Simple Summary
Lycium ruthenicum is a perennial thorny shrub belonging to the family Solanaceae and is classified as a second-grade protected plant in China. It has important ecological functions and medicinal value but is threatened by climate change. By optimizing the maximum entropy model, we estimate its current potential habitat range to be approximately 2.25 × 106 km2, predominantly distributed in northwestern China. Under future climate scenarios, its suitable habitats will gradually shrink and primarily shift northeastward as climate change progresses. These findings provide scientific support to guide the conservation of L. ruthenicum in China.
Abstract
Understanding the climate change impacts on the geographical distribution of plant species is vital for biodiversity conservation. Lycium ruthenicum, a second-grade protected plant in China, holds considerable medicinal and ecological value; however, its potential habitat distribution under climate change remains uncertain. By utilizing occurrence records and geographical and environmental data, we optimized a maximum entropy model and evaluated the current and future potential habitat suitability of L. ruthenicum in China. The main results were as follows: (1) The distribution of L. ruthenicum was primarily influenced by the precipitation of the warmest quarter, topsoil base saturation, precipitation seasonality, precipitation of the coldest quarter, and minimum temperature of the coldest month. (2) Under the current conditions, the potential suitable area of L. ruthenicum was approximately 2.25 × 106 km2 in China, predominantly distributed in Xinjiang, Qinghai, Gansu, Ningxia, and Inner Mongolia. (3) An obvious reduction in the predicted suitable area of L. ruthenicum was found under future climate scenarios, with the centroid primarily shifting northeastward. These findings highlight the potential vulnerability of this medicinally and ecologically important species and underscore the urgent need for targeted conservation strategies to ensure its long-term survival.
1. Introduction
With increasing atmospheric greenhouse gas concentrations, global average surface temperature has risen by approximately 1 °C since the pre-industrial era [1,2]. This rise in temperature has resulted in more extreme weather events, which have adversely affected biodiversity and ecosystem stability [3,4,5]. Plants, as a crucial component of terrestrial ecosystems, are particularly sensitive to climate change [6,7]. For example, climate change can alter the habitats on which plants depend, thereby influencing shifts in their original geographical distribution [8,9]. A previous study indicated that many plant species need to shift their ranges by more than 1 km/year to keep pace with climate change [10]. These distribution changes can disrupt existing ecological balances and lead to ecosystem instability [11,12]. Therefore, understanding how climate change affects the potential ranges of plant species is essential for biodiversity conservation and sustainable ecosystem management.
Species distribution models (SDMs) have become an important tool for predicting potential geographical ranges of plant species [13,14]. Commonly used SDM techniques include the maximum entropy (MaxEnt) model [15], genetic algorithm for rule-set prediction (GARP) [16], BIOCLIM program [17], generalized linear model (GLM) [18], and random forest (RF) algorithm [19]. Among these techniques, the MaxEnt model is particularly esteemed for its effectiveness and accuracy [20,21]. It handles complex interactions between species occurrence data and environmental variables, generating visual outputs such as probability distribution maps and variable importance metrics [22,23]. Nowadays, a number of works have been conducted using the MaxEnt model to analyze the environmental responses and potential habitat distributions of different plant species [24,25,26]. However, recent investigations have shown that relying on default parameters in the MaxEnt model can deteriorate the predictive performance [27,28]. In this case, selecting appropriate parameters for modeling is essential when utilizing the MaxEnt model.
Lycium ruthenicum, commonly known as Russian box thorn, is a perennial thorny shrub in Solanaceae [29]. It is widely distributed in China, Central Asia, Russia, Mongolia, India, Pakistan, and Afghanistan, even up to Mediterranean areas [30]. This species exhibits unique characteristics, including being light-loving, drought-resistant, and salinity-resistant, making it suitable for growth in desert and saline–alkali regions. It serves essential ecological functions such as windbreak, sand fixation, and soil and water conservation [31]. Meanwhile, L. ruthenicum is a medicinal and edible homologous food due to its abundant anthocyanins and other bioactive components, which can remove free radicals in the body and possess antioxidant, anti-fatigue, and immune enhancing effects [32,33]. Research on L. ruthenicum has mainly concentrated on its genetics, bioactive components, and functional applications [34]. However, a comprehensive understanding of its potential distribution and habitat suitability has not been reported. Previous studies have indicated that a variety of geographical and environmental factors jointly determine the potential ranges of plant species [24,25,26,35]. Among such factors, bioclimatic factors describing temperature and water-related annual tendencies, seasonality, and extreme climatic conditions have been frequently regarded as primary determinants [36], leading to several climate-based hypotheses on species diversity patterns [37,38,39]. Apart from climatic factors, soil properties also influence the suitable habitats of plant species. For example, unsuitable soil conditions can limit the expansion of suitable habitats for plant species [40]. For L. ruthenicum in arid and saline environments, recent field surveys indicated a contraction in its natural distribution, potentially linked to rising temperatures, altered precipitation patterns, and soil degradation [41]. Therefore, for a robust prediction of its distribution, it is crucial to consider the multiple influencing factors during the modeling process.
As one of the primary distribution areas of L. ruthenicum, the Chinese government has classified it as one of the national second-grade protected plants, which are species that are not yet facing immediate extinction but still face threats and require protection due to their ecological, economic, or cultural value, and has enacted laws and regulations to strengthen the protection of wild resources [41,42]. Recent studies indicated that the increase in temperature over China since 1900 has exceeded the global mean, highlighting its heightened sensitivity to climate change [43,44]. Given the ongoing climate change, there is still uncertainty regarding its impact on the potential habitat distribution of L. ruthenicum in China during different time periods [45,46]. We hypothesize that climate change will alter the potential distribution of this species, resulting in a contraction of its potential suitable habitat and a shift in its distribution centroid. Therefore, the research aims to (1) examine primary factors affecting the distribution of L. ruthenicum and (2) reveal the differences in its potential distribution under current and future climate scenarios. These detailed results are important for deepening our understanding of the ecological adaptations and distribution patterns of L. ruthenicum in China, thereby offering crucial insights for conservation and sustainable use strategies under climate change.
2. Materials and Methods
2.1. Data
2.1.1. Occurrence Records of L. ruthenicum
The occurrence records of L. ruthenicum were primarily sourced from the Global Biodiversity Information Facility (https://www.gbif.org/, accessed on 10 January 2020) [47], the Chinese Virtual Herbarium (https://www.cvh.ac.cn/, accessed on 10 January 2020), the National Specimen Information Infrastructure of China (http://www.nsii.org.cn/2017/home.php, accessed on 10 January 2020), and the Plant Science Data Center of China (http://www.iplant.cn/, accessed on 10 January 2020). In order to ensure data accuracy and reliability, we first removed duplicate occurrence records and those with missing longitude and latitude coordinates. Furthermore, we utilized the ENMTools program to remove the redundant occurrence data, thereby avoiding the overfitting of prediction results [48]. Finally, there were 161 records of L. ruthenicum available for further analysis (Figure 1).
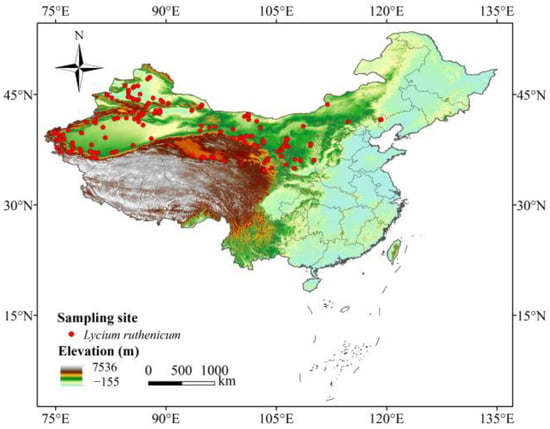
Figure 1.
Occurrence records of L. ruthenicum in China.
2.1.2. Geographical and Environmental Data
According to previous studies [8,9], we utilized a total of 40 geographical and environmental factors that can affect the distribution of L. ruthenicum in our study (Table S1). Among these, 19 bioclimatic variables were derived from https://www.worldclim.org/data/index.html (accessed on 10 January 2020), covering five periods (contemporary, 2030s, 2050s, 2070s, and 2090s) at a 2.5 min resolution. For future climate scenarios, data from shared socioeconomic pathways (SSP126: Low forcing category, radiative forcing reaches 2.6 W/m2 in 2100; SSP245: Medium forcing category, radiative forcing reaches 4.5 W/m2 in 2100; SSP370: High forcing category, radiative forcing reaches 7.0 W/m2 in 2100; and SSP585: High forcing category, radiative forcing reaches 8.5 W/m2 in 2100) were from the Beijing Climate Center Climate System Model [49].
In addition to bioclimatic variables, this study incorporated 18 soil properties from the topsoil layer and 3 topographic factors (elevation, slope, and aspect). These data were from the Harmonized World Soil Database and the National Earth System Science Data Center of China, respectively. Furthermore, we resampled the soil and topographic data into a 2.5 min resolution to match the climate data in this study.
2.2. Methods
In this study, we first screened suitable geographical and environmental variables according to the percentage contribution of variables and Pearson correlation analysis. Subsequently, we optimized parameters for the MaxEnt model using the R package ENMeval v.2.0.4. Following this, we imported the filtered occurrence records of L. ruthenicum and selected variables into the optimized MaxEnt model to predict the potential geographical distribution of L. ruthenicum in China. Finally, we conducted statistical analyses on the area of each suitability class and the centroid location of suitable areas for different periods using ArcGIS 10.2 software.
2.2.1. Correlation Analysis of Geographical and Environmental Factors
In order to avoid multicollinearity among geographical and environmental factors, the filtered occurrence records of L. ruthenicum along with 40 geographical and environmental factors were loaded into the MaxEnt model, which was calculated 10 times to assess the contribution of each factor [27]. Subsequently, the relationships among these factors were analyzed using Pearson correlation (Figure S1). When the correlation coefficient between two factors was high (|r| > 0.7), only the highest contributing factor was retained [50]. After these steps, a total of 19 factors were screened (Table 1).

Table 1.
Geographical and environmental factors used for modeling after screening.
2.2.2. Model Construction
In this study, MaxEnt v.3.4.1 was employed to investigate the potential geographical distribution of L. ruthenicum in China. According to previous studies [51,52], the model’s performance was significantly affected by the configuration of two parameters, i.e., feature combinations (FCs) and regularization multiplier (RM) [51]. Therefore, it is crucial to optimize these two parameters. Considering the available feature types including Linear (L), Quadratic (Q), Product (P), Threshold (T) and Hinge (H), six FCs were employed (L, LQ, H, LQH, LQHP, and LQHPT). Additionally, eight RM values were set, with a range from 0.5 to 4.0 in steps of 0.5, leading to 48 parameter combinations. The optimal combination was then determined using the R package ENMeval based on the metrics such as the Akaike information criterion correction (AICc) and 10% training omission rate (OR10) [52].
We imported the filtered occurrence records of L. ruthenicum along with 19 selected factors into the MaxEnt model, applied the optimal parameter combinations, and repeated the calculation 10 times. In this case, the occurrence records were divided into training and testing sets at a ratio of 75:25, with the number of iterations and background points of 500 and 10,000, respectively [53].
2.2.3. Model Evaluation and Analysis
Model prediction accuracy was assessed using the area under the Receiver Operating Characteristic (ROC) curve (AUC), which varied between 0 and 1. Generally, a higher AUC value indicates better predictive accuracy [54]. For example, an AUC value exceeding 0.9 indicates excellent accuracy.
The prediction results were categorized into four classes of potential suitable habitats using the Jenks method implemented in ArcGIS 10.2 software [55]. These classes included unsuitable area (<0.1), low suitable area (0.1–0.3), medium suitable area (0.3–0.5), and high suitable area (>0.5). Furthermore, the area of each suitability class and the centroid location of suitable areas were calculated for different periods using ArcGIS 10.2 software [56].
3. Results
3.1. Model Optimization and Evaluation
In order to improve model prediction accuracy, a total of 48 parameter combinations of FCs and RMs were evaluated using the ENMeval package. With the default parameters (RM = 1 and FC = LQHPT), the model had a delta.AICc of 348.144 (Figure 2). In contrast, the model utilizing RM = 1 and FC = LQ had the lowest delta.AICc value (i.e., delta.AICc = 0). Furthermore, the OR10 value associated with this parameter combination was much lower than that obtained with the default parameters. Therefore, the optimal parameter configuration was RM = 1 and FC = LQ.
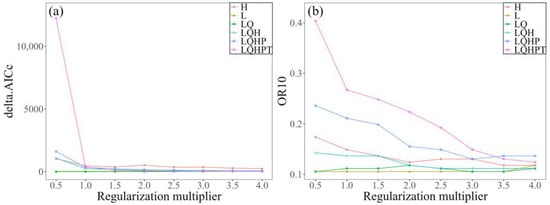
Figure 2.
Changes in (a) delta.AICc and (b) OR10 under different parameter combinations. The delta.AICc quantifies how much a model’s corrected Akaike Information Criterion (AICc) exceeds the lowest AICc found across all parameter combinations, whereas OR10 denotes the 10% training omission rate.
The MaxEnt model repeated the calculation 10 times using the optimal parameters, and then the ROC curve was generated (Figure 3). In this study, the AUC was employed to assess predictive accuracy. The average training AUC was 0.946 ± 0.003, while the average testing AUC was 0.916 ± 0.011. These results suggested that the optimized model had excellent predictive accuracy in characterizing potential suitable habitats of L. ruthenicum.
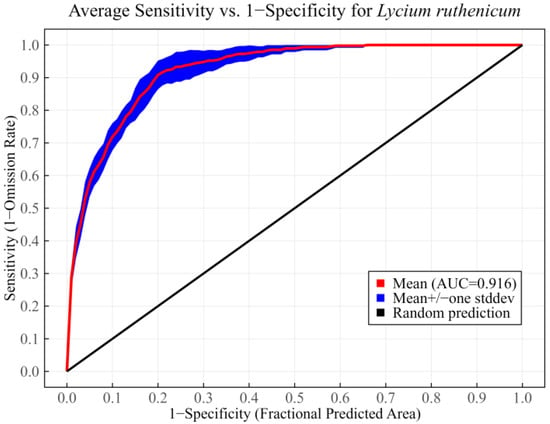
Figure 3.
The ROC curve of the optimized MaxEnt model.
3.2. Primary Influencing Factors and Response Curve Analysis
The Jackknife test was employed to analyze primary factors affecting the habitat distribution of L. ruthenicum. Table 2 shows the percentage contribution of each influencing factor. The ranking of contribution rates showed the top five factors as follows: the precipitation of warmest quarter (Bio18, 46%), topsoil base saturation (T_BS, 11.9%), precipitation seasonality (Bio15, 11.2%), the precipitation of the coldest quarter (Bio19, 8.7%), and the minimum temperature of the coldest month (Bio6, 5.8%). Together, these factors accounted for 83.6% of the total contribution. Furthermore, the cumulative permutation importance of these five factors reached 79.5%. These results suggested that the five factors primarily influenced the distribution pattern of L. ruthenicum in China.

Table 2.
The contribution rate of each influencing factor of L. ruthenicum.
Figure 4 shows response curves depicting the occurrence probability of L. ruthenicum in relation to the primary influencing factors. Interestingly, each factor showed an obvious preference for specific ranges concerning the occurrence probability of L. ruthenicum. A probability threshold of 0.3 was employed to determine the optimal range of primary influences on the suitable habitat of L. ruthenicum. As shown in Figure 4, the optimal ranges for each influencing factor were as follows: Bio18 (≤165 mm), T_BS (≥84%), Bio15 (≤105), Bio19 (≤20 mm), and Bio6 (−21.5 to −6 °C).
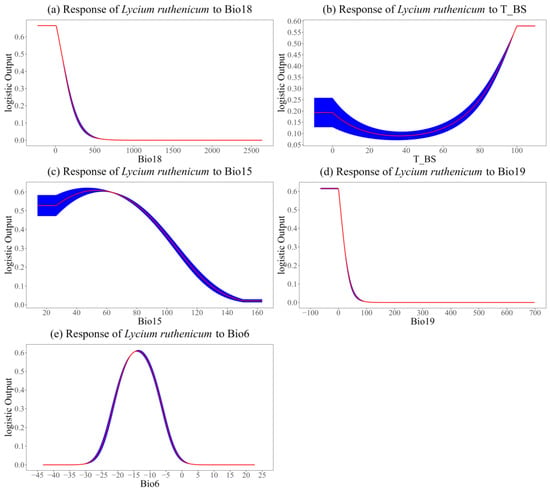
Figure 4.
Response curves of primary influencing factors. Red lines and blue areas show the average and standard deviation of 10-fold cross-validation.
3.3. Potential Geographical Distribution of L. ruthenicum Under Current Conditions
The potential habitat suitability of L. ruthenicum in China, under current conditions, were generated using the MaxEnt model, as shown in Figure 5. According to Figure 5, the potential suitable areas of L. ruthenicum were distributed in 11 provinces, predominantly in northwestern China. Approximately 0.25 × 106 km2 was identified as highly suitable, representing 11.11% of the total suitable area, and these areas were primarily distributed in Xinjiang, Qinghai, Gansu, Ningxia, and Inner Mongolia. Moderately suitable areas covered 0.56 × 106 km2, representing 24.89% of the total suitable area, and were typically adjacent to highly suitable areas. In contrast, the lowly suitable area was 1.44 × 106 km2, which constituted 64.0% of the total suitable area, and extended into regions such as Shaanxi, Shanxi, Hebei, Henan, Sichuan, and Tibet.
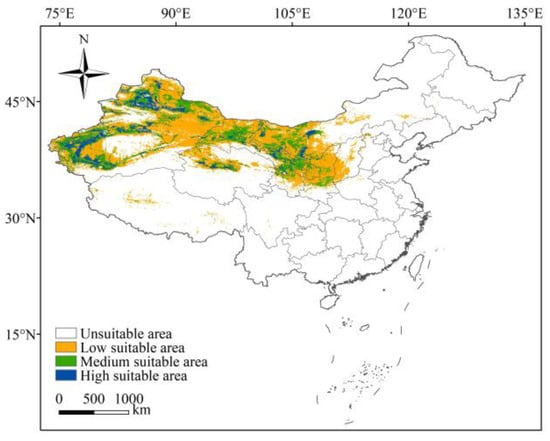
Figure 5.
The potential suitable habitats of L. ruthenicum in China under the current conditions.
3.4. Potential Geographical Distribution of L. ruthenicum Under Future Climate Scenarios
The potential habitat suitability of L. ruthenicum in China for the 2030s and 2050s was generated using the MaxEnt model based on SSP126, SSP245, SSP370, and SSP585 scenarios, as shown in Figure 6. Given the uncertainty in climate projections, the potential habitat suitability of L. ruthenicum for the 2070s and 2090s can be shown in Figure S2. The suitable distributions of L. ruthenicum under future climate scenarios showed a general similarity to its distribution under current conditions. Highly suitable areas predominantly remained in Xinjiang, Qinghai, Gansu, Ningxia, and Inner Mongolia, while suitable areas were relatively limited in Shanxi, Hebei, and Tibet. However, the area within each suitability class of L. ruthenicum exhibited varying degrees of change over time (Table 3 and Table S2). For example, the area of highly suitable habitats remained relatively stable, whereas moderate and low suitability areas decreased, resulting in an expansion of unsuitable areas of L. ruthenicum compared to contemporary levels.
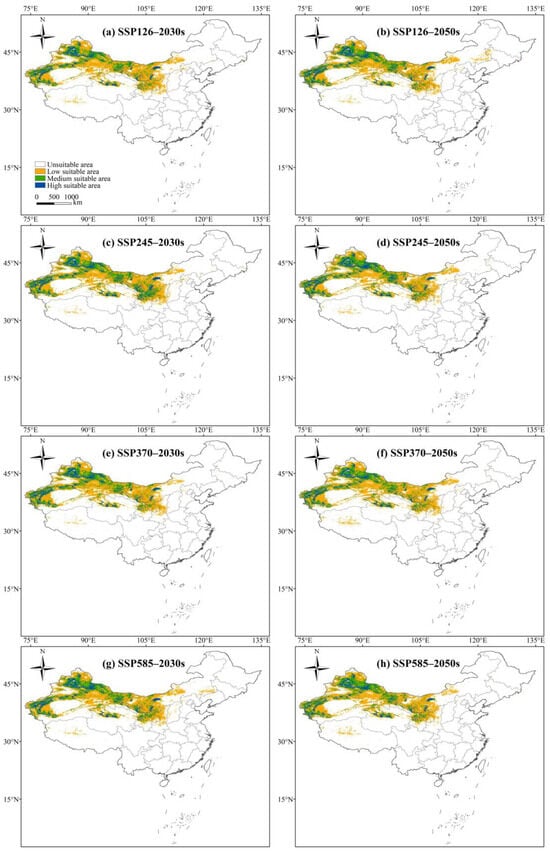
Figure 6.
The potential suitable habitats of L. ruthenicum in China under future climate scenarios. (a) The potential geographical distribution of L. ruthenicum under the SSP126 in the 2030s. (b) The potential geographical distribution of L. ruthenicum under the SSP126 in the 2050s. (c) The potential geographical distribution of L. ruthenicum under the SSP245 in the 2030s. (d) The potential geographical distribution of L. ruthenicum under the SSP245 in the 2050s. (e) The potential geographical distribution of L. ruthenicum under the SSP370 in the 2030s. (f) The potential geographical distribution of L. ruthenicum under the SSP370 in the 2050s. (g) The potential geographical distribution of L. ruthenicum under the SSP585 in the 2030s. (h) The potential geographical distribution of L. ruthenicum under the SSP585 in the 2050s. Different colors in each figure represent different suitability classes, with the meanings of the colors shown in Figure 6a.

Table 3.
The potential suitable area of L. ruthenicum in China under different periods.
The potential suitable habitats of L. ruthenicum showed obvious differences under future climate scenarios (Table 1 and Table S2). However, the overall trend indicated a shrinking distribution range compared to contemporary levels. High suitability habitats peaked at 0.28 × 106 km2 in the 2030s and 2050s under the SSP245 scenario but declined to 0.20 × 106 km2 by the 2090s under the SSP585 scenario, with a 20% reduction from contemporary levels. Moderately suitable habitats generally decreased over time, with the exception of the 2030s, under the SSP245 scenario. The most substantial decline in moderately suitable areas occurred in the 2090s under the SSP585 scenario, with a 21.4% reduction relative to contemporary levels. Furthermore, the area of low suitability habitats experienced the greatest decrease in the 2070s under the SSP585 scenario, with a 20.8% reduction compared to contemporary levels, although its area showed a consistent downward trend over time.
3.5. Centroid Shifts in the Potential Distribution of L. ruthenicum Under Climate Change
The geographical centroid of potential suitable habitats of L. ruthenicum, calculated by importing the model output into ArcGIS (Figure 7a–d and Figure S3), exhibited distinct shifts in different periods. Under the current conditions, the centroid coordinates of potential suitable habitats were recorded at 88.1553° E, 40.8586° N. Under future climate scenarios, the centroid of suitable habitats predominantly shifted northeastward over time compared to contemporary levels, with the exception of the 2090s, under the SSP370 and SSP585 scenarios. During the 2090s, migration of the centroid consistently showed a southwestward trend under the SSP370 and SSP585 scenarios (Figure S3). The furthest migration of the centroid reached 185 km in the 2030s under the SSP245 scenario, while the closest migration was recorded at 45 km in the 2050s under the SSP126 scenario.
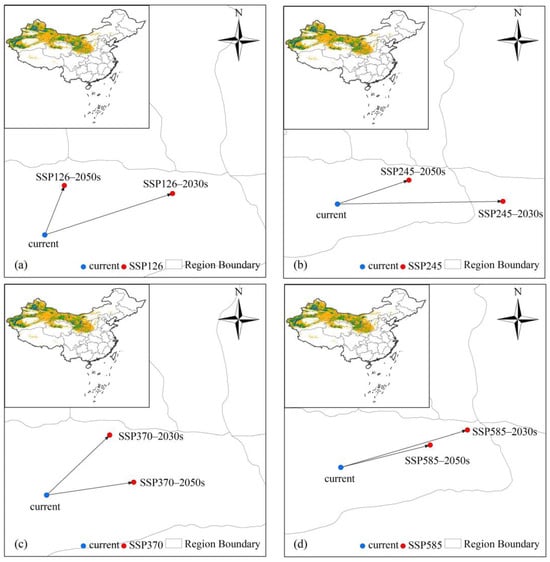
Figure 7.
The centroid migration of L. ruthenicum under future climate scenarios. (a) The centroid migration in potential suitable areas of L. ruthenicum under the SSP126 in the 2030s and 2050s. (b) The centroid migration in potential suitable areas of L. ruthenicum under the SSP245 in the 2030s and 2050s. (c) The centroid migration in potential suitable areas of L. ruthenicum under the SSP370 in the 2030s and 2050s. (d) The centroid migration in potential suitable areas of L. ruthenicum under the SSP585 in the 2030s and 2050s. The inset in the upper left corner of each figure represents the potential suitable habitats of L. ruthenicum in China under the current conditions, which is derived from Figure 5.
4. Discussion
4.1. Primary Factors Affecting the Potential Distribution of L. ruthenicum
According to the correlation analysis combined with the contribution rate of various geographical and environmental factors, we identified Bio18, T_BS, Bio15, Bio19, and Bio6 as the top five factors affecting the distribution of L. ruthenicum. Among these, Bio18, Bio15, and Bio19 were precipitation-related factors, underscoring a certain role of precipitation in reflecting the geographical distribution of L. ruthenicum. This is largely because L. ruthenicum is predominantly found in the arid desert and saline–alkali regions of northwestern China, where precipitation is extremely scarce [30,32]. It should be noted that the response curves between precipitation-related variables and the probability of species presence are likely to reflect a statistical association rather than a biologically causative relationship [52]. In addition to precipitation, temperature is also an important determinant for many plant species [57,58]. A recent study found that the semi-lethal temperatures of L. ruthenicum range from −35 to −26 °C [59], suggesting that it can thrive within the optimal range of −21.5 to −6 °C for Bio6.
In desert regions with saline and alkaline soils, soil characteristics are often one of the primary factors determining the distribution of L. ruthenicum [41]. We selected several soil factors for modeling; however, only T_BS contributed more than 5%. The result is consistent with the biological characteristics of L. ruthenicum, which possesses a well-developed root system and demonstrates adaptability to poor soil conditions, as well as tolerance to high temperatures, nutrient-poor environments, and drought [60]. Some studies pointed out that L. ruthenicum is likely adapted to deep rooting and reliance on groundwater rather than direct rainfall [61,62]. This adaptation is key to its survival in arid environments. In fact, these precipitation-related variables do not denote a direct physiological reliance on rainfall; rather, they serve as highly effective proxy indicators for the complex abiotic conditions that define its preferred habitat [35]. Therefore, the model can be used to identify the arid environmental regime that results in the specific soil conditions required for this species. The strong performance of these precipitation-related variables likely reflects their utility in mapping this broader abiotic niche. We acknowledge that this is an indirect approach and that the ideal model would incorporate direct data on groundwater depth and salinity. However, such datasets are currently unavailable. In their absence, bioclimatic variables provide a powerful and widely used proxy indicator [63].
While this study focused on the potential distribution of L. ruthenicum in China and identified its primary influencing factors, this species also occurs in other regions, such as Central Asia, Russia, Mongolia, India, Pakistan, and Afghanistan, even up to Mediterranean areas [30]. The scarcity of reliably georeferenced occurrence records in these regions precludes their inclusion in the MaxEnt model [64]. Nevertheless, the primary factors identified in China and their optimal ranges provide critical baseline parameters for preliminary habitat screening in adjacent regions. Notably, future precipitation projections exhibit complex spatial patterns that diverge from the consistent global warming trend, introducing uncertainty regarding the future potential distribution of L. ruthenicum in regions outside of China. Therefore, future transnational collaborations to compile verified occurrence records and standardized environmental datasets will be essential for evaluating the potential habitat suitability of L. ruthenicum across a broad geographic range.
4.2. Changes in the Potential Suitable Habitats of L. ruthenicum
Our findings indicate that the current potential habitat range of L. ruthenicum is approximately 2.25 × 106 km2, consistent with previous estimates that place its range primarily between 1.11 × 106 and 2.84 × 106 km2 [41,42,45,46]. The discrepancies appear to stem from different input variables and the optimized MaxEnt model. On the other hand, the current potential suitable habitats of L. ruthenicum were predominantly found in Xinjiang, Qinghai, Gansu, Ningxia, and Inner Mongolia, consistent with the distribution of occurrence records [30,32]. These regions lie in northwestern China and share similar climatic characteristics [65]. Under future climate scenarios, the predicted suitable areas of L. ruthenicum varied in different periods and scenarios, yet the overall trend pointed to a contraction. This pattern mirrors those reported for other desert plants in northwestern China [66] and is attributable to several ecological mechanisms driven by changing hydrothermal conditions. First, the projected increase in mean temperature and frequency of heatwaves may exceed the species’ photosynthetic optimum [67], causing thermal stress and cellular dysfunction, while simultaneously exacerbating water loss through transpiration [68]. Second, although L. ruthenicum relies on groundwater, the replenishment of this resource is intrinsically linked to precipitation [69]. Projected increases in precipitation seasonality and the frequency of severe droughts threaten to lower groundwater tables and intensify soil moisture deficits, potentially pushing the species beyond its hydraulic safety margin in some parts of its current range [70]. Moreover, the decline in suitable areas of L. ruthenicum was non-linear, underscoring the complex effects of climate change on habitat suitability [71]. Consistent with broader evidence [72,73], our results indicated that the centroid of suitable habitats of L. ruthenicum shifted predominantly northeastward under future climate conditions compared to the present. These distribution changes represent an adaptive response to climate warming [74], compelling L. ruthenicum to relocate to newly suitable areas.
Although our study predicted the potential distribution of L. ruthenicum under climate change, its realized distribution typically constitutes only a subset of the potential range due to intensive anthropogenic pressures [75]. For example, over-harvesting in potentially suitable areas directly threatens the growth and reproductive success of L. ruthenicum [76], while land conversion and habitat fragmentation disrupt habitat connectivity and hinder the establishment and persistence of L. ruthenicum populations [77]. As a second-grade protected plant in China, L. ruthenicum is not facing immediate extinction, but it remains under threat and requires active protection [41]. Furthermore, the Chinese government has enacted laws and regulations to restrict large-scale or destructive harvesting and to strengthen the protection of wild resources [42]. In order to reduce the negative impacts of anthropogenic pressures and better protect L. ruthenicum, we recommend integrated conservation strategies that include regulating harvests through quotas, monitoring potential suitable habitats, protecting connectivity corridors, and establishing climate-adapted cultivation in newly suitable areas, thereby maximizing the economic and ecological value of L. ruthenicum.
4.3. Limitations of the Study
This study faced limitations, primarily from three aspects. First, the issue of layer resolution was a shortfall of this study. According to the previous studies [27,78], the 2.5 min resolution of environmental factors was suitable for China as the study region. This suggested that the soil and topographic data were resampled into a 2.5 min resolution to match the climate data utilized, which could introduce uncertainties during the resampling process and affect the prediction results. Second, soil variables were held constant for both current and future modeling processes due to the lack of future datasets on soil properties [55]. We acknowledge that this assumption limits the validity of our long-term predictions. Our predictions should therefore be interpreted as the impact of climate change on the potential geographical distribution of L. ruthenicum assuming that current soil conditions remain unchanged. Third, some studies emphasized that biotic interactions may also matter at macroecological scales, and these interactions are likely to play a role in shaping the dynamic responses of species to changes in climate [79,80]. However, research on such processes on L. ruthenicum remains limited. Available findings include the following: (1) salt-tolerant rhizosphere bacteria can enhance plant growth through nitrogen fixation, phosphorus solubilization, indole-3-acetic acid production, and siderophore synthesis [81]; (2) tissue-specific endophytic fungi-colonizing root, stem, leaf, and fruit can modulate drought and disease resistance, and may produce secondary metabolites that feedback on host fitness [82]; (3) at Qingtu Lake, the terminal reach of the Shiyang River, L. ruthenicum exhibits high niche overlap with Peganum harmala L., suggesting potential interspecific competition [83]; (4) L. ruthenicum relies on insects for pollination and on birds and rodents for seed dispersal, thereby forming the mutualistic interactions [31]; (5) climate-driven increases in pest outbreaks impose additional stress on L. ruthenicum [84]. Despite these insights, fine-scale experimental datasets are still scarce and how local interactions affect broad-scale species distributions is still uncertain [80]; consequently, omitting these biotic drivers in the MaxEnt model may overestimate habitat suitability in regions experiencing inhibitory interactions. With the continuous development of big data and artificial intelligence technology [85], future work should incorporate biotic interactions and anthropogenic impacts and integrate updated environmental datasets and hybrid SDMs with dynamic models to elucidate the potential and realized distributions of L. ruthenicum at finer scales and across broader geographic ranges.
5. Conclusions
We evaluated the current and future potential habitat distribution of L. ruthenicum based on the multi-source data and an optimized MaxEnt model. By integrating variables related to temperature, precipitation, soil, and topography, five primary factors influencing habitat suitability were identified, including the Bio18 (46%), T_BS (11.9%), Bio15 (11.2%), Bio19 (8.7%), and Bio6 (5.8%). At present, potential suitable habitats were predominantly found in northwestern China, covering an area of 2.25 × 106 km2. Future climate projections indicated a general reduction in suitable habitats for L. ruthenicum, with the most significant decrease expected in the 2090s under the SSP585 scenario. These results can provide valuable insights to develop specific conservation and sustainable use strategies for climate change impacts on L. ruthenicum in China.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14101379/s1, Figure S1: Correlation diagram of geographical and environmental factors for L. ruthenicum; Figure S2: The potential suitable habitats of L. ruthenicum in the 2070s and 2090s under future climate scenarios; Figure S3: The centroid migration of L. ruthenicum in the 2070s and 2090s under future climate scenarios; Table S1: Geographical and environmental factors initially selected in this study; Table S2: The potential suitable area of L. ruthenicum in China in the 2070s and 2090s under future climate scenarios.
Author Contributions
Conceptualization, C.L. and L.Z.; methodology, C.L.; software, Y.G.; formal analysis, Y.G., Y.W., and L.Z.; data curation, Y.G., B.L. and L.Z.; writing—original draft preparation, C.L. and Y.G.; writing—review and editing, B.L., K.P.C., T.O., M.L.T., Y.W., and L.Z.; funding acquisition, C.L., Y.W., and K.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (42275029, 41801013), the Young Scientific and Technological Talents Support Project of Jiangsu Association for Science and Technology (TJ-2023-032), the Royal Society International Exchanges 2022 (IEC\NSFC\223132), the Humanities and Social Sciences Foundation of Yangzhou University (xjj2025-05), and the Qinglan Project of Yangzhou University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the reviewers and editors for their valuable comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Rawat, A.; Kumar, D.; Khati, B.S. A review on climate change impacts, models, and its consequences on different sectors: A systematic approach. J. Water Clim. Change 2024, 15, 104–126. [Google Scholar] [CrossRef]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate Change and Ecosystems: Threats, Opportunities and Solutions. Philos. Trans. R. Soc. B 2020, 375, 20190104. [Google Scholar] [CrossRef]
- Li, C.; Yao, H.; Li, Z.; Wu, F.; Liu, B.; Wu, Y.; Chun, K.P.; Octavianti, T.; Cui, X.; Xu, Y. A Bibliometric Analysis of Global Research on Climate Change and Agriculture from 1985 to 2023. Agronomy 2024, 14, 2729. [Google Scholar] [CrossRef]
- Wiens, J.J.; Zelinka, J. How many species will Earth lose to climate change? Glob. Change Biol. 2024, 30, e17125. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Wang, X.; Park, T.; Chen, C.; Lian, X.; He, Y.; Bjerke, J.W.; Chen, A.; Ciais, P.; Tømmervik, H.; et al. Characteristics, drivers and feedbacks of global greening. Nat. Rev. Earth Environ. 2020, 1, 14–27. [Google Scholar] [CrossRef]
- Higgins, S.I.; Conradi, T.; Muhoko, E. Shifts in vegetation activity of terrestrial ecosystems attributable to climate trends. Nat. Geosci. 2023, 16, 147–153. [Google Scholar] [CrossRef]
- Xue, J.; Liu, L.; Li, Y.; Zhang, Y.; Liang, S.; Chai, H. Assessing Suitable Habitats for Gerbera piloselloides (L.)Cass. in China Using an Optimized MaxEnt Model and Key Environmental Drivers. Biology 2025, 14, 769. [Google Scholar] [CrossRef]
- Liu, L.; Liang, S.; Xie, C.; Liu, J.; Zheng, Y.; Xue, J. Predicting the Potential Distribution of Aralia chinensis L. (Wild Vegetable) in China Under Different Climate Change Scenarios. Biology 2024, 13, 937. [Google Scholar] [CrossRef]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef]
- Lawlor, J.; Comte, L.; Grenouillet, G.; Lenoir, J.; Baecher, J.; Bandara, R.M.W.J.; Bertrand, R.; Chen, I.; Diamond, S.; Lancaster, L.; et al. Mechanisms, detection and impacts of species redistributions under climate change. Nat. Rev. Earth Environ. 2024, 5, 351–368. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Qazi, A.W.; Saqib, Z.; Zaman-ul-Haq, M. Trends in species distribution modelling in context of rare and endemic plants: A systematic review. Ecol. Process. 2022, 11, 40. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Huang, Z. The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol. Evol. 2022, 12, e9165. [Google Scholar] [CrossRef]
- Haase, C.G.; Yang, A.N.; McNyset, K.M.; Blackburn, J.K. GARPTools: R software for data preparation and model evaluation of GARP models. Ecography 2021, 44, 1790–1796. [Google Scholar] [CrossRef]
- Semwal, D.P.; Pandey, A.; Gore, P.G.; Ahlawat, S.P.; Yadav, S.K.; Kumar, A. Habitat prediction mapping using BioClim model for prioritizing germplasm collection and conservation of an aquatic cash crop ‘makhana’ (Euryale ferox Salisb.) in India. Genet. Resour. Crop. Evol. 2021, 68, 3445–3456. [Google Scholar] [CrossRef]
- Amindin, A.; Pourghasemi, H.R.; Safaeian, R.; Rahmanian, S.; Tiefenbacher, J.P.; Naimi, B. Predicting current and future habitat suitability of an endemic species using data-fusion approach: Responses to climate change. Rangel. Ecol. Manag. 2024, 94, 149–162. [Google Scholar] [CrossRef]
- Rahmanian, S.; Nasiri, V.; Amindin, A.; Karami, S.; Maleki, S.; Pouyan, S.; Borz, S.A. Prediction of plant diversity using multi-seasonal remotely sensed and geodiversity data in a mountainous area. Remote Sens. 2023, 15, 387. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Barber, R.A.; Ball, S.G.; Morris, R.K.A.; Gilbert, F. Target-group backgrounds prove effective at correcting sampling bias in Maxent models. Divers. Distrib. 2022, 28, 128–141. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Merow, C.; Allen, J.M.; Aiello-Lammens, M.; Silander, J.A. Improving niche and range estimates with Maxent and point process models by integrating spatially explicit information. Glob. Ecol. Biogeogr. 2016, 25, 1022–1036. [Google Scholar] [CrossRef]
- Hosseini, N.; Ghorbanpour, M.; Mostafavi, H. Habitat potential modelling and the effect of climate change on the current and future distribution of three Thymus species in Iran using MaxEnt. Sci. Rep. 2024, 14, 3641. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Xue, J.; Shi, P.; Liang, S. Habitat Suitability Shifts of Eucommia ulmoides in Southwest China Under Climate Change Projections. Biology 2025, 14, 451. [Google Scholar] [CrossRef]
- Qadir, R.Y.; Khwarahm, N.R. Current and Projected Future Spatial Distribution Patterns of Prunus microcarpa in the Kurdistan Region of Iraq. Biology 2025, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Zhao, R.F.; Wang, S.J.; Chen, S.Y. Predicting the potential habitat suitability of saussurea species in China under future climate scenarios using the optimized maximum entropy (maxent) model. J. Clean. Prod. 2024, 474, 143552. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Yin, Y.; An, W.; Qin, X.; Wang, Y.; Fan, Y.; Li, Y.; Cao, Y. Fruit ripening in Lycium barbarum and Lycium ruthenicum is associated with distinct gene expression patterns. Febs. Open Bio 2020, 10, 1550–1567. [Google Scholar] [CrossRef]
- Dhar, P.; Tayade, A.; Ballabh, B.; Chaurasia, O.P.; Bhatt, R.P.; Srivastava, R.B. Lycium ruthenicum murray: A less-explored but high-value medicinal plant from trans-himalayan cold deserts of ladakh, india. Plant Arch. 2011, 11, 583–586. [Google Scholar]
- Yisilam, G.; Wang, C.-X.; Xia, M.-Q.; Comes, H.P.; Li, P.; Li, J.; Tian, X.-M. Phylogeography and Population Genetics Analyses Reveal Evolutionary History of the Desert Resource Plant Lycium ruthenicum (Solanaceae). Front. Plant Sci. 2022, 13, 915526. [Google Scholar] [CrossRef]
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum Murr: A review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Li, S.; He, Z. A review of Lycium ruthenicum Murray: Geographic distribution tracing, bioactive components, and functional properties. Heliyon 2024, 10, e39566. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.-A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Amiri, M.; Tarkesh, M.; Jafari, R.; Jetschke, G. Bioclimatic Variables from Precipitation and Temperature Records vs. Remote Sensing-Based Bioclimatic Variables: Which Side Can Perform Better in Species Distribution Modeling? Ecol. Inform. 2020, 57, 101060. [Google Scholar] [CrossRef]
- Vega, G.C.; Pertierra, L.R.; Olalla-Tárraga, M.Á. MERRAclim, a High-Resolution Global Dataset of Remotely Sensed Bioclimatic Variables for Ecological Modelling. Sci. Data 2017, 4, 170078. [Google Scholar] [CrossRef]
- Currie, D.J.; Mittelbach, G.G.; Cornell, H.V.; Field, R.; Guegan, J.-F.; Hawkins, B.A.; Kaufman, D.M.; Kerr, J.T.; Oberdorff, T.; O’Brien, E.; et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004, 7, 1121–1134. [Google Scholar] [CrossRef]
- Turner, J.R. Explaining the global biodiversity gradient: Energy, area, history and natural selection. Basic Appl. Ecol. 2004, 5, 435–448. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, L.; Qi, S.; Jin, M.; Hu, J.; Lu, J. Effect of Habitat Factors on the Understory Plant Diversity of Platycladus Orientalis Plantations in Beijing Mountainous Areas Based on MaxEnt Model. Ecol. Indic. 2021, 129, 107917. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Yan, B.; Liu, Z. Comprehensive assessment of climate-driven distribution dynamics and anthocyanin variation in Lycium ruthenicum using MaxEnt, HPLC, and Chemometric approaches. Ind. Crops Prod. 2025, 233, 121359. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Y.; Wang, J.; Zhou, Q.; Liu, F.; Chen, Q.; Liu, F. The potential geographical distribution of Lycium ruthenicum Murr under different climate change scenarios. Chin. J. Appl. Envion. Biol. 2020, 26, 969–978. [Google Scholar]
- Yao, J.Q.; Chen, Y.N.; Guan, X.F.; Zhao, Y.; Chen, J.; Mao, W.Y. Recent climate and hydrological changes in a mountain–basin system in Xinjiang, China. Earth-Sci. Rev. 2022, 226, 103957. [Google Scholar] [CrossRef]
- Li, C.; Gu, Y.; Xu, H.; Huang, J.; Liu, B.; Chun, K.P.; Octavianti, T. Spatial Heterogeneity in the Response of Winter Wheat Yield to Meteorological Dryness/Wetness Variations in Henan Province, China. Agronomy 2024, 14, 817. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, H.; Guo, Y.; Zhao, Z.; Pang, G.; Ma, Y.; Gu, W. Impacts of Climate Change on Cultivation Suitability of Lycium ruthenicum. J. Desert Res. 2017, 37, 902–909. [Google Scholar]
- Lin, L.; Jin, L.; Wang, Z.; Cui, Z.; Ma, Y. Prediction of the potential distribution of Tibetan medicinal Lycium ruthenicum in context of climate change. China J. Chin. Mater. Med. 2017, 42, 2659–2669. [Google Scholar]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.y8ktsb (accessed on 5 October 2025).
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R Package for Comparative Ecological Biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z. BCC-CSM2-HR: A High-Resolution Version of the Beijing Climate Center Climate System Model. Geosci. Model Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, L.; Shen, H.; Guan, R.; Ge, Q.; Huang, L.; Rohani, E.R.; Ou, J.; Han, R.; Tong, X. Potentially suitable geographical area for Pulsatilla chinensis Regel under current and future climatic scenarios based on the MaxEnt model. Front. Plant Sci. 2025, 16, 1538566. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Li, S.; Yang, Q.; Li, Y.; Xiang, Y.; Yao, B. MaxEnt Modeling of Future Habitat Shifts of Itea yunnanensis in China Under Climate Change Scenarios. Biology 2025, 14, 899. [Google Scholar] [CrossRef]
- Zhang, F.-G.; Liang, F.; Wu, K.; Xie, L.; Zhao, G.; Wang, Y. The potential habitat of Angelica dahurica in China under climate change scenario predicted by Maxent model. Front. Plant Sci. 2024, 15, 1388099. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Chen, Y.; Li, C.; Ding, S.; Zhang, X.; Luo, L.; Jia, Y.; Zhao, G. Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model. Plants 2025, 14, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Xiao, H.; Li, H.; Liao, D.; Xue, Y.; Huang, X.; Su, Q.; Xiao, Y. Changes in the Distribution Range of the Genus Cardiocrinum in China Under Climate Change and Human Activities. Biology 2025, 14, 581. [Google Scholar] [CrossRef]
- He, P.; Li, J.; Li, Y.; Xu, N.; Gao, Y.; Guo, L.; Huo, T.; Peng, C.; Meng, F. Habitat protection and planning for three Ephedra using the MaxEnt and Marxan models. Ecol. Indic. 2021, 133, 108399. [Google Scholar] [CrossRef]
- Gong, L.; Li, X.; Wu, S.; Jiang, L. Prediction of potential distribution of soybean in the frigid region in China with MaxEnt modeling. Ecol. Inform. 2022, 72, 101834. [Google Scholar] [CrossRef]
- Sexton, J.P.; McIntyre, P.J.; Angert, A.L.; Rice, K.J. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 415–436. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Wu, S. MaxEnt Modeling to Estimate the Impact of Climate Factors on Distribution of Pinus densiflora. Forests 2022, 13, 402. [Google Scholar] [CrossRef]
- Qi, Y.; Geng, W.; Zhou, W.; Wang, J.; Wang, Q.; Wang, W.; Liao, K. Study on the cold resistance of two Chinese wolfberry species. Xinjiang Agr. Sci. 2016, 53, 2203–2209. [Google Scholar]
- Jalali, G.A.; Akbarian, H.; Rhoades, C.; Yousefzadeh, H. The effect of the halophytic shrub Lycium ruthenium (Mutt) on selected soil properties of a desert ecosystem in central Iran. Pol. J. Ecol. 2012, 60, 845–850. [Google Scholar]
- Wang, L.; Zhao, G.; Lilong, W.; Zhang, M.; Zhang, L.; Zhang, X.; Guanxiang, Z.; Xu, S. C:N:P Stoichiometry and Leaf Traits of Halophytes in an Arid Saline Environment, Northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef]
- Zhao, P.; Qu, J.; Xu, X.; Yu, Q.; Jiang, S.; Zhao, H. Desert vegetation distribution and species-environment relationships in an oasis-desert ecotone of northwestern China. J. Arid. Land. 2019, 11, 461–476. [Google Scholar] [CrossRef]
- Valentin, D.N.; Voyron, S.; Soteras, F.; Iriarte, H.J.; Giovannini, A.; Lumini, E.; Lugo, M.A. Modeling geographic distribution of arbuscular mycorrhizal fungi from molecular evidence in soils of Argentinean Puna using a maximum entropy approach. PeerJ 2023, 11, e14651. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, J.; Liu, L.; Zhang, K. MaxEnt Modeling and Effects of Climate Change on Shifts in Habitat Suitability for Sorbus alnifolia in China. Plants 2025, 14, 677. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Duan, G.; Wang, Z.; Zhang, Y.; Fan, G. Potentially suitable habitats of Daodi goji berry in China under climate change. Front. Plant Sci. 2024, 14, 1279019. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Potential effects of climate change in future on the distributions of 7 desert plants in China. Arid Land Geogr. 2011, 34, 70–85. [Google Scholar]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental Factors Influence Plant Vascular System and Water Regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Opoku, V.A.; Adu, M.O.; Asare, P.A.; Asante, J.; Hygienus, G.; Andersen, M.N. Rapid and low-cost screening for single and combined effects of drought and heat stress on the morpho-physiological traits of African eggplant (Solanum aethiopicum) germplasm. PLoS ONE 2024, 19, e0295512. [Google Scholar] [CrossRef]
- Fatichi, S.; Peleg, N.; Mastrotheodoros, T.; Pappas, C.; Manoli, G. An ecohydrological journey of 4500 years reveals a stable but threatened precipitation–groundwater recharge relation around Jerusalem. Sci. Adv. 2021, 7, eabe6303. [Google Scholar] [CrossRef]
- Lin, P.F.; He, Z.B.; Du, J.; Chen, L.F.; Zhu, X.; Li, J. Recent changes in daily climate extremes in an arid mountain region, a case study in northwestern China’s Qilian Mountains. Sci. Rep. 2017, 7, 2245. [Google Scholar] [CrossRef]
- Shen, T.; Yu, H.; Wang, Y.Z. Assessing the impacts of climate change and habitat suitability on the distribution and quality of medicinal plant using multiple information integration: Take Gentiana rigescens as an example. Ecol. Indic. 2021, 123, 107376. [Google Scholar] [CrossRef]
- Pauchard, A.; Milbau, A.; Albihn, A.; Alexander, J.; Burgess, T.; Daehler, C.; Englund, G.; Essl, F.; Evengård, B.; Greenwood, G.B.; et al. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: New challenges for ecology and conservation. Biol. Invasions 2016, 18, 345–353. [Google Scholar] [CrossRef]
- Vázquez-Ramírez, J.; Venn, S.E. Seeds and Seedlings in a Changing World: A Systematic Review and Meta-Analysis from High Altitude and High Latitude Ecosystems. Plants 2021, 10, 768. [Google Scholar] [CrossRef]
- Urban, M.C.; Swaegers, J.; Stoks, R.; Snook, R.R.; Otto, S.P.; Noble, D.W.A.; Moiron, M.; Hällfors, M.H.; Gómez-Llano, M.; Fior, S.; et al. When and how can we predict adaptive responses to climate change? Evol. Lett. 2024, 8, 172–187. [Google Scholar] [CrossRef]
- Bonannella, C.; Hengl, T.; Heisig, J.; Parente, L.; Wright, M.N.; Herold, M.; De Bruin, S. Forest tree species distribution for Europe 2000–2020: Mapping potential and realized distributions using spatiotemporal machine learning. PeerJ 2022, 10, e13728. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, S.-D.; Jia, Y.; Hao, L.-H.; Xiang, D.-Y.; Chen, D.-F.; Niu, S.-C. Polyploid Induction and Karyotype Analysis of Dendrobium officinale. Horticulturae 2023, 9, 329. [Google Scholar] [CrossRef]
- Wang, C.; Ma, S.; Sun, F.; Wei, B.; Nie, Y. Spatial genetic patterns of the medicinal and edible shrub Lycium ruthenicum (Solanaceae) in arid Xinjiang, China. Tree Genet. Genomes 2021, 17, 22. [Google Scholar]
- Li, Z.; Wu, Y.; Wang, R.; Liu, B.; Qian, Z.; Li, C. Assessment of Climatic Impact on Vegetation Spring Phenology in Northern China. Atmosphere 2023, 14, 117. [Google Scholar] [CrossRef]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.; Pellissier, L.; Lenoir, J.; Damgaard, C.; Dormann, C.; Forchhammer, M.; Grytnes, J.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, Y.; Teng, D.; Zhang, X.; He, X.; Zhang, Y.; Lv, G. Rhizobacterial Communities of Five Co-Occurring Desert Halophytes. PeerJ 2018, 6, e5508. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, Z.; Tang, G.; Gulinisha, S.; Zhang, L.; Zhu, J.; Tang, Q.; Chu, M.; Ghenijan, O.; Outikuer, M.; et al. Community composition and ecological function of endophytic fungi in different tissues of Lycium ruthenicum. Mycosystema 2022, 41, 1254–1267. [Google Scholar]
- Geng, D.; Zhao, P.; Chen, Y.; Zhang, Y.; Duan, X. Interspecific Association and Niche of Desert Plant Communities in Qingtu Lake, the Tail of Shiyang River. J Hydroecol. 2024, 45, 121–131. [Google Scholar]
- Liu, J.; Ali, A.; Yu, M.; Zhu, F.; Kidane, D. Risk Evaluation of Main Pests and Integrated Management in Chinese Wolfberry, Lycium barbarum L. Pak. J. Zool. 2015, 47, 21–29. [Google Scholar]
- Li, C.; Jia, J.; Wu, F.; Zuo, L.; Cui, X. County-level intensity of carbon emissions from crop farming in China during 2000–2019. Sci. Data 2024, 11, 457. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).