Simple Summary
An important strategy to mitigate global warming is to reduce methane produced by ruminants. However, the inhibition of rumen methanogenesis generally results in hydrogen accumulation, which would affect the normal fermentation in the rumen. In this study, we used a combination of two chemicals (fumarate and nitroglycerin) to mitigate the rumen methane production. Nitroglycerin inhibits the activities of methanogens. Fumarate eliminates hydrogen accumulation. In vitro rumen fermentation was used to investigate the effects of this combination on rumen fermentation, methane and hydrogen production, and microbiota. The results showed that the addition of fumarate decreased the hydrogen accumulation and increased the concentration of propionate and microbial crude protein when methanogen activities were inhibited by nitroglycerin. The bacterial and archaeal communities were altered by the addition of the two chemicals, with several taxa changed in the relative abundance. Conclusively, the combination of fumarate and nitroglycerin inhibited methane production, reduced hydrogen accumulation, improved rumen fermentation and altered rumen microbiota. This study provides an alternative way of using these chemicals in order to mitigate methane emission in ruminants.
Abstract
This study aimed to investigate the effects of fumarate and nitroglycerin on rumen fermentation, methane and hydrogen production, and microbiota. In vitro rumen fermentation was used in this study with four treatment groups: control (CON), fumarate (FA), nitroglycerin (NG) and fumarate plus nitroglycerin (FN). Real-time PCR and 16S rRNA gene sequencing were used to analyze microbiota. The results showed that nitroglycerin completely inhibited methane production and that this resulted in hydrogen accumulation. Fumarate decreased the hydrogen accumulation and improved the rumen fermentation parameters. Fumarate increased the concentration of propionate and microbial crude protein, and decreased the ratio of acetate to propionate in FN. Fumarate, nitroglycerin and their combination did not affect the abundance of bacteria, protozoa and anaerobic fungi, but altered archaea. The PCoA showed that the bacterial (Anosim, R = 0.747, p = 0.001) and archaeal communities (Anosim, R = 0.410, p = 0.005) were different among the four treatments. Compared with CON, fumarate restored Bacteroidetes, Firmicutes, Spirochaetae, Actinobacteria, Unclassified Ruminococcaceae, Streptococcus, Treponema and Bifidobacterium in relative abundance in FN, but did not affect Succinivibrio, Ruminobacter and archaeal taxa. The results indicated that fumarate alleviated the depressed rumen fermentation caused by the inhibition of methanogenesis by nitroglycerin. This may potentially provide an alternative way to use these chemicals to mitigate methane emission in ruminants.
1. Introduction
Methane (CH4) is a major contributor to global climate change [1]. About 40% of greenhouse gas emissions from livestock production can be attributed to ruminant CH4 production, which accounts for approximately 6% of global anthropogenic greenhouse gas emissions [2]. Ruminal CH4 production does not only concern greenhouse gas emissions, but also relates to energy loss for ruminants (up to 12% of the total energy intake) [3]. The rumen is rich in bacteria, protozoa, fungi and methanogens, which can ferment coarse feedstuffs to produce volatile fatty acids, carbon dioxide and CH4. Hydrogen (H2) is an important intermediate in most of those biochemical processes [4]. CH4 is generally produced through the utilization of CO2 and H2 by methanogenic archaea in the rumen [5]. The accumulation of H2 could affect the normal rumen fermentation, therefore, CH4 generation plays an important role in H2 elimination in the rumen.
Strategies like the use of feed additives, nutrition management and animal genetic improvement have been proposed for use in reducing CH4 emissions from ruminants [6,7,8]. Chemicals such as sulfate, nitrate and fumarate were studied for their potential to reduce rumen CH4 emissions [9,10,11]. The CH4 inhibitor 3-nitrooxypropanol [12,13] and the macroalga Asparagopsis taxiformis [14,15] were recently developed as promising rumen CH4-mitigating agents. However, the inhibition of methanogenic activity usually results in abnormal rumen fermentation caused by H2 accumulation. Therefore, it is necessary to devise an alternative means of eliminating H2 when inhibiting the activity of methanogens. Fumarate is a metabolic intermediate and can be reduced to succinate by H2 in the rumen. Succinate is then decarboxylated into propionate, which is a major energy source for ruminants [16]. Fumarate is a promising H2-comsuming chemical in the rumen. Nitroglycerin, targeting methanogens, has the same functional group as the chemical 3-nitrooxypropanol, which was reported as being an effective means of reducing ruminal CH4 emissions in in vitro and in vivo studies [17,18]. Its metabolic end products are propionate and ammonia in the rumen.
This study hypothesized that fumarate would alleviate the abnormal rumen fermentation when methanogenesis was inhibited by nitroglycerin. The objective of this study was to investigate the effects of fumarate and nitroglycerin on rumen fermentation, CH4 and H2 production and microbiota in an in vitro rumen trial. The results of this study could help to develop an alternative means of mitigating rumen CH4 emissions.
2. Materials and Methods
2.1. Animals
Three rumen-fistulated Chinese Hu sheep were fed on a maintenance diet for a period of 30 days. On day 31, 500 mL of rumen fluid was collected 2 h before the sheep were fed. The diet of the 3 sheep was prepared in accordance with the maintenance requirements (NY/Y 816-2004; Ministry of Agriculture of China, 2004), including 70% Chinese wild rye, 20% corn, 7% soybean meal, 1.5% CaHPO4, 0.5% stone powder, 0.5% NaCl and 0.5% additives (Vitamin and mineral mix contained the following ingredients per kilogram of diet: vitamin A, 22.5 KIU/kg; vitamin D3, 5.0 KIU/kg; vitamin E, 37.5 IU/kg; vitamin K3, 5.0 mg/kg; Mn, 63.5 mg/kg; Zn, 111.9 mg/kg; Cu, 25.6 mg/kg; and Fe, 159.3 mg/kg), and comprised 94.01% dry matter, 10.01% crude protein, 2.39% ether extract, 51.48% neural detergent fiber, 30.94% acid detergent fiber and 7.73% crude ash on a dry-matter basis. Sheep were fed a total mixed ration twice daily (08:00 and 17:00) and had free access to fresh water.
2.2. Experimental Design
In vitro rumen fermentation was carried out with a completely randomized design (CRD) for 4 treatments: control (CON), fumarate at 12 mmol/L (FA), nitroglycerin at 99 μmol/L (NG) and fumarate at 12 mmol/L plus nitroglycerin at 99 μmol/L (FN). The dosages of FN and NG used in this study were determined according to previous studies [19,20] and the results of the pre-experiments. Three replicates were prepared for each treatment. Additionally, three independent incubation runs were performed at different times [21]. Each run consisted of 4 treatments with 3 replicates and 4 blanks containing only the inoculum. The experimental procedure was conducted according to the study of Martínez-Fernández et al. [22]. The rumen fluid was collected from 3 Hu sheep before the morning feeding was performed, and this fluid was then pooled and filtered through 4 layers of cheesecloth. The filtered rumen fluid and buffer were mixed thoroughly (1:3 [vol/vol]) in a water bath at 39 °C under anaerobic conditions. Additionally, each 100 mL of the mixture was dispensed into a 180 mL serum bottle containing 1.0 g substrate and chemicals (fumarate or nitroglycerin). All serum bottles were sealed and incubated at 39 °C for 24 h at 80 rpm. After 24 h of incubation, all the fermentation flasks were taken out and put into ice water to terminate the fermentation. Samples were collected and stored for subsequent analysis. The buffer was composed of 8.75 g NaHCO3, 1.00 g NH4HCO3, 1.43 g Na2HPO4, 1.55 g KH2PO4, 0.15 g MgSO4·7H2O, 0.52 g Na2S, 0.017 g CaCl2·2H2O, 0.015 g MnCl2·4H2O, 0.002 g CoCl2·6H2O, 0.012 g FeCl3·6H2O and 1.25 mg resazurin per liter [23]. The composition of the substrate was the same as the diet provided to sheep. The substrate was dried at 65 °C for 48 h and passed through a 1 mm screen with a Wiley mill (Arthur H. Thomas, Philadelphia, PA, USA). The fumarate (disodium fumarate) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Nitroglycerin was purchased from Beijing Yimin Pharmaceutical Co., Ltd. (Beijing, China).
2.3. Sample Collection and Chemical Analysis
At the end of the fermentation, the pH value was measured using a pH meter (Ecoscan pH 5, Thermo Fisher Scientific Inc., Singapore). Then, the bottles were immediately put into ice water to stop fermentation. The supernatant of the fermentation fluid was collected and stored at −20 °C for the determination of volatile fatty acids (VFAs), lactate, microbial crude protein (MCP) and ammonia nitrogen (NH3-N). The mixture of the substrate and fermentation fluid was collected and stored at −80 °C for the analysis of the microbiota.
2.4. Sample Collection and Chemical Analysis
Gas production was assessed using a pressure transducer [24]. Methane (CH4) and hydrogen (H2) production were measured following the gas measurement procedure using a GC-TCD instrument (Agilent 7890B, Agilent Technologies Inc., Santa Clara, CA, USA). Gases were separated on packed GC columns (Porapak Q packing & MolSieve 5A packing, Agilent Technologies Inc., CA, USA) at a column temperature of 80 °C, a 200 °C injection temperature and a 200 °C TCD detector temperature. N2 was the carrier gas. The VFAs were determined according to Jin et al. [17]. Each 1.0 mL sample was mixed with 0.2 mL deproteinization–acidification solution [metaphosphoric acid (25% w/v) and crotonic acid (0.65% w/v)] before undergoing analysis via gas chromatography (Agilent 7890B instrument, Agilent Technologies Inc., CA, USA). The sample was separated using a fused silica capillary column (Supelco, Bellefonte, PA, USA) with a programmed heating process (110 °C for 3 min, 110–150 (40 °C/min)). The injection temperature was 200 °C. The flame ionization detector temperature was 220 °C. The carrier gas was nitrogen. Lactate was measured using an assay kit in accordance with the instructions of the manufacturer (Jiancheng Bioengineering Research Institute, Nanjing, China). Microbial crude protein was determined with a commercial reagent kit (BCA Protein Assay Kit, Tiandz Inc., Beijing, China) in accordance with manufacturer instructions. The concentration of NH3-N was analyzed using an indophenol method with an acidified procedure [25].
2.5. DNA Extraction and Real-Time PCR
Genomic DNA was extracted from a 1.0 mL sample using a bead-beating and phenol-chloroform–isopentanol extraction method [26]. Each DNA sample was divided into two parts to perform sequencing and real-time PCR.
Archaea, bacteria, anaerobic fungi and protozoa were quantified using an Applied Biosystems 7300 Real time PCR system (Applied Biosystems, Thermo Fisher Scientific Inc., Foster City, CA, USA). The primers for the 4 microbial populations are listed in Table A1. An SYBR® Premix Ex Tag TM (TaKaRa, Dalian, China) was used to prepare the reaction mixture. The copy number of DNA in each sample was measured in triplicate, and the average value was calculated. The external standards were prepared with plasmid DNA of clones of each microbial population. The results are expressed as the number of copies of marker genes per milliliter of fermentation liquid.
2.6. 16S rRNA Gene Sequencing and Data Analysis
The 16S rRNA genes of bacteria were amplified with a primer pair 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). The 16S rRNA genes of archaea were amplified with a primer pair Met86F (5′-GCTCAGTAACACGTGG-3′) and Met471R (5′-GWRTTACCGCGGCKGCTG-3′). The amplicons were subjected to double-ended sequencing (paired sequencing) using an Illumina MiSeq PE250 platform produced by BIOZERON Biotechnology Co., Ltd. (Shanghai, China). The raw data were stored in the Sequence Read Archive (SRA) database of the National Biotechnology Information Center (NCBI) (accession number: PRJNA913631, bacteria; PRJNA913641, archaea).
Fastp (version 0.20.0) and FLASH (version 1.2.7) were used to filter and merge 16S rRNA sequences, and the chimeras were filtered to obtain effective reads [27]. UPARSE (version 7.1) was used to pick up the operational taxonomic unit (OTU) with a 97% similarity truncation value [28]. Taxonomic assignment was performed for bacteria using RDP classifier (version 2.11) based on the SILVA database (version 138), and via the RIM-DB database for methanogens [18]. QIIME 2 was used for alpha diversity analysis. The principal coordinate analysis (PCoA) was conducted based on Bray–Curtis distance [29]. The significance of the differences among groups was assessed with ANOSIM using the vegan package in R.
2.7. Statistical Analysis
The analyses of the in vitro fermentation parameters and the real-time PCR data were performed using the MIXED procedure of SAS 9.4 version (SAS Institute, Inc., Cary, NC, USA), and the data were tested to determine their normality using the Shapiro–Wilk test of SAS. The model used for data analysis was Yijk = μ + Pi + Sj + PSij + eij, where Yijk is the observed value, μ is the overall mean, Pi is the fixed effect of treatment with nitroglycerin, Sj is the fixed effect of treatment with fumarate, PSij is the interaction effect of nitroglycerin * fumarate and eij is the random error. The variables that had non-normal distributions were analyzed using the Kruskal–Wallis test procedure. The Tukey test was used to identify differences (p < 0.05) between means.
3. Results
3.1. Total Gas, Hydrogen and Methane Production
The total gas production in FA was the highest among the four groups (p < 0.05, Table 1), and it was higher in FN than CON and NG (p < 0.05). Hydrogen was accumulated in NG and FN. Additionally, NG had the highest hydrogen production (p < 0.05). Methane was only accumulated in CON and FA. There was no methane detected in NG and FN. Methane production was higher in FA than CON (p < 0.05).

Table 1.
Gas production from 24 h in vitro fermentation.
3.2. Fermentation Characteristics
The in vitro fermentation characteristics are presented in Table 2. Total VFA was only higher in FA than NG (p < 0.05). Acetate was higher in CON and FA than NG and FN (p < 0.05). Propionate was higher in FA and FN than CON and NG (p < 0.05). The ratio of acetate/propionate in CON was the highest (p < 0.05), and it was higher in FA and NG than FN (p < 0.05). Isobutyrate in FA was the highest (p < 0.05), and it was higher in CON than NG (p < 0.05). Valerate was higher in NG and FN than CON and FA (p < 0.05). Isovalerate in FA was the highest (p < 0.05), and it was higher in CON than NG and FN (p < 0.05). Ammonia nitrogen in FA was the highest (p < 0.05). Microbial crude protein was higher in CON and FA than NG and FN (p < 0.05), and it was higher in FN than NG (p < 0.05). There were no significant differences in pH, butyrate and lactate among the four groups (p > 0.05).

Table 2.
Fermentation parameters from 24 h in vitro fermentation.
3.3. The Quantification of Protozoa, Bacteria, Anaerobic Fungi and Archaea
There was no significant difference in the abundance of bacteria, protozoa and anaerobic fungi (p > 0.530) among the four groups (Table 3). The abundance of archaea was higher in FA than CON and NG (p < 0.05), and it was higher in FN than NG (p < 0.05).

Table 3.
The abundance of protozoa, bacteria, anaerobic fungi and archaea after 24 h in vitro fermentation.
3.4. Bacterial Community
A total of 390,011 bacterial sequences remained after filtering for quality. The average length was 418 bp. A total of 2722 OTUs were identified. There was no difference in the alpha diversity indexes (p > 0.05, Table 4), except for the Shannon index (p = 0.043). There was a clear separation of clusters on the 3D-PCoA plot of bacterial populations among the four groups (Anosim, R = 0.747, p = 0.001, Figure 1A). PC1, PC2 and PC3 accounted for 53.08%, 16.04% and 10.51% of the total variance, respectively.

Table 4.
Alpha diversity of bacterial and archaea populations.
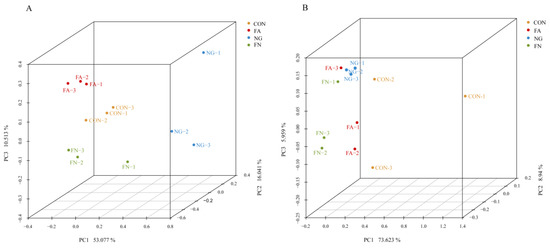
Figure 1.
(A), 3D-PCoA analysis of bacterial populations based on Bray–Curtis distance, Anosim (R = 0.747, p = 0.001); (B), 3D-PCoA analysis of archaeal populations based on Bray–Curtis distance, Anosim (R = 0.410, p = 0.005).
At the phylum level, 20 phyla were identified across all samples. The eight predominant phyla (the average relative abundances of phyla >1% in at least one group) were Bacteroidetes, Firmicutes, Proteobacteria, Spirochaetes, Actinobacteria, Candidate_division_SR1, Candidate_division_TM7 and Cyanobacteria (Table 5). The relative abundance of Bacteroidetes was higher in FN than NG (p < 0.05), but it did not show significant differences between other groups (p > 0.05). Firmicutes and Actinobacteria were the highest in NG (p < 0.05). Proteobacteria in CON and FA were higher than NG and FN (p < 0.05). Spirochaetae were higher in FA and FN than NG (p < 0.05). Candidate_division_TM7 was the highest in CON (p < 0.05). The relative abundance of Cyanobacteria showed no significant difference among groups (p > 0.05).

Table 5.
The relative abundances of bacteria at the phylum level (the average relative abundances of phylum > 1% in at least one group are presented).
A total of 307 bacterial genera were identified from all samples. The seven predominant genera (the average relative abundances of genera >2% in at least one group) were Streptococcus, Succinivibrio, Ruminobacter, Treponema, Bifidobacterium, Unclassified BS11_gut_group and Unclassified Ruminococcaceae (Table 6). The relative abundance of Streptococcus and Bifidobacterium was the highest in NG (p < 0.05). Succinivibrio was higher in CON and FA than FN (p < 0.05). Ruminobacter was higher in CON than NG and FN (p < 0.05). Treponema was higher in FN than NG (p < 0.05). Unclassified BS11_gut_group was higher in CON and FA than the other two groups (p < 0.05). Unclassified Ruminococcaceae was lower in NG than CON (p < 0.05).

Table 6.
The relative abundances of bacteria at the genus level (the average relative abundances of genus > 2% in at least one group are presented).
3.5. Archaeal Community
A total of 568, 155 archaeal sequences were obtained after quality filtering. The average length was 355 bp. No difference in the alpha diversity index was found (p > 0.05, Table 4). These sequences were clustered into 198 OTUs. There was a clear separation of clusters on the 3D-PCoA plot of archaeal populations among the four groups (Anosim, R = 0.410, p = 0.005, Figure 1B). PC1, PC2 and PC3 accounted for 73.62%, 8.94% and 5.96% of the total variance, respectively.
A total of two archaeal orders were identified from all samples. The two predominant orders (the average relative abundance of orders > 1% in at least one group) were Methanobacteriales and Methanomassiliicoccales (Table 7). The relative abundance of Methanobacteriales was the lowest in FN (p < 0.05), and it was lower in FA than CON or NG (p < 0.05). Additionally, there was no significant difference between CON and NG (p > 0.05). The relative abundance of Methanomassiliicoccales was the highest in FN (p < 0.05), and it was higher in FA than CON and NG (p < 0.05).

Table 7.
The relative abundances of archaea at the order level (the average relative abundances of orders > 1% in at least one group are presented).
A total of 47 archaeal species were identified. Six predominant species (the average relative abundance of species > 1% in at least one group) are shown in Table 8. The relative abundance of Methanobrevibacter gottschalkii clade was lower in FN than CON (p < 0.05). Group12 sp. ISO4-H5 was the highest in FN (p < 0.05). Group9 sp. ISO4-G1 was higher in FN than the other groups (p < 0.05), and it was higher in FA than CON and NG (p < 0.05).

Table 8.
The relative abundances of archaea at the species level (the average relative abundances of species > 1% in at least one group are presented).
4. Discussion
The inhibition of methanogenic activities usually results in H2 accumulation and causes depressed rumen fermentation [30]. This can affect the animal production performance. The results of this study showed that the addition of fumarate alleviated H2 accumulation and improved the depressed rumen fermentation parameters when methanogenesis was inhibited by nitroglycerin. Nitroglycerin was demonstrated to be effective at reducing rumen methane production in several in vitro and in vivo studies [17,18,20]. It was able to completely inhibit methane production and caused an accumulation of hydrogen in in vitro rumen fermentation [17,20]. Moreover, the final metabolites of nitroglycerin were propionate and ammonia in the rumen, which have no negative effect on rumen fermentation. Therefore, nitroglycerin was used to successfully establish a model of methane depression and hydrogen accumulation in this study. Nitroglycerin caused about 4.8% hydrogen of accumulation (% total gas production). The current experiment also observed that nitroglycerin inhibited methane production, but did not affect the abundance of archaea compared with the control. This result is consistent with that of a previous study [20]. In another study, an opposite result was observed, whereby the abundance of archaea declined when the methane production was depressed by nitroglycerin [17], which was consistent with the research on the other methanogenic inhibitors [31,32]. However, the mechanisms of the different results for archaeal abundance in different studies are unclear and further work is needed to elucidate this point.
Fumarate is an intermediate in the rumen metabolism and is finally reduced to propionate [33]. The reduction of fumarate has a lower H2-consuming threshold (0.02 ppm) and produces more Gibbs free energy than the methanogenesis of H2 and CO2. The fumarate reduction should be more effective than methanogenesis in the rumen [34,35,36]. Therefore, fumarate was used as a rumen CH4-mitigation agent in many previous studies [37,38,39]. However, the effects of fumarate on rumen CH4 mitigation were found to be inconsistent. Bayaru et al. [37] observed that CH4 production in steers was reduced by 23% when fumarate was added to the complete diet at 20 g/kg dry matter. In contrast, no effect was observed in steers fed barley silage and concentrate with fumarate (12 g/kg dry matter) [38] and in lambs fed dried alfalfa with fumarate (100 g/kg dry matter) [39]. Fumarate increased CH4 production in sheep fed a mixed diet [19], which is consistent with the current study. Fumarate increased the abundance of archaea, methane production and acetate with the absence of nitroglycerin. Fumarate was expected to consume H2 and reduce methane production; the increase in methane production was not expected. Fumarate can be metabolized into acetate via the malate–pyruvate pathway in the rumen [19]. In this process, there is net [H] produced (C4H4O4 + 2H2O→C2H4O2 + 2CO2 + 4H), which could account for the increase in the abundance of archaea and methane production. The increased concentration of acetate supported this speculation. Moreover, Gibbs free energy calculation shows that the production of acetate from fumarate under rumen conditions is thermodynamically feasible even at very low fumarate concentrations [35]. Fumarate was metabolized into acetate instead of propionate, which could have occurred as the microbial populations that reduce fumarate to succinate/propionate had not yet been completely established. It may also give an explanation for the inconsistent results obtained in different studies on fumarate.
Fumarate and nitroglycerin altered the relative abundance of Bacteroides and Firmicutes as well as Streptococcus and several unclassified genera to the two phyla. The relative abundance of Streptococcus was increased by nitroglycerin, but restored to the level of that in CON by the addition of fumarate. The underlying mechanism of the changes in Streptococcus is unclear. However, Streptococcus had been reported to produce bacteriocin, which could inhibit methane production [40]. Succinivibrio, belonging to Proteobaceria, produces succinate [41]. The relative abundance of Succinivibrio was decreased by the combination of fumarate and nitroglycerin. Mao et al. [42] reported that the relative abundance of Succinivibrio dextrinisolvens was increased in the rumen of goats fed disodium fumarate. It seems that the combination of the two chemicals had an opposite impact on Succinivibrio. Treponema, belonging to Spirochaetae, produces succinate, formate and acetate [43]. The relative abundance of Treponema was restored by the addition of fumarate. Jin et al. [44] observed an increase in Treponema due to disodium fumarate in an in vitro rumen fermentation. Therefore, Treponema might play a role in the restoration of the propionate concentration in FN. Ruminobacter, belonging to Proteobacteria, is associated with ruminal fiber degradation [45]. Nitroglycerin decreased the proportion of Ruminobacter, suggesting the inhibition of fiber degradation. It might be partly related to the decrease in acetate concentration caused by nitroglycerin. Bifidobacteria, belonging to the phylum of Actinobacteria, were known fermenters of starch and simple sugars [46]. The addition of nitroglycerin increased the relative abundance of Bifidobacteria, but fumarate restored it. The mechanism underlying the changes in Bifidobacteria is unclear.
The most dominant methanogens belonged to Methanobacteriales and Methanomassiliicoccales, which was consistent with the results of previous studies [17,18]. Fumarate decreased the relative abundance of Methanobacteriales and increased that of Methanomassiliicoccales. Members of the Methanobacteriales primarily use H2 and CO2 to produce CH4, which generates lower amounts of Gibbs free energy and has a higher H2-utilizing threshold than fumarate reduction [35]. The fumarate reduction might have decreased the H2 concentration, which depressed the growth of Methanobacteriales. Members of the Methanomassiliicoccales are H2-dependent methyltrophic methanogens which produce more Gibbs free energy and have a lower H2-utilizing threshold than Methanobacteriales. Moreover, the repair system of Methanomassiliicoccales seems to be more resilient than that of Methanobacteriales in the presence of nitroglycerin [47]. This might explain the changes in the relative abundance in the two methanogenic orders.
5. Conclusions
Fumarate decreased hydrogen accumulation and increased the concentration of propionate and MCP in the presence of nitroglycerin. Treatments did not affect the abundance of bacteria, protozoa and anaerobic fungi, but altered the abundance of archaea. Fumarate restored the relative abundance of several bacterial taxa to the levels in CON in the presence of nitroglycerin, but did not affect Succinivibrio, Ruminobacter and archaeal taxa. Collectively, fumarate alleviated the depressed rumen fermentation caused by the inhibition of methanogenesis by nitroglycerin. This might provide an alternative way to use those chemicals to mitigate methane emissions in ruminants. However, further studies are needed in order to evaluate the effects of nitroglycerin combined with fumarate on animal health, production performance, rumen fermentation and the microbial community in vivo.
Author Contributions
Conceptualization, W.J.; Data curation, J.L. and Y.G.; Formal analysis, S.Z., Y.G. and J.M.; Funding acquisition, S.M. and W.J.; Methodology, S.Z., Z.M., J.M. and W.J.; Project administration, S.M. and W.J.; Software, J.L. and Y.G.; Supervision, W.J.; Validation, Z.M.; Writing—original draft, J.L.; Writing—review and editing, S.M. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangsu Province Special Project for Carbon Peak & Carbon Neutral Science and Technology Innovation (BE2022309).
Institutional Review Board Statement
The experimental design and procedures were approved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China) following the requirements of the Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of China, 1988. No. SYXK (Su) 2015-0656).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the staff at Ruminant nutrition and feed engineering technology research center for their help during the experiment.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Appendix A

Table A1.
The real-time PCR primers used in this study.
Table A1.
The real-time PCR primers used in this study.
| Microbes | Primers | Sequences | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| Bacteria | F R | CCTACGGGAGGCAGCAG ATTACCGCGGCTGCTGG | 60 | [48] |
| Archaea | F R | GTGCTCCCCCGCCAATTCCT GCGGTGTGTGCAAGGAGC | 59 | [49] |
| Protozoa | F R | GCTTTCGWTGGTAGTGTATT CTTGCCCTCYAATCGTWCT | 55 | [50] |
| Fungi | F R | GAGGAAGTAAAAGTCGTAACAAGGTTTC CAAATTCACAAAGGGTAGGATGATT | 60 | [51] |
References
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.J.; Henderson, B.; Makkar, H.; Hristov, A.N.; Oosting, S. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.; Indugu, N.; Narayan, K.; Hennessy, M. Symposium review: Understanding the role of the rumen microbiome in enteric methane mitigation and productivity in dairy cows. J. Dairy Sci. 2022, 105, 8569–8585. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Johnson, D. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Ocko, I.B.; Sun, T.; Shindell, D.; Oppenheimer, M.; Hristov, A.N.; Pacala, S.W.; Mauzerall, D.L.; Xu, Y.; Hamburg, S.P. Acting rapidly to deploy readily available methane mitigation measures by sector can immediately slow global warming. Environ. Res. Lett. 2021, 16, 054042. [Google Scholar] [CrossRef]
- Eckard, R.J.; Grainger, C.; de Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Abecia, L.; Toral, P.; Martín-García, A.; Martínez, G.; Tomkins, N.; Molina-Alcaide, E.; Newbold, C.; Yáñez-Ruiz, D. Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats. J. Dairy Sci. 2012, 95, 2027–2036. [Google Scholar] [CrossRef]
- Wood, T.A.; Wallace, R.J.; Rowe, A.; Price, J.; Yáñez-Ruiz, D.R.; Murray, P.; Newbold, C.J. Encapsulated fumaric acid as a feed ingredient to decrease ruminal methane emissions. Anim. Feed. Sci. Technol. 2009, 152, 62–71. [Google Scholar] [CrossRef]
- van Zijderveld, S.; Gerrits, W.; Apajalahti, J.; Newbold, J.; Dijkstra, J.; Leng, R.; Perdok, H. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef]
- Melgar, A.; Lage, C.; Nedelkov, K.; Räisänen, S.; Stefenoni, H.; Fetter, M.; Chen, X.; Oh, J.; Duval, S.; Kindermann, M.; et al. Enteric methane emission, milk production, and composition of dairy cows fed 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Schilde, M.; von Soosten, D.; Hüther, L.; Meyer, U.; Zeyner, A.; Dänicke, S. Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Stefenoni, H.; Räisänen, S.; Cueva, S.; Wasson, D.; Lage, C.; Melgar, A.; Fetter, M.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows-Sciencedirect. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-W.; Chen, Y.-S.; Cheng, Y.-H.; Yang, C.-M.J.; Chang, C.-T.; Cheng, Y.-S. Effects of fumarate on ruminal ammonia accumulation and fiber digestion in vitro and nutrient utilization in dairy does. J. Dairy Sci. 2010, 93, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Meng, Z.; Wang, J.; Cheng, Y.; Zhu, W. Effect of nitrooxy compounds with different molecular structures on the rumen methanogenesis, metabolic profile, and methanogenic community. Curr. Microbiol. 2017, 74, 891–898. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, L.; Jin, W.; Meng, Z.; Cheng, Y.; Wang, J.; Zhu, W. Methane emission, rumen fermentation, and microbial community response to a nitrooxy compound in low-quality forage fed Hu sheep. Curr. Microbiol. 2019, 76, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, E.M.; Kohn, R.A.; Wallace, R.J.; Newbold, C.J. A meta-analysis of fumarate effects on methane production in ruminal batch cultures. J. Anim. Sci. 2007, 85, 2556–2563. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, W.; Xie, F.; Mao, S.; Cheng, Y.; Zhu, W. The role of Methanomassiliicoccales in trimethylamine metabolism in the rumen of dairy cows. Animal 2021, 15, 100259. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.; O’kiely, P.; Reynolds, C.; Schwarm, A.; Shingfield, K.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed. Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- Martinez Fernandez, G.; Abecia, L.; Arco, A.; Cantalapiedra-Hijar, G.; Martín-García, A.I.; Molina-Alcaide, E.; Kindermann, M.; Duval, S.; Yáñez-Ruiz, D.R. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J. Dairy Sci. 2014, 97, 3790–3799. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed. Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Jin, D.; Zhao, S.; Zheng, N.; Bu, D.; Beckers, Y.; Denman, S.E.; McSweeney, C.S.; Wang, J. Differences in ureolytic bacterial composition between the rumen digesta and rumen wall based on urec gene classification. Front. Microbiol. 2017, 8, 385. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Mitter, E.K.; de Freitas, J.R.; Germida, J.J. Bacterial root microbiome of plants growing in oil sands reclamation covers. Front. Microbiol. 2017, 8, 849. [Google Scholar] [CrossRef]
- Melgar, A.; Harper, M.; Oh, J.; Giallongo, F.; Young, M.; Ott, T.; Duval, S.; Hristov, A. Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 2020, 103, 410–432. [Google Scholar] [CrossRef]
- Zhou, Z.; Meng, Q.; Yu, Z. Effects of methanogenic inhibitors on methane production and abundances of methanogens and cellulolytic bacteria in in vitro ruminal cultures. Appl. Environ. Microbiol. 2011, 77, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.; Okine, E.; Guan, L.; Duval, S.; Kindermann, M.; Beauchemin, K. Effects of 3-nitrooxypropanol on methane production using the rumen simulation technique (Rusitec). Anim. Feed. Sci. Technol. 2015, 209, 98–109. [Google Scholar] [CrossRef]
- Yang, C.; Mao, S.; Long, L.; Zhu, W. Effect of disodium fumarate on microbial abundance, ruminal fermentation and methane emission in goats under different forage: Concentrate ratios. Animal 2012, 6, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Reddy, C.A. Hydrogenase activity and the H2-fumarate electron transport system in Bacteroides fragilis. J. Bacteriol. 1977, 131, 922–928. [Google Scholar] [CrossRef]
- Ungerfeld, E.; Kohn, R. The Role of Thermodynamics in the Control of Ruminal Fermentation. In Ruminant Physiology; Sejrsen, K., Hvelplund, T., Nielsen, M.O., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 55–85. [Google Scholar]
- Cord-Ruwisch, R.; Conrad, R.; Seitz, H.-J. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch. Microbiol. 1988, 149, 350–357. [Google Scholar] [CrossRef]
- Bayaru, E.; Kanda, S.; Kamada, T.; Itabashi, H.; Andoh, S.; Nishida, T.; Ishida, M.; Itoh, T.; Nagara, K.; Isobe, Y. Effect of fumaric acid on methane production, rumen fermentation and digestibility of cattle fed roughage alone. Nihon Chikusan Gakkaiho 2001, 72, 139–146. [Google Scholar] [CrossRef]
- McGinn, S.M.; Beauchemin, K.A.; Coates, T.; Colombatto, D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef]
- Molano, G.; Knight, T.W.; Clark, H. Fumaric acid supplements have no effect on methane emissions per unit of feed intake in wether lambs. Aust. J. Exp. Agric. 2008, 48, 165–168. [Google Scholar] [CrossRef]
- Lee, S.S.; Hsu, J.-T.; Mantovani, H.C.; Russell, J.B. The effect of bovicin HC5, a bacteriocin from Streptococcus bovisHC5, on ruminal methane production in vitro. FEMS Microbiol. Lett. 2002, 217, 51–55. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhang, G.; Zhu, W. Effect of disodium fumarate on ruminal metabolism and rumen bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. Anim. Feed. Sci. Technol. 2008, 140, 293–306. [Google Scholar] [CrossRef]
- Ortiz-Chura, A.; Gere, J.; Marcoppido, G.; Depetris, G.; Cravero, S.; Faverín, C.; Pinares-Patiño, C.; Cataldi, A.; Cerón-Cucchi, M.E. Dynamics of the ruminal microbial ecosystem, and inhibition of methanogenesis and propiogenesis in response to nitrate feeding to Holstein calves. Anim. Nutr. 2021, 7, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xue, C.; Liu, J.; Yin, Y.; Zhu, W.; Mao, S. Effects of disodium fumarate on in vitro rumen fermentation, the production of lipopolysaccharide and biogenic amines, and the rumen bacterial community. Curr. Microbiol. 2017, 74, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, B.; Fievez, V.; Tamminga, S.; Dewhurst, R.; van Vuuren, A.; De Brabander, D.; Demeyer, D. Milk odd- and branched-chain fatty acids in relation to the rumen fermentation pattern. J. Dairy Sci. 2006, 89, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Yohe, T.; Enger, B.; Wang, L.; Tucker, H.; Ceh, C.; Parsons, C.; Yu, Z.; Daniels, K. Short communication: Does early-life administration of a Megasphaera elsdenii probiotic affect long-term establishment of the organism in the rumen and alter rumen metabolism in the dairy calf? J. Dairy Sci. 2018, 101, 1747–1751. [Google Scholar] [CrossRef]
- Duin, E.C.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.R.; Duval, S.; Rümbeli, R.; Stemmler, R.T.; Thauer, R.K.; et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Schmitz-Esser, S.; Klevenhusen, F.; Podstatzky-Lichtenstein, L.; Wagner, M.; Zebeli, Q. Grain-rich diets differently alter ruminal and colonic abundance of microbial populations and lipopolysaccharide in goats. Anaerobe 2013, 20, 65–73. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Kirs, M.; Ronimus, R.S.; Hoskin, S.O.; Janssen, P.H. Methanogen community structure in the rumens of farmed sheep, cattle and red deer fed different diets. FEMS Microbiol. Ecol. 2011, 76, 311–326. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Karnati, S.K.R.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).