Simple Summary
Numerous studies demonstrate that the activation of transforming growth factor-β (TGF-β) signaling is a critical driving force for promoting cancer cell migration and tumor metastasis. Our recent study indicates that TGF-β1 promotes FBXO3-mediated ΔNp63α protein degradation to facilitate cancer metastasis. In this study, we show that TGF-β1 can inhibit TAp63α protein stability in a lysosome-dependent, but canonical Smad pathway-independent manner, which leads to upregulation of p53-R248W expression, and consequently results in increased pancreatic cancer cell migration.
Abstract
TGF-β signaling plays a pivotal role in promoting tumor cell migration and cancer metastasis. ΔNp63α and TAp63α are two major isoforms of p53-related p63 protein. Our recent study has shown that TGF-β1 promotes ΔNp63α protein degradation to facilitate cancer metastasis. However, whether TAp63α is involved in TGF-β1-induced cancer metastasis remains unclear. In this study, we show that, in human pancreatic cancer MIA PaCa-2 cells harboring p53-R248W allele, TGF-β1 can significantly inhibit TAp63α protein stability in a Smad pathway-independent manner. Lysosome inhibitor, chloroquine, but not proteasome inhibitor MG132, can rescue TGF-β1-induced downregulation of TAp63α protein. In addition, we show that either TGF-β1 treatment or silencing of TAp63α can dramatically increase migration of MIA PaCa-2 cells. Importantly, the restored expression of TAp63α can effectively block TGF-β1-induced migration of MIA PaCa-2 cells. Mechanistically, we show that TGF-β1 promotes TAp63α protein degradation, leading to upregulation of p53-R248W protein expression, and consequently resulting in elevated MIA PaCa-2 cell migration. Together, this study indicates that lysosomal degradation is an important way for regulating TAp63α protein fate and highlights that TGF-β1-TAp63α-mutant p53 axis is critically important in pancreatic cancer metastasis.
1. Introduction
TGF-β1 is a member of the TGF-β family which plays a pivotal role in a series of biological processes, including cell differentiation, proliferation, epithelial-mesenchymal transition (EMT), cell migration, tumor metastasis and immune escape [1,2,3]. As a ligand for TGF-β receptor, TGF-β1 binds to the TGF-β receptor and transduce signals through canonical Smad pathway or noncanonical Ras–Erk, PI3K/AKT and Rho-like GTPase pathways [4,5]. In the canonical Smad pathway, activated TGF-β receptor phosphorylates Smad2/Smad3, which then bind to Smad4 to form heteromeric Smad complexes translocated into the nucleus to transactivate downstream target genes [4,6,7]. Accumulating evidence indicates that TGF-β1 signaling can also execute its function through the regulation of protein stability. It is reported that TGF-β1 can promote iNOS protein degradation in a ubiquitin-proteasome-dependent manner during inflammatory process [8]. Furthermore, TGF-β1 can promote type I collagen protein lysosomal degradation [9]. Moreover, TGF-β1 can also facilitate MyD88 protein degradation by recruiting Smurf1 and Smurf2 [10].
p63,a p53 family member, which plays an important role in a variety of biological processes, including cell adhesion, proliferation, senescence, survival, tumor metastasis and embryonic development [11]. Derived from the alternative transcriptional start sites at the N termini and alternative splicing sites at the C termini, p63 gene encodes a series of protein isoforms [11]. ΔNp63α and TAp63α are two major isoforms of p63 family proteins. It has been well-documented that ΔNp63α is mainly expressed in epithelial cells and serves as an important tumor metastasis inhibitor [11]. The activation of PI3K/HER2/Ras can inhibit ΔNp63α gene transcription to promote cancer metastasis [12,13]. Oxidative stress suppresses cell motility and tumor metastasis via upregulation of ΔNp63α expression [14]. Our recent study demonstrates that TGF-β1 promotes FBXO3-mediated degradation of ΔNp63α to facilitate tumor metastasis [15]. On the other hand, TAp63α is mainly expressed in testicular tissues, ovarian, spermatocytes, and embryos [16,17,18,19,20,21]. It has been shown that TAp63α also plays a role in tumor metastasis. TAp63α can coordinately regulate Dicer and miR-130b to suppress tumor metastasis [22]. Furthermore, TAp63α can transcriptionally inhibit miR-19 to suppress tobacco smoke-induced lung cancer EMT [23]. Moreover, TAp63α can also transactivate miR-133b to inhibit colon cancer metastasis [24]. However, whether TAp63α is also involved in TGF-β1-induced cancer metastasis remains unclear.
In this paper, we show that TGF-β1 can significantly inhibit TAp63α protein stability in a lysosome-dependent manner, which leads to the upregulation of p53-R248W protein expression, and consequently results in increased MIA PaCa-2 cell migration.
2. Materials and Methods
2.1. Cell Culture and Drug Treatments
HEK293T (ATCC® CRL-11268™) and MIA PaCa-2 (ATCC® CRL-1420™, p53R248W) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). MIA PaCa-2 and HEK293T cells were cultured in DMEM medium (GIBCO, Rockville, MD, USA) supplemented with 10% FBS (GIBCO), 100 μg/mL streptomycin (GIBCO) and 100 units/mL penicillin (GIBCO). Cells were grown in a humidified 37 °C incubator under a 5% CO2 atmosphere. Cells at 65–75% confluence were treated with TGF-β1 or an indicated chemical compound. MG132 (M8699) was purchased from MERCK (Darmstadt, Germany). Cycloheximide (CHX, ab120093) was purchased from Abcam (Cambridge, MA, USA). TGF-β1 (GF346) and chloroquine (CLQ, C6628) were purchased from Sigma (St Louis, MO, USA).
2.2. Plasmids and Lentiviral Infection
Expression plasmids include human TAp63α and TAp63γ. Lentiviruses were amplified by transfection of HEK293T cells with pMD2.G and psPAX2 packaging plasmids and Lentiviral-based expression plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Viruses were collected at 60 h after transfection. Cells at 40–50% confluence were infected with recombinant Lentivirus encoding or an empty vector in the presence of 10 μg/mL polybrene, followed by 12 h. Lentiviral-based shRNAs specific for green fluorescent protein (GFP, GCATCAAGGTGAACTTCAA), p63 (1# CCGTTTCGTCAGAACACACAT; 2# GAGTGGAATGACTTCAACTTT) or p53 (GAGGGATGTTTGGGAGATGTA) were constructed as previous described [25].
2.3. Western Blot Analyses and Immunofluorescence Staining
Western blot analyses and immunofluorescence staining were performed as our previous described [13]. Antibodies for p63 (SC-8431), p53(SC-126) and Twist 1 (SC-15393) were purchased from Santa Cruz Biotech (Dallas, Texas, USA). Antibody for Snail1 (CST-3879), Smad3 (9523) and p-Smad3 (9520) were purchased from CST (Cambridge, MA, USA). Antibody for GAPDH (AF1186) was purchased from Beyotime (Shanghai, China).
2.4. Quantitative PCR (qPCR) Analyses
qPCR analyses were performed as our previous described [13]. qPCR primer specifics for TAp63 (F: CCCAGAGTCTTCCAGCATA; R: TTTTCGGAAGGTTCATCCAC), p53 (F: CCAACAACACCAGCTCCTCT; R: CCTCATTCAGCTCTCGGAAC) and GAPDH (F: GAGTCAACGGATTTGGTCGT; R: TTGATTTTGGAGGGATCTCG) were used.
2.5. Transwell Assay for Cell Migration
Cell migration was measured as our previous described [26]. In briefly, MIA PaCa-2 cells (3 × 105) grown in serum-free DMEM medium were seeded into transwell inner chamber. The outer chamber was filled with regular DMEM medium. Cells were incubated for 24 h. Non-migrating cells were carefully removed and migrating cells were stained with 0.1% crystal violet for 10–15 min at room temperature. Cells were photographed using a Nikon light microscope. At least 100 cells from five random fields were counted.
2.6. Statistical Analysis
GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) was used to statistical analysis. All experiments were performed at least three times in triplicate. Student’s t-test was used to assess significance.
3. Results
3.1. TGF-β1 Promotes TAp63α Protein Degradation in A Lysosome-Dependent Manner
TAp63α and ΔNp63α are two major isoforms of p63 protein. Our recent study demonstrates that TGF-β1 promotes FBXO3-mediated ΔNp63α protein proteasomal degradation to facilitate cancer metastasis [15]. However, whether TAp63α also plays a role in TGF-β1-induced tumor metastasis remains unclear. To explore this issue, we explored the effects of TGF-β1 on TAp63α expression. Human pancreatic cancer MIA PaCa-2 cells primarily expressed TAp63α isoform, as evidenced by western blot and RT-PCR (reverse transcriptional PCR) analyses (Figure 1A,B). As shown in Figure 1C, TGF-β1 treatment significantly upregulated epithelial-mesenchymal transition (EMT) markers Twist1 and Snail1 expression, as expected. Notably, TGF-β1 markedly inhibited TAp63α protein expression. Next, we explored the molecular basis by which TGF-β1 inhibits TAp63α expression. As shown in Figure 1D, TGF-β1 treatment had no effects on TAp63α mRNA levels in MIA PaCa-2 cells, suggesting TGF-β1 can’t affect TAp63α transcription. By contrast, TGF-β1 treatment significantly shortened TAp63α protein half-life in MIA PaCa-2 cells (Figure 1E,F). It is well-known that ubiquitin-proteasome and autophagy/lysosome are two major systems for protein degradation. To explore the role of proteasome and lysosome in TGF-β1-induced TAp63α protein degradation, we used proteasome inhibitor MG132 or lysosome inhibitor chloroquine (CLQ) to treat MIA PaCa-2 cells. As shown in Figure 1G, proteasome inhibitor MG132 treatment had no effect on TGF-β1-induced downregulation of TAp63α protein expression. However, TGF-β1-induced downregulation of TAp63α protein level was completely blocked by lysosome inhibitor chloroquine (CLQ) (Figure 1H). Next, we investigated whether TGF-β1 promotes TAp63α lysosomal degradation is dependent of canonical Smad pathway. As shown in Figure 1I, treatment with Smad3 inhibitor SIS3 had no effects on TGF-β1-induced downregulation of TAp63α. Moreover, we found that TGF-β1 had little effects on TAp63γ protein expression (Figure 1J). Together, these results demonstrate that TGF-β1 specifically promotes TAp63α protein lysosomal degradation in a canonical Smad-independent manner in MIA PaCa-2 cells.
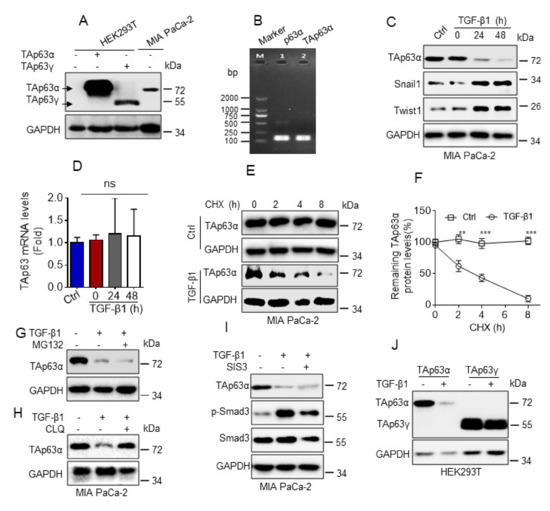
Figure 1.
TGF-β1 promotes TAp63α protein degradation in a lysosome dependent manner. (A) HEK293Tcells were transiently transfected with a vector control, TAp63α orTAp63γ. Cells were subjected to western blot analyses. (B) Reverse transcription PCR was performed to examine the mRNA transcripts of p63 in MIA PaCa-2 cells. (C,D) MIA PaCa-2 cells were treated or untreated with TGF-β1 (5 ng/mL) for an indicated time point under serum-free condition. Cells were subjected to western blot (C) or qPCR (D) analyses. (E,F) MIA PaCa-2 cells were treated with cycloheximide (CHX, 50 μg/mL) in the presence or absence of TGF-β1 (5 ng/mL) for the indicated time intervals under serum-free condition. Cells were subjected to western blot analyses (E). Western blot images were analyzed using Image Lab software, and TAp63α protein half-life was plotted as shown (F). (G,H) MIA PaCa-2 cells were treated or untreated with TGF-β1 (5 ng/mL) for 12 h prior to treatment with MG132 (10 μM) (G) or chloroquine (CLQ, 50 μM) (H) for 12 h under serum-free condition. Cells were subjected to western blot analyses. (I) MIA PaCa-2 cells were treated or untreated with 5 ng/mL TGF-β1 in the presence or absence of 3 μM SIS3 for 24 h under serum-free condition. Cells were subjected to western blot analyses. (J) HEK293T cells were transient transfected with TAp63α or TAp63γ. Cells were treated with or without 5 ng/mL TGF-β1 for 24 h under serum-free condition. Cells were subjected to western blot analyses. Data are presented as means ± s.d. **, p < 0.01; ***, p < 0.001; ns, no significance. Original images supporting all western blot results reported in Figures S1–S16.
3.2. TGF-β1 Inhibits TAp63α to Promote Pancreatic Cancer Cell Migration
Our abovementioned data show that TGF-β1 promotes TAp63α protein lysosomal degradation. Since TAp63α also serves as a key tumor metastasis suppressor, we thus, asked whether TGF-β1-induced downregulation of TAp63α contributes to pancreatic cancer cell migration. As shown in Figure 2A,B, TGF-β1 significantly increased MIA PaCa-2 cell migration, as evidenced by transwell assays. In addition, silencing of p63 by short hairpin RNA (shRNA) led to significantly increased MIA PaCa-2 cell migration (Figure 2C,E). Conversely, ectopic expression of TAp63α suppressed MIA PaCa-2 cell migration (Figure 2F–H). Importantly, TGF-β1-induced upregulation of MIA PaCa-2 cell migration was totally blocked by ectopic expression of TAp63α (Figure 2I–K). Together, these results indicate that downregulation of TAp63α plays a causative role in TGF-β1-induced MIA PaCa-2 cell migration.
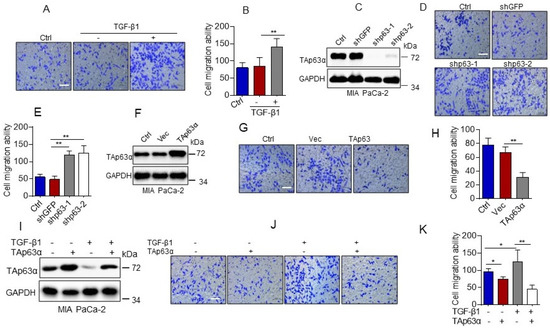
Figure 2.
TGF-β1 promotes pancreatic cancer cell migration by suppressing TAp63α. (A,B) MIA PaCa-2 cells were treated or untreated with TGF-β1 (5 ng/mL) for 36 h. Cell motility was examined by transwell assays. (C–E) MIA PaCa-2 cells stably expressing shGFP, shp63-1 or shp63-2 were subjected to Western blot analyses (C) or transwell assay for cell motility (D,E). (F–H) MIA PaCa-2 cells stably expressing a vector control (Vec) or TAp63α were subjected to Western blot analyses (F) or transwell assay for cell motility (G,H). (I–K) MIA PaCa-2 cells stably expressing TAp63α or Vec were treated or untreated with 5 ng/mL TGF-β1 for 36 h. Cells were subjected to Western blot analyses (I) or transwell assay for cell motility (J,K). Data are presented as means ± s.d. **, p < 0.01; *, p < 0.05. Original images supporting all western blot results reported in Figures S1–S16.
3.3. TGF-β1-Induced Downregulation of TAp63α Upregulates Mutant p53 Expression to Promote Pancreatic Cancer Cell Migration
Next, we examined the molecular mechanism by which TAp63α regulates MIA PaCa-2 cell migration. It has been documented that cancer-associated p53 hot-spot mutants, including R175H, R273H and R248W, possesses gain of function in promoting cancer metastasis [26]. Since MIA PaCa-2 cells carry a p53-R248W mutation [27]. We asked whether TGF-β1 affects p53-R248W protein expression in MIA PaCa-2 cells. As shown in Figure 3A, TGF-β1 significantly upregulated expression of EMT markers Twist1 and Snail1, as expected. It markedly elevated p53-R248W protein expression. Furthermore, knockdown of TAp63α by shRNAs dramatically increased p53-R248W protein expression, concomitant with increased expression of Twist1 and Snail1 proteins in MIA PaCa-2 cells (Figure 3B). qPCR analyses showed that silencing of TAp63α significantly upregulated expression of p53-R248W mRNA (Figure 3C). Immunofluorescence staining assay showed that knockdown of TAp63α dramatically led to accumulation of p53-R248W in the nucleus (Figure 3D). On the other hand, ectopic expression of TAp63α significantly inhibited expression of both p53-R248W mRNA and protein, concomitant with reduced expression of Twist1 and Snail1 proteins in MIA PaCa-2 cells (Figure 3E,F). Importantly, knockdown of TAp63α-induced upregulation of Snail1 and twist1 protein expression and increased MIA PaCa-2 cell migration were totally reversed by simultaneous knockdown of p53-R248W (Figure 3G,I). Next, we investigated whether TGF-β1 promotes p53-R248W expression via inhibiting TAp63α. As shown in Figure 3J, ectopic expression of TAp63α can totally inhibit TGF-β1-induced upregulation of p53-R248W and Snail1 protein expression. In addition, TGF-β1-induced MIA PaCa-2 cell migration can be completely suppressed by silencing of p53-R248W expression, consistent with ectopic expression of TAp63α (Figure 2I,K and Figure 3K,M). Together, these results indicate that TGF-β1 up-regulates p53-R248W expression via suppression of TAp63α, thereby facilitating MIA PaCa-2 cell migration.
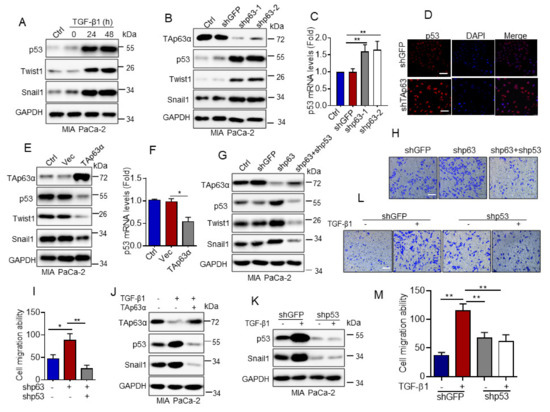
Figure 3.
TGF-β1-induced downregulation of TAp63α promotes MIA PaCa-2 cell motility via upregulation of mutant p53. (A) MIA PaCa-2 cells were treated or untreated with TGF-β1 (5 ng/mL) for an indicated time point under serum-free condition. Cells were subjected to western blot analyses. (B–D) MIA PaCa-2 cells stably expressing shGFP, shp63-1 or shp63-2 were subjected to western blot analyses (B), qPCR analyses (C) or to immuno-fluorescent staining for p53 (D). (E,F) MIA PaCa-2 cells stably expressing TAp63α or Vec were subjected to western blot (E) or qPCR analyses (F). (G–I) MIA PaCa-2-shp63 cells stably expressing shp53 were subjected to western blot analyses (G) or transwell assay for cell motility (H,I). (J) MIA PaCa-2 cells stably expressing TAp63α or Vec were treated or untreated with 5 ng/mL TGF-β1 for 36 h. Cells were subjected to western blot analyses. (K–M) MIA PaCa-2 cells stably expressing shp53 or shGFP were treated or untreated with TGF-β1 (5 ng/mL) for 36 h. Cells were subjected to western blot analyses (K) or transwell assay for cell motility (L,M). Data are presented as means ± s.d. **, p < 0.01; *, p < 0.05. Original images supporting all western blot results reported in Figures S1–S16.
4. Discussion
TGF-β signaling plays a pivotal in the regulation of EMT, cell migration and tumor metastasis [1,2,3]. It has been documented that the activation of TGF-β signaling can promote expression of Snail, Twist1, Slug, and ZEB1, which in turn, regulates the expression of N-cadherin, Vimentin and, E-cadherin, proteins critically involved in EMT [28]. Moreover, TGF-β1 can activate PI3K/AKT/mTOR signaling or promote expression of EGFR and FosB to facilitate cancer cell motility, invasion and tumor metastasis [29,30]. Furthermore, TGF-β1 has been shown to promote mutant-p53/Smad complex formation, which antagonizes TAp63 transcriptional activity to facilitate cancer metastasis [31]. Our recent study demonstrates that TGF-β1 promotes FBXO3-mediated ΔNp63α protein degradation to enhance tumor metastasis [15]. In here, we show that TGF-β1 can inhibit TAp63α protein stability in a lysosome-dependent, but canonical Smad pathway-independent manner, leading to upregulation of p53-R248W expression, and consequently resulting in increased MIA PaCa-2 cell migration (Figure 4).
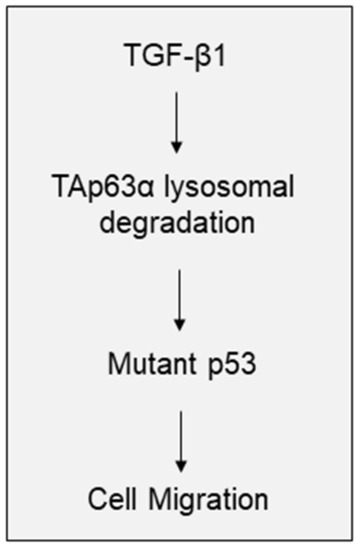
Figure 4.
A model depicts the role of TAp63α in TGF-β1-induced pancreatic cancer cell motility. TGF-β1 promotes TAp63α lysosomal degradation, which leads to the upregulation of mutant p53 expression, consequently results in pancreatic cell migration.
Accumulating evidence indicate that TGF-β signaling regulates a series of genes transcription through canonical Smad pathway or noncanonical Ras–Erk, PI3K/AKT, and Rho-like GTPase pathways [4,5]. Furthermore, it is reported that physiologic levels of TGF-β1 can stimulate paxillin mRNA translation [32]. In recent years, lots of researches demonstrated that TGF-β signaling also can regulate protein degradation. TGF-β1 can promote iNOS and MyD88 protein degradation in a proteasome-dependent manner [8,10]. Furthermore, TGF-β1 can promote type I collagen protein lysosomal degradation [9]. Our recent study demonstrates that TGF-β1 promotes ΔNp63α protein proteasomal degradation [15]. In this study, we show that TGF-β1 can facilitate TAp63α protein degradation in lysosome-dependent manner. Therefore, TGF-β signaling regulates protein expression in multiple levels, including transcription, translation and protein degradation.
Understanding how TGF-β1 facilitates TAp63α protein lysosomal degradation is not yet clear. It has been reported that the chaperone-mediated autophagy (CMA) is one of the intracellular proteolytic machineries for selective protein degradation within lysosome [33]. In the CMA process, CMA substate interacts with heat shock cognate protein 70 (Hsc70), which carries CMA substate to lysosome to degradation via lysosomal membrane protein type 2a (LAMP2A) [33]. Since TGF-β can facilitate Hsc70 and Smad2/3 interaction [34], it is plausible that TGF-β1 may promote TAp63α and Hsc70 interaction, resulting in TAp63α protein degradation in a CMA-dependent manner, a possibility needs to be further investigated.
While ΔNp63α is mainly expressed in epidermis [11], the expression of TAp63α in epidermis is extremely low [35]. Interestingly, it has been shown that the expression of TGF-β1 is high in epidermis [36]. In this study, we show that TGF-β1 inhibit TAp63α expression. Whether high levels of TGF-β1 contribute to low levels of TAp63α expression in epidermis remains to be seen.
5. Conclusions
Numerous studies demonstrate that TGF-β signaling plays an important role in promoting cancer cell migration and tumor metastasis. In this study, we show that TGF-β1 can specifically inhibit TAp63α, a p53 family member, protein stability in a lysosome-dependent, but canonical Smad pathway-independent, manner, which leads to upregulation of p53-R248W protein expression, and consequently results in pancreatic cancer cell migration. Our study reveals a new molecular mechanism by which TGF-β1 promotes cancer cell migration and demonstrates that lysosomal degradation is a novel way to regulate TAp63α protein fate.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10070597/s1, Figures S1–S16. The experimental samples, loading order, molecular weight markers, and protein quantitation are indicated. Original images supporting all western blot results reported in Figures S1–S16.
Author Contributions
Conceived and designed the experiments: G.G., Y.Y., Z.-X.J.X. Performed the experiments: G.G., J.C., D.W., Q.L., X.Y., J.W., Z.P. Analyzed the data: Y.Y. and G.G. Wrote the paper: Y.Y., G.G., Z.-X.J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82073248, 81802951, 81861148031) to Y.Y. or Z.-X.J.X.; China Postdoctoral Science Foundation (2020T130451 and 2018M631081) to Y.Y.; Zhejiang Provincial Key Discipline of Medical Science Foundation (437202002) to GHG.
Acknowledgments
We thank Kang Han for help on instruments and Yujun Zhang for helpful discussions.
Conflicts of Interest
The authors have declared that noncompeting interest exists. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| CLQ | chloroquine |
| EMT | epithelial-mesenchymal transition |
| CMA | chaperone-mediated autophagy |
References
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Jakowlew, S.B. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006, 25, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.; Massague, J. SMADs: Mediators and regulators of TGF-beta signaling. Curr. Opin. Genet. Dev. 1998, 8, 103–111. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-beta signaling from receptors to smads. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Mitani, T.; Terashima, M.; Yoshimura, H.; Nariai, Y.; Tanigawa, Y. TGF-beta1 enhances degradation of IFN-gamma-induced iNOS protein via proteasomes in RAW 264.7 cells. Nitric. Oxide. 2005, 13, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Na, H.J.; Ding, Y.; Wang, Z.; Lee, S.J.; Choi, M.E. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J. Biol. Chem. 2012, 287, 11677–11688. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, J.S.; Kim, J.H.; Jung, S.M.; Lee, J.Y.; Kim, S.J.; Park, S.H. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-beta1-induced degradation of MyD88 in TLR4 signalling. Nat. Commun. 2011, 2, 460. [Google Scholar] [CrossRef]
- Bergholz, J.; Xiao, Z.X. Role of p63 in development, tumorigenesis and cancer progression. Cancer Microenviron. 2012, 5, 311–322. [Google Scholar] [CrossRef]
- Hu, L.; Liang, S.; Chen, H.; Lv, T.; Wu, J.; Chen, D.; Wu, M.; Sun, S.; Zhang, H.; You, H.; et al. DeltaNp63alpha is a common inhibitory target in oncogenic PI3K/Ras/Her2-induced cell motility and tumor metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, E3964–E3973. [Google Scholar] [CrossRef]
- Yi, Y.; Chen, D.; Ao, J.; Zhang, W.; Yi, J.; Ren, X.; Fei, J.; Li, F.; Niu, M.; Chen, H.; et al. Transcriptional suppression of AMPKalpha1 promotes breast cancer metastasis upon oncogene activation. Proc. Natl. Acad. Sci. USA 2020, 117, 8013–8021. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Gao, Y.; Luo, Y.; Luo, H.; Wang, L.; Yi, Y.; Yuan, Z.; Jim Xiao, Z.X. Hippo kinases regulate cell junctions to inhibit tumor metastasis in response to oxidative stress. Redox. Biol. 2019, 26, 101233. [Google Scholar] [CrossRef]
- Niu, M.; He, Y.; Xu, J.; Ding, L.; He, T.; Yi, Y.; Fu, M.; Guo, R.; Li, F.; Chen, H.; et al. Noncanonical TGF-beta signaling leads to FBXO3-mediated degradation of DeltaNp63alpha promoting breast cancer metastasis and poor clinical prognosis. PLoS Biol. 2021, 19, e3001113. [Google Scholar] [CrossRef] [PubMed]
- Suh, E.K.; Yang, A.; Kettenbach, A.; Bamberger, C.; Michaelis, A.H.; Zhu, Z.; Elvin, J.A.; Bronson, R.T.; Crum, C.P.; McKeon, F. p63 protects the female germ line during meiotic arrest. Nature 2006, 444, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Bildik, G.; Acilan, C.; Sahin, G.N.; Karahuseyinoglu, S.; Oktem, O. C-Abl is not activated in DNA damage-induced and Tap63-mediated oocyte apoptosis in human ovary. Cell Death Dis. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.M.; Rossi, V.; Osterburg, S.; Smirnov, A.; Osterburg, C.; Tuppi, M.; Cappello, A.; Amelio, I.; Dotsch, V.; De Felici, M.; et al. The p63 C-terminus is essential for murine oocyte integrity. Nat. Commun 2021, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Beyer, U.; Moll-Rocek, J.; Moll, U.M.; Dobbelstein, M. Endogenous retrovirus drives hitherto unknown proapoptotic p63 isoforms in the male germ line of humans and great apes. Proc. Natl. Acad. Sci. USA 2011, 108, 3624–3629. [Google Scholar] [CrossRef]
- Marcet-Ortega, M.; Pacheco, S.; Martinez-Marchal, A.; Castillo, H.; Flores, E.; Jasin, M.; Keeney, S.; Roig, I. p53 and TAp63 participate in the recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2017, 13, e1006845. [Google Scholar] [CrossRef] [PubMed]
- Terrinoni, A.; Serra, V.; Bruno, E.; Strasser, A.; Valente, E.; Flores, E.R.; Van Bokhoven, H.; Lu, X.; Knight, R.A.; Melino, G. Role of p63 and the Notch pathway in cochlea development and sensorineural deafness. Proc. Natl. Acad. Sci. USA 2013, 110, 7300–7305. [Google Scholar] [CrossRef]
- Su, X.; Chakravarti, D.; Cho, M.S.; Liu, L.; Gi, Y.J.; Lin, Y.L.; Leung, M.L.; El-Naggar, A.; Creighton, C.J.; Suraokar, M.B.; et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 2010, 467, 986–990. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, J.; Yang, X.; Huang, C.; Zhou, L.; Meng, Z.; Li, X.; Zhong, C. TAp63alpha is involved in tobacco smoke-induced lung cancer EMT and the anti-cancer activity of curcumin via miR-19 transcriptional suppression. Front. Cell Dev. Biol. 2021, 9, 645402. [Google Scholar] [CrossRef]
- Lin, C.W.; Li, X.R.; Zhang, Y.; Hu, G.; Guo, Y.H.; Zhou, J.Y.; Du, J.; Lv, L.; Gao, K.; Zhang, Y.; et al. TAp63 suppress metastasis via miR-133b in colon cancer cells. Br. J. Cancer 2014, 110, 2310–2320. [Google Scholar] [CrossRef]
- Bergholz, J.; Zhang, Y.; Wu, J.; Meng, L.; Walsh, E.M.; Rai, A.; Sherman, M.Y.; Xiao, Z.X. DeltaNp63alpha regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene 2014, 33, 212–224. [Google Scholar] [CrossRef]
- Lv, T.; Lv, H.; Fei, J.; Xie, Y.; Lian, D.; Hu, J.; Tang, L.; Shi, X.; Wang, J.; Zhang, S.; et al. p53-R273H promotes cancer cell migration via upregulation of neuraminidase-1. J. Cancer 2020, 11, 6874–6882. [Google Scholar] [CrossRef]
- Vogiatzi, F.; Brandt, D.T.; Schneikert, J.; Fuchs, J.; Grikscheit, K.; Wanzel, M.; Pavlakis, E.; Charles, J.P.; Timofeev, O.; Nist, A.; et al. Mutant p53 promotes tumor progression and metastasis by the endoplasmic reticulum UDPase ENTPD5. Proc. Natl. Acad. Sci. USA 2016, 113, E8433–E8442. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, U.; Kowanetz, M.; Niimi, H.; Heldin, C.H.; Moustakas, A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell 2005, 16, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, J.; Fan, Y.; Wang, Z.; Tian, R.; Ji, W.; Zhang, F.; Niu, R. TGF-beta transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol. Oncol. 2018, 12, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.S.; Millena, A.C.; Khan, S.A. TGF-beta effects on prostate cancer cell migration and invasion require FosB. Prostate 2017, 77, 72–81. [Google Scholar] [CrossRef]
- Adorno, M.; Cordenonsi, M.; Montagner, M.; Dupont, S.; Wong, C.; Hann, B.; Solari, A.; Bobisse, S.; Rondina, M.B.; Guzzardo, V.; et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 2009, 137, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Stewart, J.E., Jr.; Bellis, S.L.; Benveniste, E.N.; Ding, Q.; Tachibana, K.; Grammer, J.R.; Gladson, C.L. TGF-beta1 up-regulates paxillin protein expression in malignant astrocytoma cells: Requirement for a fibronectin substrate. Oncogene 2001, 20, 7976–7986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Q.; Wang, R.; Zhu, L. Chaperone-mediated autophagy. Adv. Exp. Med. Biol. 2019, 1206, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, M.; Higashimoto, N.; Matsumura, K.; Ihara, Y. Hsc70 facilitates TGF-beta-induced activation of Smad2/3 in fibroblastic NRK-49F cells. Biochem. Biophys. Res. Commun. 2016, 477, 448–453. [Google Scholar] [CrossRef]
- Bamberger, C.; Pollet, D.; Schmale, H. Retinoic acid inhibits downregulation of DeltaNp63alpha expression during terminal differentiation of human primary keratinocytes. J. Investig. Dermatol. 2002, 118, 133–138. [Google Scholar] [CrossRef]
- Yan, L.; Cao, R.; Wang, L.; Liu, Y.; Pan, B.; Yin, Y.; Lv, X.; Zhuang, Q.; Sun, X.; Xiao, R. Epithelial-mesenchymal transition in keloid tissues and TGF-beta1-induced hair follicle outer root sheath keratinocytes. Wound Repair Regen. 2015, 23, 601–610. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).