Simple Summary
The extensive field work study between 2020 and 2023 led to the discovery of three new species of wood-inhabiting fungi; Tropicoporus pannaensis, Tropicoporus subindicus, and Tropicoporus xerophyticus from the southern part of India. The detailed descriptions and illustrations along with molecular support are presented. These discoveries may contribute to our understanding of species diversity and ecology, ultimately benefiting the society by informing the conservation efforts and exploring the potential biomolecules.
Abstract
This study aimed to investigate the morphological characteristics and phylogenetic relationships of three new species of Tropicoporus from the southern parts of India. The analyses of the ITS and nLSU regions revealed the novelty of these species, which have been named T. pannaensis, T. subindicus, and T. xerophyticus. All three species possess pileate basidiomes, a monomitic hyphal system in the context, and the presence of cystidioles and setae. However, they differ significantly in their phylogenetic placements and other morpho-taxonomic features. Tropicoporus pannaensis is characterized by a meagrely ungulate basidiome, indistinct zones, and an obtuse margin. Tropicoporus subindicus has a triquetrous basidiome and a radially cracked, crusted pileal surface with an acute margin, while T. xerophyticus is distinguished by an imbricate, perennial basidiome with an abundantly warted pileal surface. A phylogenetic tree is provided to show the placement of the three new species, along with detailed descriptions and illustrations. Additionally, a key for the identification of the Asian species of Tropicoporus is presented.
1. Introduction
Inonotus P. Karst. represents the largest and most challenging heterogeneous genus within the family Hymenochaetaceae. Segregating species within this genus has proven to be a persistent challenge for mycologists, as the traditional characteristics commonly used to delineate taxa are often inconclusive. The complex taxonomic history and the overlapping morphological features exhibited by many Inonotus species have made it difficult to establish clear and reliable species boundaries using only conventional identification methods. Additionally, intergeneric separation was known to be a polyphyletic origin, as Inonotus s.l. accommodated several homogeneous subgroups that are evident within [1,2]. Several molecular systematics, especially from the generated nLSU rDNA sequence data, were used to delimit Inonotus s.l. into four relatively smaller natural genera of monophyletic origin along with Inonotus s.s. [1]. As more molecular data were generated from East Asian and Mesoamerican origin, nearly 15 species were grouped under the Inonotus linteus complex [3,4]. Subsequently, morphological and phylogenetic analyses segregated I. linteus complex into Tropicoporus and its close evolutionary ally Sanghuangporus [5]. Tropicoporus is currently identified by the presence of resupinate, effused-reflexed to pilear surface, annual to perennial basidiomes with a homogeneous to duplex context, mono-dimitic or strictly dimitic hyphal system, hymenial setae, and basidiospores that are yellowish and have walls that range from slightly thin to thick [5]. Since then, 15 species were added under Tropicoporus. T. angustisulcatus, T. boehmeriae, T. drechsleri, T. excentrodendri, T. flabellatus, T. guanacastensis, T. hainanicus, T. lineatus, T. minus, T. nullisetus, T. ravidus, T. stratificans, T. substratificans, T. tenuis, and T. texanus [5,6,7,8,9,10,11]. Hymenial setae was reported to be absent in T. nullisetus alone [11]. According to the MycoBank database, as of September 2024, the genus Tropicoporus has 56 recognized species. Of these, 23 are newly described species, while 33 represent new taxonomic combinations. However, the phylogenetic relationships among Tropicoporus species and their geographical distribution patterns remain uncertain. To better understand the species circumscription and evolutionary history of this genus, additional systematic sampling and examination of specimens from the paleotropical region and other tropical and temperate Asian countries is required. Such comprehensive taxonomic and phylogenetic investigations will help elucidate the true diversity and biogeography of the genus Tropicoporus.
Earlier, our team reported the discovery of seven new species of Tropicoporus from the southern regions of the India. These species include T. cleistanthicola, T. indicus, T. natarajanii, T. pseudoindicus, T. subramanii, T. tamilnaduensis, and T. maritimus [12,13,14]. In the present study, we report the descriptions, illustrations, and phylogenetic analysis results for three novel species of Tropicoporus discovered in the state of Tamil Nadu, located in the southern region of India. The detailed morphological characterization and the robust phylogenetic placement of these three new taxa contribute to the growing knowledge of the diversity and evolutionary relationships within the genus Tropicoporus, particularly in the understudied mycobiota of the Indian subcontinent.
2. Materials and Methods
2.1. Sample Collection and Macro- and Micro-Taxonomic Characteristic Analyses
The basidiome samples examined in this study were collected during field surveys conducted between 2020 and 2023. The collection sites included the Veerappanur Reserve Forest in the Jawadhu Hills (coordinates: 12°54′24.9″ N, 78°87′75.6″ E), the Pennaiyar Reserve Forest in Sathanur (coordinates: 12°12′20.6172″ N, 78°53′20.2632″ E), both located within Thiruvannamalai District, and the Karaikudi (coordinates: 10°04′12.00″ N, 78°46′48.00″ E) in Sivagangai District, Tamil Nadu, India.
Morphological characteristics, including size, shape, annual or perennial, colour, texture, and margin (acute or obtuse) of basidiomes, were examined in fresh samples. Colour descriptions were based on the Methuen handbook [15]. The xanthochoric reaction in the context tissue (tissue turning permanently dark brown/black with a drop of KOH solution) was noted for fresh specimens. Other characteristic features like context (colour, homogenous, duplex with/without blackline), tube layer (length, colour, stratification, with context or not), and pores (shape and numbers per mm) were recorded.
For analyzing microscopic characteristics, tissues from dried basidiomes were taken by free-hand sections and mounted in sterile distilled water, 5% KOH solution (w/v), cotton blue (CB), and Melzer’s reagent (IK). The basidiospores, cystidiole, basidiole, and basidia were observed using phloxine stain. The microscopical features were photographed and illustrations were made as mentioned elsewhere [13]. The mean length and width of the basidiospores, their Q values (derived from an average of 30 basidiospores), and other abbreviations were used as mentioned earlier [13]. The basidiomes were deposited in the herbarium of Madras University Botany Laboratory (MUBL), Centre for Advanced Studies in Botany, University of Madras, Chennai-600 025, Tamil Nadu, India.
2.2. PCR Amplification and Phylogenetic Analyses
DNA was extracted from 50 mg of mycelium following the protocol described elsewhere [16] and was modified later [17]. The primers ITS1/ITS4 and LR0R/LR7 were used to amplify the ITS and LSU of nuclear ribosomal DNA region with the recommended thermal conditions [18,19]. The PCR products were then quantified and sequenced at Eurofins Genomics India (Karnataka, India).
Eighteen sequences were generated from the ITS and nLSU region and deposited in GenBank (Table 1). For the phylogenetic analyses, additional sequences from 71 taxa (60 nLSU and 70 ITS sequences), including Fulvifomes, Inocutis, Inonotus, Phellinus, Phylloporia, and Sanghuangporus, with an emphasis on Tropicoporus, were retrieved from GenBank (NCBI), along with Fomitiporella caryophylli (CBS 448.76) and F. neoarida (URM 80362) as outgroup (Table 1). To improve alignment similarity, the ITS and nLSU sequences were manually modified after being individually aligned in MEGA X v10.0.2 [20]. Using raxmlGUI 2.0 [21] and MrBayes 3.2.7a [22], respectively, the best-fit evolutionary model found by jModelTest 2.1.10 [23] was employed for the maximum likelihood (ML) analysis and Bayesian inference (BI) analysis. Bayesian inference was performed using two independent runs of six chains of Metropolis-coupled Markov chain Monte Carlo reconstructions for 2,000,000 generations, with tree samples obtained every 100 generations. The final sequence alignment was submitted to TreeBase (submission ID 30913; www.treebase.org).

Table 1.
Species, strain numbers, geographical locations, and corresponding GenBank accession numbers of the taxa used in this study.
3. Results
3.1. Phylogenetic Analyses
The similarity ratio of BLAST analyses for ITS and nLSU sequences of the three new species from India are summarized in Supplementary Tables S1 and S2. The total number of characters in the concatenated nLSU and ITS dataset is 1972 (1119 for nLSU and 853 for ITS), of which 1038 were constant, 833 variable, and 618 parsimony-informative. The maximum likelihood (ML) trees were constructed using raxmlGUI 2.0 [21] using the best-fit evolutionary model (GAMMA+P-Invar Model), which was estimated by jModelTest 2.1.10 [23] using 1000 rapid bootstrap inferences (BS). After 2,000,000 generations of Bayesian analysis, the average standard deviation was 0.009. A consistent tree topology was demonstrated by the phylogenetic tree built using ITS and LSU ( Supplementary Figures S1 and S2, respectively). Figure 1 shows the phylogenetic tree produced from the combined ITS and LSU datasets. Phylogenetic analyses inferred from ITS and nLSU reveal that our three new species form a sister clade with T. rudis (91% MLBS and 0.81 BI). Our three novel species clustered with allied Indian Tropicoporus taxa published earlier (0.99 BPP) and has a mono-dimitic hyphal system [12,13,14].
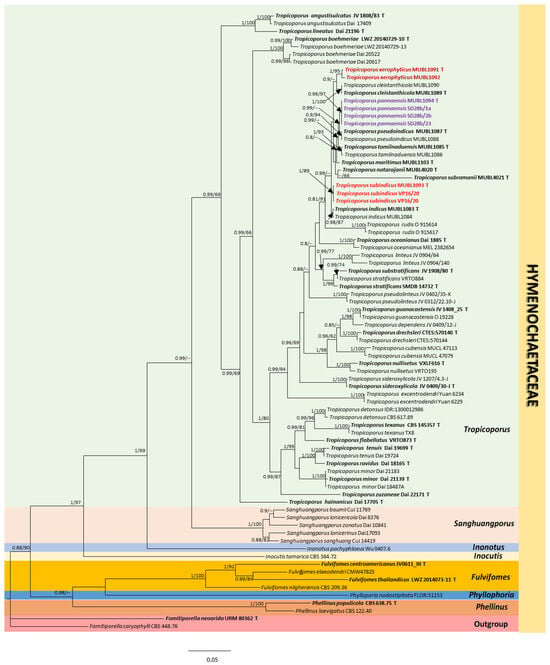
Figure 1.
Molecular phylogeny of the three new Tropicoporus species from India inferred through a Bayesian analysis of the combined ITS and LSU sequence data. The phylogenetic tree presented shows the placement of the novel taxa in relation to other known Tropicoporus species. The numbers indicated at the nodes represent the Bayesian posterior probabilities and bootstrap support values, with only those equal to or above 0.8 and 60%, respectively, being displayed. The type specimens are shown in bold, while the new Tropicoporus species are highlighted in colour and presented in bold text.
3.2. Taxonomic Characters of the Three New Species of Tropicoporus
Tropicoporus pannaensisS. Gunaseelan & M. Kaliyaperumal sp. nov.
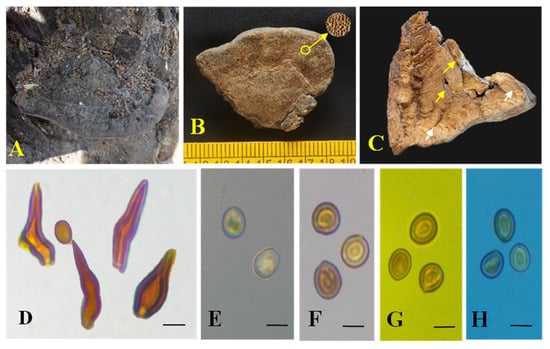
Figure 2.
Tropicoporus pannaensis (MUBL1094 holotype). (A) Holotype basidiomes. (B) Pore surface with enlarged pores. (C) Cross-section of a basidiome; yellow arrow indicates duplex context with blackline and white arrow indicates stratified tubes. (D) Hymenial setae. (E) Basidiospores in water. (F) Basidiospores in KOH. (G) Basidiospore in Melzer’s reagent. (H) Basidiospores in cotton blue. Scale bars: 5 µm (D–H).
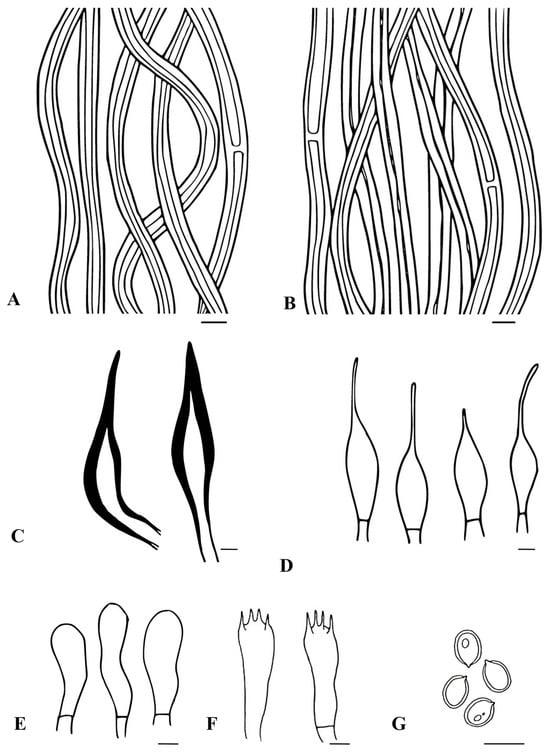
Figure 3.
Microscopic structures of Tropicoporus pannaensis (from the Holotype). (A) Hyphae from context. (B) Hyphae from trama. (C) Hymenial setae. (D) Cystidioles. (E) Basidioles. (F) Basidia. (G) Basidiospores. Scale bars: 5 µm.
Etymology: The species epithet “pannaensis” refers to the collection locality (Pennaiyar River, Sathanur Dam)
Typification: INDIA, Tamil Nadu, Thiruvannamalai District, Sathanur Dam, (12°15′60.46″ N, 78°96′05.86″ E), on the living tree (Prosopis juliflora), 29 January 2023, Sugantha Gunaseelan, SD28B (MUBL1094, Holotype).
GenBank numbers: ITS: OR515276; LSU: OR515277
Diagnosis: Tropicoporus pannaensis is characterized by applanate to meagrely ungulate, glabrous, zonate pilear surface, obtuse margin, duplex context with blackline, pores 4–7/mm, monomitic hyphal system in context, presence of cystidioles, and subglobose to broadly ellipsoid basidiospores measuring 5.3–5.8 × 4.5–5.3 μm.
Description: Basidiomes perennial, pileate, woody, light when fresh, hard when dry, lacks odour or taste. Pilei dimidiate, applanate to meagrely ungulate, projecting up to 5 cm, 6.7 cm wide, and 2.4 cm thick near attachment. Pileal surface brown (6E4) to greyish-brown (6E3), glabrous, zonate, tuberculate near the attachment. Margin obtuse, up to 3 mm thick, greyish-brown (6E3). Pore surface greyish-brown (6D3) and light brown (6D4), glancing. Pores round to angular, 4–6/mm. Context zonate, duplex with black line, brown (6D6), up to 2.4 cm thick. Tubes up to 2.5 cm long, stratified, and each stratum up to 2.4 mm, brown (6D6).
Hyphal system tissue darkening with KOH without hyphal swelling; monomitic in the context and dimitic in the trama; context: generative hyphae, thin- to thick-walled, hyaline to golden brown, occasionally branched with simple septate, 2–5 μm diam. Trama: generative hyphae, dominant, thin to thick-walled, hyaline to pale yellow, occasionally branched with septate, 2–4.5 μm diam; skeletal hyphae, thick-walled with narrow to wide lumen, yellowish-brown, unbranched, aseptate, 2–3 μm diam. Hymenial setae thick-walled, ventricose to subulate with a sharp and obtuse tip, dark brown, 14.2–28.4 × 3.8–5.6 μm. Cystidioles hyaline, thin-walled, ventricose to fusoid with elongated tapering apical portion, 9.8–23.73 × 3.8–5.2 μm. Basidia clavate to subclavate, 7.2–12 × 3–5.2 μm, with four sterigmata. Basidiole clavate, 4–11.4 × 3.1–5.2 μm. Basidiospores smooth, broadly ellipsoid to subglobose, thick-walled, pale yellow to golden yellow in water, turning golden yellow to brown in KOH, (5.3–) 5.5–5.8 × (4.5–) 4.7–5 (–5.3) μm (n = 30), Q = 1.11 (Q range 1.05–1.18), CB−, IKI−.
Habitat and distribution: Basidiomes are found on living trees of Prosopis juliflora (Fabaceae) distributed in Jawadhu Hills, Thiruvannamalai District, Tamil Nadu, India.
Tropicoporus subindicusR. Murugadoss, E. Arumugam & M. Kaliyaperumal sp. nov.
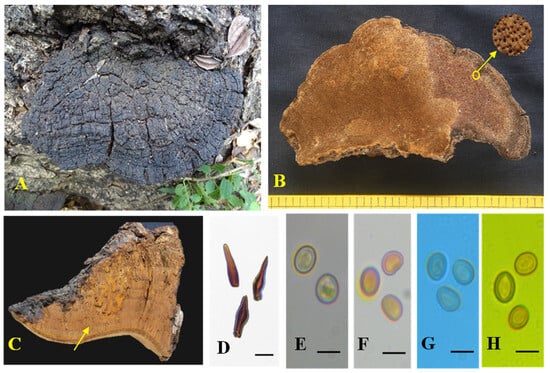
Figure 4.
Tropicoporus subindicus (MUBL1093 Holotype) (A) Basidiome (Holotype). (B) Pore surface with enlarged pores. (C) Cross-section of a basidiome; yellow arrow represents stratified tubes. (D) Hymenial setae. (E) Basidiospores in water. (F) Basidiospores in KOH. (G) Basidiospores in cotton blue. (H) Basidiospores in Melzer’s reagent. Scale bars: (D–H) = 5 µm.
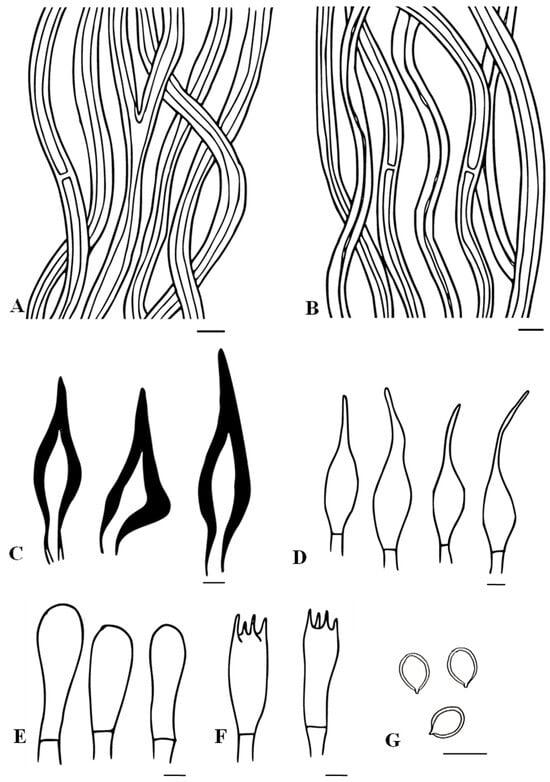
Figure 5.
Microscopic structures of Tropicoporus subindicus (from the Holotype). (A) Hyphae from context. (B) Hyphae from trama. (C) Hymenial setae. (D) Cystidioles. (E) Basidioles. (F) Basidia. (G) Basidiospores. Scale bars: 5 µm.
Etymology: The term “subindicus” refers to the tight evolutionary relationship between the species and Tropicoporus indicus.
Typification: INDIA, Tamil Nadu, Thiruvannamalai District, Veerapanur Reserve Forest, Jawadhu Hills (12°61′95.61″ N, 78°92′89.46″ E), on dead wood, 28 January 2020, Ramesh Murugadoss, VP16 (MUBL1093, Holotype)
GenBank numbers: ITS: OR519719; LSU: OR519722
Diagnosis: Tropicoporus subindicus is characterized by ungulate to triquetrous basidiome with concentrically zonate and sulcate, pilear surface radially cracked, context homogenous with monomitic hyphal system, acute margin, presence of cystidioles and setae, and subglobose to broadly ellipsoid basidiospores measuring 5–5.5 × 4.3–5.5 μm.
Holotype: MUBL1093
Description: Basidiomes perennial, pileate, woody, light in weight, hard when dry, without odour or taste. Pilei dimidiate, ungulate to triquetrous, projecting up to 8.3 cm, 20.4 cm wide, and 5.6 cm thick near attachment. Pileal surface greyish-brown (6F3) to brownish-grey (7F2), radially cracked, concentrically zonate and sulcate with crust. Margin acute, incurved towards pilear surface, >1 mm thick, dark brown (6F6). Pore surface dark brown (7E5) and brownish-orange (5C6), glancing. Pores round to angular, 4–6/mm. Context homogenous, brown (6E6), up to 1 mm thick. Tubes up to 5.5 cm long, stratified, each stratum up to 4.4 mm, light brown (6D6).
Hyphal system tissue darkens in KOH without hyphal swelling; monomitic in the context and dimitic in trama; generative hyphae, thin- to thick-walled, hyaline to golden yellow, rarely branched, simple septate, 2–5 μm diam. Trama: generative hyphae, dominant, thin- to thick-walled, hyaline to yellowish, occasionally branched, septate, 2–4.5 μm diam.; skeletal hyphae, thick-walled with narrow to wide lumen, yellowish-brown, unbranched, aseptate, 2–3 μm diam. Hymenial setae thick-walled, ventricose to subulate with a sharp and blunt tip, dark brown, 6.5–27.5 × 2.5–5.5 μm. Cystidioles thin-walled, hyaline, ventricose to fusoid with elongated tapering apical portion, 10–18 × 3–5 μm. Basidia clavate to broadly clavate, 8–10 × 3–5 μm, with four sterigmata. Basidiole clavate, 3.6–12 × 3.3–5 μm. Basidiospores broadly ellipsoid to subglobose, thick-walled, pale yellow in water, turning golden yellow to brown in KOH, CB−, IKI−, (5–) 5.3–5.5 × (4.3–) 4.5–4.8 (–5.5) μm (n = 30), Q = 1.10 (Q range 1–1.17).
Habitat and distribution: Basidiomes found on dead wood, Jawadhu Hills, Thiruvannamalai District, Tamil Nadu, India.
Tropicoporus xerophyticusE. Arumugam & M. Kaliyaperumal sp. nov.

Figure 6.
Tropicoporus xerophyticus (MUBL1091 holotype). (A) Basidiomes (Holotype). (B) Pore surface with enlarged pores. (C) Cross-section of a basidiome; yellow arrow indicates stratified tubes. (D,E) Hymenial setae. (F–I) Basidiospores: (F) Basidiospores in water. (G) Basidiopores in KOH. (H). Basidiopores in cotton blue. (I) Basidiopores in Melzer’s reagent. Scale bars: (D–I) = 5 µm.
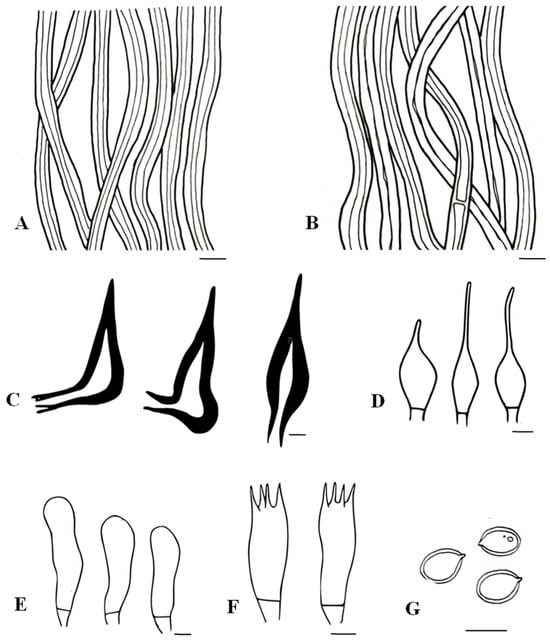
Figure 7.
Tropicoporus xerophyticus (MUBL1091 from the Holotype). (A) Contextual hyphae. (B) Tramal hyphae. (C) Hymenial setae. (D) Cystidioles. (E) Basidioles. (F) Basidia. (G) Basidiospores. Scale bars = 5 µm.
Etymology: The term “xerophyticus” refers to the dry environmental conditions in which the new species grows.
Typification: INDIA, Tamil Nadu, Karaikudi District, Tamil Nadu, (10°08′70.56″ N, 78°79′39.40″ E), on living angiosperm tree (Acacia arabica), 23 January 2023, Elangovan Arumugam, ALP18 (MUBL1091, Holotype).
GenBank numbers: ITS: OR515186; LSU: OR515187
Diagnosis: Tropicoporus xerophyticus is characterized by perennial, imbricate, broadly zonate, sulcate, deep fissures at maturity, warted, obtuse to round margin, homogenous context with monomitic hyphal system, presence of cystidioles and setae, and subglobose to broadly ellipsoid basidiospores measuring 4.5–5.5 × 4.2–5 μm.
Description: Basidiomes perennial, solitary, pileate, light when fresh, hard when dry, lacks odour or taste. Pilei dimidiate, imbricate, projecting up to 12 cm, 23 cm wide and 5 cm thick near attachment. Pilear surface greyish-brown (7F3) to dark grey (1F1), frequently warted towards margin, broadly zonate, sulcate, distinctly cracked with deep fissures at maturity. Margin yellowish-brown (5D5), obtuse to round, 1.8 cm thick. Pore surface dark brown (6F6). Pores round to angular, 3–6/mm; context brown (6D6), homogenous, up to 2.5 cm thick. Tubes brown (6E7), 2.4 cm, with intermittent context, stratified, each stratum up to 3.8 mm.
Hyphal system tissue darkens with KOH without hyphal swelling, mono-dimitic; context: generative hyphae, thin- to thick-walled, hyaline to golden yellow, occasionally branched, simple septate, 2–5 μm diam. Trama: generative hyphae, dominant, thin- to thick-walled, hyaline to yellowish, occasionally branched, septate, 2–4 μm diam.; skeletal hyphae, thick-walled with narrow to wide lumen, yellow to yellowish-brown, unbranched, aseptate, 2–3.2 μm diam. Hymenial setae thick-walled, ventricose to subulate with a sharp to blunt tip, dark brown, 12–27.5 × 4.7–5.5 μm. Cystidioles hyaline, thin-walled, fusoid, with elongated tapering apical portion 10–15.5 × 2.2–3 μm. Basidia clavate to subclavate, 8–10 × 3–5 μm, with four sterigmata. Basidiole clavate, 3–10 × 3.5–5 μm. Basidiospores smooth, subglobose to broadly ellipsoid, fairly thick-walled to thick-walled, pale yellow to golden yellow in water, turning golden yellow to brown in KOH, CB−, IKI−, (4.5–) 4.8– 5 (–5.5) × (4.2–) 4.5–4.7 (–5) μm (n = 30), Q = 1.08 (Q range 1.06–1.17).
Additional material examined: INDIA, Tamil Nadu, Karaikudi District, Tamil Nadu, 10°08′69.40″ N, 78°79′37.20″ E, on living angiosperm tree (Acacia arabica), 23 January 2023, Dr. K. Malarvizhi, ALP33 (MUBL1092, Paratype).
GenBank numbers: ITS: OR515255; LSU: OR515267
Habitat and distribution: Basidiomes are found on living trees of Acacia arabica (Fabaceae) distributed in Karaikudi, Sivagangai District, Tamil Nadu, India.
4. Discussion
In addition to comprehensive traditional taxonomic studies, phylogenetic analyses resolved the uncertainty in Inonotus s.l. [2,24,25] and delimited I. linteus complex into Inonotus s.str., Tropicoporus, and Sanghuangporus [3,4,5]. India harbours nearly four hotspots of rich vegetation; however, the members of Hymenochaetoid fungi were explored from northern parts of India and illustrated only by conventional methods [26,27,28]. In Southern India, in continuation with our earlier report from Eastern Ghats (a fragmented mountain range of lower elevation with disturbed vegetation) [13], we report two additional species of Tropicoporus. The ML and Bayesian trees depicted in this paper (Figure 1) validate the topology and are similar to previous reports [10,13,14].
Tropicoporus xerophyticus formed a sister clade with T. cleistanthicola (0.9 BPP). While analyzing the morphology of T. xerophyticus and T. cleistanthicola, the former significantly differs in the imbricate basidiome, which is broadly zonate with abundant warts; has an obtuse margin; and is distinctly cracked with deep fissures and larger pores (3–6/mm), whereas T. cleistanthicola has a pileate with an uncracked basidiome, is narrowly zonate, and has an acute margin and smaller pores (5–7/mm) [13]. Tropicoporus xerophyticus is phylogenetically distinct from other Indian allied taxa, namely T. pseudoindicus, T. tamilnaduensis, T. maritimus, T. natarajanii, and T. subramanii but are similar in mono-dimitic hyphal system alone and significantly varies in other morpho-taxonomic characters. Tropicoporus xerophyticus and T. subramanii resemble each other by having a pileate with a cracked basidiome, larger pores, and a hyphal system, but the former lacks a crust in the pileus and has an obtuse margin and smaller spores [12]. Tropicoporus xerophyticus and T. maritimus are congruous, having a pileate and broadly zonate basidiome but our new species greatly differ by having an imbricate basidiome that is cracked with deep fissures, a homogenous context, and an obtuse to round margin [14]. Tropicoporus xerophyticus and T. pseudoindicus resemble by having a cracked basidiome and an obtuse margin but the former significantly differs from the latter by having an imbricate and warted basidiome, larger pores (3–6/mm), and homogenous context; T. pseudoindicus was reported to have larger pores (6–8/mm) and a duplex context [13]. Tropicoporus xerophyticus varies from T. tamilnaduensis in having an imbricate, warted basidiome with larger pores (3–6/mm) and smaller cystidioles [13]. Though T. xerophyticus and T. rudis have a homogenous context and mono-dimitic hyphal system, the former varies from the T. rudis in basidiome characteristics and acyanophilic basidiospore, with the latter species having cyanophilic basidiospores [10]. Tropicoporus xerophyticus shares a homogenous context and distinctly cracked basidiomes with T. pesudolinteus and T. sideroxylicola, but the Indian species differs in other morphological characteristics, like having an imbricate, frequently warted, and broadly zonate basidiome [4]. Tropicoporus xerophyticus and T. stratificans are similar only in the presence of an intermittent context in the trama and the presence of cystidioles, while the latter has a resupinate and glancing basidiomes, dimitic hyphal system, and smaller basidiospores (3.5–6 × 3–4.5 μm) [7].
Tropicoporus pannaensis is phylogenetically distinct from T. pseudoindicus (0.99 BI and 57% MLBS) [13]. Morphologically, T. pannaensis differs with T. pseudoindicus in having an uncracked basidiome, larger pores (4–6/mm), and larger basidiospores (5.5–5.8 × 4.7–5), while T. pseudoindicus has a distinctly cracked basidiome, smaller pores (6–8/mm). and smaller basidiospores (4.2–5 × 4–4.5) [13]. Tropicoporus pannaensis is similar with T. tamilnaduensis, T. subramanii, and T. rudis by having pileate basidiomes and a hyphal system, but our species has uncracked basidiomes and a duplex context [10,12,13]. Tropicoporus pannaensis resembles T. maritimus with a few morphological features such as a pileate uncracked basidiome and duplex context, but the former differs in pilear surface characters such as a meagrely ungulate, frequently warted basidiome, tuberculate near attachment, and obtuse margin [14]. Tropicoporus pannaensis and T. natarajanii are similar by having an uncracked basidiome and a duplex context; however, T. pannaensis significantly varies in the glabrous pilear surface, having a broadly zonate, larger setae and smaller basidiospores. However, T. natarajanii has a velutinate azonate pileal surface with abundant tuberculate, smaller setae, and larger basidiospores [12]. Tropicoporus pannaensis and T. cleistanthicola are similar in the zonate basidiome, but our new species differ in having a glabrous basidiome, duplex context, obtuse margin, smaller cystidioles (9.8–23.73 × 3.8–5.2 μm), and larger spores. Tropicoporus cleistanthicola has a warted pileal surface, homogenous context, acute margin, larger cystidioles (7–45 × 2–5 μm), and smaller basidiospores (T. pannaensis (5.3–) 5.5–5.8 × (4.5–) 4.7–5 (–5.3) μm vs. T. cleistanthicola (4.7–) 4.9–5.2 (–5.4) × (4.2–) 4.5–4.7 (–4.9) μm) [13].
Tropicoporus pannaensis is different from T. stratificans and T. substratificans in morphological (applanate to meagrely ungulate basidiome, pores (4–6/mm) and microscopic characteristics (mono-dimitic hyphal system) [7,10]. Tropicoporus pannaensis differs from T. sideroxylicola by having uncracked and zonate basidiomes, smaller pores, a homogenous context, and a mono-dimitic hyphal system [4].
Tropicoporus subindicus is closely clustered with T. indicus but T. subindicus and these formed a sister to other Indian Tropicoporus spp. (BPP 0.99). Tropicoporus subindicus differs with T. indicus by having a radially cracked basidiome with crust, incurved margin, and is more or less concolorous with pileus colour and smaller basidiospores ((5–) 5.3–5.5 × (4.3–) 4.5–4.8 (–5.5) μm), while T. indicus is indistinctly cracked, lacks crust and an entire margin, and has distinctly yellow and larger basidiospores (5–6 × 4.2–4.9 μm) [13].
T. subindicus shares few characters with T. maritimus in having an acute incurved margin, but the former varies in having an ungulate to triquetrous, radially cracked crusted basidiome and a homogenous context [14]. Tropicoporus subindicus and T. pseudoindicus has a cracked and sulcate pilear surface (13). However, T. subindicus varies by having a concentrically zonate, acute incurved margin, homogenous context, larger pores (4–6/mm), and smaller cystidioles but T. pseudoindicus has a broadly zonate, duplex context, acute to obtuse margin, smaller pores (6–8/mm), and larger cystidioles [13]. Tropicoporus subindicus shares similar features with T. subramanii in having a cracked pilear surface, acute margin, homogenous context, and hyphal system, but our new species differs by having a concentrically zonate pileus, presence of cystidioles, and smaller basidiospores (5–) 5.3–5.5 × (4.3–) 4.5–4.8 (–5.5) μm, while T. subramanii has a deeply rimose pilear surface, absence of cystidioles, and larger basidiospores (5–) 5.3–6.4 (–6.7) × (4.5–) 4.8–5 (–5.2) μm [12]. Tropicoporus subindicus and T. natarajanii are consistent only in the hyphal system but T. subindicus significantly differs in morphological characteristics such as a cracked crust, concentrically zonate and sulcate basidiome, acute margin, and homogenous context, while T. natarajanii has an uncracked, azonate, abundant tuberculate without a crust pilear surface, obtuse margin, and duplex context [12]. Tropicoporus subindicus is similar to T. cleistanthicola in having a homogenous context and an acute margin, but T. subindicus varies in the pilear surface with a radially cracked crust, concentrically zonate sulcate basidiome, and smaller cystidioles. While T. cleistanthicola has an uncracked, narrowly zonate, warted basidiome and larger cystidioles [13]. Tropicoporus subindicus is congruous with T. tamilnaduensis in having a cracked basidiome, homogenous context, and pores but T. subindicus varies by having a concentrically zonate, crust pilear surface, acute margin and smaller cystidioles (10–18 × 3–5) μm, while T. tamilnaduensis has broadly zonate obtuse margin and larger cystidioles (10–45 × 2–5) μm [13].
We provide below the key to species of Tropicoporus in the Afro-Asian region.
Key to species of Tropicoporus in the Afro-Asian region
| 1 | Basidiomes resupinate to effused-reflexed | 2 |
| 1 | Basidiomes distinctly pileate | 8 |
| 2 | Basidiomes annual to biennial | 3 |
| 2 | Basidiomes perennial | 6 |
| 3 | Basidiospores cyanophilic | T. tenuis |
| 3 | Basidiospores acyanophilic | 4 |
| 4 | Basidiome resupinate to effused reflexed, pileal surface tomentose to hispid basidiospores > 3 μm in length | T. excentrodendri |
| 4 | Basidiome resupinate, basidiospores < 3 μm in length | 5 |
| 5 | Dissepiments lacerate, context layer present between tube layers | T. hainanicus |
| 5 | Dissepiments entire, context layer absent between tube layers | T. boehmeriae |
| 6 | Basidiomes resupinate, cystidioles present | 7 |
| 6 | Basidiomes cushion-shaped, cystidioles absent | T. ravidus |
| 7 | Pores 10–12/mm, basidiospores < 3 μm wide | T. minor |
| 7 | Pores 6–8/mm, basidiospores > 3 μm wide | T. zuzanae |
| 8 | Hyphal system strictly dimitic | T. lineatus |
| 8 | Hyphal system mono-dimitic, dimitic in trama | 9 |
| 9 | Basidiomes uncracked | 10 |
| 9 | Basidiomes cracked to rimose | 13 |
| 10 | Pilear surface warted; Pores always >5/mm | 11 |
| 10 | Pilear surface glabrous; Pores < 5/mm | 12 |
| 11 | Pilear surface azonate with warts, obtuse margin, context duplex without blackline | T. natarajanii |
| 11 | Basidiomes with infrequent warts, acute margin and homogenous context | T. cleistanthicola |
| 12 | Pilear surface indistinctly zonate, margin obtuse | T. pannaensis |
| 12 | Pilear surface broadly zonate, margin acute | T. maritimus |
| 13 | Pilear surface frequently warted with deep cracks at maturity, stratified tube with intermittent context | T. xerophyticus |
| 13 | Pilear surface cracked, lacks warts, stratified tubes without intermittent context | 14 |
| 14 | Pilear surface fulvous, velvety and cyanopilous basidiospores | T. rudis |
| 14 | Pilear surface smooth to glabrous or sulcate and acyanophilous basidiospores | 15 |
| 15 | Context duplex with black line | T. pseudoindicus |
| 15 | Context homogenous | 16 |
| 16 | Absence of cystidioles | T. subramanii |
| 16 | Presence of cystidioles | 17 |
| 17 | Obtuse margin, cystidiole more than 25 µm in length, hymenial setae not exceeding 20 µm in length | T. tamilnaduensis |
| 17 | Acute margin, cystidiole not exceeding 25 µm in length, hymenial setae more than 20 µm in length | 18 |
| 18 | Basidiomes with radially cracked, sulcate, crusted pilear surface | T. subindicus |
| 18 | Basidiomes with glabrous, irregularly cracked pilear surface without crust | T. indicus |
5. Conclusions
While previous studies on Hymenochaetoid fungi in India focused on the northern regions using only conventional taxonomic methods, this work builds on the authors’ earlier discoveries from the Eastern Ghats, reporting two additional new Tropicoporus species from Southern India. The phylogenetic relationships inferred from the ITS and nLSU sequence data place the three novel Tropicoporus taxa as a sister clade to T. rudis. Detailed morphological comparisons highlight how the new species, T. xerophyticus, T. subindicus, and T. pannaensis, differ from each other and from previously known Tropicoporus species in terms of macroscopic features like basidiome characteristics, pore sizes, and microscopic details such as, cystidioles, setae, and basidiospore dimensions. These findings contribute to the understanding of the taxonomic complexity and diversity within the genus Tropicoporus, particularly in the understudied mycobiota of southern India.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology13100770/s1, Supplementary Table S1. Similarities of ITS BLAST sequences of three new Tropicoporus species and its related species; Table S2. Similarities of LSU BLAST sequences of three new Tropicoporus species and its related species; Supplementary Figure S1. Molecular Phylogeny of three new Indian Tropicoporus species inferred from Bayesian analysis of ITS sequences. The numbers indicated at the nodes represent the Bootstrap values and Bayesian posterior probabilities that equal to or above 60% and 0.9, respectively. The type specimens are in bold; the new Tropicoporus spp. are indicated in colour and bold; Supplementary Figure S2. Molecular Phylogeny of three new Indian Tropicoporus species inferred from Bayesian analysis of nLSU sequences. The numbers indicated at the nodes represent the Bootstrap values and Bayesian posterior probabilities that equal to or above 60% and 0.9, respectively. The type specimens are in bold; the new Tropicoporus spp. are indicated in colour and bold.
Author Contributions
Conceptualization: M.K., E.A. and R.M.; Data Curation: M.K., E.A., R.M., S.G., S.C.K. and A.M.E.; Formal analysis: S.G., M.K., S.C.K. and A.M.E.; Funding acquisition: E.A., M.K., R.M., A.M.E. and P.H.R.; Investigation: M.K., E.A. and R.M.; Methodology: M.K., E.A. and R.M.; Project administration: M.K.; Resources: M.K., E.A. and R.M.; Software: M.K., S.C.K. and S.G.; Supervision: M.K. and S.C.K.; Validation: M.K., E.A., R.M. and S.C.K.; Visualization: M.K.; Writing—original draft M.K., E.A. and R.M.; Writing—review and editing: M.K., S.C.K., S.G., A.M.E. and P.H.R. All authors have read and agreed to the published version of the manuscript.
Funding
Authors Malarvizhi Kaliyaperumal and Elangovan Arumugam acknowledge the Tamil Nadu State Council for Higher Education (RGP/2019-20/MU/HECP-0040) for providing financial aid. Malarvizhi Kaliyaperumal and Sugantha Gunaseelan thank EMR-SERB, DST (EMR/2016/003078), Government of India for the financial assistance. Ramesh Murugadoss would like to acknowledge CSIR—Senior Research Fellowship (SRF-NET), New Delhi, India (09/0115(13300)/2022-EMR-I) for the financial assistance. Samantha C. Karunarathna thanks the National Natural Science Foundation of China (No. 32260004), Yunnan Revitalization Talents Support Plan (High-End Foreign Experts Program), and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River for their support. Abdallah M. Elgorban appreciates the Researchers Supporting Project number (RSP2024R56), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All holotype and paratype collections of the new species are deposited at Madras University Botany Laboratory (MUBL), Centre for Advanced Studies in Botany, University of Madras, Chennai-600 025, Tamil Nadu, India. The sequences generated during this study are deposited in NCBI GenBank. The ITS and nLSU alignment is deposited in TreeBase.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wagner, T.; Fischer, M. Proceeding towards a natural classification of the worldwide taxa Phellinus s.l. and Inonotus s.l. and phylogenetic relationships of allied genera. Mycologia 2002, 94, 998–1016. [Google Scholar] [CrossRef]
- Dai, Y.C. Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 2010, 45, 131–343. [Google Scholar] [CrossRef]
- Wu, S.H.; Dai, Y.C.; Hattori, T.; Yu, T.W.; Wang, D.M.; Parmasto, E.; Chang, H.Y.; Shih, S.Y. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Bot. Stud. 2012, 53, 135–149. [Google Scholar]
- Vlasák, J.; Li, H.J.; Zhou, L.W.; Dai, Y.C. A further study on Inonotus linteus complex (Hymenochaetales, Basidiomycota) in tropical America. Phytotaxa 2013, 124, 25–36. [Google Scholar] [CrossRef]
- Zhou, L.W.; Vlasák, J.; Decock, C.; Assefa, A.; Stenlid, J.; Abate, D.; Wu, S.H.; Dai, Y.C. Global diversity and taxonomy of the Inonotus linteus complex (Hymenochaetales, Basidiomycota): Sanghuangporus gen. nov., Tropicoporus excentrodendri and T. guanacastensis gen. et spp. nov., and 17 new combinations. Fungal Divers. 2015, 77, 335–347. [Google Scholar] [CrossRef]
- Wu, F.; Qin, W.M.; Euatrakool, O.; Zhou, L.W. Tropicoporus boehmeriae sp. nov. (Hymenochaetaceae, Basidiomycota) from Thailand, a new member of the Inonotus linteus complex. Phytotaxa 2015, 231, 73–80. [Google Scholar] [CrossRef]
- Coelho, G.; Silveira, A.O.; Antonelli, Z.I.; Yurchenko, E. Tropicoporus stratificans sp. nov. (Hymenochaetales, Basidiomycota) from southern Brazil. Phytotaxa 2016, 245, 144–152. [Google Scholar] [CrossRef]
- Brown, A.A.; Lawrence, D.P.; Baumgartner, K. Role of basidiomycete fungi in the grapevine trunk disease esca. Plant Pathol. 2019, 69, 205–220. [Google Scholar] [CrossRef]
- Salvador-Montoya, C.A.; Costa-Rezende, D.H.; Ferreira-Lopes, V.; Borba-Silva, M.A.; Popoff, O.F. Tropicoporus drechsleri (Hymenochaetales, Basidiomycota), a new species in the “Inonotus linteus” complex from Northern Argentina. Phytotaxa 2018, 338, 75–89. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.W.; Vlasak, J.; Dai, Y.C. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- de Lima, V.X.; de Oliveira, V.R.T.; de Lima-Junior, N.C.; Oliveira-Filho, J.R.C.; Santos, C.; Lima, N.; Gibertoni, T.B. Taxonomy and phylogenetic analysis reveal one new genus and three new species in Inonotus s.l. (Hymenochaetaceae) from Brazil. Cryptogam. Mycol. 2022, 43, 1–21. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, X.W.; Li, G.J.; Deng, C.-Y.; Rossi, W.; Leonardi, M.; Liimatainen, K.; Kekki, T.; Niskanen, T.; Smith, M.E.; et al. Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2024, 1717–1817. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Kezo, K.; Karunarathna, S.C.; Yang, E.; Zhao, C.; Elgorban, A.M.; Tibpromma, S.; Kaliyaperumal, M. New species of Tropicoporus (Basidiomycota, Hymenochaetales, Hymenochaetaceae) from India, with a key to Afro-Asian Tropicoporus species. MycoKeys 2024, 102, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Jurjević, Z.; Balashov, S.; De la Peña-Lastra, S.; Mateos, A.; Pinruan, U.; Rigueiro-Rodríguez, A.; Osieck, E.R.; Altés, A.; Czachura, P.; et al. Fungal Planet description sheets: 1614–1696. Fungal Syst. Evol. 2024, 13, 183–440. [Google Scholar] [CrossRef] [PubMed]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid isolation procedure for small quantities of fresh tissue. Phytochem. Bull. Bot. Soc. Am. 1987, 19, 11–15. [Google Scholar]
- Góes-Neto, A.; Loguercio-Leite, C.; Guerrero, R.T. DNA extraction from frozen field-collected and dehydrated herbarium fungal basidiome: Performance of SDS and CTAB-based methods. Biotemas 2005, 18, 19–32. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Fischer, M. Natural groups and a revised system for the European poroid Hymenochaetales (Basidiomycota) supported by nLSU rDNA sequence data. Mycol. Res. 2001, 105, 773–782. [Google Scholar] [CrossRef]
- Ryvarden, L. The genus Inonotus a synopsis. Synop. Fungorum 2005, 21, 1–149. [Google Scholar]
- Sharma, J.R. Hymenochaetaceae of India; Botanical Survey of India: Kolkata, India, 1995; 219p. [Google Scholar]
- Sharma, J.R.; Das, K.; Mishra, D. The genus Inonotus and its related species in India. Mycosphere 2013, 4, 809–818. [Google Scholar] [CrossRef]
- Tian, X.M.; Yu, H.Y.; Zhou, L.W.; Decock, C.; Vlasák, J.; Dai, Y.C. Phylogeny and taxonomy of the Inonotus linteus complex. Fungal Divers. 2013, 58, 159–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).