Phenological Stages of the Species Jacaranda mimosifolia D. Don. According to the Extended BBCH Scale
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Field Experiment and Phenological Observations
2.3. Thermal Integral Calculation
3. Results
3.1. BBCH Phenological Codification
3.2. Visual Characterization of Principal Phenophases
3.3. Principal Growth Stage 0—Germination, Sprouting, Bud Dormancy
3.4. Principal Growth Stage 1—Leaf Development
3.5. Principal Growth Stage 5—Emergence of the Flowering Organ
3.6. Principal Growth Stage 6—Flowering
3.7. Principal Growth Stage 7—Fruit Formation
3.8. Principal Growth Stage 8—Ripening and Fruit Coloring
3.9. Principal Growth Stage 9—Beginning of Dormancy
3.10. Annual Cumulative Thermal Patterns
3.11. Statistical Comparison of Thermal Requirements Between Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguirre-Becerra, H.; Pineda-Nieto, S.A.; García-Trejo, J.F.; Guevara-González, R.G.; Feregrino-Pérez, A.A.; Álvarez-Mayorga, B.L.; Rivera, D.M. Jacaranda flower (Jacaranda mimosifolia) as an alternative for antioxidant and antimicrobial use. Heliyon 2020, 6, e05802. [Google Scholar] [CrossRef] [PubMed]
- Sidjui, L.; Placide, O.; Ngosong, G.; Menkem, E.; Kouipou, R.M.; Mahiou-Leddet, V.; Herbette, G.; Boyom, F.; Ollivier, E. Secondary metabolites from Jacaranda mimosifolia and Kigelia africana (Bignoniaceae) and their anticandidal activity. Rec. Nat. Prod. 2014, 8, 307–311. [Google Scholar]
- Gilman, E.F.; Watson, D.G. Jacaranda mimosifolia. Fact Sheet ST-317 1993. Available online: https://edis.ifas.ufl.edu/publication/ST317 (accessed on 15 December 2023).
- Gachet, M.S.; Schühly, W. Jacaranda—An ehtnopharmacological and phytochemical review. J. Ethnopharmacol. 2009, 121, 14–27. [Google Scholar] [CrossRef]
- Rana, A.; Bhangalia, S.; Pratap Singh, H. A new phenylethanoid glucoside from Jacaranda mimosifolia. Nat. Prod. Res. 2012, 27, 1167–1173. [Google Scholar] [CrossRef]
- Torrico, G.; Peca, C.; Garcia, E. Leñosas Útiles de Potosí, 1st ed.; Proyecto FAO: Potosí, Bolivia, 1994; 469p. [Google Scholar]
- Kadambini, D.; Mahendru, N.; Sharma, B.P.; Shivakumar, P.; Kumar, N.; Kumar, S. Ornamental Plants of Bignoniaceae Family: Source of Bioactive Compounds with Therapeutic Applications and Ecological Services. Asian J. Environ. Ecol. 2025, 24, 79–86. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N. The genus Jacaranda (Bignoniaceae): An updated review. Pharmacogn. Commun. 2014, 4, 31–39. [Google Scholar] [CrossRef]
- Vavilov, N. Origin and Geography of Cultivated Plants, 1st ed.; Cambridge University: New York, NY, USA, 1992; 498p. [Google Scholar]
- Liao, H.; Man-Man, S.; Zhou, H.; Liu, X. Characterization of the complete chloroplast genome of Handroanthus chrysanthus (Bignonaceae). Mitochondrial DNA Part B Resour. 2022, 7, 1479–1480. [Google Scholar] [CrossRef]
- Fabris, H.A. Bignoniáceas. In Las Plantas Cultivadas en la República Argentina; Ministerio de Agricultura y Ganadería: Guatemala City, Guatemala, 1959; Volume 10, pp. 1–57. [Google Scholar]
- Fabris, H.A. Flora Argentina: Bignoniaceae. Rev. Mus. Plata 1965, 9, 273–419. [Google Scholar]
- Fabris, H.A. Bignoniaceae. Inst. Nac. Tecnol. Agropecu. 1979, 6, 504–526. [Google Scholar]
- Fabris, H.A. Bignoniaceae. Inst. Nac. Tecnol. Agropecu. 1993, 13, 226–262. [Google Scholar]
- Aguirre, Z. Pasado, presente y futuro de los “guayacanes” Handroanthus chrysanthus (Jacq.) S. O. Grose y Handroanthus billbergii (Bureau & K. Schum.) S. O. Grose, de los bosques secos de Loja, Ecuador. Arnaldoa 2015, 22, 85–104. [Google Scholar]
- Lahitte, H.J.; Hurrell, J.; Belgrano, M.; Jankowski, L.; Helova, P.; Mehltrete, K. Plantas Medicinales Rioplatenses, 1st ed.; L.O.L.A.: Buenos Aires, Argentina, 1998; 240p. [Google Scholar]
- Dimitri, M.J.; Leonardis, R.F.J.; Biloni, J.S. El Nuevo Libro del Árbol, 1st ed.; El Ateneo: Buenos Aires, Argentina, 1977; 119p. [Google Scholar]
- Macouzet, M.; Castillón, E.; Jiménez, J.; Villareal, J.; Herrera, M. Plantas Medicinales de Miquihuana, Tamaulipas, 1st ed.; Universidad Autónoma de Nueva León: Nueva León, Mexico, 2013; 146p. [Google Scholar]
- López González, G. Los Árboles y Arbustos de la Península Ibérica e Islas Baleares, 1st ed.; Mundi-Prensa: Barcelona, Spain, 2006; 1731p. [Google Scholar]
- Font Quer, P. Diccionario de Botánica, 1st ed.; Labor: Barcelona, Spain, 1953; 642p. [Google Scholar]
- Dubé, P.A.; Perry, L.P.; Vittum, M.T. Instructions for phenological observations: Lilac and honeysuckle. Vt. Agric. Exp. Stn. Bull. 1984, 692, 1–11. [Google Scholar]
- Alvarado, A.M.; Foroughbakhch, R.; Jurado, E.; Rocha, A. El cambio climático y la fenología de las plantas. Cienc. UANL 2002, 4, 493–500. [Google Scholar]
- Schwartz, M.D. Phenology: An Integrative Environmental Science, 1st ed.; Springer: New York, NY, USA, 2013; 610p. [Google Scholar]
- Cautín, R.; Agustí, M. Phenological Growth Stages of the cherimoya tree (Annona cherimola Mill.). Sci. Horti. 2005, 105, 491–497. [Google Scholar] [CrossRef]
- Ponti, R.; Sannolo, M. The importance of including phenology modelling species ecological niche. Ecography 2023, 2023, e06143. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Agustí, M.; Zaragoza, S.; Bleiholder, H.; Buhr, L.; Hack, H.; Klose, R.; Staub, R. Escala BBCH para la descripción de estadios fenológicos del desarrollo de los agrios (Gén. Citrus). Levante Agrí. 1995, 332, 189–199. [Google Scholar]
- Robin, J.; Bernard, A.; Albouy, L.; Papillon, S.; Tranchand, E.; Hebrard, M.N.; Philibert, J.B.; Barbedette, M.; Schafleitner, S.; Wenden, B.; et al. Description of Phenological Events of Persian Walnut (Junglans regia L.) according to the Extended BBCH Scale and Historical Scales. Horticulturae 2024, 10, 402. [Google Scholar] [CrossRef]
- Mabusela, M.M.; Matsiliza-Mlathi, B.; Kleynhans, R. Phenological Growth Stages of Buddleja saligna Willd. According to the BBCH Scale. Plants 2024, 13, 3542. [Google Scholar] [CrossRef]
- Qian, W.; Hu, Y.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy 2023, 13, 1328. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Betancourt, J.L.; Travers, S.E.; Pau, S.; Regetz, J.; Davies, T.J.; Kraft, N.J.B.; et al. Warming experiments underpredict plant phenological responses to climate change. Nature 2012, 485, 494–497. [Google Scholar] [CrossRef]
- Schieler, M.; Riemer, N.; Kleinhenz, B.; Saucke, H.; Veith, M.; Racca, P. SIMONTO-Pea: Phenological Models to Predict Crop Growth Stages in BBCH of Grain and Green Peas (Pisum sativum) for Temporal Pest Management. Agriculture 2023, 14, 15. [Google Scholar] [CrossRef]
- Fitter, A.H.; Fitter, R.S. Rapid changes in flowering time in British plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef]
- Smith-Ramírez, C.; Armesto, J.J.; Figueroa, J. Flowering, fruiting and seed germination in Chilean rain forest myrtaceae: Ecological and phylogenetic constraints. Plant Ecol. 1998, 136, 119–131. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, F.; Zheng, J.; Chen, L.; Hänninen, H.; Wu, J. Temperature sum models in plant spring phenology studies: Two commonly used methods have different fields of application. J. Exp. Bot. 2024, 75, 6011–6016. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Fleckinger, J. Notations phénologiques et représentations du développement des bourgeons floraux du porier. Fruit Belt 1946, 14, 41–54. [Google Scholar]
- Fleckinger, J. Les stades vegétatifs des arbres fruitiers, en raport avec les traitements. Pomol. Française 1948, 81, 81–93. [Google Scholar]
- Baggiolini, M. Les stades repères dans le développement annuel de la vigne et leur utilisation practique. Rev. Roum. Agric. 1952, 8, 10–12. [Google Scholar]
- Aubert, B.; Lossois, P. Considérations sur le phènologie des espèces fruitières arbustives. Fruits 1972, 27, 269–286. [Google Scholar]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heb, M.; Lancashire, P.; Schnock, U.; Staub, R.; Van de Boom, T.; et al. The BBCH system to coding the phenological growth stages of plants-history and publications. J. Für Kult. 2009, 61, 41–52. [Google Scholar]
- Meier, U. Die Merkblattserie 27 Entwicklungsstadien von Pflanzen der Biologischen Bundesanstalt fúr Land- und Forstwirtschaft. Nachrichtenbl. Dtsch. Pflanzenschutzd. 1985, 37, 76–77. [Google Scholar]
- Bleiholder, H.; van den Boom, T.; Langelüddeke, P.; Stauss, R. Einheitliche Codierung der phänologischen Stadien bei Kultur- und Schadpflanzen. Gesunde Pflanz. 1989, 41, 381–384. [Google Scholar]
- Lancashire, P.D.; van den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Hack, H.; Bleiholder, H.; Buhr, U.; Meier, U.; Schnock-Fricke, U.; Weber, E.; Witzenberger, A. A uniform code for phenological growth stages of mono-and dicotyledonous plants—Extended BBCH scale, general. Nachrichtenbl. Dtsch. Pflanzenschutzd. 1992, 44, 265–270. [Google Scholar]
- Hack, H.; Gall, H.; Klemke, T.; Klose, R.; Meier, R.; Stauss, R.; Witzenberger, A. The BBCH scale for phenological growth stages of potato (Solanum tuberosum L.). In Growth Stages of Mono and Dicotyledonous BBCH Monograph; Triennial Conference European Association for Potato Research: Bonn, Germany, 1992. [Google Scholar]
- Stauss, R. Compendium of Growth Stage Identification Keys for Mono and Dicotyledonous Plants. Extended BBCH Scale, 1st ed.; German Federal Biological Research for Agriculture and Forestry: Berlin, Germany, 1994; 94p. [Google Scholar]
- Acosta-Quezada, P.G.; Riofrío-Cuenca, T.; Rojas, J.; Vilanova, S.; Plazas, M.; Prohens, J. Phenological growth stages of tree tomatos (Solanum betaceum Cav.) an emerging fruit crop, according to the basic and extended BBCH scales. Sci. Hortic. 2016, 199, 216–233. [Google Scholar] [CrossRef]
- Hernández Delgado, P.M.; Aranguren, M.; Reig, C.; Fernández Galván, D.; Mesejo, C.; Martínez Fuentes, A.; Galán Saúco, V.; Agustí, M. Phenological growth stages of mango (Mangifera indica L.) according to the BBCH scale. Sci. Hortic. 2011, 130, 536–540. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Thorp, T.G.; Hormaza, J.I. Phenological growth stages of avocado (Persea americana) according to the BBCH scale. Sci. Hortic. 2013, 164, 434–439. [Google Scholar] [CrossRef]
- Herraiz, F.J.; Vilanova, S.; Plazas, M.; Gramazio, P.; Andújar, I.; Rodríguez-Burruezo, A.; Fita, A.; Anderson, G.J.; Prohens, J. Phenological growth stages of pepino (Solanum muricatum) according to the BBCH scale. Sci. Hortic. 2015, 183, 1–7. [Google Scholar] [CrossRef]
- López, I.; Salazar, D.M.; Recio, D. Fenología del Olivo: Comparación de distintas notaciones fenológicas. Frut. Prof. 2004, 145, 35–49. [Google Scholar]
- Climate Data. Available online: https://es.climate-data.org/europe/espana/comunidad-valenciana/betera-56926/ (accessed on 15 July 2024).
- Miller, P.; Lanier, W.; Brandt, S. Using Growing Degree Days to Predict Plant Stages. Mont. State Univ. Ext. Serv. 2001, 7, 1–7. [Google Scholar]
- Rauschkolb, R.; Herben, T.; Kattge, J.; Knickmann, B.; Linstädter, A.; Menzel, A.; Mora, K.; Nordt, B.; Vitasse, Y.; Weigelt, P.; et al. The performance of growing degree day models to predict spring phenology of herbaceous depends on the species’ temporal niche. Funct. Ecol. 2025, 39, 1–14. [Google Scholar] [CrossRef]
- Fornaciari, M.; Marrapodi, S.; Ruga, L.; Proietti, C.; Orlandi, F. Tree responses and temperature requirements in two central Italy phenological gardens. Int. J. Biometeorol. 2023, 67, 1607–1617. [Google Scholar] [CrossRef]
- Taghavi, T.; Rahemi, A.; Suarez, E. Development of a uniform phenology scale (BBCH) in hazelnuts. Sci. Hortic. 2022, 296, 110837. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Rötzer, T. Response of tree phenology to climate change across. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Bolton, M.; Friedl, M.A. Forecasting crop yield using remotely sensed vegetation indices and crop phenology metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]


| PSG | BBCH Code | Description | Period |
|---|---|---|---|
| 0 Germination, sprouting, and development | 00 (A) | Winter dormancy or resting period | Jan–Feb. |
| 01 | Swelling of the yolk begins | ||
| 03 | End of the yolk begins | ||
| 07 | The yolk begins to open or sprout | ||
| 09 | The bud shows green shots | Early April | |
| 1 Leaf development | 10 | First leaves separate from the shoot | Early April |
| 11 | Development of the first leaf | ||
| 12 | Development of the second leaf | Mid-April | |
| 13 | Development of the third leaf | ||
| 1… | Continuation of stages until… | ||
| 19 | Development of nine leaves or more | ||
| 5 Emergence of the flowering organ | 51 (C) | Flower organs or flower buds visible | Mid-May |
| 55 (D) | First individual buds and buds (florets) visible (unopened) | ||
| 59 | First petals (flower leaves) visible | ||
| 6 Flowering | 60 (E) | First flowers, open | Early June |
| 61 | Beginning of flowering: 10% of flowers open | ||
| 62 | 20% of open flowers | ||
| 63 | 30% of open flowers | ||
| 64 | 40% of open flowers | ||
| 65 (F) | Full flowering: 50% of flowers open | Mid-June | |
| 67 | Flowering coming to an end: most petals fallen or dry | ||
| 69 | End of flowering: fruit set visible | ||
| 7 Fruit formation | 70 | First visible fruits | Mid-July |
| 71 | Fruits reach 10% of their final size | ||
| 72 | Fruits reach 20% of their final size | ||
| 73 | Fruits reach 30% of their final size | ||
| 74 | Fruits reach 40% of their final size | ||
| 75 | Fruits reach 50% of their final size | ||
| 76 | Fruits reach 60% of their final size | ||
| 77 | Fruits reach 70% of their final size | ||
| 78 | Fruits reach 80% of their final size | ||
| 79 (H) | The fruits have reached the size appropriate to their species/variety | Mid-September | |
| 8 Ripening and fruit coloring | 81 (I) | Beginning of ripening or fruit coloring | Mid-October |
| 85 | Continuation or fruit coloring according to species/variety | Early Nov. | |
| 89 (J) | Full or harvest maturity | Late Nov. | |
| 9 Beginning of dormancy | 91 | End of wood or shoot growth, but foliage remains green | Early Dec. |
| 93 | Beginning of leaf discoloration or leaf drop | ||
| 95 | 50% of leaves discolored or fallen off | ||
| 97 | End of leaf fall. The plant is in winter dormancy or vegetative rest | Mid Dec. |
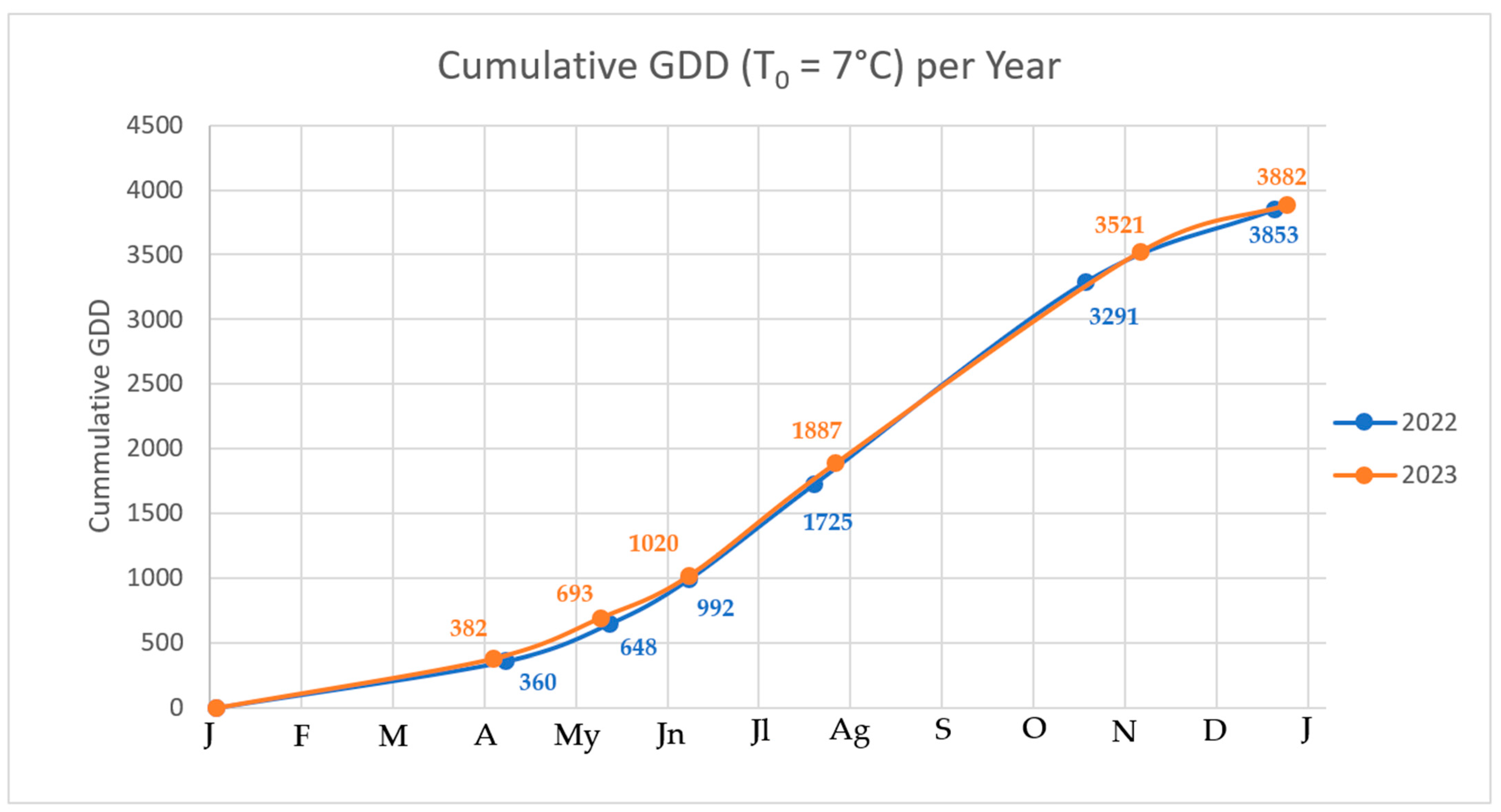
| PGS | Mean GDD 2022 | SD | CV% | Mean GDD 2023 | SD | CV% | p-Value (Wilcoxon) |
|---|---|---|---|---|---|---|---|
| 0 | 0.00 | - | - | 0.00 | - | - | - |
| 1 | 359.52 | 25.44 | 7.08 | 381.70 | 24.97 | 6.54 | 0.00063 |
| 5 | 647.12 | 28.58 | 4.42 | 693.40 | 28.86 | 4.16 | 2.3 × 10−9 |
| 6 | 991.48 | 22.74 | 2.29 | 1019.48 | 22.35 | 2.19 | 2.8 × 10−7 |
| 7 | 1725.40 | 41.69 | 2.42 | 1887.22 | 41.67 | 2.21 | 3.5 × 10−15 |
| 8 | 3290.60 | 70.30 | 2.14 | 3521.28 | 69.76 | 1.98 | 1.8 × 10−15 |
| 9 | 3852.36 | 65.55 | 1.70 | 3882.10 | 64.14 | 1.65 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandía-Ventura, I.; López-Cortés, I.; Velàzquez-Martí, B. Phenological Stages of the Species Jacaranda mimosifolia D. Don. According to the Extended BBCH Scale. Biology 2025, 14, 1569. https://doi.org/10.3390/biology14111569
Gandía-Ventura I, López-Cortés I, Velàzquez-Martí B. Phenological Stages of the Species Jacaranda mimosifolia D. Don. According to the Extended BBCH Scale. Biology. 2025; 14(11):1569. https://doi.org/10.3390/biology14111569
Chicago/Turabian StyleGandía-Ventura, Ignacio, Isabel López-Cortés, and Borja Velàzquez-Martí. 2025. "Phenological Stages of the Species Jacaranda mimosifolia D. Don. According to the Extended BBCH Scale" Biology 14, no. 11: 1569. https://doi.org/10.3390/biology14111569
APA StyleGandía-Ventura, I., López-Cortés, I., & Velàzquez-Martí, B. (2025). Phenological Stages of the Species Jacaranda mimosifolia D. Don. According to the Extended BBCH Scale. Biology, 14(11), 1569. https://doi.org/10.3390/biology14111569







