Simple Summary
Myocarditis is one of the leading causes of acute and chronic heart failure, adverse ventricular remodelling, and progression to dilated cardiomyopathy, life-threatening arrhythmias, and sudden cardiac death. According to recent guidelines, azathioprine, in association with steroids, is a cornerstone of first-line therapy regimens in biopsy-proven, autoimmune/immune-mediated, virus-negative myocarditis. Despite that the majority of published clinical studies seem to show an overall benefit of immunosuppressive therapy in the treatment of myocarditis/inflammatory cardiomyopathy, a targeted therapy has still not been standardized, and there is a need for further controlled, multicentric, clinical studies to provide further data on the efficacy and safety of IT in myocarditis. The aim of this review is to describe the pharmacological properties of azathioprine and to explore future perspectives for its usage in the cardioimmunology field.
Abstract
The use of immunosuppressive therapy (IT) in biopsy-proven, autoimmune/immune-mediated (AI), virus-negative myocarditis has become the standard of care. In particular, according to recent guidelines, azathioprine (AZA), in association with steroids, is a cornerstone of first-line therapy regimens. IT may have a crucial impact on the natural history of AI myocarditis, preventing its progression to end-stage heart failure, cardiovascular death, or heart transplantation, provided that strict appropriateness and safety criteria are observed. In particular, AZA treatment for AI virus-negative myocarditis requires the consideration of some crucial aspects regarding its pharmacokinetics and pharmacodynamics, as well as a high index of suspicion to detect its overt and/or subclinical side effects. Importantly, besides a tight teamwork with a clinical immunologist/immuno-rheumatologist, before starting IT, it is also necessary to carry out a careful “safety check-list” in order to rule out possible contraindications to IT and minimize patient’s risk. The aim of this review is to describe the pharmacological properties of AZA, as well as to discuss practical aspects of its clinical use, in the light of existing evidence, with particular regard to the new field of cardioimmunology.
1. Introduction
Myocarditis is one of the leading causes of acute and chronic heart failure, adverse ventricular remodelling, and progression to dilated cardiomyopathy (DCM), life-threatening arrhythmias, and sudden cardiac death [1,2,3,4]. It is estimated that myocarditis affects thousands of patients annually worldwide, both of adult and paediatric age, with relevant medical and social consequences. Each year, more than 300,000 patients worldwide die by DCM resulting from chronic progression of myocarditis, with a 20–50% five-year mortality in the course of DCM [1,2,3,4]. Moreover, an increase in morbidity and mortality related to myocarditis has been recorded in recent decades, probably due to better recognition of the disease [5,6,7]. In fact, myocarditis may often be underdiagnosed, since in many cases the diagnostic gold standard, i.e., endomyocardial biopsy (EMB), is not performed [8].
Myocarditis can be caused by a wide range of agents, and the most relevant clinical distinction is between infectious and noninfectious forms [1,2,3,4]. Infectious agents may cause direct cardiomyocyte injury or trigger an autoreactive cellular and humoral immune response that leads to myocardial damage with inflammation [1]. Conversely, autoimmune/immune-mediated (AI) myocarditis may occur with exclusive cardiac involvement (i.e., organ-specific autoimmune disease) or in the context of systemic immune-mediated diseases (SIDs), such as eosinophilic granulomatosis with polyangiitis (EGPA), systemic lupus erythematosus (SLE), systemic sclerosis, and others [1,2,3,4,9]. Until recent years, noninfectious causes of myocarditis were less commonly reported than infectious ones, and a trend towards an increasing diagnosis of virus-negative myocarditis forms seems to be emerging [10].
Current international guidelines recommend the use of immunosuppressive therapy (IT) in virus-negative, AI myocarditis/inflammatory cardiomyopathy refractory to standard supportive optimal medical therapy, in patients without contraindications to IT [1,2,3,4]. So far, the drugs investigated in this setting are mainly steroids, azathioprine, cyclosporine, or mycophenolate mofetil, in different combinations [9,11,12,13,14,15,16].
In particular, azathioprine (AZA) has been extensively used due to its excellent profile of efficacy and safety as an immunosuppressive agent for the treatment of a variety of SIDs, such as SLE, rheumatoid arthritis, dermatomyositis, polymyositis, systemic sclerosis, and systemic vasculitis. Other possible indications for AZA, outside cardioimmunology, are inflammatory bowel diseases or prevention of organ transplant rejection [17]. The potential applications of AZA in the cardioimmunology field range from recurrent idiopathic pericarditis refractory to standard treatment [18,19], to virus-negative, AI myocarditis and inflammatory cardiomyopathy [10,14,15]. The introduction of the use of AZA in the treatment of AI inflammatory cardiomyopathy is relatively recent, but it is supported by robust evidence in form of RCTs with excellent results [12,20].
The scope of the present article is to describe the pharmacological properties of AZA, in the light of existing evidence on its use with biopsy-proven, virus-negative, AI myocarditis/inflammatory cardiomyopathy, and to explore future perspectives for its usage in the cardioimmunology field.
2. Pharmacological Properties of Azathioprine: A Focus on Clinical Implications
AZA is an immunosuppressive agent orally administered as an inactive prodrug [17,21]. Once converted in the liver and kidneys to 6-mercaptopurine, its active form, it acts as an antimetabolite of purine bases. In particular, it interferes with DNA synthesis by incorporating purine thioanalogues into the DNA chain [17]. As a result, it inhibits the biosynthesis of nucleic acids and prevents the proliferation of immunocompetent cells. Following oral administration, AZA absorption varies between 27 and 80%, while its bioavailability decreases by about 26% if ingested with food. Moreover, AZA is degraded by xanthine oxidase in milk [17]. Therefore, this drug should be ideally administered at least one hour before or three hours after a meal or milk consumption. AZA binds to plasma proteins in a proportion of about 30% and is metabolized in the liver and kidneys, where it is rapidly broken down to 6-mercaptopurine and methylnitroimidazole [17]. 6-mercaptopurine readily enters the cells where it is transformed into purine thioanalogs [17]. It is finally converted into thiouric acid, an inactive metabolite, and, to a lesser extent, into 1-methyl-4-nitro-5-thioimidazole, which are excreted in the urine [17] (Figure 1). There are no sufficient data on the clearance and biological half-time of AZA. The half-life of 6-mercaptopurine is about 0.9 h; approximately 12% of AZA is excreted, unchanged, in the faeces, while a further 20–50% is eliminated, unchanged or as metabolites, with urine [17,22]. Notably, it may be only partially removed by dialysis.
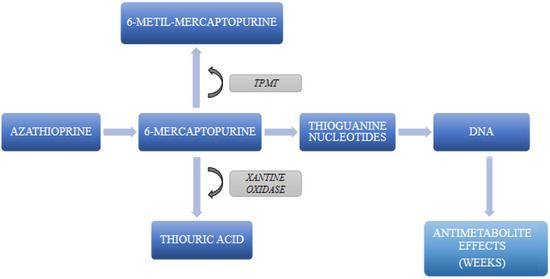
Figure 1.
Diagram summarizing the crucial steps on AZA metabolism. TPMT: thiopurine methyltransferase.
Measurement of concentrations of AZA metabolites may be used to monitor compliance [23]. Although there were conflicting results as to whether there is a direct association between AZA metabolite concentrations and the probability of disease remission, it is recommended to monitor the AZA metabolite concentration (6-TGN and 6-MMP) when treated with AZA [24,25]. AZA metabolites accumulate in lymphocytes, block the expression of cytokines, and, finally, inhibit the inflammatory response induced by T cells [26].
The metabolism of 6-mercaptopurine is mediated by several enzymes, such as thiopurine methyltransferase (TPMT), xanthine oxidase, inosine monophosphate dehydrogenase, hypoxanthine-guanine phosphoribosyl transferase, and aldehyde oxidase [17,21]. Polymorphisms of genes encoding the different enzymes involved in the metabolism of AZA and in particular, TPMT may imply an increased risk of adverse effects during treatment [27], especially agranulocytosis, which is a rare but potentially lethal side effect of the drugs that justifies the screening for TPMT before starting AZA treatment [28,29]. TPMT deficiency is an autosomal codominant trait [30] and is diagnosed with genetic testing; of note, in patients who have received a blood transfusion in the previous three months, TPMT testing can be unreliable because of TPMT activity in the transfused blood cells [31]. Several reports exist on thiopurine-induced haematological toxicity, especially myelosuppression, in patients with inherited low TPMT activity treated with standard doses of AZA [32].
AZA immunosuppressive effect usually becomes gradually apparent within several weeks of treatment; this is of particular relevance in clinical practice because other agents may be administered in combination, usually high-dose steroids, to produce a rapid immunosuppressive action. Moreover, since AZA effects on the left ventricular function may take time to become evident, patients may need temporary measures, such as a wearable cardiac defibrillator (WCD), in the meantime [33]. Finally, in case of specific histotypes of myocarditis, such as giant-cell myocarditis (GCM), which was defined as “the most fatal of autoimmune diseases” [34], AZA therapy alone may not be sufficient, especially in the acute phase; therefore, AZA is always integrated in combination IT regimens for GCM [35].
3. The Use of Azathioprine in Clinical Practice: A Pragmatic Approach
In clinical practice, AZA is usually administered as a steroid-sparing agent to maintain remission following induction therapy in AI diseases and to prevent graft rejection [17]. AZA, especially in combination with corticosteroids, is approved for the treatment of:
- multiorgan involvement in SIDs, such as SLE, rheumatoid arthritis, dermatomyositis, polymyositis, periarteritis nodosa, pemphigus vulgaris, pyoderma gangrenosum, autoimmune haemolytic anaemia, chronic refractory thrombocytopenic purpura, and autoimmune chronic hepatitis [36];
- moderate-to-severe chronic inflammatory bowel diseases (IBDs), such as Crohn’s disease or ulcerative colitis [37];
- prevention of graft rejection after kidney, heart, or liver transplantation [38].
In most cases, a standard therapeutic dose usually ranges from 1 to 2 mg/kg per day, though organ transplantation may require a higher dose [17]. The dose should be adjusted within this range depending on the patient’s individual clinical response, which becomes evident after several weeks of treatment and according to haematological and hepatic tolerance. Based on the clinical experience developed in patients with SIDs, treatment with AZA is usually prolonged over 6 months, for at least 12 months or longer. Accordingly, the same treatment duration can be adopted also for patients with biopsy-proven, virus-negative, AI myocarditis/inflammatory cardiomyopathy, even if evidence so far has focused on shorter therapy intervals [12,20]. Recent guidelines, in fact, recommend IT for at least 6–12 months in selected biopsy-proven AI myocarditis patients with unremitting HF [2]. After ruling out TPMT mutations, it is advisable to introduce AZA treatment gradually, starting with 1 mg/kg per day to test its haematological, hepatic, and pancreatic tolerance, until reaching the target dose in about two weeks. The dose should be then titrated on an individualized basis, depending on clinical response, tolerance, and additional criteria (patients >65 years of age, comorbidities, fragility, and/or body weight ≤60 kg; haematological response, particularly leukopenia; and pancreatic, hepatic, and gastrointestinal tolerance, etc.). Possible adverse effects of AZA are bone-marrow depression (leukopenia, anaemia, and thrombocytopenia), nausea, and viral and opportunistic infections; hypersensitivity reactions; pancreatitis, hepatitis, and cholestasis; and malignancies [17]. The reported adverse events of AZA are more common among patients receiving high doses of the drug (e.g., after organ transplantation) and/or with reduced tolerance due to a TPMT mutation [17]. Consequently, patients with TPMT mutations should be switched to an alternative drug.
AZA, when correctly used, is usually a safe drug. Nevertheless, focusing on its employment in the cardioimmunology field, the rate of side effects of any grade may be sizeable (up to 20%, according to literature [39]); therefore, periodic clinical and laboratory reassessment of treated patients is advisable, preferably with supervision by a clinical immunologist/rheumatologist with expertise in the field. In fact, the timing of occurrence of AZA side effects (especially subclinical liver toxicity detected by lab tests) is greatly variable and unpredictable, with onset even up to several months from drug initiation. From a practical point of view, frequent monitoring of complete blood-cell count and liver-function test is recommended during the first 4 to 8 weeks, and then every three months for the remaining treatment period after reaching the maintenance dose. In special populations (chronic kidney disease, elderly patients, and high AZA dosages), lab tests should be performed more frequently [40]. Finally, some authors suggest monitoring the level of AZA metabolites to avoid specific complications [41].
4. Existing Evidence on Azathioprine Effectiveness and Safety in Myocarditis and Inflammatory Cardiomyopathy
Current guidelines [2] suggest IT for the treatment of biopsy-proven, virus-negative, AI myocarditis/inflammatory cardiomyopathy (e.g., lymphocytic, eosinophilic, sarcoid, and giant-cell myocarditis) with severe myocardial function impairment and/or life-threatening arrhythmias [42]. A list of the results of clinical trials testing IT on AI myocarditis/inflammatory cardiomyopathy is reported in Table 1 [12,13,14,15,43,44].

Table 1.
Summary of the main characteristics of trials exploring the use of AZA, in association with prednisone, in biopsy-proven, virus-negative myocarditis/inflammatory cardiomyopathy.
The first randomized clinical trial investigating the role of AZA in the IT of myocarditis was the Myocarditis Treatment Trial in 1995. Following a 6-month treatment with cyclosporine or AZA, in combination with prednisone, when compared with placebo, this study showed no benefits. A main limitation of this study was that it did not distinguish viral from nonviral forms of myocarditis; in fact, EMBs were only analysed according to the histological Dallas criteria, without undergoing a viral genome search. In a retrospective analysis [47], the virological and immunological characterization of lymphocytic myocarditis patients treated with IT revealed a 90% responsiveness rate in virus-negative cases, whilst a viral genome was detectable in the myocardium of 85% of nonresponders. Moreover, this trial was underpowered for detecting survival differences [46].
Conversely, from the year 2000 and onwards, some single-centre studies, based on small patient populations of biopsy-proven myocarditis patients, reported beneficial effects of AZA in combination with prednisone on left ventricular (LV) ejection fraction (EF), with excellent results (improvement in up to 90% of patients), and a very high safety profile; in fact, no significant adverse events were reported [12,13].
In 2001, Wojnicz et al. randomized 84 patients with DCM and increased human leukocyte antigen (HLA) expression on EMB for a 3-month treatment with AZA and prednisone versus placebo [13]; the presence of a viral genome was not assessed. After 3 months, 71.8% of patients in the IT group versus 20.9% of patients in the placebo group met the criteria of improvement, i.e., increase in LVEF, reduction in LV diastolic dimension and volume, and reduction in New York Heart Association (NYHA) functional class (p < 0.001).
In 2009, Frustaci et al. published the first randomized, double-blind, placebo-controlled trial including patients with biopsy-proven, virus-negative myocarditis and chronic (>6 month) heart failure unresponsive to conventional therapy [12]. In the IT group, 43 patients received prednisone (1 mg/kg body weight per day for 4 weeks, followed by 0.33 mg/kg body weight for 5 months, i.e., 6 months in total) and AZA (2 mg/kg body weight for 6 months); 42 patients were randomized to the placebo group. The primary outcome was a 6-month improvement in LVEF assessed by echocardiography. Secondary objectives were the improvement of NYHA class and survival from cardiac death or heart transplantation. This study demonstrated a significant improvement of LVEF and a significant decrease in LV dimensions and volume compared with baseline in 88% of patients in the treatment group. Even patients with a severely reduced systolic function at baseline (LVEF < 20%) and severe LV adverse remodelling (LV end-diastolic diameter up to 90 mm) showed a substantial benefit from IT. A remaining minor, yet relevant, quote of 12% of patients demonstrated a stable clinical picture with no improvement of cardiac function parameters. Frustaci et al. attributed the lack of response to IT of this subset of patients to the possible lack of exclusion of presence of viruses that were not screened at baseline and to mechanisms of myocardial damage not targeted by IT. Another remarkable result is that 49% of the patients receiving IT improved by at least one NYHA class at 6 months. In contrast, none of the patients in the placebo group showed improvement of LVEF nor NYHA class. Nevertheless, at one month from baseline, 38% of patients in the placebo group showed a mild improvement of LVEF, which lasted up to 3 months, but then it declined to baseline or even lower values.
In the trials by Wojnicz and Frustaci, the duration of IT with prednisone and AZA ranged from 3 to 6 months, respectively [12,13]. As previously mentioned, both studies showed a positive effect of IT on both echocardiographic parameters and physical recovery. Recently, Chimenti et al. published a 20-year follow up of the TIMIC trial that confirmed the lasting benefit of IT, both in terms of LV function and of survival from death and heart transplant [20]. However, since the clinical effect of AZA takes 1 to 3 months to become completely evident, it is conceivable that a further improvement could be expected, prolonging the treatment to 12 months or more [48,49].
Another study by Maisch et al. (the European Study on the Epidemiology and Treatment of Cardiac Inflammatory Disease), a double-blind, randomized, placebo-controlled trial IT with prednisolone and AZA was effective in patients with AI, virus-negative, inflammatory dilated cardiomyopathy with an LVEF <45% [50]. Following 6 months of treatment, a significant improvement was observed in both LVEF and major adverse cardiac events, which was still lasting after 1 year of follow-up. Remarkably, the control group also showed some spontaneous resolution.
5. Safety Check-List before Starting IT in Biopsy-Proven AI Myocarditis/Inflammatory Cardiomyopathy
Apart from a histological confirmation of the absence of a viral genome on EMB, another fundamental prerequisite for IT in myocarditis is the verification of a lack of contraindications to IT [1]. In order to help clinicians to identify possible IT contraindications, a detailed “safety checklist” has been published [51] (Table 2). This set of investigations is intended to be performed before starting IT in all patients, aiming at the identification of absolute contraindications and individual risks related to IT [52]. Patients should be screened for common latent infections and hidden malignancies, taking into account the individual patient’s characteristics, such as ethnicity, sex, and age (for example, reproductive age, or advanced age with increased frailty profile). As previously outlined, of particular importance is the search for the mutation of TPMT before starting AZA, to prevent the appearance of secondary leukopenia/agranulocytosis. In addition, IT of AI myocarditis requires a multidisciplinary approach (i.e., cardiologists, cardiac pathologists, immunologists, rheumatologists, and other professionals) and an active involvement of the patients and their caregivers.

Table 2.
Proposed “safety check-list” prior to initiation of IT. Modified from R. Marcolongo et al. [51].
6. AZA Therapy in Cardioimmunology: Existing Evidence and Future Perspectives
Based on the pathophysiology of inflammatory heart diseases, it is assumed that a disease-specific treatment should include IT to put under control the adverse immune responses taking place in the myocardial tissue [1,10]. Most available clinical studies seem to show an overall benefit from IT in the treatment of AI, biopsy-proven, virus-negative myocarditis, but the targeted therapy has still not been specified. Published studies have mostly presented inhomogeneous patient populations (small, underpowered groups) [53] at a different disease stage (acute vs. chronic myocarditis) [14,50], often with an undefined type of cellular infiltration (e.g., lymphocytic) and/or of unknown viral presence in heart biopsy [13,53].
The nature of the above-mentioned data makes it difficult to perform a qualitative meta-analysis; therefore, those analyses that were conducted showed conflicting data [54,55]. Most of the beneficial results of IT relate to combined treatments with prednisone and AZA on top of optimal guideline-based medical therapy for HF. These results seem to be derived from the complementary pharmacokinetic properties of these medications. Prednisone usually is started at high doses to induce the rapid suppression of myocardial inflammation and the control of pathological immune response, while the immunosuppressive effect of AZA becomes gradually apparent only after several weeks of treatment, playing a crucial role in maintaining the effects of induction therapy and preventing relapses, while tapering down steroid dosage [49]. However, some crucial questions are still waiting for answers about the most appropriate moment to start IT in AI, biopsy-proven, virus-negative myocarditis/inflammatory cardiomyopathy and when to stop it. Despite that the majority of published clinical studies seem to show an overall benefit of IT in the treatment of AI, biopsy-proven, virus-negative myocarditis/inflammatory cardiomyopathy, a targeted therapy has still not been standardized, and there is a need for further controlled, preferably multicentric, clinical studies to provide further data on the efficacy and safety of IT in myocarditis [3,4,56]. Currently, a multicentric, double-blind, randomized trial (IMPROVE-MC) on a combined 12-month therapy of AZA with prednisone is ongoing in Poland [57]. The study also aims to assess the safety and long-term effects of the treatment after completing a full course of the therapy.
Further studies are needed to improve the selection of patients (i.e., based on disease aetiology, disease activity, and presence of anti-heart autoantibodies and specific inflammatory cells/cytokines) who would mostly benefit from the IT treatment [58,59]. What is more, in the future, a therapeutic-drug monitoring of thiopurine metabolites could improve clinical outcomes through dose optimization and toxicity monitoring [60].
7. Conclusions
Existing evidence shows that IT is of paramount importance in the management of biopsy-proven, virus-negative, AI myocarditis/inflammatory cardiomyopathy in patients that do not respond to conventional cardiovascular medical treatments. Azathioprine is one of the most studied drugs in this setting. If correctly managed, AZA is a safe and effective tool to modify myocarditis’s natural history, preventing its progression to DCM, end-stage HF, death, or HTx.
Author Contributions
Conceptualization, J.G., A.L.P.C. and K.O.; validation, K.O. and A.L.P.C.; investigation, K.O., J.W. and E.O.; writing—original draft preparation, K.O. and A.T.; writing—review and editing, K.O., A.T., J.W., E.O., A.S.G., A.B., R.M. and A.L.P.C.; visualization, K.O., E.O. and J.W.; supervision, A.L.P.C. and M.G.; project administration, K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analysed in this study.
Conflicts of Interest
J.G. is a researcher supporting the producer of azathioprine. K.O., A.T. and M.G. are the main investigators in the non-commercial IMPROVE-MC trial. A.L.P.C. and R.M. are members of the scientific steering committee of the non-commercial IMPROVE-MC trial.
References
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Tyminska, A.; Ozieranski, K.; Caforio, A.L.P.; Marcolongo, R.; Marchel, M.; Kaplon-Cieslicka, A.; Baritussio, A.; Filipiak, K.J.; Opolski, G.; Grabowski, M. Myocarditis and inflammatory cardiomyopathy in 2021: An update. Pol. Arch. Intern. Med. 2021, 131, 594–606. [Google Scholar] [CrossRef]
- Tyminska, A.; Ozieranski, K.; Skwarek, A.; Kaplon-Cieslicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J. Pers. Med. 2022, 12, 183. [Google Scholar] [CrossRef]
- Ozieranski, K.; Tyminska, A.; Kruk, M.; Kon, B.; Skwarek, A.; Opolski, G.; Grabowski, M. Occurrence, Trends, Management and Outcomes of Patients Hospitalized with Clinically Suspected Myocarditis-Ten-Year Perspectives from the MYO-PL Nationwide Database. J. Clin. Med. 2021, 10, 4672. [Google Scholar] [CrossRef] [PubMed]
- Ozieranski, K.; Tyminska, A.; Skwarek, A.; Kruk, M.; Kon, B.; Bilinski, J.; Opolski, G.; Grabowski, M. Sex Differences in Incidence, Clinical Characteristics and Outcomes in Children and Young Adults Hospitalized for Clinically Suspected Myocarditis in the Last Ten Years-Data from the MYO-PL Nationwide Database. J. Clin. Med. 2021, 10, 5502. [Google Scholar] [CrossRef] [PubMed]
- Ozieranski, K.; Tyminska, A.; Chabior, A.; Kruk, M.; Kon, B.; Maciejewski, C.; Opolski, G.; Grabowski, M. Sex differences in incidence, management, and outcomes in adult patients aged over 20 years with clinically diagnosed myocarditis in the last 10 years: Data from the MYOPL nationwide database. Pol. Arch. Intern. Med. 2022, 132, 1–9. [Google Scholar] [CrossRef]
- Elbadawi, A.; Elgendy, I.Y.; Ha, L.D.; Mentias, A.; Ogunbayo, G.O.; Tahir, M.W.; Biniwale, N.; Olorunfemi, O.; Barssoum, K.; Guglin, M. National Trends and Outcomes of Endomyocardial Biopsy for Patients With Myocarditis: From the National Inpatient Sample Database. J. Card. Fail. 2018, 24, 337–341. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Adler, Y.; Agostini, C.; Allanore, Y.; Anastasakis, A.; Arad, M.; Bohm, M.; Charron, P.; Elliott, P.M.; Eriksson, U.; et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur. Heart J. 2017, 38, 2649–2662. [Google Scholar] [CrossRef]
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hubner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr.; Berry, G.J.; Shabetai, R. Idiopathic giant-cell myocarditis—Natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, R.; Nowalany-Kozielska, E.; Wojciechowska, C.; Glanowska, G.; Wilczewski, P.; Niklewski, T.; Zembala, M.; Polonski, L.; Rozek, M.M.; Wodniecki, J. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: Two-year follow-up results. Circulation 2001, 104, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Kuhl, U.; Lassner, D.; Poller, W.; Westermann, D.; Pieske, B.; Tschope, C.; Schultheiss, H.P. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin. Res. Cardiol. 2016, 105, 1011–1020. [Google Scholar] [CrossRef]
- Merken, J.; Hazebroek, M.; Van Paassen, P.; Verdonschot, J.; Van Empel, V.; Knackstedt, C.; Abdul Hamid, M.; Seiler, M.; Kolb, J.; Hoermann, P.; et al. Immunosuppressive Therapy Improves Both Short- and Long-Term Prognosis in Patients With Virus-Negative Nonfulminant Inflammatory Cardiomyopathy. Circ. Heart Fail. 2018, 11, e004228. [Google Scholar] [CrossRef]
- De Luca, G.; Campochiaro, C.; Sartorelli, S.; Peretto, G.; Sala, S.; Palmisano, A.; Esposito, A.; Candela, C.; Basso, C.; Rizzo, S.; et al. Efficacy and safety of mycophenolate mofetil in patients with virus-negative lymphocytic myocarditis: A prospective cohort study. J. Autoimmun. 2020, 106, 102330. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics. Azathioprine. Available online: http://leki.urpl.gov.pl/files/43_Azathioprine_VIS_tabl_50mg.pdf (accessed on 1 January 2023).
- Vianello, F.; Cinetto, F.; Cavraro, M.; Battisti, A.; Castelli, M.; Imbergamo, S.; Marcolongo, R. Azathioprine in isolated recurrent pericarditis: A single centre experience. Int. J. Cardiol. 2011, 147, 477–478. [Google Scholar] [CrossRef]
- Imazio, M.; Lazaros, G.; Brucato, A.; Gaita, F. Recurrent pericarditis: New and emerging therapeutic options. Nat. Rev. Cardiol. 2016, 13, 99–105. [Google Scholar] [CrossRef]
- Chimenti, C.; Russo, M.A.; Frustaci, A. Immunosuppressive therapy in virus-negative inflammatory cardiomyopathy: 20-year follow-up of the TIMIC trial. Eur. Heart J. 2022, 43, 3463–3473. [Google Scholar] [CrossRef]
- Zochowska, D.; Zegarska, J.; Hryniewiecka, E.; Samborowska, E.; Jazwiec, R.; Tszyrsznic, W.; Borowiec, A.; Dadlez, M.; Paczek, L. Determination of Concentrations of Azathioprine Metabolites 6-Thioguanine and 6-Methylmercaptopurine in Whole Blood With the Use of Liquid Chromatography Combined With Mass Spectrometry. Transplant. Proc. 2016, 48, 1836–1839. [Google Scholar] [CrossRef]
- Thervet, E.; Anglicheau, D.; Toledano, N.; Houllier, A.M.; Noel, L.H.; Kreis, H.; Beaune, P.; Legendre, C. Long-term results of TPMT activity monitoring in azathioprine-treated renal allograft recipients. J. Am. Soc. Nephrol. 2001, 12, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lennard, L.; Welch, J.; Lilleyman, J.S. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: A possible indicator of non-compliance? Br. J. Cancer 1995, 72, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gokhale, R.; Kirschner, B.S. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Sanders, D.S.; Lobo, A.J.; Lennard, L. Clinical significance of azathioprine active metabolite concentrations in inflammatory bowel disease. Gut 2004, 53, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Prabha, R.; Mathew, S.K.; Joseph, A.J.; Mathew, B.S. Exposure to Azathioprine Metabolites and Clinical Outcome in Indian Patients with Crohn’s Disease. J. Pharmacol. Pharmacother. 2022, 13, 266–271. [Google Scholar] [CrossRef]
- Heckmann, J.M.; Lambson, E.M.; Little, F.; Owen, E.P. Thiopurine methyltransferase (TPMT) heterozygosity and enzyme activity as predictive tests for the development of azathioprine-related adverse events. J. Neurol. Sci. 2005, 231, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Boucher, P.; Davelu, P.; Boissonnat, P.; Champsaur, G.; Ninet, J.; Dureau, G.; Obadia, J.F.; Vallon, J.J.; Delaye, J. Thiopurine S-methyltransferase gene polymorphism is predictive of azathioprine-induced myelosuppression in heart transplant recipients. Transplantation 2000, 69, 1524–1527. [Google Scholar] [CrossRef]
- TPMT testing before azathioprine therapy? Drug Ther. Bull. 2009, 47, 9–12. [CrossRef] [PubMed]
- Evans, W.E.; Horner, M.; Chu, Y.Q.; Kalwinsky, D.; Roberts, W.M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J. Pediatr. 1991, 119, 985–989. [Google Scholar] [CrossRef]
- Ford, L.; Prout, C.; Gaffney, D.; Berg, J. Whose TPMT activity is it anyway? Ann. Clin. Biochem. 2004, 41, 498–500. [Google Scholar] [CrossRef]
- Weinshilboum, R. Thiopurine pharmacogenetics: Clinical and molecular studies of thiopurine methyltransferase. Drug Metab. Dispos. 2001, 29, 601–605. [Google Scholar] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, E.D.; Zucker, M.J.; Kramer, N. Giant cell myocarditis: Most fatal of autoimmune diseases. Semin. Arthritis. Rheum. 2000, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bang, V.; Ganatra, S.; Shah, S.P.; Dani, S.S.; Neilan, T.G.; Thavendiranathan, P.; Resnic, F.S.; Piemonte, T.C.; Barac, A.; Patel, R.; et al. Management of Patients With Giant Cell Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1122–1134. [Google Scholar] [CrossRef]
- Mathies, H. Azathioprine in rheumatology. Fortschr. Med. 1979, 97, 2215–2217. [Google Scholar]
- Bots, S.; Gecse, K.; Barclay, M.; D’Haens, G. Combination Immunosuppression in IBD. Inflamm. Bowel. Dis. 2018, 24, 539–545. [Google Scholar] [CrossRef]
- Foroncewicz, B.; Mucha, K.; Florczak, M.; Szymanska, A.; Ciszek, M.; Durlik, M.; Gorski, A.; Kieszek, R.; Kosieradzki, M.; Nazarewski, S.; et al. Long-term outcome of renal transplantation: A 10-year follow-up of 765 recipients. Pol. Arch. Intern. Med. 2019, 129, 476–483. [Google Scholar] [CrossRef]
- Peretto, G.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Sala, S.; Del Rosso, S.; Camici, P.G.; Della Bella, P.; Tresoldi, M.; Dagna, L. P4519 Subclinical toxicity of azathioprine in patients treated for autoimmune myocarditis: A single-centre experience. Eur. Heart J. 2018, 39, ehy563-P4519. [Google Scholar] [CrossRef]
- Mohammadi, O.; Kassim, T.A. Azathioprine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sheiko, M.A.; Sundaram, S.S.; Capocelli, K.E.; Pan, Z.; McCoy, A.M.; Mack, C.L. Outcomes in Pediatric Autoimmune Hepatitis and Significance of Azathioprine Metabolites. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 80–85. [Google Scholar] [CrossRef]
- Kindermann, I.; Barth, C.; Mahfoud, F.; Ukena, C.; Lenski, M.; Yilmaz, A.; Klingel, K.; Kandolf, R.; Sechtem, U.; Cooper, L.T.; et al. Update on myocarditis. J. Am. Coll Cardiol. 2012, 59, 779–792. [Google Scholar] [CrossRef]
- Jones, S.R.; Herskowitz, A.; Hutchins, G.M.; Baughman, K.L. Effects of immunosuppressive therapy in biopsy-proved myocarditis and borderline myocarditis on left ventricular function. Am. J. Cardiol. 1991, 68, 370–376. [Google Scholar] [CrossRef]
- Poloczkova, H.; Krejci, J.; Hude, P.; Ozabalova, E.; Godava, J.; Honek, T.; Zampachova, V.; Svobodova, I.; Freiberger, T.; Spinarova, L. Effect of immunosuppressive therapy in inflammatory cardiomyopathy: Data from the Czech Inflammatory Cardiomyopathy Immunosuppressive Trial. Bratisl. Lek. Listy 2022, 123, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.; Di Lenarda, A.; Dreas, L.; Silvestri, F.; Camerini, F. Immunosuppressive treatment in myocarditis. Int. J. Cardiol. 1989, 22, 329–338. [Google Scholar] [CrossRef]
- Mason, J.W.; O’Connell, J.B.; Herskowitz, A.; Rose, N.R.; McManus, B.M.; Billingham, M.E.; Moon, T.E. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 1995, 333, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Chimenti, C.; Calabrese, F.; Pieroni, M.; Thiene, G.; Maseri, A. Immunosuppressive therapy for active lymphocytic myocarditis: Virological and immunologic profile of responders versus nonresponders. Circulation 2003, 107, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Azathioprine: Drug Information. Available online: https://www.uptodate.com/contents/azathioprine-drug-information?topicRef=127933&source=see_link (accessed on 1 January 2023).
- Ozieranski, K.; Tyminska, A.; Caforio, A.L.P. Immunosuppressive therapy of myocarditis and inflammatory cardiomyopathy in the light of new data. Eur. Heart J. 2022, 43, 4758–4759. [Google Scholar] [CrossRef]
- Maisch, B.; Koelsch, S.; Hufnagel, G.; Funck, R.C.; Ruppert, V.; Pankuweit, S. Abstract 15036: Resolution of Inflammation Determines Short- and Longterm Prognosis in Myocarditis—Data from Esetcid. Circulation 2011, 124, A15036. [Google Scholar] [CrossRef]
- Marcolongo, R.; Baritussio, A.; Gianstefani, S.; Cheng, C.Y.; Iliceto, S.; Caforio, A.L.P. Clinical management and follow-up of myocarditis patients on immunosuppressive therapy. In Myocarditis; Caforio, A., Ed.; Springer: Berlin/Heidelberg, Germany; pp. 285–296.
- Baritussio, A.; Giordani, A.S.; Rizzo, S.; Masiero, G.; Iliceto, S.; Marcolongo, R.; Caforio, A.L. Management of myocarditis in clinical practice. Minerva. Cardiol. Angiol. 2022, 70, 273–284. [Google Scholar] [CrossRef]
- Hahn, E.A.; Hartz, V.L.; Moon, T.E.; O’Connell, J.B.; Herskowitz, A.; McManus, B.M.; Mason, J.W. The Myocarditis Treatment Trial: Design, methods and patients enrollment. Eur. Heart J. 1995, 16 (Suppl. O), 162–167. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Cheng, G.Y.; Shan, Z.G.; Baritussio, A.; Lorenzoni, G.; Tyminska, A.; Ozieranski, K.; Iliceto, S.; Marcolongo, R.; Gregori, D.; et al. Efficacy of immunosuppressive therapy in myocarditis: A 30-year systematic review and meta analysis. Autoimmun. Rev. 2021, 20, 102710. [Google Scholar] [CrossRef]
- Timmermans, P.; Barradas-Pires, A.; Ali, O.; Henkens, M.; Heymans, S.; Negishi, K. Prednisone and azathioprine in patients with inflammatory cardiomyopathy: Systematic review and meta-analysis. ESC Heart Fail. 2020, 7, 2278–2296. [Google Scholar] [CrossRef]
- Tyminska, A.; Ozieranski, K.; Balsam, P.; Maciejewski, C.; Wancerz, A.; Brociek, E.; Marchel, M.; Crespo-Leiro, M.G.; Maggioni, A.P.; Drozdz, J.; et al. Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction- Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries). Biology 2022, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Ozieranski, K.; Tyminska, A.; Marchel, M.; Januszkiewicz, L.; Maciejewski, C.; Glowczynska, R.; Marcolongo, R.; Caforio, A.L.; Wojnicz, R.; Mizia-Stec, K.; et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of immunosuppression in biopsy-proven virus-negative myocarditis or inflammatory cardiomyopathy (IMPROVE-MC). Cardiol. J. 2022, 29, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.S.; Matusik, P.T.; Stein, P.K. Heart rate variability in patients with systemic lupus erythematosus: A systematic review and methodological considerations. Lupus 2018, 27, 1225–1239. [Google Scholar] [CrossRef]
- Caforio, A.L.P. Myocarditis: Endomyocardial biopsy and circulating anti-heart autoantibodies are key to diagnosis and personalized etiology-directed treatment. Eur. Heart J. 2021, 42, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Tuson, S.; Yang, L.; Loomes, D. Real-World Use of Azathioprine Metabolites Changes Clinical Management of Inflammatory Bowel Disease. J. Can. Assoc. Gastroenterol. 2021, 4, 101–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).