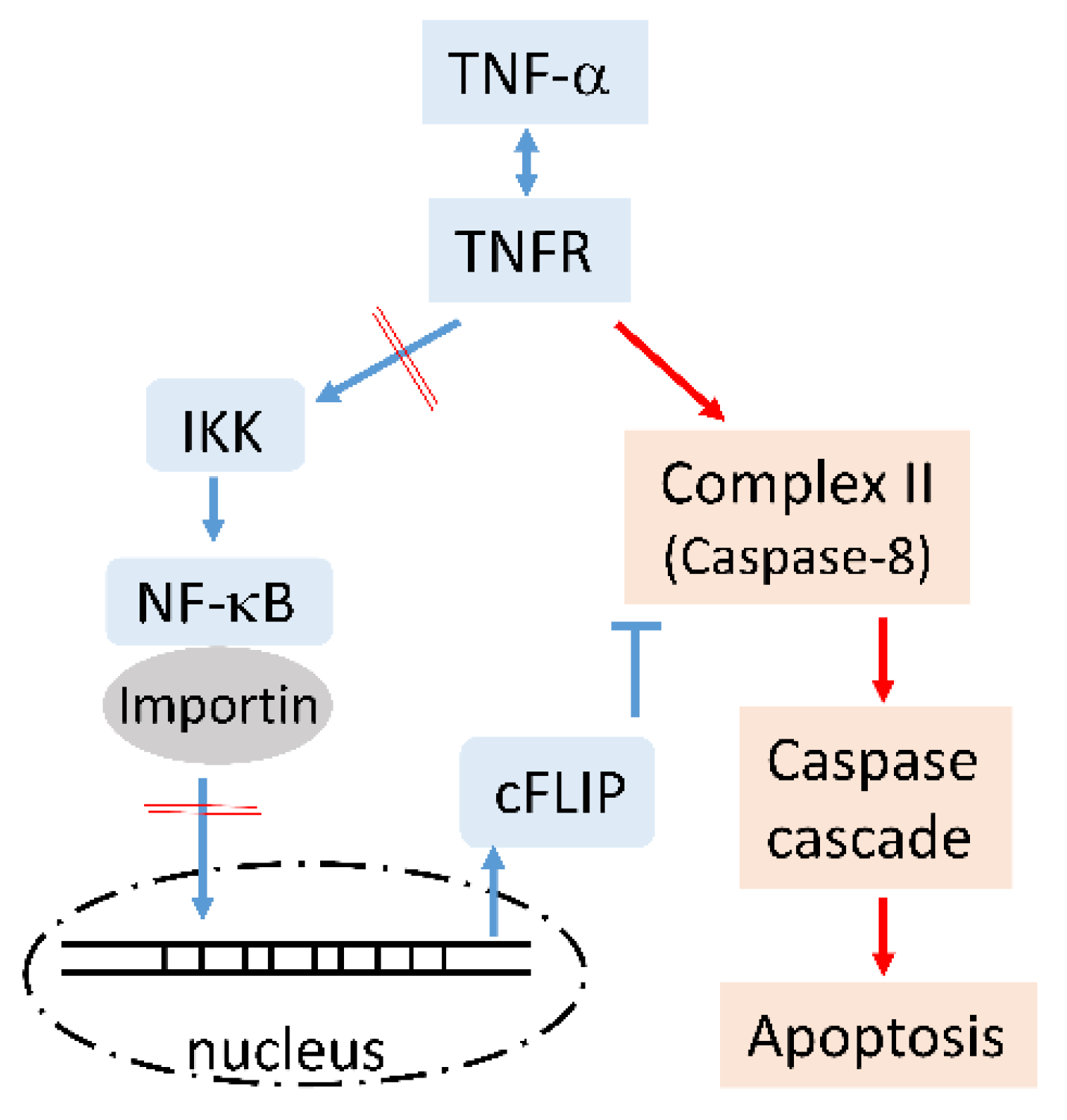
MCPIP1 Enhances TNF-α-Mediated Apoptosis through Downregulation of the NF-κB/cFLIP Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Antibodies
2.2. Cell Viability Assay
2.3. Flow Cytometry
2.4. Western Blotting
2.5. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
2.6. Immunofluorescence (IF) Assay
2.7. In Vitro Kinase Activity Assay
2.8. Electrophoretic Mobility Shift Assay (EMSA)
2.9. Statistical Analysis
3. Results
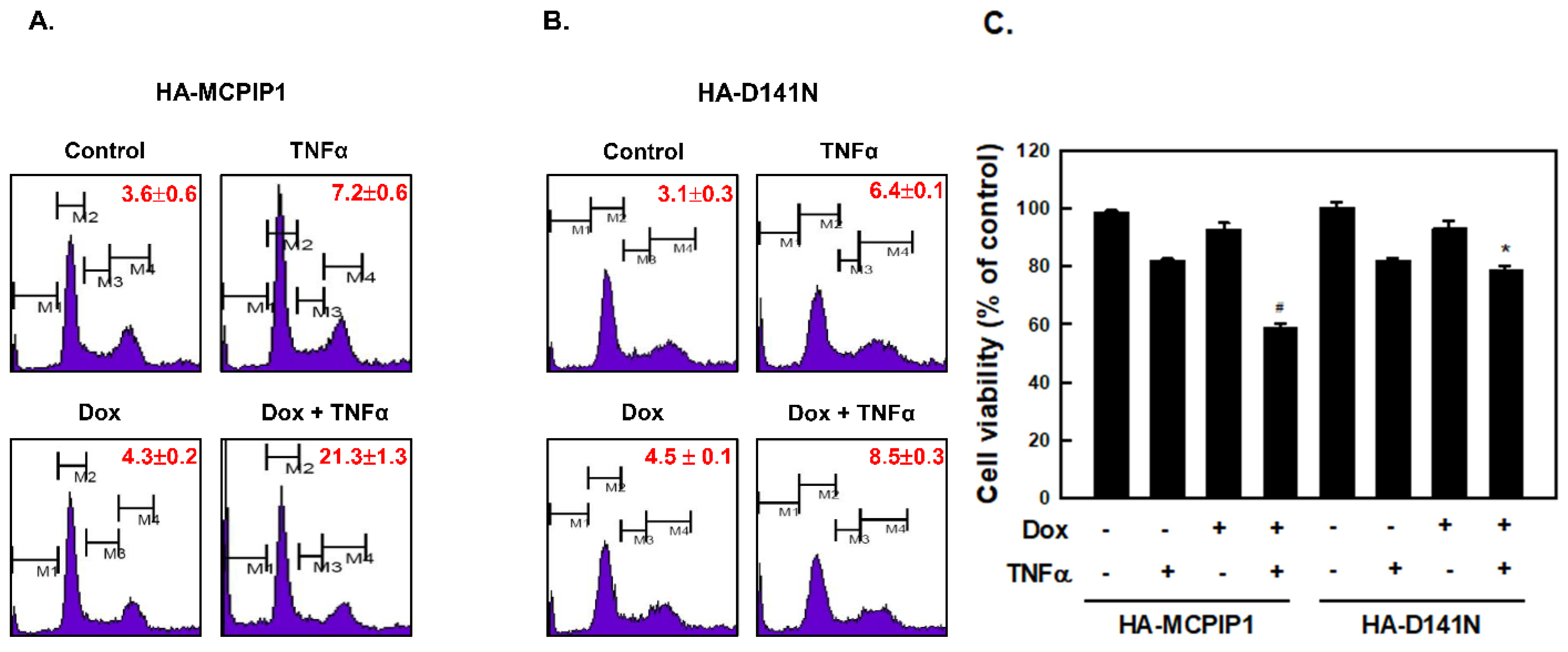
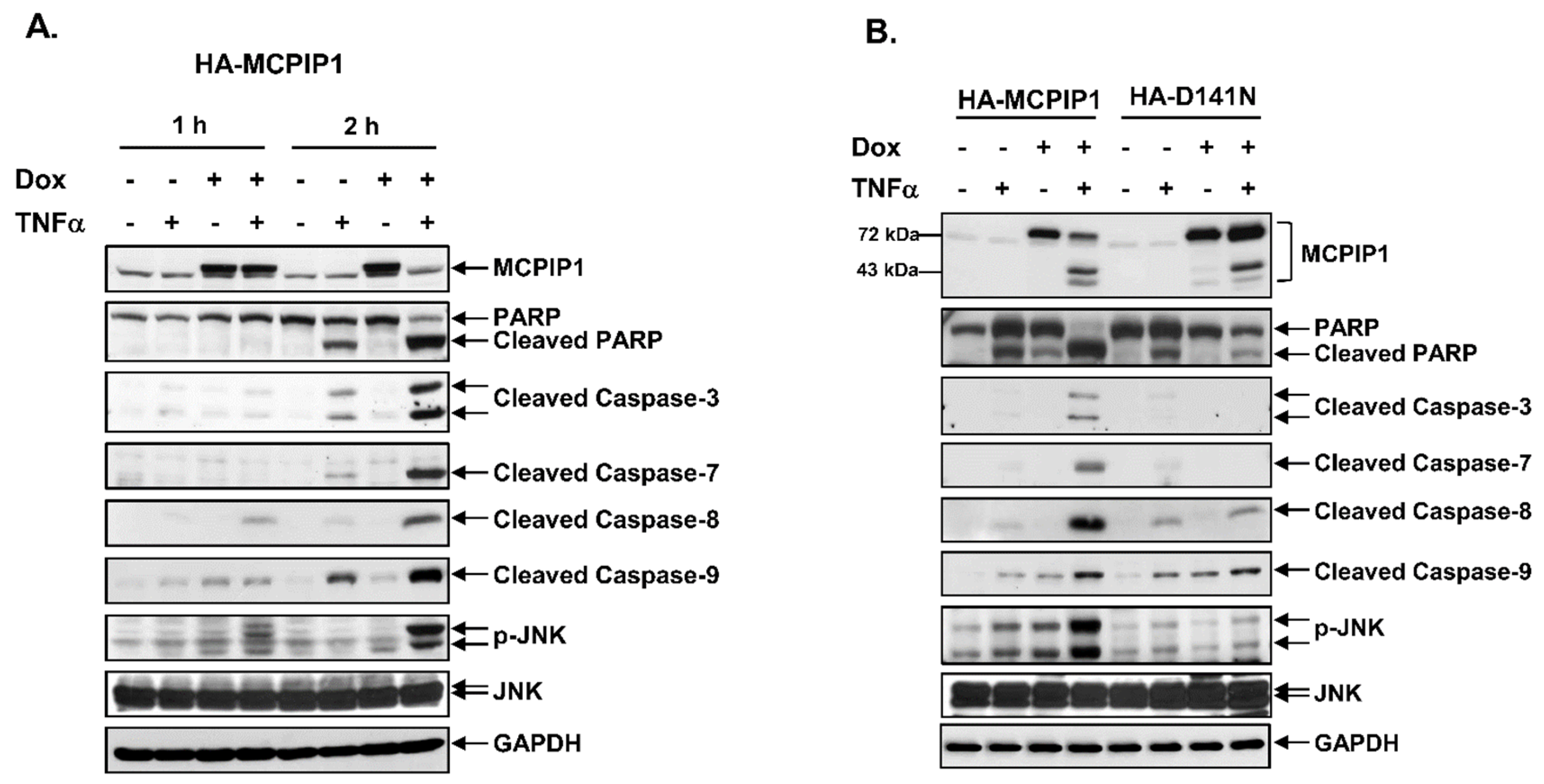
3.1. MCPIP1 Enhanced the Sub-G1 Population and Activation of the Caspase Cascade
3.2. A Pan-Caspase Inhibitor and Caspase-1 Inhibitor Reversed MCPIP1/TNF-α-Mediated Apoptosis and Caspase Activation
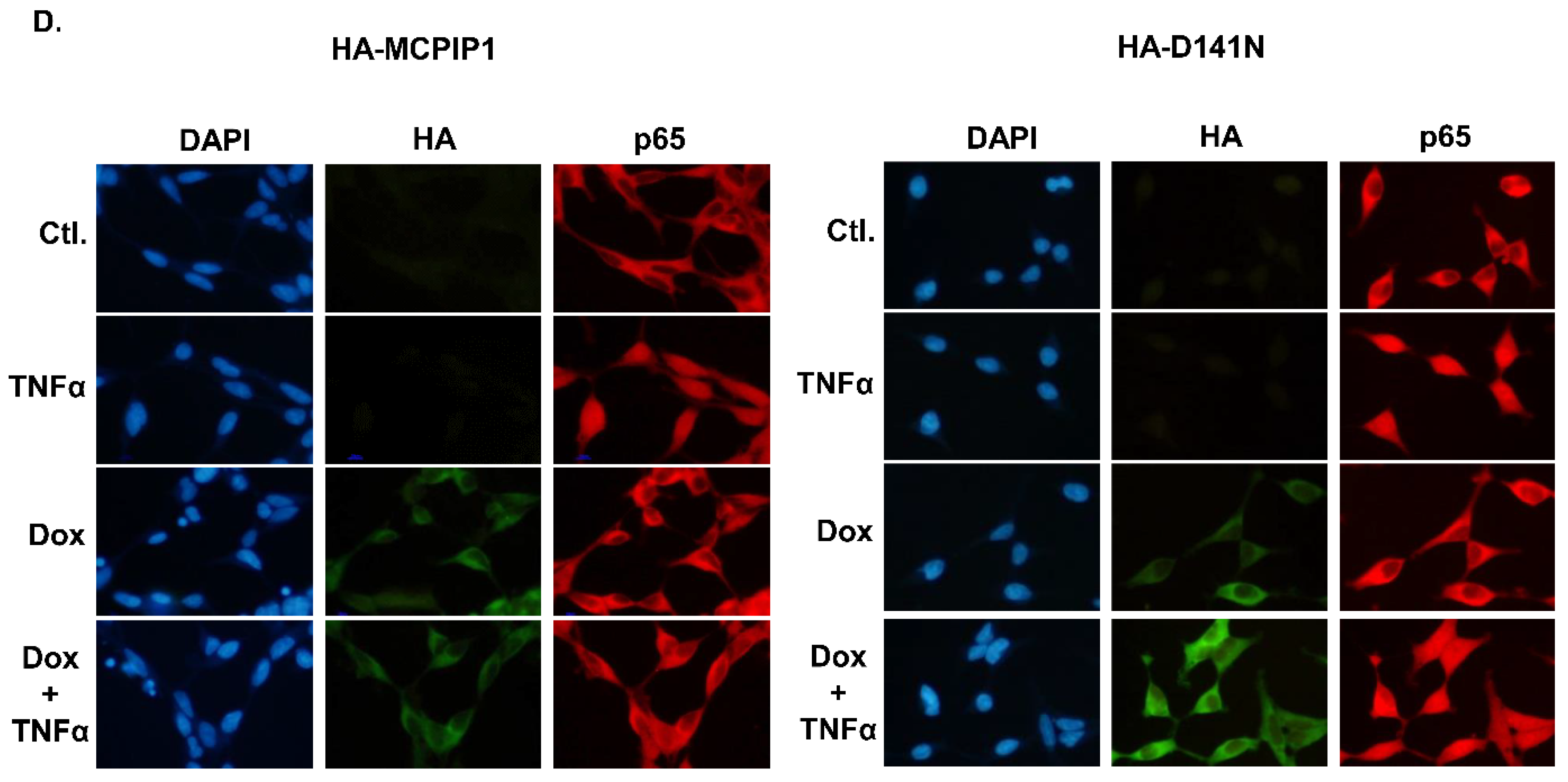
3.3. MCPIP1 Downregulated an NF-κB Activation Pathway as Well as Importin α3 and Importin α4 Expressions
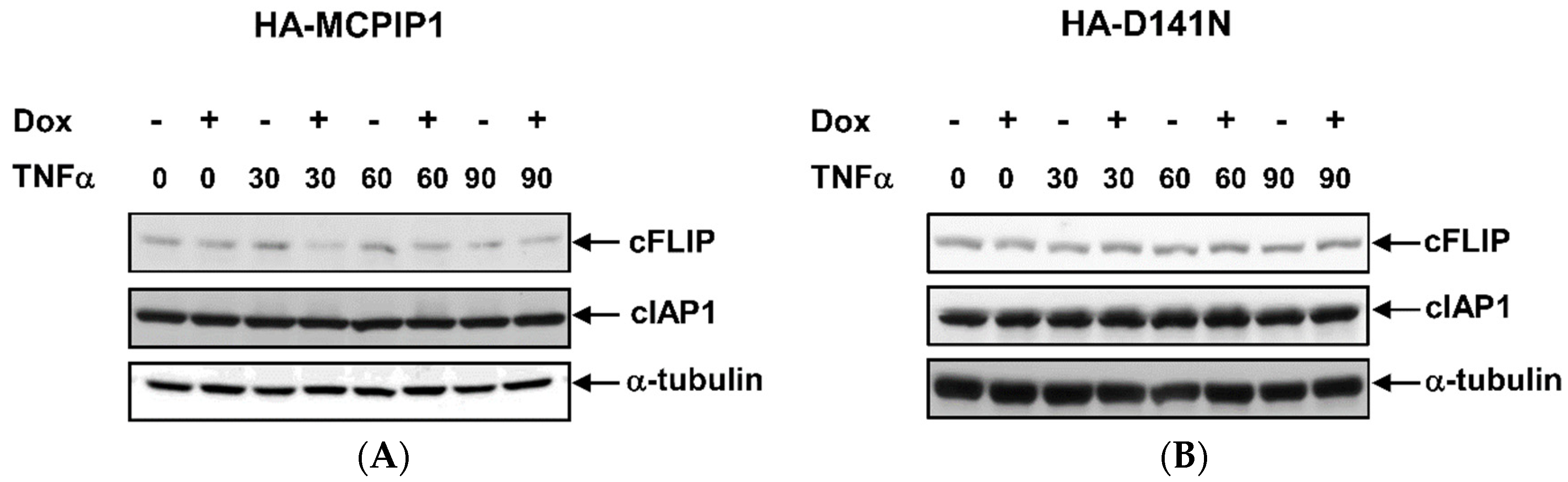
3.4. MCPIP1 Downregulates Expression of cFLIP, a Caspase-8 Inhibitor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivia, A.; Arguello, D.; Haisma, H.J. Apoptosis-inducing TNF superfamily ligands for cancer therapy. Cancers 2021, 13, 1543. [Google Scholar]
- Skaug, B.; Jiang, X.; Chen, Z.J. The role of ubiquitin in NF-κB regulatory pathways. Annu. Rev. Biochem. 2009, 78, 769–796. [Google Scholar] [CrossRef]
- Rieser, E.; Cordier, S.M.; Walczak, H. Linear ubiquitination: A newly discovered regulator of cell signalling. Trends Biochem. Sci. 2013, 38, 94–102. [Google Scholar] [CrossRef]
- Workman, L.M.; Habelhah, H. Tnfr1 signaling kinetics: Spatiotemporal control of three phases of IKK activation by posttranslational modification. Cell. Signal. 2013, 25, 1654–1664. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; O’Neill, K.L.; Gurumurthy, C.B.; Quadros, R.M.; Tu, Y.; Luo, X. Cleavage by caspase 8 and mitochondrial membrane association activate the BH3-only protein bid during trail-induced apoptosis. J. Biol. Chem. 2016, 291, 11843–11851. [Google Scholar] [CrossRef] [PubMed]
- Safa, A. c-FLIP, a master anti-apoptotic regulator. Exp. Oncol. 2012, 34, 176. [Google Scholar] [PubMed]
- Humke, E.W.; Shriver, S.K.; Starovasnik, M.A.; Fairbrother, W.J.; Dixit, V.M. Iceberg: A novel inhibitor of interleukin-1β generation. Cell 2000, 103, 99–111. [Google Scholar] [CrossRef]
- Opdenbosch, N.V.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Wang, X.; Narayanan, M.; Bruey, J.-M.; Rigamonti, D.; Cattaneo, E.; Reed, J.C.; Friedlander, R.M. Protective role of Cop in Rip2/caspase-1/caspase-4-mediated HeLa cell death. Biochim. Biophys. Acta. 2006, 1762, 742–754. [Google Scholar] [CrossRef][Green Version]
- Feltham, R.; Vince, J.E.; Lawlor, K.E. Caspase-8: Not so silently deadly. Clin. Transl. Immunol. 2017, 6, e124. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.M.-U.; Nomura, T.; Takagi, T.; Okamura, T.; Jin, W.; Shinagawa, T.; Tanaka, Y.; Ishii, S. Ubiquitination-deubiquitination by the TRIM27-USP7 complex regulates tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2013, 33, 4971–4984. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Chu, J.-S.; Chien, H.-L.; Tseng, C.-H.; Ko, P.-C.; Mei, Y.-Y.; Tang, W.-C.; Kao, Y.-T.; Cheng, H.-Y.; Liang, Y.-C. MCPIP1 suppresses hepatitis C virus replication and negatively regulates virus-induced proinflammatory cytokine responses. J. Immunol. 2014, 193, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Skalniak, L.; Mizgalska, D.; Zarebski, A.; Wyrzykowska, P.; Koj, A.; Jura, J. Regulatory feedback loop between NF-κB and MCP-1-induced protein 1 RNase. FEBS J. 2009, 276, 5892–5905. [Google Scholar] [CrossRef]
- Matsushita, K.; Takeuchi, O.; Standley, D.M.; Kumagai, Y.; Kawagoe, T.; Miyake, T.; Satoh, T.; Kato, H.; Tsujimura, T.; Nakamura, H. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 2009, 458, 1185–1190. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Liu, Y.; Qu, J.; Cao, W.; Zhang, E.; He, J.; Cai, Z. How are MCPIP1 and cytokines mutually regulated in cancer-related immunity? Protein Cell 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Musson, R.; Szukała, W.; Jura, J. MCPIP1 RNase and its multifaceted role. Int. J. Mol. Sci. 2020, 21, 7183. [Google Scholar] [CrossRef]
- Fu, M.; Blackshear, P.J. RNA-binding proteins in immune regulation: A focus on CCCH zinc finger proteins. Nature Rev. Immunol. 2017, 17, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Arase, M.; Matsuyama, H.; Choi, Y.L.; Ueno, T.; Mano, H.; Sugimoto, K.; Miyazono, K. MCPIP1 ribonuclease antagonizes Dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol. Cell 2011, 44, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, T.T. MCPIP1/regnase-i inhibits simian immunodeficiency virus and is not counteracted by Vpx. J. Gen. Virol. 2016, 97, 1693. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qiu, C.; Miao, R.; Zhou, J.; Lee, A.; Liu, B.; Lester, S.N.; Fu, W.; Zhu, L.; Zhang, L. MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, 19083–19088. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Saad, Y.; Lei, T.; Wang, J.; Qi, D.; Yang, Q.; Kolattukudy, P.E.; Fu, M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κB signaling. J. Exp. Med. 2010, 207, 2959–2973. [Google Scholar] [CrossRef]
- Niu, J.; Shi, Y.; Xue, J.; Miao, R.; Huang, S.; Wang, T.; Wu, J.; Fu, M.; Wu, Z.H. USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J. 2013, 32, 3206–3219. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Chang, C.-C.; Sheu, M.-T.; Lin, S.-Y.; Chung, C.-C.; Teng, C.-T.; Suk, F.-M. The antihistamine deptropine induces hepatoma cell death through blocking autophagosome-lysosome fusion. Cancers 2020, 12, 1610. [Google Scholar] [CrossRef]
- Suk, F.-M.; Chang, C.-C.; Lin, R.-J.; Lin, S.-Y.; Liu, S.-C.; Jau, C.-F.; Liang, Y.-C. ZFP36L1 and ZFP36L2 inhibit cell proliferation in a cyclin D-dependent and p53-independent manner. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suk, F.-M.; Chang, C.-C.; Lin, R.-J.; Lin, S.-Y.; Chen, Y.-T.; Liang, Y.-C. MCPIP3 as a potential metastasis suppressor gene in human colorectal cancer. Int. J. Mol. Sci. 2018, 19, 1350. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.H.; Hsu, S.P.; Zhong, W.B.; Liang, Y.C. Involvement of cysteine-rich protein 61 in the epidermal growth factor-induced migration of human anaplastic thyroid cancer cells. Mol. Carcinog. 2016, 55, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Sheu, M.-T.; Chen, T.-F.; Wang, Y.-C.; Hou, W.-C.; Liu, D.-Z.; Chung, T.-C.; Liang, Y.-C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 2006, 72, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Timmer, J.; Salvesen, G. Caspase substrates. Cell Death Differ. 2007, 14, 66–72. [Google Scholar] [CrossRef]
- Fagerlund, R.; Kinnunen, L.; Köhler, M.; Julkunen, I.; Melén, K. NF-κB is transported into the nucleus by importin α3 and importin α4. J. Biol. Chem. 2005, 280, 15942–15951. [Google Scholar] [CrossRef]
- Annibaldi, A.; Meier, P. Checkpoints in TNF-induced cell death: Implications in inflammation and cancer. Trends Mol. Med. 2018, 24, 49–65. [Google Scholar] [CrossRef]
- Ting, A.T.; Bertrand, M.J.M. More to life than NF-kappaB in TNFR1 signaling. Trends Immunol. 2016, 37, 535–545. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-κB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef]
- Hazawa, M.; Sakai, K.; Kobayashi, A.; Yoshino, H.; Iga, Y.; Iwashima, Y.; Lim, K.S.; Voon, D.C.-C.; Jiang, Y.-Y.; Horike, S.-i. Disease-specific alteration of karyopherin-α subtype establishes feed-forward oncogenic signaling in head and neck squamous cell carcinoma. Oncogene 2020, 39, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, Z.; Li, H.; Zhu, Y.; Sun, Z.; Zhu, A. LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol. Cancer 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, R.; Yang, Y.; Shen, X.; Shi, Q.; Lian, M.; Ma, H.; Fang, J. KPNA4 regulated by miR-548b-3p promotes the malignant phenotypes of papillary thyroid cancer. Life Sci. 2021, 265, 118743. [Google Scholar] [CrossRef]
- Li, M.; Cao, W.; Liu, H.; Zhang, W.; Liu, X.; Cai, Z.; Guo, J.; Wang, X.; Hui, Z.; Zhang, H. MCPIP1 down-regulates IL-2 expression through an ARE-independent pathway. PLoS ONE 2012, 7, e49841. [Google Scholar] [CrossRef] [PubMed]
- Saini, Y.; Chen, J.; Patial, S. The tristetraprolin family of RNA-binding proteins in cancer: Progress and future prospects. Cancers 2020, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Carballo, E.; Lai, W.S.; Blackshear, P.J. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 1998, 281, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Myer, V.E.; Fan, X.C.; Steitz, J.A. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997, 16, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Song, W.; Tromp, G.; Kolattukudy, P.E.; Fu, M. Genome-wide survey and expression profiling of CCCH-zinc finger family reveals a functional module in macrophage activation. PLoS ONE 2008, 3, e2880. [Google Scholar] [CrossRef] [PubMed]
- Bast, A.; Krause, K.; Schmidt, I.H.; Pudla, M.; Brakopp, S.; Hopf, V.; Breitbach, K.; Steinmetz, I. Caspase-1-dependent and -independent cell death pathways in burkholderia pseudomallei infection of macrophages. PLoS Pathog. 2014, 10, e1003986. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Nakajima, S.; Hosojima, S.; Nguyen, D.T.; Hattori, T.; Le, T.M.; Hori, O.; Mahib, M.R.; Yamaguchi, Y.; Miura, M. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nature Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Uehata, T.; Iwasaki, H.; Vandenbon, A.; Matsushita, K.; Hernandez-Cuellar, E.; Kuniyoshi, K.; Satoh, T.; Mino, T.; Suzuki, Y.; Standley, D.M.; et al. Malt1-induced cleavage of regnase-1 in CD4(+) helper T cells regulates immune activation. Cell 2013, 153, 1036–1049. [Google Scholar] [CrossRef]
- Jeltsch, K.M.; Hu, D.; Brenner, S.; Zöller, J.; Heinz, G.A.; Nagel, D.; Vogel, K.U.; Rehage, N.; Warth, S.C.; Edelmann, S.L.; et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat. Immunol. 2014, 15, 1079–1089. [Google Scholar] [CrossRef]
- Shinde, P.V.; Xu, H.C.; Maney, S.K.; Kloetgen, A.; Namineni, S.; Zhuang, Y.; Honke, N.; Shaabani, N.; Bellora, N.; Doerrenberg, M.; et al. Tumor necrosis factor-mediated survival of CD169+ cells promotes immune activation during vesicular stomatitis virus infection. J. Virol. 2018, 92, e01637-17. [Google Scholar] [CrossRef]
- Ruland, J.; Duncan, G.S.; Wakeham, A.; Mak, T.W. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 2003, 19, 749–758. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suk, F.-M.; Chang, C.-C.; Sun, P.-C.; Ke, W.-T.; Chung, C.-C.; Lee, K.-L.; Chan, T.-S.; Liang, Y.-C. MCPIP1 Enhances TNF-α-Mediated Apoptosis through Downregulation of the NF-κB/cFLIP Axis. Biology 2021, 10, 655. https://doi.org/10.3390/biology10070655
Suk F-M, Chang C-C, Sun P-C, Ke W-T, Chung C-C, Lee K-L, Chan T-S, Liang Y-C. MCPIP1 Enhances TNF-α-Mediated Apoptosis through Downregulation of the NF-κB/cFLIP Axis. Biology. 2021; 10(7):655. https://doi.org/10.3390/biology10070655
Chicago/Turabian StyleSuk, Fat-Moon, Chi-Ching Chang, Pei-Chi Sun, Wei-Ting Ke, Chia-Chen Chung, Kun-Lin Lee, Tze-Sian Chan, and Yu-Chih Liang. 2021. "MCPIP1 Enhances TNF-α-Mediated Apoptosis through Downregulation of the NF-κB/cFLIP Axis" Biology 10, no. 7: 655. https://doi.org/10.3390/biology10070655
APA StyleSuk, F.-M., Chang, C.-C., Sun, P.-C., Ke, W.-T., Chung, C.-C., Lee, K.-L., Chan, T.-S., & Liang, Y.-C. (2021). MCPIP1 Enhances TNF-α-Mediated Apoptosis through Downregulation of the NF-κB/cFLIP Axis. Biology, 10(7), 655. https://doi.org/10.3390/biology10070655





