Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
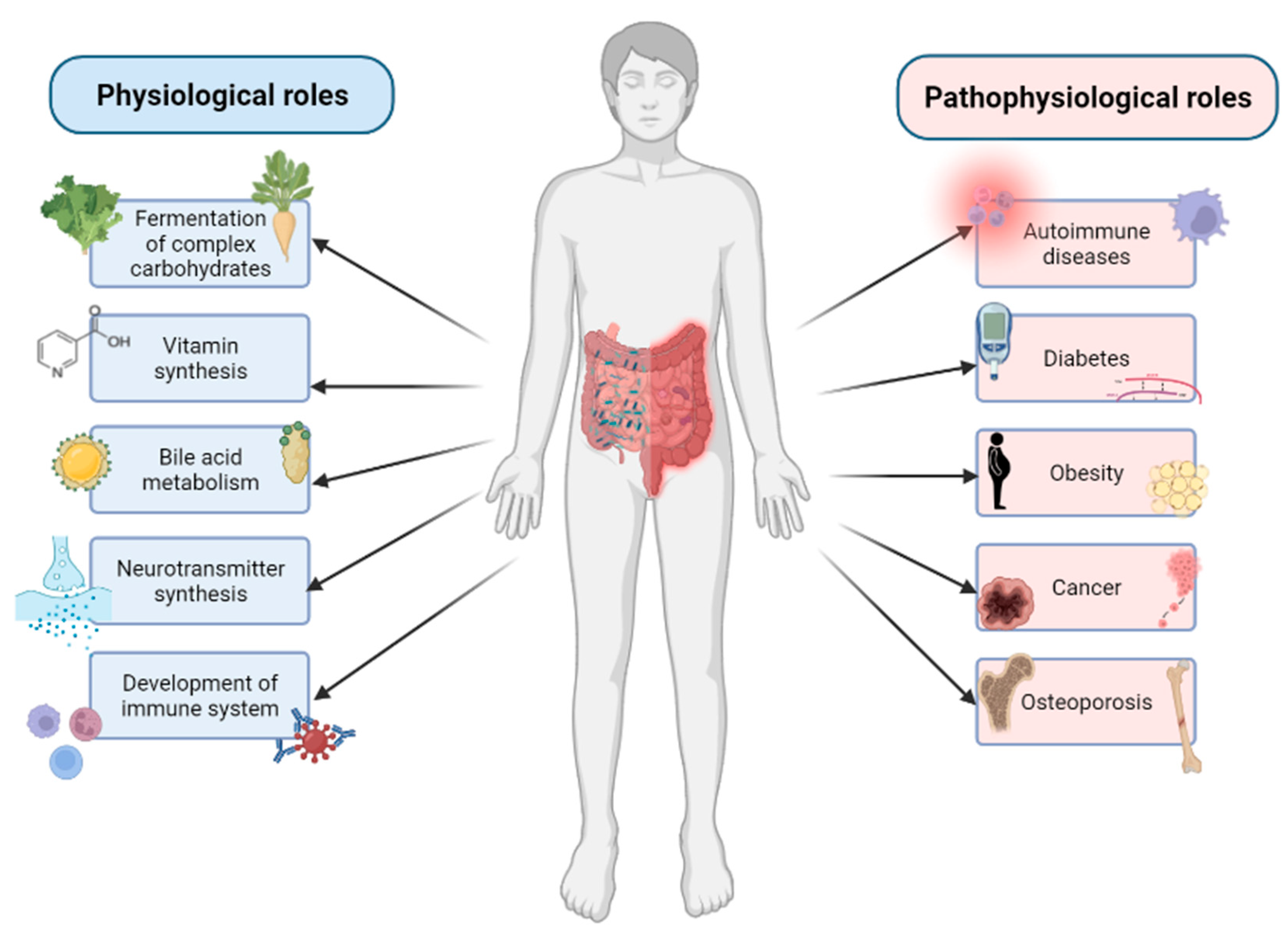
3. Composition and Functions of Gut Microbiome
4. The Role of Gut Microbiome in Bone Homeostasis
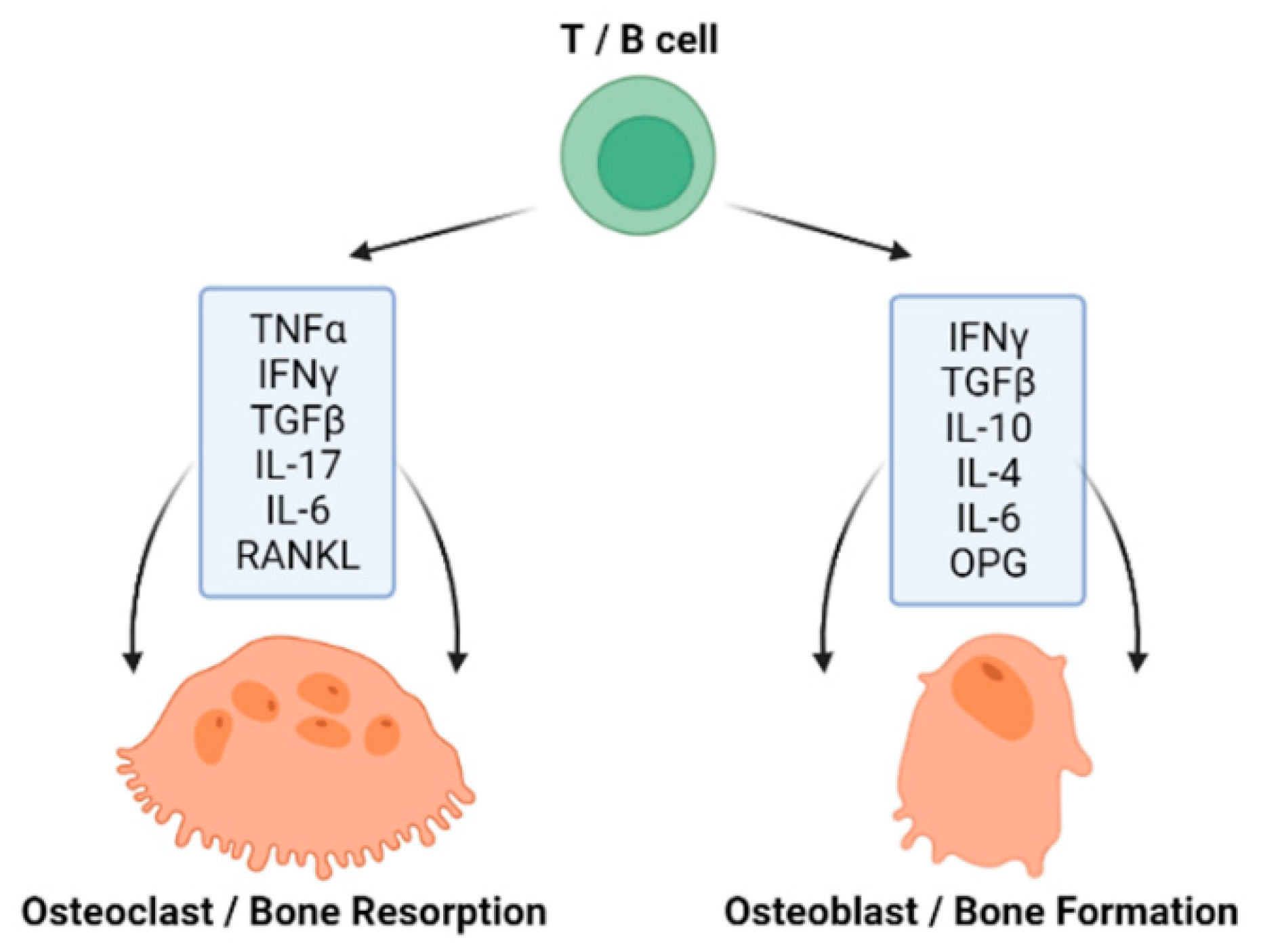
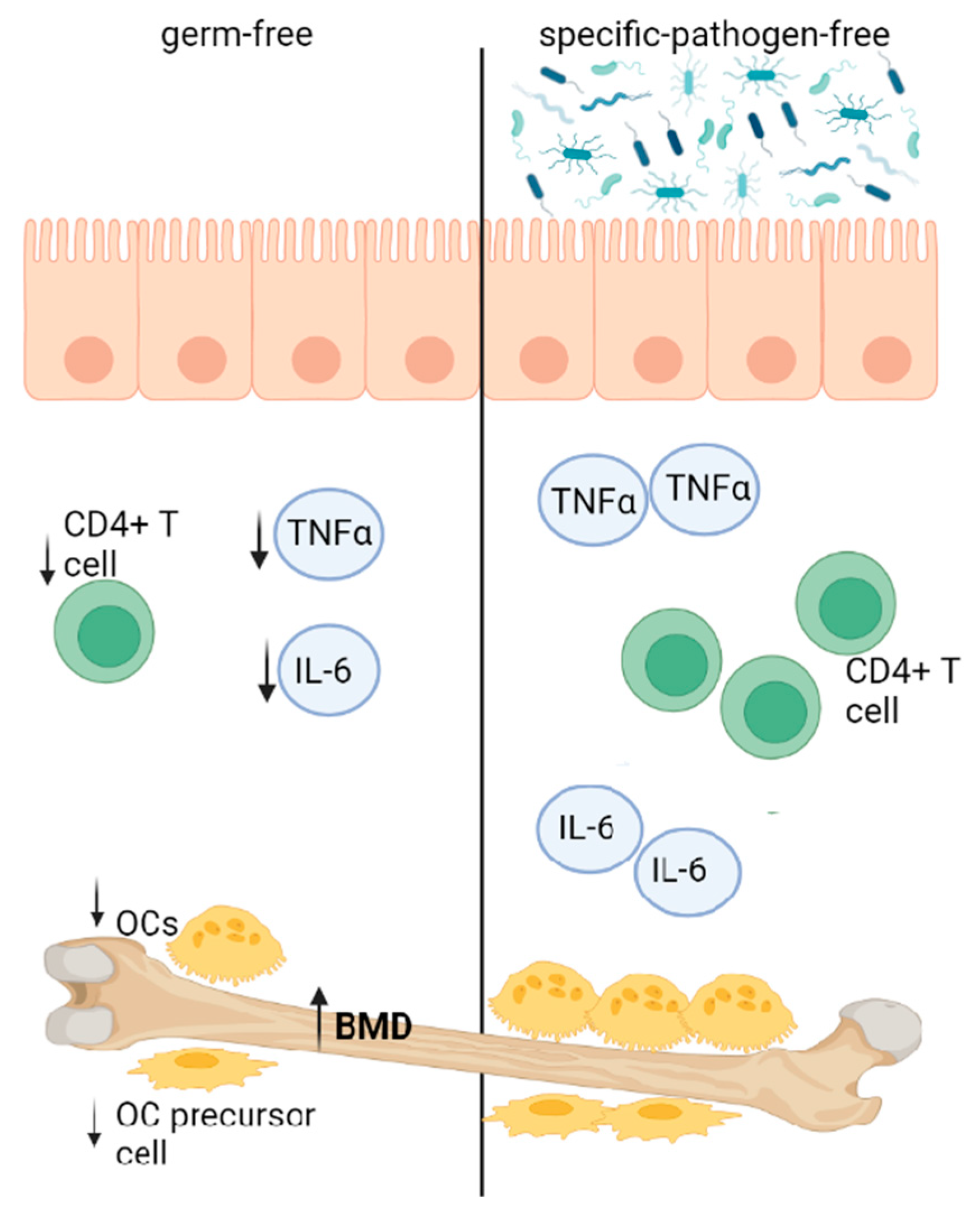
4.1. GM Influencing Bone Homeostasis via Immune Function
4.2. GM Influencing Bone Homeostasis via Short-Chain Fatty Acids Production
4.3. GM Influencing Bone Homeostasis via Absorption of Calcium
4.4. GM Influencing Bone Homeostasis via Endocrine Function
4.5. GM Influencing Bone Homeostasis via the Gut–Brain Axis
5. The Relationship between Gut Microbiome and Bone-Related Diseases
5.1. Osteoporosis
| Disease | GM Alterations Associated with the Disease | Probiotic Therapy Used | Effects of Probiotic Therapy in Studies | References |
|---|---|---|---|---|
| osteoporosis | HS: increase in Fusobacterium, Dialister, Faecalibacterium, Tolumonas, Bacteroides, Parabacteroides, Adlercreutzia, Lactobacillus; decrease in Roseburia, Clostridia, Methanobrevibacter, Romboutsia, Turicibacter, Lachnospira | AS: Lactobacillus rhamnosus, L. acidophilus, L. plantarum, L. paracasei, L. bulgaricus, L. reuteri, Bifidobacterium breve, B. longum, B. infantis, Streptococcus thermophilus HS: Lactobacillus paracasei, L. plantarum, Bacillus subtilis | AS: reduced gut permeability, intestinal and bone marrow inflammation; decrease of osteoclastogenesis and bone resorption markers HS: reduced lumbar spine BMD loss; increased total hip BMD; increased Bifidobacterium | [76,87,88,89] |
| osteoarthritis | HS: increase in Clostidium, Gemmiger, Klebsiella, Akkermansia, Lactobacillus, Betaproteobacteria, Streptococcus, Bilophila, Desulfovibrio decrease in Bifidobacterium, Faecalibacterium, Bacteroides, Prevotella, Alistipes, Clostridium, Parabacteroides, Roseburia | AS: Clostridium butyricum, Lactobacillus acidophilus, L. casei, L. fermentum, L. paracasei, Streptococcus thermophilus, Bifidobacterium longum, B. bifidum, B. breve, L. rhamnosus, L. plantarum, L. helveticus, L. salivarius | AS: preserved knee cartilage and synovial membrane; reduced fibrous tissue; decreased cartilage damage; lowered inflammatory and bone metabolism markers in serum; increased levels of IFN-γ and glycosaminoglycans; alleviated pain; increased Bifidobacterium and Roseburia; decreased Closteridium leptum, Akkemansia muciniphila | [90,91,92,93,94,95,96,97,98,99,100] |
| rheumatoid arthritis | HS: increase in Prevotella, Clostridium, Ruminococcus, Lactobacillus, Collinsella, Eggerthella decrease in Bacteroides, Haemophillus, Firmicutes, Faecalibacterium, | AS: Lactobacillus casei HS: L. acidophilus, L. casei, B. bifidum | AS: inhibited joint swelling, lowered arthritis scores, prevented bone destruction; downregulated pro-inflammatory cytokines; increased L. acidophilus HS: improved Disease Activity Score, decreased serum insulin | [101,102,103,104,105,106] |
| type 1 diabetes mellitus | HS: increase in Bacteroides, Veillonella, Alistipes, Klebsiella, Enterococcus, Clostridium, Staphylococcus, Streptococcus, Sarcina, Corynebacterium, Barnesiella, Haemophilus, Ruminococcus, Blautia decrease in Bifidobacterium, Lactobacillus, Escherichia, Prevotella, Akkermansia, Eubacterium, Roseburia, Faecalibacterium, Lachnospira | AS: Lactobacillus brevis, L. reuteri, L. lactis, L. kefiranofaciens, L. kefiri, Bifidobacterium, Streptococcus thermophilus HS: L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii, B. longum, B. infantis, B. breve, Streptococcus thermophiles | AS: reduced blood glucose levels via gamma-aminobutyric acid; elevated innate response; reduced intestinal inflammation; suppressed osteoblast Wnt pathway; stimulated secretion of anti-inflammatory cytokines HS: decrease in glycated hemoglobin, decline in total and bolus insulin; ameliorated conditions of metabolic syndrome | [107,108,109,110,111,112] |
| type 2 diabetes mellitus | HS: increase in Ruminococcus, Fusobacterium, Blautia, Bacteroides, Clostridium, Eggerthella, Escherichia decrease in Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, Roseburia, Firmicutes | AS: Lactobacillus rhamnosus, L. casei, L. plantarum, L. acidophilus, L. paracasei, Bifidobacterium bifidum HS: L. casei, L. reuteri, L. acidophilus, Bifidobacterium lactis | AS: decreased fasting and postprandial blood glucose; improved insulin sensitivity and reduced lipid accumulation by stimulating adiponectin secretion; reduced plasma lipids and pro-inflammatory cytokines HS: decreased fasting and postprandial blood glucose; reduced plasma lipids and pro-inflammatory cytokines | [109,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] |
| obesity | HS: increase in Firmicutes, Acidaminococcus, Anaerococcus, Catenibacterium, Dialister, Dorea, Escherichia-Shigella, Eubacterium, Fusobacterium, Megasphera, Prevotella, Roseburia, Streptococcus, Sutterella, Staphylococcus, Clostridium, Lactobacillus decrease in Bacteroidetes, Bifidobacterium, Eggerthella | AS: Lactobacillus gasseri, L. plantarum, L. curvatus, L. reuteri, L. acidophilus, L. paracasei, L. bulgaricus, Bifidobacterium breve, B. pseudocatenulatum, B. longum, B. infantis, Streptococcus thermophiles HS: L. acidophilus, L. rhamnosus, L. gasseri, L. salivarius, L. casei, L. amylovorus L. fermentum, L. plantarum, Enterococcus faecium, Streptococcus thermophilus, Bifidobacterium lactis | AS: decreased weight, body fat and leptin; decreased insulin resistance, triglyceridemia, cholesterolemia; increased trabecular bone volume and bone mechanical strength; improved osteoblast mineralization HS: decreased LDL cholesterol, body weight, BMI, visceral and subcutaneous fat, waist and hip circumference; increased plasma adiponectin | [130,131,132,133,134,135,136,137] |
5.2. Inflammatory Bone-Related Diseases
5.2.1. Osteoarthritis
5.2.2. Rheumatoid Arthritis
5.3. Diabetes Mellitus
5.4. Obesity
5.5. Bone Cancer
6. The Role of Gut Microbiome in Drug Response
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Awany, D.; Allali, I.; Dalvie, S.; Hemmings, S.; Mwaikono, K.S.; Thomford, N.E.; Gomez, A.; Mulder, N.; Chimusa, E.R. Host and Microbiome Genome-Wide Association Studies: Current State and Challenges. Front. Genet. 2019, 9, 637. [Google Scholar] [CrossRef]
- Sharon, G.; Garg, N.; Debelius, J.; Knight, R.; Dorrestein, P.C.; Mazmanian, S.K. Specialized Metabolites from the Microbiome in Health and Disease. Cell Metab. 2014, 20, 719–730. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef]
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: A major player in the toxicity of environmental pollutants? Npj Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Sapra, L.; Tiwari, A.; Mishra, P.K.; Sharma, S.; Srivastava, R.K. “Osteomicrobiology”: The Nexus Between Bone and Bugs. Front. Microbiol. 2022, 12, 812466. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.R.; Ward, W.E.; Comelli, E.M. Gut microbiota-bone axis. Crit. Rev. Food Sci. Nutr. 2015, 57, 1664–1672. [Google Scholar] [CrossRef]
- Gritz, E.C.; Ebhandari, V. The Human Neonatal Gut Microbiome: A Brief Review. Front. Pediatr. 2015, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M.-F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.; Rigsbee, L.; Kenche, H.; Michail, S.; Khamis, H.J.; Paliy, O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011, 77, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Cheng, J.; Ringel, Y.; Salojärvi, J.; Carroll, I.; Palva, A.; De Vos, W.M.; Satokari, R. Intestinal Microbiota in Healthy U.S. Young Children and Adults—A High Throughput Microarray Analysis. PLoS ONE 2013, 8, e64315. [Google Scholar] [CrossRef]
- McBurney, M.I.; Davis, C.; Fraser, C.M.; Schneeman, B.O.; Huttenhower, C.; Verbeke, K.; Walter, J.; Latulippe, M.E. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lankelma, J.M.; Nieuwdorp, M.; De Vos, W.M.; Wiersinga, W.J. The gut microbiota in internal medicine: Implications for health and disease. Neth. J. Med. 2015, 73, 61–68. [Google Scholar]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Reid, I.R. (Ed.) Metabolic Bone Disease; Baillière’s clinical endocrinology and metabolism; Baillière Tindall: London, UK, 1997; ISBN 978-0-7020-2340-8. [Google Scholar]
- Safadi, F.F.; Barbe, M.F.; Abdelmagid, S.M.; Rico, M.C.; Aswad, R.A.; Litvin, J.; Popoff, S.N. Bone Structure, Development and Bone Biology. In Bone Pathology; Khurana, J.S., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–50. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Z.; Guo, Y.; Qi, X.; Yang, Y.; Jia, X.; Li, R.; Shi, J.; Gao, W.; Ren, Z.; et al. Bioinspired Nanovesicles Convert the Skeletal Endothelium-Associated Secretory Phenotype to Treat Osteoporosis. ACS Nano 2022, 16, 11076–11091. [Google Scholar] [CrossRef]
- Janeway, C. (Ed.) Immunobiology: The Immune System in Health and Disease, 5th ed.; [Animated CD-ROM Inside]; Garland Publ.: New York, NY, USA, 2001; ISBN 978-0-8153-3642-6. [Google Scholar]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yu, X.-J.; Yu, L.-L.; Tian, F.-W.; Zhao, J.-X.; Zhang, H.; Chen, W.; Zhai, Q.-X. The influence of gut microbiome on bone health and related dietary strategies against bone dysfunctions. Food Res. Int. 2021, 144, 110331. [Google Scholar] [CrossRef] [PubMed]
- Titanji, K. Beyond Antibodies: B Cells and the OPG/RANK-RANKL Pathway in Health, Non-HIV Disease and HIV-Induced Bone Loss. Front. Immunol. 2017, 8, 1851. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017, 5, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; D’Amelio, P.; Faccio, R.; Brunetti, G. The Interplay between the Bone and the Immune System. Clin. Dev. Immunol. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Ohlsson, C.; Sjögren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef]
- Pacifici, R. T cells, osteoblasts, and osteocytes: Interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann. N. Y. Acad. Sci. 2015, 1364, 11–24. [Google Scholar] [CrossRef]
- MacPherson, A.J.; Martinic, M.; Harris, N.L. The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Experientia 2002, 59, 2088–2096. [Google Scholar] [CrossRef]
- Ohlsson, C.; Nigro, G.; Boneca, I.G.; Bäckhed, F.; Sansonetti, P.; Sjögren, K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell. Immunol. 2017, 317, 55–58. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.; Baron, B. The Role of Toll-Like Receptors in Autoimmune Diseases through Failure of the Self-Recognition Mechanism. Int. J. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, A.; Magrath, W.; Pugliese, B.; Stocker, N.; Westermann, P.; Heider, A.; Gehweiler, D.; Zeiter, S.; Claesson, M.J.; Richards, R.G.; et al. Butyrate Inhibits Osteoclast Activity In Vitro and Regulates Systemic Inflammation and Bone Healing in a Murine Osteotomy Model Compared to Antibiotic-Treated Mice. Mediat. Inflamm. 2021, 2021, 8817421. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 376. [Google Scholar] [CrossRef]
- Oton-Gonzalez, L.; Mazziotta, C.; Iaquinta, M.R.; Mazzoni, E.; Nocini, R.; Trevisiol, L.; D’Agostino, A.; Tognon, M.; Rotondo, J.C.; Martini, F. Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. Int. J. Mol. Sci. 2022, 23, 1500. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef]
- Mineo, H.; Hara, H.; Tomita, F. Short-chain fatty acids enhance diffusional Ca transport in the epithelium of the rat cecum and colon. Life Sci. 2001, 69, 517–526. [Google Scholar] [CrossRef]
- Bryk, G.; Coronel, M.Z.; Pellegrini, G.G.; Mandalunis, P.; Rio, M.E.; De Portela, M.L.P.M.; Zeni, S.N. Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015, 54, 913–923. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Markiewicz, L.H.; Lamparski, G.; Juśkiewicz, J. Administration of Inulin-Supplemented Gluten-Free Diet Modified Calcium Absorption and Caecal Microbiota in Rats in a Calcium-Dependent Manner. Nutrients 2017, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; A Story, J.; Macdonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, E.G.; Schoterman, M.H.C.; Muijs, T. Transgalactooligosaccharides Stimulate Calcium Absorption in Postmenopausal Women. J. Nutr. 2000, 130, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Malik, T.A.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2020, 10, 13. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate–Induced Colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-De-Moraes, A.C.; Folchetti, L.G.; Ferreira, S.R.G. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Greenstein, R.J.; Su, L.; Brown, S.T. Vitamins A & D Inhibit the Growth of Mycobacteria in Radiometric Culture. PLoS ONE 2012, 7, e29631. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, K.; Yamada, S.; Ao, M.; Matsuura, M.; Tsuji, H.; Iida, T.; Miyamoto, K.; Oka, K.; Takahashi, M.; Tanaka, K.; et al. Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease. Nutrients 2019, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y.; Wang, Y.; Ren, X.; Han, J. The impact of the intestinal microbiome on bone health. Intractable Rare Dis. Res. 2018, 7, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Karsenty, G. The two faces of serotonin in bone biology. J. Cell Biol. 2010, 191, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Michalowska, M.; Znorko, B.; Kaminski, T.; Oksztulska-Kolanek, E.; Pawlak, D. New insights into tryptophan and its metabolites in the regulation of bone metabolism. J. Physiol. Pharmacol. 2015, 66, 779–791. [Google Scholar]
- Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P.; et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J. Allergy Clin. Immunol. 2017, 139, 1525–1535. [Google Scholar] [CrossRef]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71, 307–320. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater. 2021, 10, 207–221. [Google Scholar] [CrossRef]
- Swanson, C.M.; Kohrt, W.M.; Buxton, O.M.; Everson, C.A.; Wright, K.P.; Orwoll, E.S.; Shea, S.A. The importance of the circadian system & sleep for bone health. Metabolism 2018, 84, 28–43. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef]
- He, J.; Xu, S.; Zhang, B.; Xiao, C.; Chen, Z.; Si, F.; Fu, J.; Lin, X.; Zheng, G.; Yu, G.; et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging 2020, 12, 8583–8604. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019, 593, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, Z.; Sun, J.; Huang, S.; Chen, Y.; Li, C.; Sun, X.; Xia, B.; Tian, L.; Guo, C.; et al. Gut Microbiome Reveals Specific Dysbiosis in Primary Osteoporosis. Front. Cell. Infect. Microbiol. 2020, 10, 160. [Google Scholar] [CrossRef]
- Fuhrman, B.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the Fecal Microbiome With Urinary Estrogens and Estrogen Metabolites in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer. Res. 2020, 40, 551–556. [Google Scholar] [CrossRef]
- Locantore, P.; Del Gatto, V.; Gelli, S.; Paragliola, R.M.; Pontecorvi, A. The Interplay between Immune System and Microbiota in Osteoporosis. Mediat. Inflamm. 2020, 2020, 3686749. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Jiang, T.; Fang, S.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J. Appl. Microbiol. 2016, 121, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-A.; Curiac, D.; Ahrén, I.L.; Hansson, F.; Niskanen, T.M.; Sjögren, K.; Ohlsson, C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri Treatment Prevents Bone Loss in a Menopausal Ovariectomized Mouse Model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- A Rettedal, E.; Ilesanmi-Oyelere, B.L.; Roy, N.C.; Coad, J.; Kruger, M.C. The Gut Microbiome Is Altered in Postmenopausal Women With Osteoporosis and Osteopenia. JBMR Plus 2021, 5, 10452. [Google Scholar] [CrossRef]
- Li, S.; Mao, Y.; Zhou, F.; Yang, H.; Shi, Q.; Meng, B. Gut microbiome and osteoporosis. Bone Jt. Res. 2020, 9, 524–530. [Google Scholar] [CrossRef]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447. [Google Scholar] [CrossRef]
- Favazzo, L.J.; Hendesi, H.; Villani, D.A.; Soniwala, S.; Dar, Q.-A.; Schott, E.M.; Gill, S.R.; Zuscik, M. The gut microbiome-joint connection: Implications in osteoarthritis. Curr. Opin. Rheumatol. 2020, 32, 92–101. [Google Scholar] [CrossRef]
- Binvignat, M.; Sokol, H.; Mariotti-Ferrandiz, E.; Berenbaum, F.; Sellam, J. Osteoarthritis and gut microbiome. Jt. Bone Spine 2021, 88, 105203. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, C.; Zhang, Y.; Zhang, W.; Doherty, M.; Yang, T.; Zhai, G.; Obotiba, A.D.; Lyu, H.; Zeng, C.; et al. Association Between Gut Microbiota and Symptomatic Hand Osteoarthritis: Data From the Xiangya Osteoarthritis Study. Arthritis Rheumatol. 2021, 73, 1656–1662. [Google Scholar] [CrossRef]
- Chen, J.; Wang, A.; Wang, Q. Dysbiosis of the gut microbiome is a risk factor for osteoarthritis in older female adults: A case control study. BMC Bioinform. 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Bonato, A.; Zenobi-Wong, M.; Barreto, G.; Huang, Z. A systematic review of microbiome composition in osteoarthritis subjects. Osteoarthr. Cartil. 2022, 30, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, H.; Jiang, Q.; Zhu, Y.Z. The gut microbiome as non-invasive biomarkers for identifying overweight people at risk for osteoarthritis. Microb. Pathog. 2021, 157, 104976. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.-Y.; Choi, H.-J.; Kim, M.-G.; Jeong, D.-G.; Lee, D.-G.; Yoon, J.-M.; Kang, D.-J.; Park, S.; Ji, J.-G.; Joo, I.-H.; et al. Effects of ID-CBT5101 in Preventing and Alleviating Osteoarthritis Symptoms in a Monosodium Iodoacetate-Induced Rat Model. J. Microbiol. Biotechnol. 2018, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, S.H.; Jhun, J.; Choi, J.; Jung, K.; Cho, K.H.; Kim, S.J.; Yang, C.W.; Park, S.-H.; Cho, M.-L. The Combination of Probiotic Complex, Rosavin, and Zinc Improves Pain and Cartilage Destruction in an Osteoarthritis Rat Model. J. Med. Food 2018, 21, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.Y.; Jhun, J.; Jung, K.; Park, S.-H.; Yang, C.W.; Cho, Y.; Kim, S.J.; Cho, M.-L. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol. Lett. 2018, 203, 6–14. [Google Scholar] [CrossRef]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- So, J.-S.; Song, M.-K.; Kwon, H.-K.; Lee, C.-G.; Chae, C.-S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.-H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Guo, R.; Ju, Y.; Wang, Q.; Zhu, J.; Xie, Y.; Zheng, Y.; Li, T.; Liu, Z.; Lu, L.; et al. A single bacterium restores the microbiome dysbiosis to protect bones from destruction in a rat model of rheumatoid arthritis. Microbiome 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.-K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut Pathog. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Mishra, S.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms 2019, 7, 67. [Google Scholar] [CrossRef]
- Knudsen, J.K. Gut Microbiota in Bone Health and Diabetes. Curr. Osteoporos. Rep. 2021, 18, 462–479. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Rohilla, L.; Jacob, N.; Yadav, J.; Sachdeva, N. A high potency multi-strain probiotic improves glycemic control in children with new-onset type 1 diabetes mellitus: A randomized, double-blind, and placebo-controlled pilot study. Pediatr. Diabetes 2021, 22, 1014–1022. [Google Scholar] [CrossRef]
- Groele, L.; Szajewska, H.; Szypowska, A. Effects of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: Protocol of a randomised controlled trial. BMJ Open 2017, 7, e017178. [Google Scholar] [CrossRef]
- Salgaço, M.K.; Oliveira, L.G.S.; Costa, G.N.; Bianchi, F.; Sivieri, K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl. Microbiol. Biotechnol. 2019, 103, 9229–9238. [Google Scholar] [CrossRef]
- Irwin, R.; Lin, H.; Motyl, K.J.; McCabe, L. Normal Bone Density Obtained in the Absence of Insulin Receptor Expression in Bone. Endocrinology 2006, 147, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Raisingani, M.; Preneet, B.; Kohn, B.; Yakar, S. Skeletal growth and bone mineral acquisition in type 1 diabetic children; abnormalities of the GH/IGF-1 axis. Growth Horm. IGF Res. 2017, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Krishnamra, N.; Charoenphandhu, N. Derangement of calcium metabolism in diabetes mellitus: Negative outcome from the synergy between impaired bone turnover and intestinal calcium absorption. J. Physiol. Sci. 2016, 67, 71–81. [Google Scholar] [CrossRef]
- Fatima, N.; Faisal, S.M.; Zubair, S.; Ajmal, M.; Siddiqui, S.S.; Moin, S.; Owais, M. Role of Pro-Inflammatory Cytokines and Biochemical Markers in the Pathogenesis of Type 1 Diabetes: Correlation with Age and Glycemic Condition in Diabetic Human Subjects. PLoS ONE 2016, 11, e0161548. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients With Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef]
- Greer, R.L.; Dong, X.; de Moraes, A.; Zielke, R.A.; Fernandes, G.R.; Peremyslova, E.; Vasquez-Perez, S.; Schoenborn, A.A.; Gomes, E.P.; Pereira, A.C.; et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat. Commun. 2016, 7, 13329. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Groele, L.; Szajewska, H.; Szalecki, M.; Świderska, J.; Wysocka-Mincewicz, M.; Ochocińska, A.; Stelmaszczyk-Emmel, A.; Demkow, U.; Szypowska, A. Lack of effect of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: A randomised controlled trial. BMJ Open Diabetes Res. Care 2021, 9, e001523. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L.; Hao, X.; Song, M.; Li, C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2019, 60, 670–683. [Google Scholar] [CrossRef]
- Kim, S.-W.; Park, K.-Y.; Kim, B.; Kim, E.; Hyun, C.-K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Fontané, L.; Benaiges, D.; Goday, A.; Llauradó, G.; Pedro-Botet, J. Influencia de la microbiota y de los probióticos en la obesidad. Clínica E Investig. En Arterioscler. 2018, 30, 271–279. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Montan, P.D.; Sourlas, A.; Olivero, J.; Silverio, D.; Guzman, E.; Kosmas, C.E. Pharmacologic therapy of obesity: Mechanisms of action and cardiometabolic effects. Ann. Transl. Med. 2019, 7, 393. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Voor, M.J.; Tyagi, N. Probiotics Stimulate Bone Formation in Obese Mice via Histone Methylations. Theranostics 2021, 11, 8605–8623. [Google Scholar] [CrossRef]
- Briot, K.; Geusens, P.; Em Bultink, I.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017, 28, 3301–3314. [Google Scholar] [CrossRef]
- Roux, C. Osteoporosis in inflammatory joint diseases. Osteoporos. Int. 2010, 22, 421–433. [Google Scholar] [CrossRef]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef]
- Wei, Z.; Li, F.; Pi, G. Association Between Gut Microbiota and Osteoarthritis: A Review of Evidence for Potential Mechanisms and Therapeutics. Front. Cell. Infect. Microbiol. 2022, 12, 812596. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Lyu, J.-L.; Wang, T.-M.; Chen, Y.-H.; Chang, S.-T.; Wu, M.-S.; Lin, Y.-H.; Kuan, C.-M. Oral intake of Streptococcus thermophilus improves knee osteoarthritis degeneration: A randomized, double-blind, placebo-controlled clinical study. Heliyon 2020, 6, e03757. [Google Scholar] [CrossRef]
- McInnes, I.B. The Pathogenesis of Rheumatoid Arthritis. New Engl. J. Med. 2011, 15, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Isidro, M.L. Bone Disease in Diabetes. Curr. Diabetes Rev. 2010, 6, 144–155. [Google Scholar] [CrossRef]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.; van Meurs, J.B.; Janssen, J.A.; et al. High Bone Mineral Density and Fracture Risk in Type 2 Diabetes as Skeletal Complications of Inadequate Glucose Control. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef]
- Qiu, J.; Li, C.; Dong, Z.; Wang, J. Is diabetes mellitus a risk factor for low bone density: A systematic review and meta-analysis. BMC Endocr. Disord. 2021, 21, 65. [Google Scholar] [CrossRef]
- Asokan, A.G.; Jaganathan, J.; Philip, R.; Soman, R.R.; Sebastian, S.T.; Pullishery, F. Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. J. Nat. Sci. Biol. Med. 2017, 8, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Conte, C.; Eastell, R.; Ewing, S.K.; Bauer, D.C.; Strotmeyer, E.S.; Black, D.M.; Samelson, E.J.; Vittinghoff, E.; Schwartz, A.V. Bone Turnover Markers Do Not Predict Fracture Risk in Type 2 Diabetes. J. Bone Miner. Res. 2020, 35, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Lykkeboe, S.; Handberg, A.; Vestergaard, P.; Høyem, P.; Fleischer, J.; Hansen, T.K.; Poulsen, P.L.; Laugesen, E. Glucose variability and low bone turnover in people with type 2 diabetes. Bone 2021, 153, 116159. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The Disease Burden Associated With Overweight and Obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef]
- Greco, E.; Lenzi, A.; Migliaccio, S. The obesity of bone. Ther. Adv. Endocrinol. Metab. 2015, 6, 273–286. [Google Scholar] [CrossRef]
- Schott, E.M.; Farnsworth, C.W.; Grier, A.; Lillis, J.A.; Soniwala, S.; Dadourian, G.H.; Bell, R.D.; Doolittle, M.L.; Villani, D.A.; Awad, H.; et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 2018, 3, 95997. [Google Scholar] [CrossRef]
- Biermann, J.S.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Butrynski, J.E.; Cheong, D.; Chow, W.; Curry, W.T.; Frassica, D.A.; et al. Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2013, 11, 36. [Google Scholar]
- Ferguson, J.L.; Turner, S.P. Bone Cancer: Diagnosis and Treatment Principles. Am. Fam. Physician 2018, 98, 205–213. [Google Scholar] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Wenhui, Y.; Zhongyu, X.; Kai, C.; Zhaopeng, C.; Jinteng, L.; Mengjun, M.; Zepeng, S.; Yunshu, C.; Peng, W.; Yanfeng, W.; et al. Variations in the Gut Microbiota in Breast Cancer Occurrence and Bone Metastasis. Front. Microbiol. 2022, 13, 894283. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Evolving Roles of Probiotics in Cancer Prophylaxis and Therapy. Probiotics Antimicrob. Proteins 2013, 5, 59–67. [Google Scholar] [CrossRef]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’Toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Zeibich, L.; Koebele, S.V.; Bernaud, V.E.; Ilhan, Z.E.; Dirks, B.; Northup-Smith, S.N.; Neeley, R.; Maldonado, J.; Nirmalkar, K.; Files, J.A.; et al. Surgical Menopause and Estrogen Therapy Modulate the Gut Microbiota, Obesity Markers, and Spatial Memory in Rats. Front. Cell. Infect. Microbiol. 2021, 11, 702628. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, I.Y.; Bae, E.-K.; Jeon, C.H.; Ahn, K.-S.; Cha, H.-S. Selective estrogen receptor modulator lasofoxifene suppresses spondyloarthritis manifestation and affects characteristics of gut microbiota in zymosan-induced SKG mice. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, D.; Law, H.K.-W.; Wu, Y.; Zhu, G.-H.; Huang, W.-Y.; Kang, Y. Integrative Analysis of Gut Microbiota and Fecal Metabolites in Rats after Prednisone Treatment. Microbiol. Spectr. 2021, 9, e00650-21. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-García, O.; Castro-Alarcón, N.; Pérez-Rubio, G.; Guzmán-Guzmán, I.P. DMARDs–Gut Microbiota Feedback: Implications in the Response to Therapy. Biomolecules 2020, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, X.; Chu, W. Shifts in the gut microbiota of mice in response to dexamethasone administration. Int. Microbiol. 2020, 23, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Gaurav, C.; Muhammad, U.; Chen, Y.; Li, X.; Chen, J.; Wang, Z. CUMS and dexamethasone induce depression-like phenotypes in mice by differentially altering gut microbiota and triggering macroglia activation. Gen. Psychiatry 2021, 34, e100529. [Google Scholar] [CrossRef] [PubMed]
- Kihl, P.; Krych, L.; Buschard, K.; Wesley, J.D.; Kot, W.; Hansen, A.K.; Nielsen, D.S.; Von Herrath, M.G. Oral insulin does not alter gut microbiota composition of NOD mice. Diabetes/Metab. Res. Rev. 2018, 34, e3010. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Ke, J.; An, Y.; Cao, B.; Lang, J.; Wu, N.; Zhao, D. Orlistat-Induced Gut Microbiota Modification in Obese Mice. Evidence-Based Complement. Altern. Med. 2020, 2020, 9818349. [Google Scholar] [CrossRef]
- Huang, J.; Wei, S.; Jiang, C.; Xiao, Z.; Liu, J.; Peng, W.; Zhang, B.; Li, W. Involvement of Abnormal Gut Microbiota Composition and Function in Doxorubicin-Induced Cardiotoxicity. Front. Cell. Infect. Microbiol. 2022, 12, 808837. [Google Scholar] [CrossRef]
| Disease | Selected Drugs | GM Alterations Associated with the Treatment | Interactions between the Treatment and GM | References |
|---|---|---|---|---|
| osteoporosis | bisphosphonates | not known | supposed GM-related immunosuppression via inhibition of the mevalonate pathway | [70] |
| estrogen therapy | AS: increase in Clostridium, Turicibacter, Romboutsia, Parabacteroides decrease in Kineothrix, Ruminococcus, Muribaculum | estrogen promotes microbiota, which improves T-regulatory cell function and suppresses inflammation; GM influences estrogen metabolism | [170,171] | |
| raloxifene (SERMs) | not known | lasofoxifene, a SERM not approved for clinical use, affects the composition and biodiversity of GM | [172] | |
| osteoarthritis | ibuprofen (NSAIDs) | HS: increase in families Propionibacteriaceae, Pseudomonadaceae, Puniceicoccaceae, Rikenellaceae, Acidaminococcaceae, Enterococcaceae, Erysipelotrichaceae | NSAIDs inhibit some positive effects of microbiota through enzyme inhibition. | [173] |
| prednisone (glucocorticoids) | AS: increase in Anaerobacterium decrease in Eisenbergiella, Alistipes, Clostridium | microbiota-derived SCFAs might be involved in the pharmacology of prednisone | [174] | |
| rheumatoid arthritis | methotrexate | HS: increase in Bacteroides, Faecalibacterium, Clostridia decrease in Haemophilus, Enterobacteriales, Bacteroidetes | GM includes dihydrofolate reductase enzyme, so it can modulate methotrexate metabolism; at the same time, methotrexate can modulate microbial metabolism | [175] |
| dexamethasone (glucocorticoids) | AS: increase in Lachnospiraceae, Oscillibacter, Ruminococcaceae, Ruminiclostridium, Anaerotruncus, Butyricicoccus, Enterobacteriaceae, Escherichia Shigella, Gammaproteobacteria, Enterobacteriales, Anaerofustis, Erysipelotrichaceae decrease in Lactobacillus, Enterorhabdus, Pseudomonas, Clostridium | GM directly mediates the therapeutic efficiency and side effects of glucocorticoids | [176,177] | |
| T1DM | insulin | not known | oral insulin administration had no effect on GM, possibly due to the insulin degradation in the intestine, and its low bioavailability | [178] |
| T2DM | metformin | HS: increase in Megamonas, Escherichia/Shigella, Klebsiella, Blautia, Fusobacterium, Bifidobacterium, Intestinibacter decrease in Alistipes, Bacteroidetes | metformin clinical benefits are partly mediated by bacteria-specific mechanisms such as glucose-SGLT1-sensing glucoregulatory pathway | [179,180] |
| obesity | orlistat | AS: increase in Pseudomonas, Rhodococcus, Roseburia, Acetivibrio | orlistat showed enrichment in genes involved in the endocrine and lipid metabolism including ALA metabolism; GM plays a role in the metabolism of ALA to conjugated linolenic acid, which was documented to have antiadipogenic effects | [181] |
| bone cancer | doxorubicin | AS: increase in Faecalibaculum, Dubosiella, Lachnospiraceae decrease in Allobaculum, Muribaculum, Lachnoclostridium | doxorubicin application is accompanied by variation of metabolism processes such as amino acid metabolism, glycan biosynthesis and metabolism, lipid metabolism, and other secondary metabolites | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemanova, N.; Omelka, R.; Mondockova, V.; Kovacova, V.; Martiniakova, M. Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases. Biology 2022, 11, 1402. https://doi.org/10.3390/biology11101402
Zemanova N, Omelka R, Mondockova V, Kovacova V, Martiniakova M. Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases. Biology. 2022; 11(10):1402. https://doi.org/10.3390/biology11101402
Chicago/Turabian StyleZemanova, Nina, Radoslav Omelka, Vladimira Mondockova, Veronika Kovacova, and Monika Martiniakova. 2022. "Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases" Biology 11, no. 10: 1402. https://doi.org/10.3390/biology11101402
APA StyleZemanova, N., Omelka, R., Mondockova, V., Kovacova, V., & Martiniakova, M. (2022). Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases. Biology, 11(10), 1402. https://doi.org/10.3390/biology11101402






