Simple Summary
Although fiercely defended by workers, ant societies can be infiltrated by social parasites that exploit the whole colony and its resources, instead of taking advantage of a single organism. Among them, we studied Myrmica karavajevi, a rare ant species in which the worker caste does not exist, and queens live as “inquilines” within colonies of another ant species, commonly Myrmica scabrinodis. Parasite queens entirely depend on host worker labour. They have evolved many adaptations to be admitted and integrate as members of the host ant society. Indeed, we found that, even at the pupal stage, the parasite is treated as a valuable item in the colony hierarchy, as it is rescued ahead of the colony’s own brood. Moreover, we discovered that M. karavajevi adult queens use two strategies to live undisturbed in the host nest. The parasite smells like the host queens by possessing specific odours and produces sounds similar to those emitted by M. scabrinodis ants, particularly by queens. Through these kinds of mimicking, M. karavajevi advances its rank towards the highest attainable position in the colony’s hierarchy. M. karavajevi is an outstanding example of multimodal deception, which needs a combined behavioural and molecular approach to be fully unravelled.
Abstract
Social parasitism represents a particular type of agonistic interaction in which a parasite exploits an entire society instead of a single organism. One fascinating form of social parasitism in ants is the “inquilinism”, in which a typically worker-less parasitic queen coexists with the resident queen in the host colony and produces sexual offspring. To bypass the recognition system of host colonies, inquilines have evolved a repertoire of deceiving strategies. We tested the level of integration of the inquiline Myrmica karavajevi within the host colonies of M. scabrinodis and we investigated the mechanisms of chemical and vibroacoustic deception used by the parasite. M. karavajevi is integrated into the ant colony to such an extent that, in rescue experiments, the parasite pupae were saved prior to the host’s brood. M. karavajevi gynes perfectly imitated the cuticular hydrocarbon profiles of M. scabrinodis queens and the parasite vibroacoustic signals resembled those emitted by the host queens eliciting the same levels of attention in the host workers during playback experiments. Our results suggest that M. karavajevi has evolved ultimate deception strategies to reach the highest social status in the colony hierarchy, encouraging the use of a combined molecular and behavioural approach when studying host–parasite interactions.
1. Introduction
Species interactions, e.g., mutualism or parasitism, are pivotal driving forces in evolution. The key outcome of parasite-host coevolution processes are adaptations evolved by parasitic species and counter-adaptations arising as a response in hosts. The impact of parasites could be very high as they can influence the host’s life-history traits, fitness, behaviour, or sexual choices (e.g., [1]).
Colonies of social insects, e.g., bees, wasps, termites, or ants, are attractive targets for many species of parasite, like viruses, bacteria, nematodes, mites, as well as parasitic insects and numerous species of social parasites [2]. In a strict definition, the latter group encompasses a social species that exploits host colony resources as food, shelter or reproductive effort [3]. Social parasitism occurs in all groups of social insects, but it seems to be less common in termites and widespread in ants (e.g., [3,4,5]). There are various types of social parasitism in ants from temporary parasites to obligate inquilines, reflecting different levels of parasite’s host dependence [3]. Obligate inquilines are closely related to the host species (Emery’s rule [6]) with whom they share many life-history traits [7,8]. Following the terminology presented by Buschinger [3], inquiline ants are permanent parasites that are fully dependent on their hosts and, in most cases, lack a worker caste. Therefore, the inquiline queen relies on host workers and can invest all her resources to produce sexual forms [3]. Several inquiline species are host queen-tolerant, which means that they coexist inside the same colony together with host queen(s) and all of them can reproduce. Nevertheless, the majority of sexuals are usually produced by the inquiline queen [9].
As the fitness of inquilines is wholly dependent on the host work, they have to evolve various adaptations to enter and integrate within host colonies [10]. The most important capability is to break the ant chemical communication channel which is a sophisticated code used by colony members to recognise nest-mates from non-nestmates [11,12]. This code is created by cuticular hydrocarbons (CHCs) and differences in this chemical signature play the main role as recognition cues (e.g., [11,13]). Social parasites can use various chemical strategies to mislead their hosts: chemical mimicry (active production of CHCs), camouflage (acquisition of colony odour) or chemical insignificance (reduction of specific classes of CHCs not to be detected by hosts) [13,14,15].
Besides the chemical channel, social parasites can also exploit other hosts’ communication systems like visual and acoustical cues. The latter ones have recently been studied in myrmecophilous butterflies including the Maculinea genus that encompasses only obligate social parasites of Myrmica ants [16,17,18,19,20,21,22,23]. It was demonstrated that larvae and pupae of these butterflies are able to manipulate their Myrmica hosts by mimicking vibroacoustic signals to obtain a high status in the host colony hierarchy [17].
Maculinea butterflies are only one of the many organisms found in Myrmica nests. Inquilinism as well has evolved several times in this ant genus [24,25], which is, therefore, among the best-studied models to explore invertebrate symbiotic dynamics [26]. Notwithstanding, our knowledge about interactions between inquiline ants and their hosts and about parasite adaptations is still very limited, primarily because inquilines are rare and often found in isolated patches within their host range [3]. One of such extremely rare and poorly known ant species is Myrmica karavajevi (Arnoldi, 1930). More than 80 years after the species description, M. karavajevi was reported from less than 30 localities scattered throughout nearly 20 European countries [27,28,29]. The most common host of this inquiline ant is M. scabrinodis Nylander, 1846 [30], but M. karavajevi was also found inside colonies of four other Myrmica species, all belonging to the ‘scabrinodis group’ [27]. Usually, various host species are reported from different countries [24,27]. Results of a molecular phylogenetic study by Jansen et al. [25] revealed that M. karavajevi might be classified within the ‘scabrinodis group’ (i.e., together with its host species), suggesting the existence of a relatively recent common ancestor shared with its hosts, and representing Emery’s rule in the loose sense, which means it derives from the same genus to which its hosts belong [3]. However, this inquiline ant cannot be considered as a sister species of any other Myrmica species (for further discussion see [25]).
Similar to many other inquilines of social insects, adaptations used by M. karavajevi to enter and integrate within host colonies are still unknown. The main aim of our study was to assess the level of parasite integration inside host colonies and to investigate if and how inquiline queens are able to deceive the multimodal communication channels of their hosts. Briefly, we found that inquiline immature stages are intimately integrated into the colony hierarchy to such an extent that host ants rescued M. karavajevi pupae in preference to their own brood. Besides, both chemical and acoustic communication channels of the host can be potentially corrupted by the inquiline queens (i) whose CHCs perfectly matched those of host queens, and (ii) which emit specific vibroacoustic signals partially resembling those of host queens and eliciting the same behavioural responses in the host workers as host queens.
2. Materials and Methods
2.1. Collection and Sample Maintenance
We sampled one population of Myrmica scabrinodis in Kraków, Poland (50°01′ N/19°53′ E). In the field, we searched for Myrmica scabrinodis nests. All found colonies were opened, and the brood examined in search for Myrmica karavajevi sexual pupae. At pupal stage, the parasite is easily detectable, being similar to the host workers in size but having wings. Parasitised colonies were excavated and brought to the laboratory. In the laboratory, we set up ant colonies of 100 workers in 28 cm × 15 cm × 10 cm3 Perspex containers and reared them on a diet of sugar and Drosophila larvae.
2.2. Brood Rescue Experiment
For the brood-rescue experiment, we used Brian nests [31] consisting of two adjacent chambers whose internal dimensions are 8 × 4 × 0.75 cm3 and 8 × 2 × 0.75 cm3 communicating at one end. Three Myrmica scabrinodis sclerotised worker pupae, three white gynes pupae and three Myrmica karavajevi white gyne pupae were randomly located on a 0.4 cm3 moist sponge (to preserve humidity) at the end of one compartment, which was then covered with transparent glass. Nine M. scabrinodis workers were then placed in the chamber together with the colony brood. We covered the other chamber with a dark glass. After the workers had rested for 10 min in the dark, we shone a 60 W light placed 10 cm away onto the chamber containing the worker ants and brood, to create a high level of stress which induced workers to rescue the exposed brood items and carry them into the dark chamber. We recorded the order and the time at which each item of brood was rescued. We performed three replicates for each of the three M. scabrinodis colonies used for testing the rescue behaviour and using different workers and brood. Therefore, a total of 81 brood items (27 for each of the three categories) was tested.
2.3. Chemical Analysis
The analyses of CHCs were performed using the protocol described by Csata et al. [32]. Briefly, 7 M. scabrinodis queens, 5 males, 7 workers and 7 M. karavajevi queens and 5 males belonging to three colonies were placed in separate clean glass vials and their CHCs were solvent-extracted using 200 μL of hexane for 20 min. The extracts were then stored at −20 °C until analysis. Prior to chemical analysis, 800 ng of n-eicosane (n-C20) were used as an internal standard. After evaporation under a nitrogen flow to achieve a final volume of 20 μL of heptane, we analysed 2 μL of each sample in an Agilent 7890B gas chromatograph coupled with an Agilent 7000C mass spectrometer using a Gerstel MPS autosampler. For the technical description of the GC, the program of temperatures used as well as the mass spectra setting refer to Csata et al. [32]. We analysed mass spectra by compiling previous publications and assessing fragmentation by comparing patterns obtained from the injection of standard series of n-alkanes. We manually integrated the chromatograms to calculate the area of each peak using the proportion of the sum over the area of all peaks. The absolute quantity of CHCs (ng) per ant was measured as the sum of all the peaks area divided by the peak area of the internal standard (n-C20) and multiplied by 800 (ng of the internal standard used per each sample). The resulting amount of each CHC was divided by the individual’s surface area in square millimetres to account for the different sizes of host and parasite castes. For the calculation of the surface area, we took pictures of individual ants using a Leica EZ4 W stereomicroscope. Then, the ant bodies were ideally divided into geometric areas and their dimensions were measured using the software ImageJ 1.53a (see [33] for a detailed description of the method).
2.4. Recording of Vibroacoustic Signals
After one week of settlement in laboratory conditions, we recorded the vibroacoustic emissions of individual workers (n = 7) and queens (n = 5) of Myrmica scabrinodis and M. karavajevi queens (n = 5) from three colonies. Recordings were collected with a custom recording equipment described in detail by Riva et al. [34] consisting of a 12.5 × 8 × 2 cm3 recording chamber with a moving-coil miniature microphone (sensitivity: 2.5 mV/Pa/1.0 kHz) attached through the centre (sampling rate set to 44.10 kHz). A second identical moving-coil microphone was used to record in anti-phase the ambient noise. A mixer and output amplifier (dynamic range: 5 Hz to 40 kHz; gain: 53 dB) combined the signals from the two microphone preamplifiers. Overall, the frequency ranged from 20 Hz to 20 kHz and the gain was approximately 83 dB. The equipment was powered by a 12 V gel cell battery, and the recording chamber and microphones were located inside an anechoic chamber to further reduce ambient noise and interference.
Ants were individually placed on the microphone surface within the recording chamber and recorded in the morning at room temperature (23–25 °C). Each ant was recorded for 10 min, starting 5 min after its introduction into the recording chamber.
Segments containing vibroacoustic recordings were carefully inspected, checked for clipping and digitally saved in WAV format (16-bit amplitude resolution) on a computer using Audacity v. 2.2.2 [35]. The temporal and spectral features of the signals were measured using Praat v. 6.1.05 [36].
We then randomly selected 4–7 trains per individual consisting of a variable number of pulses and measured for each pulse 15 temporal and spectral parameters (Supplementary Table S1). We then computed a pairwise Spearman rank correlation analysis on the 15 vibroacoustic parameters using R v. 4.0.3. From a pair of parameters with rs > 0.7, only one was selected for analysis. This method yielded seven vibroacoustic variables: the third quartile of the energy spectrum (Q75%, Hz; 75% of the call energy); the frequency peak (Fpeak, Hz); the frequency standard deviation (SDQ50, Hz); the relation of the frequency peak energy to the call total energy expressed as a percentage (%EFpeak); the mean intensity of the entire call represented by the root-mean-square signal level (RMS, dB), the pulse duration (Δt, s), and the pulse rate (PR, s−1; calculated as 1/tstart(x) − tstart(x + 1)).
2.5. Scanning Electron Microscopy
Six host queens and workers and five parasite queens were dissected between the postpetiole and the abdomen to reveal the stridulatory organ (SO) made of the pars stridens and the plectrum. The head and the two ant parts of each individual were mounted on the same steel stub and coated with gold, and the samples were scanned using a Cambridge Stereoscan S360 scanning electron microscope. The SEM operated at 20–25 kV.
We then measured the length (maximum distance between the basal and the apical ridge bisecting the organ in two equal parts) and width (maximum ridge length) of the pars stridens using the software ImageJ 1.53a [37] and counted the number of ridges present on the pars stridens per 100 μm.
2.6. Playback
Playback assays were carried out in three 7 × 7 × 5 cm3 artificial arenas with the speaker attached at the bottom of the box as described in detail by Sala et al. [19]. We covered the speaker with a thin layer of slightly humid soil. Ten host workers from the same M. scabrinodis colony were positioned in each arena and allowed to settle for 10 min before being played one of four vibroacoustic signals (M. scabrinodis queen, M. scabrinodis worker, M. karavajevi queen, and white noise as control). We used MP3 devices to play loops of the original recordings, adjusting volumes to the natural level (see Sala et al. [21] for detail). Each trial lasted 40 min: counts were made of all instances of antagonistic and attractive behaviours, during periods of one minute for each box, and in sequence between the four treatments, i.e., 10 min in total for each signal per trial. Each playback experiment was repeated 9 times, using fresh ants from three different M. scabrinodis colonies (i.e., three times for each colony), therefore a total of 360 workers was tested. The source of signals for each arena was randomised to control for possible positional effects. New soil was introduced, and all the equipment was cleaned with absolute alcohol between trials.
2.7. Statistical Analysis
Statistical analyses were performed with the software R v.4.0.0 [38].
Brood rescue data were analysed using the R package “coin”. Kruskal–Wallis tests were used to compare the rescue order and time (s) of different brood categories. Subsequent pairwise comparisons of median rescue order and time (s) between brood categories were made using Wilcoxon–Mann–Whitney tests. p values were calculated against a null distribution generated from data using a Monte Carlo resampling.
Partial least squares discriminant analysis (PLS-DA), implemented in the “mixOmics” R package [39], was used to determine differences or similarities between members of different castes based on their CHC profiles [40,41]. The PLS-DA is a supervised pattern recognition technique which is largely unaffected by co-variance among chemicals and small ratio between cases (samples) and variables (chemicals) [42,43]. In addition, the predict function was used to carry out a full cross validation (leave-one-out) test based on Mahalanobis distance, thus obtaining a contingency table (confusion matrix) of posterior-predicted/observed membership of all analysed samples. We reiterated this operation 999 times and calculated the average (±SE) percentages of assignment for each caste. Discriminant cuticular hydrocarbons responsible for chemical profile variation were identified according to their influence on the projection (VIP) parameter [44]. We also calculated Euclidean distances between M. karavajevi and M. scabrinodis queens on the basis of log-transformed data of epicuticular hydrocarbon concentrations (ng/mm2). The intra- and inter-colonial distances were then compared using the Welch Two Sample t-test after checking for normality.
We draw a non-metric multidimensional scaling ordination (NMDS) based on Euclidean distances using the R package “vegan” [45] on the normalised vibroacoustic parameters of signals emitted by the recorded specimens. To test for differences in vibroacoustic signals among the host (queens and workers) and parasite (queens) castes, we conducted a permutational multivariate analysis of variance with 999 permutations using the nonparametric adonis2 function implemented in the R package “vegan”. Vibroacoustic parameters were compared among castes using a linear model approach (LM, maximum likelihood fit). Tukey’s HSD test was used to calculate post-hoc comparisons on each factor in the model using the function glht from the R package “multcomp” [46].
Finally, the effect of the vibroacoustic stimulus on the sum of all worker ant behaviours and on each separate behaviour was analysed in a linear mixed-effects model with “colony” as a random factor. Post-hoc sequential comparisons among factor levels were carried out using the lsmeans function from the “lsmeans” R package [47]. A Wilcoxon-Mann-Whitney Test was used to assess differences in the SOs of host queens and workers and parasite queens.
3. Results
3.1. Social Status of Parasite and Host Brood
In the rescue assays (Figure 1a; Supplementary Table S2), M. karavajevi white pupae were saved ahead of M. scabrinodis queen white pupae (Mann-Whitney test: U = −2.363, p = 0.018) and worker sclerotised pupae (U = −4.004, p < 0.001). Among the colony brood, the worker sclerotised pupae were the last to be rescued, significantly behind the host queen unsclerotised (white) pupae (U = −2.716, p = 0.007). When we considered the rescue time (Figure 1b; Supplementary Table S2), M. scabrinodis workers took the same time to rescue the parasite and the host queen white pupae (U = −1.3058, p = 0.198) but the removal of the workers sclerotised pupae took longer compared to the other brood items (compared with parasite queen white pupae—U = −2.995, p = 0.003; compared to host queen white pupae—U = −2.1701, p = 0.031).
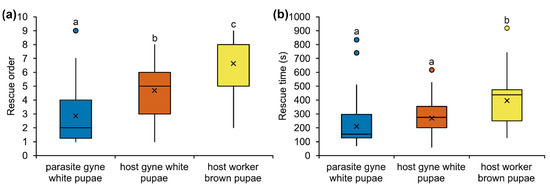
Figure 1.
Boxplots illustrate (a) the order and (b) the time (s) in which M. scabrinodis worker ants rescued M. karavajevi queen unsclerotised (white) pupae, host queen unsclerotised (white) pupae and host worker sclerotised (brown) pupae, after their nests were perturbed by exposure to light. Horizontal line = median rank of rescue, x = average value; box = 25th–75th percentiles; whiskers = minimum and maximum values; dots = outliers. Different letters indicate significantly different categories based on Wilcoxon-Mann-Whitney Test (Supplementary Table S2).
3.2. Chemical Integration
Gas-chromatography analysis revealed a total of 45 cuticular hydrocarbons present on the cuticle of the analysed specimens (Appendix A, Table A1). The branched alkanes represented the most abundant hydrocarbon class (21 peaks of which 15 monomethylated and six dimethylated alkanes), followed by alkenes (13 peaks, of which nine monoenes and four dienes) and linear alkanes (11 peaks). Overall, five peaks (n-C23, n-C25, 3-MeC23, as well as one diene and one monoene both constituted by 25 carbons) were present in a high proportion, ranging from about 65% in the case of M. scabrinodis queens, up to about 90% in the workers of the same species (Appendix A, Table A1). Interestingly, specimens of host and parasite queens showed the most complex cuticular profiles constituted by the same number (45) of hydrocarbons (Appendix A, Table A1).
PLS-DA on the log-transformed concentrations (ng/mm2) of cuticular hydrocarbons well separated M. scabrinodis worker and male specimens and M. karavajevi males (Figure 2a). On the contrary, host and parasite queen individuals partially overlapped. Full cross validation confirmed these observations with M. scabrinodis workers and males and M. karavajevi males showing 98–100% of specimens being correctly assigned while host and parasite queens having lower percentages (see contingency table in Figure 2b). M. karavajevi queens were mistaken with higher percentages for host colony queens (27% ± 9%) while M. scabrinodis queens were confused with the parasite queens in 8% ± 5% of cases. If we consider the queens (of the host and the parasite) as a whole, the error rate can reach 35% ± 14%, i.e., which in the most extreme cases translates into a misattribution percentage of 50% between host and parasite.
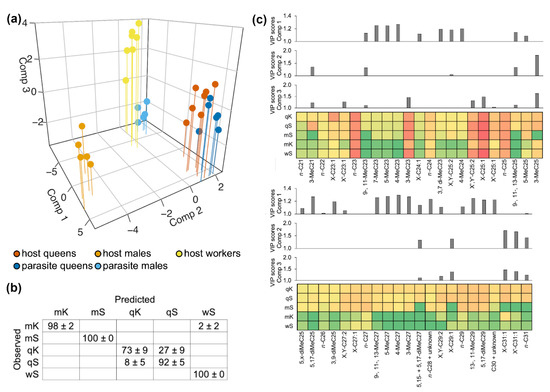
Figure 2.
(a) Representation of standardized components 1, 2 and 3 of partial least squares discriminant analysis for log-transformed concentrations (ng/mm2) of cuticular hydrocarbons found on the cuticle of parasite and host castes (qS = M. scabrinodis queen; mS = M. scabrinodis males; wS = M. scabrinodis workers; qK = M. karavajevi queen; mK = M. karavajevi males). The first three components accounted respectively for the 61%, 13% and 9% of the total variation. (b) Below, the contingency table showing percentages (± SE) of assignment for each caste after full cross validation tests is reported (qS = M. scabrinodis queen; mS = M. scabrinodis males; wS = M. scabrinodis workers; qK = M. karavajevi queen; mK = M. karavajevi males). (c) For each of the three components obtained from the PLS-DA the variable importance in projection (VIP) score plot is reported. Below the VIP score plots, the colored squares (green = minimum values; red = maximum values) represent the average log-transformed concentrations (ng/mm2) of the corresponding compounds present on the cuticle of each caste.
Overall, the first three components accounted for 83% of CHC variation. The first component contributed to separate the specimens in two groups, one including the host and parasite queens and the host males while the second including the host workers and parasitic males (Figure 2a; see also Supplementary Figure S1 where only the first two axes are represented). The variable importance plots (VIP, Figure 2c) showed that numerous branched alkanes contribute the most to explain the separation in groups observed on the first axis. On the second axis, it is worth noting the clear separation of M. scabrinodis males which have different concentrations in some methylated alkanes, e.g., 3-MeC21 and 3-MeC25 and long-chain monoenes, e.g., C29: 1 and C31: 1.
It is also interesting to note how the concentrations of some linear alkanes, e.g., n-C21, n-C22, n-C23, n-C24, n-C25, are very similar between hosts and parasites, having VIP scores below 1. Some of these linear compounds, e.g., n-C23 and n-C25, constitute a large portion of the CHC profile, with maximum values in some castes equal to 30% in the case of n-C23 and 18% for n-C25.
Finally, when we compared Euclidean distances based on chemical profiles of parasite queens and host colony queens with the distances between parasite and non-host colony queens, we found no significant differences (t-test: t = −0.91522, df = 23.025, p = 0.3696).
3.3. Vibroacoustic Integration
3.3.1. Vibroacoustic Signals
We recorded and analysed the stridulation produced by M. scabrinodis workers and queens and from M. karavajevi queens, for a total of 1204 pulses. Ants’ stridulation patterns were similar to those described in previous works carried out on the genus Myrmica [17,21,48]. The stridulations were constituted by series (trains) of a variable number of pulses (Figure 3).
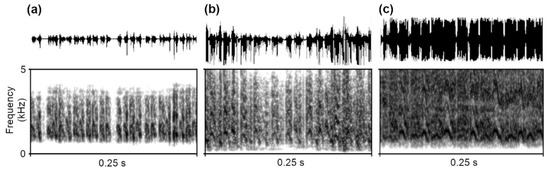
Figure 3.
Oscillograms and spectrograms of the stridulations emitted by (a) M. karavajevi queens and (b) M. scabrinodis queens and (c) M. scabrinodis workers. Spectrograms were generated in Praat using the following parameters: window shape = Gaussian, window length = 0.015 s, number of time steps = 1000, number of frequency steps = 500, dynamic range = 70 dB.
Our data showed that the vibroacoustic signals produced by the host queens and workers have similar temporal and frequency characteristics (Figure 4; Supplementary Table S3), i.e., the duration of the pulses (∆t), the frequency peak (Fpeak) and the third frequency quartile (Q75%), which differ from the signals emitted by M. karavajevi queens. The peak frequency is higher in the pulses produced by the parasitic queens, but the higher range of frequencies seems to be generally lower than in host signals (consider the third frequency quartiles). Nevertheless, some characteristics, i.e., the pulse repetition frequency (PR) and the percentage of energy (%EFpeak) allocated for the emission of the frequency peak, were similar between host and parasite ants (Figure 4). In addition, the root-mean-square (RMS) signal level resulted identical between the signals of the host and parasite queens, while the frequency standard deviation (SDQ50) differs among the three categories (qK, qS, wS; Figure 4).
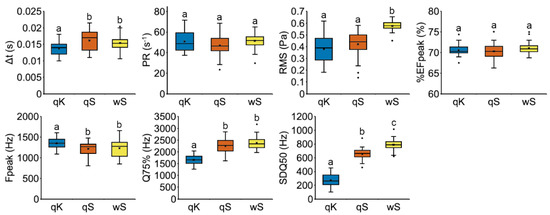
Figure 4.
Boxplots of vibroacoustic parameters of stridulation pulses emitted by M. scabrinodis queens (qS), workers (wS) and M. karavajevi queens (qK). Pulse duration (∆t), pulse repetition frequency (PR), root-mean-square (RMS), percentage of energy (%EFpeak), frequency peak (Fpeak), third frequency quartile (Q75%), frequency standard deviation (SDQ50). Horizontal lines represent median values, the boxes the first and third quartiles, whiskers maximum and minimum values, while the crosses indicate average values. Dots represent outliers. Lower-case letters above boxplots indicate pairwise significant differences between castes based on Tukey’s HSD test (Supplementary Table S3).
Non-metric multidimensional scaling ordination carried out on the vibroacoustic parameters of pulses shows overall separation among the three categories (host queen and worker, and parasite queens), confirmed by the permutational multivariate analysis of variance (F2,112 = 26.344; p = 0.0001). The signals emitted by M. karavajevi queens were statistically different from those emitted by both M. scabrinodis workers (F1,78 = 51.765; p = 0.0001) and (F1,78 = 23.261; p = 0.0001) queens. Nevertheless, the parasite signals were closer to those of the host queens than to those emitted by the worker caste of the colony. Moreover, the overall vibroacoustic signal is different between host queen and workers (F1,78 = 9.497; p = 0.0001).
3.3.2. Playback Assays
During playback experiments, worker ants did not exhibit any antagonistic or alarmed behaviour (alerting) but four benevolent responses were observed, i.e., walking, antennating, staying on the speaker, and digging.
The observed behaviours involve attraction or benevolent reactions: (1) walking—the worker was attracted to the speaker but walked over it without stopping on it; (2) staying—the workers rested on the speaker for at least 5 s; (3) antennating—the worker antennated the speaker for at least 3 s; (4) digging—the worker dug into the soil surrounding the speaker. The response called repulsion (the ant abruptly changed direction, moving away from the speaker) was observed only when the control stimulus (white noise) was played back to workers.
Generalized linear models showed that worker reactions to the four vibroacoustic stimuli were significantly different for walking, antennating, staying and for the total behaviours. Digging behaviour, which was observed on a few occasions, was never elicited by white noise (Figure 5b; Supplementary Table S4). Compared to white noise, ant vibroacoustic signals always induced significantly more instances of walking, antennating, and staying by Myrmica worker ants (0.019 < p < 0.0001). Both vibroacoustic emissions by host and parasite queens elicited the highest behavioural response in worker ants for walking, antennating, and staying, while no significant difference between the two stimuli was detected for any behaviour (Figure 5b; Supplementary Table S4).
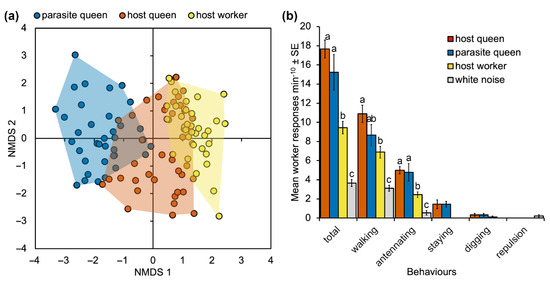
Figure 5.
(a) Non-metric multidimensional scaling ordination of vibracoustic signals emitted by M. scabrinodis workers and queens and M. karavajevi queen ants based on normalized Euclidean distances calculated using pulse parameters. Each point represents ‘average’ values of pulse parameters calculated over a train of pulses (see “Methods” for more details) (STRESS = 0.18). (b) Behavioral responses of Myrmica scabrinodis workers to vibroacoustic emissions of M. scabrinodis queens and workers, M. karavajevi queens and to one control signal (white noise). Lower-case letters above columns indicate pairwise significant differences between vibroacoustic stimuli based on Tukey’s HSD test (Supplementary Table S4).
3.3.3. Stridulatory Organs (SO)
The SO of M. karavajevi is described for the first time within the present work (Figure 6). Overall, it is very similar to the organ present in all other species of the genus Myrmica studied so far [16,17,21,48,49].
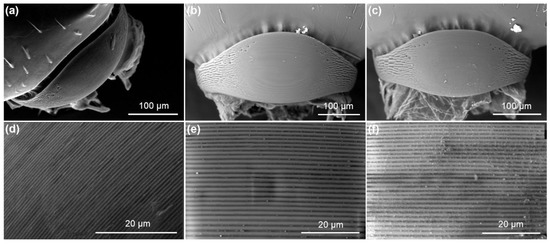
Figure 6.
Electron Scan Microscope images of the whole pars stridens and ridges of (a,d) Myrmica karavajevi queens and (b,e) M. scabrinodis queens and (c,f) workers.
However, M. karavajevi SO differs in some morphological characteristics from the SOs of the host castes. In detail, the pars stridens in M. karavajevi is smaller (width and length) than that of M. scabrinodis queens (Figure 7). In contrast, the pars stridens width is similar in M. karavajevi and host ant workers, but the latter possess a longer organ (Figure 7; Supplementary Table S5).
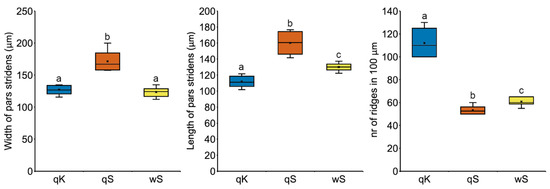
Figure 7.
Boxplots of the main characteristics measured on the stridulatory organs of the three categories, i.e., the width and length of the pars stridens and the number of ridges distributed along 100 μm (see Section 2.5 for details). Horizontal line = median rank of rescue, box = 25th–75th percentiles; whiskers = minimum and maximum values; crosses = average values. qK = parasite queen; qS = host queen; wS = host worker. Different letters indicate significantly different categories based on Wilcoxon-Mann-Whitney Test (Supplementary Table S5).
This similarity in the pars stridens to the host worker caste is explained by a similar body size of M. karavajevi queens to the adult workers. Despite this, the ridge density of the parasite’s pars stridens is much greater than that of the two host castes’ organs (Figure 7; Supplementary Table S5).
Furthermore, M. karavajevi SO differs in form. If we compare the ratio between its width and height among the three categories (parasite queen, host queens and workers), this value is statistically different (Kruskal–Wallis test: H = 6.393, df = 2, p-value = 0.041). The organ of the parasite has an elongated shape, i.e., the median value of the ratio between width and length is equal to 1.147 and is statistically different (U = 2.556, p-value = 0.0092) from the ratio measured on the host workers (median value = 0.934). The SO of host queens, on the other hand, has an almost circular shape with a median value of the ratio between width and length equal to 1.079, which does not differ from M. karavajevi (U = 0.912, p-value = 0.426), nor from workers (U = 1.4412, p-value = 0.179).
4. Discussion
Our results show that Myrmica karavajevi is intimately integrated within the colonies of its host M. scabrinodis. To achieve its acceptance within host nests, the parasite has intercepted in the course of evolution the chemical cues to fully integrate into the colonies and the acoustic signals to gain the worker’s attention and protection. The inquiline species seems to occupy the highest place in the hierarchy of the colony, a condition found in the immature stages, which are saved by host ants in preference to their own brood in rescue experiments.
Ant brood, by using different cues, contains information about development stage, caste, sex or species and adult workers can recognise it [50]. Social parasites, at which brood parasitism occurs, are under the pressure to avoid host detection and evolve strategies allowing brood to survive and develop and be treated by host workers as their own larvae and pupae. It is known that ants, as the first action after nest disturbance, rescue their brood and that the rescue order depends on social hierarchy among the different young stages, reflecting colony investment in each of these stages [48,51]. Therefore, the results of our rescue experiment suggest that M. karavajevi pupae achieve the highest rank in the colony hierarchy and they are fully integrated in the colony structure as they were the first ones taken by host workers, even before host queen pupae. As inquilinism in ants is rare and at least studied in the sense of host–parasite coevolution [3], there is no other data on ant inquilinism system to compare with. By contrast, the ecology of inquilinism was more extensively studied in parasite wasps (Hymenoptera: Vespidae) [11] and results of some studies on this insect group show that socially parasitic brood can reach high integration and rank status inside host colony. It was demonstrated that larvae of Polistes sulcifer (Zimmermann, 1930), the obligate social parasite of Polistes dominula (Christ, 1791) paper wasp, can monopolise host parental care and grow much faster compared to the host brood [52]. The acceptance and integration of parasite immature brood was also found in the case of Maculinea butterflies, which are obligate social parasites of Myrmica ants. In the rescue experiments M. rebeli (Hirschke, 1904) larvae, just after one week of integration inside the host colony, were chosen equally with the kin pupae of Myrmica host and significantly ahead of kin larvae [53].
Such high parasitic brood integration inside a host colony can be achieved by using various adaptations. Our results demonstrate the ability of M. karavajevi to break multiple communication channels of their host. The comparison of CHCs among castes revealed that M. karavajevi queens possess a complex CHC profile that is qualitatively identical to that of the host queens. Although differences in the abundances of each compound exist among castes, contributing to separating them, the chemical profile of the parasitic queens also partially overlaps with that of the host queens.
Imitation of the epicuticular profile of the host colony represents a common strategy among social parasites to overcome the efficient nestmate recognition system which acts as a barrier in eusocial insect colonies, and successfully penetrate the host fortress. Nestmate recognition is usually based on a complex mixture of hydrocarbons covering the insect’s cuticle, which primarily prevent water loss and the entry of pathogens [54], but it also plays an important role in communication, creating a bouquet of substances that characterise each colony [55].
Social parasites can imitate the host recognition code through chemical mimicry, in which the parasite actively synthesises host CHC signals, and the mimetic profile may also be the result of both passive and active acquisition of CHC compounds from the host, a strategy named camouflage [56,57,58]. Instances of camouflage were found in Temnothorax minutissimus (Smith, 1942) ants, which acquire the odour of their Temnothorax curvispinosus (Mayr, 1866) host queens by active grooming [59]. Camouflage is also found in queens of a slave-making ant species such as Polyergus samurai Yano, 1911, when usurping Formica japonica Motschoulsky, 1866 host nests. p. samurai queens obtain a colony-specific signature, which resulted in being much more similar to the chemical profile of the host queen killed. The latter similarity is likely achieved by body contact [60]. This kind of chemical strategy is largely employed in non-ant myrmecophilous species. Among other examples, larvae of a lycaenid butterfly, Niphanda fusca (Bremer & Grey, 1853) exploits Camponotus japonicus Mayr, 1866 ants by acquiring male chemical profiles [61]. However, Hojo and colleagues [61] did not exclude that some methyl-branched compounds typical of male ants can be actively synthesised. Thus, the two chemical strategies (chemical mimicry and camouflage) could occur contemporarily in the same biological system.
Evidence of chemical mimicry is scarce and primarily found in species-specific associations. The biosynthesis of mimetic compounds has been suggested in Leptothorax kutteri Buschinger, 1966 to enhance the exploitation of L. acervorum (Fabricius, 1793) host ants [62]. The majority of studies highlighting the existence of chemical mimicry involved myrmecophilous species other than ants, such as lycaenids of the genus Maculinea. These larvae gain access to the host colony resources by the active production of mimetic compounds. M. rebeli caterpillars synthesise CHCs which are peculiar of their primary host ants Myrmica schencki Viereck, 1903, but which would pinpoint the parasite as a “stranger” in other Myrmica species. Thus, their biosynthesis is basically suppressed when M. rebeli is adopted in non-host Myrmica nests.
Parasites also use a third strategy, chemical insignificance, which is based on a general reduction or a scanty presence of informative CHCs (i.e., n-alkanes) as respectively shown in Brachymyrmex thief ants [11] and in Acromyrmex insinuator Schultz et al., 1998 exploiting its host A. echinatior (Forel, 1899) [63,64].
To understand whether mimetic compounds are biosynthesised or acquired, parasites are usually experimentally isolated from the host colony for a certain time, so that they can reveal their own CHC profile [65,66,67]. In our experiment, we could not do so due to the paucity of sampled nests and the analysed individuals. However, we cannot exclude both strategies to be adopted by the parasite. Indeed, we can hypothesise that M. karavajevi queens may acquire their mimetic CHC profiles living inside the host colonies, coming into contact with the host individuals, and being fed by the workers of the host colony. On the other hand, one clue that may suggest the active biosynthesis of mimetic compounds is related to the size of the parasite. Indeed, the body surface area (see Supplementary Table S6) of parasitic queens is equal to half the body surface (40.8 mm2 ± 3.5 mm2) of M. scabrinodis queens (84.7 mm2 ± 5.1 mm2) and similar to that of the host workers (43.0 mm2 ± 6.1 mm2). The fact that the amount (ng) of hydrocarbons per area is similar between parasitic and host queens suggests that the parasite may be able to synthesize double (or nearly double) amounts of hydrocarbons. It is unlikely for the parasitic queens to acquire such amounts of hydrocarbons by contact, and it is equally unlikely that from simple trophallaxis, used in ant colonies to exchange food or colony odours, queens can then extrude or heavily spread epicuticular compounds on their cuticle in such an amount to mimic the host queens. In addition, when we tested if the CHC profiles of inquilines follow those of the hosts at the colony-level (see Section 3.2), by comparing the Euclidean distances based on the CHC profiles of M. karavajevi queens with host queens from the same colony and different colonies, we did not find any difference. These results suggest that all inquiline queens are chemically similar to host queens in general, but with no obvious differences among colonies, hence pointing towards the mimicry strategy for which the CHC similarity is innate.
However, there are still many doubts about how the recognition process works in eusocial insects. For instance, it is not clear whether some signals are more informative than others and whether the simple presence/absence of one or more compounds can be decisive in the recognition of nestmates or whether CHC concentrations are also used for discriminating between nestmate and non-nestmates [68,69]. Some works have emphasized the quality of cuticular hydrocarbons by attributing different importance for the purposes of intra- and interspecific communication to the three classes of hydrocarbons, i.e., linear alkanes, branched alkanes, and alkenes. In certain experiments, the supplementation of linear alkanes to non-nestmates does not cause a defensive reaction in the individuals of the host colony, while the alkanes and branched alkenes do [70,71]. Furthermore, the greater the quantity of these complex hydrocarbons, the stronger the defensive reaction of the hosts becomes [72]. Recently, however, the role of linear alkanes has been revised in another key. In colonies of M. scabrinodis, infected with the ectoparasitic fungus Rickia wasmannii Cavara, 1899, infected individuals showed a percentage increase of some linear alkanes, i.e., n-C23 and n-C24, and the same individuals were attacked less than uninfected individuals in which the epicuticular profile was unchanged [32]. At the same time a decrease of another methylated alkane was also observed, i.e., 3-MeC23, which has suggested that the linear CHC and its methylated counterpart may be part of a recognition mechanism that, once discovered by some social parasites, may have favoured the integration and exploitation of Myrmica colonies.
In our study, the queens of the parasite have high percentage levels of n-C23 (15.81 ± 5.31) and lower percentage levels of the methylated counterpart, i.e., 3-MeC23 (8.37 ± 1.98). It is therefore possible that the parasite, besides having developed an overall similarity towards the host queen CHC profile, may rely on only some compounds for its deception system, perhaps linear alkanes, whose increase seems to have the ability to reduce the aggressiveness in the workers of M. scabrinodis. This fact appears to be even more evident for the parasitic males that besides possessing CHC profiles, closer to that of the workers of the colony (Figure 1a), reach percentage levels of n-C23 equal to 30% which, in addition to those of n-C25, constitute half of their epicuticular profile. Such a strategy could be beneficial for the parasite males, who find themselves flickering inside the host colony, and must avoid being attacked by the M. scabrinodis nurses with the brood chambers.
Besides an efficient imitation of the overall chemical profile of their hosts (or the use of certain compounds such as linear alkanes) to fool or appease Myrmica ants, M. karavajevi queens are also able to break a second communication code by mimicking the host queen’s stridulations.
Indeed, vibroacoustic communication is extensively used by ants from different subfamilies, e.g., Myrmicinae, Ponerinae [73], both inside and outside their nests (see [74] for a review), therefore it is likely that intruders might produce signals directed to disrupt this type of communication. This ability has been observed in a few other social parasites such as butterflies [17,21,74] and beetles [75]. An increasing body of evidence suggests that vibroacoustic emissions are not only attractive cues but rather are used to signal the social rank within the colony, at least in some Myrmicinae ants. The studies performed on species belonging to Myrmica [17] and Pheidole [75] genera reveal that ants are capable of producing caste-specific vibroacoustic signals, which can elicit several distinct responses in nest-mates.
The analysis of similarities performed on the vibroacoustic signal emitted by different M. scabrinodis castes and M. karavajevi queens (Figure 5) confirms that host queens and workers overall produce distinct sounds and reveals that the parasite signals better resemble those of host queens rather than workers. The ability to produce signals matching those of the highest rank caste can be partially explained by the morphology of the stridulatory organ (SO). The pars stridens shape of M. karavajevi SO is much more similar to host queen SO than to workers SO, although the ridge numbers strongly differ among the three categories (host queens and workers and parasite queens). The width and height of the SO differs between host and parasitic queens due to the size of the body, but the shape, described by the relationship between width and height, is similar. On the contrary, the shape of the SO of the queens of M. karavajevi and the host workers is different, but the individual of these two castes have the same body size. If we compare the morphology with the characteristics of the signals produced, the difference in the number of ridges, higher in the parasitic queens, could explain the diversity of the frequency parameters found in the analysis of the acoustic emissions.
However, it is still unknown whether ants perceive the whole vibroacoustic signal or if one or few components may be more informative than others [34]. In our behavioural experiments, we played back the full repertoire of the whole recordings, thus assessing the overall effect of acoustic emissions on the ant colony. During playback experiments, we found that the queen-like sounds produced by the parasite and the queen host vibroacoustic emissions cause the highest rate of benevolent response in workers, exactly as observed for Maculinea butterfly parasite [17]. Unlike what is known in the latter host-parasite systems, the information available on the life cycle of M. karavajevi is scanty. Therefore, we do not know if adult stages of the parasite are indeed treated like royalty in the host ant colony, as it happens for Maculinea larvae in Myrmica nests [65].
M. karavajevi’s ability to break both the chemical and acoustic code of the host is not surprising. Indeed, there is a close phylogenetic relationship between the parasite and the host species. In some cases, host and parasite are closely related sister species and follow the strict Emery’s rule, while in other cases they belong to the same genus, following the relaxed version of Emery’s rule, as happens in the case of M. karavajevi and M. scabrinodis.
In addition, M. karavajevi can use several host species and some studies have hypothesized that some sort of local sympatric speciation has occurred within populations [25,76,77], which led to the formation of cryptic genetic lines, each specialized on a particular species of Myrmica of the scabrinodis-group, which all colonize warm but relatively humid environments [27].
The close phylogenetic origin of many obligate social parasites with their hosts indicates that they share many traits of evolutionary history and have developed strategies to efficiently exploit the host system, deriving from ancestral phenotypic traits [8,10,78]. Thus, such examples are particularly valuable for exploring the mechanistic basis, such as molecular regulatory processes, of social traits that are subtly (but measurably) different between host and parasite. When closely related species of parasites and hosts are compared, it is difficult to assess if the similarity in certain phenotypic traits, e.g., CHCs or vibroacoustic parameters, is due to adaptation or to common ancestry. Some hints could be provided by comparing the chemical profile of another member of the scabrinodis-group, M sabuleti Meinert, 1861 [79]. As M. karavajevi shares a common ancestry with both M. scabrinodis and M. sabuleti, we could hypothesise that a higher chemical similarity of the parasite to its host might be due to the coevolutionary dynamics rather than to phylogenetic closeness. In the work by Guillem and colleagues [79], indeed, the CHC compounds used to differentiate M. scabrinodis and M. sabuleti, these two closely related species, are two methylated alkanes, i.e., 3-MeC23 and 5-MeC25, present in greater proportions only in one of the two species, respectively. If we compare the relative proportions reported in Guillem et al. [79] with our data, M. karavajevi queens possess the 3-MeC23, the characteristic compound of M. scabrinodis, in higher proportions (10%), while the relative proportion of 5-MeC25, characteristic of M. sabuleti, is close to 1%, suggesting a closer adaptation towards M. scabrinodis. However, this remains to be formally tested. As suggested by Jansen et al. [25], M. karavajevi may even represent a complex of cryptic species, each adapted to a specific host ant, but a comparison between multiple host and parasite populations would be necessary.
Overall, the results from rescue experiments suggest that M. karavajevi, at least as gyne pupae, achieves a high social status in the colony hierarchy. However, further research is needed to unravel if this royal treatment is also gained by the adult parasite queens and obtained through a supernormal stimulus (sensu Tinbergen [80]), as our acoustical and chemical data seem to suggest.
5. Conclusions
A plethora of distinct signals (chemical, vibroacoustic, tactile and visual) concur to a fine multimodal communication in ants, which is at the base of the complex social structure of the colony. Nevertheless, we have poor knowledge about how parasites might manipulate ant responses and behaviours by means of multiple cues. It is likely that chemical and vibroacoustic communications have evolved independently, but in strictly obligate parasites these two channels may have started to function in a multimodal way to let the parasite achieve the highest level of integration within the colony.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10070654/s1, Figure S1: Representation of standardized components 1 and 2 of partial least squares discriminant analysis for log-transformed concentrations (ng/cm2) of cuticular hydrocarbons, Table S1: List of the 15 vibroacoustic parameters, Table S2: Results of Kruskall-Wallis test and Wilcoxon-Mann-Whitney Test on rescue order and time (s) of brood items within Myrmica scabrinodis colonies, Table S3: Results of linear models on the vibroacoustic parameters, Table S4: Results of generalized linear models of M. scabrinodis worker responses to different vibroacoustic stimuli, Table S5: Results of Kruskall-Wallis test and Wilcoxon-Mann-Whitney test on the main features of the stridulatory organs, Table S6: Mean (± SD) values of body surface (mm2) for each caste.
Author Contributions
Conceptualization, L.P.C., F.B., and M.W.; methodology, L.P.C., F.B., P.Ś., and M.W.; formal analysis, L.P.C.; investigation, L.P.C. and F.B.; writing—original draft preparation, L.P.C., F.B., and M.W.; writing—review and editing, L.P.C., F.B., P.Ś., and M.W.; visualization, L.P.C.; supervision, L.P.C.; project administration, L.P.C.; funding acquisition, L.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
L.P.C. was supported by the National Science Center, Poland, under grant Polonez UMO−2016/23/P/NZ8/04254. This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska Curie grant agreement No 665778.

Institutional Review Board Statement
To obtain the data presented in the manuscript it was necessary to involve animals, however, both model species are not under protection and during the specimen collection only small fractions of the ant nests were gathered to preserve the integrity of the colonies.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are openly available in the GitHub repository at: https://github.com/lcasacci/Casacci_et_al_2021_kara.git (accessed on 22 June 2021).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Relative proportions ± SD of each cuticular hydrocarbon constituting the profile of Myrmica scabrinodis queens, workers and males and M. karavajevi queens and males. Compounds reported in bold contribute overall to a large proportion of the cuticular profile.
Table A1.
Relative proportions ± SD of each cuticular hydrocarbon constituting the profile of Myrmica scabrinodis queens, workers and males and M. karavajevi queens and males. Compounds reported in bold contribute overall to a large proportion of the cuticular profile.
| Myrmica scabrinodis | Myrmica karavajevi | |||||
|---|---|---|---|---|---|---|
| nr | CHC | Queens | Workers | Males | Queens | Males |
| 1 | n-C21 | 0.14 ± 0.11 | 0.12 ± 0.08 | 0.06 ± 0.01 | 0.42 ± 0.15 | 0.12 ± 0.04 |
| 2 | 3-MeC21 | 5.64 ± 5.22 | 0.11 ± 0.13 | --- | 0.71 ± 0.16 | 0.14 ± 0.09 |
| 3 | n-C22 | 0.28 ± 0.1 | 0.3 ± 0.08 | 0.35 ± 0.11 | 0.64 ± 0.18 | 0.32 ± 0.09 |
| 4 | X-C23:1 | 0.72 ± 0.19 | 0.55 ± 0.33 | 0.86 ± 0.3 | 7.28 ± 1.72 | 1.1 ± 0.15 |
| 5 | X’-C23:1 | 0.85 ± 0.26 | 0.67 ± 0.34 | 0.87 ± 0.14 | 1.02 ± 0.14 | 0.16 ± 0.1 |
| 6 | n-C23 | 8.4 ± 2.63 | 19.43 ± 7.17 | 11.76 ± 1.11 | 15.81 ± 5.31 | 30.93 ± 4.91 |
| 7 | 9-, 11-MeC23 | 0.2 ± 0.08 | --- | --- | 0.3 ± 0.06 | --- |
| 8 | 7-MeC23 | 0.19 ± 0.08 | 0.02 ± 0.01 | 0.22 ± 0.1 | 0.28 ± 0.07 | 0.02 ± 0.01 |
| 9 | 5-MeC23 | 0.17 ± 0.11 | 0.02 ± 0.01 | 0.25 ± 0.15 | 0.31 ± 0.06 | 0.02 ± 0.01 |
| 10 | 4-MeC23 | 0.17 ± 0.08 | --- | 0.16 ± 0.13 | 0.27 ± 0.05 | --- |
| 11 | 3-MeC23 | 9.93 ± 1.63 | 14.2 ± 6.14 | 12.78 ± 1.92 | 8.37 ± 1.98 | 8.06 ± 1.04 |
| 12 | X-C24:1 | 0.18 ± 0.09 | 0.03 ± 0.03 | 0.16 ± 0.12 | 0.27 ± 0.11 | 0.04 ± 0.02 |
| 13 | n-C24 | 0.83 ± 0.16 | 1.07 ± 0.53 | 1 ± 0.16 | 1.52 ± 0.41 | 1.83 ± 0.53 |
| 14 | 3,7 di-MeC23 | 0.29 ± 0.11 | 0.07 ± 0.04 | 0.23 ± 0.12 | 0.47 ± 0.09 | 0.04 ± 0.03 |
| 15 | X,Y-C25:2 | 0.41 ± 0.15 | 0.05 ± 0.02 | 0.66 ± 0.17 | 0.39 ± 0.08 | 0.02 ± 0.01 |
| 16 | 4-MeC24 | 0.3 ± 0.06 | 0.06 ± 0.04 | 0.31 ± 0.17 | 0.44 ± 0.08 | 0.04 ± 0.02 |
| 17 | X’,Y’-C25:2 | 11.66 ± 9.84 | 9.99 ± 4.83 | 6.13 ± 1.6 | 3.84 ± 3.05 | 4.09 ± 1.62 |
| 18 | X-C25:1 | 30.97 ± 8.84 | 42.77 ± 10.37 | 37.45 ± 5.28 | 21.66 ± 4.89 | 26.16 ± 3.7 |
| 19 | X’-C25:1 | 2.12 ± 0.54 | 2 ± 1.15 | 2.64 ± 0.62 | 1.82 ± 0.69 | 1.32 ± 0.4 |
| 20 | n-C25 | 4.8 ± 1.54 | 5.19 ± 3.99 | 7.18 ± 1.06 | 5.69 ± 1.06 | 17.59 ± 4.68 |
| 21 | 9-, 11-, 13-MeC25 | 0.56 ± 0.26 | --- | --- | 1.19 ± 0.53 | --- |
| 22 | 5-MeC25 | 1.17 ± 0.27 | 0.32 ± 0.19 | 1.34 ± 0.31 | 1.26 ± 0.41 | 0.25 ± 0.15 |
| 23 | 3-MeC25 | 2.1 ± 0.56 | 1.28 ± 0.75 | 1.9 ± 0.68 | 2.31 ± 0.67 | |
| 24 | 5,x-diMeC25 | 0.29 ± 0.1 | 0.07 ± 0.06 | 0.53 ± 0.36 | 0.36 ± 0.1 | 0.04 ± 0.02 |
| 25 | 5,17-diMeC25 | 0.56 ± 0.27 | --- | 0.52 ± 0.17 | 0.41 ± 0.16 | --- |
| 26 | n-C26 | 0.37 ± 0.13 | 0.1 ± 0.08 | 0.34 ± 0.23 | 0.63 ± 0.16 | 0.13 ± 0.09 |
| 27 | 3,9-diMeC25 | 0.32 ± 0.18 | 0.01 ± 0.01 | 0.86 ± 0.58 | 0.57 ± 0.22 | 0.02 ± 0.01 |
| 28 | X,Y-C27:2 | 1.13 ± 0.59 | 0.16 ± 0.1 | 1.16 ± 0.3 | 0.94 ± 0.62 | 0.15 ± 0.19 |
| 29 | X-C27:1 | 1.1 ± 0.39 | 0.54 ± 0.51 | 1.13 ± 0.16 | 1.18 ± 0.54 | 0.26 ± 0.23 |
| 30 | n-C27 | 0.87 ± 0.22 | 0.31 ± 0.25 | 1.54 ± 0.51 | 1.24 ± 0.46 | 0.8 ± 0.42 |
| 31 | 9-, 11-, 13-MeC27 | 0.57 ± 0.21 | 0.02 ± 0.01 | 0.83 ± 0.42 | 1.06 ± 0.56 | --- |
| 32 | 5-MeC27 | 0.43 ± 0.11 | --- | 0.78 ± 0.36 | 1.15 ± 0.57 | --- |
| 33 | 4-MeC27 | 0.42 ± 0.08 | --- | 0.53 ± 0.28 | 0.93 ± 0.26 | --- |
| 34 | 3-MeC27 | 0.5 ± 0.18 | --- | 0.96 ± 0.55 | 0.93 ± 0.31 | --- |
| 35 | 5,15- + 5,17-diMeC27 | 0.74 ± 0.22 | --- | 0.97 ± 0.24 | --- | |
| 36 | n-C28 + unknown | 0.32 ± 0.12 | --- | 0.77 ± 0.38 | 0.73 ± 0.22 | 0.02 ± 0.01 |
| 37 | X,Y-C29:2 | 1.27 ± 0.7 | --- | 0.99 ± 0.54 | 1.1 ± 0.42 | 0.31 ± 0.12 |
| 38 | X-C29:1 | 1.17 ± 0.36 | 0.12 ± 0.09 | --- | 1.01 ± 0.32 | 0.02 ± 0.01 |
| 39 | n-C29 | 1.09 ± 0.24 | 0.15 ± 0.11 | 1.59 ± 0.56 | 1.89 ± 0.73 | 0.53 ± 0.21 |
| 40 | 13-, 11-MeC29 | 1.35 ± 0.23 | 0.05 ± 0.04 | 1.57 ± 0.51 | 1.72 ± 0.61 | 0.08 ± 0.06 |
| 41 | 5,17-diMeC29 | 1.44 ± 0.34 | 0.04 ± 0.04 | 1.22 ± 0.36 | 1.77 ± 0.53 | 0.25 ± 0.19 |
| 42 | n-C30 + unknown | 0.54 ± 0.15 | 0.02 ± 0.02 | 0.27 ± 0.23 | 0.92 ± 0.34 | 0.03 ± 0.01 |
| 43 | X-C31:1 | 1.7 ± 1.15 | 0.11 ± 0.12 | --- | 1.76 ± 0.67 | 2.54 ± 1.89 |
| 44 | X’-C31:1 | 0.66 ± 0.15 | 0.04 ± 0.04 | --- | 1.1 ± 0.47 | 0.25 ± 0.08 |
| 45 | n-C31 | 1.08 ± 0.43 | 0.03 ± 0.04 | --- | 1.77 ± 0.51 | 0.02 ± 0.01 |
References
- Michalakis, Y. Parasitism and the evolution of life-history traits. In Ecology and Evolution of Parasitism; Thomas, F., Guégan, J.-F., Renaud, F., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 19–30. [Google Scholar]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, Princeton, NJ, USA, 1998; p. 392. [Google Scholar]
- Buschinger, A. Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 219–235. [Google Scholar]
- Grüter, C.; Jongepier, E.; Foitzik, S. Insect societies fight back: The evolution of defensive traits against social parasites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170200. [Google Scholar] [CrossRef]
- Forel, A. La parabiose chez les fourmis. Bull. Soc. Vaud. Sc. Nat. 1898, 34, 380–384. [Google Scholar]
- Emery, C. Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biol. Zentralbl. 1909, 29, 352–362. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Springer: Berlin/Heidelberg, Germany, 1990; p. 732. [Google Scholar]
- Lowe, R.M.; Ward, S.A.; Crozier, R.H. The evolution of parasites from their hosts: Intra–and interspecific parasitism and Emery’s rule. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1301–1305. [Google Scholar] [CrossRef]
- Brandt, M.; Foitzik, S.; Fischer-Blass, B.; Heinze, J. The coevolutionary dynamics of obligate ant social parasite systems—between prudence and antagonism. Biol. Rev. 2005, 80, 251–267. [Google Scholar] [CrossRef]
- Cini, A.; Sumner, S.; Cervo, R. Inquiline social parasites as tools to unlock the secrets of insect sociality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180193. [Google Scholar] [CrossRef]
- Lenoir, A.; d’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef]
- d’Ettorre, P.; Moore, A.J. Chemical communication and the coordination of social interactions in insects. In Sociobiology of Communication: An Interdisciplinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 81–96. [Google Scholar]
- Nash, D.R.; Boomsma, J.J. Communication between hosts and social parasites. In Sociobiology of Communication: An Interdisciplinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 55–79. [Google Scholar]
- van Zweden, J.; d’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 222–243. [Google Scholar]
- Barbero, F. Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Bonelli, S.; Thomas, J.A.; Balletto, E.; Schonrogge, K. Acoustical mimicry in a predatory social parasite of ants. J. Exp. Biol. 2009, 212, 4084–4090. [Google Scholar] [CrossRef]
- Barbero, F.; Thomas, J.A.; Bonelli, S.; Balletto, E.; Schonrogge, K. Queen Ants Make Distinctive Sounds That Are Mimicked by a Butterfly Social Parasite. Science 2009, 323, 782–785. [Google Scholar] [CrossRef]
- Thomas, J.A.; Schonrogge, K.; Bonelli, S.; Barbero, F.; Balletto, E. Corruption of ant acoustical signals by mimetic social parasites: Maculinea butterflies achieve elevated status in host societies by mimicking the acoustics of queen ants. Commun. Integr. Biol. 2010, 3, 169–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Settele, J.; Barbero, F.; Musche, M.; Thomas, J.A.; Schönrogge, K. Singing the blues: From experimental biology to conservation application. J. Exp. Biol. 2011, 214, 1407–1410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbero, F.; Patricelli, D.; Witek, M.; Balletto, E.; Casacci, L.P.; Sala, M.; Bonelli, S. Myrmica ants and their butterfly parasites with special focus on the acoustic communication. Psyche 2012, 2012, 725237. [Google Scholar] [CrossRef]
- Sala, M.; Casacci, L.P.; Balletto, E.; Bonelli, S.; Barbero, F. Variation in Butterfly Larval Acoustics as a Strategy to Infiltrate and Exploit Host Ant Colony Resources. PLoS ONE 2014, 9, e094341. [Google Scholar] [CrossRef]
- Barbero, F.; Casacci, L.P. Butterflies that trick ants with sound. Phys. Today 2015, 68, 64–65. [Google Scholar] [CrossRef]
- Casacci, L.P.; Bonelli, S.; Balletto, E.; Barbero, F. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Evol. 2019, 7, 454. [Google Scholar] [CrossRef]
- Radchenko, A.; Elmes, G.W. A taxonomic revision of the socially parasitic Myrmica ants (Hymenoptera: Formicidae) of the Palaearctic region. Ann. Zool. 2003, 217–243. [Google Scholar]
- Jansen, G.; Savolainen, R.; Vepsäläinen, K. Phylogeny, divergence-time estimation, biogeography and social parasite–host relationships of the Holarctic ant genus Myrmica (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 2010, 56, 294–304. [Google Scholar] [CrossRef]
- Witek, M.; Barbero, F.; Marko, B. Myrmica ants host highly diverse parasitic communities: From social parasites to microbes. Insectes Soc. 2014, 61, 307–323. [Google Scholar] [CrossRef]
- Radchenko, A.; Elmes, G. Myrmica Ants (Hymenoptera: Formicidae) of the Old World; Natura Optima Dux Foundation: Warsaw, Poland, 2010; p. 789. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsalainen, K. Ants of Poland—With Reference to the Myrmecofauna of Europe; Natura Optima Dux Foundation: Warsaw, Poland, 2012; p. 496. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags - und Vertriebsgesellschaft: Tauer, Germany, 2018; p. 408. [Google Scholar]
- Witek, M.; Babik, H.; Czechowski, W.; Czechowska, W. Myrmica karavajevi (Arn.) (Hymenoptera: Formicidae) in Poland: A species not as rare as it is thought to be. Fragm. Faunist. 2013, 56, 17–24. [Google Scholar] [CrossRef]
- Wardlaw, J.; Elmes, G.; Thomas, J. Techniques for studying Maculinea butterflies: I. Rearing Maculinea caterpillars with Myrmica ants in the laboratory. J. Insect Conserv. 1998, 2, 79–84. [Google Scholar] [CrossRef]
- Csata, E.; Timuş, N.; Witek, M.; Casacci, L.P.; Lucas, C.; Bagnères, A.-G.; Sztencel-Jabłonka, A.; Barbero, F.; Bonelli, S.; Rákosy, L.; et al. Lock-picks: Fungal infection facilitates the intrusion of strangers into ant colonies. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- von Beeren, C.; Schulz, S.; Hashim, R.; Witte, V. Acquisition of chemical recognition cues facilitates integration into ant societies. BMC Ecol. 2011, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Barbero, F.; Bonelli, S.; Balletto, E.; Casacci, L.P. The acoustic repertoire of lycaenid butterfly larvae. Bioacoustics 2017, 26, 77–90. [Google Scholar] [CrossRef]
- Audacity Team. Audacity®: Free Audio Editor and Recorder [Computer application]. 2021. Available online: https://www.audacityteam.org/ (accessed on 20 May 2018).
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer. Available online: https://www.fon.hum.uva.nl/praat/ (accessed on 1 April 2019).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 5 May 2021).
- González, I.; Lê Cao, K.; Déjean, S. MixOmics: Omics Data Integration Project. Available online: http://www.math.univ-toulouse.fr/~biostat/mixOmics (accessed on 4 May 2020).
- Doyle, N.; Swain, D.; Roberts, J.; Cozzolino, D. The use of qualitative analysis in food research and technology: Considerations and reflections from an applied point of view. Food Anal. Methods 2017, 10, 964–969. [Google Scholar] [CrossRef]
- Roberts, J.; Cozzolino, D. An overview on the application of chemometrics in food science and technology—An approach to quantitative data analysis. Food Anal. Methods 2016, 9, 3258–3267. [Google Scholar] [CrossRef]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Bookstein, F. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evol. Biol. 2011, 38, 100–114. [Google Scholar] [CrossRef]
- Indahl, U.G.; Liland, K.H.; Næs, T. Canonical partial least squares—A unified PLS approach to classification and regression problems. J. Chemom. 2009, 23, 495–504. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Package ‘Vegan’. Community Ecology Package, Version 2.5-7. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 4 May 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Least-squares means: The R Package lsmeans. J. Stat. Softw. 2016, 69. [Google Scholar] [CrossRef]
- Casacci, L.P.; Thomas, J.A.; Sala, M.; Treanor, D.; Bonelli, S.; Balletto, E.; Schönrogge, K. Ant pupae employ acoustics to communicate social status in their colony’s hierarchy. Curr. Biol. 2013, 23, 323–327. [Google Scholar] [CrossRef]
- Castro, S.; Àlvarez, M.; Munguira, M. Morphology of the stridulatory organs of Iberian myrmicine ants (Hymenoptera: Formicidae). Ital. J. Zool. (Modena) 2015, 82, 387–397. [Google Scholar] [CrossRef]
- Schultner, E.; Pulliainen, U. Brood recognition and discrimination in ants. Insectes Soc. 2020, 67, 11–34. [Google Scholar] [CrossRef]
- Thomas, J.A.; Elmes, G.W. Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol. Entomol. 1998, 23, 457–464. [Google Scholar] [CrossRef]
- Cervo, R.; Macinai, V.; Dechigi, F.; Turillazzi, S. Fast growth of immature brood in a social parasite wasp: A convergent evolution between avian and insect cuckoos. Am. Nat. 2004, 164, 814–820. [Google Scholar] [CrossRef]
- Thomas, J.; Wardlaw, J. The capacity of a Myrmica ant nest to support a predacious species of Maculinea butterfly. Oecologia 1992, 91, 101–109. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.-G. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: New York, NY, USA, 2010; p. 492. [Google Scholar]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Dettner, K.; Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994, 39, 129–154. [Google Scholar] [CrossRef]
- Akino, T.; Knapp, J.J.; Thomas, J.A.; Elmes, G.W. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 1999, 266, 1419–1426. [Google Scholar] [CrossRef]
- Akino, T. Chemical strategies to deal with ants: A review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News. 2008, 11, 173–181. [Google Scholar]
- Johnson, C.A. Slave-maker ant competition for a shared host and the effect on coevolutionary dynamics. Ecol. Monogr. 2008, 78, 445–460. [Google Scholar] [CrossRef]
- Tsuneoka, Y.; Akino, T. Chemical camouflage of the slave-making ant Polyergus samurai queen in the process of the host colony usurpation (Hymenoptera: Formicidae). Chemoecology 2012, 22, 89–99. [Google Scholar] [CrossRef]
- Hojo, M.K.; Wada-Katsumata, A.; Akino, T.; Yamaguchi, S.; Ozaki, M.; Yamaoka, R. Chemical disguise asparticular caste of host ants in the ant inquiline parasite Niphanda fusca (Lepidoptera: Lycaenidae). Proc. R. Soc. B Biol. Sci. 2009, 276, 551–558. [Google Scholar] [CrossRef]
- Franks, N.; Blum, M.; Smith, R.K.; Allies, A.B. Behavior and chemical disguise of cuckoo ant Leptothorax kutteri in relation to its host Leptothorax acervorum. J. Chem. Ecol. 1990, 16, 1431–1444. [Google Scholar] [CrossRef]
- Lambardi, D.; Dani, F.R.; Turillazzi, S.; Boomsma, J.J. Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 2007, 61, 843–851. [Google Scholar] [CrossRef]
- Nehring, V.; Evison, S.E.F.; Santorelli, L.A.; D’Ettorre, P.; Hughes, W.O.H. 2011: Kin-informative recognition cues in ants. Proc. R. Soc. B. 2011, 278, 1942–1948. [Google Scholar] [CrossRef]
- Thomas, J.A.; Elmes, G.W.; Sielezniew, M.; Stankiewicz-Fiedurek, A.; Simcox, D.J.; Settele, J.; Schonrogge, K. Mimetic host shifts in an endangered social parasite of ants. Proc. R. Soc. Lond. B 2013, 280. [Google Scholar] [CrossRef]
- Scarparo, G.; d’Ettorre, P.; Di Giulio, A. Chemical deception and structural adaptation in Microdon (Diptera, Syrphidae, Microdontinae), a genus of hoverflies parasitic on social insects. J. Chem. Ecol. 2019, 45, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Schönrogge, K.; Wardlaw, J.C.; Peters, A.J.; Everett, S.; Thomas, J.A.; Elmes, G.W. Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J. Chem. Ecol. 2004, 30, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Bagnères, A.-G.; Lorenzi, M.C. Chemical deception/mimicry using cuticular hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, Blomquist, G.J., Bagneres, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 282–323. [Google Scholar]
- Uboni, A.; Bagnères, A.-G.; Christidès, J.-P.; Lorenzi, M.C. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 2012, 58, 1259–1264. [Google Scholar] [CrossRef]
- Bonavita-Cougourdan, A.; Theraulaz, G.; Bagnères, A.-G.; Roux, M.; Pratte, M.; Provost, E.; Clément, J.-L. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. 1991, 100B, 667–680. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Destri, S.; Spencer, S.H.; Turillazzi, S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 2001, 62, 165–171. [Google Scholar] [CrossRef]
- Cini, A.; Gioli, L.; Cervo, R. A quantitative threshold for nest-mate recognition in a paper social wasp. Biol. Lett. 2009, 5, 459–461. [Google Scholar] [CrossRef]
- Masoni, A.; Frizzi, F.; Nieri, R.; Casacci, L.; Mazzoni, V.; Turillazzi, S.; Santini, G. Ants modulate stridulatory signals depending on the behavioural context. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schonrogge, K.; Barbero, F.; Casacci, L.P.; Settele, J.; Thomas, J.A. Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim. Behav. 2017, 134, 249–256. [Google Scholar] [CrossRef]
- Di Giulio, A.; Maurizi, E.; Barbero, F.; Sala, M.; Fattorini, S.; Balletto, E.; Bonelli, S. The Pied Piper: A Parasitic Beetle’s Melodies Modulate Ant Behaviours. PLoS ONE 2015, 10, e0130541. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, R.; Vepsäläinen, K. Sympatric speciation through intraspecific social parasitism. Proc. Natl. Acad. Sci. USA 2003, 100, 7169–7174. [Google Scholar] [CrossRef] [PubMed]
- Vepsäläinen, K.; Ebsen, J.; Savolainen, R.; Boomsma, J. Genetic differentiation between the ant Myrmica rubra and its microgynous social parasite. Insectes Soc. 2009, 56, 425–437. [Google Scholar] [CrossRef]
- Huang, M.H.; Dornhaus, A. A meta-analysis of ant social parasitism: Host characteristics of different parasitism types and a test of Emery’s rule. Ecol. Entomol. 2008, 33, 589–596. [Google Scholar] [CrossRef]
- Guillem, R.M.; Drijfhout, F.P.; Martin, S.J. Using chemo-taxonomy of host ants to help conserve the large blue butterfly. Biol. Conserv. 2012, 148, 39–43. [Google Scholar] [CrossRef]
- Tinbergen, N. The Study of Instinct; Pygmalion Press, an imprint of Plunkett Lake Press: Lexington, KY, USA, 2020; p. 19441. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).