Behavioral Responses of the Bumblebee Bombus terrestris to Volatile Compounds from Blueberries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Collection and Analysis of Headspace Volatiles from Vaccinium spp.
2.3. Gas Chromatography–Mass Spectrometry (GC–MS)
2.4. Electroantennography (EAG)
2.5. Y-Tube Olfactometer Choice-Behavioral Assays
2.6. Data Analysis
3. Results
3.1. Analysis of the Types and Contents of Volatiles
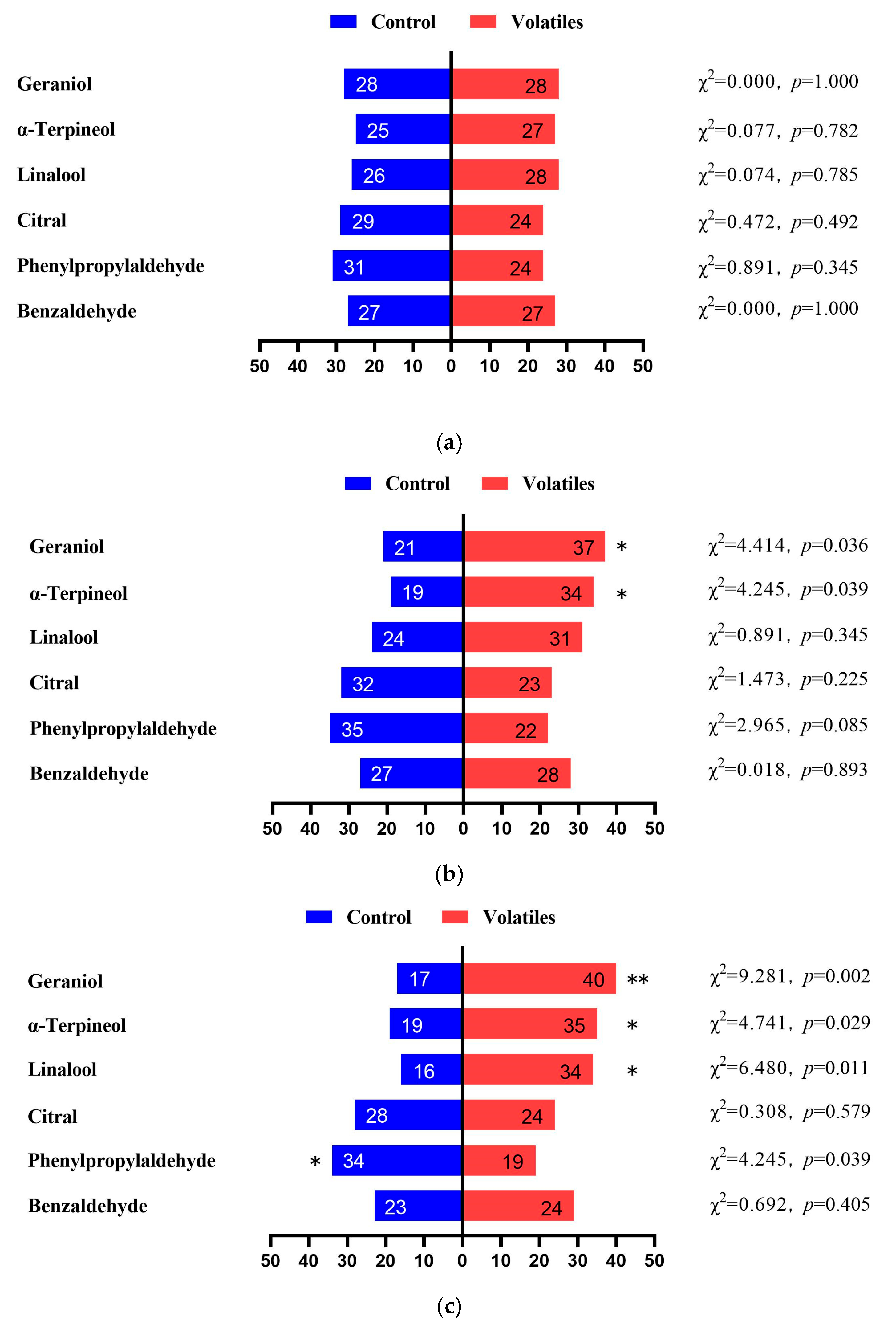
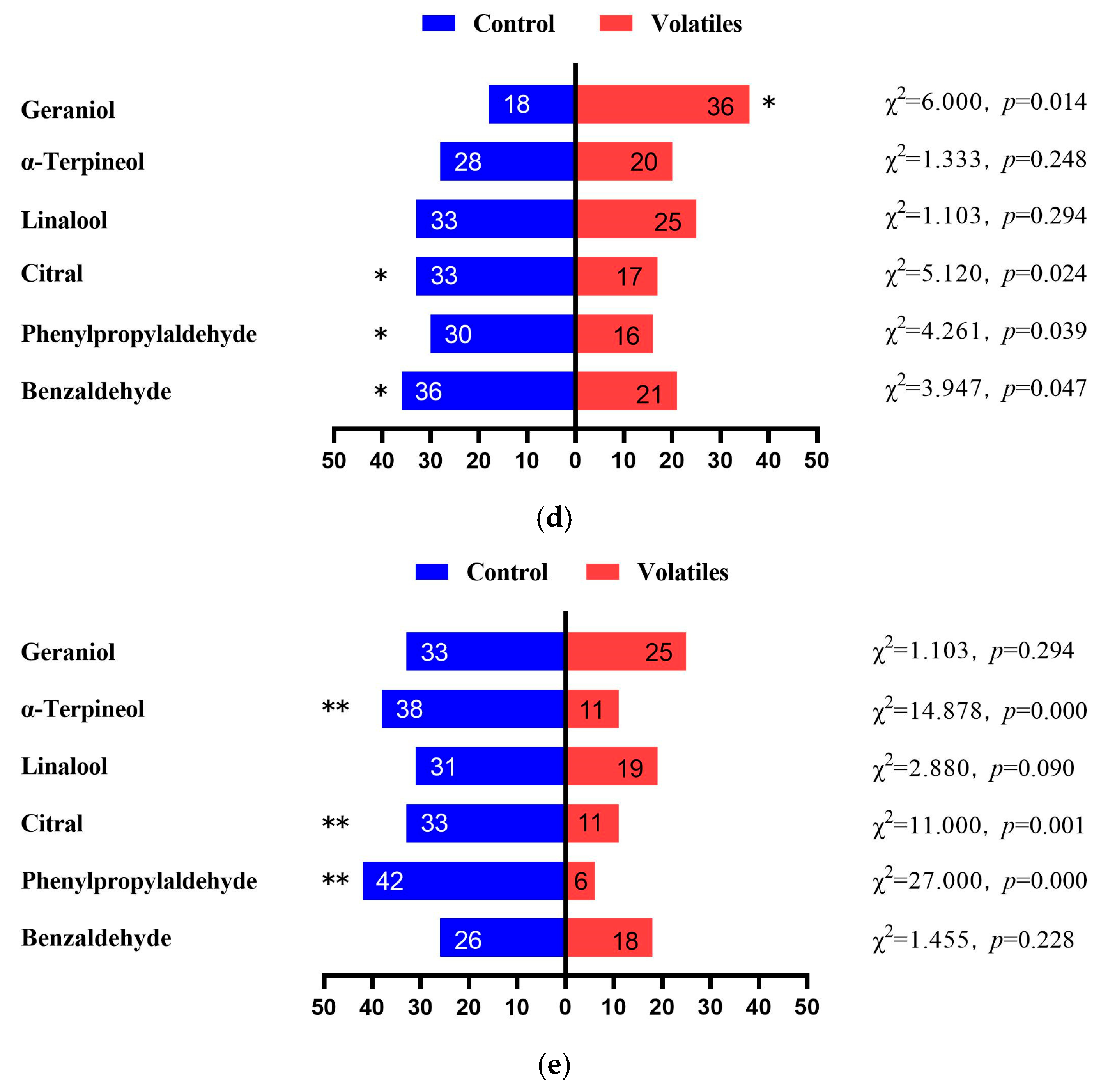
3.2. EAG Responses
3.3. Choice Behavioral Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Jiang, J.; Pang, Z.; Ma, W.; Jiang, Y.; Fu, Y.; Liu, Y. Tracking existing factors directly affecting the reproduction of bumblebees: Current knowledge. Insects 2024, 15, 654. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Williams, P.H.; Vaissière, B.E.; Zhou, Z.; Gai, Q.; Dong, J.; An, J. Managed bumblebees outperform honeybees in increasing peach fruit set in China: Different limiting processes with different pollinators. PLoS ONE 2015, 10, e0121143. [Google Scholar] [CrossRef]
- Symington, H.A.; Glover, B.J. Strawberry varieties differ in pollinator-relevant floral traits. Ecol. Evol. 2024, 14, e10914. [Google Scholar] [CrossRef]
- Pfister, S.C.; Eckerter, P.W.; Schirmel, J.; Cresswell, J.E.; Entling, M.H. Sensitivity of commercial pumpkin yield to potential decline among different groups of pollinating bees. R. Soc. Open Sci. 2017, 4, 170102. [Google Scholar] [CrossRef]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef]
- Wakamura, S.; Arakaki, N.; Moriyama, D.; Kanayama, S.; Oike, M.; Kimura, A.; Wajima, S.; Ono, H.; Yasui, H. Does the orchid Luisia teres attract its male chafer pollinators (Scarabaeidae: Protaetia pryeri pryeri) by sexual deception? Chemoecology 2020, 30, 49–57. [Google Scholar] [CrossRef]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Colazza, S.; Peri, E.; Cusumano, A. Chemical ecology of floral resources in conservation biological control. Annu. Rev. Entomol. 2023, 68, 13–29. [Google Scholar] [CrossRef]
- Lyu, Z.; Zhou, T.; Sun, M.; Feng, M.; Guo, W.; Nie, L.; Song, Y.; Men, X.; Li, L.; Yu, Y. Exploratory comparison of flower visiting behavior and pollination ability of mason bees, bumble bees, and honey bees. J. Econ. Entomol. 2023, 116, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.M.; Combes, S.A.; Hopkins, R. Dispensing pollen via catapult: Explosive pollen release in mountain laurel (Kalmia latifolia). Am. Nat. 2018, 191, 767–776. [Google Scholar] [CrossRef]
- Knauer, A.C.; Schiestl, F.P. Bees use honest floral signals as indicators of reward when visiting flowers. Ecol. Lett. 2015, 18, 135–143. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Sater, H.; Lyrene, P.; Amadeu, R.R.; Sims, C.A.; Tieman, D.M.; Munoz, P.R. Terpene volatiles mediates the chemical basis of blueberry aroma and consumer acceptability. Food Res. Int. 2022, 158, 111468. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Marti-Anders, C.; Álvarez, M.D.; Escribano, M.I.; Merodio, C.; Romero, I. Are the blueberries we buy good quality? comparative study of berries purchased from different outlets. Foods 2023, 12, 2621. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Cromie, J.; Ternest, J.J.; Komatz, A.P.; Adunola, P.M.; Azevedo, C.; Mallinger, R.E.; Muñoz, P.R. Genotypic variation in blueberry flower morphology and nectar reward content affects pollinator attraction in a diverse breeding population. BMC Plant Biol. 2024, 24, 814. [Google Scholar] [CrossRef]
- Miñarro, M.; García, D. Complementary contribution of wild bumblebees and managed honeybee to the pollination niche of an introduced blueberry crop. Insects 2021, 12, 595. [Google Scholar] [CrossRef]
- Bobiwash, K.; Uriel, Y.; Elle, E. Pollen foraging differences among three managed pollinators in the highbush blueberry (Vaccinium corymbosum) agroecosystem. J. Econ. Entomol. 2018, 111, 26–32. [Google Scholar] [CrossRef]
- Estravis-Barcala, M.C.; Palottini, F.; Macri, I.; Nery, D.; Farina, W.M. Managed honeybees and South American bumblebees exhibit complementary foraging patterns in highbush blueberry. Sci. Rep. 2021, 11, 8187. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Gao, F.; Chen, M.; Jiang, Y.; Zhao, H.; Ma, W. Electrophysiological and behavioral responses of apis mellifera and bombusterrestris to melon flower volatiles. Insects 2022, 13, 973. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Kessler, A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol. 2010, 188, 393–402. [Google Scholar] [CrossRef]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honestsignalling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef]
- Howell, A.D.; Alarcón, R. Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Anim. Behav. 2007, 74, 199–205. [Google Scholar] [CrossRef]
- Raman, V.; Wang, M.; Avula, B.; Lee, J.; Manfron, J.; Khan, I.A. Chemical mimicry in the corpse flower: Floral odor and phytochemical profiles of Amorphophallus titanum (becc.) becc. Biochem. Syst. Ecol. 2025, 118, 104920. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Johnson, S.D. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.L. The sweet smell of success: Floral scent affects pollinator attraction and seed fitness inHesperis matronalis. Funct. Ecol. 2009, 23, 480–487. [Google Scholar] [CrossRef]
- Spaethe, J.; Brockmann, A.; Halbig, C.; Tautz, J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Die Naturwissenschaften 2007, 94, 733–739. [Google Scholar] [PubMed]
- Yang, M.; Deng, G.C.; Gong, Y.B.; Huang, S.Q. Nectar yeasts enhance the interaction between Clematis akebioides and its bumblebee pollinator. Plant Biol. 2019, 21, 732–737. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Shen, J.; Zhao, H.; Ma, W.; Jiang, Y. Differences in EAG response and behavioral choices between honey bee and bumble bee to tomato flower volatiles. Insects 2022, 13, 987. [Google Scholar] [CrossRef]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; McCartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2017, 220, 750–759. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Parra, L.; Quiroz, A.; Isaacs, R. Variation in highbush blueberry floral volatile profiles as a function of pollination status, cultivar, time of day and flower part: Implications for flower visitation by bees. Ann. Bot. 2011, 107, 1377–1390. [Google Scholar] [CrossRef]
- Silva, F.A.N.; da Silva, A.A.; de Sousa Fernandes, N.; Rodrigues, T.H.; Canuto, K.M.; do Nascimento, R.F.; de Brito, E.S.; deAragão, F.A.S.; Freitas, B.M.; Zocolo, G.J. Evaluation of Headspace Solid-Phase Microextraction Gas Chromatography-MassSpectrometry for the characterization of volatile organic compounds from Melon (Cucumis melo L.) Flowers. Chromatographia 2018, 81, 1231–1239. [Google Scholar] [CrossRef]
- Huang, W.; Vallejo-Marín, M.; Inouye, D.W.; Yang, C.F.; Ye, Z.M. Bumblebees’ flower preferences are associated with floral abundance and buzz frequency when buzz-pollinating co-flowering plants. Entomol. Gen. 2024, 44, 133–141. [Google Scholar] [CrossRef]
- Kreiman, G.; MaBouDi, H.; Shimazaki, H.; Giurfa, M.; Chittka, L. Olfactory learning without the mushroom bodies: Spiking neural network models of the honeybee lateral antennal lobe tract reveal its capacities in odour memory tasks of varied complexities. PLoS Comput. Biol. 2017, 13, e1005551. [Google Scholar]
- Lösel, P.D.; Monchanin, C.; Lebrun, R.; Jayme, A.; Relle, J.J.; Devaud, J.M.; Heuveline, V.; Lihoreau, M. Natural variability in bee brain size and symmetry revealed by micro-CT imaging and deep learning. PLoS Comput. Biol. 2023, 19, e1011529. [Google Scholar] [CrossRef]
- Joerges, J.; Kuettner, A.; Galizia, C.G.; Menzel, R. Representations of odours and odour mixtures visualized in the honeybee brain. Nature 1997, 387, 285–287. [Google Scholar] [CrossRef]
- Galizia, C.G.; Menzel, R. Odour perception in honeybees: Coding information in glomerular patterns. Curr. Opin. Neurobiol. 2000, 10, 504–510. [Google Scholar] [CrossRef]
- Galizia, C.G.; Mcilwrath, S.L.; Menzel, R. A digital three-dimensional atlas of the honeybee antennal lobe based on optical sections acquired by confocal microscopy. Cell Tissue Res. 1999, 295, 383–394. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; GolsBeatriz, R.; Lago-NúezSergio, B.; Rasmann, S.; Röder, G.; Soengas, P.; Vázquez-González, C.; Cartea, M.E. Insect herbivory but not plant pathogen infection drive floral volatile-mediated indirect effects on pollinators and plant fitness in Brassica rapa. J. Ecol. 2024, 112, 402–415. [Google Scholar] [CrossRef]
- Clement, W.M. Flower color, a factor in attractiveness of alfalfa clones for honeybees. Crop Sci. 1965, 5, 267–268. [Google Scholar] [CrossRef]
- Kauffeld, N.M.; Sorensen, E.L. Interrelations of Honeybee Preference and Alfalfa Clones and Flower Color, Aroma, Nectar Volume and Sugar Concentration; Agricultural Experiment Station, Kansas State University of Agriculture and Aplied Science: Manhattan, NY, USA, 1971; p. 14. [Google Scholar]
- Dötterl, S.; Milchreit, K.; Schäffler, I. Behavioural plasticity and sex differences in host finding of a specialized bee species. J. Comp.Physiol. A 2011, 197, 1119–1126. [Google Scholar] [CrossRef]
- Balkenius, A.; Rosen, W.; Kelber, A. The relative importance of olfaction and vision in a diurnal and a nocturnal hawkmoth. J. Comp. Physiol. A 2006, 192, 431–437. [Google Scholar] [CrossRef]
- Wenzell, K.E.; Skogen, K.A.; Fant, J.B. Range-wide floral trait variation reflects shifts in pollinator assemblages, consistent with pollinator-mediated divergence despite generalized visitation. Oikos 2023, 2023, e09708. [Google Scholar] [CrossRef]
- Dötterl, S.; Glück, U.; Jürgens, A.; Woodring, J.; Aas, G. Floral reward, advertisement and attractiveness to honey bees in dioecious Salix caprea. PLoS ONE 2014, 9, e93421. [Google Scholar] [CrossRef]
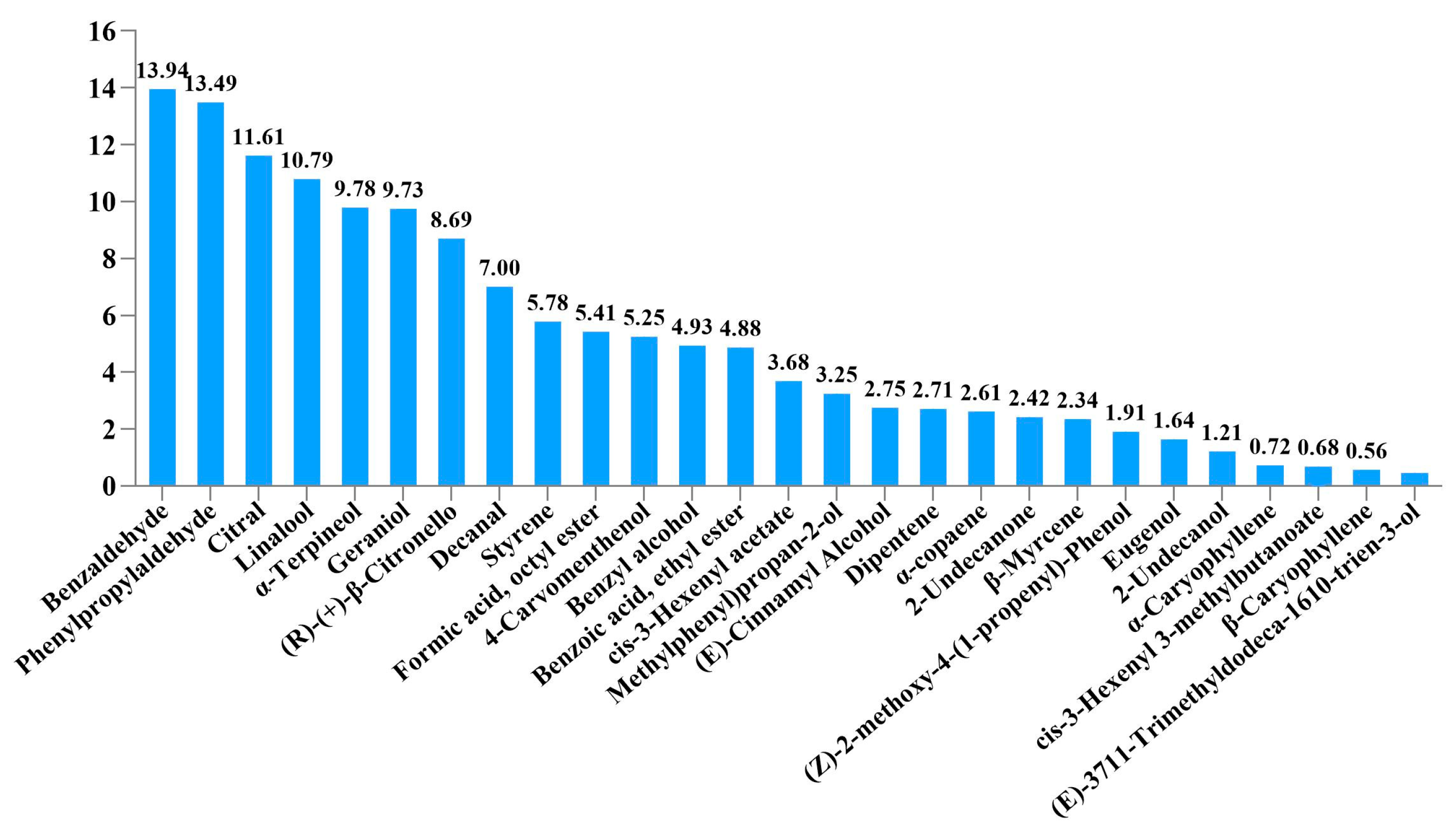

| CM | Compounds | CAS Number | Retain Time (s) | Relative Content (%) |
|---|---|---|---|---|
| Alcohols | ||||
| Benzyl alcohol | 100-51-6 | 9.028 | 0.92 | |
| Linalool | 78-70-6 | 10.419 | 25.93 | |
| 4-Carvomenthenol | 562-74-3 | 11.727 | 2.36 | |
| 2-(4-Methylphenyl)propan-2-ol | 1197-01-9 | 11.848 | 1.06 | |
| α-Terpineol | 98-55-5 | 11.948 | 3.92 | |
| (R)-(+)-β-Citronello | 1117-61-9 | 12.511 | 6.07 | |
| Geraniol | 106-24-1 | 12.923 | 5.78 | |
| 2-Undecanol | 1653-30-1 | 13.569 | 3.32 | |
| (E)-Cinnamyl Alcohol | 4407-36-7 | 13.702 | 0.42 | |
| (E)-3,7,11-Trimethyldodeca-1,6,10-trien-3-ol | 40716-66-3 | 16.985 | 0.42 | |
| Aldehydes | ||||
| Benzaldehyde | 100-52-7 | 7.153 | 1.79 | |
| Phenylacetaldehyde | 122-78-1 | 9.194 | 1.64 | |
| Phenylpropylaldehyde | 104-53-0 | 11.461 | 0.64 | |
| Decanal | 112-31-2 | 12.123 | 0.43 | |
| Citral | 5392-40-5 | 12.702 | 0.58 | |
| Esters | ||||
| cis-3-Hexenyl acetate | 3681-71-8 | 8.424 | 1.20 | |
| Formic acid, octyl ester | 112-32-3 | 9.803 | 1.26 | |
| Benzoic acid, ethyl ester | 93-89-0 | 11.598 | 6.21 | |
| cis-3-Hexenyl 3-methylbutanoate | 35154-45-1 | 12.59 | 1.80 | |
| Ketones | ||||
| 2-Undecanone | 112-12-9 | 13.452 | 2.06 | |
| Aromatic compounds | ||||
| m-Cymene | 535-77-3 | 8.769 | 2.92 | |
| Eugenol | 97-53-0 | 14.406 | 2.89 | |
| methoxy-4-(1-propenyl)-Phenol | 5912-86-7 | 15.631 | 0.46 | |
| Olefins | ||||
| Styrene | 100-42-5 | 4.812 | 14.28 | |
| β-Myrcene | 123-35-3 | 8.024 | 2.09 | |
| Dipentene | 138-86-3 | 8.857 | 3.87 | |
| α-Cubebene | 17699-14-8 | 14.319 | 0.44 | |
| α-copaene | 3856-25-5 | 14.706 | 0.99 | |
| (-)-β-Bourbonene | 5208-59-3 | 14.844 | 0.75 | |
| β-Caryophyllene | 87-44-5 | 15.319 | 1.61 | |
| α-Caryophyllene | 6753-98-6 | 15.761 | 0.81 | |
| γ-Muurolene | 30021-74-0 | 16.015 | 0.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhang, J.; Hu, Z.; Cao, Y.; Mayo, K.H.; Liu, D.; E, M. Behavioral Responses of the Bumblebee Bombus terrestris to Volatile Compounds from Blueberries. Biology 2025, 14, 1570. https://doi.org/10.3390/biology14111570
He Y, Zhang J, Hu Z, Cao Y, Mayo KH, Liu D, E M. Behavioral Responses of the Bumblebee Bombus terrestris to Volatile Compounds from Blueberries. Biology. 2025; 14(11):1570. https://doi.org/10.3390/biology14111570
Chicago/Turabian StyleHe, Yun, Jiaru Zhang, Ziyang Hu, Yingxue Cao, Kevin H. Mayo, Duo Liu, and Mingju E. 2025. "Behavioral Responses of the Bumblebee Bombus terrestris to Volatile Compounds from Blueberries" Biology 14, no. 11: 1570. https://doi.org/10.3390/biology14111570
APA StyleHe, Y., Zhang, J., Hu, Z., Cao, Y., Mayo, K. H., Liu, D., & E, M. (2025). Behavioral Responses of the Bumblebee Bombus terrestris to Volatile Compounds from Blueberries. Biology, 14(11), 1570. https://doi.org/10.3390/biology14111570






