Neurobiochemical Effects of a High-Fat Diet: Implications for the Pathogenesis of Neurodegenerative Diseases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. High-Fat Diet and Body Metabolism
4. Inflammation in the Nervous System as a Possible Consequence of HFD
5. Effects of a High-Fat Diet on the Central Nervous System
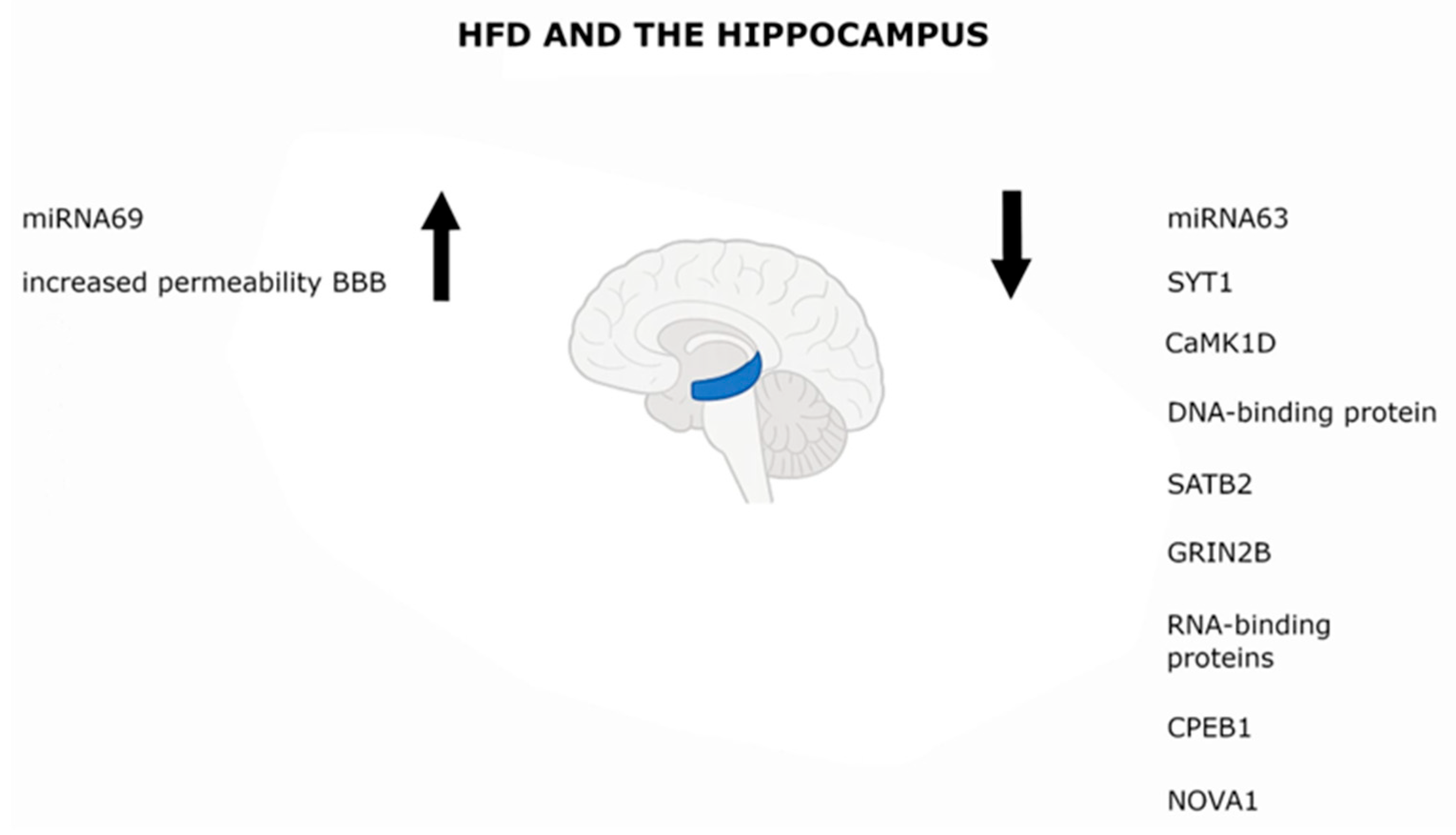
5.1. Hippocampus
5.2. Hypothalamus
5.3. Other Brain Structures Sensitive to HFD
5.4. Microglia
5.5. Astrocytes
5.6. Neurons
5.7. Dopaminergic System
5.8. Cholinergic System
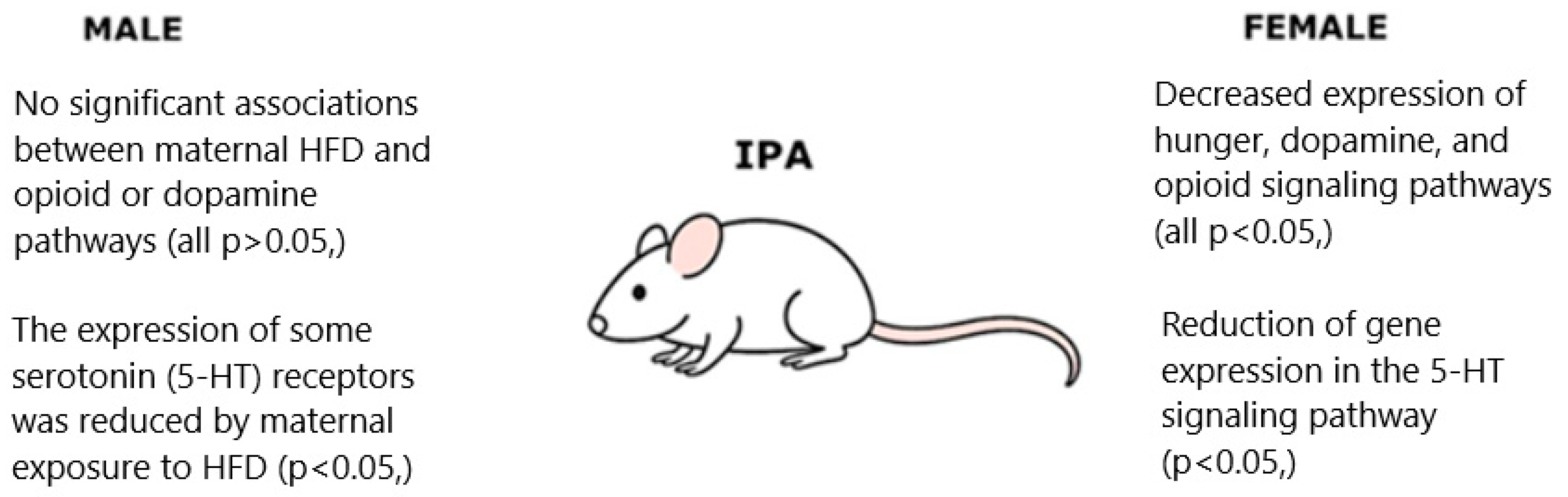
5.9. Influence of Maternal HFD on Offspring
6. High-Fat Diet and Neurodegenerative Diseases
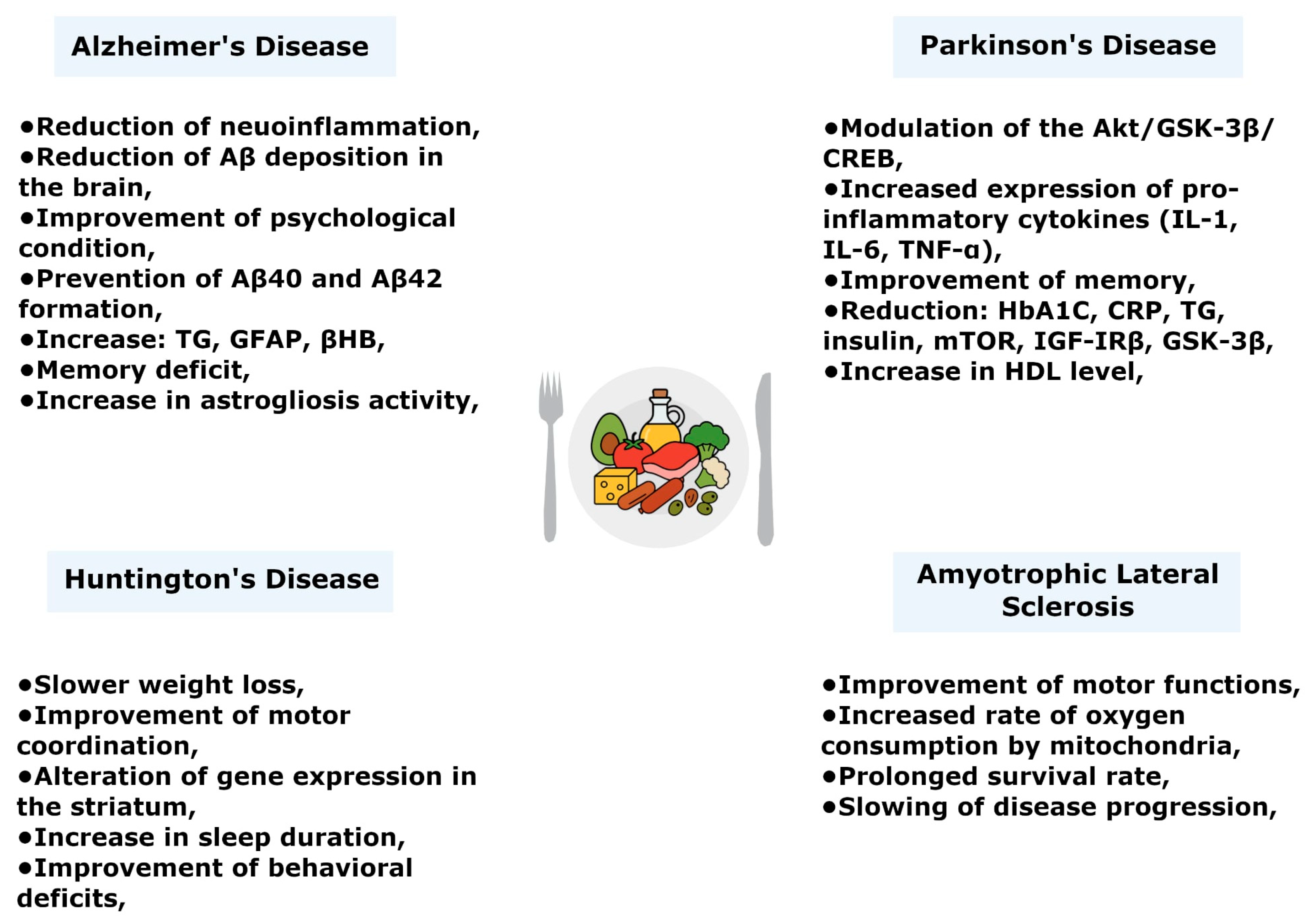
6.1. Alzheimer’s Disease
6.2. Parkinson’s Disease
6.3. Huntington’s Disease
6.4. Amyotrophic Lateral Sclerosis (ALS)
7. Conclusions
8. Perspectives and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AgRP | agouti-related peptide |
| ALS | amyotrophic lateral sclerosis |
| AMPK | AMP-activated protein kinase |
| BDNF | brain-derived neurotrophic factor |
| BrAce | breath acetone |
| CAG | cytosine–adenine–guanine trinucleotide repeat |
| CD38 | cyclic ADP-ribose hydrolase 1 |
| CD68 | cluster of differentiation 68 |
| CNS | central nervous system |
| CPEB1 | cytoplasmic polyadenylation element-binding protein 1 |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| CaMK1D | calcium/calmodulin-dependent protein kinase I delta |
| D1/D2 | dopamine D1/D2 receptors |
| DA | dopamine |
| DAT | dopamine transporter |
| DIO | diet-induced obesity |
| EMR1 | EGF-like module-containing mucin-like hormone receptor 1 (F4/80) |
| GFAP | glial fibrillary acidic protein |
| GLUT1 | glucose transporter type 1 |
| GLUT3 | glucose transporter type 3 |
| GRIN2B | glutamate ionotropic receptor NMDA type subunit 2B |
| HD | Huntington’s disease |
| HDL | high-density lipoprotein |
| HbA1c | glycated hemoglobin |
| HFD | high-fat diet |
| IBA1 | ionized calcium-binding adapter molecule 1 |
| IDE | insulin-degrading enzyme |
| IFN-γ | interferon gamma |
| IKKβ | inhibitor of kappa-B kinase beta |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IRS2 | insulin receptor substrate 2 |
| JNK | c-Jun N-terminal kinase |
| KB | ketone bodies |
| KD | ketogenic diet |
| LDL | low-density lipoprotein |
| MCP-1 | monocyte chemoattractant protein-1 (CCL2) |
| MCT | medium-chain triglyceride |
| MCT1 | monocarboxylate transporter 1 |
| MMP12 | matrix metalloproteinase-12 |
| MyD88 | myeloid differentiation primary response 88 |
| NAD+ | nicotinamide adenine dinucleotide (oxidized form) |
| NAMPT | nicotinamide phosphoribosyl transferase |
| NAc | nucleus accumbens |
| NF-κB | nuclear factor kappa-B |
| NOVA1 | neuro-oncological ventral antigen 1 |
| NO | nitric oxide |
| PD | Parkinson’s disease |
| PFC | prefrontal cortex |
| POMC | proopiomelanocortin |
| ROS | reactive oxygen species |
| SATB2 | special AT-rich sequence-binding protein 2 |
| SFA | saturated fatty acids |
| SN | substantia nigra |
| SNc | substantia nigra pars compacta |
| SYT1 | synaptotagmin-1 |
| TBI | traumatic brain injury |
| TG | triglycerides |
| TH | tyrosine hydroxylase |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor alpha |
| UCP2 | uncoupling protein 2 |
| UFA | unsaturated fatty acids |
| VTA | ventral tegmental area |
| mTOR | mechanistic (mammalian) target of rapamycin |
| βHB | β-hydroxybutyrate |
References
- Lawrence, M. Fundamentals of a Healthy and Sustainable Diet. Nutr. J. 2024, 23, 150. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef]
- Song, M.; Bai, Y.; Song, F. High-Fat Diet and Neuroinflammation: The Role of Mitochondria. Pharmacol. Res. 2025, 212, 107615. [Google Scholar] [CrossRef]
- Eickelberg, V.; Rimbach, G.; Seidler, Y.; Hasler, M.; Staats, S.; Lüersen, K. Fat Quality Impacts the Effect of a High-Fat Diet on the Fatty Acid Profile, Life History Traits and Gene Expression in Drosophila Melanogaster. Cells 2022, 11, 4043. [Google Scholar] [CrossRef]
- Petersen, K.S.; Maki, K.C.; Calder, P.C.; Belury, M.A.; Messina, M.; Kirkpatrick, C.F.; Harris, W.S. Perspective on the Health Effects of Unsaturated Fatty Acids and Commonly Consumed Plant Oils High in Unsaturated Fat. Br. J. Nutr. 2024, 132, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.I. Saturated Fats, Dairy Foods and Cardiovascular Health: No Longer a Curious Paradox? Nutr. Bull. 2022, 47, 407–422. [Google Scholar] [CrossRef]
- Zemer, A.; Samaei, S.; Yoel, U.; Biderman, A.; Pincu, Y. Ketogenic Diet in Clinical Populations-a Narrative Review. Front. Med. 2024, 11, 1432717. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The Ketogenic Diet for the Treatment of Childhood Epilepsy: A Randomised Controlled Trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Zupec-Kania, B.A.; Amark, P.E.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Helen Cross, J.; Dahlin, M.G.; et al. Optimal Clinical Management of Children Receiving the Ketogenic Diet: Recommendations of the International Ketogenic Diet Study Group. Epilepsia 2009, 50, 304–317. [Google Scholar] [CrossRef]
- Pfeifer, H.H.; Lyczkowski, D.A.; Thiele, E.A. Low Glycemic Index Treatment: Implementation and New Insights into Efficacy. Epilepsia 2008, 49 (Suppl. 8), 42–45. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Sun, K.; Choi, Y.T.; Yu, C.C.W.; Nelson, E.A.S.; Goh, J.; Dai, S.; Hui, L.L. The Effects of Ketogenic Diets and Ketone Supplements on the Aerobic Performance of Endurance Runners: A Systematic Review. Sports Health 2025, 17, 906–918. [Google Scholar] [CrossRef]
- Hagström, H.; Hagfors, L.N.; Hedelin, R.; Brunström, M.; Lindmark, K. Low Carbohydrate High Fat-Diet in Real Life; A Descriptive Analysis of Cardiovascular Risk Factors. Int. J. Cardiol. Cardiovasc. Risk Prev. 2025, 25, 200384. [Google Scholar] [CrossRef]
- Sharma, S. High Fat Diet and Its Effects on Cognitive Health: Alterations of Neuronal and Vascular Components of Brain. Physiol. Behav. 2021, 240, 113528. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Sokoloff, L. Regulation of Cerebral Metabolic Rate. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott-Raven: New York, NY, USA, 1999. [Google Scholar]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F. Ketone Bodies, Potential Therapeutic Uses. IUBMB Life 2001, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Lei, B.; Li, J.; Wang, R. Effects of a Low-Carbohydrate High-Fat Diet Combined with High-Intensity Interval Training on Body Composition and Maximal Oxygen Uptake: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10740. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High Fat Diet, Gut Microbiome and Gastrointestinal Cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.K.; Saw, N.L.; Woods, C.E.; Vidano, L.M.; Blumenfeld, S.E.; Lam, R.K.; Chu, E.K.; Reading, C.; Shamloo, M. Impact of High-Fat Diet on Cognitive Behavior and Central and Systemic Inflammation with Aging and Sex Differences in Mice. Brain Behav. Immun. 2024, 118, 334–354. [Google Scholar] [CrossRef]
- Ullah, R.; Rauf, N.; Nabi, G.; Yi, S.; Yu-Dong, Z.; Fu, J. Mechanistic Insight into High-Fat Diet-Induced Metabolic Inflammation in the Arcuate Nucleus of the Hypothalamus. Biomed. Pharmacother. 2021, 142, 112012. [Google Scholar] [CrossRef]
- Brouns, F. Overweight and Diabetes Prevention: Is a Low-Carbohydrate–High-Fat Diet Recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef]
- McGaugh, E.; Barthel, B. A Review of Ketogenic Diet and Lifestyle. Mo. Med. 2022, 119, 84–88. [Google Scholar]
- Borowicz-Reutt, K.; Krawczyk, M.; Czernia, J. Ketogenic Diet in the Treatment of Epilepsy. Nutrients 2024, 16, 1258. [Google Scholar] [CrossRef]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic Diet: An Effective Treatment Approach for Neurodegenerative Diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef] [PubMed]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Dilliraj, L.N.; Schiuma, G.; Lara, D.; Strazzabosco, G.; Clement, J.; Giovannini, P.; Trapella, C.; Narducci, M.; Rizzo, R. The Evolution of Ketosis: Potential Impact on Clinical Conditions. Nutrients 2022, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Dement. Geriatr. Cogn. Disord. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef]
- Masood, W.; Annamaraju, P.; Khan Suheb, M.Z.; Uppaluri, K.R. Ketogenic Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Morris, G.; Puri, B.K.; Carvalho, A.; Maes, M.; Berk, M.; Ruusunen, A.; Olive, L. Induced Ketosis as a Treatment for Neuroprogressive Disorders: Food for Thought? Int. J. Neuropsychopharmacol. 2020, 23, 366–384. [Google Scholar] [CrossRef]
- Harvey, C.J.D.C.; Schofield, G.M.; Williden, M.; McQuillan, J.A. The Effect of Medium Chain Triglycerides on Time to Nutritional Ketosis and Symptoms of Keto-Induction in Healthy Adults: A Randomised Controlled Clinical Trial. J. Nutr. Metab. 2018, 2018, 2630565. [Google Scholar] [CrossRef]
- Weber, I.C.; Braun, H.P.; Krumeich, F.; Güntner, A.T.; Pratsinis, S.E. Superior Acetone Selectivity in Gas Mixtures by Catalyst-Filtered Chemoresistive Sensors. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2020, 7, 2001503. [Google Scholar] [CrossRef]
- Marfatia, K.; Ni, J.; Preda, V.; Nasiri, N. Is Breath Best? A Systematic Review on the Accuracy and Utility of Nanotechnology Based Breath Analysis of Ketones in Type 1 Diabetes. Biosensors 2025, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Grisotto, C.; Taïlé, J.; Planesse, C.; Diotel, N.; Gonthier, M.-P.; Meilhac, O.; Couret, D. High-Fat Diet Aggravates Cerebral Infarct, Hemorrhagic Transformation and Neuroinflammation in a Mouse Stroke Model. Int. J. Mol. Sci. 2021, 22, 4571. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. Int. J. Mol. Sci. 2023, 24, 1144. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Q.; Chen, Y.; Du, H.; Zhao, Y. Leptin Limits Hepatic Lipid Accumulation and Inflammation via Vagal Activation of the JAK2-STAT3/AMPK Pathway. J. Nutr. Biochem. 2024, 134, 109748. [Google Scholar] [CrossRef]
- Arita, S.; Inagaki-Ohara, K. High-Fat-Diet-Induced Modulations of Leptin Signaling and Gastric Microbiota Drive Precancerous Lesions in the Stomach. Nutrition 2019, 67–68, 110556. [Google Scholar] [CrossRef]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the Hippocampus Contributes to Memory, Navigation and Cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef]
- Zhuang, H.; Yao, X.; Li, H.; Li, Q.; Yang, C.; Wang, C.; Xu, D.; Xiao, Y.; Gao, Y.; Gao, J.; et al. Long-Term High-Fat Diet Consumption by Mice throughout Adulthood Induces Neurobehavioral Alterations and Hippocampal Neuronal Remodeling Accompanied by Augmented Microglial Lipid Accumulation. Brain Behav. Immun. 2022, 100, 155–171. [Google Scholar] [CrossRef]
- Abedi, A.; Foroutan, T.; Mohaghegh Shalmani, L.; Dargahi, L. Sex-Specific Effects of High-Fat Diet on Rat Brain Glucose Metabolism and Early-Onset Dementia Symptoms. Mech. Ageing Dev. 2023, 211, 111795. [Google Scholar] [CrossRef]
- Escalona, R.; Larqué, C.; Cortes, D.; Vilchis, R.; Granados-Delgado, E.; Sánchez, A.; Sánchez-Bringas, G.; Lugo-Martínez, H. High-Fat Diet Impairs Glucose Homeostasis by Increased P16 Beta-Cell Expression and Alters Glucose Homeostasis of the Progeny in a Parental-Sex Dependent Manner. Front. Endocrinol. 2023, 14, 1246194. [Google Scholar] [CrossRef]
- Yao, X.; Yang, C.; Jia, X.; Yu, Z.; Wang, C.; Zhao, J.; Chen, Y.; Xie, B.; Zhuang, H.; Sun, C.; et al. High-Fat Diet Consumption Promotes Adolescent Neurobehavioral Abnormalities and Hippocampal Structural Alterations via Microglial Overactivation Accompanied by an Elevated Serum Free Fatty Acid Concentration. Brain Behav. Immun. 2024, 119, 236–250. [Google Scholar] [CrossRef]
- Osorio-Gómez, D.; Perez, C.I.; Salcedo-Tello, P.; Hernández-Matias, A.; Hernández-Ramírez, S.; Arroyo, B.; Pacheco-López, G.; Gutierrez, R.; Bermúdez-Rattoni, F.; Guzmán-Ramos, K.; et al. Early-Life and Chronic Exposure to High-Fat Diet Alters Noradrenergic and Glutamatergic Neurotransmission in the Male Rat Amygdala and Hippocampus under Cognitive Challenges. J. Neurosci. Res. 2024, 102, e25360. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A Four-Day Western-Style Dietary Intervention Causes Reductions in Hippocampal-Dependent Learning and Memory and Interoceptive Sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive Impairment Following High Fat Diet Consumption Is Associated with Brain Inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Park, M.; Choi, J.; Park, K.-Y.; Chung, H.Y.; Lee, J. A High-Fat Diet Impairs Neurogenesis: Involvement of Lipid Peroxidation and Brain-Derived Neurotrophic Factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, V.; Merino, B.; Del Olmo, N.; Frago, L.M.; Chowen, J.A.; Ruiz-Gayo, M.; Cano González, V. Effect of a High-Fat Diet and Leptin on STAT3 Phosphorylation in Hippocampal Astrocytes. Neuroreport 2023, 34, 30–37. [Google Scholar] [CrossRef]
- Butler, M.J.; Sengupta, S.; Muscat, S.M.; Amici, S.A.; Biltz, R.G.; Deems, N.P.; Dravid, P.; Mackey-Alfonso, S.; Ijaz, H.; Bettes, M.N.; et al. CD8+ T Cells Contribute to Diet-Induced Memory Deficits in Aged Male Rats. Brain Behav. Immun. 2023, 109, 235–250. [Google Scholar] [CrossRef]
- Atak, S.; Boye, A.; Peciña, S.; Liu, Z.-X. High-Fat-Sugar Diet Is Associated with Impaired Hippocampus-Dependent Memory in Humans. Physiol. Behav. 2023, 268, 114225. [Google Scholar] [CrossRef]
- Spinelli, M.; Spallotta, F.; Cencioni, C.; Natale, F.; Re, A.; Dellaria, A.; Farsetti, A.; Fusco, S.; Grassi, C. High Fat Diet Affects the Hippocampal Expression of miRNAs Targeting Brain Plasticity-Related Genes. Sci. Rep. 2024, 14, 19651. [Google Scholar] [CrossRef]
- Williams, G.; Bing, C.; Cai, X.J.; Harrold, J.A.; King, P.J.; Liu, X.H. The Hypothalamus and the Control of Energy Homeostasis: Different Circuits, Different Purposes. Physiol. Behav. 2001, 74, 683–701. [Google Scholar] [CrossRef]
- De Paula, G.C.; Simões, R.F.; Garcia-Serrano, A.M.; Duarte, J.M.N. High-Fat and High-Sucrose Diet-Induced Hypothalamic Inflammation Shows Sex Specific Features in Mice. Neurochem. Res. 2024, 49, 3356–3366. [Google Scholar] [CrossRef]
- Marcos, P.; Sánchez, M.L.; Coveñas, R. Neuropeptides in the Hypothalamus. Vitam. Horm. 2025, 127, 1–50. [Google Scholar] [CrossRef]
- Wang, X.; Ge, A.; Cheng, M.; Guo, F.; Zhao, M.; Zhou, X.; Liu, L.; Yang, N. Increased Hypothalamic Inflammation Associated with the Susceptibility to Obesity in Rats Exposed to High-Fat Diet. Exp. Diabetes Res. 2012, 2012, 847246. [Google Scholar] [CrossRef]
- Sonnefeld, L.; Rohmann, N.; Geisler, C.; Laudes, M. Is Human Obesity an Inflammatory Disease of the Hypothalamus? Eur. J. Endocrinol. 2023, 188, R37–R45. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Charmandari, E.; Bargiota, A.; Vlahos, N.F.; Mastorakos, G.; Valsamakis, G. The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients 2021, 13, 498. [Google Scholar] [CrossRef]
- Lopes, P.K.F.; de Oliveira Costa, S.; de Paula Simino, L.A.; Chaves, W.F.; Silva, F.A.; Costa, C.L.; Milanski, M.; Ignacio-Souza, L.M.; Torsoni, A.S.; Torsoni, M.A. Hypothalamic Inflammation and the Development of an Obese Phenotype Induced by High-Fat Diet Consumption Is Exacerbated in Alpha7 Nicotinic Cholinergic Receptor Knockout Mice. Food Res. Int. 2024, 176, 113808. [Google Scholar] [CrossRef]
- Mendes, N.F.; Jara, C.P.; Zanesco, A.M.; de Araújo, E.P. Hypothalamic Microglial Heterogeneity and Signature under High Fat Diet–Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 2256. [Google Scholar] [CrossRef]
- Lizarbe, B.; Cherix, A.; Duarte, J.M.N.; Cardinaux, J.-R.; Gruetter, R. High-Fat Diet Consumption Alters Energy Metabolism in the Mouse Hypothalamus. Int. J. Obes. 2019, 43, 1295–1304. [Google Scholar] [CrossRef]
- Poon, K. Behavioral Feeding Circuit: Dietary Fat-Induced Effects of Inflammatory Mediators in the Hypothalamus. Front. Endocrinol. 2020, 11, 591559. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, D.; Wang, L.; Król, E.; Mazidi, M.; Li, L.; Huang, Y.; Niu, C.; Liu, X.; Lam, S.M.; et al. Maternal High Fat Diet in Lactation Impacts Hypothalamic Neurogenesis and Neurotrophic Development, Leading to Later Life Susceptibility to Obesity in Male but Not Female Mice. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2305472. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 Receptors in Addiction-like Reward Dysfunction and Compulsive Eating in Obese Rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Cone, J.J.; Chartoff, E.H.; Potter, D.N.; Ebner, S.R.; Roitman, M.F. Prolonged High Fat Diet Reduces Dopamine Reuptake without Altering DAT Gene Expression. PLoS ONE 2013, 8, e58251. [Google Scholar] [CrossRef]
- Fordahl, S.C.; Jones, S.R. High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chem. Neurosci. 2017, 8, 290–299. [Google Scholar] [CrossRef]
- Valdivia, S.; Patrone, A.; Reynaldo, M.; Perello, M. Acute High Fat Diet Consumption Activates the Mesolimbic Circuit and Requires Orexin Signaling in a Mouse Model. PLoS ONE 2014, 9, e87478. [Google Scholar] [CrossRef]
- Naneix, F.; Tantot, F.; Glangetas, C.; Kaufling, J.; Janthakhin, Y.; Boitard, C.; Smedt-Peyrusse, V.D.; Pape, J.R.; Vancassel, S.; Trifilieff, P.; et al. Impact of Early Consumption of High-Fat Diet on the Mesolimbic Dopaminergic System. eNeuro 2017, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, M.J.; Han, J.; Kim, S.J.; Ryu, I.; Ju, X.; Ryu, M.J.; Chung, W.; Oh, E.; Kweon, G.R.; et al. A High-Fat Diet Induces a Loss of Midbrain Dopaminergic Neuronal Function That Underlies Motor Abnormalities. Exp. Neurobiol. 2017, 26, 104–112. [Google Scholar] [CrossRef]
- Bousquet, M.; St-Amour, I.; Vandal, M.; Julien, P.; Cicchetti, F.; Calon, F. High-Fat Diet Exacerbates MPTP-Induced Dopaminergic Degeneration in Mice. Neurobiol. Dis. 2012, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Jang, E.-H.; Park, C.-S.; Kang, J.-H. Enhanced Susceptibility to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Neurotoxicity in High-Fat Diet-Induced Obesity. Free Radic. Biol. Med. 2005, 38, 806–816. [Google Scholar] [CrossRef]
- Lowe, C.J.; Reichelt, A.C.; Hall, P.A. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn. Sci. 2019, 23, 349–361. [Google Scholar] [CrossRef]
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral Changes in Male Mice Fed a High-Fat Diet Are Associated with IL-1β Expression in Specific Brain Regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Guillemot-Legris, O.; Masquelier, J.; Everard, A.; Cani, P.D.; Alhouayek, M.; Muccioli, G.G. High-Fat Diet Feeding Differentially Affects the Development of Inflammation in the Central Nervous System. J. Neuroinflamm. 2016, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell. Neurosci. 2021, 15, 1–14. [Google Scholar] [CrossRef]
- Kim, J.D.; Yoon, N.A.; Jin, S.; Diano, S. Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding. Cell Metab. 2019, 30, 952–962. [Google Scholar] [CrossRef]
- Baufeld, C.; Osterloh, A.; Prokop, S.; Miller, K.R.; Heppner, F.L. High-Fat Diet-Induced Brain Region-Specific Phenotypic Spectrum of CNS Resident Microglia. Acta Neuropathol. 2016, 132, 361–375. [Google Scholar] [CrossRef]
- Henry, R.J.; Barrett, J.P.; Vaida, M.; Khan, N.Z.; Makarevich, O.; Ritzel, R.M.; Faden, A.I.; Stoica, B.A. Interaction of High-Fat Diet and Brain Trauma Alters Adipose Tissue Macrophages and Brain Microglia Associated with Exacerbated Cognitive Dysfunction. J. Neuroinflamm. 2024, 21, 113. [Google Scholar] [CrossRef]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef]
- Davis, A.B.; Lloyd, K.R.; Bollinger, J.L.; Wohleb, E.S.; Reyes, T.M. Adolescent High Fat Diet Alters the Transcriptional Response of Microglia in the Prefrontal Cortex in Response to Stressors in Both Male and Female Mice. Stress Amst. Neth. 2024, 27, 2365864. [Google Scholar] [CrossRef]
- Douglass, J.D.; Dorfman, M.D.; Fasnacht, R.; Shaffer, L.D.; Thaler, J.P. Astrocyte IKKβ/NF-κB Signaling Is Required for Diet-Induced Obesity and Hypothalamic Inflammation. Mol. Metab. 2017, 6, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Sa, M.; Park, M.G.; Lee, C.J. Role of Hypothalamic Reactive Astrocytes in Diet-Induced Obesity. Mol. Cells 2022, 45, 65–75. [Google Scholar] [CrossRef]
- Wang, Y.; Hsuchou, H.; He, Y.; Kastin, A.J.; Pan, W. Role of Astrocytes in Leptin Signaling. J. Mol. Neurosci. 2015, 56, 829–839. [Google Scholar] [CrossRef]
- Park, J.W.; Park, S.E.; Koh, W.; Jang, W.H.; Choi, J.H.; Roh, E.; Kang, G.M.; Kim, S.J.; Lim, H.S.; Park, C.B.; et al. Hypothalamic Astrocyte NAD+ Salvage Pathway Mediates the Coupling of Dietary Fat Overconsumption in a Mouse Model of Obesity. Nat. Commun. 2024, 15, 2102. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Pham, K.; Gammons, J.W.; Sutherland, D.; Liu, Y.; Smith, A.; Kaczorowski, C.C.; O’Connell, K.M.S. Diet Composition, Not Calorie Intake, Rapidly Alters Intrinsic Excitability of Hypothalamic AgRP/NPY Neurons in Mice. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Hayes, A.M.R.; Lauer, L.T.; Kao, A.E.; Sun, S.; Klug, M.E.; Tsan, L.; Rea, J.J.; Subramanian, K.S.; Gu, C.; Tanios, N.; et al. Western Diet Consumption Impairs Memory Function via Dysregulated Hippocampus Acetylcholine Signaling. Brain Behav. Immun. 2024, 118, 408–422. [Google Scholar] [CrossRef]
- Kang, J.; Park, M.; Oh, C.-M.; Kim, T. High-Fat Diet-Induced Dopaminergic Dysregulation Induces REM Sleep Fragmentation and ADHD-like Behaviors. Psychiatry Res. 2023, 327, 115412. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Wei, W.-Y.; Tsai, K.-J.; Wang, L.-C. High Fat Diet Suppresses Peroxisome Proliferator-Activated Receptors and Reduces Dopaminergic Neurons in the Substantia Nigra. Int. J. Mol. Sci. 2020, 21, 207. [Google Scholar] [CrossRef]
- Estes, M.K.; Bland, J.J.; Ector, K.K.; Puppa, M.J.; Powell, D.W.; Lester, D.B. A High Fat Western Diet Attenuates Phasic Dopamine Release. Neurosci. Lett. 2021, 756, 135952. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The Cholinergic System in Aging and Neuronal Degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Berry, A.S.; Harrison, T.M. New Perspectives on the Basal Forebrain Cholinergic System in Alzheimer’s Disease. Neurosci. Biobehav. Rev. 2023, 150, 1–26. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.; Kwon, Y.H. Long-Term Effects of Maternal Fat Consumption on the Brain Transcriptome of Obesogenic Diet-Fed Young Adult Mice Offspring. J. Nutr. 2024, 154, 1532–1539. [Google Scholar] [CrossRef]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The Impact of Maternal High-Fat Diet on Offspring Neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, Y.; Kumon, M.; Wada, E.; Onodera, M.; Mochizuki, H.; Wada, K. Maternal Obesity Impairs Hippocampal BDNF Production and Spatial Learning Performance in Young Mouse Offspring. Neurochem. Int. 2010, 57, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Frankowska, M.; Surówka, P.; Gawlińska, K.; Borczyk, M.; Korostyński, M.; Filip, M.; Smaga, I. A Maternal High-Fat Diet during Pregnancy and Lactation Induced Depression-like Behavior in Offspring and Myelin-Related Changes in the Rat Prefrontal Cortex. Front. Mol. Neurosci. 2023, 16, 1303718. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.M.; Kievit, P.; Kirigiti, M.A.; Bauman, L.A.; Baquero, K.; Blundell, P.; Dean, T.A.; Valleau, J.C.; Takahashi, D.L.; Frazee, T.; et al. Maternal High-Fat Diet and Obesity Impact Palatable Food Intake and Dopamine Signaling in Nonhuman Primate Offspring. Obesity 2015, 23, 2157–2164. [Google Scholar] [CrossRef]
- Kretschmer, T.; Turnwald, E.-M.; Janoschek, R.; Zentis, P.; Bae-Gartz, I.; Beers, T.; Handwerk, M.; Wohlfarth, M.; Ghilav, M.; Bloch, W.; et al. Maternal High Fat Diet-Induced Obesity Affects Trophoblast Differentiation and Placental Function in Mice†. Biol. Reprod. 2020, 103, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Cordner, Z.A.; Khambadkone, S.G.; Boersma, G.J.; Song, L.; Summers, T.N.; Moran, T.H.; Tamashiro, K.L.K. Maternal High-Fat Diet Results in Cognitive Impairment and Hippocampal Gene Expression Changes in Rat Offspring. Exp. Neurol. 2019, 318, 92–100. [Google Scholar] [CrossRef]
- da Silva, R.K.B.; de Vasconcelos, D.A.A.; da Silva, A.V.E.; da Silva, R.P.B.; de Oliveira Neto, O.B.; Galindo, L.C.M. Effects of Maternal High-Fat Diet on the Hypothalamic Components Related to Food Intake and Energy Expenditure in Mice Offspring. Life Sci. 2022, 307, 120880. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, M.; Comin, C.H.; Fernández de Cossío, L.; Lacabanne, C.; Freitas-Andrade, M.; González Ibáñez, F.; Raman-Nair, J.; Wakem, M.; Chakravarty, M.; Costa, L. da F.; et al. Maternal High-Fat Diet in Mice Induces Cerebrovascular, Microglial and Long-Term Behavioural Alterations in Offspring. Commun. Biol. 2022, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Zarnowska, I.M. Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients 2020, 12, 2616. [Google Scholar] [CrossRef]
- Rubio, C.; López-Landa, A.; Romo-Parra, H.; Rubio-Osornio, M. Impact of the Ketogenic Diet on Neurological Diseases: A Review. Life 2025, 15, 71. [Google Scholar] [CrossRef]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. Bethesda Md 2017, 8, 804–811. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146–1165. [Google Scholar] [CrossRef]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef]
- Rong, L.; Peng, Y.; Shen, Q.; Chen, K.; Fang, B.; Li, W. Effects of Ketogenic Diet on Cognitive Function of Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2024, 28, 100306. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A Ketogenic Diet Reduces Amyloid Beta 40 and 42 in a Mouse Model of Alzheimer’s Disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef]
- Lippi, S.L.; Barkey, R.E.; Rodriguez, M.N. High-Fat Diet Negatively Affects Brain Markers, Cognitive Behaviors, and Noncognitive Behaviors in the rTg4510 Tau Mouse Model. Physiol. Behav. 2023, 271, 1–14. [Google Scholar] [CrossRef]
- Gannon, O.J.; Robison, L.S.; Salinero, A.E.; Abi-Ghanem, C.; Mansour, F.M.; Kelly, R.D.; Tyagi, A.; Brawley, R.R.; Ogg, J.D.; Zuloaga, K.L. High-Fat Diet Exacerbates Cognitive Decline in Mouse Models of Alzheimer’s Disease and Mixed Dementia in a Sex-Dependent Manner. J. Neuroinflammation 2022, 19, 110. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Kip, E.; Parr-Brownlie, L.C. Reducing Neuroinflammation via Therapeutic Compounds and Lifestyle to Prevent or Delay Progression of Parkinson’s Disease. Ageing Res. Rev. 2022, 78, 101618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The Anti-Inflammatory Effect of Preventive Intervention with Ketogenic Diet Mediated by the Histone Acetylation of mGluR5 Promotor Region in Rat Parkinson’s Disease Model: A Dual-Tracer PET Study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional Ketosis for Mild Cognitive Impairment in Parkinson’s Disease: A Controlled Pilot Trial. Clin. Park. Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef]
- Tidman, M. Effects of a Ketogenic Diet on Symptoms, Biomarkers, Depression, and Anxiety in Parkinson’s Disease: A Case Study. Cureus 2022, 14, e23684. [Google Scholar] [CrossRef] [PubMed]
- Biju, K.C.; Hernandez, E.T.; Stallings, A.M.; Felix-Ortiz, A.C.; Hebbale, S.K.; Norton, L.; Mader, M.J.; Clark, R.A. Resistance to High-Fat Diet-Induced Weight Gain in Transgenic Mice Overexpressing Human Wild-Type α-Synuclein: A Model for Metabolic Dysfunction in Parkinson’s Disease. Res. Sq. 2024, 1, 1–26. [Google Scholar] [CrossRef]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s Disease: Diagnosis and Management. Pract. Neurol. 2022, 22, 32–41. [Google Scholar] [CrossRef]
- Cepeda, C.; Tong, X.-P. Huntington’s Disease: From Basic Science to Therapeutics. CNS Neurosci. Ther. 2018, 24, 247–249. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s Disease: From Molecular Pathogenesis to Clinical Treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Ross, J.L.; Kawamura, M.; Ruiz, T.L.; Geiger, J.D.; Masino, S.A. A Ketogenic Diet Delays Weight Loss and Does Not Impair Working Memory or Motor Function in the R6/2 1J Mouse Model of Huntington’s Disease. Physiol. Behav. 2011, 103, 501–507. [Google Scholar] [CrossRef]
- Whittaker, D.S.; Tamai, T.K.; Bains, R.S.; Villanueva, S.A.M.; Luk, S.H.C.; Dell’Angelica, D.; Block, G.D.; Ghiani, C.A.; Colwell, C.S. Dietary Ketosis Improves Circadian Dysfunction as Well as Motor Symptoms in the BACHD Mouse Model of Huntington’s Disease. Front. Nutr. 2022, 9, 1034743. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Tran, C.; Hwang, L.; Deng, G.; Jung, M.E.; Faull, K.F.; Levine, M.S.; Cepeda, C. Partial Amelioration of Peripheral and Central Symptoms of Huntington’s Disease via Modulation of Lipid Metabolism. J. Huntingt. Dis. 2016, 5, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Varghese, M.; Vempati, P.; Dzhun, A.; Cheng, A.; Wang, J.; Lange, D.; Bilski, A.; Faravelli, I.; Pasinetti, G.M. Caprylic Triglyceride as a Novel Therapeutic Approach to Effectively Improve the Performance and Attenuate the Symptoms Due to the Motor Neuron Loss in ALS Disease. PLoS ONE 2012, 7, e49191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Y.; Zhu, Y.; Jin, Q. Association between Dietary Patterns and the Prognosis of Amyotrophic Lateral Sclerosis in China: A Cross-Sectional Study. Front. Nutr. 2024, 11, 1437521. [Google Scholar] [CrossRef]
| Brain Structure | Model | Sex/Species/Strain | Age (When Diet Started/at Assessment) | Effect of HFD | Ref. |
|---|---|---|---|---|---|
| Hippocampus | High-fat, refined-sugar (HFS) diet vs. low-fat complex carbohydrate (LFCC) | Female, Rat, Fisher 344 | Start: 2 months old; assessed after 2 mo, 6 mo, or 2 years of diet | Impaired hippocampal-dependent memory; altered synaptic markers) | [51] |
| Hippocampus | High-fat diet (HFD) model of insulin resistance | Mouse (strain not specified in the main article text visible in PDF); “male mice” stated | Age not specified in the visible Methods; behavioral tests at end of diet regimen | ↓ SYT1, CaMK1D, GRIN2B, SATB2, CPEB1, NOVA1 | [55] |
| Hippocampus | Maternal HFD (32% fat) | Male offspring, Mouse, C57BL/6J | Dams: 5 wk; offspring E18, P21, P70 | ↓ BDNF, impaired dendritic development, ↓ spatial learning in young | [100] |
| Nucleus accumbens (NAc), VTA, medial prefrontal cortex | Periadolescent HFD exposure | Male, Rat, Long-Evans | Start: PND 21; assessed PND 110–120 | Reduced D2 receptor binding; impaired dopamine function | [71] |
| Hypothalamus, microglia | HFD with Ucp2 manipulation | Male and female, Mouse | Adult | UCP2 mediates microglial inflammation and obesity | [79] |
| Hypothalamus, microglia | HFD (short- and long-term) | Male, Mouse, C57BL/6J | Start: 100–120 d | Increased microglial activation, altered plasticity | [80] |
| Substantia nigra (SN), VTA | Chronic HFD (60% kcal fat, 20 wk) | Male, Mouse, C57BL/6J | Start: ~3 wk; assessed ~26 wk | Loss of dopaminergic neurons, ↓ PPARα/β/γ, dendritic spine loss, anxiety and cognitive deficits | [93] |
| Prefrontal cortex (PFC) | Maternal HFD (37% kcal fat) | Both sexes, Macaque (M. fuscata) | Dams 3.5–9.5 yr; offspring 7.5 and 13 mo | ↑ Palatable food intake; ↓ DA fiber density, ↓ D1/D2 receptors | [102] |
| Cortex (vascular system), microglia | Maternal HFD (60% kcal fat) | Both sexes offspring, Mouse, C57BL/6N | Dams: 6 wk; offspring P21–P85 | Hypervascularization, altered microglia–vascular interactions, ↑ stereotypic behaviors | [106] |
| Hippocampus, amygdala | Short-term HFD (60% kcal fat, 3 d) | Male, Rat, F344 × BN F1 | 3 mo and 24 mo | Aged rats: memory deficits, ↑neuroinflammation; mediated by CD8+ T cells | [53] |
| Hippocampus (DG, CA1) | HFD (45% kcal fat, 12 wk) or HSD (34% sucrose, 18 wk) | Male, Mouse, C57BL/6J; Cx3cr1^GFP/+ | Start: 8 wk; assessed after 12–18 wk | Cognitive deficits, dendritic spine loss, microglial activation and synaptic engulfment | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srokowska, M.; Żwierełło, W.; Wszołek, A.; Gutowska, I. Neurobiochemical Effects of a High-Fat Diet: Implications for the Pathogenesis of Neurodegenerative Diseases. Biology 2025, 14, 1317. https://doi.org/10.3390/biology14101317
Srokowska M, Żwierełło W, Wszołek A, Gutowska I. Neurobiochemical Effects of a High-Fat Diet: Implications for the Pathogenesis of Neurodegenerative Diseases. Biology. 2025; 14(10):1317. https://doi.org/10.3390/biology14101317
Chicago/Turabian StyleSrokowska, Marta, Wojciech Żwierełło, Agata Wszołek, and Izabela Gutowska. 2025. "Neurobiochemical Effects of a High-Fat Diet: Implications for the Pathogenesis of Neurodegenerative Diseases" Biology 14, no. 10: 1317. https://doi.org/10.3390/biology14101317
APA StyleSrokowska, M., Żwierełło, W., Wszołek, A., & Gutowska, I. (2025). Neurobiochemical Effects of a High-Fat Diet: Implications for the Pathogenesis of Neurodegenerative Diseases. Biology, 14(10), 1317. https://doi.org/10.3390/biology14101317







