Abstract
The world has recently witnessed the dire consequences of microbial infections in the form of the spread of COVID 19. Like viruses, bacterial infections too are a serious global health concern, especially because of the evolution of multidrug-resistant (MDR) bacterial populations. MDR bacteria are a result of the mindless use and misuse of antibiotics all over the world. Hence, there arises a need to find alternative strategies to effectively combat bacterial infections; all the more so for MDR bacterial infections. A lot of research has been conducted to find alternative antibacterial strategies such as phage therapy, the synthesis of antimicrobial peptides, the development of CRISPR-Cas systems, the incorporation of pro- and pre-biotics into our food or as supplements, and the development of bactericidal nanotechnology and antibacterial materials. Of these many strategies, this review focusses on the last one—the development of antibacterial materials. This article explores the potential of plant-derived natural colorants to serve as effective antibiotic materials to be used in various industries ranging from food, textile, paper, and leather to the pharmaceutical industry. Some major advantages of developing plant-derived natural colorants into antibacterial materials is that many of them possess inherent medicinal properties, they are biocompatible, non-toxic for humans, and biodegradable, and hence environment friendly. Many plant-derived natural colorants, like curcumin, indigo, lawsone, emodin, etc., have already been well studied for their antimicrobial properties. This review article aims at integrating some relevant studies to offer a cohesive overview of the current state of knowledge on the antibacterial properties of plant-derived natural colorants.
Keywords:
natural colorants; plant derived; antibacterial materials; carotenoids; flavonoids; curcumin; lawsone; indigo; neem; pomegranate 1. Introduction
The world has recently witnessed the extent of damage microbes can do to human health. The spread of COVID 19 brought the whole world’s activities to a halt. Instances like these prompt scientists to explore effective antimicrobial strategies. Like viruses, bacteria are another class of microbes which can easily trigger large-scale outbreaks [1]. Unlike viruses, not all bacteria are bad for human health. Only pathogenic bacteria are the primary culprits behind many human diseases and fatalities [2]. Traditionally, oral and intravenous antibiotics have been the most common treatments for bacterial infections [3]. In the last few decades, antibiotics have been rampantly used for almost every other disease. Without knowing whether bacteria are involved in the disease, or exactly which bacteria are involved, doctors would prescribe common antibiotics at every visit just to “cover all bases”. For a very long time, antibiotics have been misused too; for example, antibiotics have been prescribed for the common cold, which is caused by a virus. And, in countries where the use of antibiotics is not regulated by the government, people have senselessly used over-the-counter antibiotics for almost every common ailment [4,5]. This mindless use and misuse of antibiotics has led to the unintentional evolution of multidrug-resistant bacteria [6,7,8]. As bacteria evolved to resist existing antibiotics, it became increasingly difficult to treat infections effectively. This situation has prompted researchers to explore new antibacterial strategies including, but not limited to, the phage therapy [9,10], the synthesis of antimicrobial peptides [11], the CSIPR-Cas systems [12,13,14], nanotechnology [15], incorporation of probiotics and prebiotics [16,17], and development of antibacterial materials [18,19,20]. This review aims at bringing together the relevant information present in the scientific literature about plant-derived natural colorants that could potentially serve as a new class of antibacterial materials. Antibacterial materials refer to a type of functional material with the property of killing or inhibiting microorganisms, specifically bacteria [21]. There are more than 100,000 commercially available synthetic and natural dyes already known for their antibacterial properties [22]. This comprehensive review presents detailed information on “plant-derived” natural colorants with studied and proven antibacterial activity. It should be noted that some of the well-studied plant-derived natural colorants are now known by their main chemical component—for example, “curcumin” rather than “turmeric”—but many other natural colorants not widely known or well studied in the literature are still known by their natural plant/flower/fruit names like “marigold”, “pomegranate”, or “neem”. The naming convention followed is as revealed in the literature. The information has been synthesized and organized after conducting detailed searches of pertinent studies and articles concerning specifically the antibacterial properties of these natural colorants. The hope is that this article can serve as a handy resource for researchers planning to explore plant-derived natural colorants as antibacterial materials.
Natural colorants specifically refers to a class of colorants obtained from natural sources, without using any chemical treatments [23]. These sources can range from plants (indigo, henna, turmeric, etc.) to insects (like carmine red from cochineal bugs or royal purple from seashells) and minerals (azurite blue, iron oxide, malachite, lapis lazuli, etc.). Many of these natural colorants are known to be safe for the environment since they are derived from Nature. Many of these (though not all) are known to be non-toxic and are known for their medicinal properties. Although there exist thousands of plant-derived natural colorants, not all have been scientifically studied [24]. Due to their additional environment-friendly and medicinal values, many plant-derived natural colorants have become the focus of recent scientific studies [25]. To be fair, we should pay attention to some disadvantages that come with the use of natural colorants like their less-than-optimal extraction techniques, their seasonal/regional availability, economic viability, color fastness, etc. [26]. Synthetic dyes, on the other hand, are composed of organic and inorganic chemicals [27,28], mostly synthesized in a lab. Most of the synthesis procedures are optimized for large-scale production so they are economically more viable. For these reasons, they find significant use in the textile, pharmaceutical, food, printing, and leather industries [29,30]. In comparison to their natural counterparts, they possess stronger color fastness and higher intensity [31]. Many synthetic dyes have also been explored as antibacterial materials [32,33,34,35]. However, they come with their own disadvantages, a few of which include them being environmental pollutants [36] and hazardous to health, especially while using synthetic food dyes [37]. In addition to them being environmentally toxic, many of them are known carcinogens [38,39]. Hence, in this review, we focused our attention primarily on “plant-derived” natural colorants. In the following sections, the salient features, mechanisms of action (wherever available), and pros and cons of the use of some naturally occurring colorants also serving as antibacterial materials is presented.
2. Natural Colorants
As mentioned earlier, natural colorants are materials capable of imparting colors to a medium and are specifically derived from various natural sources ranging from plants and rocks to aquatic animals. Colors have fascinated humans from ancient times, and they have used different sources present in Nature to derive them. It is only over the last century or so, with the advancement of science, that colorants have started being synthesized in labs. Before that, plants, rocks, minerals, seashells, animals, and other -natural sources were used to derive colorants [40]. The preference towards natural colorants is due to their many medicinal properties, along with the presence of color in the material [24]. If we focus our attention on just one country at a time, then in India itself there are at least hundreds of plants that are used to extract natural colors inherently containing medicinal properties. More in-depth study of different cultures around the world is certain to bring forth thousands of other such natural sources of colorants. Many of the known natural colorants are known to be non-toxic, non-allergic, and biodegradable in nature as they are derived from natural sources [41]. Because of these desirable traits, they find wide application in the textile industry [42,43,44], in cosmetics [45], and in the food industry [46]. Though artificial or lab-synthesized colorants have their own strong merits, natural colorants are the preference of the day due to their non-toxicity to humans and the environment [47]. In the following sections, we review a variety of plant-derived natural colorants from the most commonly found to the newer, less explored colorants possessing antibacterial activity.
Table 1 below gives an overview of all the plant-derived natural colorants presented in detail in this review.

Table 1.
The plant-derived natural colorants covered in detail in this review, with their sources, the common names for the major active chemical components, and their IUPAC name, respectively.
2.1. Curcumin (Turmeric)
Curcumin deserves the first mention due to its use since ancient times to the re-emergence of its popularity currently. Curcumin, a bright yellow compound, is the main active ingredient of turmeric and hence the source of its natural yellow color. Turmeric is a perennial, tropical plant belonging to the family of Zingiberacea. It is popularly known as “Haldi” in India [48] and holds great cultural and traditional significance. It is an integral part of Indian cooking and is widely used in cultural rituals. Its popularity in Western countries, especially in the last decade or two, stems from its countless medicinal properties mentioned in Ayurveda, the Indian medical science. The practice of Yoga the world over has spread the principles of Ayurveda around the world along with it. From an Ayurvedic point of view, turmeric is a spice that is very commonplace and yet has immense health benefits. These benefits have prompted scientists to research and prove the medicinal value of turmeric as mentioned in ancient Indian texts. Applying chemical principles, the active ingredient of turmeric, curcumin, was first extracted by Vogel and Pelletier in 1842 [49]. Its structural characterization was then completed by Lampe and Milobedeska in 1910 [50]. The same group synthesized (via the natural-product synthesis route) curcumin in a lab and confirmed the structural analysis that had been proposed earlier [51]. The yellow extract, curcumin, further contains three compounds known as curcumin, demethoxy curcumin (DMC, lacking one methoxy group from the original curcumin), and bis-demethoxy curcumin (BDMC, lacking two methoxy groups). The structures of all three compounds are shown in Figure 1. They were extracted from turmeric and separated using the column chromatography technique by Srinivasan et al. Their respective ratio was found to be, 17: 3: 77 in the extract examined [52,53].
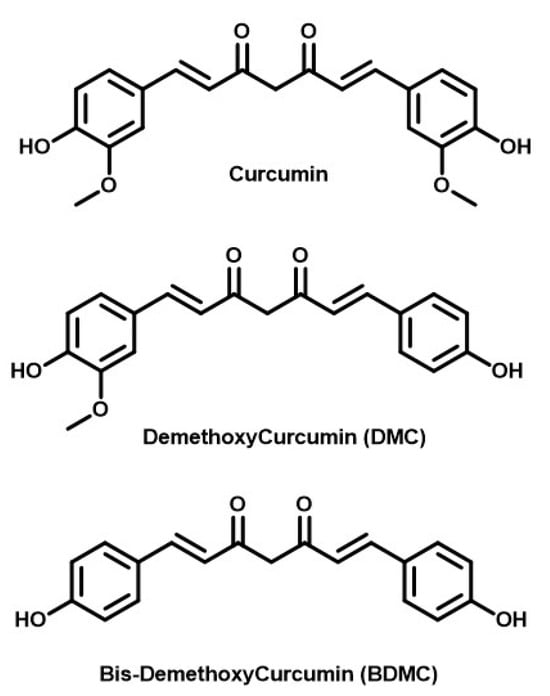
Figure 1.
Structures of relevant curcuminoids.
Structurally, the aromatic ring with a hydroxyl group, a methoxy group on the ortho (next to hydroxy) position, and an ethylene substituent on the para (straight across) position is called a feruloyl group (derived from ferulic acid). Curcumin and its other two derivatives contain two feruloyl moieties connected with a seven-carbon chain. This seven-carbon aliphatic chain is hydrophobic in nature, which makes curcumin and its derivatives fat-soluble. The hydroxyl group on the aromatic ring makes it partially water-soluble. Given the structures of the three curcuminoids, one can easily say that BDMC would be the most water-soluble component of turmeric extract. These curcuminoids, however, are readily soluble in almost all polar organic solvents like ethanol, methanol, acetone, and DMSO [54]. The seven-membered aliphatic chain also consists of two ketone groups and is conjugated. The high conjugation is the reason why curcumin absorbs the maximum amount of light (λmax) at 430 nm and emits a bright yellow color. The two ketone groups can exist in bis-keto form in acidic, neutral, and solid-state forms; whereas, the enol state is prominent in the alkaline phase [55]. Hence, curcumin is capable of showing keto-enol tautomerism at different pHs [56]. The phenolic hydroxyl groups are the major active sites of curcumin responsible for phenomena like H and electron transfer [57]. Due to its polyphenolic constitution, it is considered to be a powerful ingredient in traditional herbal practices [58]. In Ayurveda, turmeric is well known for its use as an anti-inflammatory substance and is excellent for skin disorders. In both Ayurveda and Chinese traditional medicine, it is considered to be a carminative and a bitter digestive. In Unani medicine, it is considered to be a blood purifier and a safe herb to consume [59]. Numerous studies in modern science have proven the antimicrobial properties of turmeric. It shows broad-spectrum antibacterial activity against many Gram-positive and -negative bacteria [60,61,62,63,64,65,66]. In the following section, some representative examples of its efficacy as an antibacterial colorant are presented.
In one of the studies, curcumin in conjunction with photodynamic therapy (PDT) was studied against S. mutans, a bacterium which is considered to be a major pathogen causing dental caries. S. mutans is resistant to many antibiotics. These studies showed that the total number of bacterial cells decreased, though the effect on their biofilm was minimal after the use of the curcumin–PDT method [67,68,69,70]. The same conclusions were drawn by other research groups [61,71]. The technique of using curcumin along with PDT proved beneficial in eradicating other bacteria like E. coli and L. innocua. In a study concerning food safety, de Oliveira et al. [72,73] proved that curcumin in the presence of UVA light can kill certain bacteria present in cooked food. In another study, curcumin was found to be a stronger bactericidal substance than porphyrin [74]. In one research experiment, Gayani et al. used a natural curcuminoid-intercalated layered double-hydroxide nanohybrid against biofilms made by Staphylococcus aureus, P. aeruginosa, and Enterococcus faecalis. After the treatment of these biofilms with the aforementioned nanohybrid, a prominent reduction in cell density was observed. The amount of extracellular polymeric substances produced around these biofilms also decreased [75]. In an important study, the effect of using curcumin on the pathogenic bacteria causing urinary tract infections was studied using the light microscopy, confocal laser scanning microscopy, and microliter plate methods. It was shown that the thickness of the biofilm treated with curcumin drastically decreased, in addition to the disruption of the biofilm architecture [76]. The study also showed that curcumin having antiadhesion effect on H. pylori, can suppress the swimming of bacteria in the urinary tract, and can block cluster formation when used at higher concentrations [77]. In another biochemical study, curcumin was found to block the effects of UV radiation, acting as a SOS functional inhibitor, hence inhibiting the reactivation of bacterial phages of Salmonella typhimurium and E. coli [78]. Curcumin has a very interesting aspect of antibacterial activity. It avoids the development of drug resistance in bacterial cells. This aspect is of great value when compared to other antibiotics that, after some time, are rendered useless due to the drug resistance developed by bacteria. Curcumin has been tested for its support of other antibiotics of the ß-lactam family (like penicillin and methicillin) at a sub-inhibitory dose. The use of curcumin alongside these antibiotics increases bacterial susceptibility and helps kill the bacteria more effectively [79]. As mentioned in the first study discussed above, curcumin can be used in conjunction with PDT. In PDT, curcumin acts as a natural photosensitizer, absorbing light at 457 nm [80]. The mechanism of action is the autooxidation of curcumin. As we noted earlier, there are phenols present in curcumin. The presence of this functional group makes it easier for curcumin to auto-oxidize [81]. Other mechanisms of action of curcumin acting as an antimicrobial agent are its ability to bind to the micro-tubulins of micro-organisms and hence inhibiting cell division, disrupting RNA to interrupt further protein synthesis, and finally deactivating the active sites present in proteins and enzymes [82]. At physiological pH, curcumin self-oxidizes to give a broad variety of electrophilic and nucleophilic metabolites. Chemical analysis/characterization of these metabolites—for example, quinone methide, peroxy radical, endoperoxides, etc.—can further help our understanding of the different pathways of its action against microbes [83]. Curcumin and the oil extracted from turmeric exhibit strong inhibiting action on several bacteria like Streptococci, Staphylococci, Lactobacillus, and Helicobacter pylori [84]. In a study conducted to test the efficacy of curcumin in oral health, it was found that a 1% curcumin solution, when used as a subgingival irrigant, significantly reduced gum bleeding and redness in comparison with standard oral rinse solutions like chlorhexidine and saline solution [85,86]. For a detailed review of the bacteriostatic mechanism of curcumin and studies of its bacteriostatic applications, review article [57] should be referred to. This article contains in depth discussion of the different mechanism pathways of curcumin. As the effect of common antibiotics has been challenged due to rampant antibiotic resistance, it is always good to find materials/compounds which can support these antibiotics in a synergistic way by offering an alternative pathway to kill the bacteria. In that context, curcumin has been found to lower the dose of many common antibiotics when used in addition to them [87]. Biocompatibility is another factor when considering any natural colorant intended to be used by humans, especially as a medicine [88]. One research study proved that a dose of 8000 mg per day of curcumin, when taken orally for three months, is a safe dosage for humans [89]. While applying curcumin and other active components of turmeric, some disadvantages should also be kept in mind. The phenolic groups and the enolate form of curcuminoids can chelate strongly with iron; this may result in a lower absorption of iron in the blood, leading to iron deficiency [90]. Another drawback of using curcumin is its lower bioavailability and higher rate of metabolism, meaning that curcumin may be effective as an antibacterial agent at a lower dose in vitro, but a higher in vivo dose would be needed [91]. One also has to note that, as stated earlier, curcuminoids have lower solubility in water and are fat soluble; this property may interfere with its absorption in the gastrointestinal tract [92]. The easy way out, as commonly practiced in Indian cooking, is to use it with oil. In a lab setting though, these drawbacks can be taken care of by developing curcumin nanoparticles, incorporating metals, or using other supportive therapeutic agents. As an example, in one experiment, Banerjee et al. modified the structure of curcumin by turning it into a metal complex. This increased its stability in biological media and enhanced its photocytotoxicity to effectively kill cancer cells [93]. Much wisdom on enhancing its bioavailability could be derived from studying its use in Indian medicine and cooking.
2.2. Lawsone (Henna)
Lawsone is another widely available and used natural colorant, especially as a cosmetic. Henna leaves are the main source of lawsone. The powder and paste of henna leaves have been used as a cosmetic color for staining the hands and conditioning and coloring hair [94,95]. Henna has extensive cultural value in India and in neighboring and Middle Eastern countries. It is used on special occasions, like weddings, engagements, etc., to such an extent that henna application is considered an art form. Apart from its cultural value, lawsone from henna leaves has been shown to possess many medicinal properties as well. The chemical structure of the most active component of henna leaves, lawsone, is shown in Figure 2. The chemical name of lawsone is 2-hydroxy-1,4-naphthoquinone. Its chemical and physical properties were confirmed by Xavier et al. [96,97]. Lawsone is described as Natural Orange 6 (CI 75480) [98], which acts as a substantive dye for keratin and imparts an orange color due to the presence of the -OH (auxochrome) group in its naphthoquinone structure [99,100,101,102].
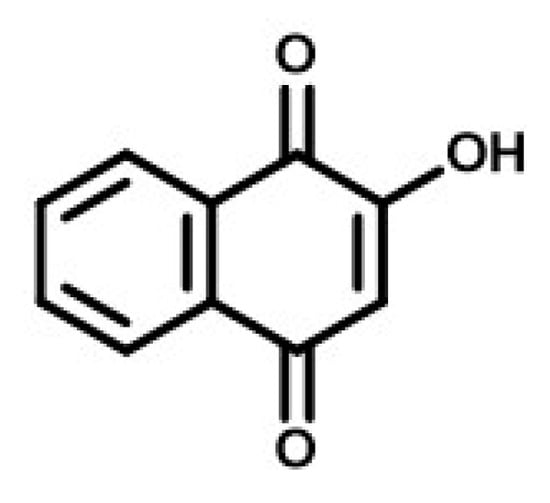
Figure 2.
Chemical structure of Lawsone derived from henna leaves.
Here, we have presented evidence of the antibacterial properties shown by lawsone. In one study, a low concentration (10%) of the extracts from henna leaves was shown to be active against the bacteria E. coli and S. aureus and the fungus C. albicans. When compared to commercial antimicrobials, a higher concentration of lawsone was even more effective at inhibiting the growth of C. albicans [103]. Episiotomy is a common procedure during childbirth, but it is also the most painful and infection-prone procedure. In a study conducted by Zibanejad et al., an ointment made from henna leaves helped in faster healing of the wound by reducing the diameter of the cutaneous wound over time. This observation was attributed to the antibacterial and anti-inflammatory properties of the lawsone present in henna leaves [104]. In another study, henna extracts were tested against the activity of several strains of bacteria (both Gram-positive and Gram-negative), using the agar well diffusion method. The bacteria included in the test ranged from Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Vibrio cholerae, Shigella flexneri, Klebsiella pneumoniae, Vibrio cholerae, and Enterobacter aerogenes to Bacillus subtilis, Bacillus megaterium, Bacillus fusifirmis, Staphylococcus aureus, Streptococcus faecalis, Streptococcus pyogenes, and Streptococcus pneumonia. The ethanolic extract tested positive for all of the strains, meaning that it was effective at combatting most of the bacteria, with the highest activity shown against Vibrio cholerae, with a zone of inhibition of 24.5 mm. S. faecalis recorded the highest zone of inhibition for Gram-positive bacteria at 26.3 mm [105]. In yet another study, the methanolic extract of henna leaves collected from a region of Yemen showed antibacterial activity against three different strains of bacteria [106]. The study compared the methanolic extract’s activity to that of the aq. extract. And, it was found that the methanolic extract is much stronger than the aq. extract in terms of its activity against the tested bacteria. The reason, the authors proposed is the presence of free hydroxyl groups present in the methanolic solution responsible for binding to the carbohydrates and proteins present in the bacterial cell wall and inactivating the active sites of the enzymes. In addition to the antibacterial properties of the lawsone present in henna leaves, henna leaves have also been shown to possess antitumor [107], anthelmintic [108], and antioxidant [109] properties. Due to their cooling effect, they have been shown to heal burn wounds [110]. They have also been shown to be UV-protective [94] and possess other antimicrobial properties as well [111].
When applying any natural colorant in industries serving humans, it is important to test its toxicity. Studies have shown that lawsone from henna leaves is non-toxic, and this has been well proven by its wide use, especially to color palms (hand). One has to note that palms are used for dealing with the cooking, eating, etc., of food. That could imply that small amounts of lawsone may be non-consequential in terms of its internal consumption. A study conducted by Kirkland and Marzin assessed the genotoxicity of lawsone and proved that it is non-toxic for humans [112,113,114]. Stains of henna are hard to remove from clothes, and this could prove beneficial for its utility in the textile, wood, and paper industry. Its antibacterial and antifungal properties can be applied in developing medicinal shampoos and conditioners, or natural hair colors.
2.3. Emodin
Anthraquinones are one class of natural colorants which give bright purple, maroon, and blueish shades of red and are derived from many different parts of the plants. Hence, they have found many uses as natural colorants [115]. Emodin (the chemical structure of which is shown in Figure 3) is one specific kind of anthraquinone known for its color and medicinal properties. It is found in various parts of many plants like Senna alata (Cassia alata) [116], Rumex abyssinicus [117], Odontites serotine [118], Reynoutria japonica [119], and Polygonum cuspidatum [120]. Although, the most common source of emodin for us is from rhubarb [121].
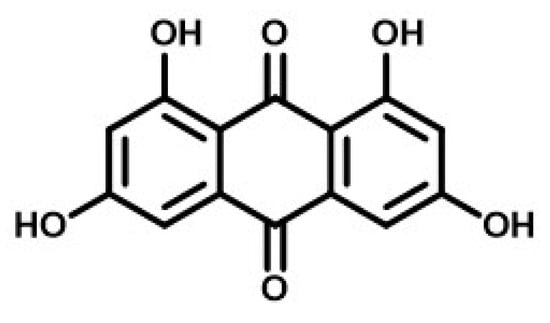
Figure 3.
Chemical Structure of Emodin.
To start the discussion on the antibacterial properties of emodin, Shifa et al. [122] isolated two kinds of anthraquinones, emodin and chrysophenol, from the roots of the herb Rumex abyssinicus (of African origin) and showed that they can cure various diseases. Specifically, emodin was shown to be a strong inhabitant of S. aureus (the growth inhibition zone was 23 mm). It also showed inhibition activity against the Gram-negative strains of P. aeruginosa and E. coli. Minimum inhibitory concentration (MIC) measures the minimum amount of antibacterial agent required to inhibit the growth of the bacteria in question. An earlier study showed that emodin was active against the methicillin-resistant S. aureus (MRSA), with an MIC value of 4 μg/mL. The incorporation of an iodine atom into the aromatic ring of emodin reduced the MIC values from two- to four-fold. Chlorine and bromine substitution, on the other hand, showed no significant change in the activity of emodin derivatives. Although, these latter derivatives were shown to have a higher cytotoxic activity observed against Vero cells (IC50 in the range 9.7–18.7 μM). Similar results were obtained by Promgool et al. in a study conducted with emodin [123]. They obtained the MIC values of emodin against the Gram-positive bacterial strains of Bacillus cereus TISTR 687 as being 16 μg/mL, those against methicillin-resistant S. aureus (MRSA)-SK1 as being 4 μg/mL, and those against S. aureus TISTR 1466 as being 16 μg/mL. On the other hand, the MIC values for the Gram-positive bacteria tested were 128 μg/mL for P. aeruginosa TISTR 781 and Salmonella typhimurium TISTR780. Haemophilus parasuis is the bacterium which causes Glässer’s disease. Emodin was found to have an MIC of 32 μg/mL and an MBC (minimum bactericidal concentration) of 64 μg/mL, respectively [124]. Chukwujekwu et al. found emodin (extracted from the roots of Cassia occidentalis) to be active against Bacillus subtilis and S. aureus with MIC values of 7.8 × 10−3 and 3.9 × 10−3 mg/mL, respectively [125]. However, emodin was shown to be inactive against two Gram-negative bacteria, Klebsiella pneumoniae and E. coli, even at higher concentrations of 5.0 × 10−3 mg/mL. When applying these natural colorants as antibacterial materials, especially in the pharmaceutical industry, investigating the limit of safe dosage is important. A study showed that, when different doses of emodin (20, 40, and 80 mg/kg) were administered to mice for 12 weeks, no pathophysiological disorders in the major organs were observed [126], hence proving emodin to be safe for mammal consumption (though not yet proven safe for humans). This section focused on the particularly interesting and hence deeply studied anthraquinone derivative emodin. In further sections we will focus on other major classes of natural colorants.
2.4. Flavonoids
Another class of natural colorants widely available in Nature is flavonoids. Flavonoids are compounds that are present in many fruits, flowers, and herbs, and are responsible for their vibrant colors and medicinal properties [127]. Apart from plant sources, flavonoids are also found in honey and propolis, which are synthesized by bees using flowers and herbs. Flavones and flavanols, present in citrus fruits, onions, and tea leaves, offer yellow and red shades. Whereas the isoflavones found in soybeans and other legumes can contribute to yellow and orange shades. Flavonoids are widely used in the food industry to impart natural color to flavored drinks. They also find their uses in the cosmetic and textile industries. The chemical structures of some common flavonoids are shown in Figure 4. The basic skeleton consists of three rings labeled as A, B, and C. The position and constituents of these rings determine which particular kind of flavonoid it is. In total, there are fourteen flavonoids known chemically [128]. However, the most studied flavonoids are the six represented in the following figure.
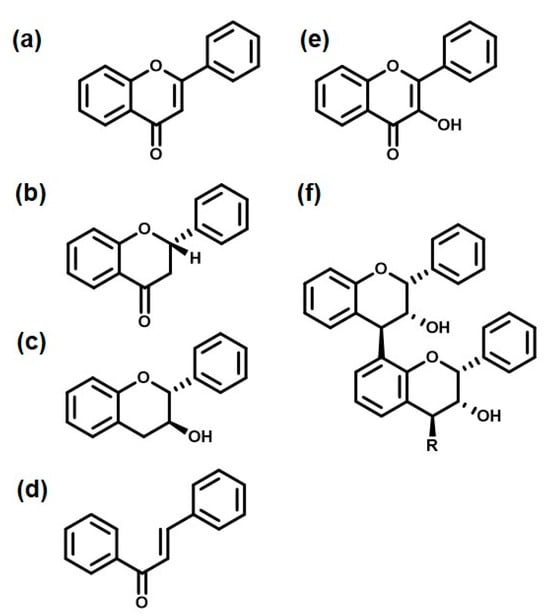
Figure 4.
Six classes of flavonoids: (a) Flavone, (b) Flavanone, (c) Flavan-3-ol (catechin), (d) Chalcone, (e) Flavanol, and (f) Flavolan.
Some relevant studies pertaining to the antibacterial activity of flavonoids are presented here. Research on the antibacterial properties of flavonoids has been gathering interest since the 1980s. The research carried out in the last two decades has mainly elucidated three different mechanisms of action of their serving as antibiotics [129,130]. The first mechanism is concerned with the damage caused by flavonoids to the cytoplasmic membrane of bacterial cells [131], followed by flavonoids reducing the membrane’s fluidity [132]. The other two pathways of inhibiting bacterial growth were the inhibition of nucleic acid synthesis in bacteria [133,134,135] and the inhibition of energy metabolism by flavonoids [136]. In addition to inhibiting the growth of bacteria, flavonoids have also been studied as augmenting the activity of antibiotics, specifically flavan-3-ols. Flavan-3-ols, also known as epicatechins (Figure 4c), have been shown to reduce the minimum inhibition concentration values of β-lactam antibiotics against strains of S. aureus by 500 times [137]. Another relevant study showed that isoflavones [138] and chalcones [139] can inhibit the production of the enzyme urease, which is secreted by the pathogenic bacteria H. pylori, in the stomach at lower pH levels. Killing bacteria or inhibiting bacterial growth are not the only ways to solve the problems caused by bacteria. The toxins secreted by bacteria remain in the system long after they have been killed [140]. Some studies have shown that flavonoids can help with metabolizing and excreting these toxins. For example, Choi et al. showed that polymerized catechin can negate the effect of α-toxin secreted by the bacteria S. aureus in vivo and in vitro [141]. The isoflavone genistein also inhibited exotoxin [142]. Oh et al. [142] showed that the pretreatment of HeLa cells with genistein protected them from the Vibrio vulnificus toxin RtxA1. Genistein also had a protective effect against V. vulnificus infection in vivo, as demonstrated using CD-1 mice [142]. Many studies have proven that the ability of bacteria to cause disease is largely dependent on the kind and amounts of enzymes and toxins they can release into the host body. Flavonoids have been shown to inhibit the release of these toxins. In a study conducted by Shah et al., the authors proved that epicatechin gallate prevents the secretion of coagulase and α-toxin [143].
2.5. Carotenoids
The yellow, orange, and red colors found in Nature are commonly due to a class of compounds collectively known as carotenoids. Their organic structure is composed of highly unsaturated long chains (hence the bright colors) and because of this they are sparingly soluble in water and are mostly fat-soluble. Carotenoids can be derived from various natural sources like plants, fungi, bacteria, and algae [144]. Carotenoids, being readily available, have been a favorite source of natural colorants for the food and textile industries. Since the variety of carotenoids is vast, as are their different sources, a major section is devoted to them. In this section, we cover various carotenoids extracted from different plant sources and exhibiting unique characteristics, cultural attributes, and antimicrobial properties.
2.5.1. Crocus (Saffron)
The first carotenoid we present here is crocus. It is commonly known as saffron. Crocus consist of the dried stigma and styles of the flower of the plant Crocus sativus Linn. It is a very expensive herb used in cosmetics and the food industry. Depending on the variety, source, and quantity of the saffron, it can impart a yellow or red color. It has great medicinal value in Ayurveda. Paying attention to its chemistry, crocus contains pigments like crocin, crocetin, picrocrocin, and safranal. Crocin is the main ingredient of crocus, which imparts it with a bright yellow–red color. The molecular formula of crocin is C44H64O24, and its structure is shown in Figure 5 [145]. Crocin is the glycosylated esters of a dicarboxylic acid, and its partial solubility in water is due to the hydroxyl groups present in the two sugar units, gentibiose and neapolitanose (the two rings at each end) [146]. The middle part of the structure consists of seven conjugated double bonds; it is this part which is responsible for its bright orange–red color and its antioxidant properties.
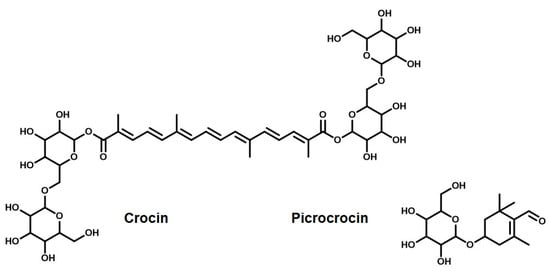
Figure 5.
Active ingredients of saffron (crocin and picrocrocin).
In one of the earlier studies, crocin-soaked disks were incubated with various microbial species. Of the many species tested, crocin showed antimicrobial activity against only S. epidermidis, a pathogenic organism [147], showing its valuable use in cosmetics, especially as a face cream. Due to its excellent inhibition properties against S. epidermidis, it was used in other similar studies [148,149,150,151]. In another study, Jinous et al., reported the antibacterial activity of saffron at higher concentrations (1000–31.2 mg/mL) [150]. Though saffron may not have extraordinary antibacterial activity, yet it has extensive medicinal values. And, we thought it would be beneficial for the readers to have this information. The source of this information is uncertain, but saffron has been used in folk medicine as an antispasmodic, eupeptic, gingival sedative, anticatarrhal, nerve sedative, carminative, diaphoretic, expectorant, stimulant, stomachic, aphrodisiac, and emmenagogue. Its active constituents have anticonvulsant, antidepressant, anti-inflammatory, and antitumor properties; it acts as a radical scavenger as well as exhibits learning- and memory-improving properties by promoting the diffusivity of oxygen in different tissues. In a conclusive study, the two active ingredients of saffron—crocin and safranal—were shown to have antidepressant effects [24]. Apart from that, the crocin, picrocrocin, and safranal have been shown to inhibit the growth of human cancer cells (HeLa cells) in vitro [152]. Carotenoids, in general, are well known for promoting eye health. Saffron is no different, it has been shown that crocin and its analogs increase the blood flow in the retina and choroid as well as facilitating retinal functional recovery, and it could be used to treat ischemic retinopathy and/or age-related macular degeneration [153].
2.5.2. Lycopene (Tomato)
Another important carotenoid is lycopene. It is a favorite natural colorant in the food industry, mainly due its bright color and nutritional value [154]. Lycopene is found in various fruits like tomatoes, melons, papaya, and red bell peppers [155]. Tomatoes contain the highest amount of lycopene [156], making up 65–98% of the total amount of carotenoids in tomatoes. The other carotenoids present in tomatoes, in addition to lycopene, are α- and β-carotene, lutein, zeaxanthin, and b cryptoxanthin. Β-carotene, the yellow pigment of the carrot, is an isomer of lycopene [24,157]. The chemical structure of lycopene is shown in Figure 6a. Lycopene consists of highly unsaturated long-chain alkenes. The conjugated double bonds are responsible for its bright red color and its antioxidant activity.
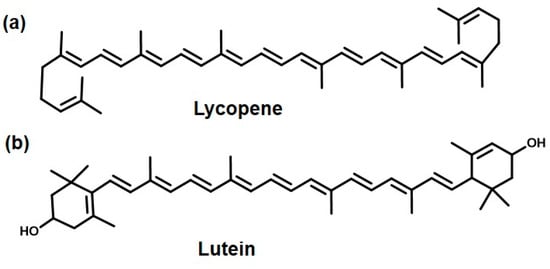
Figure 6.
Carotenoids as natural colorants: (a) Lycopene (tomato) and (b) Lutein (marigold).
The general information about the antibacterial and antioxidant properties of lycopene are covered in this reference [158]. In one of the studies, using the agar well diffusion method, it was shown that lycopene and lycopene selenium nanoparticles showed significant activity against S. aureus strains. After treating the biofilms produced by S. aureus with different MIC values (½, ¼ and 1/8) of lycopene selenium nanoparticles, the numbers decreased from 19 to 11, 16, and 18, respectively. Moreover, these treatments reduced the percentage of strong biofilm-forming isolates from 25% to 5%, 10%, and 15%, respectively [159]. In another study, lycopene extracts showed potent oral antimicrobial activity, particularly against S. aureus and C. albicans. This study has been confirmed by further studies as well [159,160]. Al Oqaili et al. assert that this antimicrobial activity of tomato extract is attributed to the presence of other active ingredients in addition to lycopene [161]. The mechanism of action was further investigated by Lee et al. They proposed that the antibacterial function of lycopene could be due to it inducing ROS-mediated DNA damage, particularly involving hydroxyl radicals [162].
2.5.3. Capsanthin and Capsorubin (Paprika)
Another class of common carotenoids used as natural colorants comes from paprika. Paprika, also known as sweet pepper, belongs to the family of Solanaceae and is the most cultivated species of the genus capsicum. The natural red color of paprika is widely used in food products like meat, dairy, cheese, soups, sauces, and snacks. The active ingredients of paprika are known for their antioxidant, antiviral, antimicrobial, anticancer, and anti-inflammatory properties [163]. The main active ingredients of paprika which impart it with its particularly strong red color and the above medicinal properties are capsanthin and capsorubin—see Figure 7. They are collectively called capsaicinoids [24,164].
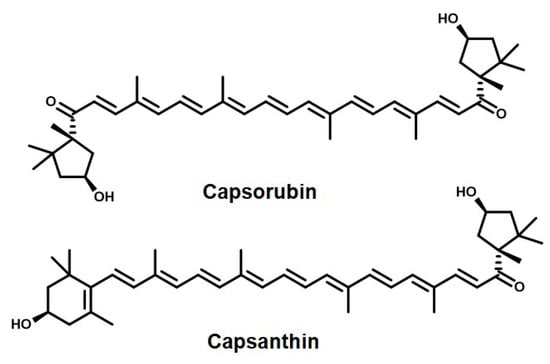
Figure 7.
The naturally occurring pigments responsible for the red color of paprika.
In a study, paprika extracts using organic solvents of different polarities were tested for their antimicrobial, antibiofilm, and anti-Quorum sensing (QS) activity against several pathogenic microorganisms and for their possible toxic effects using an in vivo model (Galleria mellonella L.). The non-polar extracts (hexanes and chloroform) were found to be most active in inhibiting the biofilm formation of the eight bacteria tested, with inhibition percentages between 22 and 88% for a concentration of 100 μg/mL, in addition to them being potent inhibitors of the biofilm of S. aureus. In another experiment, polystyrene surfaces (the material of the “to-go” boxes) were coated with the extracts from paprika. It was observed that the growth and biofilm formation of Staph. aureus decreased significantly. It was also observed that the extract also interferes with hemolysin and coagulase activities. The extract was also shown to inhibit the biofilm formation and enhanced mortality of Gram-negative bacteria. These studies proved the age-old use of paprika as a preservative, especially for pickles and sauces [165].
2.5.4. Lutein (Marigold)
Let us move to another interesting carotenoid called lutein, derived from marigold flowers. Historians have reported the use of marigold in India, China, and Indonesia for its medicinal and cultural value [166]. The different classes of bioactive compounds found in marigold consist of flavonoids [167], phenols [168], and carotenoids, of which lutein (Figure 6b) is the chief component [169]. This is the component which imparts it with its deep golden yellow color and pungent smell [24,170]. Because of this, marigold is widely used as a natural colorant, especially in the textile (cotton, wool, and silk) industry. Lutein, as is apparent from its chemical structure, consist of long-chain conjugated double-bond hydrocarbon, which makes it fat-soluble. The only polarity is imparted due to the two hydroxyl groups on the cyclohexene rings on each end. These two hydroxyl groups are not enough to make it even sparingly water-soluble [171]. The other components of marigold are galenine, lycopene, α-carotene, β-carotene, and v-carotene [172]. In some studies, one of the other components of marigold flower petals, patulitrin, was shown to be an effective bactericidal [173,174]. In other studies, the marigold extract was shown to have antibacterial activity against the growth of the pathogenic bacteria E. coli, B. cereus, and S. aureus [167,175]. Some other components found in marigold extracts like laricitrin and glycosides also showed potent antibacterial activity [176,177]. In one interesting study, soap was prepared using marigold flowers. It was shown that this soap was quite effective at inhibiting the growth of E. coli and S. aureus [178]. The proposed mechanism for this observation could be inhibition of bacterial nucleic acid synthesis. In yet another study, marigold’s active components were extracted with different organic solvents and tested against many bacterial strains like Alcaligens faecalis, Bacillus cereus, Campylobacter coli, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Streptococcus mutans, and Streptococcus pyogenes. The extracts were shown to be active against all of the tested strains but they were most active against Klebsiella pneumoniae (29.50 mm). Hence, apart from lutein, patulitrin has also been shown to be an important component responsible for antibacterial activity [174]. In another study, marigold flowers were shown to have the maximum inhibitory activity against the Neisseria gonorrhoeae strain [179]. In addition to its antibacterial properties [178], marigold has been shown to possess antimicrobial [180], antimalarial [181], antihyperlipidemic [182], and anti-inflammatory [183] effects. In addition to its rampant use in the textile industry, these properties make it a very promising candidate to be used in the cosmetics, food, and medicine industries.
2.5.5. Bixin and Norbixin (Annatto)
The last carotenoids we discuss in this sub-class of natural colorants are bixin and norbixin. These compounds are responsible for the bright red color of annatto, making it a widely used natural dye. These compounds are extracted from the seeds of the plant Bixandicant L. [24,184]. Annatto has found wide applications, from the coloring and bleaching of dairy products to the use as a natural colorant in the food industry, especially in bakeries for cream-based deserts [185,186,187,188]. The latter mentioned use could be attributed to its chemical structure (Figure 8), which contains highly conjugated long-chain hydrocarbons, readily soluble in fat. The yellowish red dye is extracted from the seeds found in the fruit of the plant B. orellana [189]. Apart from bixin and norbixin, the other important components of annatto extracts are cryptoxanthin, lutein, zeaxanthin, and methylbixin [190,191,192].
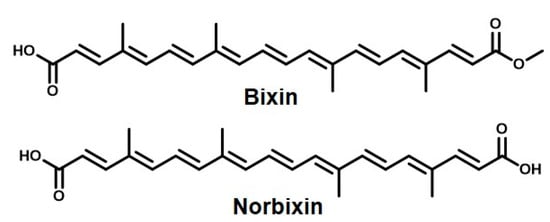
Figure 8.
The main active ingredients of annatto, responsible for its color and antibacterial properties.
In the following section, are presented some studies which prove the efficacy of annatto extracts as a potent antibacterial material. In one study, Fleischera et al. showed that the extracts from the leaves and seeds of annatto had strong antimicrobial activity against bacterial and fungal strains [193]. The leaves were shown to have stronger (MIC = 1000 μg/mL) and wider activity. Although, the greatest activity was shown to be against the Salmonella typhi (MIC = 31.25 μg/mL) and Acinetobacter species (MIC = 31.25 μg/mL). These MIC values were compared to the antibiotic streptomycin (9 ± 0.3 mm and 20 ± 0.2 mm) as a reference [194]. The ethanol extract of the annatto leaves showed antibacterial activity against P. aeruginosa (MIC = 512 μg/mL) and B. cereus (MIC = 4096 μg/mL); in this case, the bacteriocin drug niacin was used as a reference [195]. In another study, Braga et al. showed that the extracts were active against Cryptococcus neoformans with an MIC value of 78.0 μg/mL, compared to standard Amphotericin-B with an MIC value of 0.078 μg/mL [196]. They also proved that the ethanolic extracts showed broad-spectrum antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Streptococcus pyogenes, Salmonella typhi, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. This study substantiated its use in oral health for sore throat gargling [193]. There are numerous studies that showed the antibacterial activity of annatto extracts; for instance, the ethanol extract of the leaves was shown to exhibit antimicrobial activity against Bacillus pumilus [197]. The dried leaves were analyzed for active chemical compounds, and it was found that a sesquiterpenes (Bixaghane) along with ellagic acid, 7-bisulfate luteolin, 8-bisulfate hypoluteolin, 7-glucoside luteolin, and bixorellin account for the potent antimicrobial activity of this plant [198]. 1H NMR, thin-layer chromatography (TLC), liquid chromatography (LC)/photodiode array/mass spectrometry (LC/PDA/MS) analyses were used to characterize the chemical structures of the active components bixin and norbixin [199]. In another study, different organic solvents like ethanol, methanol, acetone, and DMSO were used to extract the active components of annatto leaves and seeds and these were tested against different bacteria like E. coli, K. pneumonia, P. aeruginosa, B. subtilis, B. cereus, and S. aureus by using a disk diffusion assay and antibiotic tetracycline as a standard. The DMSO and acetone extracts were found to be the strongest bacterial growth inhibitors [200]. All of these studies proved that the natural colorants bixin and norbixin derived from annatto were excellent candidates for antibacterial agents to be used in the food, textile, and pharmaceutical industries.
2.6. Indigo
Indigo is a well-known natural dye that produces a blue color. Indigo, as a natural colorant of textiles, has been used in India since ancient times and its popularity soon spread in China through the Silk Route [201]. China applied this natural dye not just in the textile industry but in calligraphy as ink, in paintings, and most importantly in pottery, hence the world famous “blue china” crockery. In India, indigo powder is still commonly used in every household as a finishing product on white clothes and as an additive in limewash for interior and exterior wall paint. Indigo is obtained from the stems and leaves of the tree Baphicacanthus cusia (Neel) Bremek. The product is used in the form of either paste or, for more convenient use, as a powder. The active ingredients responsible for the blue color of this dye and for its medicinal properties are indigo and indirubin (Figure 9). Modern researchers, especially in the textile industry, proved indigo’s many medicinal properties, like it being anti-inflammatory, antioxidant, an immune regulator, antimicrobial, etc. [202,203].
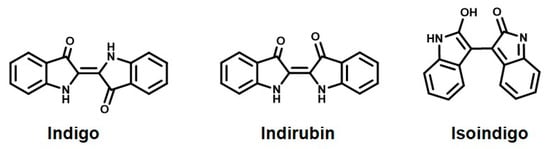
Figure 9.
The chemicals responsible for the natural blue color of indigo.
Herein, we discuss the studies conducted on the antibacterial properties of indigo. In one of the earlier studies, the ethyl acetate extract of indigo proved to be a strong inhabitant of methicillin-resistant Gram-positive bacteria like Staphylococcus aureus, Staphylococcus epidermidis, and Staphylococcus aureus [204]. The mechanism of action was that by producing superoxide, indigo caused oxidative damage to augment cell death in bacteria [205]. The mechanism of action of indigo was studied in the presence as well as the absence of light. In the absence of light, indigo showed antibacterial effects against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Proteus vulgaris, and Candida albicans, with a minimum inhibitory concentration [MIC] of 240 μm. But in the presence of light, the MIC decreased by half, showing that indigo is a stronger antibacterial agent in the presence of light [205]. This could be due to the enhancement of superoxide formation in the presence of light. Another active ingredient of indigo, indirubin, was also shown to inhibit Staphylococcus aureus and enhanced the activity of ciprofloxacin. The mechanism of action could be attributed to the inhibition of the NorA efflux pump in Staphylococcus aureus [206]. In another study, an organic extract of indigo was tested against various strains of S. aureus. It showed antibacterial activity against all strains, but particularly high inhibition zones were observed for two MRSA strains (UFPEDA670 and UFPEDA672). Interestingly, these two strains had a nasty profile. They were extracted from two different sources and were found to be multidrug-resistant (resistant to oxacillin, cefoxitin, erythromycin, and clindamycin). The same study showed that the acetone extract showed the best activity. Also, the study proved the additive antibacterial effect of indigo in conjunction with erythromycin [207]. Another study showed that all parts of the tree, including the roots, bark, leaves, and flowers, show strong antibacterial activity against Staphylococcus aureus [208]. In yet another study, the active ingredients extracted from indigo leaves with organic solvents showed major antimicrobial activity against twenty-two microbial species including bacteria and fungi. Using the infusion method, the aq. extract of the leaves showed inhibition zones with 10 mm/diameter (mm d−1) for S. aureus, 14 mm d−1 and 12 mm d−1 for T. rubrum (LM-09, LM-13), and 12 mm d−1 for M. canis (filamentous fungal), respectively. Dahot et al. showed that the aq. extract from the leaves of indigo had strong antibacterial activity against both Gram-positive and Gram-negative bacteria [209]. A further study from the literature also showed that the ethanolic extracts of indigo have potent antibacterial activity against S. aureus and P. aeruginosa and antifungal activity against Enterococcus faecalis and E. coli [210]. The same conclusions were drawn from other studies [211]. In one of the studies, the ethyl acetate extract of indigo was tested against various strains of Staphylococcus. And, it was found that MRSA was the most sensitive bacterium. But neither indigo nor tryptanthrin were able to completely inhibit the growth of the MRSA bacterium [203]. These were some examples of the general antibacterial properties of indigo. It should be noted that the antibacterial properties of indigo are not just of tremendous use in the textile, paper, pottery, and paint industry but in the cosmetic industry too, as indigo powder is used as a hair dye after using henna on hair, to override the orange shade provided by it and impart a black color to the hair.
2.7. Neem
Neem leaves and bark are one of Nature’s many blessings. The red–brown color extracted from the bark of the tree has been used as a natural colorant. The strong antimicrobial properties of neem have been known about since ancient times [212]. Neem has been shown to contain at least one hundred and forty bioactive compounds used primarily for their pesticidal properties [213]. The neem plant is a native of India and its chief active ingredient is azadirachtin (Figure 10), hence the scientific name Azadirachta Indica. Some of the other chief bioactive components of neem are sodium nimbinate, nimbin, nimbolin, nimbidol, gedunin, and solanine; whereas, neem leaves contain compounds like nimbolide, 7-deacetyl-7-benzoylazadiradione, 17-hydroxyazadiradione, amino acids, nimbandol, and nimbolide [214].
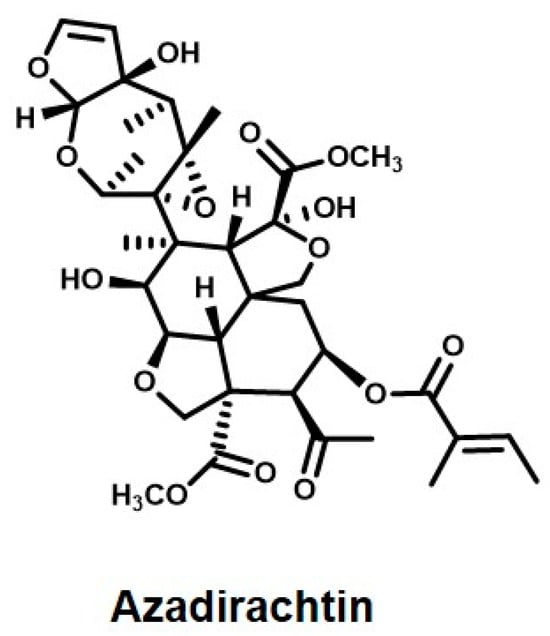
Figure 10.
Active ingredient of Neem.
Another class of bioactive compounds found in Neem are tricyclic diterpenoids like margolone, margolonone, and isomargolonone. These compounds have been shown to exhibit profound antibacterial activity [215,216]. Just like the leaves and bark of the neem tree, the seeds of the neem fruit are extremely beneficial. The oil extracted from the seeds contains nimbidin and nimbolide as its chief ingredients; these have been shown to have strong antifungal, antimalarial, and antibacterial properties. They have been shown to inhibit Mycobacterium [217,218]. It is for this reason, that neem seed oil is very widely used as a pesticide in Indian farming. When the extracts containing azadirachtin, quercetin, and β-sitosterol derived from the bark, seeds, and leaves were compared for their antibacterial and antifungal properties, it was found that the leaves had the highest concentration and hence the highest activity [219]. From time immemorial, neem bark has been used as a natural brush with which to brush teeth in India. With the appearance of plastic toothbrushes and synthetic toothpastes, this is no longer the case. But, a research study has proven the antibacterial efficacy of neem bark in endodontic infections [220]. This study was just scratching the surface of the potency of the antimicrobial properties of neem in dental science. Another study showed the potent antibacterial activity of the methanolic extracts of neem bark against polymicrobial dental biofilm [221]. Earlier studies had shown that different concentrations of the aqueous extracts of neem had a detrimental effect on S. mutans [222]. In yet another study, the ethanolic extract of neem leaves at 10 mg/mL concentration was shown to be significantly more effective against S. mutans than the active ingredient of the synthetic antibacterial mouthwash, Chlorhexidine [223]. These examples have proven the age-old use of neem as an effective daily material for taking care of oral health. With these studies, there is hope that the pharmaceutical companies manufacturing toothpastes containing synthetic lab-produced chemicals might try to replace them with neem extracts as the chief component of the toothpaste.
In the context of nanomedical science, nano emulsions of neem have been shown to inhibit pathogenic bacteria like V. vulnificus at a very low concentration [224]. In another study, this concentration was determined to be 150 μg/mL [225]. Although aqueous neem extracts also showed antibacterial activity (MIC) at 6 mg/mL, the nanoemulsions showed antibacterial activity against V. vulnificus. at even lower concentrations of 150 μg/mL [226].
2.8. Pomegranate
Pomegranate is a common fruit used worldwide. The medicinal value of pomegranate is evident in a saying, which means ‘one pomegranate and hundreds sick’. The scientific name of pomegranate is Punica granatum and it belongs to the family Punicaceae [227]. A class of natural dyes, anthocyanins, specifically pelargonidin glycosides, delphinidin, and cyanidin, are common components of pomegranate. These specific anthocyanins are responsible for the attractive red–purple color of pomegranate juice. Also, since they are phenolic in nature, they are readily soluble in water [24,228]. The chief components include punicalagin (Figure 11), punicalin, gallagic, and ellagic acids, and alkaloids like isopelletierine [229].
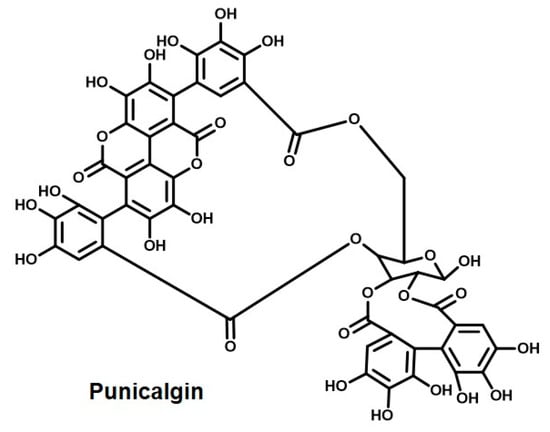
Figure 11.
Punicalgin—the active ingredient of the natural colorant from pomegranate.
Since biblical times, pomegranate have been used for its medicinal value. The Egyptians used pomegranates to treat a number of different infections [230]. In Ayurveda, the rind of the fruit and the bark of the pomegranate tree have been used to treat diarrhea and dysentery [231]. The dye made from pomegranate juice has been reported to possess strong antimicrobial properties due to the presence of large amounts of tannins in it [232]. Pomegranate is also used as a bactericide and stimulant [233]. It has been found that the pomegranate peel used to dye wool, in addition to using mordants like potash alum, ferrous sulfate, stannous chloride, etc., is effective against bacteria like E. coli, S. aureus, and C. albicans [234]. In a study conducted by Lee et al. to improve the antimicrobial properties of cotton dyed with pomegranate peel extract, the authors mentioned the use of two methods to extract the dye. In these methods, they used aqueous and ethanolic solutions and mordants like alum and copper sulfate. The bacteria E. coli, S. aureus, and Klebsiella pneumoniae are common germs which grow rapidly on cotton fabric. Lee et al. found that the ethanolic solution was resistant to the growth of these bacteria. They also found that the bacteria-resistant properties reduced to zero after 10 washes [235]. In another study, three strains of E. coli were used to test the viability of the antibacterial properties of extracts from pomegranate [236]. It was shown that the aqueous extract of pomegranate was quite effective against one particular strain of E. coli, O157:H7. The MIC and the minimal bactericidal concentration values were found to be 0.19 and 0.39 g/mL, respectively. On the other hand, the ethanolic extract of pomegranate had MICs of 0.49 to 1.95 mg/mL and MBCs of 1.95 to 3.91 mg/mL against the same bacterial strain, proving that the ethanolic extract was a more effective bactericidal [237]. This enhancement in the antibacterial property could be due to the inherent antimicrobial property of ethanol. The antibacterial activity of pomegranate extract was compared to the antibacterial activity of the antibiotic ampicillin using the agar well diffusion method. It was found that the pomegranate extract showed higher activity compared to the reference concentration–response curve of ampicillin [238].
Pomegranate extracts have been found to be quite effective against many food- and waterborne pathogenic bacteria like Salmonella typhi (S. typhi) [238,239], Vibrio cholerae [240,241], Yersinia enterocolitica [242], Shigella spp. [240,243], and Listeria monocytogenes (L. monocytogenes) [242,244,245]. In a study conducted in Peru, pomegranate peel extract and tea infusions were found to be effective in treating cholera [241].
The antibacterial properties of the pomegranate extracts found their use in the food industry. In a study focusing on the use of the antibacterial properties of pomegranate extract in the meat industry, it was shown that 250 µg/mL of pomegranate peel was effective at inhibiting antibiotic-resistant strains of Salmonella typhimurium and S. aureus on the surface of meat [246]. In a related study, it was shown that curing raw chicken breasts in 0.02% of pomegranate juice did not only enhance its color, but also reduced the protein oxidation, resisted bacterial growth, and increased sensory acceptability for up to 12 days when refrigerated at 4 °C [247]. Using pomegranate juice to cure cheese also resulted in reduced lipid oxidation, hence improving the shelf life of the cheese. In the food industry, chefs love to incorporate a pomegranate-based sour sauce with lettuce, spring onion, and parsley. A study found that this incorporation had an antimicrobial effect on the naturally present bacterial flora (S. aureus and E. coli O157:H7) of the above greens, hence proving the usefulness of using pomegranate extract in salad dressings [248]. In a very interesting study, a hydrolysable tannin-rich pomegranate by-product (POMx) was incubated with fecal bacteria. Dibenzopyranone-type urolithins were produced as a result. This compound enhanced the growth of “good” gut bacteria like Bifidobacterium spp. And Lactobacillus [249]. This study proved how beneficial pomegranate juice/extracts can be for gut health. In a similar study, POMx inhibited the growth of pathogenic bacteria like clostridia and Staphylooccus aureus, but supported the growth of Bifidobacterium breve and Bifidobacterium infantis [250]. Pomegranate has been shown to be useful to livestock and not just humans—pomegranate extract has been shown to be effective against the bacterial strains of the rumen in lactating cows [251]. Herein were presented various examples of how pomegranate can be used not only as a natural colorant, but that its antibacterial properties could be a blessing to various industries, especially the meat and dairy industries.
Table 2 below summarizes the relevant antibacterial studies of the “plant-derived” natural colorants covered in this article.

Table 2.
Names, active chemical components, relevant antibacterial studies, and associated references of “plant-derived” natural colorants presented in this review.
3. Conclusions
Fortunately, with the advancement of science and technology in recent years, researchers worldwide have paid attention to the problems that come with the use of synthetic dyes, such as them being environmental pollutants, medically unsafe in the food and cosmetic industries, and so forth. Since then, a number of studies have been conducted to test the viability of natural colorants in terms of replacing synthetic dyes [103,252,253,254,255]. Natural colorants, as they are derived from natural sources, are mostly safe to use in cosmetics and the food industry. As they are biocompatible, non-toxic, and environment friendly, they have brought about a revolution in the textile industry [256,257,258]. Natural colorants can be used to impart color not only to textiles but, as mentioned earlier, foods, cosmetics, leather, paper, ink, medicine, etc. Hence, the use of natural colorants has immense potential in many industries. Apart from imparting color, many natural colorants have been identified as possessing medicinal value, especially as antimicrobials. In this review, we specifically focused on the antibacterial properties of plant-derived natural colorants.
The reason for focusing on the antibacterial properties of natural colorants is simple—if we are to use a natural colorant in the above-mentioned industries, then why not utilize its antibacterial properties as well. Due to the overuse and misuse of antibiotics, antibiotic resistance is a common problem today. Scientists all over the world are working tirelessly on alternative antibacterial strategies. Natural colorants with antibacterial properties can act as a new range of antibacterial materials. While it is impossible to include all known antibacterial natural colorants in one review article, this review presents examples of commonly found and used natural colorants acting as antibacterial agents.
Extensive research conducted on the antibacterial properties of these natural colorants has been collected, organized, and summarized here, in the hope that this review article can serve as a fundamental resource for researchers planning to use plant-derived natural colorants in various industries, such as the food, cosmetics, medicine, paper, leather, wood, and textiles industries, and simultaneously utilize their antibacterial functions.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Kong, Y.; Jiang, Q.; Zhang, F.; Yang, Y. Small Molecular Fluorescent Probes: Application Progress of Specific Bacteria Detection and Antibacterial Phototherapy. Chem. Asian J. 2023, 18, e202300178. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Miao, L.; Li, X.L.; Xu, Z. Development of fluorescent probes targeting the cell wall of pathogenic bacteria. Coord. Chem. Rev. 2021, 429, 213646. [Google Scholar] [CrossRef]
- Deusenbery, C.; Wang, Y.; Shukla, A.A. Recent Innovations in Bacterial Infection Detection and Treatment. ACS Infect. Dis. 2021, 7, 695–720. [Google Scholar] [CrossRef]
- Shen, S.; Huang, Y.; Yuan, A.; Lv, F.; Liu, L.; Wang, S. Electrochemical Regulation of Antibacterial Activity Using Ferrocene-Containing Antibiotics. CCS Chem. 2021, 3, 129–135. [Google Scholar] [CrossRef]
- Raja Lakshmi, P.; Nanjan, P.; Kannan, S.; Shanmugaraju, S. Recent advances in luminescent metal–organic frameworks (LMOFs) based fluorescent sensors for antibiotics. Coord. Chem. Rev. 2021, 435, 213793. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, Z.; Liu, X.; Li, J.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Feng, X.; et al. Overcoming Multidrug-Resistant MRSA Using Conventional Aminoglycoside Antibiotics. Adv. Sci. 2020, 7, 1902070. [Google Scholar] [CrossRef]
- Ndieyira, J.W.; Watari, M.; Barrera, A.D.; Zhou, D.; Vogtli, M.; Batchelor, M.; Cooper, M.A.; Strunz, T.; Horton, M.A.; Abell, C.; et al. Nanomechanical detection of antibiotic-mucopeptide binding in a model for superbug drug resistance. Nat. Nanotechnol. 2008, 3, 691–696. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef] [PubMed]
- Fowoyo, P.T. Phage Therapy: Clinical Applications, Efficacy, and Implementation Hurdles Microbiol. Open 2024, 18, 18742858281566. [Google Scholar] [CrossRef]
- Sawa1, T.; Moriyama, K.; Kinoshita, M. Current status of bacteriophage therapy for severe bacterial infections. J. Intensive Care Med. 2024, 12, 44. [Google Scholar] [CrossRef]
- Nayab, S.; Aslam, M.A.; Rahman, S.; Sindhu, Z.D.; Sajid, S.; Zafar, N.; Razaq, M.; Kanwar, R. Amanullah Review of Antimicrobial Peptides: Its Function, Mode of Action and Therapeutic Potential. Int. J. Pept. Res. Ther. 2022, 28, 46. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Li, J.; Lu, A.; Liang, C. CRISPR/Cas systems: Delivery and application in gene therapy. Front. Bioeng. Biotechnol. 2022, 10, 942325. [Google Scholar] [CrossRef]
- Azeez, S.S.; Hamad, R.S.; Hamad, B.K.; Shekha, M.S.; Bergsten, P. Advances in CRISPR-Cas technology and its applications: Revolutionising precision medicin. Front. Genome Ed. 2022, 6, 1509924. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, S.A.; Barth, Z.K.; Makarova, K.S.; Wolf, Y.I.; Brover, V.; Peters, J.E.; Koonin, E.V. Widespread CRISPR-derived RNA regulatory elements in CRISPR-Cas systems. Nucleic Acids Res. 2023, 51, 8150–8168. [Google Scholar] [CrossRef] [PubMed]
- Moradialv, M.; Asri, N.; Jahdkaran, M.; Beladi, M.; Houri, H. Advancements in Nanoparticle-Based Strategies for Enhanced Antibacterial Interventions. Cell Biochem. Biophys. 2024, 82, 3071–3090. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Bedani, R.; Saad, S.M.I. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: An update for current perspectives and future challenges. Br. J. Nutr. 2015, 114, 1993. [Google Scholar] [CrossRef]
- Bedair, H.M.; Hamed, M.; Mansour, F.R. Antibacterial and antifungal activities of natural deep eutectic solvents. Appl. Microbiol. Biotechnol. 2024, 108, 515. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Jiao, Z.; Gao, L.; Dai, X.; Cheng, L.; Wang, Y.; Yan, L.T. Designing antibacterial materials through simulation and theory. J. Mater. Chem. B 2024, 12, 9155. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, D.; Duan, H. Recent advances in responsive antibacterial materials: Design and application scenarios. Biomater. Sci. 2023, 11, 356. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, X.; Li, Z.; Zheng, Y.; Nie, J.J.; Cui, Z.; Liang, Y.; Zhu, S.; Chen, D.; Wu, S. Recent progress of photo-excited antibacterial materials via chemical vapor deposition. Chem. Eng. J. 2022, 437, 135401. [Google Scholar] [CrossRef]
- Silveira, E.; Marques, P.P.; Silva, S.S.; Lima-Filho, J.L.; Porto, A.; Tambourgi, E. Selection of Pseudomonas for Industrial Textile Dyes Decolourization. Intern. Biodeter. Biodegrad. 2009, 63, 230–235. [Google Scholar] [CrossRef]
- Kadolph, S. Natural Dyes: A Traditional Craft Experiencing New Attention. Delta Kappa Gamma Bull. 2008, 75, 14. [Google Scholar]
- Chengaiah, B.; Rao, K.M.; Kumar, K.M.; Alagusundaram, M.; Chetty, C. Medicinal importance of natural dyes—A review. Int. J. PharmTech Res. 2010, 2, 144–154. [Google Scholar]
- Hussein, S.A.M.; Barakat, H.H.; Merfort, I.; Nawwar, M.A.M. Tannins from the leaves of Punica granatum. Phytochemistry 1997, 45, 819–823. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Beulega, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Colrants and Preservatives: A Review, a Demand and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Fobiri, G.K. Synthetic Dye Application in Textiles: A Review on the Efficacies and Toxicities Involved. Tex. Leath. Rev. 2022, 5, 180–198. [Google Scholar] [CrossRef]
- Soni, I.; Kumar, P.; Sharma, S.; Jayaprakash, G.K. A Short Review on Electrochemical Sensing of Commercial Dyes in Real Samples Using Carbon Paste Electrodes. Electrochem 2021, 2, 274–294. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.Y.; Wen, J.C.; Gollakota, A.R.K.; Shu, C.M.; Lin, K.Y.A. Adsorption of Reactive Red 195 from aqueous medium using Lotus (Nelumbo nucifera) leaf powder chemically modified with dimethylamine: Characterization, isotherms, kinetics, thermodynamics, and mechanism assessment. Int. J. Phytoremediat. 2022, 24, 131–144. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M. Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2020; pp. 162–169. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, D.; Khan, T.A. Recent Advances in Synthetic Dyes. In Innovative and Emerging Technologies for Textile Dyeing and Finishing; Scrivener Publishing LCC: Beverly, MA, USA, 2021; pp. 91–111. [Google Scholar]
- Russell, C. Understanding Antibacterial Action and Resistance; Ellis Horwood: London, UK; New York, NY, USA, 1996. [Google Scholar]
- Cooper, R. A Review of the Evidence for the Use of Topical Antimicrobial Agents in Wound Care. World Wide Wounds 2004, 1, 1–11. [Google Scholar]
- Wainwright, M. Acridine-a neglected antibacterial chromophore. J. Antimicrob. Chemother. 2001, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, S.; Churchward, G.; Bozia, J.; Stojilokivic, I.; Anic, S. Light Activated Antiviral Materials and Devices and Methods for Decontaminating Virus Infected Environments. U.S. Patent Application No. 11/598,549, 11 October 2007. [Google Scholar]
- Ratna; Padhi, B.S. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–945. [Google Scholar] [CrossRef]
- Miller, M.D.; Steinmaus, C.; Golub, M.S. Potential impacts of synthetic food dyes on activity and attention in children: A review of the human and animal evidence. Environ. Health 2022, 21, 45. [Google Scholar] [CrossRef]
- Novotny, C.; Dias, N.; Kapanen, A.; Malachova, K.; Vandrovcova, M.; Itavarra, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Bhatnagar, P. Mutagenicity assessment of textile dyes from Sanganer (Rajasthan). J. Environ. Biol. 2007, 28, 123–126. [Google Scholar]
- Hernandez-Ceruelos, A.; Madrigal-Bujaidar, E.; de La cruz, C. Inhibitory effect of chamomile essential oil on the sister chromatid exchanges induced by daunorubicin and methyl methanesulfonate in mouse bone marrow. Toxicol. Lett. 2002, 135, 103. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Che, J.; Yang, X. A recent (2009–2021) perspective on sustainable color and textile coloration using natural plant resources. Heliyon 2022, 8, e10979. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Nazarpoor, K.; Karimi, L. Eco-friendly dyeing of wool using natural dye from weld as co-partner with synthetic dye. J. Clean. Prod. 2011, 19, 1045–1051. [Google Scholar] [CrossRef]
- Nateri, A.S. Reusing wastewater of madder natural dye for wool dyeing. J. Clean. Prod. 2011, 19, 775–781. [Google Scholar] [CrossRef]
- Kole, P.L.; Jadhav, H.R.; Thakurdesai, P.; Nagappa, A.N. Cosmetics Potential of Herbal Extracts. Nat. Prod. Rad. 2005, 4, 315–321. [Google Scholar]
- MacDougall, D.B. Color in Food Improving Quality; Woodhead Publishing Ltd.: Cambridge, UK, 2002. [Google Scholar]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections—A review. Photodiagn. Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lawande, S.A. Antimicrobial Activity of Turmeric Extracts Against Oral Pathogens. J. Pharm. Biomed. Sci. 2013, 27, 586–591. [Google Scholar]
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H.; Ahn, K.S.; Sethi, G.; Sandur, S.K. Curcumin—Biological and Medicinal Properties; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Lampe, V.; Milobedzka, J. Studien über curcumin. Berichte Dtsch. Chem. Ges. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Srinivasan, K.R. A chromatographic study of the curcuminoids in Curcuma longa L. J. Pharm. Pharmacol. 1953, 5, 448–457. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
- Damyeh, M.S.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Orteca, G.; Sinnes, J.P.; Rubagotti, S.; Iori, M.; Capponi, P.; Piel, M.; Rosch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2019, 204, 110954–110963. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huanga, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, 2000171. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Sood, S. Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 2013, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.D.; Neelakantan, P. Curcumin- Pharmacological actions and its role in dentistry. Asian J. Pharmaceut. Res. Health Care 2014, 6, 19–22. [Google Scholar]
- Khan, A.M.; Abid, O.U.R.; Mir, S. Assessment of biological activities of chitosan Schiff base tagged with medicinal plants. Biopolymers 2019, 111, 23338. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Tosati, J.V.; Tikekar, R.V.; Monteiro, A.R.; Nitin, N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157:H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
- Tortik, N.; Steinbacher, P.; Maisch, T.; Spaeth, A.; Plaetzer, K. A comparative study on the antibacterial photodynamic efficiency of a curcumin derivative and a formulation on a porcine skin model. Photochem. Photobiol. Sci. 2016, 15, 187–195. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Zhang, J.H.; Chuang, W.C.; Yu, K.H.; Huang, X.B.; Lee, Y.C. An in Vitro Study on the Effect of Combined Treatment with Photodynamic and Chemical Therapies on Candida albicans. Int. J. Mol. Sci. 2018, 19, 337. [Google Scholar] [CrossRef]
- Najafi, S.; Khayamzadeh, M.; Paknejad, M.; Poursepanj, G.; Fard, M.J.K.; Bahador, A. An In Vitro Comparison of Antimicrobial Effects of Curcumin-Based Photodynamic Therapy and Chlorhexidine, on Aggregatibacter actinomycetemcomitans. J. Lasers Med. Sci. 2016, 7, 21–25. [Google Scholar] [CrossRef]
- Saitawee, D.; Teerakapong, A.; Morales, N.P.; Jitprasertwong, P.; Hormdee, D. Photodynamic therapy of Curcuma longa extract stimulated with blue light against Aggregatibacter actinomycetemcomitans. Photodiagn. Photodyn. Ther. 2018, 22, 101–105. [Google Scholar] [CrossRef]
- Wu, J.; Mou, H.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q.J. Photodynamic effect of curcumin on Vibrio parahaemolyticus. Photodiagnosis Photodyn. Ther. 2016, 15, 34–39. [Google Scholar] [CrossRef]
- Panhoca, V.H.; Florez, F.; Junior de Faria, N.B.; Rastelli, A.N.; Tanomaru, J.; Kurachi, C. Evaluation of Antimicrobial Photodynamic Therapy against Streptococcus mutans Biofilm in situ. J. Contemp. Dent. Pract. 2016, 17, 184–191. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Lin, M.; Santos-Pinto, L.; Duarte, S. Photodynamic antimicrobial chemotherapy on Streptococcus mutans using curcumin and toluidine blue activated by a novel LED device. Lasers Med. Sci. 2015, 30, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.; Santos-Pinto, L.; Lin, M.; Duarte, S. Steptococcus Mutans Photoinactivation by Combination of Short Exposure of a Broad Spectrum Visible Light and Low Concentrations of Photosenstizers Photomed. Laser Surg. 2014, 32, 175–180. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Tonon, C.C.; Spolidorio, D.M.P.; Bagnato, V.S.; Giusti, J.S.M.; Santos-Pinto, L. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagn. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Manoil, D.; Filieri, A.; Gameiro, C.; Lange, N.; Schrenzel, J.; Wataha, J.C. Flow cytometric assessment of Streptococcus mutans viability after exposure to blue light-activated curcumin. Photodiagn. Photodyn. Ther. 2014, 11, 372–379. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.M.; Jeong, S.H.; Chung, K.H.; Kim, B.I. Antibacterial photodynamic therapy with curcumin and Curcuma xanthorrhiza extract against Streptococcus mutans. Photodiagn. Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Tosati, J.V.; de Oliveira, E.F.; Oliveira, J.V.; Nitin, N.; Monteiro, A.R. Light-activated antimicrobial activity of turmeric residue edible coatings against cross-contamination of Listeria innocua on sausages. Food Control 2018, 84, 177–185. [Google Scholar] [CrossRef]
- Bonifacio, D.; Martins, C.; David, B.; Lemos, C.; Neves, M.; Almeida, A. Photodynamic inactivation of Listeria innocua biofilms with food-grade photosensitizers: A curcumin-rich extract of Curcuma longa vs. commercial curcumin. J. Appl. Microbiol. 2018, 125, 282–294. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Wijesinghe, G.K.; Kumarage, S.; Abayaweera, G.; Samarakoon, S.R.; Perera, I.C.; Kottegoda, N.; Weerasekera, M.M. Effect of natural curcuminoids-intercalated layered double hydroxide nanohybrid against Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis: A bactericidal, antibiofilm, and mechanistic study. Microbiol. Open 2019, 8, 00723. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef]
- Oda, Y. Inhibitory effect of curcumin on SOS functions induced by UV irradiation. Mutat. Res. 1995, 348, 67–73. [Google Scholar] [CrossRef]
- Tonon, C.C.; Paschoal, M.A.; Correia, M.; Spolidorio, D.M.P.; Bagnato, V.S.; Giusti, J.S.M.; Santos-Pinto, L. Comparative effects of photodynamic therapy mediated by curcumin on standard and clinical isolate of Streptococcus mutans. J. Contemp. Dent. Pract. 2015, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Kim, S.B.; Kong, R.; Choi, J.G.; Kim, Y.C.; Shin, D.W.; Kang, O.H.; Kwon, D.Y. Curcumin Reverse Methicillin Resistance in Staphylococcus aureus. Molecules 2014, 19, 18283–18295. [Google Scholar] [CrossRef] [PubMed]
- Penha, C.B.; Bonin, E.; da Silva, A.F.; Hioka, N.; Zanqueta, E.B.; Nakamura, T.U.; de Abreu Filho, B.A.; Zanetti Campanerut-Sa, P.A.; Graton Mikcha, J.M. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. Food Sci. Technol. 2017, 73, 198–202. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic Action of LED-Activated Curcumin against Staphylococcus aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling curcumin degradation: Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Biswas, K.; Bandhopadhyay, U.; Banerjee, R. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Waghmare, P.F.; Chaudhary, A.U.; Karhadkar, V.M.; Jamkhande, A.S. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: A clinical and microbiological study. J. Contemp. Dent. Pract. 2011, 12, 221–222. [Google Scholar] [CrossRef]
- Suhag, A.; Dixit, J.; Dhan, P. Role of Curcumin as a Subgingival Irrigant: A Pilot Study. Periodontal Pract. Today 2007, 4, 115–121. [Google Scholar]
- Deepika, M.S.; Thangam, R.; Vijayakumar, T.S.; Sasirekha, R.; Vimala, R.T.V.; Sivasubramanian, S.; Arun, S.; Babu, M.D.; Thirumurugan, R. Antibacterial synergy between rutin and florfenicol enhances therapeutic spectrum against drug resistant Aeromonas hydrophila. Microb. Pathog. 2019, 135, 103612–103625. [Google Scholar] [CrossRef]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial Activity of Curcumin Against Periodontopathic Bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; Montellano, P.R.O. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Shete, M.; Sonune, S.; Singh, P.; Shete, A.; Karande, P.; Chougule, Y. Curcumin: A Wonder Therapy for Oral Diseases. Eur. J. Biomed. Pharm. Sci. 2016, 6, 622–626. [Google Scholar]
- Imran, M.; Ullah, A.; Saeed, F.; Nadeem, M.; Arshad, M.U.; Suleria, H.A.R. Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1271–1293. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Prasad, P.; Hussain, A.; Khan, I.; Kondaiah, P.; Chakravarty, A.R. Remarkable photocytotoxicity of curcumin in HeLa cells in visible light and arresting its degradation on oxovanadium(IV) complex formation. Chem. Commun. 2012, 48, 7702–7704. [Google Scholar] [CrossRef]
- Dweck, A.C. Natural ingredients for colouring and styling. Int. J. Cosmet. Sci. 2002, 24, 287–302. [Google Scholar] [CrossRef]
- Rao, Y.M.; Shayeda; Sujatha, P. Formulation and Evaluation of Commonly used Natural Hair Colorants. Nat. Prod. Rad. 2008, 7, 45–48. [Google Scholar]
- Xavier, M.R.; Santos, M.M.S.; Queiroz, M.G.; De Lima Silva, M.S.; Goes, A.J.S.; De Morais, M.A. Lawsone, a 2-hydroxy-1,4-naphthoquinone from Lawsonia inermis (henna), produces mitrochondrial dysfunctions and triggers mitophagy in Saccharomyces cerevisiae. Mol. Biol. Rep. 2020, 47, 1173–1185. [Google Scholar] [CrossRef]
- Mahkam, M.; Nabati, M.; Kafshboran, H.R. Isolation, identification and characterization of lawsone from henna leaves. Iran Chem. Commun. 2014, 2, 34–38. [Google Scholar]
- Color Index, 3rd ed.; Society of Dyers and Colourists: Bradford, UK, 1971; Volume 4, p. 4632.
- Bechtold, T. Natural colorants e quinoid, naphthoquinoid and anthraquinoid dyes. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 153–156. [Google Scholar]
- Gulrajani, M.L.; Gupta, D.B.; Agarwal, V.; Jain, M. Some studies on natural yellow dyes, Part III: Quinones: Henna, Dolu. Indian Text. J. 1992, 102, 76–83. [Google Scholar]
- Mayer, F.; Cook, A.H. The Chemistry of Natural Colouring Matters; Reinhold Publishing Corporation: New York, NY, USA, 1943; p. 105. [Google Scholar]
- Mondal, M.I.H.; Sayeed, M.A.; Newaz, M.O.; Salam, M.A. Isolation of a natural dye isolated from the leaves of Lawsonia inermis Linn and its dyeing characteristics onto silk. Colourage 2009, 57, 52–56. [Google Scholar]
- Yusuf, M.; Ahmad, A.; Shahid, M.; Khan, M.I.; Khan, S.A.; Manzoor, N.; Mohammada, F. Assessment of colorimetric, antibacterial and antifungal properties of woollen yarn dyed with the extract of the leaves of henna (Lawsonia inermis). J. Clean. Prod. 2012, 27, 42–50. [Google Scholar] [CrossRef]
- Zibanejad, S.; Miraj, S.; Rafieian Kopaei, M.M. Healing effect of Quercus persica and Lawsonia inermis ointment on episiotomy wounds in primiparous women. J. Res. Med. Sci. 2020, 25, 11. [Google Scholar] [CrossRef]
- Dahake, P.R.; Kamble, S.I. Study on Antimicrobial Potential and Preliminary Phytochemical Screening of Lawsonia inermis Linn. Int. J. Pharm. Sci. Res. 2015, 6, 3344–3350. [Google Scholar]
- Ali, K.S.; Al-hood, F.A.; Obad, K.; Alshakka, M. Phytochemical Screening And Antibacterial Activity of Yemeni Henna (Lawsonia inermis) Against Some Bacterial Pathogens. IOSR J. Pharm. Biol. Sci. 2016, 11, 24–27. [Google Scholar]
- Ozaslan, M.; Zumutdal, M.E.; Daglioglu, K.; Kilic, I.H.; Karagoz, I.D.; Kalender, M.E.; Tuzcu, M.; Colak, O.; Cengiz, B. Antitumoral Effect of L. inermis in Mice with EAC. Int. J. Pharmacol. 2009, 4, 263–267. [Google Scholar] [CrossRef]
- Bairagi, G.B.; Kabra, A.O.; Mandade, R.J. Anthelmintic Activity of Citrus medica L. leaves in Indian Adult Earthworm. Int. J. Res. Pharmaceut. Biomed. Sci. 2011, 2, 237–240. [Google Scholar]
- Mikhaeil, B.R.; Badria, F.A.; Matooq, G.T.; Amer, M.A.A. Antioxidant and immunomodulatory constituents of henna leaves. Natureforsch 2004, 59, 468–476. [Google Scholar] [CrossRef]
- Muhammad, H.S.; Muhammad, S. The use of Lawsonia inermis linn. (henna) in the management of burn wound infections. Afr. J. Biotechnol. 2005, 4, 934–937. [Google Scholar]
- Saadabi, M.A.A. Evaluation of Lawsonia inermis L. (Sudanese Henna) Leaf extracts as an antimicrobial agent. Res. J. Biol. Sci. 2007, 2, 419–423. [Google Scholar]
- Kirkland, D.; Marzin, D. An assessment of the genotoxicity of 2-hydroxy-1,4-naphthoquinone, the natural dye ingredient of Henna. Mutat. Res. 2003, 537, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, D.; Marzin, D. 2-Hydroxy-1,4-naphthoquinone, the natural dye of Henna, is non-genotoxic in the mouse bone marrow micronucleus test and does not produce oxidative DNA damage in Chinese hamster ovary cells. Mutat. Res. 2004, 560, 41–47. [Google Scholar] [CrossRef]
- Kirkland, D.J.; Henderson, L.; Marzin, D.; Muller, L.; Parry, J.M.; Speit, G.; Tweats, D.J.; Williams, G.M. Testing strategies in mutagenicity and genetic toxicology: An appraisal of the guidelines of the European Scientific Committee for Cosmetics and Non-Food Products for the evaluation of hair dyes. Mutat. Res. 2005, 588, 88–105. [Google Scholar] [CrossRef]
- Stompor-Goracy, M. The Health Benefits of Emodin, a Natural Anthraquinone Derived from Rhubarb-A Summary Update. Int. J. Mol. Sci. 2021, 22, 9522. [Google Scholar] [CrossRef]
- Chimi Fotso, S.; Tadjong, A.T.; Tsopgni, W.D.T.; Lenta, B.N.; Nkenfou, C.N.; Wansi, J.D.; Toze, F.A.A. Chemical constituents and antimicrobial activities of some isolated compounds from the Cameroonian species of Senna alata (Cassia alata L. Roxb synonym, The plant list 2013). (Leguminosae). Trends Phytochem. Res. 2021, 5, 37–43. [Google Scholar]
- Kengne, I.C.; Feugap, L.D.T.; Njouendou, A.J.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Tamokou, J.D.D. Antibacterial, antifungal and antioxidant activities of whole plant chemical constituents of Rumex abyssinicus. BMC Complement. Med. Ther. 2021, 21, 164. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, L.; Wang, H.; Chang, X.; Ren, S.; Lai, H.; Liu, L. Phytochemical profiles and antioxidant, anticholinergic, and antidiabetic activities of Odontites serotina (Lam.) Dum. Eur. J. Integr. Med. 2021, 44, 101340. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Frac, M.; Oszust, K.; Woch, M.W.; Zubek, S. Invasive plant Reynoutria japonica produces large amounts of phenolic compounds and reduces the biomass but not activity of soil microbial communities. Sci. Total Environ. 2021, 767, 145439. [Google Scholar] [CrossRef]
- Yu, M.; Chen, T.T.; Zhang, T.; Jia, H.M.; Li, J.J.; Zhang, H.W.; Zou, Z.M. Anti-inflammatory constituents in the root and rhizome of Polygonum cuspidatum by UPLC-PDA-QTOF/MS and lipopolysaccharide-activated RAW264.7 macrophages. J. Pharm. Biomed. Anal. 2021, 195, 113839. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.Y.; Huang, S.Q.; Zou, X.Q.; Zhong, X.B.; Yang, Y.F.; Zhang, Y.T.; Mi, Z.C.; Zhang, Y.S.; Huang, Z.G. Drug-containing serum of rhubarb-astragalus capsule inhibits the epithelial-mesenchymal transformation of HK-2 by downregulating TGF-β1/p38MAPK/Smad2/3 pathway. J. Ethnopharmacol. 2021, 280, 114414. [Google Scholar] [CrossRef]
- Shifa, M.; Abdissa, D.; Asere, T.G. Chemical Constituents of Rumex abyssinicus Roots and Evaluation of its Antibacterial Activities. J. Turk. Chem. Soc. A 2021, 8, 21–46. [Google Scholar] [CrossRef]
- Promgool, T.; Pancharoen, O.; Deachathai, S. Antibacterial and antioxidative compounds from Cassia alata Linn. J. Sci. Technol. 2014, 36, 459–463. [Google Scholar]
- Li, L.; Song, X.; Yin, Z.; JIa, R.; Li, Z.; Zhou, X.; Zou, Y.; Li, L.; Yin, L.; Yue, G. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microb. Res. 2016, 186–187, 139–145. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Coombes, P.H.; Mulholland, D.A.; Staden, J.S. Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis. Afr. J. Bot. 2006, 72, 295–297. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; Enos, R.T.; VanderVeen, B.N.; Velazquez, K.T.; Kelly, B.; McDonald, S.; Cotham, W.; Chatzistamou, I.; Nagarkatti, M.; Fan, D. Safety of natural anthraquinone emodin: An assessment in mice. BMC Pharmacol. Toxicol. 2021, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Bernard, F.X.; Sable, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob. Agents Chemother. 1997, 41, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Shah, S.; Anderson, J.C.; Hara, Y.; Hamilton-Miller, J.M.T.; Taylor, P.W. Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 2004, 23, 462–467. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Shi, D.H.; Li, H.Q.; Zhang, L.N.; Xu, C.; Zhu, H.L. Polyphenols based on isoflavones as inhibitors of Helicobacter pylori urease. Bioorg. Med. Chem. 2007, 15, 3703–3710. [Google Scholar] [CrossRef]
- Ansari, F.L.; Umbreen, S.; Hussain, L.; Makhmoor, T.; Nawaz, S.A.; Lodhi, M.A. Syntheses and biological activities of chalcone and 1,5-benzothiazepine derivatives: Promising new free-radical scavengers, and esterase, urease, and alpha-glucosidase inhibitors. Chem. Biodivers. 2005, 2, 487–496. [Google Scholar] [CrossRef]
- Garrett, D.O.; McDonald, L.A.C.; Wanderley, A.; Wanderley, C.; Miller, P.; Carr, J. An Outbreak of Neonatal Deaths in Brazil Associated with Contaminated Intravenous Fluids. J. Infect. Dis. 2002, 186, 81–86. [Google Scholar] [CrossRef]
- Choi, O.; Yahiro, K.; Morinaga, N.; Miyazaki, M.; Noda, M. Inhibitory effects of various plant polyphenols on the toxicity of Staphylococcal α-toxin. Microb. Pathog. 2007, 42, 215–224. [Google Scholar] [CrossRef]
- Oh, D.R.; Kim, J.R.; Kim, Y.R. Genistein inhibits Vibrio vulnificus adhesion and cytotoxicity to HeLa cells. Arch. Pharm. Res. 2010, 33, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The polyphenol (−)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Lett. Appl. Microbiol. 2008, 46, 181–185. [Google Scholar] [CrossRef]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Prod. Bioprospect. 2020, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Recio, M.C.; Ginger, R.M.; Manz, S. An Update Review of Saffron and its Active Constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Licón, C.C.; Carmona, M.; Rubio, R.; Molina, A.; Berruga, M.I. Preliminary study of saffron (Crocus sativus L. stigmas) color extraction in a dairy matrix. Dyes Pigments 2012, 92, 1355–1360. [Google Scholar] [CrossRef]
- Hatta, M.; Othman, R.; Mat Ali, Q.A.; Mohd Hassan, N.; Ramya, R.; Wan Sulaiman, W.S.; Mohd Latiff, N.H.; Mohd Kashim, M.I.A. Carotenoids composition, antioxidant and antimicrobial capacities of Crocus sativus L. stigma. Food Res. 2023, 7, 337–343. [Google Scholar] [CrossRef]
- Licón, C.; Carmona, M.; Llorens, S.; Berruga, M.I.; Alonso, G.L. Potential healthy effects of saffron spice (Crocus sativus L. stigmas) consumption. Funct. Plant Sci. Biotech. 2010, 4, 64–73. [Google Scholar]
- Vahidi, H.; Kamalinejad, M.; Sedaghati, N. Antimicrobial Properties of Croccus sativus L. Iran. J. Pharm. Sci. 2002, 1, 33–35. [Google Scholar]
- Jinous, A.; Elahe, D.M.; Arash, M.; Rezvan, M.; Mojdeh, H. In-Vitro Evaluation of Crocus sativus L. Petals and Stamens as Natural Antibacterial Agents Against Food-Borne Bacterial Strains. Iran. J. Pharm. Sci. 2013, 9, 69–82. [Google Scholar]
- Okmen, G.; Kardas, S.; Bayrak, D.; Arslan, A.; Cakar, H. The Antibacterial Activities of Crocus Sativus against Mastitis Pathogens and its Antioxidant Activities. World J. Pharm. Pharm. Sci. 2016, 5, 146–156. [Google Scholar]
- Escribano, J.; Alonso, G.L.; Coca-Prados, M.; Fernandez, J.A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996, 100, 23–30. [Google Scholar] [CrossRef]
- Xuan, B.J. Effects of crocin analogs on ocular blood flow and retinal function. Ocul. Pharmacol. Ther. 1999, 15, 143–152. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and Beyond Color: Comparitive Overview of Functional Qulaity of Tomato and Watermelon Fuits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Preventin. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; El-Masry, T.A.; El-Nagar, M.M.F.; El Zahaby, E.I.; Gaballa, M.M.S.; El-Bouseary, M.M. Investigating the Antibacterial, Antioxidant, and Anti-Inflammatory Properties of a Lycopene Selenium Nano-Formulation: An In Vitro and In Vivo Study. Pharmaceuticals 2024, 17, 1600. [Google Scholar] [CrossRef]
- Divyadharsini, V.; Uma Maheswari, T.S.R. Assessment of Antimicrobial Activity of Lycopene, Vitamin E, and Lycopene-Vitamin E Combination Against Staphylococcus aureus, Streptococcus mutans, Enterococcus faecalis, and Candida albicans: An In Vitro Study. Cureus 2023, 15, 42419. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, I.S.; Lee, D.G. Damage to the cytoplasmic membrane and cell death caused by lycopene in Candida albicans. J. Microbiol. Biotechnol. 2007, 17, 1797–1804. [Google Scholar] [PubMed]
- Al-Oqaili, R.M.; Mohammed, B.B.; Salman, I.M.; Asaad, D.A. Antibacterial Activity of Hibiscus rosa-sinensis Extract and Synergistic Effect with Amoxicillin against some Human Pathogens. Food Sci. Qual. Manag. 2014, 27, 12–17. [Google Scholar]
- Lee, W.; Lee, D.G. Lycopene-induced hydroxyl radical causes oxidative DNA damage in Escherichia coli. J. Microbiol. Biotechnol. 2014, 24, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Cantrill, R. Paprika extract. Chem. Tech. Assess. CTA 2008, 11, 2. [Google Scholar]
- Molina, R.D.I.; Campos-Silva, R.; Díaz, M.A.; Macedo, A.J.; Blázquez, M.A.; Alberto, M.R.; Arena, M.E. Inhibition of bacterial virulence factors of foodborne pathogens by paprika (Capsicum annuum L.) extracts. Food Control 2022, 133, 108568. [Google Scholar] [CrossRef]
- Singh, Y.; Gupta, A.; Kannojia, P. Tagetes erecta (Marigold)—A review on its phytochemical and medicinal properties. Curr. Med. Drug Res. 2020, 4, 201. [Google Scholar] [CrossRef]
- Mekvimol, T.; Poonthona, G.; Chaipunna, C.; Pumipuntu, N. Antimicrobial activity of marigold (Tagetes erecta), mulberry (Morus indica), and red shallot (Allium ascalonicum) extracts against Streptococcus agalactiae. Int. J. One Health 2020, 6, 56–60. [Google Scholar] [CrossRef]
- Pramitha, D.A.I.; Sibarani, J.; Suaniti, M. Sifat fisikokimia hand and body cream dengan pemanfaatan ekstrak etanol bunga gemitir (Tagetes erecta L.) dan bunga pacar air merah (Impatiens balsamina L.) dari limbah canang. Indones E-J. Appl. Chem. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Zhang, H.; Chen, X.; Liang, F.; Qin, H.; Zhang, Y.; Cong, R.; Xin, H.; Zhang, Z. Carotenoid metabolite and transcriptome dynamics underlying flower color in marigold (Tagetes erecta L.). Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef]
- Philip, T.; Berry, J.W. A Process for the Purification of Lutein-Fatty Acid Esters from Marigold Petals. J. Food Sci. 1976, 41, 163–164. [Google Scholar] [CrossRef]
- Dixit, P.; Tripathi, S.; Verma, N.K. A Brief Study on Marigold (Tagetes Species): A review. Int. Res. J. Pharm. 2013, 4, 43–48. [Google Scholar]
- Vasudevan, P.; Kashyap, S.; Sharma, S. Tagetes: A multipurpose plant. Bioresour. Technol. 1997, 62, 29–35. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Saha, P.; Jain, R.; Malhotra, S.; Saleh, M.A. Antibacterial Activity of Leaf Extract of Mexican Marigold against Different Gram Positive and Gram Negative Bacterial Strains. J. Pharm. Res. 2012, 5, 4201–4203. [Google Scholar]
- Rhama, S.; Madhavan, S. Antibacterial Activity of the Flavonoid, patulitrin isolated from the flowers of Tagetes erecta L. Int. J. PharmTech Res. 2011, 3, 1407–1409. [Google Scholar]
- Jafari, B.; Ahmadizadeh, C. Antibacterial Effects of Marigold (Tagetes patula) Extracts on Postharvest Diseases. Electron. J. Biol. 2017, 13, 348–352. [Google Scholar]
- Mandias, I.I.; Yamlean, P.; Abdullah, S.S. Formulation and Test of Antibacterial Activity Mask Peel-Off Gel Fraction of Cocoa Fruit Skin (Theobroma cacao L.) Against Staphylococcus aureus as an anti-acne. Pharmacon 2022, 11, 1813–1824. [Google Scholar]
- Verma, V.; Joshi, C.P.; Agarwal, A.; Soni, S.; Kataria, U. A Review on Pharmacological Aspects of Pyrimidine Derivatives. J. Drug Deliv. Ther. 2020, 10, 358–361. [Google Scholar] [CrossRef]
- Cahyaningrum, P.L.; Yuliari, S.A.M.; Mediastari, A.P.A. Effectiveness of Marigold Flower (Tagetes erecta L.) Soap Preparation Against Staphylococcus aureus and Escherichia coli. J. Muhammadiyah Med. Lab. Technol. 2020, 3, 11–24. [Google Scholar] [CrossRef]
- Patrick, R.S.; Marijo, C.; Sandra, R. Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 2011, 38, 81–88. [Google Scholar] [CrossRef]
- Padalia, H.; Chand, S. Antimicrobial Efficacy of Different Solvent Extracts of Tagetes erecta L. Flower, Alone and in Combination with Antibiotics. Appl. Microbiol. 2015, 1, 1000106. [Google Scholar] [CrossRef]
- Edy, H.J.; Parwanto, M.L.E. Pemanfaatan tanaman Tagetes erecta Linn. dalam kesehatan. J. Biomed. Dan Kesehat. 2019, 2, 77–80. [Google Scholar] [CrossRef]
- Kresnapati, I.N.B.A.; Khaerunnisa, S.; Safitri, I. Ethanol Extract of Marigold Flower (Tagetes erecta L.) Decreases The Total Cholesterol, Low Density Lypoprotein (LDL), Malondialdehyde (MDA), and Apoliprotein B (APOB) on Hyperlipidemia Rat Models. Folia Med. Indones. 2021, 57, 245–249. [Google Scholar] [CrossRef]
- Selvam, S.I.; Joicesky, S.M.B.; Dashli, A.A.; Vinothini, A.; Premkumar, K. Assessment of anti bacterial, anti inflammation and wound healing activity in Wistar albino rats using green silver nanoparticles synthesized from Tagetes erecta leaves. J. Appl. Nat. Sci. 2021, 13, 343–351. [Google Scholar] [CrossRef]
- Collins, P. Natural Colours—A Question of Stability. Food Ingred. Eur. Conf. Proc. 1992, 60. [Google Scholar]
- Islam, S.; Rather, L.J.; Mohammad, F. Phytochemistry, biological activities and potential of annatto in natural colorant production for industrial applications—A review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef]
- Kang, E.J.; Campbell, R.E.; Bastian, E.; Drake, M.A. Invited review: Annatto usage and bleaching in dairy foods. J. Dairy Sci. 2010, 93, 3891–3901. [Google Scholar] [CrossRef]
- Venugopalan, A.; Giridhar, P.; Ravishankar, G.A. Food, Ethanobotancal and Diversified Applications of Bixa orellana L.: A Scope for its Improvemnet Through Biotechnological Mediation. Ind. J. Fundament. Appl. Life Sci. 2011, 1, 9–31. [Google Scholar]
- Scotter, M. The chemistry and analysis of annatto food colouring: A review. Food Addit. Contam. 2009, 26, 1123–1145. [Google Scholar] [CrossRef]
- Alonso, J. Tratado de Fitofarmacosy Nutraceuticos; Corpus: Rosario, Argentina, 2004; ISBN 9789871860630. [Google Scholar]
- Mercadante, A.Z.; Steck, A.; Pfander, H. Isolation and structure elucidation of minor carotenoids from annatto (Bixa orellana L.) seeds. Phytochemistry 1997, 46, 1379–1383. [Google Scholar] [CrossRef]
- Rao, P.G.P.; Jyothirmayi, T.; Balaswamy, K.; Satyanarayana, A.; Rao, D.G. Effect of processing conditions on the stability of annatto (Bixa orellana L.) dye incorporated into some foods. LWT-Food Sci. Technol. 2005, 38, 779–784. [Google Scholar] [CrossRef]
- Tirimanna, A.S.L. Study of the Carotenoid Pigments of Bixa orellana L. Seeds by, Thin layer Chromatography. Mikrochim. Acta 1981, 1–2, 11. [Google Scholar] [CrossRef]
- Fleischera, T.C.; Ameadea, E.P.K.; Mensaha, M.L.K.; Sawerb, I.K. Antimicrobial activity of the leaves and seeds of Bixa orellana. Fitoterapia 2003, 74, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.T.; Dinesh, M.G.; Satyan, R.S.; Chandrasekaran, B.; Rose, C. Leaf and Seed extracts of Bixa orellana L. exert anti-microbial activity against bacterial pathogens. J. Appl. Pharm. Sci. 2011, 1, 116–120. [Google Scholar]
- Viuda-martos, M.; Ciro-go’mez, G.L.; Ruiz-navajas, Y.; Zapatamontoya, J.E.; Sendra, E.; Pérez-Álvarez, J.A. In vitro Antioxidant and Antibacterial Activities of Extracts from Annatto (Bixa orellana L.) Leaves and Seeds. J. Food Saf. 2012, 32, 399–406. [Google Scholar] [CrossRef]
- Braga, F.G.; Bouzada, M.L.M.; Fabri, R.L.; Matos, M.; Moreira, F.O.; Scio, E. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007, 111, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Castello, M.; Phatak, A.; Chandra, N.; Sharon, M. Antimicrobial activity of crude extracts from plant parts and corresponding calli of Bixa orellana L. Ind. J. Exp. Bio. 2002, 40, 1378–1381. [Google Scholar]
- Solkar, L.V.; Kakkar, K.K.; Chakre, O.J. Second Supplement to Glossary of Indian Medicinal Plants with Active Principles, Part-1 (A-K); CSIR, Orient Longmann: New Delhi, India, 1992. [Google Scholar]
- Galindo-Cuspinera, V.; Lubran, M.B.; Rankin, S.A. Comparison of volatile compounds in water- and oil-soluble annatto (Bixa orellana L.) extracts. J. Agric. Food Chem. 2002, 50, 2010–2015. [Google Scholar] [CrossRef]
- Stohs, S.J. Safety and efficacy of shilajit (mumie, moomiyo). Phytother. Res. 2014, 28, 956–960. [Google Scholar] [CrossRef]
- Naganuma, M. Treatment with indigo naturalis for inflammatory bowel disease and other immune diseases. Immunol. Med. 2019, 42, 16–21. [Google Scholar] [CrossRef]
- Yu, H.; Li, T.N.; Ran, Q. Strobilanthes cusia (Nees) Kuntze, a multifunctional traditional Chinese medicinal plant, and its herbal medicines: A comprehensive review. J. Ethnopharmacol. 2020, 265, 113325. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.R.; Li, A.; Leu, Y.L. An in vitro study of the antimicrobial effects of indigo naturalis prepared from Strobilanthes formosanus Moore. Molecules 2013, 18, 14381–14396. [Google Scholar] [CrossRef]
- Andreazza, N.L.; de Lourenço, C.C.; Stefanello, M. Photodynamic antimicrobial effects of bis-indole alkaloid indigo from Indigofera truxillensis Kunth (Leguminosae). Lasers Med. Sci. 2015, 30, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.; Ramasamy, M.; Savarimuthu, I.; Paulraj, M.G. Indirubin potentiates ciprofloxacin activity in the NorA efflux pump of Staphylococcus aureus. Scand. J. Infect. Dis. 2010, 42, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Brawley, D.N.; Sauer, D.B.; Li, J.; Zheng, X.; Koide, A.; Jedhe, G.S.; Suwatthee, T.; Song, J.; Liu, Z.; Arora, P.S.; et al. Structural basis for inhibition of the drug efflux pump NorA from Staphylococcus aureus. Nat. Chem. Biol. 2022, 18, 706–712. [Google Scholar] [CrossRef]
- Bhat, S.A.; Zargar, M.I.; Wani, S.D.; Mohiuddin, I.; Masoodi, M.H.; Shakeel, F.; Ali, M.; Mehdi, S. In-vitro evaluation of Indigofera heterantha extracts for antibacterial, antifungal and anthelmintic activities. J. Pharm. Health Care Sci. 2024, 10, 7. [Google Scholar] [CrossRef]
- Leite, S.P.; Vieira, J.R.C.; de Medeiros, P.L.; Leite, R.M.P.; Lima, V.L.M.; Xavier, H.S.; Lima, E.O. Characterization of Morinda citrifolia L. (noni) fruit. Adv. Access Publ. CAM 2006, 3, 261–265. [Google Scholar]
- Dahot, M.U. Antibacterial and antifungal activity of small protein of Indigofera oblongifolia leaves. J. Ethnopharmacol. 1999, 64, 277–282. [Google Scholar] [CrossRef]
- Ali, A.N.; Attif, O.A.; Mohammed, M.I. Herbal medicine in two Yemeni provinces. Yemen Med. J. 1999, 3, 13–20. [Google Scholar]
- Esimone, C.O.; Adikwu, M.U.; Muko, K.N. Antimicrobial properties of Indigofera dendroides leaves. Fitoterapia 1999, 70, 517–520. [Google Scholar] [CrossRef]
- El–Khatib, E.M.; Ali, N.F.; El–Mohamedy, R.S.R. Influence of Neen Oil Pretreatment on the Dyeing and Antimicrobial Properties of Wool and Silk Fibers with Some Natural Dyes. Arab. J. Chem. 2020, 13, 1094–1104. [Google Scholar] [CrossRef]
- Reshma, A.; Priyadarisini, V.; Amutha, K. Sustainable Antimicrobial finishing of fabrics using Natural Bioactive Agents—A Review. Int. J. Life Sci. Pharm. Res. 2018, 8, 10–20. [Google Scholar] [CrossRef]
- Rani, J.; Singh, S. Antimicrobial Properties of Herbal Dyes of Indian Medicinal Plants. Tex. Leather Rev. 2022, 5, 199–222. [Google Scholar] [CrossRef]
- Reddy, Y.R.R.; Kumari, C.K.; Lokanatha, O.; Mamatha, S.; Reddy, C.D. Antimicrobial activity of Azadirachta indica (neem) leaf, bark and seed extracts. Int. J. Res. Phytochem. Pharmacol. 2013, 3, 1–4. [Google Scholar]
- Pennington, T.D. Sapotaceae. In Flora Neotropica; New York Botanical Garden Press: New York, NY, USA, 1990. [Google Scholar]
- Rojanapo, W.; Suwanno, S.; Somaree, R.; Glinsukon, T.; Thebtaranonth, Y. Mutagenic and AntibacterialActivity Testing of Nimbolide and Nimbic Acid. J. Sci. Soc. Thailand 1985, 11, 177–181. [Google Scholar] [CrossRef]
- Khalid, S.A.; Duddect, H.; Gonzalez-Sierra, M.J. Isolation and characterization of an antimalarial agent of the neem tree Azadirachta indica. J. Nat. Prod. 1989, 52, 922–927. [Google Scholar] [CrossRef]
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: A review. Curr. Med. Chem. Anticancer Agents 2005, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, D.; Kumar, D.; Singh, G.; Swami, G.; Rathore, M.S. Formulation, Development, and Evaluation of Herbal Effervescent Mouthwash Tablet Containing Azadirachta Indica (Neem) and Curcumin for the Maintenance of Oral Hygiene. Recent Pat. Drug Deliv. Formul. 2020, 14, 145–161. [Google Scholar] [CrossRef]
- Mistry, K.S.; Sanghvi, Z.; Parmar, G.; Shah, S.; Pushpalatha, K. Antibacterial efficacy of Azadirachta indica, Mimusops elengi and 2% CHX on multispecies dentinal biofilm. J. Conserv. Dent. 2015, 18, 461–466. [Google Scholar] [CrossRef]
- Subramaniam, S.K.; Siswomihardjo, W.; Sunarintyas, S. The effect of different concentrations of Neem (Azadiractha indica) leaves extract on the inhibition of Streptococcus mutans (In vitro). Dent. J. 2005, 38, 176–179. [Google Scholar] [CrossRef]
- Christy, S.; Nivedhitha, M.S. Antimicrobial Efficacy of Azadirachta indica against Streptococcus mutans—An In vitro Study. Asian J. Pharm. Technol. 2019, 9, 149–153. [Google Scholar] [CrossRef]
- Witebsky, F.G.; Maclowry, J.D.; French, S.S. Broth dilution minimum inhibitory concentrations: Rationale for use of selected antimicrobial concentrations. J. Clin Microbiol. 1979, 9, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, J.; Makwana, P.; Suresh Kumar, R.S.; Sundaramoorthy, R.; Mukherjee, A.; Chandrasekaran, N. Antibacterial activity of neem nanoemulsion and its toxicity assessment on human lymphocytes in vitro. Int. J. Nanomed. 2015, 10, 77–86. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, M.; Kim, J.; Balasundaram, C.; Jawahar, S.; Heo, M. Identification and Antimicrobial Activity of Combined Extract from Azidirachta Indica and Ocimum sanctum. Isr. J. Aquac. 2010, 62, 85–95. [Google Scholar]
- Tutak, M.; Acar, G.; Akman, O. Natural Dyeing Properties of Wool fabrics by Pomegranate (Punica granatum) Peel. Tekst. Ve Konfeksiyon 2014, 24, 81–85. [Google Scholar]
- Seeram, N.P.; Zhang, Y.; Reed, J.D.; Krueger, C.G.; Vaya, J. Pomegranate phytochemicals. In Pomegranates: Ancient Roots to Modern Medicine; CRC Press: Boca Raton, FL, USA; Taylor and Francis Book: Abingdon, UK, 2006; pp. 3–29. [Google Scholar]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef]
- Farmahan, H.L. Pomegranate. In Recent Trends in Horticulture in the Himalayas; Jindal, K.K., Sharma, R.C., Eds.; Indus: New Dehli, India, 2004; p. 139. [Google Scholar]
- Jayaprakasha, G.K.; Negi, P.S.; Jena, B.S. Antimicrobial activities of pomegranate. In Pomegranates: Ancient Roots to Modern Medicine; Seeram, N.P., Schulman, R.N., Heber, D., Eds.; CRC Press: New York, NY, USA, 2006; p. 168. [Google Scholar]
- Khare, C.P. Indian Herbal Remedies, Rational Western Therapy, Ayurvedic and Other Traditional Usage; Springer: Berlin/Heidelberg, Germany, 2004; pp. 390–392. [Google Scholar]
- Nair, R.; Chanda, S. Antimicrobial activity of Terminalia catappa, Manilkara zapota and Piper betel leaf extract. Indian J. Pharm. Sci. 2008, 70, 390–393. [Google Scholar]
- Shahid, M.; ul-Islam, S.; Rather, L.J.; Manzoor, N.; Mohammad, F. Simultaneous shade development, antibacterial, and antifungal functionalization of wool using Punica granatum L. Peel extract as a source of textile dye. J. Nat. Fibers 2019, 16, 555–566. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hwang, E.K.; Baek, Y.M.; Lee, M.S.; Lee, D.J.; Jung, Y.J.; Kim, H. Deodorizing and Antibacterial Performance of Cotton, Silk and Wool Fabrics Dyed with Punica granatum L. Extracts. Fibers Polym. 2013, 14, 1445–1453. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.; Lortheeranuwat, A.; Jeeju, W.; Sririrak, T.; Phongpaichit, S.; Supawita, T. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157:H7. J. Ethnopharm. 2004, 94, 49–54. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.P.; Limsuwan, S. Medicinal plant extracts as anti-Escherichia coli O157:H7 agents and their effects on bacterial cell aggregation. J. Food Prot. 2006, 69, 2336–2341. [Google Scholar] [CrossRef]
- P’erez, C.; Anesini, C. In vitro antibacterial activity of Argentine folk medicinal plants against Salmonella typhi. J. Ethnopharm. 1994, 44, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Khullar, N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytother. Res. 2004, 18, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Mathabe, M.C.; Nikolova, R.V.; Lall, N.; Nyazema, N.Z. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. J. Ethnopharm. 2006, 105, 286–293. [Google Scholar] [CrossRef]
- Guevara, J.M.; Chumpitaz, J.; Valencia, E. The in vitro action of plants on Vibrio cholerae. Rev. Gastroenterol. Peru 1994, 14, 27–31. [Google Scholar]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Alan’ıs, A.D.; Calzada, F.; Cervantes, J.A.; Torres, J.; Ceballos, G.M. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J. Ethnopharm. 2005, 100, 153–157. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef]
- Lucas, D.L.; Were, L.M. Antimicrobial activity of chitosan against Campylobacter spp. and other microorganisms and its mechanism of action. J. Food Prot. 2009, 72, 2508–2516. [Google Scholar] [CrossRef]
- Tayel, A.; El-Tras, W.; Moussa, S.; El-Sabbagh, S. Surface decontamination and quality enhancement in meat steaks using plant extracts as natural biopreservatives. Foodborne Pathog. Dis. 2012, 9, 755–761. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Naveena, B.M.; Muthukumar, M.; Girish, P.S.; Kondaiah, N. Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4 °C). Meat Sci. 2011, 88, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Karabiyikli, S.; Kisla, D. Inhibitory effect of sour pomegranate sauces on some green vegetables and kisir. Int. J. Food Microbiol. 2012, 155, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef]
- Jami, E.; Shabtay, A.; Nikbachat, M. Effects of adding a concentrated pomegranate-residue extract to the ration of lactating cows on in vivo digestibility and profile of rumen bacterial population. J. Dairy Sci. 2012, 95, 5996–6005. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Gokhale, A.V.; Bodake, U.M.; Pathade, G.R. Cotton Dyeing with Natural Dye Extracted from Pomegranate (Punica granatum) Peel. Univ. J. Environ. Res. Technol. 2011, 1, 135–139. [Google Scholar]
- Luan, F.; Xu, X.; Liu, H.; Cordeiro, M.N. Review of quantitative structure-activity/property relationship studies of dyes: Recent advances and perspectives. Color. Technol. 2013, 129, 173–186. [Google Scholar] [CrossRef]
- Swamy, V.N.; Gowda, K.N.; Sudhakar, R. Extraction and dyeing conditions of natural dye from flowers of Plumeria rubra L. on textiles and fastness properties. Indian J. Trad. Knowl. 2016, 15, 278–284. [Google Scholar]
- Wang, H.; Tang, Z.; Zhou, W. A method for dyeing cotton fabric with anthocyanin dyes extracted from mulberry (Morus rubra) fruits. Color. Technol. 2016, 132, 222–231. [Google Scholar] [CrossRef]
- Shahid, M.; Ahmad, A.; Yusuf, M.; Khan, M.I.; Khan, S.A.; Manzoor, N. Dyeing, fastness and antimicrobial properties of woolen yarns dyed with gallnut (Quercus infectoria Oliv.) extract. Dyes Pigments 2012, 95, 53–61. [Google Scholar] [CrossRef]
- Sarkar, A.K. An evaluation of UV protection imparted by cotton fabrics dyed with natural colorants. BMC Dermatol. 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Mohammad, F.; Shabbir, M. Natural Colorants: Historical, Processing and SustainableProspects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).