Simple Summary
The mangrove ecosystem is an important resource for the survival of thousands of species of animals and plants. It has high ecological and economic value and has made great contributions to the protection and maintenance of marine and terrestrial ecological environment. However, due to climatic and human factors, the mangrove ecosystem has been destroyed and its ecological functions have been damaged. As an important indicator species, fishes have also been affected, with changes in their population and nutritional structure. This study summarized the mangrove fish in China and compiled a relatively complete list of mangrove fish. In addition, the biogeographic characteristics of Chinese mangrove fish were analyzed, filling the gap of national mangrove fish distribution data. Completion of this study contributed to a better understanding of the role of mangroves in maintaining the stability and diversity of fish communities, which was of great value for the management and conservation of regional and global biodiversity.
Abstract
Mangroves are among the most productive marine and coastal ecosystems and play an important role in maintaining the stability and diversity of fish communities. To explore the structure of mangrove fish communities in China, we compiled previous studies, monographs, and two databases on 54 mangrove areas published in the past 30 years. Mangrove fish communities in China comprised Osteichthys (597 species) and Chondrichthyes (14 species), representing 611 species in 344 genera, 117 families, and 28 orders. Perciformes were the predominant taxon, with 350 species in 52 families, accounting for 57% of the total species richness. Reef fish accounted for 29.62%. With regard to feeding groups, there were 328 carnivorous species (53.68%), 214 omnivorous species (35.02%), 41 herbivorous species (6.71%), and 28 detritivores species (4.58%). Classified by body size, 57.61% were small-sized, 24.22% medium-sized, and 18.17% were large-sized fishes. A total of 5.23% (32 species) of these mangrove fish are currently on IUCN red lists, i.e., 2 species are critically endangered, 4 are endangered, 12 are vulnerable, and 14 are near threatened. Cluster analyses shows that Chinese mangroves fish were divided into two categories, i.e., coastal mangrove and island mangrove type. This is closely related to the distribution of reef fish. Moreover, the number of fish species showed a strong positive correlation with mangrove area, but not with latitude. The main reasons may be the subtropical and tropical geographic locations, as well as the characteristics of the South China Sea and the Taiwan Warm Current. The size and integrity of mangrove area are crucial to the local ecosystems; thus, protecting and restoring mangroves is of great significance to large-scale ecosystem-stability and local biodiversity.
1. Introduction
Mangroves play an important role in national and regional economic development, supporting 30% of global commercial fishery [,]. Mangrove ecosystems are characterized by high productivity, high biodiversity, and complex structures. These habitats provide shelter, feeding and spawning grounds for fish, and doubling the biomass of some commercially important fish species [,,]. In addition, mangroves also make considerable contributions to ecosystem stability and functioning, which is crucial for the safety of human life in such areas. As a barrier protecting coastal systems, mangroves play an important role in reducing wave impact, filtering harmful substances, and sequestering carbon [,,,]. Globally, mangroves are mainly distributed in four tropical geographic regions, i.e., the Indo-West Pacific, Eastern Pacific, Western Atlantic, and Eastern Atlantic []. Among them, the Asian region of the Indo-West Pacific comprises the largest and most diverse mangrove areas, accounting for about 42% of the global mangrove area [].
Due to human impact, especially wetland reclamation, pollution through aquaculture, and urbanization, mangroves have experienced severe degradation and area decline. Global mangrove areas are estimated to have decreased by 30–50% in the past 50 years [,]. The Sustainable Development Goals established by the United Nations are closely related to the protection of mangroves, and China has also launched a series of projects for the protection and restoration of mangroves [,]. It is worth noting that not only trees but also important ecological functions such as fish, waterbirds, benthic animals, and mangrove-associates should be considered for the restoration of mangroves [,,].
Fish are an important component of mangrove ecosystems where they occur at multiple trophic levels. The fish community structure can reflect major and minor changes in the ecological environment, and it is an important indicator of mangrove ecosystem stability and functioning [,,]. However, the current status of mangrove fish communities does not make for optimistic prospects. Overexploitation and disproportionate targeting of fish at high trophic levels will affect fish population structures and life history process []. The trophic imbalance of mangrove fish communities and combined adverse effects will continue to affect ecosystem health and reduce ecosystem stability []. Approximately 4.1 million people work in mangrove-related fishery, of which Asia has the highest fishing intensity []. Although research on Chinese mangrove fish has been initiated, it is typically limited to small areas, for example, Hainan Wenchang [], Quanzhou Bay [], Beilun Estuary [], Maowei Sea []; however, large-scale processes, structures, and changes in Chinese mangrove waters are unclear. Therefore, the research on fish in mangrove areas in China has important theoretical and practical significance for the protection of mangrove ecosystems in Asia and in the world.
This study was conducted to describe the structural characteristics and distribution pattern of Chinese mangrove fish communities. Specifically, (1) we summarized the species composition, dietary characteristics, habitat type, and endangerment degree of Chinese mangrove fish; (2) we studied the fish community structure in 54 mangrove areas in China on a large spatial scale and analyzed the distribution pattern of mangrove fish in various geographic regions of China; (3) we assessed the extent to which mangrove area and latitude predict fish communities in Chinese mangroves, and we examined the reasons for changes in fish community structures over a gradient. These insights contribute to a better understanding of the role of mangroves in maintaining the stability and diversity of fish communities, with important outcomes regarding the management and conservation of regional and global biodiversity [,].
2. Materials and Methods
2.1. Study Areas
The study area comprised documented Chinese mangrove areas. The currently known distribution locations of Chinese mangrove fish ranged from the southernmost Qingmei Port to the northernmost Tanshui River, with a total of 54 mangrove areas (Table 1, Figure 1). The provinces or administrative regions involved included Guangdong, Guangxi, Hainan, Fujian, Hong Kong, Taiwan, and Macau. To achieve general representativeness of the data and analysis accuracy, we divided the 54 mangrove areas into 16 regions by geographic location, and the areas with less survey data were excluded. These areas experience a subtropical and tropical marine monsoon climate with an annual average temperature of 21–25 °C []. The tides tend to vary, with regular semi-diurnal tides in Fujian, irregular semi-diurnal tides in Guangdong, regular diurnal tides in Guangxi, and irregular semi-diurnal tides and irregular diurnal tides in Hainan and Taiwan. Water salinity shows considerable variation owing to changes in rainfall and river runoff, and it is higher in summer and lower in winter, ranging from 0.7 to 34.4‰ [,].

Table 1.
Distribution of study areas.
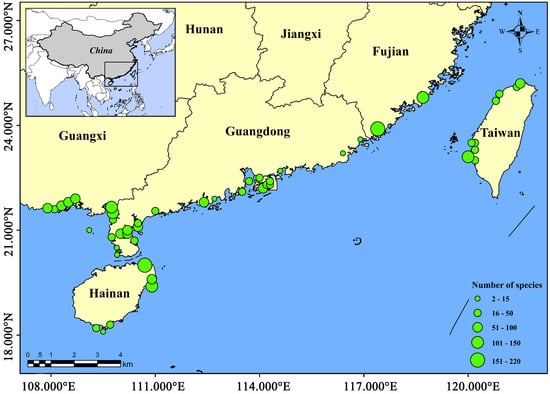
Figure 1.
Distribution of fish populations in different study areas. The green circle in the figure is the number of fish species.
2.2. Data acquisition
2.2.1. List of Mangrove Fish Species
First, all the mangrove areas in China were identified. Second, all the research data on mangrove fish in China were collected. Third, classified according to the geographical position, the size and latitude of the mangrove areas were determined and the fish list of different regions compiled. Fourth, the data of habitat type, feeding habits, conservation status, and body size of various fish were found according to the database. Finally, the list and related data of mangrove fish in China were obtained. Statistical data on species composition, morphological characteristics, and community structure of fishes in mangrove areas of China were used from historical research. Fish data were obtained from two sources: (1) published studies, regional inventories, reports, and monographs (Table 1); (2) the Fishbase Database (https://www.fishbase.se/search.php, accessed on 26 October 2022) and the Fish Database of Taiwan (https://fishdb.sinica.edu.tw/chi/home.php, accessed on 26 October 2022). Synonyms in the compiled fish list were adjusted, and undetermined species were eliminated to ensure accuracy of data analysis, thus a comparably complete list of Chinese mangrove fish was produced (Table S1).
2.2.2. Habitat Types
The habitat types of fish species on the final list were obtained from the Fishbase Database (https://www.fishbase.se/search.php), and reef-associated fish were analyzed separately.
2.2.3. Feeding Habits
Fish were assigned to four feeding groups, namely herbivores, carnivores, detritivores, and omnivores [].
2.2.4. Conservation Status
The conservation status of each fish species was obtained from the International Union of Conservation of Nature and Natural Resources Red List (IUCN Red List) (https://www.iucnredlist.org/, accessed on 26 October 2022), using the categories critically endangered (CR), endangered (EN), vulnerable (VU), near threatened (NT), least concern (LC), data deficient (DD), and not evaluated (NE).
2.2.5. Body Size
The maximum length of fish (regardless of sex) is obtained from the Fishbase Database (https://www.fishbase.se/search.php). According to the maximum total length, fish were considered small-sized (maximum length < 35 cm), medium-sized (65 cm > maximum length ≥ 35 cm), or large-sized (maximum length ≥ 65 cm) [].
2.2.6. The size and Latitude of the Mangrove
The size and latitude of the mangrove study areas were derived from published studies, regional inventories, reports, and monographs. In addition, the mangrove size data was normalized in logarithmic form during the analysis [].
2.3. Data Analyses
PRIMER 5.0 and R v.4.2.1 (package ggpolt2) [] (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/, accessed on 26 October 2022.) were used for cluster analysis (CLUSTER), nonparametric multidimensional analysis (NMDS), and species contributions to similarity (SIMPER) analysis. SPSS v.24 (IBM, Armonk, NY, USA) was used for Pearson correlation analysis, Spearman correlation analysis and similarity analysis (ANOSIM). The fish community structure was investigated using methods such as graphical analysis and multivariate analysis with PRIMER 5.0 and R 4.2.1 software. The number of fish species per mangrove area was counted, and Bray–Curtis similarity matrices were produced based on the number of fish species per order and through square root-transformation. Then, clustering using CLUSTER and nonparametric multidimensional scaling (NMDS) were used for further analyses. After several iterations, the optimal result was obtained. The judgment standard was lower stress value. It is generally believed that stress < 0.2 has explanatory significance []. The difference of fish community composition in different mangrove regions was analyzed as described previously []. SPSS v. 24 was used to analyze the correlation between the area and latitude of each mangrove area and the community characteristics. Before statistical analyses, the data were tested for homogeneity of variance and normal distribution. When these conditions were met, Pearson’s correlation analysis was performed, otherwise Spearman’s correlation analysis was used. Linear regression models were fitted to analyze the relationship between mangrove area and latitude and fish community characteristics (including: number of species, habitat types, feeding habits and body size) []. Analysis of similarities (ANOSIM) was used to test the significance of differences in fish composition in different regions, and species contributions to similarity (SIMPER) analysis was used to calculate the similarity of fish composition in different regions and the average contribution rate of species to similarity [].
3. Results
3.1. Fish Community Composition
Excluding undetermined species, Chinese mangrove fish were found to belong to the classes Osteichthys (597 species) and Chondrichthyes (14 species), with a total of 611 species in 344 genera, 117 families, and 28 orders. Perciformes were the predominant taxon, with 350 species in 52 families, accounting for 57% of the total species richness. The second most numerous orders were Clupeiformes (43 species), Pleuronectiformes (34 species), Anguilliformes (26 species), Tetraodontiformes (24 species), Mugiliformes (19 species), Scorpaeniformes (20 species), Beloniformes (17 species), and Cypriniformes (15 species). The remaining orders comprised fewer than 10 species, each, i.e., Torpediniformes, Myliobatiformes, Orectolobiformes, Rajiformes, Carcharhiniformes, Cichliformes, Gasterosteiformes, Myctophiformes, Pegasiformes, Elopiformes, Synbranchiformes, Gobiesociformes, Cyprinodontiformes, Stomiiformes, Siluriformes, Gonorhynchiformes, Aulopiformes, Gadiformes, and Atheriniformes (Figure 2).
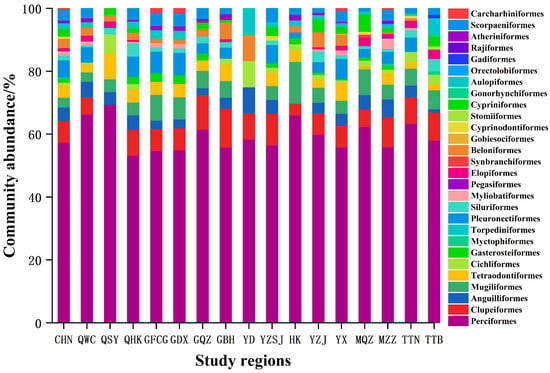
Figure 2.
Relative abundance of fish community composition at the order levels across different mangrove regions. CHN: CHINA; QWC: Wenchang, Hainan; QSY: Sanya, Hainan; QHK: Haikou, Hainan; GFCG: Fangchenggang, Guangxi; GDX: Dongxing, Guangxi; GQZ: Qinzhou, Guangxi; GBH: Beihai, Guangxi; YD: Eastern Guangdong; YZSJ: Pearl River Delta; HK: Hong Kong; YX: Western Guangdong; YZJ: Zhanjiang, Guangdong; MQZ: Quanzhou, Fujian; MZZ: Zhangzhou, Fujian; TTN: Tainan, Taiwan; TTB: Taipei, Taiwan.
The number of fish species in each mangrove regions were the largest in Western Guangdong, with 263 species. Followed by Zhangzhou, Fujian, Haikou, Hainan, and Wenchang, Hainan, more than 150 species. However, there were fewer fish species in Qinzhou, Guangxi, Fangchenggang, Guangxi, Dongxing, Guangxi, Pearl River Delta, Hong Kong, Sanya, Hainan, and Eastern Guangdong, all fewer than 100 species (Table 1, Figure 1). The composition of fish communities differed between mangrove regions. The first dominant order of different mangrove fish communities was the same in each study area, i.e., Perciformes, accounting for more than 53.2% (Figure 2). However, the second dominant orders differed, i.e., Clupeiformes in Wenchang, Hainan, Haikou, Hainan, Qinzhou, Guangxi, Beihai, Guangxi, Pearl River Delta, Zhangzhou, Fujian, Tainan, Taiwan, and Taipei, Taiwan, Tetraodontiformes in Sanya, Hainan, and Mugiliformes in Fangchenggang, Guangxi, Dongxing, Guangxi, Hong Kong, and Quanzhou, Fujian (Figure 2). The composition of fish orders varies in different mangrove regions. Moreover, Myctophiformes, Torpediniformes, Pegasiformes, Synbranchiformes, Gobiesociformes, Stomiiformes, Gonorhynchiformes, Orectolobiformes, Gadiformes, and Rajiformes are the least distributed orders and only exist in one region (Figure 2).
3.2. Fish Community Characteristics in the Study Regions
3.2.1. Habitat Type
According to the Fishbase database, the reef fish accounts for 29.62% of the mangroves in China. Among the different mangrove regions, the proportion of reef fish in Hong Kong was the highest (45.28% of all fish species in the region), followed by Wenchang, Hainan (39.88%), Sanya, Hainan (36.73%), Tainan, Taiwan (35.11%), Eastern Guangdong (33.33%), and Taipei, Taiwan (30%); in the remaining regions, reef fish accounted for less than 30% (Figure 3A).
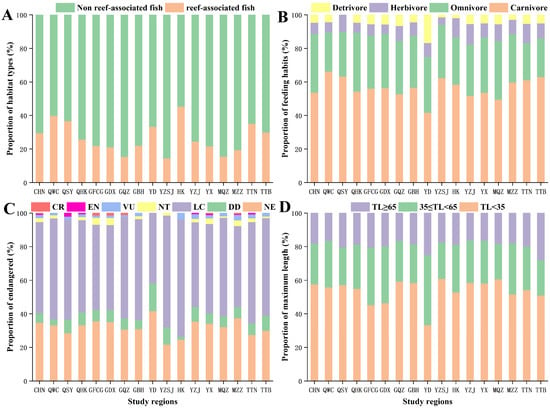
Figure 3.
Distribution characteristics of fish community structure in different regions. (A) Habitat types of fishes in different regions. (B) Feeding habits of fishes in different regions. (C) Endangered of fishes in different regions. (D) Maximum length of fishes in different regions. CHN: CHINA; QWC: Wenchang, Hainan; QSY: Sanya, Hainan; QHK: Haikou, Hainan; GFCG: Fangchenggang, Guangxi; GDX: Dongxing, Guangxi; GQZ: Qinzhou, Guangxi; GBH: Beihai, Guangxi; YD: Eastern Guangdong; YZSJ: Pearl River Delta; HK: Hong Kong; YX: Western Guangdong; YZJ: Zhanjiang, Guangdong; MQZ: Quanzhou, Fujian; MZZ: Zhangzhou, Fujian; TTN: Tainan, Taiwan; TTB: Taipei, Taiwan.
3.2.2. Feeding Habits
Throughout the range of mangroves in China, carnivorous fish accounted for 53.68% of all species, followed by omnivores (35.02%), herbivores (6.71%), and detritivores (4.58%). The proportion of herbivores and detritivores fishes was similar and significantly smaller than that of carnivorous and omnivores fishes. Thus, dietary characteristics of fish communities were similar in all mangrove regions (Figure 3B).
3.2.3. Conservation Status of Fishes in Different Regions
After identifying the conservation status of fish species in the Fishbase Database, 5.23% (32 species) of the species living in Chinese mangroves were threatened, of which critically endangered species accounted for 0.33% (2 species), endangered for 0.65% (4 species), vulnerable for 1.96% (12 species), and near threatened species accounted for 2.29% (14 species). Similar patterns were detected in various mangrove regions, where the proportion of endangered fish is high: Zhangzhou, Fujian (7.58%), Dongxing, Guangxi (7.04%), Fangchenggang, Guangxi (6.85%), Western Guangdong (6.46%), Zhanjiang, Guangdong (5.44%), and Tainan, Taiwan (5.34%). The proportion of endangered fish in other regions was slightly lower, yet considerable, such as: Haikou, Hainan (4.09%), Sanya, Hainan (4.08%), Hong Kong (3.77%), Beihai, Guangxi (3.54%), Wenchang, Hainan (3.07%), Taipei, Taiwan (3.00%), Quanzhou, Fujian (2.75%), Qinzhou, Guangxi (2.19%), and Pearl River Delta (1.45%) (Figure 3C).
3.2.4. Fish Body Size
Overall, the proportion of small-sized fishes was the highest (57.61%), followed by that of medium-sized fishes (24.22%) and large-sized fishes (18.17%). In each mangrove area, the fish community was dominated by small-sized fishes (>45%), and the proportion of large-sized fishes was the smallest (approximately 20%) (Figure 3D).
3.3. Distribution Patterns of Fish Communities
Cluster analysis of fish communities in Chinese mangroves showed that at a similarity level of 30%, 16 mangrove regions were divided into 4 groups; Eastern Guangdong only was assigned to group A; Sanya, Hainan, and Hong Kong were assigned to group B; Wenchang, Hainan, Taipei, Taiwan, and Tainan, Taiwan were assigned to group C; Quanzhou, Fujian, Haikou, Hainan, Western Guangdong, Zhanjiang, Guangdong, Zhangzhou, Fujian, Pearl River Delta, Fangchenggang, Guangxi, Dongxing, Guangxi, Qinzhou, Guangxi, and Beihai, Guangxi were assigned to group D (Figure 4). The results of the NMDS analysis (Figure 5) also supported the clustering (stress = 0.117 < 0.2). The SIMPER similarity percentage reflects the intra-group similarity and inter-group dissimilarity, as well as key species with larger contribution rates. As group A comprised only one region, SIMPER intragroup similarity analysis was performed only on groups B, C, and D. The results of intra-group similarity showed that the intra-group similarity of group B was 31.37%, and the highest contribution rate of Periophthalmus modestus was 93.75%; the similarity within group C was 34.54%, of which Acentrogobius caninus had the highest contribution rate of 90.15%; the similarity within group D was 40.30%, among which Megalops cyprinoides had the highest contribution rate of 90.13%. The results of the SIMPER between groups show that, except for the lower degree of dissimilarity between groups C and D (72.71%), dissimilarity between groups generally exceeded 75% (Table 2). The ANOSIM test results were comparable, with a Global R range of 0–1 (the closer R is to 1, the greater the difference). The R value between groups C and D was 0.71, whereas that between the other groups were larger, indicating significant differences between the four groups (Table 2).
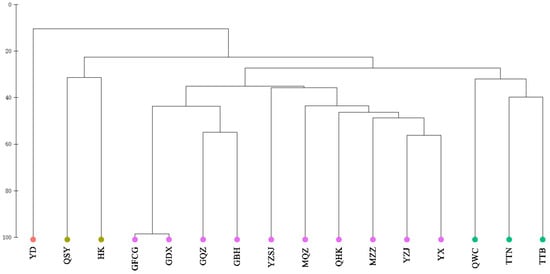
Figure 4.
Cluster diagram of fish community structure in mangrove of China. YD: Eastern Guangdong; QSY: Sanya, Hainan; HK: Hong Kong; GFCG: Fangchenggang, Guangxi; GDX: Dongxing, Guangxi; GQZ: Qinzhou, Guangxi; GBH: Beihai, Guangxi; YZSJ: Pearl River Delta; MQZ: Quanzhou, Fujian; QHK: Haikou, Hainan; MZZ: Zhangzhou, Fujian; YZJ: Zhanjiang, Guangdong; YX: Western Guangdong; QWC: Wenchang, Hainan; TTN: Tainan, Taiwan; TTB: Taipei, Taiwan.
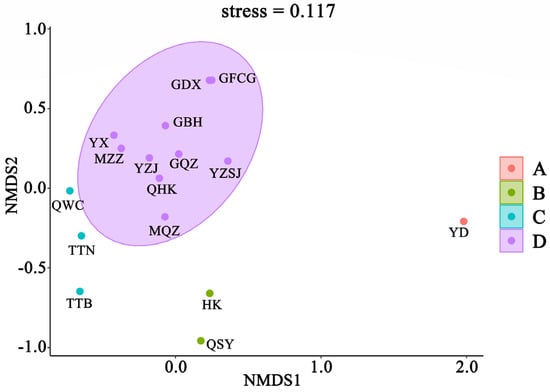
Figure 5.
Nonparametric multivariate sequence of fish community structure in mangrove of China. YD: Eastern Guangdong; QSY: Sanya, Hainan; HK: Hong Kong; GFCG: Fangchenggang, Guangxi; GDX: Dongxing, Guangxi; GQZ: Qinzhou, Guangxi; GBH: Beihai, Guangxi; YZSJ: Pearl River Delta; MQZ: Quanzhou, Fujian; QHK: Haikou, Hainan; MZZ: Zhangzhou, Fujian; YZJ: Zhanjiang, Guangdong; YX: Western Guangdong; QWC: Wenchang, Hainan; TTN: Tainan, Taiwan; TTB: Taipei, Taiwan.

Table 2.
ANOSIM and SIMPER analysis between groups.
3.4. Correlation of fish Community Characteristics with Size and Latitude
The total number of fish species was positively correlated with the size of mangrove size (r = 0.71, p < 0.01) (Figure 6A), while the proportion of reef fish showed a significant negative correlation with mangrove size (r = −0.46, p < 0.01) (Figure 6B). Further detailed analyses of the fish community results showed that the size of mangroves was also correlated with fish feeding habits and was significantly positively correlated with the proportion of carnivorous fish (r = 0.42, p < 0.05) (Figure 6C). The proportion of omnivores was negatively correlated (r = −0.44, p < 0.01) (Figure 6D). The correlations of mangrove size with the proportion of herbivores and that of detritivores were not significant (r = 0.15 and r = −0.16, respectively) (Figure 6E,F). With the increase of mangrove size, the proportion of fish size changed, and the proportion of small-sized fish and large-sized fish showed a decreasing, yet non-significant, trend (r = −0.10 and r = −0.24, respectively) (Figure 6G,I), while there was an increasing, yet non-significant, trend in medium-sized fish (r = 0.22) (Figure 6H).
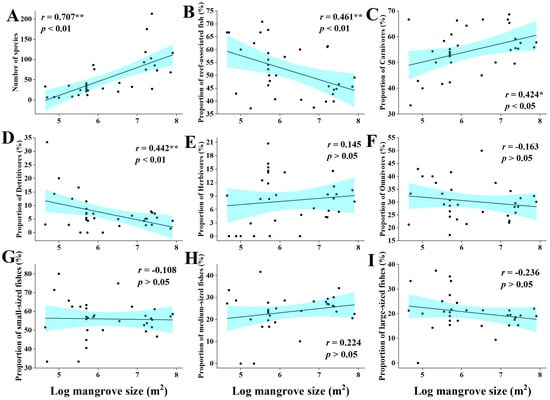
Figure 6.
Relationship between mangrove size and characteristics of fish communities. (A) The relationship between number of fish species and the mangrove size. (B) The relationship between reef-associated fish and the mangrove size. (C) The relationship between carnivores and the mangrove size. (D) The relationship between detritivores and the mangrove size. (E) The relationship between herbivores and the mangrove size. (F) The relationship between omnivores and the mangrove size. (G) The relationship between small-sized fishes and the mangrove size. (H) The relationship between medium-sized fishes and the mangrove size. (I) The relationship between large-sized fishes and the mangrove size. (**: Extremely Significant, p < 0.01. *: Significant, p < 0.05).
The correlation of fish community indicators with latitude of mangroves and was less pronounced than that with mangrove size. With respect to fish feeding habits, the proportion of omnivores was positively correlated with latitude (r = 0.38, p < 0.05) (Figure 7D), while the proportions of carnivores, detritivores, and herbivores showed non-significant trends of correlation with mangrove latitude (r = −0.22; r = −0.12; and r = 0.15, respectively) (Figure 7C,E,F). Other fish community indicators (number of species, habitat types, and fish size) were also not correlated with mangrove latitude (Figure 7A,B,G–I).
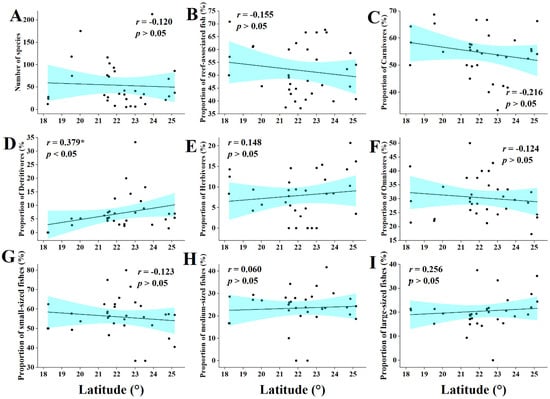
Figure 7.
Relationship between mangrove latitude and characteristics of fish communities. (A) The relationship between number of fish species and the mangrove latitude. (B) The relationship between reef-associated fish and the mangrove latitude. (C) The relationship between carnivores and the mangrove latitude. (D) The relationship between detritivores and the mangrove latitude. (E) The relationship between herbivores and the mangrove latitude. (F) The relationship between omnivores and the mangrove latitude. (G) The relationship between small-sized fishes and the mangrove latitude. (H) The relationship between medium-sized fishes and the mangrove latitude. (I) The relationship between large-sized fishes and the mangrove latitude. (*: Significant, p < 0.05).
4. Discussion
4.1. Mangrove Fish Community Composition in China
With this study, we summarized the population structure of mangrove fish in China. The number of mangrove fish species in China is markedly higher than that in other countries, including New Caledonia (262 species) [], the Sultanate of Oman (177 species) [], and Vietnam (258 species) []. This high mangrove fish diversity is mostly attributable to the expansive coastline and advantageous geographical location []. In the present study, Perciformes was the predominant order in all regions, which was consistent with the results of studies conducted in other mangrove systems, such as in the Sultanate of Oman [], the Philippines [], and Panama []. In addition, we found that the number of fish species in the order Clupeiformes by far exceeded that in other orders, and most of them were tenacious, euryhaline, and eurythermal [,]. The numbers of herbivorous and detritivores fish species were comparably lower in the mangrove regions of China, which is consistent with low abundances but high biomass of herbivorous fish in mangroves in other geographical regions [,,]. Herbivorous fish play a connecting role in their respective ecosystems, as they control the growth of large algae through grazing (i.e., top-down effects) and they regulate the populations of larger carnivorous fish as their prey (bottom-up effects). However, low abundances of herbivorous fish species may result in low elasticity of ecosystems [], as herbivore populations can buffer adverse effects that disturb ecosystem functioning at lower and higher trophic levels. Moreover, numerous marine fish species and their respective habitats are severely threatened by climate change, overfishing, alien invasion, and environmental pollution []. Since 1600, 90 non-indigenous species have been introduced into the South China Sea, 32 of which are fish, some of which have successfully invaded brackish ecosystems, such as mangroves and estuarine wetlands. The primary pathways of introduction are through aquaculture, followed by shipping, ecological restoration, and biocontrol [,,]. Mangroves constitute important habitats of many endangered fish. A total of 32 threatened fish species in Chinese mangroves, accounting for 5.23% of the total fish species diversity. However, many mangroves have been severely degraded in recent years due to overexploitation and pollution (including heavy metals, PCBs, microplastics, etc.) [,]. Therefore, awareness must be raised for the protection and restoration of mangrove habitats.
4.2. Fish Community Distribution Patterns among Different Mangrove Regions
The 16 mangrove regions examined in the current study were divided into four groups through cluster analysis at fish species level. Mangrove fish communities in China were thus divided into two categories: coastal mangrove type (groups A and D) and island mangrove type (groups B and C) [].The proportion of reef fish made a considerable contribution to the results, and they represented a larger proportion in fish communities of island mangroves (35%) than in those of coastal mangroves (20%). This is likely due to the stronger ocean circulation, clearer water, and more suitable environment for coral growth in sea areas surrounding island reef mangroves, which promotes connectivity between mangrove and coral reef ecosystems [,]. Therefore, coastal mangroves and reef mangroves are characterized by their respective unique fish fauna. It is worth noting that according to the investigation on habitat types of mangrove fish in China, reef fishes accounted for 29% of the total. In particular, Hong Kong (45%) had the largest proportion of reef fishes in mangrove regions. Meanwhile, the fish community was generally dominated by small-sized fishes, accounting for 57% of the total number. This result was consistent with those of previous studies on mangroves as fish shelters, feeding grounds, and spawning grounds, and it further confirmed the importance of connectivity of mangroves with coral reefs [,,]. Connectivity of mangrove and coral reef ecosystems has been demonstrated in studies in Europe [], East Asia [], Southeast Asia [], and the Southwest Atlantic []. Studies in Caribbean waters found that the biomass of several species in coral reefs linked to mangroves has more than doubled []. Du, Xie, Wang, Chen, Liu, Liao, and Chen [] described the connectivity of fish communities on the mangrove-seagrass-coral reef continuum in Wenchang, China, and emphasized the importance of mangroves as habitats for juvenile fish. Through such connectivity and exchange functions, mangroves can produce and export large quantities of fish, including commercially important species; therefore, stable functioning of mangrove ecosystems facilitates stability of fishery resources and ensures diversity of fish communities in mangrove and coral reef ecosystems [].
The distribution patterns of fish are the result of long-term adaptation of fish populations to the environment, and the composition of fish communities across different areas with similar habitat types is remarkably similar []. In coral reefs, such similarity of fish communities between geographically distant reef areas is higher than that between geographically close reef areas and shelf areas []. Comparably, even though the mangrove regions of Wenchang City and Taiwan are geographically distant from each other, both are typical mangrove ecosystems with strong connectivity with coral reefs, and both are affected by the South China Sea Warm Current and the Kuroshio branch [,]. The environmental conditions of these two mangrove sites are relatively similar, which may explain why their fish communities are also relatively similar, and they were categorized in the same group. In addition, the Haikou mangrove is a reef mangrove, which is geographically close to Zhanjiang, and these two sites are connected through hydrology and human transport activities. Fish inhabiting these two sites exhibit habitat migration and elicit close genetic exchange at some stage in their life cycle, which may explain why the Haikou mangroves are divided into separate inshore mangroves [].
4.3. Fish Community Characteristics in Different Size and Latitudes
We found that the number of fish species was positively correlated with mangrove size, which is consistent with respective findings in Malaysia [], Indonesia [], Vietnam [], and the Caribbean []. Habitat heterogeneity is an important factor for biodiversity [], and with increasing habitat complexity, the ecological niche breadth increases and thus the number of species [,]. Mangroves are highly complex ecosystems, mostly because (1) the forms of plants in mangrove regions are diverse; (2) fallen leaves, sediments, and tides can substantially and rapidly change the turbidity of the water; and (3) owing to inflow from rivers, the water salinity range may vary considerably [,,]. Vaslet et al. [] investigated the habitat trophic-level structure of the Guadeloupe mangrove coastline and found that food availability strongly affects mangrove fish community composition. Fallen leaves and sediments can provide large amounts of nutrients and constitute rich food sources for some fish species through the detritus food chain []. Wu, et al. [] collected mangrove species data at 70 sites in China and found that mangrove species composition exhibited a nested structure. Nesting of species communities was significantly correlated with habitat size, and nesting patterns were frequent on islands [,]. Therefore, the focus of conservation efforts should be placed on protecting mangroves covering large size, which provide more habitat space and thus sustain more fish [,]. Londono et al. [] investigated a mangrove size in the Southern Caribbean and found that mangrove size was the main factor affecting fish richness, which was significantly and positively correlated with the catch of common fish. However, it is insufficient to rely solely on mangrove size when judging habitats regarding the importance of protection. Moreover, functional integrity and plant density are important factors affecting fish diversity. Tran and Fischer [] studied the relationship between habitat fragmentation and fish diversity in mangroves of CaMau Province, Vietnam, and fish diversity in mangroves with higher fragmentation was 1.78-fold lower than that in less fragmented mangroves. Sitorus et al. [] found a very strong correlation between mangrove density and fish diversity in mangroves of North Sumatra. Taken together, protection and restoration of complete large-size mangroves were key to protecting the fish resources in these regions.
Latitude is frequently considered an important variable affecting species richness, and fish richness changes over the latitude [,]. The correlation between species composition and latitude has been confirmed in the Great Barrier Reef [], Mexico [], and California []. However, in our study, the number of species of mangrove fishes was not correlated with latitude, probably because the range of latitude was not sufficiently large for such effects to occur. A survey by Zintzen, et al. [] showed that fish community structures in New Zealand were strongly correlated with latitude (29.15–50.91 degrees S). Holland et al. [] studied the relationship between the trophic structure of coral reef fish in eastern Australia and latitude (29–44 degrees S) and found that fish biomass decreased towards higher latitude. However, in the current study, the latitude of the examined systems was 18–25 degrees S, thus covering a comparatively narrow range. Most importantly, water temperature is lower at higher latitudes, which also affected fish communities. Travers, Potter, Clarke, Newman, and Hutchins [] found that changes in the structure of inshore fish populations on soft substrates and at coral reefs on the tropical west coast of Australia were driven by differences in water temperature across latitudes. However, mangroves in China are located in the tropics and subtropics. Due to the South China Sea Warm Current and the Taiwan Warm Current, the average water temperature in the mangrove regions were 21–25 °C [,,]. Therefore, the species composition of mangrove fish in Chinese waters is less affected by latitude differences. Similar results were produced in previous studies. For example, Kulbicki et al. [] analyzed the biogeography of Chaetodontidae in the western and central Pacific areas and found that Chaetodontidae diversity was not significantly correlated with latitude. Roberts surveyed coral reef fish communities in the Saudi Arabian Red Sea between 26.8 to 18.6 degrees N and found only subtle changes in fish communities along the latitude gradient, which were closely related to environmental conditions. More challenging environmental conditions may increase homogeneity among species communities [].
There are two major limitations in this study that could be addressed in future research. First, the study focused on the distribution of mangrove fish in China at large spatial scales. Only two variables, which are latitude and mangrove size, were analyzed and small-scale variables for the potential effect of fish communities including temperature, tides levels, salinities, depths, mangrove community composition, human influences, pollutant discharge, and urbanization were ignored. Therefore, it is of great significance to study these small-scale variables in future research. Second, this study did not analyze the changes of mangrove fish communities between different time periods. Since the data span is 30 years, it is of great significance to analyze the changes of mangrove fish communities in different time periods in future studies.
5. Conclusions
We summarized the distribution patterns of mangrove fish in China, and we describe the biogeographic characteristics of Chinese mangrove fish and present a relatively complete list of Chinese mangrove fish. The insights produced here will be of importance for decision makers with respect to regional and global mangrove conservation and restoration. Further, our results fill a gap in national mangrove fish species distribution data. Considering the grave adverse effects of anthropogenic disturbance and climate change on these sensitive ecosystems, our study constitutes an important reference for the protection and restoration of global mangrove ecosystems, and it provides data support for the protection of mangrove fish communities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11121696/s1, Table S1: The checklist of mangrove fishes in China.
Author Contributions
Conceptualization, T.W. and Y.L.; methodology, T.W., Y.L. and J.Z.; software, T.W., C.L. (Chunhou Li), and J.Z.; validation, C.L. (Chunran Li), J.S. and P.W.; formal analysis, T.W., Y.L. and J.Z.; investigation, T.W., Y.L. and J.Z.; resources, T.W., Y.L. and C.L. (Chunhou Li); data curation, T.W. and J.Z.; writing—original draft preparation, T.W., Y.L. and J.Z.; writing—review and editing, T.W., Y.L. and C.L. (Chunhou Li); visualization, T.W. and J.Z.; supervision, T.W., Y.L. and C.L. (Chunhou Li) All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Guangdong Basic and Applied Basic Research Foundation (2019B1515120065), National Key R&D Program of China (2019YFD0901201; 2019YFD0901204; 2018YFD0900803); National Natural Science Foundation of China (31702351); Fundamental and Applied Fundamental Research Major Program of Guangdong Province (2019B030302004-05); Science and Technology Planning Project of Guangdong Province (2019B121201001); Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD16); Financial Fund of the Ministry of Agriculture and Rural Affairs, P. R. of China (NFZX2021); Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (No. 2021SD04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Part of the data presented in this study are available in the Supplementary Material. The remaining data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to have influenced the work reported here.
References
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- Tran, L.X.; Fischer, A. Spatiotemporal changes and fragmentation of mangroves and its effects on fish diversity in Ca Mau Province (Vietnam). J. Coast. Conserv. 2017, 21, 355–368. [Google Scholar] [CrossRef]
- Alongi, D.M. Impact of global change on nutrient dynamics in mangrove forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Ouyang, X.G.; Guo, F. Patterns of Mangrove Productivity and Support for Marine Fauna. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer: Cham, Switzerland, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Anneboina, L.R.; Kumar, K.K. Economic analysis of mangrove and marine fishery linkages in India. Ecosyst. Serv. 2017, 24, 114–123. [Google Scholar] [CrossRef]
- Twilley, R.R.; Castañeda–Moya, E.; Rivera–Monroy, V.H.; Rovai, A. Productivity and Carbon Dynamics in Mangrove Wetlands. In Mangrove Ecosystems: A Global Biogeographic Perspective; Springer: Cham, Switzerland, 2017; pp. 113–162. [Google Scholar]
- Njana, M.A.; Zahabu, E.; Malimbwi, R.E. Carbon stocks and productivity of mangrove forests in Tanzania. South. For. J. For. Sci. 2018, 80, 217–232. [Google Scholar] [CrossRef]
- Zhao, P.H.; Sanganyado, E.; Wang, T.Y.; Sun, Z.W.; Jiang, Z.Y.; Zeng, M.R.; Huang, Z.X.; Li, Y.F.; Li, P.; Bi, R. Accumulation of nutrients and potentially toxic elements in plants and fishes in restored mangrove ecosystems in South China. Sci. Total Environ. 2022, 838, 155964. [Google Scholar] [CrossRef]
- Sánchez–Núñez, D.A.; Pineda, J.E.M.; Osorio, A.F. From local-to global-scale control factors of wave attenuation in mangrove environments and the role of indirect mangrove wave attenuation. Estuar. Coast. Shelf Sci. 2020, 245, 106926. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.; Meynecke, J.O.; Pawlik, J.; Penrose, H.; Sasekumar, A. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Worthington, T.A.; Andradi–Brown, D.A.; Bhargava, R.; Buelow, C.; Bunting, P.; Duncan, C.; Fatoyinbo, L.; Friess, D.A.; Goldberg, L.; Hilarides, L. Harnessing Big Data to Support the Conservation and Rehabilitation of Mangrove Forests Globally. One Earth 2020, 2, 429–443. [Google Scholar] [CrossRef]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The state of the world’s mangrove forests: Past, present, and future. Ann. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef]
- Aburto–Oropeza, O.; Ezcurra, E.; Danemann, G.; Valdez, V.; Murray, J.; Sala, E. Mangroves in the Gulf of California increase fishery yields. Proc. Natl. Acad. Sci. USA 2008, 105, 10456–10459. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Q.; Wang, W.Q. Some thematic issues for mangrove conservation in China. J. Xiamen Univ. (Nat. Sci.) 2017, 56, 323–330. [Google Scholar]
- Bosire, J.O.; Dahdouh–Guebas, F.; Walton, M.; Crona, B.I.; Lewis, R.R.; Field, C.; Kairo, J.G.; Koedam, N. Functionality of restored mangroves: A review. Aquat. Bot. 2008, 89, 251–259. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Cadier, C.; Bayraktarov, E.; Piccolo, R.; Adame, M.F. Indicators of Coastal Wetlands Restoration Success: A Systematic Review. Front. Mar. Sci. 2020, 7, 600220. [Google Scholar] [CrossRef]
- Vaslet, A.; Phillips, D.L.; France, C.; Feller, I.C.; Baldwin, C.C. The relative importance of mangroves and seagrass beds as feeding areas for resident and transient fishes among different mangrove habitats in Florida and Belize: Evidence from dietary and stable-isotope analyses. J. Exp. Mar. Biol. Ecol. 2012, 434, 81–93. [Google Scholar] [CrossRef]
- Soria–Barreto, M.; Fernandez, R.G.; Ramos, H.E.R.; Brito, R. The fish community in Gulf of Mexico mangroves, a response to hydrological restoration. Lat. Am. J. Aquat. Res. 2021, 49, 507–519. [Google Scholar] [CrossRef]
- Reis, J.A.; Harvey, E.S.; Giarrizzo, T. Impacts of small-scale fisheries on mangrove fish assemblages. ICES J. Mar. Sci. 2019, 76, 153–164. [Google Scholar] [CrossRef]
- Sandilyan, S.; Kathiresan, K. Mangrove conservation: A global perspective. Biodivers. Conserv. 2012, 21, 3523–3542. [Google Scholar] [CrossRef]
- Zu Ermgassen, P.S.; Mukherjee, N.; Worthington, T.A.; Acosta, A.; Araujo, A.R.D.R.; Beitl, C.M.; Castellanos-Galindo, G.A.; Cunha-Lignon, M.; Dahdouh-Guebas, F.; Diele, K.; et al. Fishers who rely on mangroves: Modelling and mapping the global intensity of mangrove-associated fisheries. Estuarine Coast. Shelf Sci. 2020, 247, 106975. [Google Scholar] [CrossRef]
- Xie, M.L. Fishconnectivityin Mangrove–Seagrass–Coral Reef Continuum—A Case Study in Wenchang, Hainan Province. Master’s Thesis, Shantou University, Shantou, China, 2019. [Google Scholar]
- Guo, Z.M.; Chen, J.; Ju, P.L.; Fang, C.; Du, J.G.; Yang, S.Y.; Chen, M.R.; Xiao, J.M. Fish Biodiversity and Ecological Support for Fishes in Quanzhou Bay Mangrove Zone. J. Xiamen Univ. (Nat. Sci.) 2016, 55, 9. [Google Scholar]
- Lan, D.Z. Status Quo of Marine Resources and Environment in Beilun Estuary; Xiamen University Press: Xiamen, China, 2013. [Google Scholar]
- Chang, T.; Wu, Z.Q.; Huang, L.L.; Geng, J.J.; Zhu, Z.J.; Wu, W.L. Community structures of fish larvae and its relation with environmental factors in the mangrove of Maoweihai Bay. J. Appl. Oceanogr. 2015, 034, 219–226. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Zou, Q.; Chang, T.; Zhang, N.; Huang, L.-L. Seasonal dynamics of the juvenile fish community structure in the Maowei Sea mangroves. PLoS ONE 2018, 13, e0192426. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.M.; Wang, Z.M.; Mao, D.H.; Huang, C.L.; Lu, C.Y. Spatial–temporal changes of China’s mangrove forests over the past 50 years: An analysis towards the Sustainable Development Goals (SDGs). Chin. Sci. Bull. 2021, 66, 3886–3901. [Google Scholar] [CrossRef]
- Chen, J. Distribution Pattern of Taxonomic Diversity of Fish Community in Four Typical Mangrove Areas, China; Xiamen University: Xiamen, China, 2017. [Google Scholar]
- Chen, I.; Weng, C.J.; Chen, Y.R.; Huang, S.P.; Wen, Z.H.; Jang–Liaw, N.H.; Tsai, T.H. The checklist of inland–water and mangrove fish fauna of Kinmen Island, Fujian Province, Taiwan with comments on ecological conservation of native fishes. J. Mar. Sci. Technol. 2013, 21, 42. [Google Scholar] [CrossRef]
- Meng, Y. The Characteristics of Heavy Metal Pollution and Reason Analysis of Common Fishes in Different Mangrove Forests. Master’s Thesis, Xiamen University, Xiamen, China, 2017. [Google Scholar]
- Jiang, C.P. The Change of Fish Community Structure and Its Stress Factors Analysing in Zhangjiang Estuary Mangrove Area. Master’s Thesis, Xiamen University, Xiamen, China, 2019. [Google Scholar]
- Zhong, H.Q. The Main Community Structure Characteristics of Fishes in Zhangjiang Estuary Mangrove National Nature Reserve; Xiamen University: Xiamen, China, 2015. [Google Scholar]
- Feng, J.X. Effects of the Invasion and Ecological Control of Spartina alterniflora on the Foodweb of Mangrove Wetlands. Ph.D. Thesis, Xiamen University, Xiamen, China, 2013. [Google Scholar]
- Li, G.R. Ecological Study of Wetlands in Guangxi. Master’s Thesis, Guangxi Normal University, Guilin, China, 2008. [Google Scholar]
- Liu, C. Research on the Diversity of Juvenile Fish and Its Contribution to Fishery Resources in Shankou Mangrove Ecosystem; Xiamen University: Xiamen, China, 2012. [Google Scholar]
- Liu, C.; Hu, W.J.; Chen, M.R.; Yang, S.Y. Juvenile Fish Diversity in Shankuo Mangrove Reserve and Their Recruitment to the Fishery Resources. J. Xiamen Univ. (Nat. Sci.) 2013, 52, 273–280. [Google Scholar]
- Fan, H.Q.; Chen, G.H.; He, B.Y. Mangrove Coastal Wetland in Shankou and Its Management; China Ocean Press: Beijing, China, 2005. [Google Scholar]
- He, B.Y. Comparative Study on the Ecology of Mangrove Fishes Between Two Bay of Guangxi. Mar. Sci. Bull. 1999, 18, 28–35. [Google Scholar]
- Fan, H.Q.; Wei, S.Q.; He, B.Y. The Seasonal Dynamics of Nekton Assemblages in Mangrove fringed Tidal Waters of Yingluo Bay, Guangxi. Guangxi Sci. 1998, 005, 46–51. [Google Scholar]
- Huang, D.L. Fish Diversity and Their Association to Environmental Variables such as Tide in Qinzhou Harbor Mangroves, Guangxi Province; Guilin University of Technology: Guilin, China, 2013. [Google Scholar]
- Huang, L.L.; Huang, D.L.; Wu, Z.Q.; Kang, B.; Chen, Z.B. Temporal variation of fish diversity and assemblages and their associations to environmental variables in the mangrove of Qinzhou Harbor, Guangxi Province, China. Turk. J. Fish. Aquat. Sci. 2016, 16, 297–310. [Google Scholar] [CrossRef]
- Huang, D.L.; Wu, Z.Q.; Huang, L.L.; Chang, T.; Geng, J.J.; Wu, W.L. The Fish Species Diversity Analysis in Mangrove of Maowei Gulfin Qinzhou, Guangxi Province. Trans. Oceanol. Limnol. 2013, 004, 135–142. [Google Scholar]
- Chang, T.; Wu, Z.Q.; Huang, L.L.; Huang, D.L.; Geng, J.J.; Zhu, Z.J.; Wu, W.L.; LI, F.H. Species Composition and Diversity of Fish Larvae and Juveniles in the Creek of Mangrove of Maowei Gulf, Guangxi Province. Trans. Oceanol. Limnol. 2014, 007, 52–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Wang, W.; Li, Y.; Wang, M. Distribution of fish among Avicennia and Sonneratia microhabitats in a tropical mangrove ecosystem in South China. Ecosphere 2019, 10, e02759. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Z.; Shi, F.; Wang, W. Are vegetated areas of mangroves attractive to juvenile and small fish? The case of Dongzhaigang Bay, Hainan Island, China. Estuarine Coast. Shelf Sci. 2009, 85, 208–216. [Google Scholar] [CrossRef]
- Yan, S.Z. Effects of Mangrove Microhabitat Heterogeneity on Fish to Fish Diversity; Xiamen University: Xiamen, China, 2011. [Google Scholar]
- Huang, Z.Y. The Application of Energy Signature Technology in Mangrove Energy Flux and Attractive of Mangrove to Fish Abstract. Master’s Thesis, Xiamen University, Xiamen, China, 2008. [Google Scholar]
- Mi, X.F. Mangrove Restoration by Water Quality Improvement: A Case of Dongzhaigang Bay, Hainan, China; Xiamen University: Xiamen, China, 2017. [Google Scholar]
- Ding, Y.P. Distribution Pattern of Fish in Different Microhabitats of a Vicennia Marina Forest and Sonneratia Apetala Forest; Xiamen University: Xiamen, China, 2015. [Google Scholar]
- Wang, M.; Zhang, J.H.; Shi, F.S. Investigation on Fishing Gear and Catch in Mangrove Area of Dongzhaigang, Hainan. Fish. Sci. Technol. Inf. 2007, 34, 6–9. [Google Scholar]
- Wang, M.; Wang, W.Q.; Lin, G.S. Sanya Mangrove; Science Press: Beijing, China, 2019. [Google Scholar]
- Du, J.G.; Xie, M.L.; Wang, Y.Y.; Chen, Z.H.; Liu, W.H.; Liao, J.J.; Chen, B. Connectivity of fish assemblages along the mangrove–seagrass–coral reef continuum in Wenchang, China. Acta Oceanol. Sin. 2020, 39, 43–52. [Google Scholar] [CrossRef]
- Guo, Z.M. Study of Fish Community Structure and Ecological Function of Mangrove Ecosystem in Qinglan Mangrove Area, Hainan. Master’s Thesis, Xiamen University, Xiamen, China, 2017. [Google Scholar]
- Tzeng, W.N.; Wang, Y.T. Structure, composition and seasonal dynamics of the larval and juvenile fish community in the mangrove estuary of Tanshui River, Taiwan. Mar. Biol. 1992, 113, 481–490. [Google Scholar] [CrossRef]
- Lin, H.J.; Shao, K.T. Seasonal and diel changes in a subtropical mangrove fish assemblage. Bull. Mar. Sci. 1999, 65, 775–794. [Google Scholar]
- Kuo, S.R.; Lin, H.J.; Shao, K.T. Fish assemblages in the mangrove creeks of northern and southern Taiwan. Estuaries 1999, 22, 1004–1015. [Google Scholar] [CrossRef][Green Version]
- Tsai, C.H.; Wang, Y.K.; Tsai, S.T.; Wu, S.H. Seasonal and diel changes of the fish assemblage employing the fyke nets in a subtropical mangrove estuary of Puzih river, Taiwan. J. Mar. Sci. Technol. 2015, 23, 109–116. [Google Scholar] [CrossRef]
- Kuo, S.R.; Lin, H.J.; Shao, K.T. Seasonal changes in abundance and composition of the fish assemblage in Chiku Lagoon, southwestern Taiwan. Bull. Mar. Sci. 2001, 68, 85–99. [Google Scholar]
- Nip, T.H.; Wong, C.K. Juvenile fish assemblages in mangrove and non–mangrove soft–shore habitats in eastern HongKong. Zool. Stud. 2010, 49, 760–778. [Google Scholar] [CrossRef]
- Ming, N.H. Ecology of Fishes in Mangrove and Non–mangrove Habitats in Eastern HongKong; The Chinese University of HongKong: HongKong, China, 2005. [Google Scholar]
- Wu, Y.M.; Zheng, P.S.; Liu, N.; Tang, Y.J.; Cui, Z.M.; Huang, P.P.; Huang, F.; Wang, X.P. Diversity of fish species in Guangdong mangrove areas. Acta Sci. Nat. Univ. Sunyatseni 2018, 57, 110–120. [Google Scholar] [CrossRef]
- He, X.L.; Ye, N.; Xuan, L.Q. Investigation of Fishes in Mangrove Areas of Leizhou Peninsula. J. Guangdong Ocean. Univ. 2003, 23, 3–10. [Google Scholar]
- Ye, N.; Chen, X.H.; Liu, H.L. Investigation on Toxic Gobyon the West Coast of Zhanjiang. Ocean. Fish. 2007, 12, 45–47. [Google Scholar]
- Liao, J. Species Diversity of Larvae Fish in Mangrove of Leizhou Peninsula and the Response of Oryzias Curvinotus to Nonylphenol. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2017. [Google Scholar]
- Bai, Q. Molecular Classification of Fishes in the Eastern of Leizhou Mangrove. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2014. [Google Scholar]
- Zhang, S.; Liao, J.; Bai, Q.; Chen, C.; Guo, Y.S.; Liu, C.W.; Wang, Z.D. COI Barcode–assisted species diversity study on mangrove fish in Leizhou Peninsula. Oceanol. Limnol. Sin. 2016, 47, 663–672. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, C.Q.; Liao, J.; Guo, Y.S.; Liu, C.W.; Wang, Z.D. Molecular Systematics of Mangrove Oxudercine Gobies (Gobiidae: Oxudercinae) Based on DNA Barcodes. Genom. Appl. Biol. 2017, 36, 3219–3228. [Google Scholar] [CrossRef]
- Chen, Z.S. China Gulf Chronicle; China Ocean Press: Beijing, China, 1999; Volume 10. [Google Scholar]
- Chen, C. Classification and Diversity of Fishes in the Mangrove at Western Zhanjiang. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2014. [Google Scholar]
- Ye, N.; Wu, X.D.; Zhang, W. Survey of Fishes in Gaoqiao Mangrove Zone in Zhanjiang. J. Guangdong Ocean. Univ. 2007, 27, 55–61. [Google Scholar]
- Wu, X.D. Fish survey in Lianjiang Gaoqiao Mangrove National Nature Reserve. For. Sci. Technol. 2008, 37–40. [Google Scholar] [CrossRef]
- Huang, J.S.; Koongolla, J.B.; Li, H.X.; Lin, L.; Pan, Y.F.; Liu, S.; He, W.H.; Maharana, D.; Xu, X.R. Microplastic accumulation in fish from Zhanjiang mangrove wetland, South China. Sci. Total Environ. 2020, 708, 134839. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhu, G.P.; Wang, Z.L.; Ye, N. Length-weight relationships of four fish species from mangrove of Zhanjiang, China. J. Appl. Ichthyol. 2017, 34, 167–168. [Google Scholar] [CrossRef]
- Yi, Z.Q. Study on Contribution of Organic Carbon Sources to Fishes in Mangrove Ecosystem in Zhanjiang Harbor; Guangdong Ocean University: Zhanjiang, China, 2010. [Google Scholar]
- Liu, J.L.; Li, H.L.; Tang, Y.J.; Xie, L.N.; Zhong, J.Y.; Huang, Q.Y.; Deng, G.M.; Liu, N. Heavy metal pollution and risk analysis to human in economic fish of mangrove wetland in Qi’ao island, Zhuhai. Ecol. Sci. 2017, 36, 186–195. [Google Scholar] [CrossRef]
- Cai, L.Z. Zoobenthic Ecology in Shenzhen Bay; Xiamen University Press: Xiamen, China, 2014. [Google Scholar]
- Liu, L.N. Effects on the Environment of Tidal Flat from Introduced Sonneratia apetala Mangrove in Shenzhen Bay, China. Ph.D. Thesis, SunYat-sen University, Guangzhou, China, 2017. [Google Scholar]
- Chen, G.Z.; Miao, S.Y.; Zhang, J.H. Ecologic Study on the Mangrove Forest in Futian Nature Reserve, Shenzhen, China. Acta Sci. Nat. Univ. Sunyatseni 1996, 35, 294–300. [Google Scholar]
- Chen, G.Z.; Wang, Y.J.; Huang, Q.L. A study on the biodiversity and protection in Futian National Nature Reserve of mangrove sand birds, Shenzhen. Biodivers. Sci. 1997, 5, 104–111. [Google Scholar]
- Liang, H. Study on the Dynamic of Bio Community Structure and Biodiversity in Taipa–Coloane Reclamation Site, Macao. Ph.D. Thesis, Jinan University, Guangzhou, China, 2007. [Google Scholar]
- Jennings, S.; Grandcourt, E.M.; Polunin, N. The effects of fishing on the diversity, biomass and trophic structure of Seychelles’ reef fish communities. Coralreefs 1995, 14, 225–235. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Quan, Q.M.; Xiao, Y.Y.; Wu, P.; Li, C.H. Species composition characteristics analysis of Qilianyu reef fishes of Xisha Islands. J. Fish. Sci. China 2022, 29, 102–117. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Baoyin, T.; Bao, Y. Estimation and Climate Factor Contribution of Aboveground Biomass in Inner Mongolia’s Typical/Desert Steppes. Sustainability 2019, 11, 6559. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package‘ggplot2’. Computer Software. Available online: http://ggplot2 (accessed on 12 August 2022).
- Dexter, E.; Rollwagen-Bollens, G.; Bollens, S.M. The trouble with stress: A flexible method for the evaluation of nonmetric multidimensional scaling. Limnol. Oceanogr. Methods 2018, 16, 434–443. [Google Scholar] [CrossRef]
- Xie, L.; Yin, C. Seasonal variations of soil fungal diversity and communities in subalpine coniferous and broadleaved forests. Sci. Total Environ. 2022, 846, 157409. [Google Scholar] [CrossRef]
- Bai, J. Species Diversity and Conservation of Fish and Benthic Fauna in Upper Reaches of the Jinsha River. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar]
- Kulbicki, M.; Wantiez, L.; Thollot, P.; Tham, G.M. Functional and Taxonomic Overlap in Shore Fish Assemblages in a Tropical Seascape. Diversity 2022, 14, 310. [Google Scholar] [CrossRef]
- AlJufaili, S.M.; Jawad, L.A.; Park, J.M.; AlSariri, T.S.; AlBalushi, B.Y. Fish diversity of mangrove ecosystems in Sultanate of Oman. Cah. Biol. Mar. 2021, 62, 235–249. [Google Scholar] [CrossRef]
- Hong, P.N.; San, H.T. Mangroves of Vietnam; IUCN: Gland, Switzerland, 1993; Available online: https://portals.iucn.org/library/efiles/documents/WTL-006.pdf (accessed on 26 October 2022).
- Fan, Q.D.; Liang, L.K.; Liang, F.; Sun, X.F. Research Progress on Coastline Change in China. J. Coast. Res. 2020, 99, 289–295. [Google Scholar] [CrossRef]
- Salmo, S.G.; Tibbetts, I.R.; Duke, N.C. Nekton communities as indicators of habitat functionality in Philippine mangrove plantations. Mar. Freshw. Res. 2018, 69, 477–485. [Google Scholar] [CrossRef]
- Castellanos–Galindo, G.A.; Baos, R.A.; Zapata, L.A. Mangrove–associated fish assemblages off the southern Panama Bight region (tropical eastern Pacific). Neotrop. Ichthyol. 2021, 19, e210025. [Google Scholar] [CrossRef]
- Hoque, M.M.; Kamal, A.H.M.; Idris, M.H.; Ahmed, O.H.; Saifullah, A.S.M.; Billah, M.M. Status of some fishery resources in a tropical mangrove estuary of Sarawak, Malaysia. Mar. Biol. Res. 2015, 11, 834–846. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Garrard, S.L.; DeLeon, P.S.; Cullen, L.C.; Smith, D.J.; Sloman, K.A.; Bell, J.J. Structuring of Indo-Pacific fish assemblages along the mangrove–seagrass continuum. Aquat. Biol. 2009, 5, 85–95. [Google Scholar] [CrossRef]
- Shi, F.S.; Wang, M.; Wang, W.Q.; Song, C.H. A review of the relationship between mangrove sandfishes. Mar. Sci. 2005, 54–59. [Google Scholar]
- Seemann, J.; Yingst, A.; Stuart–Smith, R.D.; Edgar, G.J.; Altieri, A.H. The importance of sponges and mangroves in supporting fish communities on degraded coral reefs in Caribbean Panama. PeerJ 2018, 6, e4455. [Google Scholar] [CrossRef] [PubMed]
- Allard, H.; Ayling, A.M.; Shears, N.T. Long-term changes in reef fish assemblages after 40 years of no-take marine reserve protection. Biol. Conserv. 2021, 265, 109405. [Google Scholar] [CrossRef]
- Xiong, W.; Shen, C.Y.; Wu, Z.X.; Lu, H.S.; Yan, Y.R. A brief overview of known introductions of non-native marine and coastal species into China. Aquat. Invasions 2017, 12, 109–115. [Google Scholar] [CrossRef]
- Wang, H.; Xie, D.; Bowler, P.A.; Zeng, Z.F.; Xiong, W.; Liu, C.L. Non-indigenous species in marine and coastal habitats of the South China Sea. Sci. Total Environ. 2020, 759, 143465. [Google Scholar] [CrossRef]
- Xiong, W.; Sui, X.Y.; Liang, S.H.; Chen, Y.F. Non–native fresh water fish species in China. Rev. Fish Biol. Fish. 2015, 25, 651–687. [Google Scholar] [CrossRef]
- Lei, J.; Liao, Y.Y.; Tang, W.; Xie, D.; Wang, T.; Xiong, W.; Bowler, P.A. Fish biodiversity in Zhanjiang Mangroves National Nature Reserve, China. Turk. J. Zool. 2022, 46, 74–77. [Google Scholar] [CrossRef]
- Liang, S.C. Mangrove Resources of Guangxi and Their Sustainable Utilization. Mar. Sci. Bull. 1999, 18, 77–83. [Google Scholar]
- Loiola, M.; Cruz, I.C.S.; Lisboa, D.S.; Mariano–Neto, E.; Leao, Z.; Oliveira, M.D.M.; Kikuchi, R.K.P. Structure of marginal coral reef assemblages under different turbidity regime. Mar. Environ. Res. 2019, 147, 138–148. [Google Scholar] [CrossRef]
- Mumby, P.J.; Edwards, A.J.; Arias–Gonzalez, J.E.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. MMangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef]
- Faunce, C.H.; Serafy, J.E. Mangroves as fish habitat: 50 years of field studies. Mar. Ecol. Prog. Ser. 2006, 318, 1–18. [Google Scholar] [CrossRef]
- Vorsatz, L.D.; Pattrick, P.; Porri, F. Ecological scaling in mangroves: The role of microhabitats for the distribution of larval assemblages. Estuarine, Coast. Shelf Sci. 2021, 253, 107318. [Google Scholar] [CrossRef]
- Whitfield, A.K. The role of seagrass meadows, mangrove forests, saltmarshes and reedbeds as nursery areas and food sources for fishes in estuaries. Rev. Fish Biol. Fish. 2017, 27, 75–110. [Google Scholar] [CrossRef]
- Perry, D.; Staveley, T.A.; Gullström, M. Habitat Connectivity of Fish in Temperate Shallow-Water Seascapes. Front. Mar. Sci. 2018, 4, 440. [Google Scholar] [CrossRef]
- Irawan, A.; Supriharyono, S.; Hutabarat, J.; Ambariyanto, A. Seagrass beds as the buffer zone for fish biodiversity in coastal water of Bontang City, East Kalimantan, Indonesia. Biodiversitas, J. Biol. Divers. 2018, 19, 1044–1053. [Google Scholar] [CrossRef]
- Bastos, R.F.; Lippi, D.L.; Gaspar, A.L.B.; Yogui, G.T.; Frédou, T.; Garcia, A.M.; Ferreira, B.P. Ontogeny drives allochthonous trophic support of snappers: Seascape connectivity along the mangrove-seagrass-coral reef continuum of a tropical marine protected area. Estuarine, Coast. Shelf Sci. 2021, 264, 107591. [Google Scholar] [CrossRef]
- Mumby, P.J. Connectivity of reef fish between mangroves and coral reefs: Algorithms for the design of marine reserves at seascape scales. Biol. Conserv. 2006, 128, 215–222. [Google Scholar] [CrossRef]
- Serafy, J.E.; Shideler, G.S.; Araújo, R.J.; Nagelkerken, I. Mangroves Enhance Reef Fish Abundance at the Caribbean Regional Scale. PLoS ONE 2015, 10, e0142022. [Google Scholar] [CrossRef] [PubMed]
- Singkran, N.; Meixler, M.S. Influences of habitat and land cover on fish distributions along a tributary to Lake Ontario, New York. Landsc. Ecol. 2008, 23, 539–551. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y.R.; Ai, H.; Dong, L.N.; Li, N.N.; Li, X.; Gao, T.X. Large scale distribution patterns of taxonomic diversity of fish in coral reef waters, South China Sea. J. Fish. Sci. China 2011, 18, 619–628. [Google Scholar] [CrossRef]
- Ma, Y.L. Observation and Research on the Circulation in the Northern Part of the South China Sea; Dalian Ocean University: Dalian, China, 2014. [Google Scholar]
- Shi, F.S. Study on Fish Ecology in Dongzhaigang National Mangrove Reserve, Hainan; Xiamen University: Xiamen, China, 2005. [Google Scholar]
- Blaber, S.J.M. Mangrove sandfishes: Issues of diversity, dependence, and dogma. Bull. Mar. Sci. 2007, 80, 457–472. [Google Scholar]
- Lengyel, S.; Mester, B.; Szabolcs, M.; Szepesvary, C.; Szabo, G.; Polyak, L.; Boros, Z.; Mizsei, E.; Malnas, K.; Mero, T.O.; et al. Restoration for variability: Emergence of the habitat diversity paradigm in terrestrial ecosystem restoration. Restor. Ecol. 2020, 28, 1087–1099. [Google Scholar] [CrossRef]
- Nordhaus, I.; Hadipudjana, F.A.; Janssen, R.; Pamungkas, J. Spatio–temporal variation of microbenthic communities in the mangrove–fringed Segara Anakan lagoon, Indonesia, affected by anthropogenic activities. Reg. Environ. Chang. 2009, 9, 291–313. [Google Scholar] [CrossRef]
- Dias, R.M.; Tofoli, R.M.; da Silva, J.C.B.; Gomes, L.C.; Agostinho, A.A. Effects of habitat complexity on trophic interactions of three congeneric fish species. Aquat. Ecol. 2022, 56, 877–889. [Google Scholar] [CrossRef]
- Travers, M.J.; Potter, I.C.; Clarke, K.R.; Newman, S.J.; Hutchins, J.B. The inshore fish faunas over soft substrates and reefs on the tropical west coast of Australia differ and change with latitude and bioregion. J. Biogeogr. 2010, 37, 148–169. [Google Scholar] [CrossRef]
- Vaslet, A.; Bouchon–Navaro, Y.; Louis, M.; Bouchon, C. Fish assemblages in a mangrove shoreline lagoon of Guadeloupe (FWI): Spatial and temporal distribution patterns along environmental gradients. Cybium 2010, 34, 115–127. [Google Scholar]
- Wu, Y.T.; Ricklefs, R.E.; Huang, Z.J.; Zan, Q.J.; Yu, S.X. Winter temperature structures mangrove species distributions and assemblage composition in China. Glob. Ecol. Biogeogr. 2018, 27, 1492–1506. [Google Scholar] [CrossRef]
- Caetano, V.; Camana, M.; Dala–Corte, R.B.; Melo, A.S. Scale–sensitive stream slope drives nested fish trait–based diversity. Aquat. Ecol. 2021, 55, 1051–1063. [Google Scholar] [CrossRef]
- Pelaez, O.; Pavanelli, C.S. Environmental heterogeneity and dispersal limitation explain different aspects of β-diversity in Neotropical fish assemblages. Freshw. Biol. 2018, 64, 497–505. [Google Scholar] [CrossRef]
- Chen, C.W.; Xu, A.C.; Ding, P.; Wang, Y.P. The small-island effect and nestedness in assemblages of medium- and large-bodied mammals on Chinese reservoir land-bridge islands. Basic Appl. Ecol. 2019, 38, 47–57. [Google Scholar] [CrossRef]
- Londono, L.A.S.; Leal–Florez, J.; Blanco–Libreros, J. FLinking mangroves and fish catch: A correlational study in the southern Caribbean Sea (Colombia). Bull. Mar. Sci. 2020, 96, 415–429. [Google Scholar] [CrossRef]
- Sitorus, H.; Lesmana, I.; Tarigan, R. Relationship of mangrove density with fish diversity in the waters of mangrove area in Lubuk Ker tang Village, Langkat District of North Sumatera. Int. J. Fish Aquat. Stud. 2017, 5, 266–271. [Google Scholar]
- Patankar, V.; D’Souza, E.; Marathe, A. Latitude and live coral cover in dependently affect Chaetodontid and Pomacanthid fish community distribution in the Andaman and Nicobar archipelago, India. Mar. Biodivers. 2019, 49, 235–245. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Abdallah, M. Spatial distribution of fifty ornamental fish species on coral reefs in the Red Sea and Gulf of Aden. ZooKeys 2014, 367, 33–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lowe, J.R.; Payet, S.D.; Harrison, H.B.; Hobbs, J.P.A.; Hoey, A.S.; Taylor, B.M.; Sinclair–Taylor, T.H.; Pratchett, M.S. Regional versus latitudinal variation in the life-history traits and demographic rates of a reef fish, Centropyge bispinosa, in the Coral Sea and Great Barrier Reef Marine Parks, Australia. J. Fish Biol. 2021, 99, 1602–1612. [Google Scholar] [CrossRef]
- Payan–Alcacio, J.A.; DelaCruz–Aguero, G.; Cruz–Escalona, V.H.; Moncayo–Estrada, R. Fish communities in high-latitude mangrove in north-western Mexico. Acta Ichthyol. et Piscat. 2021, 51, 1–11. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Kim, H.J.; Watson, W.; DiLorenzo, E.; Sugihara, G. Climate–driven changes in abundance and distribution of larvae of oceanic fishes in the southern California region. Glob. Chang. Biol. 2009, 15, 2137–2152. [Google Scholar] [CrossRef]
- Zintzen, V.; Anderson, M.J.; Roberts, C.D.; Harvey, E.S.; Stewart, A.L. Effects of latitude and depth on the beta diversity of New Zealand fish communities. Sci. Rep. 2017, 7, 8081. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.M.; Smith, J.A.; Everett, J.D.; Verges, A.; Suthers, I.M. Latitudinal patterns in trophic structure of temperate reef-associated fishes and predicted consequences of climate change. Fish Fish. 2020, 21, 1092–1108. [Google Scholar] [CrossRef]
- Kulbicki, M.; Bozec, Y.M.; Green, A. Implications of biogeography in the use of butterflyfishes (Chaetodontidae) as indicators for Western and Central Pacific areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, S109–S126. [Google Scholar] [CrossRef]
- Roberts, M.B.; Jones, G.P.; McCormick, M.I.; Munday, P.L.; Neale, S.; Thorrold, S.; Robitzch, V.S.N.; Berumen, M.L. Homogeneity of coral reef communities across 8 degrees of latitude in the Saudi Arabian Red Sea. Mar. Pollut. Bull. 2016, 105, 558–565. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).