Abstract
This paper focuses on the study of the famous royal purple dye. It aims to present a holistic approach by researching historical evidence, both for its use and its production, to highlight the importance of the dye within the Greek area. As a substantial part of information concerning the dyeing procedure of purple dye has been lost during the ages, it is crucial to establish points of documentation and identification. The latter can be achieved through chemical analysis, but as this dye is found on precious’s cultural heritage items, which cannot always be sampled, a non-destructive approach should be considered as more appropriate. At first, the history of the dye purple is presented within the Greek area. Then, samples of purple dye are created based on traditional recipes from the Greek area, in order to compose a profile with the characteristics of purple using non-destructive and imaging techniques, thus emphasizing the importance of applying these techniques for the study of dyes on textiles. The results of the experiments show differences in behavior between the pure gland and the dyed samples, as well as the intensity of the color depending on the dyeing procedure.
1. Introduction
Purple dye stands as one of the most legendary and revered dyes of antiquity, with its production in Greek territories traceable from ancient times to the present day. Known by various names—including Tyrian purple, royal purple, imperial purple, and imperial dye—this reddish-purple natural pigment has long symbolized power, royalty, and divinity [1,2,3].
Tyrian purple was reserved exclusively for priests, kings, emperors, and magistrates, elevating it to a status of elite distinction. Garments dyed with this color were so valuable that their cost often surpassed that of precious metals. Its brilliance and durability endured through all weather conditions and wear, making it not only luxurious but remarkably resilient [1,3,4].
Purple dye is widely regarded as a milestone in the history of textile chemistry and remains one of the most iconic dyes ever produced. Its influence on the ancient Mediterranean economy was profound, spanning from the Bronze Age through to the fall of the Byzantine Empire. The dye’s exceptional range—from vivid purples to deep reds—and its resistance to fading made Tyrian purple garments highly coveted and extraordinarily expensive [4,5].
1.1. Historic Background
Mentions of purple dye date back to the early second millennium BCE. Its high value resulted in the existence of many true and false purple dyes. Some sources questioned whether the dye was plant-based or derived from animal matter. Dioscorides (40–90 CE) classified purple dye as botanical in origin, a view similarly held by Pliny the Elder (23–79 CE). Theophrastus (c. 371–287 BCE), however, proposed that the dye referenced by these authors was produced by an algal species—yielding a vivid and aesthetically pleasing pigment, albeit inferior to the renowned Tyrian purple. This distinction records that in antiquity there existed more than one red dye, trying to copy the purple color [1,6,7].
Aristotle, in his treatise On Colors, identified purple dye as animal-derived and emphasized its exceptional stability, noting that it required no mordanting—a rare property among natural dyes. Although modern research confirms that Tyrian purple was extracted from marine mollusks of the genus Murex, ancient scholars remained divided on its botanical versus zoological origins [1,4,8].
Several legends attempt to present the discovery of Tyrian purple. One narrative describes a fisherman working for a Phoenician king whose dog, after playing with a Murex bandaris shell, had its mouth stained red. Upon attempting to clean the dog, the fisherman observed the brilliance and permanence of the dye. Another story, recorded by the 2nd-century Greek grammarian Julius Pollux, recounts Hercules encountering the nymph Tyros and her dog by the seashore. The dog, having bitten into a seashell, had its mouth and nose stained purple. Enchanted by the hue, Tyros requested a garment dyed in the same color.
These stories reflect the intertwined cultural claims of Phoenician and Greek civilizations regarding the origins of purple dye. Both societies not only asserted their role in its discovery but also became prominent exporters of the dye throughout their colonial networks [1,3].
Archeological evidence supports the antiquity of purple dye production. Excavations have uncovered remnants of dye workshops dating to approximately 1800 BCE, along with inscriptions in Linear B that reference the dye’s significance. Furthermore, records from the Assyrian monarchy in the 9th century BCE document tax payments made in purple-dyed wool. Two distinct terms appear in these records: argamannu, referring to red-dyed textiles, and takiltu, denoting violet-dyed fabrics [1,2,3,4].
The widespread use and appreciation of purple-dyed textiles in antiquity underscores their cultural, economic, and symbolic significance, particularly as tributes and spoils of war. The prophet Ezekiel records that commanders of the Assyrian army were adorned in Tyrian purple [1], highlighting its association with elite status and military prestige. During the Bronze Age, Minoan textiles and purple-dyed goods represented one of the most esteemed commodities in international trade [9].
Literary sources further attest to the prominence of Tyrian purple in ancient society. In Homer’s Iliad, Agamemnon is described as possessing a mantle dyed in Tyrian purple, while Hector’s funerary urn was draped in the same luxurious fabric (Il. B221, 19,225). In the Odyssey, Odysseus receives a purple mantle from Penelope as a farewell gift and is later depicted wearing one on the island of the Phaeacians (Od. 8.84, 19,225). Telemachus is also described wearing a purple mantle (Od. 4.115, 4.154), and palace women are noted to have worn garments dyed in purple (Il. 24.644–645).
By the 6th century BCE, several prominent centers of purple dye production had emerged. The Persians, renowned for their opulence, were avid consumers of purple-dyed garments, which were regarded as symbols of wealth and prestige. On the island of Lesvos, at the dawn of the 6th century BCE, purple dye was considered the most valuable offering a mortal could present to a deity. Prior to the Battle of Marathon (Location close to Athens, where a great battle between Greeks and Persians took place) in 490 BCE, wearing Tyrian purple garments was a common means of displaying affluence and social power.
Spartan (Sparta is a place in the south Greece. It was famous for its military educational system. Lycurgus is traditionally regarded as the lawgiver who established the Spartan strict military-oriented society and its unique governance system) law, as codified by Lycurgus, explicitly prohibited the use of Tyrian purple wool garments, deeming them excessively luxurious. Alexander the Great restricted the use of purple dye to royal attire and gifts for favored officials. He acquired vast quantities of purple textiles as spoils during his campaigns, particularly in Persian territories. One such fabric, reportedly found in the royal vault of Darius and produced in Hermione (a town in the Peloponnese), (Figure 1) was valued at approximately 5000 talents—an extraordinary sum, considering that Alexander’s annual military expenditures ranged between 4000 and 7000 talents. Plutarch (Alexander 36) and Diodorus Siculus (17.70) both record that despite being 180 years old, the garment retained its vivid coloration and quality.
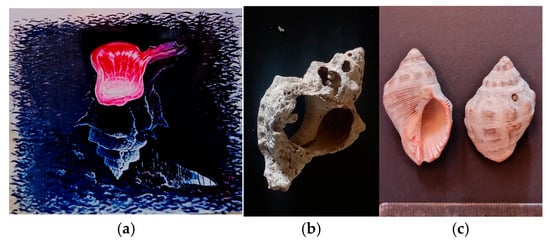
Figure 1.
Tyrian purple seashell. (a) Fresco painting in a traditional tavern in Hermioni, where ancient ruins of manufacturing still exist (photo A. Tsatsarou) (b) Murex Trunculus shell broken to the point where the gland is placed. The shell was found in the ruins of within the archeological site Bisti, Hermione, Greece. (photo A. Tsatsarou) (c) Seashell Murex Haemastoma (photo A. Tsatsarou).
Following Alexander’s death, the exclusivity surrounding purple dye diminished. Its use was democratized, and individuals who could afford it were permitted to own garments dyed in Tyrian purple [10,11].
In the centuries following its initial prominence, Tyrian purple gained widespread recognition and was adopted by the Romans as a potent symbol of imperial authority and elite status. Pliny the Elder notes that by the 2nd century BCE, Roman philosophers began to associate the dye with the decline of both the Roman Empire and democratic ideals. Emperors such as Caligula and Nero (Roman Emperors notorious of their insanity and cruelty) restricted its use exclusively to the imperial palace, reinforcing its role as a marker of centralized power and privilege. During this period, the price of Tyrian purple reached unprecedented levels, reflecting its rarity and prestige [6].
In the Byzantine era (330–1453 CE), Royal Purple became the definitive emblem of imperial sovereignty. Members of the imperial family were said to be “born in the purple” and “die in the purple,” signifying that every aspect of the emperor’s private quarters was adorned in this revered hue [3]. Byzantine sources, including the Book of Ceremonies by Emperor Constantine VII Porphyrogenitus, provide detailed accounts of the symbolic functions and prescribed uses of purple-dyed fabrics and ceremonial garments within the court.
Emperor Constantine the Great centralized control over Tyrian purple production, instituting strict regulations on its distribution. According to Walter [12], the export of purple-dyed textiles beyond Byzantine borders was prohibited without explicit imperial authorization. One notable incident illustrates the political weight of this restriction: a group of foreign diplomats received valuable purple-dyed garments as gifts but failed to obtain the necessary export permissions. Upon attempting to leave the empire, they were arrested for smuggling—underscoring the dye’s strategic and symbolic importance.
The production of Tyrian purple was a labor-intensive and odorous process, involving the extraction of pigment from the hypobranchial glands of various marine mollusks, including Murex trunculus (also known as Hexaplex trunculus) (Figure 1), Purpura lapillus, Helix ianthina, and especially Murex brandaris. These shellfish inhabited relatively deep waters and were captured using baited traps suspended from floats. Thousands of specimens were crushed to extract the dye, which was then left to ferment and bake in the sun. The resulting liquid produced a spectrum of hues ranging from pink to violet. Due to the pungent odor associated with the process, dye workshops—such as the one in Sidon—were located at considerable distances from urban centers. The Sidonian production was located 14 km south of the city at Sarepta [4,12].
Pliny the Elder (Known as Gaius Plinius Secundus. He was a Roman author, naturalist, and military commander) later described an enhanced extraction method involving the boiling of shellfish glands with salt over a period of three days. Whole fleeces were then immersed in the solution to achieve the desired coloration, reflecting the evolving sophistication of dyeing techniques in the Roman period.
Tyrian purple, historically valued above gold by weight, remains a subject of enduring fascination due to its cultural, economic, and technological significance. Its legacy as a symbol of power and prestige continues to resonate in scholarly discourse and public imagination alike. However, the marine mollusks responsible for producing this dye—primarily species of the genus Murex—now face existential threats due to anthropogenic pressures [1].
Environmental degradation, particularly pollution and climate change, has severely impacted the habitats of these sea snails. The decline of Murex populations underscores the urgency of preserving these ancient biological sources, not only for their ecological value but also for their historical and scientific importance. Conservation efforts aimed at protecting marine biodiversity are essential to safeguarding the legacy of Tyrian purple and the natural organisms that made its production possible [4].
1.2. Historic Evidence of the Dye Procedure During the Ages
The production of purple textiles in antiquity adhered to a meticulously organized sequence of operations, encompassing the acquisition of mollusk shells, extraction of the hypobranchial glands, preparation of the dye, execution of the dyeing process, and ultimately, the trade of the finished textiles. This structured chain reflects the complexity and specialization inherent in ancient dyeing practices.
Archeological evidence indicates that well-established dyeing centers often included dedicated laboratory spaces equipped with essential chemicals, vessels, instruments, and standardized dye samples. Such facilities have been identified at multiple ancient sites (Figure 2a), suggesting a high degree of technical sophistication. Notably, many of the vessels recovered from these locations resemble beehives in shape and construction.

Figure 2.
(a) A water cistern which was used to gather rain water for the purple dye workshop found within the archeological site Bisti, Hermione Greece. (A. Tsatsarou) (b) pieces of broken shells used as mortar in the Byzantine castles. (A. Tsatsarou).
Honey appears to have played a functional role in the dyeing process. It was likely used to preserve the freshness of the dye and may have served as a natural reducing agent, contributing to the chemical stability and intensity of the final pigment. These findings underscore the advanced empirical knowledge possessed by ancient dyers and the integration of organic materials into their chemical processes [3,4].
The initial stage of Tyrian purple production involved the manual breaking of harvested seashells to extract the hypobranchial gland, the source of the dye. Daily operations at dyeing centers reportedly processed between 5000 and 10,000 mollusks, yielding approximately 10 to 20 kg of dye per day. Given the labor-intensive nature of the process and the vast quantity of raw material required, the resulting dye was exceptionally valuable—often priced on par with, or exceeding, the value of gold and silver.
Due to its high economic and symbolic worth, all stages of production were conducted within secure and controlled environments. Both the dye and the finished textiles were stored in vaults to prevent theft and ensure preservation. As the garments were intended for sale, dyers needed to remain informed about market fluctuations and consumer demand, indicating a sophisticated understanding of trade dynamics and pricing strategies.
It is noteworthy that the by-products of this process—the broken and emptied shells—were repurposed in later periods. Archeological and textual evidence suggests that these remnants were incorporated into construction materials, serving as components in mortar or road surfacing during the Roman and Byzantine eras. This practice reflects both the resourcefulness of ancient industries and the broader integration of dye production into urban infrastructure. (Figure 2b and Figure 3) [3,4].

Figure 3.
A path (b) in the archeological site in Bisti, Hermione Greece, where the ground is covered with empty broken shells not gravel (a). (A. Tsatsarou).
The expertise surrounding the production of Tyrian purple was not static but evolved dynamically over centuries, shaped by accumulated experience and the distinct environmental and logistical challenges of each region. Every phase of the production process—from the initial harvesting of mollusks in coastal zones to the final trade of the dyed textiles—underwent continual refinement in both spatial organization and technical execution [10,12].
This transmission of knowledge was neither casual nor open; rather, it was a carefully preserved tradition, passed through generations and often confined to specialized communities. The dyeing techniques, chemical processes, and trade practices associated with Tyrian purple were considered a form of intellectual capital—an asset of immense value. As such, mastery of this knowledge was regarded as a form of wealth and was frequently treated as a closely guarded secret (Figure 4).
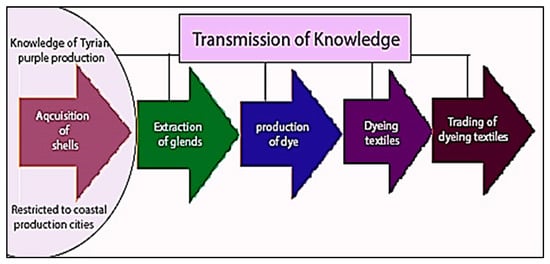
Figure 4.
A model showing schematically how the knowledge of Tyrian purple dyeing process evolved through the different stages of production from seashells to the final customer. While most stages of production could be spatially dispersed, the collection and harvesting of seashells remained geographically constrained. Specifically, the fishing stage consistently occurred in close proximity to the shoreline, reflecting its inherent dependence on coastal access. (A. Tsatsarou).
The persistent development and protection of this expertise underscore the cultural and economic significance of Tyrian purple across the ancient Mediterranean world. It reflects not only the sophistication of early textile chemistry but also the strategic importance of artisanal knowledge in sustaining long-distance trade and elite consumption.
Tyrian purple, a pigment of exceptional historical value, is notably insoluble in water, necessitating complex chemical manipulation to render it suitable for textile dyeing. According to Pliny the Elder, ancient dyers achieved solubility by converting the dye into a leuco-indigo form through alkaline reduction. Among the most accessible alkaline agents in antiquity were fermented urine and sodium hydroxide (NaOH) [13], with additional compounds such as sodium carbonate (Na2CO3), sourced from Egypt, and calcium hydroxide (Ca(OH)2) also employed in the process.
Historical accounts suggest that urine—sometimes collected from children in exchange for small bronze coins—was a common additive due to its ammonia content, which facilitated the necessary pH conditions for reduction [11]. The dyeing procedure involved immersing textile fibers in the leuco-compound solution, allowing them to absorb the reduced dye. Once saturated, the fibers were exposed to sunlight, where rapid oxidation—often occurring in under ten minutes in the Mediterranean climate—transformed the color into its characteristic purple hue. To prevent premature oxidation during the dyeing phase, vessels used in the process were typically made of lead, which helped maintain the chemical stability of the solution. The dyeing cycle could be repeated multiple times to intensify the color and achieve the desired saturation.
The procedure was accompanied by a notably pungent odor, frequently compared to garlic, which contributed to the spatial organization of dyeing facilities. During the Byzantine period, the guild responsible for Tyrian purple production—known as the katavlattades—was deliberately situated outside urban centers to mitigate the impact of the odor on city inhabitants [10,14].
1.3. Chemistry and Structure of the Dye Purple
Tyrian purple is classified as a reducing organic dye, chemically identified as 6,6′-dibromoindigo—a compound that is inherently insoluble in water (Figure 5a). To render it suitable for textile dyeing, ancient practitioners employed reducing agents such as sodium dithionite (Na2S2O4), various sugars, and iron salts to convert the dye into its soluble yellow leuco-form, known as leuco-dibromoindigo.
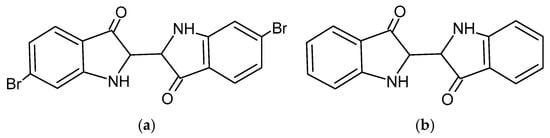
Figure 5.
(a) Molecule of red insoluble Tyrian purple dye. (b) Molecule of indigo (blue) (chem sketch drawn by A. Tsatsarou).
This leuco-compound is highly unstable when exposed to sunlight, ultraviolet radiation, or ambient light. Upon exposure, atmospheric oxygen facilitates the oxidation of the leuco-form, restoring it to its original indigo structure (Figure 5b). This transformation is central to the dyeing process, wherein textile fibers are first saturated in the reduced solution and then exposed to light to develop the final color.
The phenomenon of photo-debromination—where bromine atoms are removed under light exposure—was observed and experimentally explored in antiquity, indicating a sophisticated empirical understanding of photochemical reactions. These insights reflect the advanced chemical knowledge embedded in ancient dyeing practices and the nuanced manipulation of organic compounds to achieve durable and vibrant coloration [15,16].
The chemical products derived from Murex trunculus are notably complex, yielding a range of indigoid compounds including indigo, indirubin, monobromoindigo, and dibromoindigo (Figure 6). The specific composition of these pigments can vary depending on biological and environmental factors, such as the gender of the mollusk and the season in which it is harvested. These variables contribute to the diversity of hues and intensities observed in Tyrian purple textiles [2].
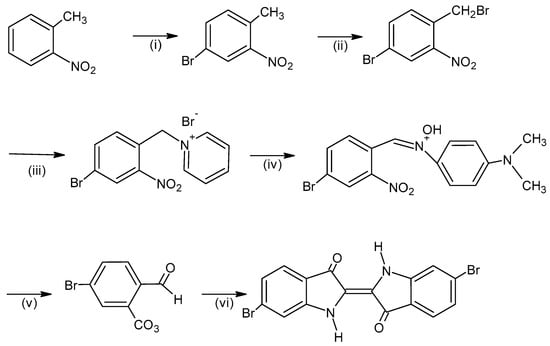
Figure 6.
Synthesis of 6,6′-dibromoindigo. The reagents are: (i) Br2, Fe, (ii) N-bromosuccinimide, (iii) pyridine, (iv) 4-nitroso-N, N’-dimethylaniline, (v) aqueous sulfuric acid and (vi) CH2NO2, CH3ONa, Na2S2O4 (chem sketch drawn by A. Tsatsarou).
The full sequence of dye production—from mollusk extraction to pigment transformation and textile application—is illustrated in Figure 7. This process reflects a sophisticated understanding of organic chemistry in antiquity, where empirical knowledge of biological variability and environmental conditions informed the refinement of dyeing techniques [2].
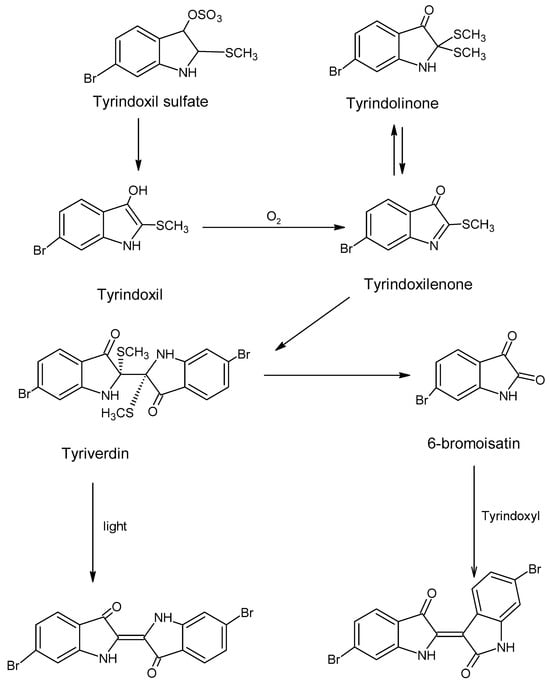
Figure 7.
The generation of the purple color (chem sketch drawn by A. Tsatsarou).
1.4. Chemical Analyses and Documentation Methods
Given its historical prominence, Tyrian purple has been identified on a wide array of archeological artifacts, underscoring its widespread use and cultural significance. To confirm the presence of this dye and to investigate its molecular composition, researchers have employed a range of analytical techniques.
Traditional separation methods, which typically require physical sampling, have been instrumental in isolating and characterizing the dye compounds. Spectroscopic methods, such as Raman spectroscopy, UV-Vis spectrophotometry, and X-ray fluorescence, have also supported the research of the purple dye. However, due to the fragile nature of many ancient textiles and artifacts, there has been a growing emphasis on non-destructive imaging techniques. These methods allow the inspection of an object and the acquisition of information without compromising the integrity of the object.
The shift toward non-invasive diagnostics reflects both technological advancements and a heightened awareness of conservation ethics in cultural heritage research. As a result, the study of Tyrian purple continues to evolve, balancing scientific inquiry with the preservation of irreplaceable historical materials.
1.4.1. Analytical and Spectroscopic Methods
A wide range of analytical techniques—both spectroscopic and separation-based—have been employed to investigate dyes and pigments present on cultural heritage objects suspected to contain Tyrian purple [15,17].
Among the spectroscopic techniques applied are Fourier Transform Infrared Spectroscopy (FTIR) [18], Ultraviolet-Visible (UV-Vis) Spectroscopy [19,20], Raman and micro-Raman Spectroscopy [21,22], Photon-Induced X-ray Emission Spectroscopy (PIXE) [22], Fiber Optic Reflectance Spectroscopy (FORS) [23], Scanning Electron Microscopy with Energy Dispersive X-ray Analysis (SEM-EDX), and Cathodoluminescence. These methods have proven effective in characterizing the chemical structure and composition of purple dyes only requiring a tiny amount of sample without compromising the entire integrity of the objects under study.
In cases where sampling was permitted, separation techniques have also been employed, including High-Performance Liquid Chromatography (HPLC) [24,25], Thin Layer Chromatography (TLC), paper chromatography, and ion-exchange chromatography. Spectrometric methods such as Mass Spectrometry (MS) [26] have yielded valuable insights into the molecular profiles of the dye compounds.
UV-Vis spectroscopy has played a critical role in identifying the molecular structure of standard molluscan dyes, such as 6,6′-dibromoindigo [17]. By utilizing the photodiode array detector integrated into HPLC systems, researchers have successfully recorded UV-Vis spectra of various indigoid compounds [19]. Notably, Tyrian purple in solution exhibits an absorption maximum at approximately 585 nm for 1,1,2,2-tetrachloroethane and 590,5 nm for xylene, which shifts to 520 nm upon application as a dye—an important diagnostic feature in pigment analysis [15,27].
Comparative analysis of FTIR spectra from archeological samples and synthetic standards of 6,6′-dibromoindigotin and indigotin has revealed that the mollusk species used in dye production significantly influences the final color outcome. Specifically, Murex trunculus yields approximately equal proportions of 6,6′-dibromoindigotin (purple) and indigotin (blue), resulting in a composite purple hue. In contrast, Murex brandaris produces a dye composed almost exclusively of 6,6′-dibromoindigotin, yielding a more saturated purple coloration [19].
The compound dibromoindigo exhibits distinct spectroscopic features, including characteristic peaks for the NH stretch at 3300 cm−1 and the C=O stretch at 1630 cm−1. However, due to its poor solubility in conventional solvents at ambient temperatures, dibromoindigo cannot be readily analyzed using Nuclear Magnetic Resonance (NMR) spectroscopy unless it is chemically derivatized or reduced [18].
Advanced analytical techniques such as X-ray Fluorescence (XRF), Fiber Optic Reflectance Spectroscopy (FORS), and molecular fluorescence analysis have been applied to selected areas of the Codex Brixianus, a 5th-century manuscript produced in Northern Italy comprising 418 folios, all written in purple ink [22]. While trace amounts of bromine were detected—suggesting the possible presence of Tyrian purple—the majority of spectral data indicated the use of alternative organic colorants, such as folium, orchil, or lichen-based dyes.
Pliny the Elder provides historical context for this practice, noting that orchil was commonly used as a base dye for wool textiles, with small quantities of Tyrian purple added to achieve the desired hue. This layering technique reflects both the economic constraints and aesthetic preferences of the period, as well as the ingenuity of ancient dyers in manipulating available resources to replicate prestigious colors [3].
High-Performance Liquid Chromatography (HPLC) has proven to be a pivotal analytical tool in the study of ancient Tyrian purple, enabling researchers to isolate and identify its complex biochemical constituents. HPLC was employed in the analysis of a historical dyeing vat containing remnants of Tyrian purple [19], where the formation of indigoid compounds from Murex trunculus was elucidated. The technique also facilitated the detection of purple dye on a two-thousand-year-old textile from Masada [19], and enabled comparative analysis of a purple pigment found on a stone jar attributed to Darius I, alongside samples from contemporary mollusks [17,18].
Koren, successfully utilized HPLC to isolate and characterize the various components of Tyrian purple. A more advanced application of HPLC, incorporating a diode array detector, was later introduced [20,28]. This method requires only minimal sample quantities and significantly enhances the probability of accurate substance identification. Through this approach, the presence of Tyrian purple was confirmed at Tel Shiqmona, Israel, and the predominance of Hexaplex trunculus over other mollusk species in local dye production was established. Similar findings were reported in the analysis of purple-dyed wool fibers from Timna Valley, Israel [29].
The complexity of purple dye precursors has also been demonstrated through paper chromatography, which was used in a comparative study of ethanol extracts from various mollusk species. The resulting stain patterns were indicative of species-specific chemical profiles [14,30,31]. Thin Layer Chromatography (TLC) and ion-exchange chromatography have likewise been successfully employed to separate dye compounds immediately following gland extraction—prior to any exposure to air—thereby preserving the integrity of the analytes for further study [14].
Mass spectrometry (MS) has emerged as a valuable analytical technique for the detection of dibromoindigo in dyed materials, owing to its ability to produce a distinct molecular signature [14,19,30]. This method enables precise identification of dye compounds, even in minute quantities, and has contributed significantly to the characterization of Tyrian purple in archeological contexts [14,19,30].
1.4.2. Imaging Techniques
Imaging techniques offer non-destructive and non-invasive means of analyzing cultural heritage objects, including textiles, as they capture the images in fragments of seconds, minimizing exposure to any harmful radiations. Each modality provides complementary data that, when integrated, enhances the understanding of an artifact’s structural, chromatic, and chemical properties [28]. Despite their widespread application in heritage diagnostics, imaging techniques have not yet been systematically employed for the documentation of Tyrian purple.
Photographic and imaging methods [32,33,34,35,36] are routinely used for inspection, testing, and documentation of artworks and historical objects. These include:
- -
- Visible Color Imaging (VIS)
- -
- Ultraviolet-Induced Visible Luminescence Imaging (UVL)
- -
- Multispectral Imaging (MSI)
- -
- Hyperspectral Imaging (HSI), often enhanced through spectral cube (SC) acquisitions
- -
- False Color Infrared Reflectography (IRRFC)
These techniques are particularly valuable for textile analysis, offering insights into dye distribution, pigment layering, and material degradation without compromising the integrity of the object [35]. Their potential application to Tyrian purple documentation represents a promising avenue for future research in heritage science.
Visible imaging (VIS) and Ultraviolet-Induced Luminescence (UVL) are non-destructive diagnostic techniques widely employed in the documentation and analysis of cultural heritage objects, including textiles. These imaging modalities provide valuable visual and chemical insights while preserving the integrity of the artifacts under investigation.
In visible imaging (VIS), the object is recorded as perceived by the human eye, capturing its true colors and current condition. This method serves as a baseline reference for future comparative studies, conservation assessments, and historical documentation.
Ultraviolet Luminescence (UVL) [31,32,33,36,37,38,39] operates on the principle that ultraviolet radiation can induce luminescence in certain materials, including pigments and dyes. When exposed to UV photons, matter enters an excited state; as this state is inherently unstable, the absorbed energy is gradually released as photons of longer wavelengths—typically within the visible spectrum—according to Stokes’ law. The emitted luminescence can be observed with the naked eye and recorded using conventional photographic equipment, provided that appropriate barrier filters are used to eliminate parasitic ultraviolet and infrared radiation.
Not all substances fluoresce under UV exposure. Materials that absorb UV radiation without emitting visible light appear darker, while those that do fluoresce appear lighter. Moreover, the specific wavelengths of fluorescence emission vary among substances, resulting in distinct colors on the imaging detector. These variations can reveal differences in chemical composition, even among visually similar materials, thereby enabling preliminary identification and classification of dyes and pigments.
Hyperspectral Imaging (HSI) captures images of an object across discrete spectral channels spanning the 380–1000 nm range, enabling detailed band-to-band comparisons that reveal variations in material behavior and composition. This capability is particularly valuable in the analysis of cultural heritage objects, where subtle differences in pigment or substrate can indicate restoration interventions, preparatory sketches, or underlying structural conditions [37,40]
The near-infrared (NIR) component of HSI allows radiation to penetrate paint layers, facilitating the visualization of subsurface features. As hyperspectral cameras have evolved to include a greater number of spectral bands and faster acquisition speeds, they now offer both qualitative and quantitative spectral reflectance data, which are critical for pigment identification [41,42]. These systems can operate in multiple modes—including ultraviolet (UV), visible, NIR-reflectance, and UV-fluorescence—expanding their diagnostic utility [43,44].
HSI technology generates a series of successive images of the same spatial area, each corresponding to a distinct spectral band. These images are compiled into a three-dimensional dataset known as a spectral cube [33]. Each cube contains pixel-specific spectral information, resulting in highly accurate and reproducible reflectance spectra [42,43,44,45,46,47].
An advanced application of spectral cubes involves excitation at 365 nm, producing spectrofluorimetric data that capture the fluorescence behavior of the surface under investigation. This technique has been successfully applied to the analysis of purple-dyed areas in illuminated manuscripts and codices [20,23,48], offering new insights into the composition and preservation of historical dyes such as Tyrian purple [20,24,48].
False Color Infrared (FCIR) Imaging is a diagnostic technique based on the simultaneous recording of infrared and selected visible wavelengths reflected from an object’s surface [33,34,47,49]. Unlike conventional imaging, which captures blue, green, and red light, FCIR imaging records green, red, and infrared radiation (typically around 800 nm). The resulting composite image—termed “false color”—visually represents the object’s behavior in the infrared region, offering insights into material composition and surface characteristics.
Images at individual wavelengths can be acquired using modified digital SLR cameras equipped with appropriate filters, and subsequently combined using image processing software to generate the false color output. Additionally, multispectral and hyperspectral imaging systems are capable of producing false color images, often with enhanced spectral resolution and diagnostic precision [22].
Similar to Ultraviolet-Induced Luminescence (UVL), FCIR imaging is particularly effective in differentiating pigments that appear visually identical but possess distinct chemical compositions. These differences influence how materials absorb and reflect infrared radiation, allowing for qualitative discrimination. However, it is important to note that false color imaging alone cannot provide definitive pigment identification, as multiple substances may exhibit similar false color responses [22,50]
Given that a substantial portion of historical data concerning Tyrian purple has been lost over time, it is imperative to establish robust methods for documentation and identification. While chemical analysis remains the most reliable approach for confirming the presence of specific dyes, the non-destructive nature of imaging techniques makes them especially valuable when working with fragile or culturally significant artifacts that cannot be sampled.
The objective of this study is to investigate the properties of Tyrian purple through the application of non-destructive imaging techniques on samples derived both from pure mollusk gland extracts and traditional dyeing recipes from the Greek region. In parallel, historical research into the dye’s production and use is essential to contextualize its cultural and technological importance within the broader framework of Greek heritage.
2. Materials and Methods
The experimental component of this study focused on the characterization of Tyrian purple pigment through non-destructive analytical techniques. The initial and foundational step involved the collection of Murex seashells and the preparation of dye samples, which were subsequently analyzed using a combination of spectroscopic and imaging methods, following established protocols designed to preserve sample integrity.
To complement the primary investigation, two supplementary experiments were conducted. The first aimed to quantify the ratio between the total weight of harvested seashells, the extracted hypobranchial glands, and the resulting dried pigment. This experiment highlighted the extreme disproportionality inherent in Tyrian purple production, underscoring the labor-intensive nature and rarity of the dye.
The second experiment examined the chromatic transformation and rate of color development during the application of the gland extract onto wool fabric. This dynamic observation provided insights into the oxidation process and the behavior of the dye under ambient conditions, contributing to a deeper understanding of its chemical properties and practical application.
2.1. Sample Preparation and Dying Procedure
The initial phase of the experimental procedure involved the collection of live Murex sea snails, which were maintained in optimal conditions to preserve the integrity of the dye-producing glands. Ensuring the mollusks remained healthy prior to extraction was critical, as premature stress or delayed death can trigger metabolic processes that degrade the pigment precursors. If the mollusk does not expire immediately during the procedure, the resulting dye may exhibit reduced strength, purity, and yield.
All dyeing procedures were conducted at the Color Chemistry Laboratory of the West Attica University, under controlled conditions designed to replicate traditional methods while enabling precise documentation and analysis. This setting provided the necessary infrastructure for both the preparation of dye samples and the application of non-destructive analytical techniques.
2.1.1. Experiment 1-Pigment Ratio of the Purple Seashells
The objective of this experiment was to determine the approximate ratio between the number of Murex trunculus shells, the weight of the extracted hypobranchial glands, and the final yield of dried pigment. A total of sixty-two (62) specimens were collected with the assistance of local fishermen from the Hermioni region, where these mollusks are frequently caught incidentally in fishing nets but are not commercially utilized.
The total weight of the shells was approximately 2 kg. Of the collected specimens, fifty-five (55) were medium to large in size (approximately 5 cm), and seven (7) were notably larger (approximately 8 cm). The extraction process was conducted with care to maximize gland recovery. The procedure, carried out by two individuals over a span of 20 min, involved one person crushing the shells while the other isolated the mucus glands from the surrounding tissue [1,11,51].
Following extraction, the total weight of the glands was recorded at 67 g. After removal of residual tissue, the net weight of the purified glands was 32 g. These were subsequently dried in a furnace at a controlled temperature of 40 °C, yielding a final dried pigment mass of 12 g.
This experiment highlights the disproportionate relationship between the quantity of raw material and the amount of usable dye, underscoring the labor-intensive nature and rarity of Tyrian purple production.
2.1.2. Experiment 2-Rate and Change of Color
Wool was selected as the substrate for dye application due to its historical prevalence in the Hellenic region and its superior affinity for dibrominated indigotin compared to indigotin [24,51]. This affinity enhances the likelihood of achieving a purer and more saturated purple hue, aligning with both traditional practices and chemical compatibility [52,53].
Three wool samples, each measuring 5 × 4 cm (designated as samples A, B, and C), were prepared for the experiment. A single porphyrin gland was applied to each sample, ensuring full surface coverage. To prevent premature oxidation of the dye precursors, the samples were immediately immersed in water, which was gradually heated to 70 °C and maintained for 30 min. Following thermal treatment, the samples were exposed to direct sunlight to initiate the oxidation process.
The final coloration observed was a pinkish hue, which is likely attributable to the dilution or partial oxidation of 6,6′-dibromoindigo. This outcome suggests that the concentration and exposure conditions may influence the chromatic development of Tyrian purple, warranting further investigation into the kinetics and stability of the dye under varying environmental parameters [11,52].
2.1.3. Experiment 3-Purple Dyeing Process
In a subsequent phase of the experimental study, 150 specimens of Murex trunculus were processed, yielding 84.34 g of mucus glands, following the previously described extraction protocol. A dyebath was prepared using a gland-to-distilled water ratio of 1:5. The solution was heated to 60 °C for 30 min, during which four pre-soaked wool fabric samples (each weighing 1 g) were immersed to enhance dye absorption.
The samples remained in the dyebath at an elevated temperature of 70 °C for one hour. Upon completion, they were removed and allowed to dry under direct sunlight to facilitate oxidation. One sample was set aside, while the remaining three were re-immersed in the dyebath. This cycle was repeated three times, with one sample removed after each iteration, resulting in a gradation of dye exposure across the samples.
Following the dyeing process, all but one of the two triple-dyed samples were subjected to a reduction treatment. The reduction solution consisted of anhydrous sodium sulfate (Na2H2SO4) (Merck) at a concentration of 1 g/L, applied in a 1:20 ratio of solution to dyed material. The samples were heated in this solution for 15 min at 40 °C.
This multi-step procedure was designed to evaluate the cumulative effect of repeated dyeing and reduction on color intensity, stability, and fabric saturation, contributing to a deeper understanding of Tyrian purple’s behavior under controlled experimental conditions [1,52].
2.2. Application of Imaging and Spectroscopic Techniques and Instrumentation
All non-destructive diagnostic imaging techniques were conducted at the Laboratory for Non-Destructive Testing at the University of West Attica. The facility was specifically configured to support the requirements of advanced imaging procedures, including the creation of a controlled dark environment essential for techniques such as ultraviolet-induced luminescence, hyperspectral imaging, and false color infrared imaging.
The protocols employed during the experiments were based on standardized methodologies developed and refined within the laboratory over the past two decades. These long-established procedures ensured consistency, reliability, and reproducibility across all imaging sessions, while also safeguarding the integrity of the cultural heritage samples under investigation.
Visible reflectance imaging was carried out using a Nikon D800 digital single lens reflex camera (DSLR) equipped with an AF-S Nikkor 24–70 mm 1:2.8 G ED lens. A copy stand was employed to support the textile samples. Two J78 halogen lamps were used for symmetrical illumination.
UVL was carried out by a Nikon D800 digital single lens reflex camera (DSLR) equipped with an AF-S Nikkor 24–70 mm 1:2.8 G ED lens and a copy stand was employed to support the textile samples. Two Blacklight bulb lights Philips were used for excitation radiation. Barrier filter KODAK 2E and B&W UV/IR CUT filter were used to enable recording of only the visible region and to balance excess blue radiation from the light source. The procedure was carried out in a light isolated room, in complete darkness.
For HSI, two different cameras were used namely MuSIS HS and MUSES9-HS. MuSIS HS model 2009 (Forthphotonics Hellas S.A. now Dysis, Heraklion, Hellas) is equipped with a CCD 1/200 progressive scan sensor (1600 × 1200 pixels, 8 bits, 15 fps) with 34 selectable spectral bands in the range of 400–1000 nm for B&W and color imaging. Its standard illumination sources were two J78 halogen lamps. Calibration was carried out with a white card. IRRFC images were acquired. MUSES9-HS (Spectricon, Chania, Hellas) has a full frame CCD detector (spatial resolution 370–1000 nm, 6.4 megapixel) and an InGaAs detector (Allied Vision, Stadtroda, Germany), (spatial resolution 1000–1700 nm VGA 0,.3 megapixel), B&W and color imaging. It comes with its own illumination sources comprising different wavelengths LED controlled by the HSI software. Calibration was carried out with a white card. Two kinds of spectral cubes were acquired, i.e., Vis-IR (400–1000 nm) and Ultra Violet induced visible fluorescence (400–700 nm). From the cubes, reflectance spectra and fluorimetric spectra were obtained. UV LED lamp with radiation power 14,250 mW and max spectral emission: 365 nm. For each sample multiple spectra were obtained from the cubes for statistical reasons.
FORS was carried out using the optical visible spectrometer (300–900 nm) type S2000 of the company OceanOptics (CCD, 2048 channels, optical barrier 600 g/mm, 2nd class filter, 25 μm split), with bifurcated fiber optics QR200-7-UV/VIS-BX, Ocean Optics and FCB-UV400-2, Avantes, and a stabilized tungsten-halogen lamp for fiber optics (Avantes HL-2000). For the acquisition and recording of the spectra, the software SpectraSuite version 5.1 was used. Calibration was carried out with a standard (Spectralon, WS-2 of OceanOptics), which is considered as 100% reflectance standard within the visible region of the spectrum.
3. Results and Discussion
The quantity of the dried dye obtained by the first experimental procedure, 12 g purple dye out of 2 kg seashells, certainly explains its high prestige and regard amongst other dyes. The small amount of the final dye, in conjunction with the difficulty to keep seashells alive until their gland is extracted as well as the entire chemical procedure contribute to and justify the dye’s title of “royal purple”.
In the second experimental procedure, where crude glandular fluid was applied directly to wool samples, notable irregularities in color distribution were observed. Areas where the gland was more forcefully pressed exhibited darker, concentrated staining, while the remaining surface showed a more uniform but less intense coloration. This variability highlights the influence of manual application and gland size on chromophore density and dye saturation.
It is important to note that the final hue achieved through direct gland application may differ significantly from that produced via standardized dyeing protocols involving chemical additives. The oxidation process was visually dynamic: upon initial application, the wool samples appeared yellowish, gradually transitioning through green and blue stages before stabilizing into a deep purple. This transformation, driven by exposure to light and air, was accompanied by a pungent odor, consistent with historical accounts of Tyrian purple production.
Further variation in dye intensity was attributed to the size of the glands used. Sample A, derived from a smaller mollusk, exhibited limited coloration due to reduced chromophore content, whereas samples B and C—sourced from larger specimens—demonstrated broader and more saturated dye coverage. In all cases, one gland per sample was used, following the protocol recommended by G. Moatsos [1].
In the third experimental procedure, a dyebath was prepared using a quantity of glandular material sufficient to produce a light purple hue. Historically, achieving a deep and saturated Tyrian purple required the processing of vast quantities of Murex mollusks to dye only a limited number of textiles. Due to practical constraints, such volumes were not attainable in the present study, resulting in a paler coloration.
To replicate historical dyeing practices and evaluate the impact of repeated dyeing cycles, the experiment included single, double, and triple immersion treatments. Additionally, a triple-dyed sample was prepared without undergoing chemical reduction, in order to assess the influence of reduction on final color intensity. The unpleasant odor frequently cited in historical sources [8] was also noted during this phase of experimentation.
Upon initial observation, all samples—regardless of the number of dyeing cycles—appeared to exhibit similar hues and intensity. However, closer inspection revealed that repeated dyeing subtly deepened the color, a nuance more evident when samples were viewed collectively in the composite image (Appendix A Figure A1, Figure A2, Figure A3, Figure A4 and Figure A5). This finding supports the claims of Moatsos (1936) and Gatsos (1998), who documented that ancient dyers reused dyebaths multiple times to intensify shades or produce lighter tones from residual dye [1,11].
Imaging techniques provided consistent visual data across all dyed samples, confirming their diagnostic value in assessing dye distribution and chromophore concentration. Ultraviolet-induced visible fluorescence revealed a characteristic violet coloration, resulting from the absorption of UV radiation at 365 nm and subsequent fluorescence emission peaking between 420 and 450 nm. This spectral behavior is indicative of the presence of indigoid compounds, particularly dibromoindigo.
Samples subjected to multiple dyeing cycles exhibited slightly darker fluorescence, suggesting a cumulative increase in chromophore density. This observation aligns with historical accounts of repeated dyeing to achieve deeper hues and supports the hypothesis that layering enhances pigment saturation.
Infrared reflectance false color imaging produced bright yet pale orange representations of the samples, with intensity levels correlating directly to the number of dyeing cycles. The progressive enhancement in false color intensity reflects subtle but measurable changes in material composition and dye absorption.
These findings demonstrate that, despite minimal visual differences to the naked eye, advanced imaging modalities can detect and document incremental variations in dye application. Such techniques offer valuable insights into ancient dyeing methodologies and contribute to the broader understanding of historical textile production and preservation.
The comparative analysis of the two experimental dyeing methodologies yielded distinct and informative results regarding the chromatic behavior and imaging profiles of Tyrian purple. Samples treated with raw glandular fluid exhibited a markedly deeper and more saturated purple hue, though the coloration was localized and uneven. In contrast, samples subjected to the dyebath process displayed a more uniform distribution of color, albeit with a paler overall intensity.
This divergence was further substantiated through ultraviolet-induced visible fluorescence imaging. Gland-treated samples demonstrated stronger fluorescence, with a more intense and darker violet emission, while dyed samples showed weaker fluorescence and a lighter violet hue. At the excitation wavelength of 365 nm, differential absorption was observed across all samples, correlating with dye concentration. Fluorescence emission consistently peaked around 450 nm, validating the selection of representative images for Table 1.

Table 1.
The purple samples under the various imaging techniques.
Infrared reflectance false color imaging reinforced these findings. Dyed samples produced a pale, light orange false color, whereas gland-treated samples yielded a vivid and bright orange response. These variations reflect the differing chromophore densities and dye application methods, offering insight into the material behavior of Tyrian purple under various treatment conditions.
All imaging data presented in Table 1 were derived from a single, unprocessed composite photograph containing all samples captured simultaneously. This approach ensured consistency and reliability in comparative analysis. Full-resolution images are available in the Appendix A for further examination.
Reflectance spectral analysis provided valuable insights into the chromatic behavior of both gland-applied and dyebath-treated samples, as illustrated in Figure 8. In the visible region, samples treated with crude gland material exhibited similar spectral profiles. However, in the red region, samples B and C displayed elevated reflectance curves, suggesting a stronger contribution of red wavelengths to their final coloration. This correlates with their more intense red-purple appearance, in contrast to sample A, which covered a smaller surface area, was less saturated, and exhibited weaker reflectance in the red-infrared region.
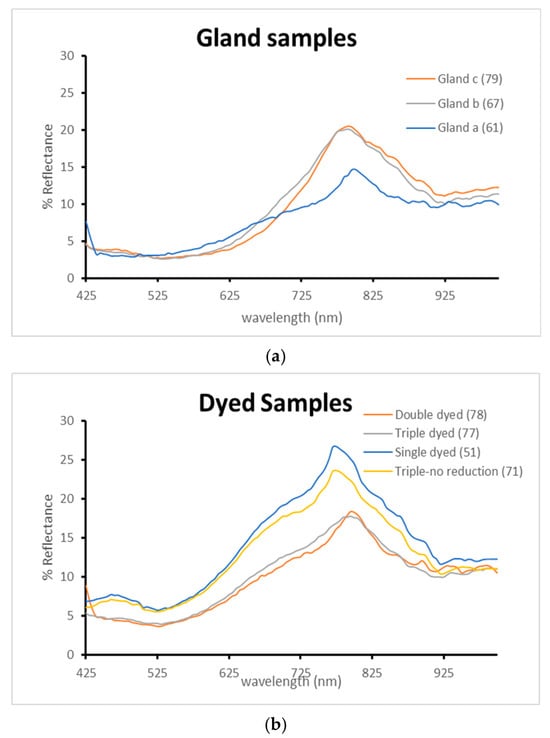
Figure 8.
Reflectance spectra comparison. (a): Applied gland samples. (b): Dyed samples.
In comparison, the dyed samples (Figure 8b) demonstrated higher overall reflectance values than the gland-applied samples, consistent with the more diluted pigment concentration inherent in the dyebath method. Despite this, the dyed samples shared similar spectral morphology, indicating a consistent hue across treatments, albeit with lighter tonal values.
Among the dyed samples, the single-dyed specimen exhibited the highest reflectance, while the double-dyed sample showed reduced reflectance due to increased pigment absorption. Interestingly, the double and triple-dyed samples presented nearly identical spectra, suggesting that the dyebath was chemically depleted by the third immersion and contributed minimally to further color enhancement.
The most revealing result emerged from the triple-dyed sample that did not undergo chemical reduction. Its reflectance profile closely resembled that of the single-dyed sample, indicating that without the reduction step, the dye fails to stabilize into its deeper chromatic form. Consequently, the sample retained a pale purple tone, underscoring the critical role of reduction in achieving the full saturation and permanence of Tyrian purple.
Fluorescence analysis revealed a consistent elevation around 430 nm across all samples, aligning with the violet coloration observed under ultraviolet-induced visible fluorescence. This spectral feature confirms the presence of indigoid compounds and supports the visual documentation obtained through imaging techniques.
It is important to note that, in general, Tyrian purple samples do not exhibit high fluorescence intensity, which is in accordance with the recorded UVL images. The morphology of the curves is representable of the deep dark bluish purple fluorescence color that has been observed in the corresponding fluorescence images. The gland-applied samples demonstrated low fluorescence levels, which can be attributed to the localized and highly concentrated pigment areas. This concentration likely limits the uniform excitation and emission of fluorescence across the sample surface.
The dyed samples (Figure 9b) presented similar fluorescence curves, indicating comparable chromophore distribution and dye absorption. However, the triple-dyed sample that did not undergo chemical reduction showed noticeably lower fluorescence intensity. This suggests that the absence of the final stabilizing step in the dyeing process compromises the development and fixation of the dye, resulting in diminished chromophore activation and a paler overall appearance.
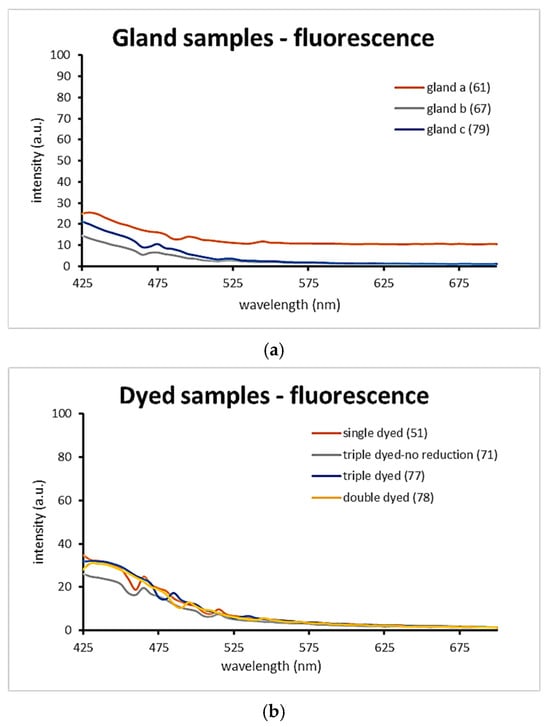
Figure 9.
Fluorescence intensity spectra comparison of the dyed samples. (a): Applied gland samples. (b): Dyed samples.
These findings reinforce the importance of reduction in achieving stable and saturated Tyrian purple coloration and demonstrate the sensitivity of fluorescence techniques in detecting subtle variations in dye chemistry and application.
4. Conclusions
Tyrian purple—often referred to as “royal purple”—is among the most historically revered and chemically unique dyes to have survived through the ages. Its production, which ceased centuries ago, has left significant gaps in the historical and technical record. Given that this dye is found on culturally significant artifacts that cannot always be sampled, the development of non-destructive documentation methods is essential for its study and preservation.
Due to the secrecy surrounding and extremely high price of Tyrian purple production in antiquity, the present research sought to recover and experimentally validate historical accounts of dyeing practices within the Greek region. The experimental procedures aimed to test and interpret the surviving literature while also exploring the chemical and optical behavior of the dye. Notably, Aristotle’s observation regarding photooxidation was confirmed: exposure of the crude gland to sunlight initiated a gradual color transformation, progressing from transparency to deep purple.
A series of wool samples were prepared using single, double, and triple dyeing cycles, and analyzed through both imaging and spectroscopic techniques. These included visible reflectance, ultraviolet reflectance at 365 nm, ultraviolet-induced visible fluorescence, and infrared reflectance false color imaging. Across all methods, the dyed samples exhibited consistent optical characteristics, though visible reflectance spectroscopy proved particularly effective in differentiating between dyeing stages and sample types.
Hyperspectral imaging contributed twofold in the study. Spectral analysis revealed clear distinctions between crude gland applications and processed dye samples, indicating that while both originate from the same biological source, the dyeing procedure alters the material’s spectral profile. Fluorimetric measurements showed only minor differences between crude and dyed samples, with similar fluorescence behavior observed across the various dyeing techniques. This suggests that the core chromophoric compounds remain largely unaffected by the number of dyeing cycles.
Future experimental work may focus on increasing the concentration of dye solutions to achieve deeper and more saturated coloration, thereby further elucidating the chemical dynamics of this legendary pigment.
Author Contributions
Conceptualization, A.T., A.K. and A.G.A.; methodology, A.T. and A.A.K.; validation, A.T. and A.A.K.; formal analysis, A.T. and A.A.K.; investigation, A.T. and A.A.K.; resources, A.T. and A.G.A.; writing—original draft preparation, A.T. and A.A.K.; writing—review and editing, A.T., N.M.B., A.K., A.G.A. and A.A.K.; visualization, A.T. and A.A.K.; supervision A.K., A.G.A. and N.M.B.; project administration, A.T., A.K., A.G.A., N.M.B. and A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, A.T., upon reasonable request.
Acknowledgments
The authors would like to thank the fishermen of the Hermione area, who donated the seashells for the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
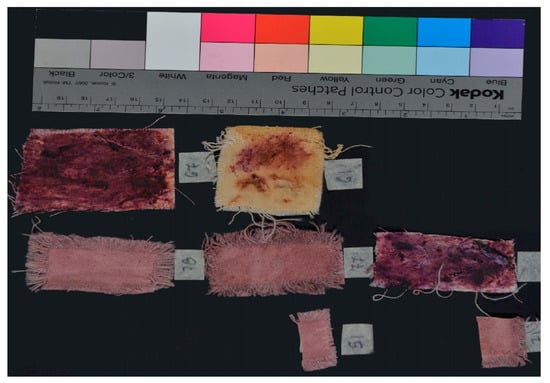
Figure A1.
Visible image of the samples (VIS).
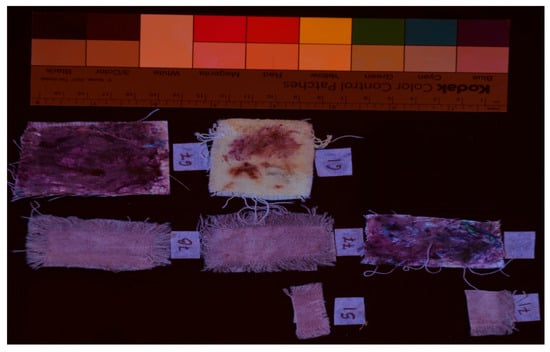
Figure A2.
Ultraviolet induced visible fluorescence image of the samples (UVL).
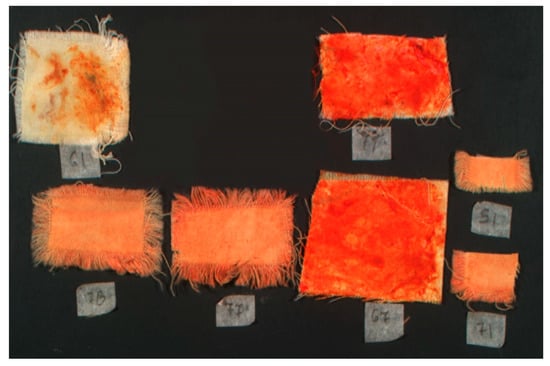
Figure A3.
Infrared reflectance false color image of the samples (IRRFC).
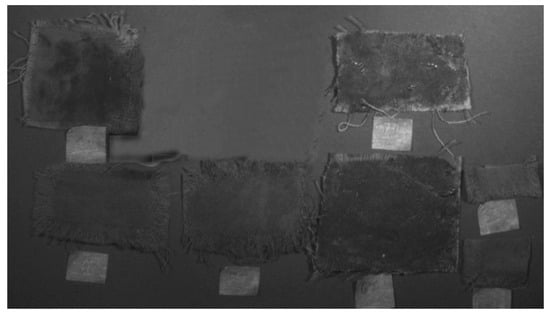
Figure A4.
Ultraviolet reflectance image of the samples (UVR-365 nm).
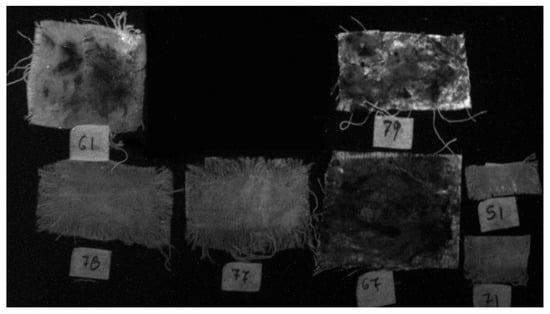
Figure A5.
Ultraviolet induced visible fluorescence image of the samples (UVL-450 nm).
References
- Moatsos, G. H Porphyra; Self-Publishing: Alexandria, Egypt, 1936. [Google Scholar]
- Cooksey, C.J. Making Tyrian Purple, Dyes in History and Archaeology; Textile Research Associates: Osaka, Japan, 1994; Volume 13, pp. 7–13. [Google Scholar]
- Tsatsarou, A.; Boussias, C. Purple Dyes Made from Shellfish in Antiquity-Historical Aspects and Process. In Proceedings of the 1st International Scientific Conference, Tripoli, eRA 1, Tripoli, Greece, 16–17 September 2006. [Google Scholar]
- Gkatsos, B. H Ton Ermioneon Polis; Gkatsos, B.: Piraeus, Greece, 1996; pp. 245–256. [Google Scholar]
- Protopappas, S.; Gatsos, B. The famous Purple of Hermionis and its technology. Arxaiologia Kai Technes 2003, 89, 90. [Google Scholar]
- Pliny the Elder, Natural History; Book 9, 61 The Loeb Classical Library; Harvard University Press: Cambridge, MA, USA, 1952.
- Jensen, B.W. The Leiden and Stockolm Papiri, Greco-Egyptian Chemical Documents from the Early 4th Century; Oesper Collections in the History of Chemistry; University of Cincinnati: Cincinnati, OH, USA, 2008. [Google Scholar]
- Pikkoloς, Ν. Aristoteles, Peri zoon istoriai; Firmin Didot Freres, Fils et Cie: Paris, France, 1863. [Google Scholar]
- Korai, A. Stravonos, Geografikon; Everartou, I. M.: Paris, France, 1819. [Google Scholar]
- Carannante, A. Archaeomalacology and Purple-dye. In State Of the Art and New Prospects of Research; Universidad de Cádiz: Andalusia, Spain, 2014; pp. 273–282. [Google Scholar]
- Ziderman, I.I. Purple Dyes Made from Shellfish in Antiquity. Soc. Dye. Colour. 1986, 16, 46–52. [Google Scholar] [CrossRef]
- Walter, G. H Kathimerini Zoi sto Vizadio; Oceanis: Athens, Greece, 1970. [Google Scholar]
- Sukenik, N.; Iluz, D.; Amar, Z.; Varvak, A.; Bar, S. New Evidence of the Purple-Dye Industry at Tel Shiqmona, Israel. Archaeometry 2017, 59, 775–785. [Google Scholar] [CrossRef]
- Sukenik, N.; Iluz, D.; Amar, Z.; Varvak, A.; Shamir, O.; Ben-Yosef, E. Early evidence of royal purple dyed textile from Timna Valley (Israel). PLoS ONE 2021, 16, e0245897. [Google Scholar] [CrossRef]
- Cooksey, C.J. Tyrian Purple: 6,6′-Dibromoindigo and Related Compounds. Molecules 2001, 6, 736–769. [Google Scholar] [CrossRef]
- Karapanagiotis, I. A Review on the Archaeological Chemistry of Shellfish Purple. Sustainability 2019, 11, 3595. [Google Scholar] [CrossRef]
- Clark, R.J.; Cooksey, C.J.; Daniels, M.A.; Withnall, R. Indigo, woad, and Tyrian Purple: Important vat dyes from antiquity to the present. Endeavour 1993, 17, 191–199. [Google Scholar] [CrossRef]
- Koren, Z.C. Archaeo-chemical analysis of Royal Purple on a Darius I stone jar. Microchim. Acta 2007, 162, 381–392. [Google Scholar] [CrossRef]
- McGovern, P.E.; Michel, R.H. Royal Purple dye: Tracing the chemical origins of the industry. Anal. Chem. 1985, 57, 1514A–1522A. [Google Scholar]
- Koren, Z.C. A New HPLC-PDA Method for the Analysis of Tyrian Purple Components. In Dyes in History and Archaeology; Archetype Publications: London, UK, 2008; Volume 21, pp. 26–35. [Google Scholar]
- Aceto, M.; Idone, A.; Agostino, A.; Fenoglio, G.; Gulmini, M.; Baraldi, P.; Crivello, F. Non-invasive investigation on a VI century purple codex from Brescia, taly. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Margariti, C.; Protopapas, S.; Allen, N.; Vishnyakov, V. Identification of purple dye from molluscs on an excavated textile by non-destructive analytical techniques. Dye. Pigment. 2013, 96, 774–780. [Google Scholar] [CrossRef]
- McGovern, P.E.; Lazar, J.; Michel, R.H. The analysis of indigoid dyes by mass spectrometry. J. Soc. Dyers Colour. 1990, 106, 22–25. [Google Scholar] [CrossRef]
- Maynez-Rojas, M.A.; Casanova-González, E.; Ruvalcaba-Sil, J.L. Identification of natural red and purple dyes on textiles by Fiberoptics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 239–250. [Google Scholar] [CrossRef]
- Koren, Z.C. The Unprecedented Discovery of the Royal Purple Dye on the Two Thousand Year-Old Royal Masada Textile. In The Textile Specialty Group Postprints, Volume Seven; American Institute for Conservation of Historic and Artistic Works: Washington, DC, USA, 1997; pp. 23–34. [Google Scholar]
- Wouters, J. A new method for the analysis of blue and purple dyes in textiles. In Dyes in History and Archaeology; 10th Annual Meeting National Gallery, London, UK; Textile Research Associates: Clarksville, TN, USA, 1991. [Google Scholar]
- Lech, K.; Fornal, E. A Mass Spectrometry-Based Approach for Characterization of Red, Blue, and Purple Natural Dyes. Molecules 2020, 25, 3223. [Google Scholar] [CrossRef]
- Koren, Z.E. Photochemical Vat Dyeings ofthe Biblical ‘Purple’ Tekhelet and Argaman Dyes. In Paper Delivered at Dyes in History and Archaeology 13th Symposium; Royal Museum of Scotland: Edinburgh, SA, Australia, 1994. [Google Scholar]
- Baker, J.T. Tyrian purple. Ancient dye, a modern problem. Endeavour 1974, 33, 11–17. [Google Scholar] [CrossRef]
- Głowacki, E.; Leonat, L. Ambipolar organic field effect transistors and inverters with the natural material Tyrian Purple. AIP Adv. 2011, 1, 042132. [Google Scholar] [CrossRef]
- Aceto, M.; Melo, J. Identification of the Purple Dye on the Vienna Genesis; Böhlau Verlag: Cologne, Germany, 2020; p. 103. [Google Scholar]
- Baker, J.T.; Sutherland, M.D. Pigments of marine animals VIII. Precursors of 6,6′-Dibromo-indigotin (Tyrian Purple) from the mollusc Dicathais Orbita Gmelin. Tetrahedron Lett. 1968, 9, 43–46. [Google Scholar] [CrossRef]
- Alexopoulou, A.; Kaminari, A.; Moutsatsou, A.; Banou, P. Imaging Techniques in the Study of Cultural Heritage [Postgraduate Textbook]; Kallipos: Athens, Greece, 2024. [Google Scholar] [CrossRef]
- Alexopoulou, A.; Chryssoulakis, Y. Science and Works of Art; Gonis: Athens, Greece, 1993. [Google Scholar]
- Alexopoulou, A.; Kaminari, A.A. The evolution of imaging techniques in the study of manuscripts. Manuscr. Cult. 2014, 7, 58–68. [Google Scholar]
- Payne, E. Imaging Techniques in Conservation. J. Conserv. Mus. Stud. 2012, 10, 17–29. [Google Scholar] [CrossRef]
- Banou, P.; Alexopoulou, A.; Kaminari, A.A. Photographic and technical examination: A valuable tool for the conservation treatment of works of art on paper and parchment. In Collective Edition Works of Art on Parchment and Paper, Interdisciplinary Approaches; Natasa, G., Tomazic, J.V., Eds.; Slovenian Ministry of Culture and the Archives of the Republic of Slovenia: Ljubljana, Slovenia, 2019; pp. 217–225. [Google Scholar]
- Dyer, J.; Tamburini, D.; O’Connell, E.R.; Harrison, A. A multispectral imaging approach integrated into the study of Late Antique textiles from Egypt. PLoS ONE 2018, 13, e0204699. [Google Scholar] [CrossRef]
- Kaminari, A.; Kanakari, O.; Rompakis, P.; Moutsatsou, A.; Alexopoulou, A. Ultraviolet fluorescence photography on works of art: Can digital era reach and surpass traditional film quality? A first approach. In Proceedings of the 5th International Conference on NDT of HSNT-IC MINDT, Eugenides Foundation, Athens, Greece, 20–22 May 2013. [Google Scholar]
- Cosentino, A. Practical notes on ultraviolet technical photography for art examination. Conserv. Patrim. 2015, 21, 53–62. [Google Scholar] [CrossRef]
- Webb, K. UV-Induced Visible Luminescence for Conservation Documentation. In Conservation 360°; Fuster-Lopez, L., Stols-Witlox, M., Picollo, M., Eds.; UV-Vis Luminescence Imaging Techniques; Universitat Politècnica de València: Valencia, Spain, 2020. [Google Scholar]
- Liang, H.; Keita, K.; Peric, B.; Vajzovic, T. Pigment identification with optical coherence tomography and multispectral imaging. In Proceedings of the OSAV’2008, The 2nd Int. Topical Meeting on Optical Sensing and Artificial Vision, St. Petersburg, Russia, 12 May 2008; pp. 33–42. [Google Scholar]
- Delaney, J.K.; Dooley, K.A.; Radpour, R.; Kakoulli, I. Macroscale multimodal imaging reveals ancient painting production technology and the vogue in Greco-Roman Egypt. Sci. Rep. 2017, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Balas, C.; Papadakis, V.; Papadakis, N.; Papadakis, A.; Vazgiouraki, E.; Themelis, G. A novel hyper-spectral imaging apparatus for the non-destructive analysis of objects of artistic and historic value. J. Cult. Herit. 2003, 4, 330–337. [Google Scholar] [CrossRef]
- Daniel, F.; Mounier, A.; Pérez-Arantegui, J.; Pardos, C.; Prieto-Taboada, N.; Fdez-Ortiz de Vallejuelo, S.; Castro, K. Hyperspectral imaging applied to the analysis of Goya paintings in the Museum of Zaragoza (Spain). Microchem. J. 2016, 126, 113–120. [Google Scholar] [CrossRef]
- Villafana, T.E.; Delaney, J.K.; Warren, W.S.; Fischer, M.C. High-resolution, three-dimensional imaging of pigments and support in paper and textiles. J. Cult. Herit. 2016, 20, 583–588. [Google Scholar] [CrossRef]
- Kaminari, A.; Liritzis, I.; Alexopoulou, A. Fibre Optics Reflectance Spectroscopy for the differentiation of clays from ceramics. In Proceedings of the Case Study of Kastrouli, Greece, 43rd International Symposium on Archaeometry (ISA), Lisbon, Portugal, 16–20 May 2022. [Google Scholar]
- Moon, T.; Schilling, M.; Thirkettle, S. A note on the use of false-color infrared photography in conservation. Stud. Conserv. 1992, 37, 42–52. [Google Scholar] [CrossRef]
- Kaminari, A.; Sianoudis, I.; Alexopoulou, A. Iron Gall Inks on papers and copies: The possibility of achieving differentiation based on the use of Fibre Optics Reflectance Spectroscopy (FORS). In Proceedings of the 8th National NDT Conference: The Contribution of Non-Destructive Testing for the Safety of Small and Large Works and Constructions, The Hellenic Society of Non-Desrtuctive Testing, Athens, Greece, 8–9 May 2015. [Google Scholar]
- Adourakis, G. Krete-Laography; Heliniki Ethniki Grammi: Athens, Greece, 1991; p. 189. [Google Scholar]
- Guckelsberger, M. Purple Murex Dye in Antiquity; Gottskálk Jensson, University of Iceland: Sæmundargata, Iceland, 2013. [Google Scholar]
- Tsatsarou, A.; Mpiniari, K. Natural Dyes with Royal Purple. Graduation Thesis, Grade 10/10; Technological Institute of Piraeus, Textiles Dep. Aigaleo: Aigaleo, Greece, 1999. [Google Scholar]
- Marín-Aguilera, B.; Iacono, F.; Gleba, M. Colouring the Mediterranean: Production and Consumption of Purple-dyed Textiles in Pre-Roman Times. J. Mediterr. Archaeol. 2018, 31, 127–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
































