Simple Summary
The Upper Jurassic deposits of the Lastres Formation crop out on the Asturian coast (northwest of the Iberian Peninsula), in the so-called “The Dinosaur Coast”. This formation presents a high abundance of dinosaur remains and other vertebrates. Despite the deep knowledge about its fauna and environment, practically nothing is known about the plant communities that formed the landscape of the region at the end of the Jurassic. We present here the first palynological data of the Lastres Fm., identifying a rich and abundant palynological assemblage formed by more than 60 different taxa. The presence of some taxa with biostratigraphic value suggests a Kimmeridgian-Tithonian age for this formation. On the other hand, the botanical affinities of the taxa found indicate that the vegetation of the “The Dinosaur Coast” of Asturias would not be homogeneous, but would be formed by a mosaic of different plant communities that would adapt to the variety of environments present in the region. The presence of forest areas probably represented a protected environment as well as a food source for herbivorous dinosaurs. Analysis of charcoalified wood remains suggests that palaeofires were relatively recurrent in the study area.
Abstract
Abundant fossils of vertebrates (mainly footprints and bones of dinosaurs) and numerous invertebrates occur in the Upper Jurassic deposits of the Lastres Formation in the Asturias region, North of Spain. However, no palynological study has been published from this geological formation; therefore, much palaeoenvironmental and palaeoecological information is still unknown. In this study, a total of 62 morphospecies, belonging to 49 different morphogenera were identified, including pollen, spores, algae remains, fungi spores, dinoflagellates, foraminifera, and scolecodonts from four different locations on the Asturian coast. Spores are the dominant group of palynomorphs, both in diversity and abundance, contrasting with the minor diversity of pollen grains. The age of some key taxa indicates that the palynological assemblage cannot be older than the Kimmeridgian, suggesting a Kimmeridgian-Tithonian age. The botanical and environmental affinities of the pollen and spores indicate the presence of different plant assemblages, including plant communities from humid areas such as the margin of rivers and small freshwater ponds that were dominated by bryophytes and ferns, and a coastal plant community that would inhabit arid areas and would be dominated by gymnosperms and some pteridophytes. The SEM analyses of wood remains show the abundance of charcoalified remains suggesting that wildfires were usual in “The Dinosaur Coast” of Asturias during the Kimmeridgian.
Keywords:
palynology; plant communities; wildfires; Late Jurassic; “The Dinosaur Coast”; North Spain 1. Introduction
“The Dinosaur Coast” of Asturias in the Northwest of Spain extends over several kilometers of Jurassic deltaic deposits exposed along the Asturian coast. Its Upper Jurassic deposits, specifically those from the Lastres Formation, are a benchmark in the study of the coastal fauna of this period of time and an important touristic attraction in the region. The Lastres Formation has been widely studied both sedimentologically [1,2,3,4,5] and palaeontologically [6,7,8,9,10,11,12,13]. The Lastres Formation yields a rich, diverse, and abundant faunal assemblage. The fossils published so far include different groups of dinosaurs [10,11,12,13] but other vertebrate remains and tracks have been found, such as turtles [8], crocodylomorphs [3,9], or pterosaurs [13]. In addition, these deposits are also rich in invertebrates such as ammonites [6,14,15], bivalves [16], or ostracods [7,17].
This diversity of fossils of animals that characterizes the Lastres Formation contrasts with the very scarce knowledge about their plant communities. Few studies concerning palaeobotany of this formation have been published, including wood remains of conifers attributed to Protocupressinoxylon sp. [2] and to Protocupressinoxylon purbeckensis [18]. So far, no palynological studies have been published on the Lastres Formation.
Although the Lastres Formation is usually referred to as Kimmeridgian, its age is still controversial. Some biostratigraphical data have suggested an early Kimmeridgian age for the upper part of the Lastres Formation based on ammonites [6,14,15], but other authors considered a late Kimmeridgian age for the lower and middle part and a Tithonian age for the upper part of the formation based on both ammonites and ostracods [5,7,17]. Consequently, to solve this stratigraphic conundrum, it is necessary to provide new biostratigraphical data; in this context, palynostratigraphy could be a useful tool.
The references to Jurassic palynological studies from the Iberian Peninsula are scarce and mostly focused on Lower and Middle Jurassic deposits. In Portugal there are some studies from the Algarve Basin in Pliensbachian to Kimmeridgian outcrops [19,20,21], and from the Lusitanian Basin in Pliensbachian to Bajocian marine stratigraphic levels [22], in marine Pliensbachian-Toarcian deposits [23,24,25] and from the Callovian-Oxfordian boundary [26]. In addition, there are other studies focused on Upper Jurassic deposits from the Oxfordian of Leiria [27], the Oxfordian-Kimmeridgian of Porto de Mos [19], and from the Upper Oxfordian to Tithonian of the Lusitanian Basin [28].
In Spain, there are only a few palynological studies from Upper Jurassic deposits, including the palynology from the Bathonian-Oxfordian stratigraphic levels in the Iberian Range [20,29,30] and the Lower Kimmeridgian deposits from Jaen and Teruel provinces [31]. Recently, some palynological studies were focused on the J/K boundary in the Galve area from Teruel [32], and the Cameros Basin in Burgos [33]. More specifically, in the studied area, few palaeobotanical studies were performed: a preliminary palynological study of the Vega Fm. [34] and a recent macroflora discovery in the Lastres Fm. [35].
This scarcity of palynological studies in the studied area, especially for Upper Jurassic deposits, raises many questions about the evolution and distribution of plant communities in the Iberian Peninsula during the Late Jurassic. Our work represents the first palynological data from the Lastres Fm. of special interest due to its vertebrate fossil abundance, also providing new data on the knowledge of the plant communities of this period. Therefore, the main objectives of this work are: (1) to characterize the palynological assemblage from the Lastres Fm.; (2) to provide new biostratigraphic data improving the chronostratigraphic resolution of the formation; and (3) to discuss the palaeoecological and palaeoenvironmental implications of this new palynological assemblage.
2. Geographical and Geological Context
The Upper Jurassic sedimentary rocks of Asturias crop out along the coast between Gijón and Ribadesella (Figure 1 and Figure 2). The succession is more than 600 m thick, and consists of three lithostratigraphic units, which yielded an important sample of dinosaur footprints and skeletal remains (Vega, Tereñes, and Lastres formations, see Figure 2).
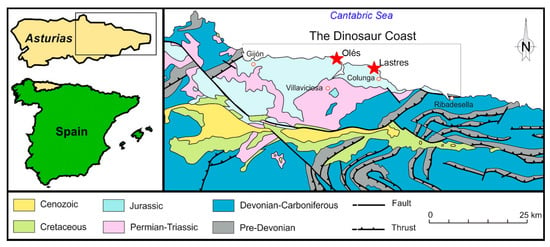
Figure 1.
Geological and geographical map from the Asturias coast. The studied locations (Oles and Lastres) are indicated with red stars.
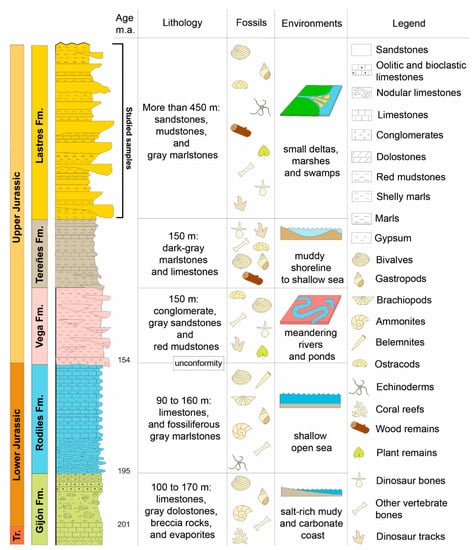
Figure 2.
Lithostratigraphical and palaeoenvironmental context of “The Dinosaur Coast” of Asturias.
The Lastres Formation represents a splendid Jurassic example of a fluvial-dominated lagoonal delta sourced mostly by high-sinuosity rivers. It is characterized by alternating grey sandstones, mudstones, marls and occasional conglomerate levels [3,36]. Although the main environments of the delta system are well represented along the formation, all the selected samples with palynomorphs are included mostly in the delta plain and locally in the delta abandonment facies.
The apparent absence of tides in this restricted lagoonal delta prevents the distinction between the upper and lower delta plain but it is possible to differentiate between a subaerial well-drained delta plain and a subaqueous poorly drained delta plain (Figure 3).
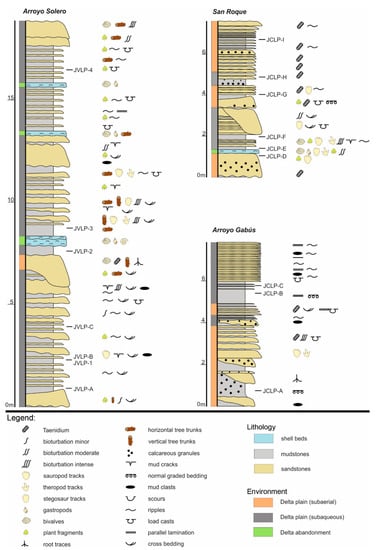
Figure 3.
Stratigraphic sections of the Lastres Formation indicating the locations of the samples.
The siliciclastic sedimentation was repeatedly interrupted by carbonate shell beds, representing the typical delta abandonment facies. These last facies (shell beds) formed during transgressive periods driven by regional or local increases in tectonic subsidence (normal faults related to contemporary rifting processes), eustatic sea-level rise or lessening/stopping in sediment supply (i.e., avulsion processes). During the Kimmeridgian-Tithonian, the Iberian Peninsula was a big island between the Euroasiatic, African and American Plates.
3. Material and Methods
For this work, three different sections from the Lastres Fm. were selected according to the ones that are more complete sedimentologically and chronologically: Arroyo Solero in Oles (Villaviciosa, Asturias) and Arroyo Gabús and San Roque, both in Lastres (Colunga, Asturias). For the palynological study, the samples were taken from levels with fine-grain sediment and rich in organic matter. A total of 16 palynological samples were collected, seven from Arroyo Solero (JVLP1, JVLP2, JVLP3, JVLP4, JVLPA, JVLPB, and JVLPC), three from Arroyo Gabús (JCLPA, JCLPB, JCLPC), and six from San Roque (JCLPD, JCLPE, JCLPF, JCLPG, JCLPH, and JCLPI).
The samples were analysed using standard palynological techniques [37] at the Palynology Laboratory of the Department of Marine Geosciences at the University of Vigo. Briefly, 10–15 g of material was crushed and treated with hydrochloric (HCl 10%) and hydrofluoric acid (HF 70%) in order to dissolve carbonates and remove silicate components, followed by application of hot 10% HCl to dissolve silica gel formed during HF treatment. The residue was sieved using a nylon filter with a mesh of 10 µm; the organic residue was washed with distilled water and sprayed on a coverslip using cellosize (hydroxyethyl cellulose), dried, and mounted on a microscope slide. For the SEM observation (Figure 4), the organic residue was dried and covered with gold. The palynological slides were observed and photographed under a Leica ICC50W (Leica Camera, Wetzlar, Alemania) optical microscope at 1000× magnification (Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9). Some palynomorphs and small wood remains present in the slides were re-examined under the Scanning Electron Microscope (SEM) JEOL JSM6010LA (JEOL Ldt., Tokyo, Japan) at CACTI (Centro de Apoio Científico-Tecnolóxico á Investigación, University of Vigo). The position of each illustrated palynomorph is given in the figure captions according to the “England Finder” graticule.
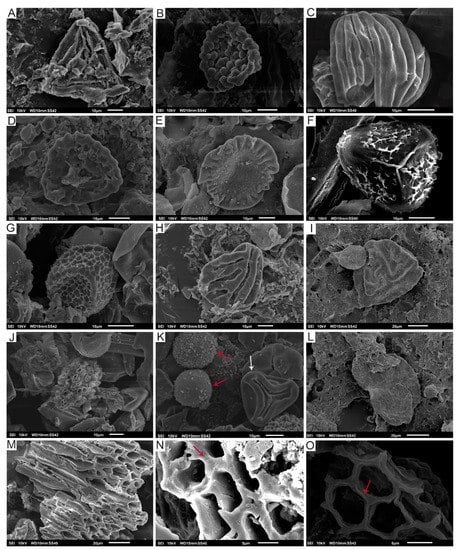
Figure 4.
Palynomorphs from the Lastres Fm. under SEM: (A) Cicatricosisporites sp., SEM-V1b-Lastres; (B) Leptolepidites sp., SEM-VA-Lastres; (C) Contignisporites cooksonii, SEM-V1-Lastres; (D) Klukisporites sp., SEM-CA4-Lastres; (E) Callialasporites dampieri, SEM-V1-Lastres; (F) cf. Camarazonosporites rudis, SEM-V1b-Lastres; (G) Retitriletes clavatoides, SEM-V1b-Lastres; (H) Striatella balmei, SEM-V3-Lastres; (I) Striatella scanica, SEM-CAb-Lastres; (J) Botryococcus sp., SEM-V1-Lastres; (K) Dictyophyllidites harrisii (white arrow); pirite (red arrows), SEM-V1-Lastres; (L) Pinuspollenites sp., SEM-CA3-Lastres; (M) Remains of wood with almost totally fused cell walls, SEM-V1-Lastres; (N) Detail of fused cell walls in wood (see red arrow), SEM-V1b-Lastres; (O) Unfused cell walls in wood (see red arrow), SEM-VB-Lastres.
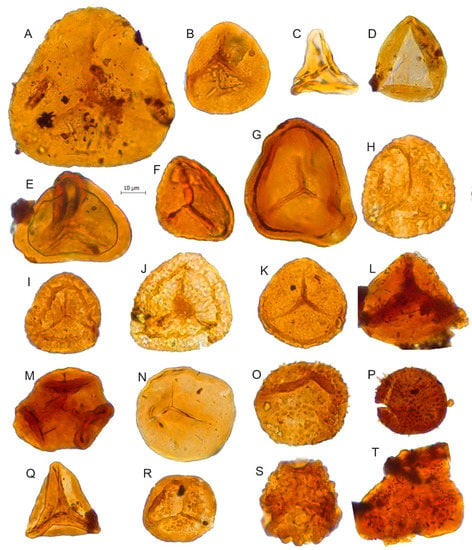
Figure 5.
Scale bar: 10 µm. (A) Cyathidites australis, JVLP-2-4-H160; (B) Cyathidites minor, JCLP-B-4-O270; (C) Gleicheniidites senonicus, JVLP-C-2-T372; (D) Deltoidospora sp., JVLP-A-1-J054; (E) Murospora sp., JVLP-B-2-C281; (F) Matonisporites equiexinus, JVLP-3-1-D510; (G) Matonisporites sp., JVLP-3-1-Q430; (H) cf. Camarazonosporites rudis, JVLP-3-1-J360; (I) Staplinisporites caminus, JVLP-3-1-G440; (J) Staplinisporites sp., JVLP-C-2-R280; (K) Stereisporites antiquasporites, JVLP-1-2-N470; (L) Biretisporites potoniaei, JVLP-A-1-N211; (M) Cibotiumspora jurienensis, JVLP-3-2-L240; (N) Todisporites minor, JVLP-3-2-F502; (O) Baculatisporites comaumensis, JVLP-1-2-H083; (P) Apiculatisporites sp., JVLP-4-1-L222; (Q) Dictyophyllidites, mortonii, JVLP-2-4-K370; (R) Nevesisporites vallatus, JVLP-3-1-G221; (S) Spore indeterminate, JVLP-B-2-F070; (T) Obtusisporites sp., JVLP-A-3-O431.
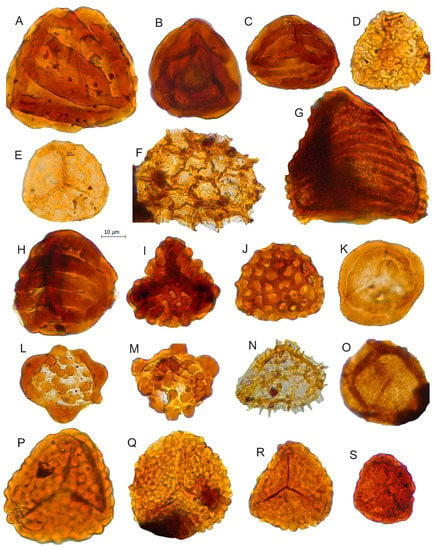
Figure 6.
Scale bar: 10 µm. (A) Striatella balmei, JVLP-2-2-V421; (B) Striatella seebergensis, JVLP-1-1-X413; (C) Striatella cooksoniae, JVLP-3-2-F332; (D) Retitriletes pseudoreticulatus, JVLP-B-2-M422; (E) Retitriletes sp., JVLP-2-4-M252; (F) cf. Retitriletes sp., JVLP-2-4-U373; (G) Contignisporites globulentus, JVLP-1-2-N340; (H) Contignisporites fornicatus, JVLP-3-2-M503; (I) Ischyosporites marburgensis, JVLP-2-2-B220; (J) Klukisporites lacunus, JVLP-3-2-K531; (K) Polycingulatisporites crenelatus, JVLP-3-1-W300; (L) Patellasporites distaverrucosus, JCLP-B-4-K402; (M) Patellasporites cf. distaverrucosus, JVLP-3-2-M380; (N) Neoraistriskia sp. JVLP-B-1-S420; (O) Osmundacidites wellmanii, JCLP-C-1-L400; (P) Leptolepidites verrucatus, JVLP-3-2-J261; (Q) Leptolepidites major, JVLP-B-2-H393; (R) Leptolepidites sp., JVLP-3-1-k410; (S) Leptolepidites crassibalteus, JVLP-C-1-G340.
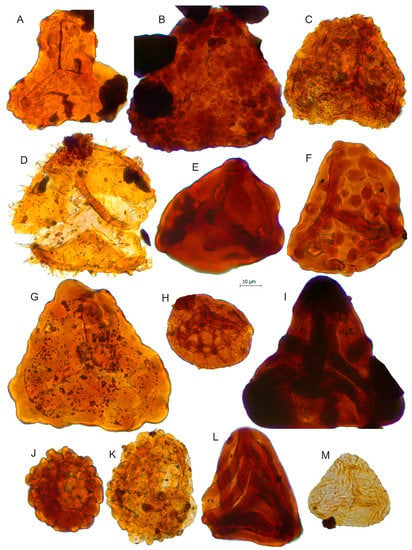
Figure 7.
Scale bar: 10 µm. (A) Impardecispora apiverrucata, JVLP-A-1-C114; (B) Impardecispora sp., JCLP-B-4-R260; (C) Concavissimisporites variverrucatus, JVLP-3-1-f464; (D) Pilosisporites trichopapillosus, JVLP-B-2-P401; (E) Trilobosporites sp.1, JVLP-3-1-H190; (F) Concavissimisporites montuosus, JVLP-3-2-J390; (G) Trilobosporites cf. canadensis, JVLP-1-2-L272; (H) Triporoletes sp.?; (I) Trilobosporites sp.2, JCLP-C-2-K350; (J) Cerebropollenites macroverrucosus, JVLP-3-2-G420; (K) Cerebropollenites cf. mesozoicus, JVLP-2-4-O340; (L) Cicatricosisporites cf. pseudotripartitus, JVLP-3-1-S331; (M) Cicatricosisporites sinuosus, JVLP-A-2-P051.
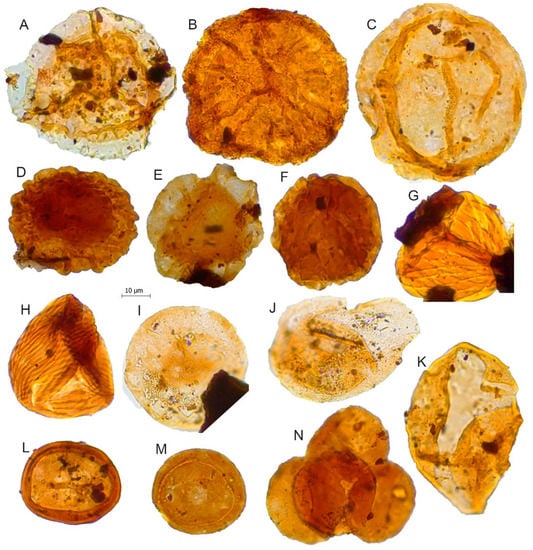
Figure 8.
Scale bar: 10 µm. (A) Aequitriradites spinulosus, JVLP-A-M260; (B) Densoisporites velatus, JVLP-A-EX-G271; (C) Araucariacites australis, JVLP-1-2-J494; (D) Callialasporites segmentatus, JCLP-E-1-U120; (E) Callialasporites trilobatus, JVLP-3-1-V240; (F) Callialasporites microvelatus, JVLP-3-1-K494; (G) Ruffordiaspora sp.1, JVLP-A-EX-Q121; (H) Ruffordiaspora australiensis, JCLP-C-1-D280; (I) Exesipollenites tumulus, JVLP-3-1-G391; (J) Perinopollenites elatoides, JVLP-3-1-G490; (K) Cycadopites follicularis, JVLP-2-4-K310; (L) Classopollis classoides, JCLP-E-2-N254; (M) Classopollis simplex, JVLP-3-2-D421; (N) Tetrad of Classopollis sp., JVLP-2-4-E210.
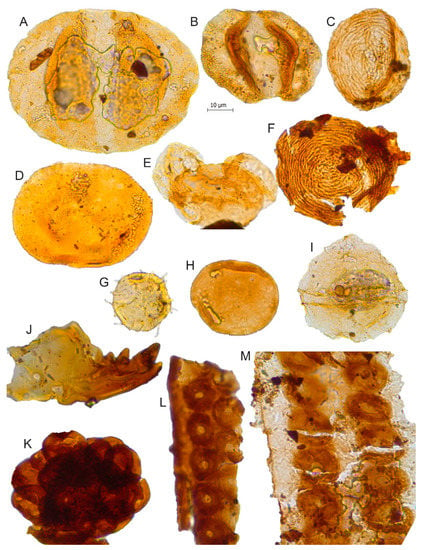
Figure 9.
Scale bar: 10 µm. (A) Alisporites grandis, JVLP-3-1-Q240; (B) Alisporites cf. similis, JVLP-3-1-D132; (C) Chomotriletes minor, JVLP-2-2-J132; (D) Tasmanites sp., JVLP-3-1-E460; (E) Pinuspollenites sp., JVLP-2-4-T262; (F) Chomotriletes fragilis, JVLP-A-1-L272; (G) Michrystridium sp.1, JVLP-1-1-F490; (H) Spheripollenites sp. JVLP-2-2-C352; (I) Gonyaulacaceae gen. et sp. indet., JVLP-3-2-M501; (J) Scolecodont (annelid jaw), JCLP-C-1-D370; (K) Cluster of spores, JVLP-C-2-F490; (L) Tracheid: Biseriate alternate pits (Araucarioxylon?), JCLP-E-1-C260; (M) Tracheid: Biseriate pits, JCLP-E-1-G431.
The relative dominance of the palynomorphs was analysed as an entire unity for all the samples because of the lack of sedimentary rate continuity and the frequent reworked levels in these sections. The palynomorph dominance data in this work, should be understood as qualitative more than quantitative.
The botanical affinities of the palynomorphs found in the Lastres Fm. are shown in the Table 1 after a selection of references where the relation between the palynomorph and its producer is indicated.

Table 1.
Palynomorphs identified in the Lastres Fm., with their botanical affinities.
4. Results
All the palynological samples yielded palynomorphs, showing a high diversity with different levels of preservation. The samples taken from Colunga municipality (JCLP) presented an acceptable richness and preservation, while the samples collected in the Villaviciosa municipality (JVLP) presented, in general, greater richness and preservation due to taphonomical processes. A total of 62 different (morpho)species were identified, belonging to at least 49 (morpho)genera (see Table 1). The different stratigraphic levels showed very similar compositions among them. This fact, in addition to the complexity to correlate the different sections of the Lastres Fm. based on its sedimentology, led us to consider all the studied palynological samples as a single assemblage.
The diversity of this palynoflora present sporo-pollen dominance, where the dominant groups are the spores of pteridophytes and bryophytes (representing half of the identified palynomorphs), while the other half corresponded to pollen from different groups of gymnosperms. Less than 5% of palynomorphs are non-pollen-palynomorphs (NPPs), including dinoflagellates, algae, and fungi spores. During the palynological analysis, different wood fragments, unidentifiable cuticle remains, and phytoclasts with different grades of opacity were also found.
The relative abundance of the taphonomy in this assemblage is dominated by Spheripollenites psilatus, which is usually linked to conifers. The second most abundant palynomorph was Leptolepidites followed by bisaccate pollen of the genus Alisporites, pollen from Classopollis spp., and trilete spores of Cyathidites spp. The other genera identified had a minor representation of relative abundance.
5. Discussion
5.1. Palynostratigraphical Implications
Most of the taxa present in the Lastres Fm. have a wide biostratigraphic range, and many of the genera found are very common and representative of palynological assemblages from the Upper Jurassic and Lower Cretaceous of Europe, such as Leptolepidites, Callialasporites, Classopollis, Concavissimisporites, Retitriletes, Striatella, Cyathidites, Contignisporites, or Dyctiophyllidites. Nevertheless, some of the genera, such as Cicatricosisportes, Ruffordiaspora, Pilosisporites, or Aequitriradites, have species with a more restricted range.
The first occurrences of Cicatricosisporites-Ruffordiaspora complex in the fossil record occur in the Upper Jurassic. Dettmann and Clifford [46] suggested that their first records could take place in the Oxfordian or even in the Callovian. However, the first clear appearances of Cicatricosisporites in Europe and Africa occur in the Kimmeridgian of Norway [64], Ethiopia [65], and Egypt [66]. The first occurrence of Cicatricosisporites in the Iberian Peninsula has been placed in Lower Kimmeridgian deposits from Segura de la Sierra in Jaen province, Spain [31].
The Cicatricosisporites-Ruffordiaspora morphospecies present in this palynological assemblage are C. cf. pseudotripartitus, C. sinuosus, and R. (C.) australiensis. The older occurrences of Ruffordiaspora australiensis were found in the Kimmeridgian of North America [67,68], the North Atlantic offshore [69], Egypt [70], and other parts of Europe [71,72]. In the Iberian Peninsula, it is also known from the Kimmeridgian deposits of Portugal [73]. The first occurrence of Cicatricosisporites pseudotripartitus is in the Kimmeridgian of Egypt [66] while, in the Iberian Peninsula, it is only found in Cretaceous deposits, more specifically in the Berriasian of the Cameros Basin in Northern Spain [33]. However, although the distal face of the specimen from the Lastres Formation (see Figure 7L) is compatible with C. pseudotripartitus, the proximal face is not observable, so we cannot be sure if it corresponds to this species, therefore we prefer to be cautious with its biostratigraphic utility. The presence of Cicatricosisporites sinuosus is a bit more problematic in the Upper Jurassic Lastres Formation since its presence is more common in the Cretaceous [74]. Currently, its first occurrence is in the Purbeck Group (Tithonian-Berriasian) of England, where the holotype of this species was found [75], so this record from Asturias would be the first record of this taxon in pre-Tithonian deposits.
The first appearance of Aequitriradites spinulosus was in the Upper Jurassic, and in Europe, the oldest record was found in the Tithonian of Romania [76]. However, in the Iberian Peninsula this taxon was only recorded in Cretaceous deposits, having its first occurrence in this region in the lowermost Berriasian of the Galve sub-Basin in the Teruel province [32] and the Early Berriasian of the Cameros Basin in Burgos province [33]. It was also frequently found along the Early Cretaceous in the Valanginian-Barremian of Burgos province [77], the Lower Valanginian of the NW Iberian offshore [78], the Barremian from Maestrazgo Basin [79] and Peñacerrada in Alava province [80], and also in the Hauterivian, Barremian and Aptian from Portugal [81,82]. Consequently, the presence of A. spinulosus in the Kimmeridgian strata of the Lastres Formation represents the oldest record of this species in Europe.
The oldest record of Impardecispora apiverrucata apparently occurs in the Bathonian of Iran (see plate 1, Figure 8 in [83]), nevertheless the quality of the illustration provided by these authors does not allow to check the correct identification of this palynomorph. Anyway, the first appearance in the Iberian Peninsula is from the Tithonian-Berriasian deposits of Porto Pinheiro and Vale Painho in Portugal [29,84] and in the Berriasian of the Cameros Basin in Spain [33]. In Europe, the presence of this morphospecie in pre-Tithonian deposits is rare, and its presence in the Lastres Fm. constitutes the oldest record in the Iberian Peninsula.
The genus Patellasporites is relatively common in the Cretaceous of the Iberian Peninsula [79,84,85], while the oldest record of this genus in this region comes from the Tithonian deposits of Portugal [28]. Therefore, the presence of P. distaverrucosus in the studied levels represents the oldest record of this genus in the Iberian Peninsula and the first Jurassic record for this morphospecies worldwide.
The oldest record of Pilosisporites trichopapillosus comes from the Upper Kimmeridgian of Canada [86,87], but this species only becomes common from the Berriasian onwards. In northern Europe, the oldest evidence of this species is in the Berriasian [71,88], and in the Iberian Peninsula its first occurrence was found in Vale Painho, Portugal [84,85].
Previous studies have indicated that the age of the Lastres Fm. is clearly Upper Jurassic, and the formation was assigned to the late Lower Kimmeridgian (Cymodoce chronozone) by Olóriz et al. [6] based on the presence of some ammonites. On the other hand, the middle part of the Lastres Fm. was referred to the Upper Kimmeridgian (Eudoxus chronozone), also with ammonites by Dubar and Mouterde [15] and Suárez-Vega [14]. Otherwise, Ramírez del Pozo [17] suggests a Portlandian (=late Tithonian) age for the upper part of this formation. Moreover, recent interpretations also suggest a late Kimmeridgian age for the lower and middle part of the formation and an early Tithonian age for its upper part based on the ostracod assemblages [7]. Additionally, González-Fernández et al. [5], based on correlation of sedimentary sequences, suggested a Kimmeridgian-early Tithonian age for the lower and middle part of the Lastres Fm. and a Tithonian age for the upper part, and the presence of Protocupressinoxylon purbeckensis in the Tereñes and Lastres formations is in tune with the Kimmeridgian-Tithonian age for these strata [18].
Most of the taxa found in the palynological assemblage of the Lastres Fm. deposits present a wide stratigraphic range that encompasses much of the Jurassic and Cretaceous periods. However, no evidence was found to suggest a Cretaceous age. The eventual presence of some taxa such as Cicatricosisporites cf. pseudotripartitus, C. sinuosus, R. (C.) australiensis, Aequitriradites spinulosus, Impardecispora apiverrucata, Patellasporites distaverrucosus, and Pilosisporites trichopapillosus, are not compatible with pre-Kimmeridgian deposits, having affinities with the classical Tithonian palynofloras (or relatively close to the Jurassic-Cretaceous boundary). However, the presence of ammonites in one of the studied sections [6] shows that the age of the lower part of the Lastres Fm., is probably late Early Kimmeridgian even though our palynological assemblage presents more Tithonian than Kimmeridgian affinities. The Lastres Fm. extends for tens of kilometres along the Asturian coast, so further palynological and biostratigraphic studies are necessary in new sections of this formation in order to make a more detailed interpretation of its age range.
5.2. Palaeoenvironment and Palaeoecology
This palynological assemblage from the Lastres Formation is mainly dominated by palynomorphs of continental origin (spores fundamentally). However, prasinophytes, dinoflagellate cysts, and scolecodonts probably of autochthonous origin, have also been found indicating that the depositional setting was a transitional environment with marine influence like a paralic sedimentary environment. This interpretation is consistent with a deltaic environment previously reported for the Lastres Fm. by other authors [3,36] that is in tune with the occasional presence of remains of open marine ammonites in the studied sections [6]. The possibility of an open marine depositional setting close to the coast is not consistent with this palynological assemblage, since palynological slides are plenty of well-preserved cuticles and wood remains with little transport. In addition, the presence of tree trunks preserved in situ in life position and also the presence of different remains of macroflora [2] reinforce this interpretation of a transitional (paralic) environment.
The quality of preservation of the palynomorphs is not homogeneous. This could be explained because some of them probably suffer long-distance transport representing allochthonous pollen/spores, which is the case with some poorly preserved taxa such as Pilosisporites and Aequitriradites, with affinities related to ferns and mosses, respectively [40]. These two groups of plants are less tolerant to salinity, living in more protected and continental environments. On the other hand, most of the bisaccate pollen grains present a deficient to bad preservation and they could be allochthonous in origin. Abbink [53] and Abbink et al. [52] argue that most of the bisaccate pollen (such as Alisporites) could have their origin in upland SEGs (sporomorph eco-group) which is consistent with previous interpretation since these upland areas would be located several kilometres away from the depositional setting.
On the other hand, in addition to the parautochthonous or allochthonous prasinophytes, dinoflagellate cysts, scolecodonts, and foraminiferal test lining, the presence of unseparated tetrads of Classopollis sp. is suggestive of a little transport from the parent-plant that is likely representative of the autochthonous flora. Classopollis, is a pollen commonly related to tree-size conifers belonging to the Cheirolepidiaceae family, that show xerophytic and halophytic adaptations inhabiting disturbed environments as they are coastal areas [52,55]. In particular, Volkheimer et al. [60] considered Classopollis from the Lajas Formation in the Middle Jurassic of the Neuquén Basin (Argentina) as a thermophilic coastal proxy related to extraordinary flooding in well-drained soils of the delta plain.
The mixture of taxa related to different botanical affinities and associated with different types of environments, such as marine-influenced brackish environments (dinoflagellate cyst, foraminiferal test linings, prasinophytes, etc.), coastal (Classopollis spp. was one of the more abundant palynomorphs with Araucariacites, as well as Callialasporites; Exesipollenites are also present), high-sinuosity rivers/lowlands (most of the fern and bryophyte spores; see Table 1) and uplands (Alisporites was the third most common genera in the Lastres assemblage) suggests that during the Late Jurassic the Asturian coast dominated by deltas would be composed of different subenvironments each of them dominated by different botanical communities. Taking into account previous sedimentological data and observations during the collection of the palynological samples, we have drawn an interpretation of the most probable distribution of the plant communities in the Asturian environments of the Upper Jurassic, based on the classification in SEGs (Sporomorphs Eco-groups; sensu [52,53]) of the different palynomorphs (Figure 10).

Figure 10.
Palaeoreconstruction of “The Dinosaur Coast” in the Kimmerdigian-Tithonian of Asturias, taking into account the new palaeobotanical data about the Lastres Fm. (Artist: Jose Luis Suso).
On the one hand, there are a group of palynomorphs that would be related to upland eco-groups, as would be the case of the bisaccate pollen such as Alisporites (the third most frequent genus in the assemblage) or Pinuspollenites. These palynomorphs present worse preservation than other taxa which could indicate an allochthonous origin, which would be consistent with upland areas [52,53] that were further away from the depositional setting. Most of these groups of bisaccate forms have been related to conifers of families Podocarpaceae and Pinaceae [41], which would inhabit areas of higher altitude.
Many of the taxa present in the Lastres Fm. show affinities with lowland SEG (e.g., Cicatricosisporites, Concavissimisporites, Cycadopytes, Contignisporites, Deltoidosora, Striatella, Gleicheniidites, Impardecispora, Ischyosporites, Matonisporites, Monosulcites, Osmundacidites, Perinopollenites, or Trilobosporites). These lowland communities probably would be vegetation close to freshwater swamps and ponds in flood plains, with combination of taxa adapted to drier and wetter environmental conditions [52,53], likely without influence of salt water. In the case of the Lastres Fm., these plant communities would be placed in the lower delta plain, which is an area with little marine influence dominated by swampy conditions and areas of freshwater. Most types of ferns need shady and humid environments to grow, and hence they would be located closer to the freshwater masses. However, other taxa of this SEG could be located in less humid areas of the lowland. For example, the ferns of the Family Matoniaceae (represented in this assemblage by both form-genera Dictyophyllidites and Matonisporites) currently inhabit the mountain slopes in the Malaysian Archipelago and present cuticles that make them resistant to differences of temperature during the day [89,90]. Nevertheless, the distribution of this family of ferns during the Mesozoic was wider and its possible habitats were more diverse [90]. It is also the case of the ferns of the family Gleicheniaceae, represented in the Lastres Fm. by the genus Gleicheniidites, which are resistant to direct sunlight. These ferns are currently common in subtropical regions [89]. Although most of the taxa of this type of plant community would correspond to pteridophytes (see botanical affinities in Table 1), woody plants from other groups such as Cycadophytes [41] or taxodiaceous conifers would also be common in Late Jurassic communities [52,91].
Some species related to river SEG would be placed associated with freshwater fluvial channels [52,53], such as Leptolepidites spp. (the second most abundant taxon), or Stereisporites and Staplinisporites, which are related to Bryophytes and Lycopodiaceae-Selaginellaceae [41]. These plant groups present high water requirements consistent with riverbank communities that offered constant humidity and were possibly periodically submerged [52,53].
Finally, in the Lastres Fm., the palynomorphs related to coastal environments are abundant (Coastal SEG), corresponding to pollen or spore plant producers that would grow close to the coast possibly supporting conditions of certain salinity. Taking into account the sedimentology of the study area, these plant communities were possibly located in the delta plain and the palynomorphs related to this SEG would be Classopollis (=Corollina), Callialasporites, Araucariacites, and Exesipollenites [52,53]. Exesipollenites presents affinities with the family Cupressaceae and both Callialasporites and Araucariacites have been related to Araucaraceae conifers [65,66,69,73]. Classopollis was one of the most abundant pollen grains, clearly related to Cheirolepidiaceae [63]. This is a group of abundant and diversified conifers in the Mesozoic, many of which would be adapted to coastal conditions and with tendency to establish extensive forests in tropical to subtropical lowland areas [63]. Although their ecological affinities are controversial, they have been widely related to thermophilus and drought-resistant shrubs and trees [52,53]. In the Lastres Fm., Cheirolepidiaceae was already known due to the presence of wood remains attributed to Protocupressinoxylon purbeckensis [18,92], a tree-like taxon (about 13 m in altitude) that has also been associated with coastal ecosystems and with a certain tolerance to salinity (spray or salty soils) [18,93].
On the other hand, the most abundant palynomorph, Spheripollenites, presents an ambiguous biological affinity [94,95], frequently assigned to gymnosperm pollen. It was related to the inner bodies of Cheirolepidiaceae-Cupressaceae pollen [52,53,96] while some authors have associated it with other families such as Cupressaceae [97] or Araucariaceae [74]. The presence of a large number of Spherinopollenites spp., as occurs in the Lastres Fm. together with a large number of Classopollis, was related in some cases to a more developed annual dry season [29,52,53,63,98]. The interpretation of a seasonal climate during the Kimmeridgian in the Asturias area is also supported by the presence of several palynomorphs linked with different climate and environmental conditions. These climatic interpretations are partially consistent with previous data for the underlying Lower Kimmeridgian Vega Formation based on palaeosoil studies. The sediments of Vega Fm. were deposited during a subhumid to semiarid seasonal climate [99], and these conditions are also compatible with the palynological assemblage found in the Lastres Fm. At the same time, this is in tune with the palaeoecological conditions observed for the Upper Jurassic of other regions in Northern Spain according to the palaeobotanical macro remains [100]. In addition, similarities in the fauna assemblages [99,101] and in the palaeoenvironmental conditions between the Upper Jurassic formations from Asturias and the Morrison Fm. (western North America) have been found [99]. In the Morrison Fm. and in the Vega Fm., a seasonally variable precipitation regime was interpretated, although the Morrison Formation would represent a drier and warmer environment [99].
Moreover, the abundant tracks and remains of giant plant-eating dinosaurs such as sauropods, stegosaurs, and ornithopods in the studied strata of the Lastres Fm. [3,13,101] suggest the presence of abundant and diverse vegetation. Therefore, the denser vegetation areas (possibly the plant communities associated with river channels and ponds or swamps in the most protected area of the delta; see Figure 10) served as food sources for these groups of dinosaurs.
5.3. Evidence of Wildfires
The detailed analysis of the samples in the scanning electron microscope (SEM) allowed us to identify the frequent presence of charcoalified wood remains. Small fragments showing homogenised cell walls (see Figure 4M,N) occur when the wood is exposed to the fire. Some of the remains are composed of secondary xylem with wood cells that present both empty cell lumina and walls with homogenised middle lamellas (Figure 4M) indicating high temperatures during the combustion process ranging from 220–230 °C [102] to 300–325 °C [103] depending on the different studies and the possible oxygen ingress on the fire system [104]. Nevertheless, some of the charcoalified remains still preserve a thin separation between lamellas (Figure 4M) while other records show middle lamellas that are not fused (Figure 4O) indicating a variety of regimes of wildfires in the Kimmeridgian palaeoenvironments of Asturias during the deposition of the Lastres Formation. In addition, the presence of these charcoalified remains in all the studies samples suggests that the wildfires were recurrent in this formation.
6. Conclusions
The studied sections from the Lastres Fm. in northwestern Spain reveal a very homogeneous palynofloral assemblage, in terms of diversity, along the different stratigraphical levels. A total of 62 morphospecies and 49 morphogenera of palynomorphs have been identified, including pteridophyte and bryophyte spores, gymnosperm pollen, acritarchs, dinoflagellate cysts, marine, and freshwater algae, scolecodonts, as well as cuticle and wood debris. The relative dominance of continental palynomorphs and the presence of some dinoflagellate cysts, Prasinophyceae, and scolecodonts suggest a transitional depositional setting, with an occasional marine influence, but with indicators of freshwater, compatible with interdistributary depositional environments. The age of some key taxa indicates that the palynological assemblage cannot be older than the Kimmeridgian, indicating a Kimmeridgian-Tithonian age. The botanical and environmental affinities of the pollen and spores suggest the presence of different vegetation, including plant communities in humid areas such as riverbanks (riparian) and small freshwater ponds (dominated by bryophytes and ferns) and a coastal plant community that would inhabit more arid areas (dominated by gymnosperms and some pteridophytes), where these masses of vegetation probably offered food and protection to some of the herbivorous dinosaurs of the Lastres Fm. The mixed climatic preferences of the studied taxa suggest a seasonal environment for the Kimmeridgian of Asturias, in tune with previous palaeontological and sedimentological studies. In addition, the dominance of some palynomorphs is indicative of arid and warm conditions at least during some periods or seasons. Finally, the SEM analyses of microscopical wood remains indicate the presence of wildfires during the Kimmeridgian in “The Dinosaur Coast” affecting the plant communities in this zone.
Author Contributions
Conceptualization, A.A.S., L.P. and J.C.G.-R.; methodology, A.A.S. and J.B.D.; validation, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D.; formal analysis, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D.; investigation, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D.; resources, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D.; data curation, A.A.S.; writing—original draft preparation, A.A.S.; writing—review and editing, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D.; visualization, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D.; supervision, A.A.S.; funding acquisition, A.A.S., L.P., I.R.-B., J.C.G.-R. and J.B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia, Innovación y Universidades’ (grant number PGC2018-094034-B-C22) and by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (grant number XDB26000000). A.A.S. and I.R.-B. are financially supported by the Galician Government (grants ED481A-2019/243 and ED481A-2020/175). A.A.S. was also supported by mobility grants from the Universidade de Vigo to take samples in the field. The financial support for L.P. and J.C.G.-R. was provided by Sociedad Pública de Gestión y Promoción Turística y Cultural del Principado de Asturias.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The palynological slides are stored in the Department of Marine Geosciences of the Vigo University (Spain).
Acknowledgments
We want to thank Denise Pons, Jean Dejax and Dario de Franceschi from Muséum National d’Histoire Naturelle de Paris and Sorbonne Université and Uxue Villanueva from UNAM for their advice and orientation in the identification of some palynomorphs and in the interpretation of wood remains and to Jose Luis Suso for the artistic reconstruction of this Jurassic landscape. We also thank the project PGC2018-094034-B-C22 of the ‘‘Ministerio de Ciencia, Innovación y Universidades’’ of Spain and the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB26000000) for their support. Artai Santos and Iván Rodríguez-Barreiro were awarded with a fellowship from the Galician Government (Department of Culture, Education and University Planning) supported by the European Social Fund (Refs.: ED481A-2019/243 and ED481A-2020/175, respectively). The financial support for Laura Piñuela and Jose Carlos García-Ramos was provided by Sociedad Pública de Gestión y Promoción Turística y Cultural del Principado de Asturias. We would like to thank the editor and three reviewers for their thoughtful comments and efforts toward improving this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valenzuela, M.; García-Ramos, J.C.; Suárez de Centi, C. The Jurassic sedimentation in Asturias (N Spain). Trab. Geol. 1986, 16, 121–132. [Google Scholar]
- Valenzuela, M.; Díaz-González, T.E.; Gutiérrez-Villarias, M.G.; Suárez de Centi, C. La Fm. Lastres del Kimmeridgiense de Asturias: Sedimentología y estudio paleobotánico inicial. Cuad. Geol. Ibér. 1998, 24, 141–172. [Google Scholar]
- García-Ramos, J.C.; Piñuela, L.; Lires, J. Atlas del Jurásico de Asturias; Ediciones Nobel: Oviedo, Spain, 2006. [Google Scholar]
- García-Ramos, J.C.; Piñuela, L.; Uzqueda, H.; Poblet, J.; Bulnes, M.; Alonso, J.L.; Suárez-Vega, L.C. Travertinos ricos en oncoides asociados a paleomanantiales y lagos efímeros próximos a fallas sinsedimentarias en el Jurásico Superior de Asturias. In Proceedings of the Comunicaciones del V Congreso del Jurásico de España, Museo del Jurásico de Asturias, Colunga, Spain, 8–11 September 2010; pp. 83–91. [Google Scholar]
- González-Fernández, B.; Menéndez-Casares, E.; Vicedo, V.; Aramburu, C.; Caus, E. New insights about the Upper Jurassic—Lower Cretaceous sedimentary successions from Asturias (NW Iberian Peninsula). J. Iber. Geol. 2014, 40, 409–430. [Google Scholar] [CrossRef]
- Olóriz, F.; Valenzuela, M.; Garcia-Ramos, J.C.; Suárez de Centi, C. The first record of the genus Eurasenia (Ammonitina) from the Upper Jurassic of Asturias (Northern Spain). Geobios 1988, 21, 741–748. [Google Scholar] [CrossRef]
- Schudack, M.; Schudack, U. New biostratigraphical data for the Upper Jurassic of Asturias (northern Spain) based on Ostracoda. Rev. Esp. Micropaleontol. 2002, 34, 1–18. [Google Scholar]
- Avanzini, M.; García-Ramos, J.C.; Lires, J.; Menegon, M.; Piñuela, L.; Fernández, L.A. Turtle tracks from the Late Jurassic of Asturias, Spain. Acta Palaeontol. Pol. 2005, 50, 743–755. [Google Scholar]
- Avanzini, M.; Piñuela, L.; Ruiz-Omeñaca, J.I.; García-Ramos, J.C. The crocodile track Hatcherichnus, from the Upper Jurassic of Asturias (Spain). NM Mus. Nat. Hist. Sci. Bull. 2010, 51, 89–92. [Google Scholar]
- Avanzini, M.; Piñuela, L.; García-Ramos, J.C. Late Jurassic footprints reveal walking kinematics of theropod dinosaurs. Lethaia 2011, 45, 238–252. [Google Scholar] [CrossRef]
- Ruiz-Omeñaca, J.I.; Piñuela, L.; García-Ramos, J.C. Una vértebra de un pequeño ornitópodo (Dinosauria: Ornithischia) del Kimmeridgiense (Formación Lastres) de Tazones (Villaviciosa, Asturias). Geogaceta 2007, 42, 83–86. [Google Scholar]
- Ruiz-Omeñaca, J.I.; Piñuela, L.; García-Ramos, J.C. El primer diente de ornitópodo del Jurásico Superior de España (Asturias). Geogaceta 2010, 48, 83–86. [Google Scholar]
- Piñuela Suárez, L. Huellas de dinosaurios y otros reptiles del Jurásico Superior de Asturias. Ph.D. Thesis, Department of Geology, University of Oviedo, Oviedo, Spain, 2015. [Google Scholar]
- Suárez Vega, L.C. Estratigrafía del Jurásico de Asturias. Cuad. Geol. Ibér. 1974, 3, 1–368. [Google Scholar]
- Dubar, G.; Mouterde, R. Extension du Kimméridgien marin dans les Asturies depuis Ribadesella jusqu’à Gijón. C. R. Acad. Sci. París Sér. D 1957, 244, 99–101. [Google Scholar]
- Delvene, G.; Munt, M.C.; Piñuela, L.; García-Ramos, J.C. New Unionida (Bivalvia) from the Kimmeridgian (Late Jurassic) of Asturias, Spain, and their palaeobiogeographical implications. Pap. Palaeontol. 2016, 2, 265–285. [Google Scholar] [CrossRef]
- Ramírez del Pozo, J.R. Bioestratigrafía y microfacies del Jurásico y Cretácico del Norte de España (región Cantábrica). Acta Geol. Hisp. 1969, 4, 49–59. [Google Scholar]
- Philippe, M.; Billon-Bruyat, J.P.; García-Ramos, J.C.; Bocat, L.; Gomez, B.; Piñuela, L. New occurrences of the wood Protocupressinoxylon purbeckensis Francis: Implications for terrestrial biomes in southwestern Europe at the Jurassic/Cretaceous boundary. Palaeontology 2010, 53, 201–214. [Google Scholar] [CrossRef]
- Mohr, B.A.R.; Schmidt, D. The Oxfordian/Kimmeridgian boundary in the region of Porto de Mós (Central Portugal): Stratigraphy, facies and palynology. N. Jb. Geol. Paläontol. Abh. 1988, 176, 245–267. [Google Scholar]
- Smelror, M. Biogeography of Bathonian to Oxfordian (Jurassic) dinoflagellates: Arctic, NW Europe and circum-Mediterranean regions. Palaeogeogr. Palaeoclimtol. Palaeoecol. 1993, 102, 121–160. [Google Scholar] [CrossRef]
- Borges, M.E.N.; Riding, J.B.; Fernandes, P.; Pereira, Z. The Jurassic (Pliensbachian to Kimmeridgian) palynology of the Algarve Basin and the Carrapateira outlier, southern Portugal. Rev. Palaeobot. Palynol. 2011, 163, 190–204. [Google Scholar] [CrossRef]
- Davies, E.H. The miospore and dinoflagellate cyst oppel-zonation of the Lias of Portugal. Palynology 1985, 9, 105–132. [Google Scholar] [CrossRef]
- Palliani, R.B.; Riding, J.B. Biostratigraphy, provincialism and evolution of European Early Jurassic (Pliensbachian to early Toarcian) dinoflagellate cysts. Palynology 2003, 27, 179–214. [Google Scholar] [CrossRef]
- Oliveira, L.C.V.; Dino, R.; Duarte, L.V.; Perilli, N. Calcareous nannofossils and palynomorphs from Pliensbachian–Toarcian boundary in the Lusitanian Basin, Portugal. Rev. Bras. Paleontol. 2007, 10, 5–16. [Google Scholar] [CrossRef]
- Barrón, E.; Comas-Rengifo, M.J.; Duarte, L.V. Palynomorph succession of the Upper Pliensbachian-Lower Toarcian of the Peniche section (Portugal). Comun. Geol. 2013, 100, 55–61. [Google Scholar]
- Barrón, E.; Azerêdo, A.C. Palynology of the Jurassic (Callovian-Oxfordian) succession from Pedrógão (Lusitanian Basin, Portugal). Palaeoecological and palaeobiogeographical aspects. N. Jb. Geol. Paläont. Abh. 2003, 227, 259–286. [Google Scholar] [CrossRef]
- van Erve, A.; Mohr, B. Palynological investigations of the Late Jurassic microflora from the vertebrate locality Guimarota coal mine (Leiria, Central Portugal). N. Jb Geol. Paläont. Mh. 1988, 4, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L. Upper Jurassic (Upper Oxfordian–Tithonian) palynostratigraphy from the Lusitanian Basin (Portugal). Mem. Acad. Cienc. Lisboa Classe Sci. 1998, 37, 49–77. [Google Scholar]
- Mohr, B.A.R. New palynological information on the age and environment of Late Jurassic and Early Cretaceous vertebrate localities of the Iberian Peninsula (eastern Spain and Portugal). Berl. Geowiss. Abh. 1989, 106, 291–301. [Google Scholar]
- Smelror, M.; Århus, N.; Meléndez, G.; Lardies, M.D. A reconnaissance study of Bathonian to Oxfordian (Jurassic) dinoflagellates and acritarchs from the Zaragoza region (NE Spain) and Figueira da Foz (Portugal). Rev. Esp. Micropaleontol. 1991, 23, 47–82. [Google Scholar]
- Van Erve, A.W.; Besems, R.E.; Love, C.F. A palynological investigation of some Lower Kimmeridgian deposits from Spain. J. Micropalaeontology 1988, 7, 217–232. [Google Scholar] [CrossRef]
- Santos, A.A.; Villanueva-Amadoz, U.; Royo-Torres, R.; Sender, L.M.; Cobos, A.; Alcalá, L.; Diez, J.B. Palaeobotanical records associated with the first dinosaur defined in Spain: Palynostratigraphy, taxonomy and palaeoenvironmental remarks. Cretac. Res. 2018, 90, 318–334. [Google Scholar] [CrossRef]
- Rodríguez-Barreiro, I.; Santos, A.A.; Arribas, M.E.; Mas, R.; Arribas, J.; Villanueva-Amadoz, U.; Torcida, F.; Diez, J.B. The Jurassic–Cretaceous transition in the West Cameros Basin (Tera Group, Burgos, Spain): Sedimentological and palynostrati-graphical insights. Cretaceous Res. 2022, 139, 105300. [Google Scholar] [CrossRef]
- Barrón, E. Las sucesiones margo-calcáreas marinas del Jurásico Inferior y las series fluviales del Jurásico Superior. Acantilados de la playa de Vega (Ribadesella). Guía de campo (Excursión A). In Proceedings of the V Congreso del Jurásico de España, Colunga, Spain, 8–11 September 2010. [Google Scholar]
- Santos, A.A.; Sender, L.M.; Piñuela, L.; García-Ramos, J.C.; Diez, J.B. First evidence of Ricciaceae in the Jurassic of the Iberian Peninsula (Asturias, NW Spain): Ricciopsis asturicus sp. nov. Bot. Lett. 2022, 169, 557–567. [Google Scholar] [CrossRef]
- García-Ramos, J.C.; Piñuela, L.; Lires, J. Guía del Jurásico de Asturias. Rutas por los Yacimientos de Huellas de Dinosaurios; Zinco Comunicación: Gijón, Spain, 2004. [Google Scholar]
- Wood, G.D.; Gabriel, A.M.; Lawson, J.C. Palynological techniques-processing and microscopy. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists: Dallas, TX, USA, 1996; pp. 29–50. [Google Scholar]
- Cookson, I.C.; Dettmann, M.E. Cretaceous “Megaspores” and a Closely Associated Microspore from the Australian Region. Micropaleontology 1958, 4, 39. [Google Scholar] [CrossRef]
- Cookson, I.C.; Dettmann, M.E. Reappraisal of the Mesozoic microspore genus Aequitriradites. Palaeontology 1961, 4, 425–427. [Google Scholar]
- Schneider, A.C.; Heimhofer, U.; Heunisch, C.; Mutterlose, J. From arid to humid–The Jurassic–Cretaceous boundary interval in northern Germany. Rev. Palaeobot. Palynol. 2018, 255, 57–69. [Google Scholar] [CrossRef]
- Filatoff, J. Jurassic palynology of the Perth Basin, Western Australia. Palaeontogr. Abt. B 1975, 154, 1–120. [Google Scholar]
- Hubbard, R.N.L.B.; Boulter, M.C. Mid Mesozoic floras and climates. Palaeontology 1997, 40, 43–70. [Google Scholar]
- Hubbard, R.N.L.B.; Boulter, M.C. Phytogeography and paleoecology in western Europe and eastern Greenland near the Triassic-Jurassic boundary. Palaios 2000, 15, 120–131. [Google Scholar] [CrossRef]
- Delcourt, A.F.; Sprumont, G. Les spores et grains de pollen du Wealdien du Hainaut. Mem. Sóc. Belge Géol. 1955, 4, 1–73. [Google Scholar]
- Galloway, J.M.; Tullius, D.N.; Evenchick, C.A.; Swindles, G.T.; Hadlari, T.; Embry, A. Early Cretaceous vegetation and climate change at high latitude: Palynological evidence from Isachsen Formation, Arctic Canada. Cretac. Res. 2015, 56, 399–420. [Google Scholar] [CrossRef]
- Dettmann, M.E.; Clifford, H.T. Phylogeny and biogeography of Ruffordia, Mohria and Anemia (Schizaeaceae) and Ceratopteris (Pteridaceae): Evidence from in situ and dispersed spores. Alcheringa 1992, 16, 269–314. [Google Scholar] [CrossRef]
- Potonié, R. Synopsis der Gattungen der Sporae dispersae: III. Teil: Nachträge Sporites, Fortsetzung Pollenites mit Generalregister zu Teil I–III; Amt für Bodenforschung: Hannover, Germany, 1960; Volume 39. [Google Scholar]
- Potonié, R.; Gelletich, J. Über Pterodophyten-Sporen einer eozänen Braunkohle aus Dorog in Ungarn. Sipz-ber. Ges. Naturf. Fr. Berlin 1933, 33, 517–528. [Google Scholar]
- Cittert, J.V.K.-V.; Van Der Burgh, J. The flora from the Kimmeridgian (upper jurassic) of Culgower, Sutherland, Scotland. Rev. Palaeobot. Palynol. 1989, 61, 1–51. [Google Scholar] [CrossRef]
- Cittert, J.H.V.K.-V. A review of the matoniaceae based on in situ spores. Rev. Palaeobot. Palynol. 1993, 78, 235–267. [Google Scholar] [CrossRef]
- McArthur, A.D.; Jolley, D.W.N.; Hartley, A.J.; Archer, S.G.; Lawrence, H.M. Palaeoecology of syn-rift topography: A Late Jurassic footwall island on the Josephine Ridge, Central Graben, North Sea. Palaeogeogr. Palaeoclim. Palaeoecol. 2016, 459, 63–75. [Google Scholar] [CrossRef]
- Abbink, O.A.; Van Konijnenburg-Van Cittert, J.H.A.; Visscher, H. A sporomorph ecogroup model for the Northwest European Jurassic-Lower Cretaceous: Concepts and framework. Neth. J. Geosci. 2004, 8, 17–31. [Google Scholar]
- Abbink, O.A. Palynological Investigations in the Jurassic of the North Sea Region; LPP Contribution Series 8; LPP Foundation: Utrecht, The Netherlands, 1998. [Google Scholar]
- Groot, J.J.; Groot, C.R. Plant microfossils from Aptian, Albian and Cenomanian deposits of Portugal. Com. Servs. Geol. Port. 1961, 46, 133–171. [Google Scholar]
- Balme, B.E. Fossil in situ spores and pollen grains: An annotated catalogue. Rev. Palaeobot. Palynol. 1995, 87, 81–323. [Google Scholar] [CrossRef]
- Potonié, R. Versuch der Einordnung der fossilen Sporae dispersae in das phylogenetische System der Pflanzenfamilien: I. Teil Thallophyta bis Gnetales: II. Teil Angiospermae. Forsch. Landes Nordrh.-Westfal. 1967, 1761, 1–130. [Google Scholar]
- Filatoff, J.; Price, P.L. A pteridacean spore lineage in the Australian Mesozoic. Mem. Ass. Austral. Palaeontol. 1988, 5, 89–124. [Google Scholar]
- Playford, G. Palynology of Lower Cretaceous (Swan River) strata of Saskatchewan and Manitoba. Palaeontology 1971, 14, 533–565. [Google Scholar]
- Kvaček, J.; Mendes, M.M. Callialastrobus sousai gen. et sp. nov.; a new araucariaceous pollen cone from the Early Cretaceous of Catefica (Lusitanian Basin, western Portugal) bearing Callialasporites and Araucariacites pollen. Rev. Palaeobot. Palynol. 2020, 283, 104313. [Google Scholar] [CrossRef]
- Volkheimer, W.; Quattrocchio, M.E.; Cabaleri, N.G.; Narváez, P.L.; Rosenfeld, U.; Scafati, L.; Melendi, D.L. Environmental and climatic proxies for the Cañadón Asfalto and Neuquén basins (Patagonia, Argentina): Review of Middle to Upper Jurassic continental and near coastal sequences. Rev. Bras. Paléontol. 2015, 18, 71–82. [Google Scholar] [CrossRef]
- Van Konijnenburg-Cittert, J.H. Dicksoniaceous spores in situ from the Jurassic of Yorkshire, England. Rev. Palaeobot. Palynol. 1989, 61, 273–301. [Google Scholar] [CrossRef]
- Dejax, J.; Pons, D.; Yans, J. Palynology of the dinosaur-bearing Wealden facies in the natural pit of Bernissart (Belgium). Rev. Palaeobot. Palynol. 2007, 144, 25–38. [Google Scholar] [CrossRef]
- Alvin, K. Cheirolepidiaceae: Biology, structure and paleoecology. Rev. Palaeobot. Palynol. 1982, 37, 71–98. [Google Scholar] [CrossRef]
- Thusu, B.; Vigran, J.O. Middle–Late Jurassic (Late Bathonian–Tithonian) palynomorphs. J. Micropalaeontol. 1985, 4, 113–129. [Google Scholar] [CrossRef]
- Santos, A.A.; Jain, S.; Diez, J.B. Upper Jurassic palynology from the Blue Nile Basin (Ethiopia). Rev. Palaeobot. Palynol. 2020, 285, 104361. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Ela, N.A.; Kholeif, S. Palynostratigraphy of Jurrasic to Lower Cretaceous sequences from the Eastern Desert of Egypt. J. Afr. Earth Sci. 2001, 32, 269–297. [Google Scholar] [CrossRef]
- Brideaux, W.W.; Fisher, M.J. Upper Jurassic-Lower Cretaceous dinoflagellate assemblages from Arctic Canada. Geol. Surv. Can. Bull. 1976, 259, 1–53. [Google Scholar]
- Williams, G.L. Dinoflagellate and spore stratigraphy of the Mesozoic-Cenozoic, offshore eastern Canada. Geol. Surv. Can. Pap. 1975, 74, 107–161. [Google Scholar]
- Gradstein, F.M.; Williams, G.L.; Jenkins, W.A.M.; Ascoli, P. Mesozoic and Cenozoic stratigraphy of the Atlantic continental margin, eastern Canada. Canada’s Continental Margins and Offshore Petroleum Exploration. Mem 1975, 4, 103–131. [Google Scholar]
- Schrank, E. Palaeozoic and Mesozoic palynomorphs from northeast Africa (Egypt and Sudan) with special reference to Late Cretaceous pollen and dinoflagellates. Berl. Geowiss. Abh. Reihe A Geol. Paläontologie 1987, 75, 249–310. [Google Scholar]
- Dörhöfer, G.; Norris, G. Discrimination and correlation of highest Jurassic and lowest Cretaceous terrestrial palynofloras in north-west Europe. Palynology 1977, 1, 79–93. [Google Scholar] [CrossRef]
- Higgs, K.T.; Jones, G.L. Palynological evidence for Mesozoic karst at Piltown, Co. Kilkenny. Proc. Geol. Assoc. 2000, 111, 355–362. [Google Scholar] [CrossRef]
- Riley, L.A. A palynological investigation of Upper Jurassic basal Cretaceous sediments from England, France and Iberia. Ph.D. Thesis, The Open University, Milton Keynes, UK, 1975. [Google Scholar]
- Legrand, J.; Pons, D.; Nishida, H.; Yamada, T. Barremian palynofloras from the Ashikajima and Kimigahama formations (Choshi Group, Outer Zone of south-west Japan). Geodiversitas 2011, 33, 87–135. [Google Scholar] [CrossRef]
- Hunt, C.O. Miospores from the Portland Stone Formation and the power part of the Purbeck Formation (Upper Jurassic/Lower Cretaceous) from Dorset, England. Pollen Spores 1985, 27, 419–451. [Google Scholar]
- Avram, E.; Szasz, L.; Antonescu, E.; Baltreš, A.; Iva, M.; Melinte, M.; Neagu, T.; Rādan, S.; Tomescu, C. Cretaceous terrestrial and shallow marine deposits in northern South Dobrogea (SE Rumania). Cretac. Res. 1993, 14, 265–305. [Google Scholar] [CrossRef]
- Villanueva-Amadoz, U.; Diez, J.B.; Pérez-Arlucea, M.; Bercovici, A.; Cascales-Miñana, B.; Ferrer, J.; Huerta, P.; Sánchez-Pellicer, R.; Sender, L.M.; Torcida Fernández-Baldor, F. Determinación de la edad y deducciones paleoecológicas de los yacimientos de dinosaurios de Vallazmorra (Burgos) en base a datos palinológicos. In Proceedings of the Actas de las V Jornadas Internacionales sobre Paleontología de Dinosaurios y su Entorno, Salas de los Infantes, Burgos, Spain, 16–18 September 2012; pp. 207–214. [Google Scholar]
- Taugourdeau-Lantz, J. Stratigraphic implications of Early Cretaceous spores and pollen grains at Holes 638B, 638C, and 641C, Leg 103, off the Iberian margin, eastern North Atlantic. In Proceedings of the Ocean Drilling Program, Scientific Results; Boillot, G., Winterer, E.L., Meyer, A.W., Audrey, W., Applegate, J., Baltuck, M., Bergen, J.A., Comas, M.C., Davies, T.A., Dunham, K.W., et al., Eds.; Ocean Drilling Program: College Station, TX, USA, 1988; Volume 103, pp. 419–428. [Google Scholar]
- Villanueva-Amadoz, U.; Santisteban, C.; Santos-Cubedo, A. Age determination of the Arcillas de Morella Formation (Maestrazgo Basin, Spain). Hist. Biol. 2015, 27, 389–397. [Google Scholar] [CrossRef]
- Barrón, E.; Comas-Rengifo, M.J.; Elorza, L. Contribuciones al estudio palinológico del Cretácico Inferior de la Cuenca Vasco-Cantábrica: Los afloramientos ambarígenos de Peñacerrada (España). Coloq. Paleontol. 2001, 52, 135–156. [Google Scholar]
- Mendes, M.M.; Barrón, E.; Dinis, P.; Rey, J.; Batten, D.J. A new palynoflora from upper Barremian–lower Aptian rocks at Casal do Borracho, Torres Vedras, western Portugal, and its palaeoecological significance. Cretaceous Res. 2018, 90, 363–374. [Google Scholar] [CrossRef]
- Mendes, M.M.; Polette, F.; Cunha, P.P.; Dinis, P.; Batten, D.J. A new Hauterivian palynoflora from the Vale Cortiço site (central Portugal), and its palaeoecological implications for western Iberia. Acta Palaeobot. 2019, 59, 215–228. [Google Scholar] [CrossRef]
- Sajjadi, F.; Hashemi, H.; Dehbozorgi, A. Middle Jurassic palynomorphs of the Kashafrud Formation, Koppeh Dagh Basin, Northeastern Iran. Micropaleontology 2007, 53, 391–408. [Google Scholar] [CrossRef][Green Version]
- Mendes, M.M.; Dinis, J.; Pais, J.; Friis, E.M. Early cretaceous flora from Vale Painho (Lusitanian basin, western Portugal): An integrated palynological and mesofossil study. Rev. Palaeobot. Palynol. 2011, 166, 152–162. [Google Scholar] [CrossRef]
- Rodríguez-Barreiro, I.; Villanueva-Amadoz, U.; Santos, A.A.; Diez, J.B. Palynostratigraphical dating of the Lower Cretaceous Peñaferruz Formation, San Pedro de Antromero Beach (Asturias Region, northwestern Iberian Peninsula). Geobios 2018, 51, 579–589. [Google Scholar] [CrossRef]
- Bujak, J.P.; Williams, G.L. Jurassic palynostratigraphy of offshore eastern Canada. In Developments in Palaeontology and Stratigraphy; Swain, F.M., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; Volume 6, pp. 321–339. [Google Scholar]
- Barss, M.S.; Bujak, J.P.; Williams, G.L. Palynological zonation and correlation of sixty-seven wells, eastern Canada. Geol. Surv. Can. Pap. 1979, 78, 1–24. [Google Scholar]
- Norris, G. Palynology of the Jurassic-cretaceous boundary in southern England. Geosci. Man 1970, 1, 57–65. [Google Scholar] [CrossRef]
- Abbink, O.A.; Colloman, J.H.; Riding, J.B.; Williams, P.D.B.; Wolfard, A. Biostratigraphy of Jurassic–Cretaceous boundary strata in the Terschelling Basin, the Netherlands. Proc. Yorkshire Geol. Soc. 2001, 53, 275–302. [Google Scholar] [CrossRef]
- Passalia, M.; Iglesias, A.; Varela, A.; Santamarina, P.; Poiré, D.; Richiano, S. The fern Konijnenburgia alata in the mid-Cretaceous of Patagonia, and the Matoniaceae fossil record. Cretac. Res. 2018, 89, 264–278. [Google Scholar] [CrossRef]
- Van Konijnenburg-Van Cittert, J.H.A. In situ gymnosperm pollen from the Middle Jurassic of Yorkshire. Acta Bot. Neerl. 1971, 20, 1–97. [Google Scholar]
- Watson, J.; Alvin, K.L. The cheirolepidiaceous conifers Frenelopsis occidentalis Heer and Watsoniocladus valdensis (Seward) in the Wealden of Germany. Cretac. Res. 1999, 20, 315–326. [Google Scholar] [CrossRef]
- Francis, J.E. The dominant conifer of the Jurassic Purbeck Formation. Palaeontology 1983, 26, 277–294. [Google Scholar]
- Palliani, R.B.; Mattioli, E.; Riding, J.B. The response of marine phytoplankton and sedimentary organic matter to the early Toarcian (Lower Jurassic) oceanic anoxic event in northern England. Mar. Micropaleontol. 2002, 46, 223–245. [Google Scholar] [CrossRef]
- Galasso, F.; Schmid-Röhl, A.; Feist-Burkhardt, S.; Bernasconi, S.M.; Schneebeli-Hermann, E. Changes in organic matter com-position during the Toarcian Oceanic Anoxic Event (T-OAE) in the Posidonia Shale Formation from Dormettingen (SW-Germany). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 569, 110327. [Google Scholar] [CrossRef]
- Krupnik, J.; Ziaja, J.; Barbacka, M.; Feldman-Olszewska, A.; Jarzynka, A. A palaeoenvironmental reconstruction based on palynological analyses of Upper Triassic and Lower Jurassic sediments from the Holy Cross Mountains region. Acta Palaeobot. 2014, 54, 35–65. [Google Scholar] [CrossRef]
- Mendes, M.M.; Vajda, V.; Cunha, P.P.; Dinis, P.; Svobodová, M.; Doyle, J.A. A Lower Cretaceous palynoflora from Carregueira (Lusitanian Basin, westernmost Iberia): Taxonomic, stratigraphic and palaeoenvironmental implications. Cretac. Res. 2021, 130, 105036. [Google Scholar] [CrossRef]
- Santos, A.A.; Wang, X.; Fu, Q.; Diez, J.B. First palynological data from the Jurassic South Xiangshan Formation (Nanjing area, China). Geobios 2018, 51, 559–570. [Google Scholar] [CrossRef]
- Gutierrez, K.; Sheldon, N.D. Paleoenvironmental reconstruction of Jurassic dinosaur habitats of the Vega Formation, Asturias, Spain. GSA Bull. 2011, 124, 596–610. [Google Scholar] [CrossRef]
- Santos, A.A.; Nel, A.; Rodríguez-Barreiro, I.; Sender, L.M.; Wappler, T.; Diez, J.B. Insect and Plant Diversity in Hot-Spring Ecosystems during the Jurassic-Cretaceous Boundary from Spain (Aguilar Fm.; Palencia). Biology 2022, 11, 273. [Google Scholar] [CrossRef]
- Lockley, M.G.; García-Ramos, J.C.; Piñuela, L.; Avanzini, M. A review of vertebrate track assemblages from the Late Jurassic of Asturias, Spain with comparative notes on coeval ichnofaunas from the western USA: Implications for faunal diversity in siliciclastic facies assemblages. Oryctos 2008, 8, 53–70. [Google Scholar]
- Jones, T.P.; Chaloner, W.G. Fossil charcoal, its recognition and palaeoatmospheric significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 97, 39–50. [Google Scholar] [CrossRef]
- Scott, A.C. Charcoal recognition, taphonomy and uses in palaeoenvironmental analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 11–39. [Google Scholar] [CrossRef]
- Glasspool, I.J.; Scott, A.C. Identifying past fire events. In Fire Phenomena and the Earth System: An Interdisciplinary Guide to Fire Science; Belcher, C.M., Ed.; John Wiley and Sons: Chichester, UK, 2013; pp. 179–206. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).