Highlights
What are the main findings?
- The significance of molecular re-assembled fibres in tissue engineering bio-composite development relies on different advantages such as the possibility to develop through a cost-effective production route, efficient biodegradability, improved liquid absorbance property, high chemical stability, etc.
- However, significant challenges persist in the successful application of regenerated fibres, which include difficulty in processing, production scale-up-related challenges, etc.
What is the implication of the main finding?
- The present study highlights the important applications and limitations associated with the successful utilisation of molecularly reassembled regenerated fibres.
- Current work also provides important recommendations for future research, considering the expanding scope for developing innovative biomaterials in the field of tissue engineering.
Abstract
Due to their interesting physicochemical and bioactive properties, regenerated fibres (including cellulose and collagen regenerated fibres) have been considered attractive biomaterials for biomedical applications. These regenerated fibres have an altered molecular arrangement compared to the native fibres and exhibit unique properties. Despite their distinctive structural characteristics, a meagre amount of research explores their potential for the development of tissue-engineering bio-composites. This work focuses on exploring the promise of cellulose and collagen-based regenerated fibres in tissue-regeneration bio-composite development. Initially, the work investigates the similarities and dissimilarities between the collagen and cellulose structures, which are linked to their specific properties, such as crystallinity, chemical characteristics, and mechanical properties. It then delves deeper into their molecular structural reassembly and various aspects of the already reported bio-composites developed using them. Finally, their promise in the development of tissue-engineering bio-composites is explored through a meticulous comparative analysis of their advantages and challenges. It was found that efficient biodegradability is one of the key advantages of regenerated fibres, whereas difficulty in processing presents a significant disadvantage. Despite these facts, regenerated fibres can incorporate enhanced and desired properties into the bio-composite matrix, which could lead to tissue-specific bio-regenerative applications.
1. Introduction
Tissue engineering and regenerative treatment strategies involve significant development of bio-composites with diverse biological properties [1]. The raw materials of these bio-composite materials often include naturally derived biomaterials like collagen and cellulose-based fibres. One of the important advantages of these fibre-based biomaterials is their unique architecture and abundant availability in nature [2]. Due to their renewability, environmental friendliness, and cost-effectiveness, collagen and cellulose-based fibres have long been considered promising biomaterials for the development of tissue engineering composites [3].
Despite several advantages, these fibre-based biomaterials face significant drawbacks such as uncontrolled stability and degradation, and limited bioactivity [4]. Natural fibres possess significant ‘self-assembly’ properties [5], and the aforementioned limitations could be efficiently addressed through the development of regenerated fibres through the utilisation of their ‘molecular self-assembly’ property. This molecular reassembly has been generated within certain solvent systems (like alkaline solutions, ionic liquids) during the development of regenerated fibres.
It has been reported that ‘regenerated fibres’ with improved physicomechanical and chemical properties play a significant role in the development of high-quality tissue regeneration biomaterials [6,7]. However, the utilisation of the regeneration fibres is limited in the context of tissue engineering biomaterial development.
This work focuses on the exploration of the promise of cellulose and collagen-based regenerated fibres in tissue regeneration and bio-composite development. The novelty of the current work involves (i) addressing different challenges and limitations of the successful utilisation of these regenerated fibres; (ii) possible recommendations through addressing those limitations for improvement of their existing property for effective utilisation. This knowledge will not only facilitate a clearer understanding of the significance and potential role of molecularly reassembled regenerated cellulose and collagen fibres but also provide potential solutions for their sustainable and efficient use in improving the properties of tissue engineering bio-composites. Previous research has shown that natural fibres can act as fibrous fillers, which improve the overall mechanical properties of a biocomposite [8]. The current review describes the significance of molecular reassembly in fibre structure to introduce important properties (like viscoelasticity) in the tissue engineering biocomposites, which explains the relevance of the present work.
This work begins with the identification of the similarities and dissimilarities of the collagen and cellulose structures, which are linked to their specific properties such as crystallinity, chemical characteristics, and mechanical properties. It then delves deeper into their molecular structural reassembly and examines various aspects of previously reported bio-composites developed using these materials. Finally, their promise and potential in the development of tissue-engineering bio-composites are explored through a meticulous analysis of their advantages and challenges. This study not only highlights the significant applications and limitations associated with the successful utilisation of molecularly reassembled regenerated fibres but also provides important recommendations for future research, considering the expanding scope for developing innovative biomaterials in the field of tissue engineering.
2. Methodology
This work utilised a comprehensive literature survey from different databases such as Web of Science core collections, ScienceDirect, and Google Scholar through a careful search using keywords like ‘Molecular rearranged fibres’, ‘regenerated fibres in tissue engineering application’, ‘regenerated fibres for tissue engineering bio-composite’, ‘regenerated cellulose and collagen fibres’, within the custom range between 1990 and 2025. The current systematic review follows the four important steps of PRISMA 2020 protocols, which involve database search, title/abstract screening, removing all duplicates, reports searched for retrieval, reports not retrieved, full text screening, reports excluded, and included studies. Finally, their promise in the development of tissue-engineering bio-composites is explored through a meticulous analysis of their advantages and challenges.
Historically, the first man-made regenerated fibre (i.e., regenerated cellulose fibres) was used in the textile industry in the 1850s [9]. The concept of regenerated fibres (i.e., regenerated protein fibres) was also elaborated in Rules and Regulations Under the Textile Fibre Products Identification Act (1959) by the Federal Trade Commission USA as ‘a manufactured fibre in which the fibre-forming substance is composed of any regenerated naturally occurring proteins’ [10]. Over time, regenerated fibres have been gradually used in various biomedical applications, including wound healing and tissue engineering. This work carefully surveyed the utilisation of regenerated fibres in the development of different tissue engineering bio-composites by analysing the importance of the fibre’s molecular rearrangement properties. In addition, the present work provides an in-depth analysis of the existing challenges and promises of the regenerated fibres in tissue engineering bio-composite development.
3. Properties of Native Cellulose and Collagen Fibres
Before delving into the significance of molecular self-assembly and regenerated fibres in the field of tissue engineering, it is necessary to understand the structural attributes and properties of the native fibres. Hence, this section provides an overview of the different types of native fibres, which are discussed below:
3.1. Supramolecular Arrangement
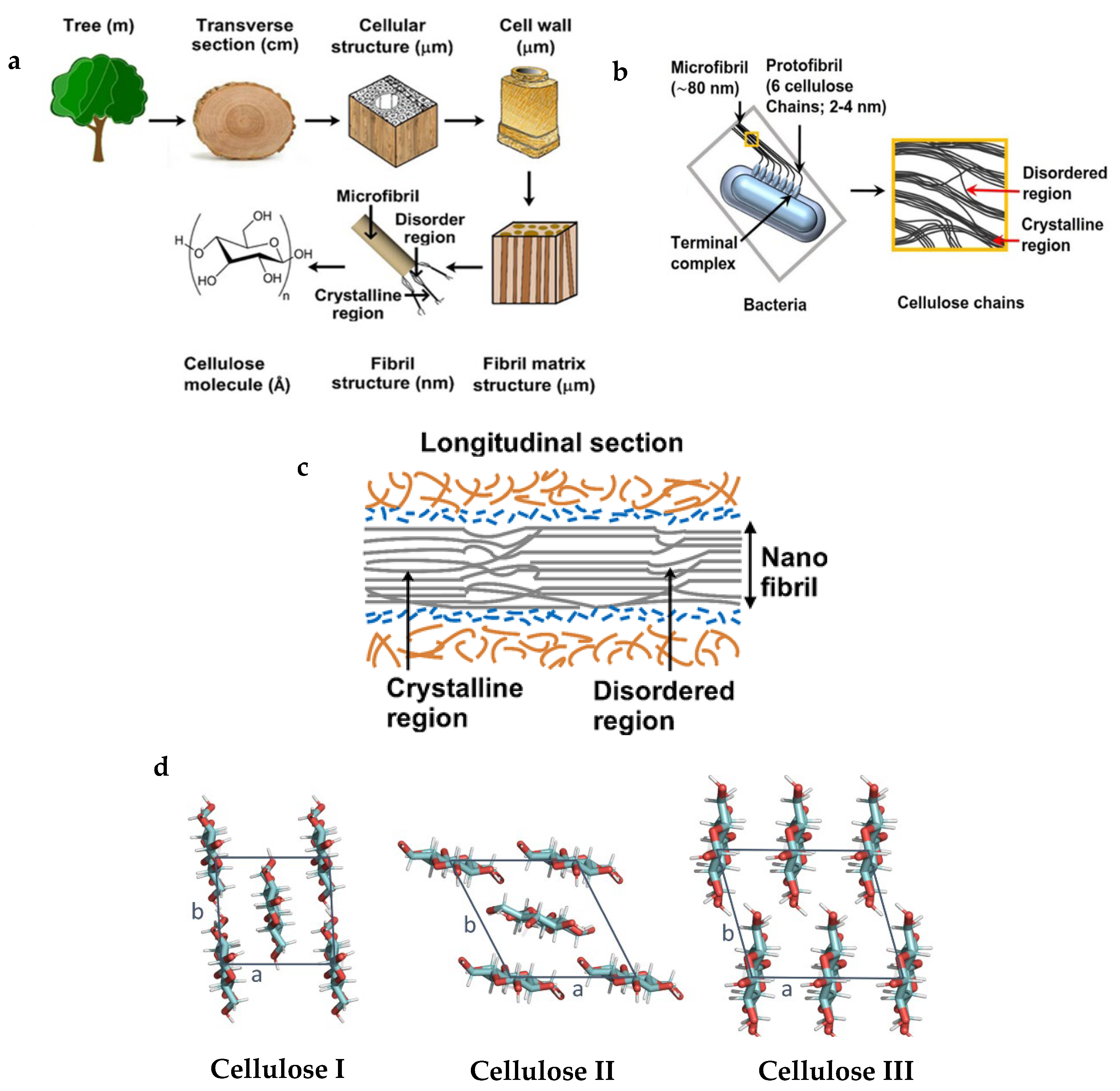
A unique supramolecular arrangement is one of the important properties of native fibres like collagen and cellulose. Collagen’s structural organisation starts at the primary sequence level, where its α-chains are composed of approximately 1000 amino acids in a repeating glycine-X-Y pattern (Figure 1), in which X and Y are frequently proline or hydroxyproline. This primary structure is highly conserved across species and accounts for the regularity and compactness of the molecule at every level of assembly. Glycine is the only residue small enough to fit into the interface of the three chains, while the cyclic nature of proline and hydroxyproline restricts chain flexing, promoting a straight, rod-like structure [11,12]. Three α-chains intertwine to form the triple helix, commonly referred to as tropocollagen. This superstructure is approximately 300 nm long and 1.5 nm in diameter, creating a rigid and elongated molecular rod [13]. The arrangement results from every third residue being glycine, enabling tight packing in the core, while proline and hydroxyproline face outward, conferring resistance to tensile stress and unwinding. Physically, the triple helix is stabilised by numerous hydrogen bonds, resulting in high axial stiffness, and further protected from enzymatic degradation and adverse environmental conditions due to the dense wrapping of the three chains [11]. Collagen fibrils are formed through the self-assembly of tropocollagen molecules into a highly organised quarter-staggered array, in which each ~300 nm long tropocollagen molecule is offset from its neighbours by ~67 nm. The fibril is a quasi-crystalline structure in which microfibrils stack with precise spatial registry, leading to anisotropic mechanical properties: the fibril is much stiffer and stronger along its length than across its diameter [14]. Within the fibril, lateral associations are stabilised and the periodic pattern extended, creating distinct domains: gap regions, where mineralisation often initiates in bone, and overlap regions, which offer greater density and resistance to deformation. Fibrils thus provide high-tensile endurance and enable the transmission of load between the nanoscale building blocks and the macroscale tissue [15]. Finally, collagen fibrils are grouped into fibre bundles, reaching dimensions ranging from sub-micrometres to several micrometres in diameter, forming the recognisable wavy bundles in tendons, skin, and other connective tissues [14].
The structure of cellulose is complex due to its hierarchical arrangement. Structurally, cellulose consists of anhydroglucose units (AGU) united through β-1,4 glycosidic linkages. These AGUs ultimately develop a cellulose microfibrillar structure. The glucose monomer of these AGU has a flipped conformation to its immediate adjacent glucose (of another AGU) and forms networks through intra- and intermolecular hydrogen bonds resulting in the crystalline zones in the microfibrils [16]. Cellulose generally has a few different crystalline polymorphs, like I, II, and III. The native structure of plant cellulose is predominantly Cellulose I polymorph, which is composed of different crystal units like Cellulose Iα and Cellulose Iβ [17,18,19]. The primary source of cellulose is plants. The plant’s primary cell wall contains cellulose fibres embedded within a matrix of hemicellulose and pectin. The secondary cell wall layer also comprises hemicellulose and lignin. The cellulose fibrils are randomly situated in the plane of the primary cell wall layer, whereas the cellulose fibrils are wrapped in ‘helical’ orientation with varying patterns in different secondary layers, i.e., S1, S2, and S3. The S2 layer is responsible for the high thickness of the entire cell wall in plants like the Norway Spruce (Picea abies). This S2 layer comprises the highest fraction of cellulose microfibrils, whereas in the S1 layer, cellulose microfibrils are generally wrapped circumferentially around the cell wall. The cellulose microfibrils in the S1 layer are wrapped in a helical conformation, whereas in the S2 layer, the microfibrils are wrapped with a 10–30° vertical angle. The microfibrillar arrangement of the S3 layer has also been found to be helical [20]. On the other hand, cellulose of certain bacteria, like the genus Gluconobacter, produces ribbon-like cellulose. These bacteria first produce protofibrils of glucose chains, which are then secreted by the bacterial cell wall and produce microfibrils, which then form these ribbon-like cellulose fibres [21,22].
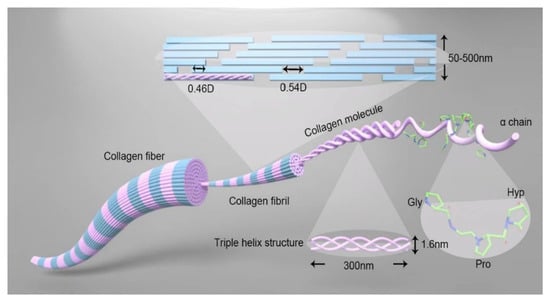
Figure 1.
Diagrammatic representation of collagen ultrastructure. [Represented from Collagen and Leather 5, Chen et al. [17] Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials—a review, Copyright (2023), SPRINGER NATURE, CC BY licence].
Figure 1.
Diagrammatic representation of collagen ultrastructure. [Represented from Collagen and Leather 5, Chen et al. [17] Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials—a review, Copyright (2023), SPRINGER NATURE, CC BY licence].
3.2. Chemical Attributes
Water plays a multifaceted and essential role in the chemistry and biology of collagen and cellulose. Both biomolecules are highly hydrated, with water molecules forming intricate hydrogen-bond networks. These networks contribute directly to stability, mediate intermolecular spacing, and modulate the kinetics of fibril self-assembly. Hydrogen bonds between collagen/cellulose and water molecules significantly outnumber other physicochemical interactions. Changes in hydration or solvent isotope composition can alter nucleation rates, fibril morphology, and the mechanical properties of collagen matrices [11,23].
Table 1 describes the differential chemical nature of native fibres, like collagen and cellulose, which is important to understand their inherent properties. This is important for future research, as this information will, in turn, help the researchers to modify and influence the molecular rearrangement for the development of regenerated fibres.

Table 1.
Chemical comparison of collagen and cellulose.
3.3. Structural and Physical Properties of Native Fibres
Both collagen and cellulose form microfibrillar structures, but they do so through fundamentally different mechanisms, reflective of their distinct chemical compositions—collagen being a protein and cellulose a polysaccharide. Despite these differences, microfibrils serve a similar biological function in both systems, providing structural integrity and mechanical strength to tissues.
Collagen exhibits a partially crystalline hierarchical structure. X-ray diffraction patterns of collagen, especially from tendons and other connective tissues, show prominent reflections corresponding to a periodic axial repeat of approximately 67 nanometres, termed the D-band periodicity [28]. The D-band is a hallmark of the collagen fibril crystallinity and defines the repeating structural motif at the nanoscale. The type of collagen affects the precise characteristics of the D-band periodicity. For instance, type I collagen fibrils, typical in skin, tendon, and bone, exhibit a consistent axial spacing, whereas other collagen types, such as type II found in cartilage and type III in reticular fibres, may show variations in periodicity or fibril morphology due to differences in polypeptide chain composition and assembly kinetics [11]. These variations influence tidiness and packing order, thus affecting crystallinity. The fibrils are semi-crystalline, possessing ordered domains interspersed with less ordered or hydrated regions, contributing to mechanical flexibility while maintaining tensile strength. Studies reveal that collagen fibril crystallinity can be modulated by pH, ionic strength, and enzymatic treatments. Lowering pH disrupts intermolecular interactions, decreasing the crystallinity and increasing the lateral swelling of fibrils [28].
Cellulose fibrils are not ideally crystalline; however, they contain crystallites or crystalline zones which are separated by amorphous regions (Figure 2a,b). According to Ioelovich et al., 2016 [29], cellulose comprises ‘mesomorphous-crystalline nanofibril with the paracrystalline monomolecular surface layer’. These crystalline regions have developed through a process where monomolecular glucan chain sheets were formed through van der Waals forces, then these sheets assemble into small crystals through H-bonding, and thereby these small crystals congregate to form crystalline microfibrils [30] (Figure 2a,b). The structure of the cellulose chain involving a twofold helix shows a periodicity of 10.36 Å due to the 4C1 structural confirmation and the β(1→4) glycosidic bond [31]. The macromolecular structure of cellulose contains free hydroxyl groups that are responsible for intermolecular and intramolecular hydrogen bonds, which develop the ordered crystalline structure (Figure 2c). The density of the crystalline phase of cellulose is 1.59 g·cm−3.
Cellulose generally has different crystalline polymorphs, like I, II, and III. Regenerated fibres like Cellulose II and III consist of a regenerated crystal form of cellulose [23] (Figure 2d). In the native and synthetic fibre structure of cellulose, the chains are found arranged in elementary cellulose fibrils with an approximate length between 0.1 and 0.2 μm, with a lateral dimension of 0.0015 μm to 0.0035 μm. These cellulose fibrils are then further convolved together into cellulose microfibrils with around 0.1 μm width and 0.1–1.0 μm length [24,32,33,34]. These crystalline regions are responsible for many of cellulose’s characteristic properties, including its mechanical strength, resistance to enzymatic degradation, and low solubility in water and organic solvents. The crystalline structure of cellulose is well established and can be observed using techniques such as X-ray diffraction, which yields sharp diffraction peaks indicative of a repeating lattice.
Both cellulose and collagen exhibit significant mechanical properties in regard to their application in biomedical science. The structure of cellulose fibres involves crystalline and amorphous regions, which have a significant impact on their high mechanical properties. The elastic modulus of cellulose fibres has been found in the range between 20 and 30 GPa; however, depending on the type of cellulose, this value can be increased to 138 GPa [35]. This molecular arrangement resists deformation and allows cellulose materials to bear mechanical loads. On the other hand, in the molecular and fibrillar levels, isolated collagen molecules show a nonlinear viscoelastic behaviour with a relatively high Young’s modulus ranging approximately from 6 to 16 GPa under strains up to 20%, reflecting their intrinsic stiffness [36]. When molecules assemble into fibrils, the effective stiffness decreases significantly to around 0.9 GPa to 140 MPa for isolated fibrils, depending on test conditions and strain levels, indicating that fibrillar assembly modulates mechanical properties [37,38]. Moreover, collagen fibrils exhibit intrinsic viscoelasticity that can be modelled by mechanistic frameworks such as the Maxwell–Weichert model, which incorporates multiple relaxation times, reflecting complex intra- and intermolecular relaxation processes [37].
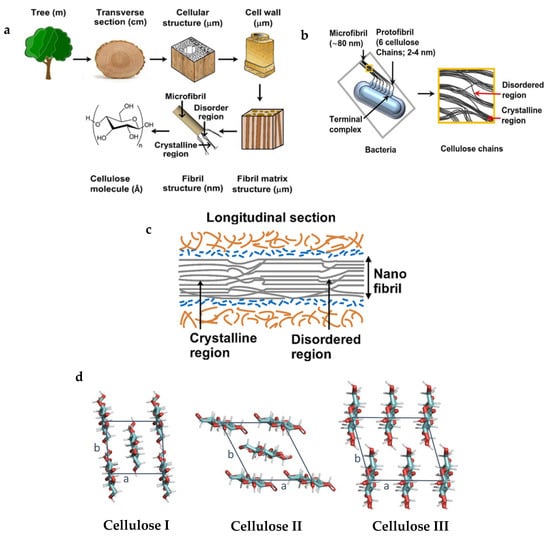
Figure 2.
Diagrammatic representation of cellulose. (a) Hierarchical structure of plant cellulose [Represented from Cellulose, 28, Seddiqi et al. [39] Cellulose and its derivatives: towards biomedical applications, 1893–1931, Copyright (2021), SPRINGER, CC BY licence]; (b) bacterial cellulose nanofibrils [Represented from Cellulose, 28, Seddiqi et al. [39] Cellulose and its derivatives: towards biomedical applications, 1893–1931, Copyright (2021), SPRINGER, CC BY licence]; (c) longitudinal microstructure cellulose nanofibrils showing crystalline regions [Represented from Cellulose, 28, Seddiqi et al. [39] Cellulose and its derivatives: towards biomedical applications, 1893–1931, Copyright (2021), SPRINGER, CC BY licence]; (d) unit cell structure of native cellulose (Cellulose I) and regenerated cellulose (Cellulose II, III) [Represented from Carbohydrate Polymers, 343, Khodayari et al. [23] Advancing plant cell wall modelling: Atomistic insights into cellulose, disordered cellulose, and hemicelluloses—A review, 122415, Copyright (2024), ELSEVIER, CC BY licence].
Figure 2.
Diagrammatic representation of cellulose. (a) Hierarchical structure of plant cellulose [Represented from Cellulose, 28, Seddiqi et al. [39] Cellulose and its derivatives: towards biomedical applications, 1893–1931, Copyright (2021), SPRINGER, CC BY licence]; (b) bacterial cellulose nanofibrils [Represented from Cellulose, 28, Seddiqi et al. [39] Cellulose and its derivatives: towards biomedical applications, 1893–1931, Copyright (2021), SPRINGER, CC BY licence]; (c) longitudinal microstructure cellulose nanofibrils showing crystalline regions [Represented from Cellulose, 28, Seddiqi et al. [39] Cellulose and its derivatives: towards biomedical applications, 1893–1931, Copyright (2021), SPRINGER, CC BY licence]; (d) unit cell structure of native cellulose (Cellulose I) and regenerated cellulose (Cellulose II, III) [Represented from Carbohydrate Polymers, 343, Khodayari et al. [23] Advancing plant cell wall modelling: Atomistic insights into cellulose, disordered cellulose, and hemicelluloses—A review, 122415, Copyright (2024), ELSEVIER, CC BY licence].
4. Molecular Self-Assembly and Regenerated Fibre
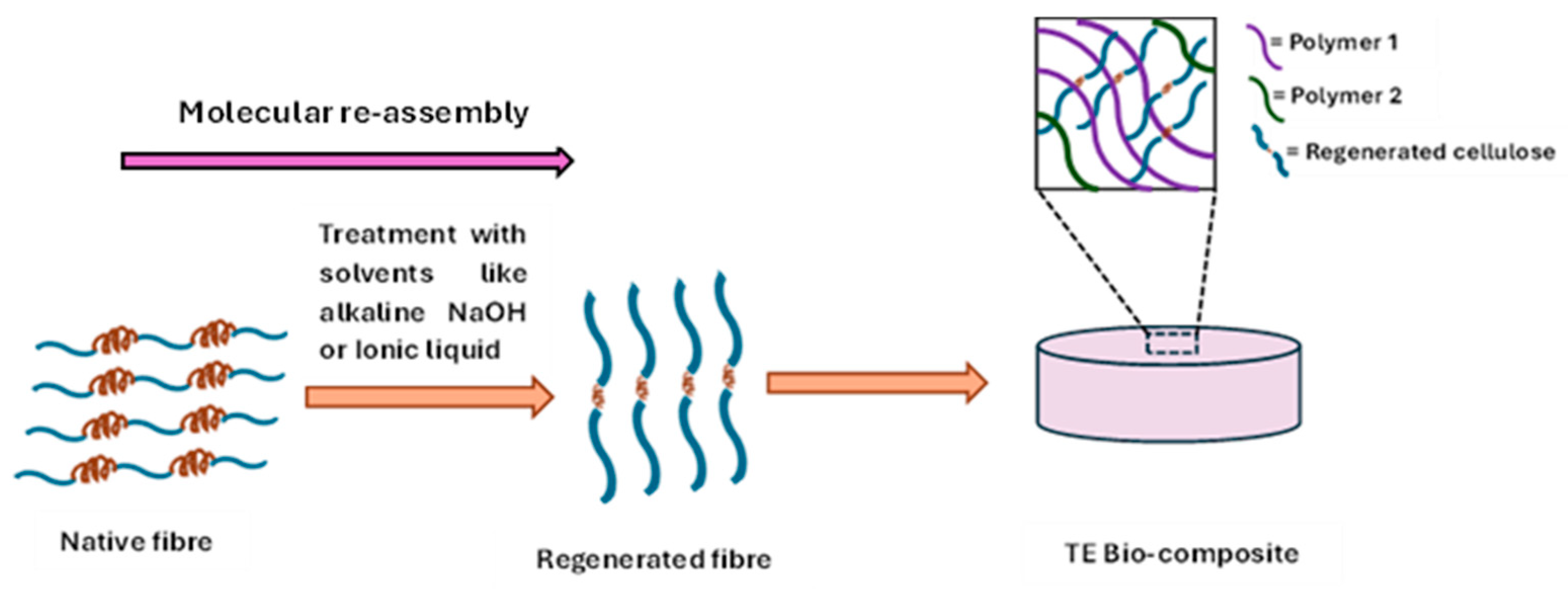
Molecular self-assembly is a significant biological phenomenon, where fibres like collagen and cellulose can form various complex structures with high physicochemical properties, such as mechanical properties [40]. This phenomenon resulted in the development of regenerated fibres (Figure 3).
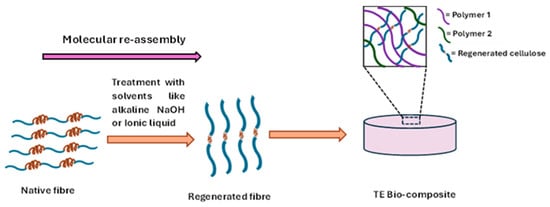
Figure 3.
Regenerated fibres for tissue engineering (TE) bio-composite development.
4.1. Molecular Reassembly and the Regenerative Process
The regenerated fibres developed through utilising different solvents like alkali solution, ionic liquid, in which the fibre exhibits the molecular reassembly. In case of cellulose fibres, molecular reassembled Cellulose II polymorph has been developed by the mercerisation process in the derivatising solutions like aqueous NaOH, NaOH/Urea solution from native cellulose I. This mercerisation process polymer causes the disruption of the hydrogen bond in Cellulose II, which ultimately results in a molecular conformational change in the polymer chains, which involves an antiparallel rearrangement of the directions of the reducing ends with respect to the axis of the cellulose microfibrils [41]. On the other hand, regenerated collagen fibres have been generated with the derivatising solvent like 1-ethyl-3-methylimidazolium acetate/sodium salt solvent in which the hydrogen bonds in the native collagen structure are first disrupted and then reformed when the solvent is removed [42].
4.2. Structural Properties of Regenerated Fibres
Regenerated fibres like Cellulose II consist of a ‘regenerated crystal form of cellulose’ harbouring a monoclinic unit cell with P21 symmetry [23]. Additionally, another polymorph of cellulose, i.e., Cellulose III, can also be prepared through treatment of cellulose I and II with liquid ammonia, which shows a similar conformation to the unit cell with P21 symmetry [23]. The molecular reassembly of native cellulose into regenerated cellulose results in the rotation of hydroxymethyl groups on the cellulose chain, ultimately causing differential patterns of hydrogen bonding [23]. The conformation of the hydroxymethyl groups on the cellulose chain is mainly divided into three forms, i.e., trans-gauche (tg), gauche-trans (gt) and gauchegauche (gg). The native cellulose I generally showed tg conformation, whereas the molecular reassembled regenerated cellulose II exhibited gt conformation in its structure. Moreover, molecular rearrangement of Cellulose II also involved the change in orientation of hydroxymethyl groups and inter- and intra-chain hydrogen bonding patterns. Cellulose-I contains two intramolecular hydrogen bonds at (O) 5-(OH) 31 and (OH) 2-(O) 61 positions and an interchain hydrogen bond in between O) 6- (O) 311 positions. The molecular rearrangement in Cellulose II causes the development of intra-chain hydrogen bonding at (OH)3-(O)51 and an intermolecular hydrogen bond at (OH)6-(O)211 [43]. Moreover, in Cellulose II, an inter-sheet interaction is also found between (OH)2 (corner chain) ± (O)211 (centre chain), which is absent in native cellulose [43].
On the other hand, collagen fibre assembly in vivo involves processing of tropocollagen molecules that self-assemble into fibrils stabilised by enzymatic covalent crosslinking. The staggered arrangement of collagen results from specific intermolecular interactions, including hydrophobic, electrostatic, and hydrogen bonding forces, guiding the lateral and longitudinal packing necessary to form nanoscale fibrillar structures [13]. In vitro collagen fibre assembly provides a controlled environment to systematically study the structural parameters that influence fibre morphology and properties. Factors such as pH, temperature, ionic strength, collagen concentration, and the presence of telopeptides substantially affect fibrillogenesis kinetics and the final fibril structure [44]. Ionic strength variations modulate fibril characteristics as well [45]. On the other hand, proteoglycans (PGs), including large aggregating species such as hyaluronan and aggrecan, as well as small leucine-rich proteoglycans (SLRPs) such as decorin, are vital regulators of collagen fibrillogenesis and fibre assembly. Proteoglycans incorporated in vitro alter fibrillogenesis kinetics and fibril morphology quantitatively; versican, a large chondroitin sulphate PG, accelerates fibrillogenesis and enhances collagen compaction by cells, whereas aggrecan exerts inhibitory effects on fibril growth, highlighting functional diversity amongst PGs [46]. Decorin slows fibril lateral growth, producing fibrils with smaller and more uniform diameters, which is essential for mechanical uniformity and tissue function. The concentration of such PGs can modulate tensile strength and stiffness of the assembled fibres, with reported strength increases by factors of up to 1.5 due to high molecular weight chondroitin sulphate PGs [47].
5. Tissue Engineering Biomaterials and Molecular Self-Assembled Collagen–Cellulose
Tissue engineering biomaterials developed with molecular self-assembled collagen and cellulose fibres are engineered by utilising their hierarchical fibrous structures. Chemical modifications of the native fibres target the reactive groups in both polymers to improve interfacial adhesion and structural cohesion. The resulting materials possess enhanced mechanical strength, biocompatibility, moisture handling, and functionality, making them ideal for wound healing dressings, drug delivery systems, and sustainable material alternatives in various industries. The preparation of composites with collagen and cellulose fibres typically requires chemical modifications to facilitate compatibility and interfacial adhesion between the two inherently different macromolecules. Collagen’s triple helical protein structure presents a surface rich in functional groups such as amine, carboxyl, and hydroxyl groups, whereas cellulose, composed of β-1,4-linked glucose units, offers numerous hydroxyls capable of hydrogen bonding. To obtain a highly efficient biomaterial, crosslinking or coupling agents such as glutaraldehyde, genipin, or carbodiimide-based chemistries can be used to covalently link collagen and cellulose within the biomaterial matrix. Modification involving the surface activation of the fibres improves their reactivity. For example, in collagen–cellulose fibre-based biomaterial, the oxidation of cellulose to introduce aldehyde groups facilitates Schiff base formation with amino groups on collagen, enhancing bonding and composite integrity [48].
Structurally, molecular reassembled collagen/cellulose fibres often display a dense, well-integrated morphology characterised by hydrogen bonding networks and covalent crosslinks that reinforce the scaffold architecture. Moreover, collagen enhances the bioactivity of the composite, making it more conductive to cell attachment and proliferation, while cellulose maintains high tensile strength and water interaction properties [48]. Functionally, these composites exhibit different important properties like high mechanical properties, hydrophilicity and water retention capacity. This synergistic interaction makes these materials excellent candidates for wound dressings that require moisture retention, biocompatibility, and mechanical protection during tissue healing. The following table (Table 2) depicts the characteristics of different molecular self-assembled collagen and/or cellulose-based bio-composites in tissue engineering applications:

Table 2.
Biomaterials developed with regenerated cellulose and collagen containing molecularly reassembled fibres.
6. Advantages and Challenges of Molecular Re-Assembled Cellulose and Collagen Fibres in Terms of Tissue Engineering Bio-Composite Development
Tissue engineering biomaterials utilise different properties of molecular reassembled fibre material, like regenerated cellulose and collagen, to enhance their functional properties. The molecular reassembled fibres, like regenerated cellulose, have different advantages over native fibres, such as: (a) possibility to develop through a cost-effective production route: Regenerated fibres could be developed through applying different solvent systems [62,63,64,65], which are low-cost (Table 3) and thus facilitate a cost-effective production route.

Table 3.
Different solvent systems for cost-effective production of regenerated fibres [62,63,64,65].
(b) Efficient biodegradability: The regenerated or molecular re-assembled fibres showed high degradability than the native fibre [7]; (c) improved liquid absorbance property: due to modified molecular arrangement in the molecular structure, regenerated or molecular re-assembled fibres facilitates improved liquid absorption property of the biomaterial [7]; (d) high chemical stability: Due to altered molecular re-arrangement in the chemical structure developed in derivatizing solutions like ironic liquid, the regenerated fibres demonstrates high chemical stability [66]; (e) environmental sustainability: regenerated or molecular re-assembled fibres like cellulose II have high environmental sustainability [7]; (f) can be used as excellent reinforcement material: regenerated fibres like cellulose II can be used as excellent reinforcement material for development of a bio-composite [67]; (g) high uniformity and adhesion properties: regenerated fibres, such as cellulose, exhibit greater uniformity and adhesion properties than native fibres [68]; (h) chemically modifiable for antimicrobial efficiency: regenerated fibres like cellulose can be chemically modified to incorporate antimicrobial efficiency into tissue engineering bio-composites.
Despite several advantages, significant challenges persist in the successful application of regenerated fibres. These include: (a) difficulty in processing: the preparation of regenerated cellulose or collagen fibres requires the use of specific solvents such as ionic liquids and urea-based solvent systems [56,61] to dissolve the fibres. This process is often challenging to obtain homogenous dissolved fibres like cellulose [69]; (b) production scale up related challenge: low recovery from solvents and scaling up related to the fibre (like cellulose) dissolution procedure have remained challenges for their production [70]; (c) inadequate durability of fibres: In general, regenerated fibres have moderate durability than the native fibres. The change in crystallinity and altered chemical arrangement in the regenerated cellulose fibres decreases its overall durability [7]; (d) high brittleness of the developed composites: bio-composite developed with regenerated fibres has low mechanical property which ultimately make the bio-composite brittle [71,72]; (e) possibility to induce undesired tissue damage: Due to the processing of the native fibres with harsh chemicals like NaOH or ionic liquids, regenerated fibres (such as cellulose) could induce undesired tissue damage in ‘nonbuffered clinical conditions (e.g., inadequate tissue perfusion)’ [73]; (f) unwanted immunogenicity and low cell adhesion property: collagen sourced from different sources can show immunogenicity [74]. On the other hand, the cell adhesion property can be found to be very low in cellulose fibres [75]. Regenerated fibres developed from these fibres could also exhibit similar properties that need to be addressed to be used efficiently for tissue engineering applications.
7. Perspective for Future Research
Biological materials in living organisms, many of which are proteins, exhibit a complex hierarchical organisation. The most interesting fact about cellulose and collagen-based materials is that they are abundant in nature and present in a unique hierarchical arrangement. This hierarchical arrangement includes microfibrils with different orientations and properties, such as crystallinity. Scientists have been utilising these properties to efficiently use these fibre-based materials to develop highly functional tissue engineering bio-composites. Future research is needed to explore their efficiency further in tissue engineering applications. The aspects for future research could be as follows: (a) analysis of in-depth chemical properties: molecularly rearranged regenerated fibres were being produced through utilising different derivatising solutions. The molecular rearrangement and their stability resulting from different derivatising solvent treatment must be studied thoroughly. This will explain the variety and variability of different physicochemical properties (like mechanical properties) of the fibres developed in different solvent systems. By using this information, different crosslinking strategies could be introduced in the polymeric system that will enhance the bio-composite’s overall stability; (b) utilisation of machine learning and AI for in-depth analysis of reinforcement property in bio-composites: fibre-like materials can act as an excellent reinforcement filler in polymeric bio-composites [8]. Careful study of regenerated fibres with altered molecular re-arrangements and their contribution to reinforcing a polymeric system could provide important insights related to the overall mechanical property of the bio-composites. Machine learning and AI-based simulation study could be beneficial for this; (c) biofunctionalisation efficiency: The process of regenerated fibre development involves the use of different solutions like ionic liquid or alkali-based solution. These process results in the activation of different functional groups like hydroxyl (-OH) groups, amine (-NH2) groups in the building molecules of the fibre (like glucose for cellulose and peptide containing amino acid moieties in collagen), which can be further utilised to introduce novel chemical properties in the fibres for specific purposes like antimicrobial efficiency for tissue engineering application; (d) utilisation of doping material to improve the mechanical property: the molecular re-arrangement of the regenerated fibres often make them mechanically weak, which will result low mechanically durable bio-composites. Different doping materials like ceramics (like calcium phosphates, calcium carbonate) could be utilised to increase the overall mechanical property of the biomaterial; (e) environmental sustainability and enhanced bio-applicability: As the development of molecular rearranged regenerated fibres involves harsh derivatizing solutions like alkali/urea based solution, which has a significant environmental and health implications, hence it is necessary to explore further solvent systems to process and produce these fibres in a sustainable manner. (f) utilisation of different sustainable sources of native fibres to develop regenerated fibres: Further research is needed for the development of regenerated fibres from the native fibres found in sources like algae (algal cellulose) and collagen from marine sources. This will further facilitate the possibility of utilisation of the differential properties of these fibres in tissue regeneration applications.
8. Conclusions
Natural fibres like cellulose and collagen can be obtained from cost-effective sources; however, their scope of applicability in tissue engineering is challenging. Uncontrolled degradability and unwanted immunogenicity are the main challenges that limit their application in tissue engineering. To increase the applicability of these fibre-based materials in diverse biomedical applications, different processing methods have been employed. Regenerated fibres are the result of this processing of the native fibres and have a different molecular arrangement than the native fibres. The successful application of these regenerated fibres in the development of tissue engineering bio-composites depends on factors such as physical and chemical stability, as well as bio-functionality. These properties can be increased and optimised by modifying these fibres primarily through alterations of their molecular structure, utilising different chemical derivatives. The chemical incorporation of bioactive groups will also facilitate interesting opportunities for interaction with other components in the bio-composites. Hence, these regenerated fibres will not only positively influence the stability of the polymer matrix but also provide necessary bioactive signals to the cells for tissue regeneration. Further studies on the chemical and physiological properties of these regenerated fibres developed through different derivatising solvents (such as alkali and ionic solvents) are necessary to explore their promising applicability in tissue engineering bio-composites.
9. Summary
The present work indicated the significance of molecular reassembled regenerated fibres (collagen and cellulose) in tissue engineering by comparing them with native fibres. Regenerated fibres were being prepared through the utilisation of derivative solvent systems, which facilitated stable molecular re-arrangements in the fibre’s molecular orientations. Different researchers have been utilising modified fibre materials for fabricating different tissue engineering biomaterials; however, their functional application is still challenging. The following table (Table 4) summarises the present work, indicating the advantages and present challenges, and future research perspectives

Table 4.
A summary of the significance of the molecular self-reassembled regenerated fibres in tissue engineering bio-composite development.
Author Contributions
K.S.-A.—literature search, Interpretation, original draft preparation; P.B.—conceptualisation, analysis, interpretation, editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
EU Marie Skłodowska-Curie Individual Fellowship July 2023-June 2025 [MultiphaseGTR, Grant Agreement No. 101108847] (P.B.), Contratos Predoctorales de Formación en Investigación en Salud 2023 [FI23/00239], Instituto de Salud Carlos III (ISCIII) (K.S.A).
Data: Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
P.B also acknowledges the Postdoctoral Fellowship received (July 2025 onwards) from Marie Skłodowska-Curie Actions Grant Agreement [Grant Agreement No. 101081457] and in part from CÚRAM, Research Ireland Centre for Medical Devices [under Grant Reference 13/RC/2073_P2] for providing necessary support. P.B also acknowledges the Wallenberg Wood Science Centre, Department of Chemistry and Chemical Engineering, Chalmers University of Technology, where the idea of the work was conceived for providing the Wallenberg Wood Science Centre Postdoctoral Fellowship 2021–2022.
Conflicts of Interest
The authors declare no conflicts of interest.
AI Statement
An artificial intelligence tool (specifically OpenAI’s ChatGPT, Model: GPT-4o mini beta version, University of Oslo, Oslo, Norway) was used exclusively to assist with grammar correction and punctuation. This use complies with applicable regulations, including the European Commission’s Proposal for a Regulation on Artificial Intelligence (Artificial Intelligence Act, 2021) (Document 52021PC0206). All substantive content, data analysis, and conclusions remain the original work of the authors.
References
- Cota Quintero, J.L.; Ramos-Payán, R.; Romero-Quintana, J.G.; Ayala-Ham, A.; Bermúdez, M.; Aguilar-Medina, E.M. Hydrogel-Based Scaffolds: Advancing Bone Regeneration Through Tissue Engineering. Gels 2025, 11, 175. [Google Scholar] [CrossRef]
- Li, X.; Sim, D.; Wang, Y.; Feng, S.; Longo, B.; Li, G.; Andreassen, C.; Hasturk, O.; Stout, A.; Yuen, J.S.K.; et al. Fiber-Based Biomaterial Scaffolds for Cell Support towards the Production of Cultivated Meat. Acta Biomater. 2025, 191, 292–307. [Google Scholar] [CrossRef]
- Prasad, V.; Alliyankal Vijayakumar, A.; Jose, T.; George, S.C. A Comprehensive Review of Sustainability in Natural-Fiber-Reinforced Polymers. Sustainability 2024, 16, 1223. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, Applications and Challenges of Natural Biomaterials in Tissue Engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Schiller, T.; Scheibel, T. Bioinspired and Biomimetic Protein-Based Fibers and Their Applications. Commun. Mater. 2024, 5, 56. [Google Scholar] [CrossRef]
- Thongnok, K.; Torgbo, S.; Sallehuddin, N.; Maarof, M.; Khamplod, T.; Fauzi, M.B.; Sukyai, P. Polydopamine-Coated Regenerated Cellulose-Bioceramic Composite Scaffolds for Enhanced Bone Tissue Engineering. Mater. Chem. Phys. 2025, 341, 130891. [Google Scholar] [CrossRef]
- Varnaitė-Žuravliova, S.; Baltušnikaitė-Guzaitienė, J. Properties, Production, and Recycling of Regenerated Cellulose Fibers: Special Medical Applications. J. Funct. Biomater. 2024, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Saha, N.; Saha, P. Swelling and Rheological Study of Calcium Phosphate Filled Bacterial Cellulose-Based Hydrogel Scaffold. J. Appl. Polym. Sci. 2020, 137, 48522. [Google Scholar] [CrossRef]
- Woodings, C. A brief history of regenerated cellulose fibres. In Regenerated Cellulose Fibres; Woodings, C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 1–21. [Google Scholar]
- Rules and Regulations Under the Textile Fiber Products Identification Act by USA Federal Trade Commission, Source: 24 FR 4480, 2 June 1959, in § 303.7 Generic Names and Definitions for Manufactured Fibers. Available online: https://www.ecfr.gov/current/title-16/part-303/section-303.7 (accessed on 28 September 2025).
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Orgel, J.P.R.O.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar Structure of Type I Collagen in Situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and Mechanical Quality of the Collagen–Mineral Nano-Composite in Bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen Fibril Formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Pedersen, G.B.; Blaschek, L.; Frandsen, K.E.H.; Noack, L.C.; Persson, S. Cellulose Synthesis in Land Plants. Mol. Plant. 2023, 16, 206–231. [Google Scholar] [CrossRef]
- Chen, Q.; Pei, Y.; Tang, K.; Albu-Kaya, M.G. Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials—A review. Collagen Leather 2023, 5, 20. [Google Scholar] [CrossRef]
- Delmer, D.; Dixon, R.A.; Keegstra, K.; Mohnen, D. The Plant Cell Wall—Dynamic, Strong, and Adaptable—Is a Natural Shapeshifter. Plant Cell 2024, 36, 1257. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review of Analytical Methods in Pretreatment of Lignocelluloses: Composition, Imaging, and Crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. The Hierarchical Structure and Mechanics of Plant Materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Sumathi, S. Biosynthesis and Assemblage of Extracellular Cellulose by Bacteria. In Handbook of Environmental Materials Management; Springer: Cham, Switzerland, 2018; pp. 1–43. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Khodayari, A.; Hirn, U.; Spirk, S.; Ogawa, Y.; Seveno, D.; Thielemans, W. Advancing Plant Cell Wall Modelling: Atomistic Insights into Cellulose, Disordered Cellulose, and Hemicelluloses—A Review. Carbohydr. Polym. 2024, 343, 122415. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a Glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Svendsen, K.H.; Koch, M.H. X-ray Diffraction Evidence of Collagen Molecular Packing and Cross-linking in Fibrils of Rat Tendon Observed by Synchrotron Radiation. EMBO J. 1982, 1, 669–674. [Google Scholar] [CrossRef]
- Ioelovich, M. Peculiarity of Phase Transitions of Cellulose Nano Crystallites. ChemXpress 2016, 9, 106. [Google Scholar]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Brown, R.M.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Pérez, S.; Mazeau, K. Conformations, Structures, and Morphologies of Celluloses From. In Polysaccharides; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 41–68. [Google Scholar]
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional Cellulose-Based Hydrogels as Extracellular Matrices for Tissue Engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of Bacterial Cellulose in Food. Food Hydrocoll 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Krässig, H.A. Cellulose: Structure, Accessibility and Reactivity; Gordon & Breach: Philadelphia, PA, USA, 1993. [Google Scholar]
- Quesada Cabrera, R.; Meersman, F.; McMillan, P.F.; Dmitriev, V. Nanomechanical and Structural Properties of Native Cellulose under Compressive Stress. Biomacromolecules 2011, 12, 2178–2183. [Google Scholar] [CrossRef]
- Gautieri, A.; Redaelli, A.; Buehler, M.J.; Vesentini, S. Age- and Diabetes-Related Nonenzymatic Crosslinks in Collagen Fibrils: Candidate Amino Acids Involved in Advanced Glycation End-Products. Matrix Biol. 2014, 34, 89–95. [Google Scholar] [CrossRef]
- Shen, Z.L.; Kahn, H.; Ballarini, R.; Eppell, S.J. Viscoelastic Properties of Isolated Collagen Fibrils. Biophys. J. 2011, 100, 3008–3015. [Google Scholar] [CrossRef]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquidaz, P. Mechanical Properties of Collagen Fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Xu, M.; Hu, M.; Zhang, R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels 2024, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Delepierre, G.; Eyley, S.; Thielemans, W.; Weder, C.; Cranston, E.D.; Zoppe, J.O. Patience Is a Virtue: Self-Assembly and Physico-Chemical Properties of Cellulose Nanocrystal Allomorphs. Nanoscale 2020, 12, 17480–17493. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Dan, W.; Dan, N.; Gu, Z. Evaluation of 1-Ethyl-3-Methylimidazolium Acetate Based Ionic Liquid Systems as a Suitable Solvent for Collagen. J. Appl. Polym. Sci. 2013, 130, 2245–2256. [Google Scholar] [CrossRef]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Jamaluddin, J.; Awasthi, M.K.; Sarsaiya, S. A Review on Systematic Study of Cellulose. J. Appl. Nat. Sci. 2010, 2, 330–343. [Google Scholar] [CrossRef]
- Achilli, M.; Mantovani, D. Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of PH, Temperature and Ionic Strength on Gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef]
- Harris, J.R.; Soliakov, A.; Lewis, R.J. In Vitro Fibrillogenesis of Collagen Type I in Varying Ionic and PH Conditions. Micron 2013, 49, 60–68. [Google Scholar] [CrossRef]
- Chen, D.; Smith, L.R.; Khandekar, G.; Patel, P.; Yu, C.K.; Zhang, K.; Chen, C.S.; Han, L.; Wells, R.G. Distinct Effects of Different Matrix Proteoglycans on Collagen Fibrillogenesis and Cell-Mediated Collagen Reorganization. Sci. Rep. 2020, 10, 19065. [Google Scholar] [CrossRef]
- Garg, A.K.; Berg, R.A.; Silver, F.H.; Garg, H.G. Effect of Proteoglycans on Type I Collagen Fibre Formation. Biomaterials 1989, 10, 413–419. [Google Scholar] [CrossRef]
- Prasong, W.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Ito, H. Improvement of Interlayer Adhesion and Heat Resistance of Biodegradable Ternary Blend Composite 3D Printing. Polymers 2021, 13, 740. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated Cellulose Nanofiber Reinforced Chitosan Hydrogel Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated Cellulose Nanofibers from Cellulose Acetate: Incorporating Hydroxyapatite (HAp) and Silver (Ag) Nanoparticles (NPs), as a Scaffold for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.; Hua, W.; Li, P.; Wang, X. 3D Porous Poly(Lactic Acid)/Regenerated Cellulose Composite Scaffolds Based on Electrospun Nanofibers for Biomineralization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124048. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile Fabrication of Electrospun Regenerated Cellulose Nanofiber Scaffold for Potential Bone-Tissue Engineering Application. Int. J. Biol. Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Sousa Gomes, P. Novel Cellulose/Hydroxyapatite Scaffolds for Bone Tissue Regeneration: In Vitro and in Vivo Study. J. Tissue Eng. Regen. Med. 2018, 12, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- De Araújo Júnior, A.M.; Braido, G.; Saska, S.; Barud, H.S.; Franchi, L.P.; Assunção, R.M.N.; Scarel-Caminaga, R.M.; Capote, T.S.O.; Messaddeq, Y.; Ribeiro, S.J.L. Regenerated Cellulose Scaffolds: Preparation, Characterization and Toxicological Evaluation. Carbohydr. Polym. 2016, 136, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.I.N.; Sankar, S.; Kashif, P.M.; Basha, S.K.H.; Sastry, T.P. Evaluation of Biomaterial Containing Regenerated Cellulose and Chitosan Incorporated with Silver Nanoparticles. Int. J. Biol. Macromol. 2015, 72, 680–686. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-Based Scaffold Materials for Cartilage Tissue Engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, X.; Zhao, H.; Xia, S.; Liu, W.; Bai, H.; Lv, F.; Zheng, X.; Huang, Y.; Gu, Q.; et al. Synthesis of Easily-Processable Collagen Bio-Inks Using Ionic Liquid for 3D Bioprinted Liver Tissue Models with Branched Vascular Networks. Sci. China Chem. 2023, 66, 1489–1499. [Google Scholar] [CrossRef]
- Wang, X.; Wu, T.; Wang, W.; Huang, C.; Jin, X. Regenerated Collagen Fibers with Grooved Surface Texture: Physicochemical Characterization and Cytocompatibility. Mater. Sci. Eng. C 2016, 58, 750–756. [Google Scholar] [CrossRef]
- Amsaveni, M.; Anumary, A.; Ashokkumar, M.; Chandrasekaran, B.; Thanikaivelan, P. Green Synthesis and Characterization of Hybrid Collagen-Cellulose-Albumin Biofibers from Skin Waste. Appl. Biochem. Biotechnol. 2013, 171, 1500–1512. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, X.; Tang, K.; Liu, J.; Ma, Z.; Zhao, Q. Dissolution and Regeneration of Collagen Fibers Using Ionic Liquid. Int. J. Biol. Macromol. 2012, 51, 440–448. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, C.L.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 39, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yamagishi, N.; Gotoh, Y.; Potthast, A.; Rosenau, T. High performance cellulose fibers regenerated from 1-butyl-3-methylimidazolium chloride solution: Effects of viscosity and molecular weight. J. Appl. Polym. Sci. 2020, 137, 48681. [Google Scholar] [CrossRef]
- Tu, H.; Li, X.; Liu, Y.; Luo, L.; Duan, B.; Zhang, R. Recent progress in regenerated cellulose-based fibers from alkali/urea system via spinning process. Carbohydr. Polym. 2022, 296, 119942. [Google Scholar] [CrossRef]
- Yang, K.; Shan, J.; Wang, X.; Zhang, J.; Tian, G.; Yang, D.; Yang, J.; Ma, J. Characteristics of cellulose solutions prepared with three non-derivatizing solvents and comparison of regenerated fiber properties. J. Mol. Liq. 2025, 437, 128443. [Google Scholar] [CrossRef]
- Armir, N.A.Z.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Othman, M.H.C.; Zakaria, S. Regenerated Cellulose Products for Agricultural and Their Potential: A Review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef] [PubMed]
- Santamala, H.; Livingston, R.; Sixta, H.; Hummel, M.; Skrifvars, M.; Saarela, O. Advantages of Regenerated Cellulose Fibres as Compared to Flax Fibres in the Processability and Mechanical Performance of Thermoset Composites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 377–385. [Google Scholar] [CrossRef]
- Fazeli, M.; Islam, S.; Baniasadi, H.; Abidnejad, R.; Schlapp-Hackl, I.; Hummel, M.; Lipponen, J. Exploring the Potential of Regenerated Ioncell Fiber Composites: A Sustainable Alternative for High-Strength Applications. Green Chem. 2024, 26, 6822–6835. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Wang, L.; Chen, L.; Jin, Z.; Zhang, Q. A Cost-Effective and Chemical-Recycling Approach for Facile Preparation of Regenerated Cellulose Materials. Nano Lett. 2024, 24, 9074–9081. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Liyanage, S.; Abidi, N.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Wu, K.; Liu, D.; Gong, F.; Lei, C.; Fu, Q. Addressing the Challenge of Fabricating a High Content Regenerated Cellulose/Nanomaterial Composite: The Magical Effect of Urea. Green Chem. 2020, 22, 4121–4127. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Yang, S.G.; Li, L.; Yang, B.; Huang, H.D.; Yan, D.X.; Zhong, G.J.; Xu, L.; Li, Z.M. Ultralight Cellulose Porous Composites with Manipulated Porous Structure and Carbon Nanotube Distribution for Promising Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 40156–40167. [Google Scholar] [CrossRef]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of Regenerated and Non-Regenerated Oxidized Cellulose Hemostatic Agents. Eur. Surg. Acta Chir. Austriaca 2013, 45, 213–220. [Google Scholar] [CrossRef]
- Fang, Z.; Lu, C.; Du, W.; Wang, X.; Yang, H.; Shi, M.; Liu, T.; Xie, Y.; Wang, S.; Xu, X.; et al. Injectable Self-Assembled Dual-Crosslinked Alginate/Recombinant Collagen-Based Hydrogel for Endometrium Regeneration. Int. J. Biol. Macromol. 2023, 236, 123943. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.d.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).