zhang, Y.; Guo, A.; Zhang, Y. Intercellular transport of Tau protein and β-amyloid mediated by tunneling nanotubes. Am. J. Transl. Res. 2021, 13, 12509–12522. [Google Scholar] [PubMed]
- Valappil, D.K.; Mini, N.J.; Dilna, A.; Nath, S. Membrane interaction to intercellular spread of pathology in Alzheimer’s disease. Front. Neurosci. 2022, 16, 936897. [Google Scholar] [CrossRef]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tan, K.S.; Zhang, X.; Sun, A.Y.; Sun, G.Y.; Lee, J.C. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell Sci. 2005, 118, 3695–3703. [Google Scholar] [CrossRef]
- Abounit, S.; Wu, J.W.; Duff, K.; Victoria, G.S.; Zurzolo, C. Tunneling nanotubes: A possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 2016, 10, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Sun, H.; Chen, N.H.; Yuan, Y.H. Tunneling nanotubes: A novel pharmacological target for neurodegenerative diseases? Pharmacol. Res. 2021, 170, 105541. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Pardo, J.A.; Sanchez-Ferro, A.; Monje, M.H.G.; Pavese, N.; Obeso, J.A. Onset pattern of nigrostriatal denervation in early Parkinson’s disease. Brain 2022, 145, 1018–1028. [Google Scholar] [CrossRef]
- Cramb, K.M.L.; Beccano-Kelly, D.; Cragg, S.J.; Wade-Martins, R. Impaired dopamine release in Parkinson’s disease. Brain 2023, 146, 3117–3132. [Google Scholar] [CrossRef]
- Siderowf, A.; Lang, A.E. Premotor Parkinson’s disease: Concepts and definitions. Mov. Disord. 2012, 27, 608–616. [Google Scholar] [CrossRef]
- Bendor, J.T.; Logan, T.P.; Edwards, R.H. The function of α-synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef]
- Grassi, D.; Howard, S.; Zhou, M.; Diaz-Perez, N.; Urban, N.T.; Guerrero-Given, D.; Kamasawa, N.; Volpicelli-Daley, L.A.; LoGrasso, P.; Lasmezas, C.I. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E2634–E2643. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef]
- Power, J.H.; Barnes, O.L.; Chegini, F. Lewy Bodies and the Mechanisms of Neuronal Cell Death in Parkinson’s Disease and Dementia with Lewy Bodies. Brain Pathol. 2017, 27, 3–12. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Mutlu, E.; Dodiya, H.B.; Daian, D.; Jaglin, J.A.; Kordower, J.H. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 2012, 27, 709–715. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Park. Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.; Feng, J. Emerging role of microglia in inter-cellular transmission of α-synuclein in Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1411104. [Google Scholar] [CrossRef]
- Cook, L.; Verbrugge, J.; Schwantes-An, T.H.; Schulze, J.; Foroud, T.; Hall, A.; Marder, K.S.; Mata, I.F.; Mencacci, N.E.; Nance, M.A.; et al. Parkinson’s disease variant detection and disclosure: PD GENEration, a North American study. Brain 2024, 147, 2668–2679. [Google Scholar] [CrossRef]
- Lubbe, S.J.; Escott-Price, V.; Gibbs, J.R.; Nalls, M.A.; Bras, J.; Price, T.R.; Nicolas, A.; Jansen, I.E.; Mok, K.Y.; Pittman, A.M.; et al. Additional rare variant analysis in Parkinson’s disease cases with and without known pathogenic mutations: Evidence for oligogenic inheritance. Hum. Mol. Genet. 2016, 25, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; de Vries, R.L.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 2010, 107, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.S.; Li, L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.A.; Kitada, T.; Shen, J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl. Acad. Sci. USA 2008, 105, 11364–11369. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Lu, Y.; Duan, C.; Gao, G.; Lu, L.; Yang, H. Pink1 interacts with α-synuclein and abrogates α-synuclein-induced neurotoxicity by activating autophagy. Cell Death Dis. 2017, 8, e3056. [Google Scholar] [CrossRef]
- Iqbal, A.; Baldrighi, M.; Murdoch, J.N.; Fleming, A.; Wilkinson, C.J. Alpha-synuclein aggresomes inhibit ciliogenesis and multiple functions of the centrosome. Biol. Open 2020, 9, bio054338. [Google Scholar] [CrossRef]
- Rui, Q.; Ni, H.; Li, D.; Gao, R.; Chen, G. The Role of LRRK2 in Neurodegeneration of Parkinson Disease. Curr. Neuropharmacol. 2018, 16, 1348–1357. [Google Scholar] [CrossRef]
- Khan, S.S.; Sobu, Y.; Dhekne, H.S.; Tonelli, F.; Berndsen, K.; Alessi, D.R.; Pfeffer, S.R. Pathogenic LRRK2 control of primary cilia and Hedgehog signaling in neurons and astrocytes of mouse brain. eLife 2021, 10, e67900. [Google Scholar] [CrossRef] [PubMed]
- Dhekne, H.S.; Yanatori, I.; Gomez, R.C.; Tonelli, F.; Diez, F.; Schule, B.; Steger, M.; Alessi, D.R.; Pfeffer, S.R. A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. eLife 2018, 7, e40202. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Jaimon, E.; Lin, Y.E.; Nikoloff, J.; Tonelli, F.; Alessi, D.R.; Pfeffer, S.R. Loss of primary cilia and dopaminergic neuroprotection in pathogenic LRRK2-driven and idiopathic Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2024, 121, e2402206121. [Google Scholar] [CrossRef]
- Lin, Y.E.; Jaimon, E.; Tonelli, F.; Pfeffer, S.R. Pathogenic LRRK2 mutations cause loss of primary cilia and Neurturin in striatal parvalbumin interneurons. Life Sci. Alliance 2025, 8, e202402922. [Google Scholar] [CrossRef] [PubMed]
- Minakaki, G.; Menges, S.; Kittel, A.; Emmanouilidou, E.; Schaeffner, I.; Barkovits, K.; Bergmann, A.; Rockenstein, E.; Adame, A.; Marxreiter, F.; et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy 2018, 14, 98–119. [Google Scholar] [CrossRef]
- Fussi, N.; Hollerhage, M.; Chakroun, T.; Nykanen, N.P.; Rosler, T.W.; Koeglsperger, T.; Wurst, W.; Behrends, C.; Hoglinger, G.U. Exosomal secretion of α-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Abounit, S.; Bousset, L.; Loria, F.; Zhu, S.; de Chaumont, F.; Pieri, L.; Olivo-Marin, J.C.; Melki, R.; Zurzolo, C. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 2016, 35, 2120–2138. [Google Scholar] [CrossRef]
- Dilsizoglu Senol, A.; Samarani, M.; Syan, S.; Guardia, C.M.; Nonaka, T.; Liv, N.; Latour-Lambert, P.; Hasegawa, M.; Klumperman, J.; Bonifacino, J.S.; et al. α-Synuclein fibrils subvert lysosome structure and function for the propagation of protein misfolding between cells through tunneling nanotubes. PLoS Biol. 2021, 19, e3001287. [Google Scholar] [CrossRef]
- Hase, K.; Kimura, S.; Takatsu, H.; Ohmae, M.; Kawano, S.; Kitamura, H.; Ito, M.; Watarai, H.; Hazelett, C.C.; Yeaman, C.; et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 2009, 11, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Scheiblich, H.; Eikens, F.; Wischhof, L.; Opitz, S.; Jungling, K.; Cserep, C.; Schmidt, S.V.; Lambertz, J.; Bellande, T.; Posfai, B.; et al. Microglia rescue neurons from aggregate-induced neuronal dysfunction and death through tunneling nanotubes. Neuron 2024, 112, 3106–3125.e8. [Google Scholar] [CrossRef] [PubMed]
- Melachroinou, K.; Divolis, G.; Tsafaras, G.; Karampetsou, M.; Fortis, S.; Stratoulias, Y.; Papadopoulou, G.; Kriebardis, A.G.; Samiotaki, M.; Vekrellis, K. Endogenous Alpha-Synuclein is Essential for the Transfer of Pathology by Exosome-Enriched Extracellular Vesicles, Following Inoculation with Preformed Fibrils in vivo. Aging Dis. 2024, 15, 869–892. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, X.; Li, F.; Yu, Y.; Chen, Z.; Liu, Z.; Wang, Z.; Xu, H.; Yang, W. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget 2017, 8, 15539–15552. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Liu, D.; Guo, J.J.; Su, J.H.; Svanbergsson, A.; Yuan, L.; Haikal, C.; Li, W.; Gouras, G.; Li, J.Y. Differential seeding and propagating efficiency of α-synuclein strains generated in different conditions. Transl. Neurodegener. 2021, 10, 20. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef] [PubMed]
- Neylan, T.C. Neurodegenerative disorders: George Huntington’s description of hereditary chorea. J. Neuropsychiatry Clin. Neurosci. 2003, 15, 108. [Google Scholar] [CrossRef]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Mende-Mueller, L.M.; Toneff, T.; Hwang, S.R.; Chesselet, M.F.; Hook, V.Y. Tissue-specific proteolysis of Huntingtin (htt) in human brain: Evidence of enhanced levels of N- and C-terminal htt fragments in Huntington’s disease striatum. J. Neurosci. 2001, 21, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- White, J.A., II; Anderson, E.; Zimmerman, K.; Zheng, K.H.; Rouhani, R.; Gunawardena, S. Huntingtin differentially regulates the axonal transport of a sub-set of Rab-containing vesicles in vivo. Hum. Mol. Genet. 2015, 24, 7182–7195. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhu, G.; Liu, X.; Li, H. Mutant Huntingtin Causes a Selective Decrease in the Expression of Synaptic Vesicle Protein 2C. Neurosci. Bull. 2018, 34, 747–758. [Google Scholar] [CrossRef]
- Vitet, H.; Bruyere, J.; Xu, H.; Seris, C.; Brocard, J.; Abada, Y.S.; Delatour, B.; Scaramuzzino, C.; Venance, L.; Saudou, F. Huntingtin recruits KIF1A to transport synaptic vesicle precursors along the mouse axon to support synaptic transmission and motor skill learning. eLife 2023, 12, e81011. [Google Scholar] [CrossRef]
- Hoffner, G.; Soues, S.; Djian, P. Aggregation of expanded huntingtin in the brains of patients with Huntington disease. Prion 2007, 1, 26–31. [Google Scholar] [CrossRef]
- Riguet, N.; Mahul-Mellier, A.L.; Maharjan, N.; Burtscher, J.; Croisier, M.; Knott, G.; Hastings, J.; Patin, A.; Reiterer, V.; Farhan, H.; et al. Nuclear and cytoplasmic huntingtin inclusions exhibit distinct biochemical composition, interactome and ultrastructural properties. Nat. Commun. 2021, 12, 6579. [Google Scholar] [CrossRef]
- Duyao, M.P.; Auerbach, A.B.; Ryan, A.; Persichetti, F.; Barnes, G.T.; McNeil, S.M.; Ge, P.; Vonsattel, J.P.; Gusella, J.F.; Joyner, A.L.; et al. Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science 1995, 269, 407–410. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nagai, Y. Protein Misfolding and Aggregation as a Therapeutic Target for Polyglutamine Diseases. Brain Sci. 2017, 7, 128. [Google Scholar] [CrossRef]
- Nollen, E.A.; Garcia, S.M.; van Haaften, G.; Kim, S.; Chavez, A.; Morimoto, R.I.; Plasterk, R.H. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc. Natl. Acad. Sci. USA 2004, 101, 6403–6408. [Google Scholar] [CrossRef]
- Kaliszewski, M.; Knott, A.B.; Bossy-Wetzel, E. Primary cilia and autophagic dysfunction in Huntington’s disease. Cell Death Differ. 2015, 22, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.; Kreiner, G.; Kaminska, K.; Wood, A.J.; Kirsch, J.; Tucker, K.L.; Parlato, R. Targeted Depletion of Primary Cilia in Dopaminoceptive Neurons in a Preclinical Mouse Model of Huntington’s Disease. Front. Cell. Neurosci. 2019, 13, 565. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.M.; Katsanis, N. Microtubule transport defects in neurological and ciliary disease. Cell. Mol. Life Sci. CMLS 2005, 62, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.; Tebbe, L.; Weber, C.; Messaddeq, N.; Morle, L.; Kessler, P.; Wolfrum, U.; Trottier, Y. A novel function of Huntingtin in the cilium and retinal ciliopathy in Huntington’s disease mice. Neurobiol. Dis. 2015, 80, 15–28. [Google Scholar] [CrossRef]
- Ma, R.; Kutchy, N.A.; Chen, L.; Meigs, D.D.; Hu, G. Primary cilia and ciliary signaling pathways in aging and age-related brain disorders. Neurobiol. Dis. 2022, 163, 105607. [Google Scholar] [CrossRef]
- Volos, P.; Fujise, K.; Rafiq, N.M. Roles for primary cilia in synapses and neurological disorders. Trends Cell Biol. 2025, 35, 6–10. [Google Scholar] [CrossRef]
- Lattao, R. Centrosomes and cilia in neurodegeneration: Main actors or mere spectators? Open Biol. 2025, 15, 240317. [Google Scholar] [CrossRef]
- Liu, J.P.; Zeitlin, S.O. The long and the short of aberrant ciliogenesis in Huntington disease. J. Clin. Investig. 2011, 121, 4237–4241. [Google Scholar] [CrossRef]
- Maiuri, T.; Woloshansky, T.; Xia, J.; Truant, R. The huntingtin N17 domain is a multifunctional CRM1 and Ran-dependent nuclear and cilial export signal. Hum. Mol. Genet. 2013, 22, 1383–1394. [Google Scholar] [CrossRef]
- Atwal, R.S.; Desmond, C.R.; Caron, N.; Maiuri, T.; Xia, J.; Sipione, S.; Truant, R. Kinase inhibitors modulate huntingtin cell localization and toxicity. Nat. Chem. Biol. 2011, 7, 453–460. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Overbye, A.; Brech, A.; Torgersen, M.L.; Jakobsen, I.S.; Sandvig, K.; Llorente, A. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell. Mol. Life Sci. 2016, 73, 4717–4737. [Google Scholar] [CrossRef]
- Trajkovic, K.; Jeong, H.; Krainc, D. Mutant Huntingtin Is Secreted via a Late Endosomal/Lysosomal Unconventional Secretory Pathway. J. Neurosci. 2017, 37, 9000–9012. [Google Scholar] [CrossRef]
- Drummond, M.L.; Li, M.; Tarapore, E.; Nguyen, T.T.L.; Barouni, B.J.; Cruz, S.; Tan, K.C.; Oro, A.E.; Atwood, S.X. Actin polymerization controls cilia-mediated signaling. J. Cell Biol. 2018, 217, 3255–3266. [Google Scholar] [CrossRef]
- Nager, A.R.; Goldstein, J.S.; Herranz-Perez, V.; Portran, D.; Ye, F.; Garcia-Verdugo, J.M.; Nachury, M.V. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 2017, 168, 252–263.e214. [Google Scholar] [CrossRef]
- Sharma, M.; Subramaniam, S. Rhes travels from cell to cell and transports Huntington disease protein via TNT-like protrusion. J. Cell Biol. 2019, 218, 1972–1993. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Abounit, S.; Marzo, L.; Danckaert, A.; Chamoun, Z.; Roux, P.; Zurzolo, C. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J. Cell Sci. 2013, 126, 3678–3685. [Google Scholar] [CrossRef] [PubMed]
- Ananbeh, H.; Novak, J.; Juhas, S.; Juhasova, J.; Klempir, J.; Doleckova, K.; Rysankova, I.; Turnovcova, K.; Hanus, J.; Hansikova, H.; et al. Huntingtin Co-Isolates with Small Extracellular Vesicles from Blood Plasma of TgHD and KI-HD Pig Models of Huntington’s Disease and Human Blood Plasma. Int. J. Mol. Sci. 2022, 23, 5598. [Google Scholar] [CrossRef] [PubMed]
- Danna, R. Ciliary Dysfunction and Protein Propagation in AD, PD, and HD. 2025. Available online: https://BioRender.com/jasqo6c (accessed on 4 December 2025).
- Martinez-Vicente, M.; Talloczy, Z.; Wong, E.; Tang, G.; Koga, H.; Kaushik, S.; de Vries, R.; Arias, E.; Harris, S.; Sulzer, D.; et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010, 13, 567–576. [Google Scholar] [CrossRef]
- Folger, A.; Wang, Y. The Cytotoxicity and Clearance of Mutant Huntingtin and Other Misfolded Proteins. Cells 2021, 10, 2835. [Google Scholar] [CrossRef]
- Saba, J.; Couselo, F.L.; Bruno, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Neuroinflammation in Huntington’s Disease: A Starring Role for Astrocyte and Microglia. Curr. Neuropharmacol. 2022, 20, 1116–1143. [Google Scholar] [CrossRef]
- Disatnik, M.H.; Joshi, A.U.; Saw, N.L.; Shamloo, M.; Leavitt, B.R.; Qi, X.; Mochly-Rosen, D. Potential biomarkers to follow the progression and treatment response of Huntington’s disease. J. Exp. Med. 2016, 213, 2655–2669. [Google Scholar] [CrossRef]
- Jia, Q.; Li, S.; Li, X.J.; Yin, P. Neuroinflammation in Huntington’s disease: From animal models to clinical therapeutics. Front. Immunol. 2022, 13, 1088124. [Google Scholar] [CrossRef]
- Stoberl, N.; Donaldson, J.; Binda, C.S.; McAllister, B.; Hall-Roberts, H.; Jones, L.; Massey, T.H.; Allen, N.D. Mutant huntingtin confers cell-autonomous phenotypes on Huntington’s disease iPSC-derived microglia. Sci. Rep. 2023, 13, 20477. [Google Scholar] [CrossRef]
- Deng, J.; Koutras, C.; Donnelier, J.; Alshehri, M.; Fotouhi, M.; Girard, M.; Casha, S.; McPherson, P.S.; Robbins, S.M.; Braun, J.E.A. Neurons Export Extracellular Vesicles Enriched in Cysteine String Protein and Misfolded Protein Cargo. Sci. Rep. 2017, 7, 956. [Google Scholar] [CrossRef]
- Lee, M.; Liu, T.; Im, W.; Kim, M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur. J. Neurosci. 2016, 44, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Choi, J.H.; Kim, M.S. Primary Cilia as a Signaling Platform for Control of Energy Metabolism. Diabetes Metab. J. 2018, 42, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, R.; Matsuda, Y.; Ijaz, F.; Ikegami, K. Circadian oscillation in primary cilium length by clock genes regulates fibroblast cell migration. EMBO Rep. 2023, 24, e56870. [Google Scholar] [CrossRef]
- Yang, D.J.; Hong, J.; Kim, K.W. Hypothalamic primary cilium: A hub for metabolic homeostasis. Exp. Mol. Med. 2021, 53, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- DeLong, K.; Sheu, S.H. Serotonin signaling at cilia synapses. Curr. Opin. Neurobiol. 2025, 92, 102994. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levalahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Ornish, D.; Madison, C.; Kivipelto, M.; Kemp, C.; McCulloch, C.E.; Galasko, D.; Artz, J.; Rentz, D.; Lin, J.; Norman, K.; et al. Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: A randomized, controlled clinical trial. Alzheimers Res. Ther. 2024, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Zhang, M.Y.; Wang, Z.L.; Deng, J.H.; Bao, Y.P.; Shi, J.; Lu, L.; Shi, L. Associations among sleep quality, sleep duration, and Alzheimer’s disease biomarkers: A systematic review and meta-analysis. Alzheimers Dement. 2025, 21, e70096. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Cao, W.; Lin, J.; Xiang, W.; Liu, J.; Wang, B.; Liao, W.; Jiang, T. Physical Exercise-Induced Astrocytic Neuroprotection and Cognitive Improvement Through Primary Cilia and Mitogen-Activated Protein Kinases Pathway in Rats With Chronic Cerebral Hypoperfusion. Front. Aging Neurosci. 2022, 14, 866336. [Google Scholar] [CrossRef]
- Fuller, O.K.; Whitham, M.; Mathivanan, S.; Febbraio, M.A. The Protective Effect of Exercise in Neurodegenerative Diseases: The Potential Role of Extracellular Vesicles. Cells 2020, 9, 2182. [Google Scholar] [CrossRef]
- Fang, X.; Han, D.; Cheng, Q.; Zhang, P.; Zhao, C.; Min, J.; Wang, F. Association of Levels of Physical Activity With Risk of Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2018, 1, e182421. [Google Scholar] [CrossRef]
- Frese, S.; Petersen, J.A.; Ligon-Auer, M.; Mueller, S.M.; Mihaylova, V.; Gehrig, S.M.; Kana, V.; Rushing, E.J.; Unterburger, E.; Kagi, G.; et al. Exercise effects in Huntington disease. J. Neurol. 2017, 264, 32–39. [Google Scholar] [CrossRef]
- Avila-Villanueva, M.; Gomez-Ramirez, J.; Maestu, F.; Venero, C.; Avila, J.; Fernandez-Blazquez, M.A. The Role of Chronic Stress as a Trigger for the Alzheimer Disease Continuum. Front. Aging Neurosci. 2020, 12, 561504. [Google Scholar] [CrossRef]
- Mo, C.; Renoir, T.; Hannan, A.J. Effects of chronic stress on the onset and progression of Huntington’s disease in transgenic mice. Neurobiol. Dis. 2014, 71, 81–94. [Google Scholar] [CrossRef]
- Xia, D.; Xiong, M.; Yang, Y.; Wang, X.; Chen, Q.; Li, S.; Meng, L.; Zhang, Z. Chronic stress induces depression-like behaviors and Parkinsonism via upregulating α-synuclein. npj Park. Dis. 2025, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, Y.; Liu, J.; Peng, Y.; Wang, D.; Li, L.; Hu, X.; Li, J.; Wang, L.; Chu, J.; et al. Primary ciliary protein kinase A activity in the prefrontal cortex modulates stress in mice. Neuron 2025, 113, 1276–1289.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, C.; Xiong, X.; Yang, Y.; Zhang, J. Extracellular vesicles: New horizons in neurodegeneration. eBioMedicine 2025, 113, 105605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, M.; Wang, S.; Chu, S.; Zhang, Z.; Chen, N. Tunneling nanotubes: The transport highway for astrocyte-neuron communication in the central nervous system. Brain Res. Bull. 2024, 209, 110921. [Google Scholar] [CrossRef]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People with Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, F.; Marconi, A.; Tagliaferri, A.; Manzoni, G.M.; Gatto, R.; Fabiani, V.; Gragnano, G.; Rossi, G.; Volpato, E.; Banfi, P.; et al. Meditation training for people with amyotrophic lateral sclerosis: A randomized clinical trial. Eur. J. Neurol. 2017, 24, 578–586. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef]
- Maddalena, A.; Papassotiropoulos, A.; Muller-Tillmanns, B.; Jung, H.H.; Hegi, T.; Nitsch, R.M.; Hock, C. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to β-amyloid peptide42. Arch. Neurol. 2003, 60, 1202–1206. [Google Scholar] [CrossRef]
- Andreasen, N.; Minthon, L.; Davidsson, P.; Vanmechelen, E.; Vanderstichele, H.; Winblad, B.; Blennow, K. Evaluation of CSF-tau and CSF-Aβ42 as diagnostic markers for Alzheimer disease in clinical practice. Arch. Neurol. 2001, 58, 373–379. [Google Scholar] [CrossRef]
- Heyer, S.; Simon, M.; Doyen, M.; Mortada, A.; Roch, V.; Jeanbert, E.; Thilly, N.; Malaplate, C.; Kearney-Schwartz, A.; Jonveaux, T.; et al. (18)F-FDG PET can effectively rule out conversion to dementia and the presence of CSF biomarker of neurodegeneration: A real-world data analysis. Alzheimers Res. Ther. 2024, 16, 182. [Google Scholar] [CrossRef]
- Ou, Y.N.; Xu, W.; Li, J.Q.; Guo, Y.; Cui, M.; Chen, K.L.; Huang, Y.Y.; Dong, Q.; Tan, L.; Yu, J.T.; et al. FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: A longitudinal study. Alzheimers Res. Ther. 2019, 11, 57. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Przybelski, S.A.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Petersen, R.C.; Jack, C.R., Jr. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain 2007, 130, 1777–1786. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yang, Y.; Cai, H.; Ao, Z.; Xing, Y.; Li, K.; Yang, K.; Guan, W.; Friend, J.; et al. Extracellular vesicle-based point-of-care testing for diagnosis and monitoring of Alzheimer’s disease. Microsyst. Nanoeng. 2025, 11, 65. [Google Scholar] [CrossRef]
- Singh, R.; Rai, S.; Bharti, P.S.; Zehra, S.; Gorai, P.K.; Modi, G.P.; Rani, N.; Dev, K.; Inampudi, K.K.; Vishnu, V.Y.; et al. Circulating small extracellular vesicles in Alzheimer’s disease: A case-control study of neuro-inflammation and synaptic dysfunction. BMC Med. 2024, 22, 254. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernandez-Sapiens, M.A.; Gutierrez-Mercado, Y.K.; Sandoval-Avila, S.; Gomez-Pinedo, U.; Marquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, J.; Gu, G.; Han, X.; Zhang, Q.; Zhang, W. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J. Neurochem. 2020, 154, 502–518. [Google Scholar] [CrossRef]
- Liu, Y.; Huber, C.C.; Wang, H. Disrupted blood-brain barrier in 5xFAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 525, 192–196. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019, 95, 236–244. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Zhang, Q.Y.; Tan, J.; Xie, H.Q. Techniques for increasing the yield of stem cell-derived exosomes: What factors may be involved? Sci. China Life Sci. 2022, 65, 1325–1341. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Tariot, P.N.; Farlow, M.R.; Grossberg, G.T.; Graham, S.M.; McDonald, S.; Gergel, I.; Memantine Study, G. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. JAMA 2004, 291, 317–324. [Google Scholar] [CrossRef]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Heng, N.; Malek, N.; Lawton, M.A.; Nodehi, A.; Pitz, V.; Grosset, K.A.; Ben-Shlomo, Y.; Grosset, D.G. Striatal Dopamine Loss in Early Parkinson’s Disease: Systematic Review and Novel Analysis of Dopamine Transporter Imaging. Mov. Disord. Clin. Pract. 2023, 10, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, W.; Li, X.; Dutta, S.; Castle, A.R.; Liu, Y.; Sahoo, A.; Lam, C.L.; Gatford, N.J.F.; Hu, M.T.; et al. Single extracellular vesicle detection assay identifies membrane-associated α-synuclein as an early-stage biomarker in Parkinson’s disease. Cell Rep. Med. 2025, 6, 101999. [Google Scholar] [CrossRef]
- Hauser, R.A.; Hsu, A.; Kell, S.; Espay, A.J.; Sethi, K.; Stacy, M.; Ondo, W.; O’Connell, M.; Gupta, S. IPX066 ADVANCE-PD investigators. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: A phase 3 randomised, double-blind trial. Lancet Neurol. 2013, 12, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Weaver, F.M.; Follett, K.; Stern, M.; Hur, K.; Harris, C.; Marks, W.J., Jr.; Rothlind, J.; Sagher, O.; Reda, D.; Moy, C.S.; et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. JAMA 2009, 301, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lotvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Esteves, M.; Abreu, R.; Fernandes, H.; Serra-Almeida, C.; Martins, P.A.T.; Barao, M.; Cristovao, A.C.; Saraiva, C.; Ferreira, R.; Ferreira, L.; et al. MicroRNA-124-3p-enriched small extracellular vesicles as a therapeutic approach for Parkinson’s disease. Mol. Ther. 2022, 30, 3176–3192. [Google Scholar] [CrossRef]
- Diaz Reyes, M.; Gatti, S.; Delgado Ocana, S.; Ortega, H.H.; Banchio, C. Neuroprotective effect of NSCs-derived extracellular vesicles in Parkinson’s disease models. Sci. Rep. 2025, 15, 6092. [Google Scholar] [CrossRef]
- Bean, L.; Bayrak-Toydemir, P. American College of Medical Genetics and Genomics Standards and Guidelines for Clinical Genetics Laboratories, 2014 edition: Technical standards and guidelines for Huntington disease. Genet. Med. 2014, 16, e2. [Google Scholar] [CrossRef]
- Ranen, N.G.; Stine, O.C.; Abbott, M.H.; Sherr, M.; Codori, A.M.; Franz, M.L.; Chao, N.I.; Chung, A.S.; Pleasant, N.; Callahan, C.; et al. Anticipation and instability of IT-15 (CAG)n repeats in parent-offspring pairs with Huntington disease. Am. J. Hum. Genet. 1995, 57, 593–602. [Google Scholar]
- Fusilli, C.; Migliore, S.; Mazza, T.; Consoli, F.; De Luca, A.; Barbagallo, G.; Ciammola, A.; Gatto, E.M.; Cesarini, M.; Etcheverry, J.L.; et al. Biological and clinical manifestations of juvenile Huntington’s disease: A retrospective analysis. Lancet Neurol. 2018, 17, 986–993. [Google Scholar] [CrossRef]
- Soylu-Kucharz, R.; Sandelius, A.; Sjogren, M.; Blennow, K.; Wild, E.J.; Zetterberg, H.; Bjorkqvist, M. Neurofilament light protein in CSF and blood is associated with neurodegeneration and disease severity in Huntington’s disease R6/2 mice. Sci. Rep. 2017, 7, 14114. [Google Scholar] [CrossRef]
- Zeun, P.; Scahill, R.I.; Tabrizi, S.J.; Wild, E.J. Fluid and imaging biomarkers for Huntington’s disease. Mol. Cell. Neurosci. 2019, 97, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Vauleon, S.; Schutz, K.; Massonnet, B.; Gruben, N.; Manchester, M.; Buehler, A.; Schick, E.; Boak, L.; Hawellek, D.J. Quantifying mutant huntingtin protein in human cerebrospinal fluid to support the development of huntingtin-lowering therapies. Sci. Rep. 2023, 13, 5332. [Google Scholar] [CrossRef]
- Caron, N.S.; Banos, R.; Yanick, C.; Aly, A.E.; Byrne, L.M.; Smith, E.D.; Xie, Y.; Smith, S.E.P.; Potluri, N.; Findlay Black, H.; et al. Mutant Huntingtin Is Cleared from the Brain via Active Mechanisms in Huntington Disease. J. Neurosci. 2021, 41, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Niemela, V.; Landtblom, A.M.; Blennow, K.; Sundblom, J. Tau or neurofilament light-Which is the more suitable biomarker for Huntington’s disease? PLoS ONE 2017, 12, e0172762. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef]
- Harris, G.J.; Codori, A.M.; Lewis, R.F.; Schmidt, E.; Bedi, A.; Brandt, J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington’s disease. Brain 1999, 122, 1667–1678. [Google Scholar] [CrossRef]
- Denis, H.L.; Lamontagne-Proulx, J.; St-Amour, I.; Mason, S.L.; Weiss, A.; Chouinard, S.; Barker, R.A.; Boilard, E.; Cicchetti, F. Platelet-derived extracellular vesicles in Huntington’s disease. J. Neurol. 2018, 265, 2704–2712. [Google Scholar] [CrossRef]
- Huntington Study, G. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology 2006, 66, 366–372. [Google Scholar] [CrossRef]
- Lee, S.T.; Im, W.; Ban, J.J.; Lee, M.; Jung, K.H.; Lee, S.K.; Chu, K.; Kim, M. Exosome-Based Delivery of miR-124 in a Huntington’s Disease Model. J. Mov. Disord. 2017, 10, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Belloni, E.; Gaudenz, K.; Jay, P.; Berta, P.; Scherer, S.W.; Tsui, L.C.; Muenke, M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat. Genet. 1996, 14, 357–360. [Google Scholar] [CrossRef]
- Spassky, N.; Han, Y.G.; Aguilar, A.; Strehl, L.; Besse, L.; Laclef, C.; Ros, M.R.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 2008, 317, 246–259. [Google Scholar] [CrossRef]
- Jaafar Marican, N.H.; Cruz-Migoni, S.B.; Borycki, A.G. Asymmetric Distribution of Primary Cilia Allocates Satellite Cells for Self-Renewal. Stem Cell Rep. 2016, 6, 798–805. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, L.E.; Verbitsky, M.; Blesa, J.; Jackson-Lewis, V.; Paredes, D.; Tillack, K.; Phani, S.; Kramer, E.R.; Przedborski, S.; Kottmann, A.H. Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron 2012, 75, 306–319. [Google Scholar] [CrossRef]
- Sun, F.; Mao, X.; Xie, L.; Ding, M.; Shao, B.; Jin, K. Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell 2013, 12, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Halleskog, C.; Schulte, G. Pertussis toxin-sensitive heterotrimeric Gαi/o proteins mediate WNT/β-catenin and WNT/ERK1/2 signaling in mouse primary microglia stimulated with purified WNT-3A. Cell. Signal. 2013, 25, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, W.; Guo, L.; Zhao, L.; Sun, S.; Li, H. The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea. Cell Tissue Res. 2021, 386, 281–296. [Google Scholar] [CrossRef]
- Ma, X.; Drannik, A.; Jiang, F.; Peterson, R.; Turnbull, J. Crosstalk between Notch and Sonic hedgehog signaling in a mouse model of amyotrophic lateral sclerosis. Neuroreport 2017, 28, 141–148. [Google Scholar] [CrossRef]
- Bae, J.E.; Kang, G.M.; Min, S.H.; Jo, D.S.; Jung, Y.K.; Kim, K.; Kim, M.S.; Cho, D.H. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson’s disease model. Cell Death Dis. 2019, 10, 952. [Google Scholar] [CrossRef]
- Wu, C.L.; Chen, S.D.; Hwang, C.S.; Yang, D.I. Sonic hedgehog mediates BDNF-induced neuroprotection against mitochondrial inhibitor 3-nitropropionic acid. Biochem. Biophys. Res. Commun. 2009, 385, 112–117. [Google Scholar] [CrossRef]
- Cerrotti, G.; Buratta, S.; Latella, R.; Calzoni, E.; Cusumano, G.; Bertoldi, A.; Porcellati, S.; Emiliani, C.; Urbanelli, L. Hitting the target: Cell signaling pathways modulation by extracellular vesicles. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 527–552. [Google Scholar] [CrossRef] [PubMed]
- Cerpa, W.; Farias, G.G.; Godoy, J.A.; Fuenzalida, M.; Bonansco, C.; Inestrosa, N.C. Wnt-5a occludes Aβ oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol. Neurodegener. 2010, 5, 3. [Google Scholar] [CrossRef]
- Hall, A.C.; Lucas, F.R.; Salinas, P.C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 2000, 100, 525–535. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Wu, Q.; Shi, J. Dendrobium nobile Lindl. Alkaloids Ameliorate Aβ25-35-Induced Synaptic Deficits by Targeting Wnt/β-Catenin Pathway in Alzheimer’s Disease Models. J. Alzheimers Dis. 2022, 86, 297–313. [Google Scholar] [CrossRef]
- Lucas, F.R.; Salinas, P.C. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 1997, 192, 31–44. [Google Scholar] [CrossRef]
- Laksitorini, M.D.; Yathindranath, V.; Xiong, W.; Hombach-Klonisch, S.; Miller, D.W. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci. Rep. 2019, 9, 19718. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Mohanbhai, S.J.; Tiwari, V.; Chaturvedi, R.K.; Khurana, S.; Shukla, S. Axin-2 knockdown promote mitochondrial biogenesis and dopaminergic neurogenesis by regulating Wnt/β-catenin signaling in rat model of Parkinson’s disease. Free Radic. Biol. Med. 2018, 129, 73–87. [Google Scholar] [CrossRef]
- Vargas, J.Y.; Loria, F.; Wu, Y.J.; Cordova, G.; Nonaka, T.; Bellow, S.; Syan, S.; Hasegawa, M.; van Woerden, G.M.; Trollet, C.; et al. The Wnt/Ca2+ pathway is involved in interneuronal communication mediated by tunneling nanotubes. EMBO J. 2019, 38, e101230. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Inestrosa, N.C. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Tsai, C.W.; Deak, F.; Rogers, J.; Penuliar, M.; Sung, Y.M.; Maher, J.N.; Fu, Y.; Li, X.; Xu, H.; et al. Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron 2014, 84, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Castillon, C.C.M.; Gebis, K.K.; Bartom, E.T.; d’Azzo, A.; Contractor, A.; Savas, J.N. Notch receptor-ligand binding facilitates extracellular vesicle-mediated neuron-to-neuron communication. Cell Rep. 2024, 43, 113680. [Google Scholar] [CrossRef]
- Grandbarbe, L.; Michelucci, A.; Heurtaux, T.; Hemmer, K.; Morga, E.; Heuschling, P. Notch signaling modulates the activation of microglial cells. Glia 2007, 55, 1519–1530. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Yu, M.; Yang, F.; Tu, M.; Xu, H. Notch Signaling Regulates Microglial Activation and Inflammatory Reactions in a Rat Model of Temporal Lobe Epilepsy. Neurochem. Res. 2018, 43, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Barr, M.M. Ciliary Extracellular Vesicles: Txt Msg Organelles. Cell. Mol. Neurobiol. 2016, 36, 449–457. [Google Scholar] [CrossRef]
- Smith, C.E.L.; Lake, A.V.R.; Johnson, C.A. Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, A Little More Actin Please. Front. Cell Dev. Biol. 2020, 8, 622822. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Cao, M.; Chen, M.; Liu, M.; Xie, S. Cytoskeletal networks in primary cilia: Current knowledge and perspectives. J. Cell. Physiol. 2022, 237, 3975–3983. [Google Scholar] [CrossRef]
- Zhang, C.T.; Wang, J.; Wang, W.Y. Wnt signaling in synaptogenesis of Alzheimer’s disease. Ibrain 2023, 9, 316–325. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef]
- Smith-Geater, C.; Hernandez, S.J.; Lim, R.G.; Adam, M.; Wu, J.; Stocksdale, J.T.; Wassie, B.T.; Gold, M.P.; Wang, K.Q.; Miramontes, R.; et al. Aberrant Development Corrected in Adult-Onset Huntington’s Disease iPSC-Derived Neuronal Cultures via WNT Signaling Modulation. Stem Cell Rep. 2020, 14, 406–419. [Google Scholar] [CrossRef]
- Chen, G. The Interplay Between EVs, TNTs, and Ciliary Signaling Pathways: Shh, Wnt, and Notch. 2025. Available online: https://BioRender.com/3h40i7a (accessed on 4 December 2025).
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly, Y.M.; Gidlof, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Lipinski, M.M.; Zheng, B.; Lu, T.; Yan, Z.; Py, B.F.; Ng, A.; Xavier, R.J.; Li, C.; Yankner, B.A.; Scherzer, C.R.; et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2010, 107, 14164–14169. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lin, M.G.; Stowe, T.R.; Chen, S.; Zhu, M.; Stearns, T.; Franco, B.; Zhong, Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 2013, 502, 254–257. [Google Scholar] [CrossRef]
- Spektor, A.; Tsang, W.Y.; Khoo, D.; Dynlacht, B.D. Cep97 and CP110 suppress a cilia assembly program. Cell 2007, 130, 678–690. [Google Scholar] [CrossRef]
- Arora, K.; Lund, J.R.; Naren, N.A.; Zingarelli, B.; Naren, A.P. AC6 regulates the microtubule-depolymerizing kinesin KIF19A to control ciliary length in mammals. J. Biol. Chem. 2020, 295, 14250–14259. [Google Scholar] [CrossRef]
- Morleo, M.; Vieira, H.L.A.; Pennekamp, P.; Palma, A.; Bento-Lopes, L.; Omran, H.; Lopes, S.S.; Barral, D.C.; Franco, B. Crosstalk between cilia and autophagy: Implication for human diseases. Autophagy 2023, 19, 24–43. [Google Scholar] [CrossRef]
- Bae, J.E.; Jang, S.; Kim, J.B.; Hyung, H.; Park, N.Y.; Kim, Y.H.; Kim, S.H.; Kim, S.H.; Ha, J.M.; Oh, G.S.; et al. Enhanced primary ciliogenesis via mitochondrial oxidative stress activates AKT to prevent neurotoxicity in HSPA9/mortalin-depleted SH-SY5Y cells. Mol. Brain 2023, 16, 41. [Google Scholar] [CrossRef]
- Rivagorda, M.; Romeo-Guitart, D.; Blanchet, V.; Mailliet, F.; Boitez, V.; Barry, N.; Milunov, D.; Siopi, E.; Goudin, N.; Moriceau, S.; et al. A primary cilia-autophagy axis in hippocampal neurons is essential to maintain cognitive resilience. Nat. Aging 2025, 5, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, M.C.; Al Saedi, A.; Fulzele, S.; Hamrick, M.W. Extracellular Vesicles as Communicators of Senescence in Musculoskeletal Aging. JBMR Plus 2022, 6, e10686. [Google Scholar] [CrossRef]
- Mohieldin, A.M.; Pala, R.; Beuttler, R.; Moresco, J.J.; Yates, J.R., III; Nauli, S.M. Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12086. [Google Scholar] [CrossRef]
- Davis, D.M.; Sowinski, S. Membrane nanotubes: Dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Cordero Cervantes, D.; Zurzolo, C. Peering into tunneling nanotubes-The path forward. EMBO J. 2021, 40, e105789. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Austefjord, M.W.; Gerdes, H.H.; Wang, X. Tunneling nanotubes: Diversity in morphology and structure. Commun. Integr. Biol. 2014, 7, e27934. [Google Scholar] [CrossRef]
- Eltom, K.; Mothes, T.; Libard, S.; Ingelsson, M.; Erlandsson, A. Astrocytic accumulation of tau fibrils isolated from Alzheimer’s disease brains induces inflammation, cell-to-cell propagation and neuronal impairment. Acta Neuropathol. Commun. 2024, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Risner, M.L.; Ribeiro, M.; McGrady, N.R.; Kagitapalli, B.S.; Chamling, X.; Zack, D.J.; Calkins, D.J. Neutral sphingomyelinase inhibition promotes local and network degeneration in vitro and in vivo. Cell Commun. Signal. 2023, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ellman, D.G.; Fang, S.; Bak, S.T.; Norgard, M.O.; Svenningsen, P.; Andersen, D.C. Transfer of cardiomyocyte-derived extracellular vesicles to neighboring cardiac cells requires tunneling nanotubes during heart development. Theranostics 2024, 14, 3843–3858. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Nahacka, Z.; Oliveira, G.L.; Brisudova, P.; Dubisova, M.; Dvorakova, S.; Miklovicova, S.; Dalecka, M.; Puttrich, V.; Grycova, L.; et al. The adaptor protein Miro1 modulates horizontal transfer of mitochondria in mouse melanoma models. Cell Rep. 2025, 44, 115154. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Huang, N.; Meng, W.; Liu, Y.; Li, J.; Li, C.; Wang, W.; Lai, Y.; Zhao, Y.; Ma, Z.; et al. Beyond mitochondrial transfer, cell fusion rescues metabolic dysfunction and boosts malignancy in adenoid cystic carcinoma. Cell Rep. 2024, 43, 114652. [Google Scholar] [CrossRef]
- Chakraborty, R.; Belian, S.; Zurzolo, C. Hijacking intercellular trafficking for the spread of protein aggregates in neurodegenerative diseases: A focus on tunneling nanotubes (TNTs). Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 27–43. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front. Mol. Biosci. 2017, 4, 50. [Google Scholar] [CrossRef]
- Palese, F.; Rakotobe, M.; Zurzolo, C. Transforming the concept of connectivity: Unveiling tunneling nanotube biology and their roles in brain development and neurodegeneration. Physiol. Rev. 2025, 105, 1823–1865. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).