Abstract
UV/ozone (UVO)-assisted formation of self-assembled monolayer (SAM) of 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDS) was prepared on a glass surface. The effect of UVO exposure time on surface roughness and hydrophilicity was investigated through goniometer and atomic force microscope (AFM), and deposition time-dependent SAM quality was detected by AFM and X-ray photoelectron spectroscopy (XPS). The glass surface became smooth with UVO radiation after 10 min, and the hydrophilicity was also improved after the treatment. Confirmed by surface topography detection and chemical composition analysis, a high-quality SAM can be formed rapidly on glass with 10 min UVO treatment followed by 2 h deposition in PFDS solution. Excellent tribological performances of SAM coated with UVO treatment glass were demonstrated by friction and wear tests on AFM compared to film-deposited glass without UVO treatment and original glass. The study sheds a light on preparing high-quality lubrication and antiwear self-assembled films on the surface of engineering materials.
1. Introduction
The excellent performance of self-assembled monolayer (SAM) has attracted attention worldwide [1,2,3,4]. A range of materials such as silicon, mica, copper, and aluminum alloys can be used as substrates for SAMs [5,6,7,8]. However, traditional hydroxylation of the substrates for the preparation of SAM is dependent on chemical reaction in strong oxidizing acid solution, such as piranha solution [4,9]. Strong chemically active solution will inevitably cause damage, including oxidation and corrosion of a material’s surface, especially in the case of metal surfaces. An alternative method for the hydroxylation or chemical activation of material surfaces would push the application of SAM into wide-range engineering fields.
UV/ozone (UVO) treatment has been used to remove surface contaminants effectively on various materials [10]. Compared with other methods such as hydrofluoric (HF) solution and high velocity air jets, UVO treatment generates little toxic or harmful gases and no waste liquid during the cleaning process, and can even produce near-atomically clean surfaces in air or in a vacuum system at ambient temperature. Aside from surface cleaning, UVO can also be employed in surface modification and rapid nanofabrication on the basis of selective etching [11,12,13]. It is noted that material surface after UVO treatment exhibits more hydrophilic tendencies, which may be attributed to the hydroxylation of surface atoms during processing [14,15], and the hydroxyl group is believed to be one of the most chemically reactive groups. Many methods have been provided for material surface hydroxylation, including UV/ozone treatment, O2 plasma, and post-anodization, to bind hydroxyl groups to other molecules [16]. UVO-treated material substrates are likely to facilitate by forming high-quality tribological SAMs enabling materials to withstand severe working conditions [17]. However, few studies have involved the adsorption mechanism of SAM film on UVO-treated substrate. It is noted that the quality of SAM is dependent on the bonding state between the film-forming molecules and the substrate and compact structures of the SAM [4]. Generally, friction and wear tests are proven to be the effective way for evaluating the film quality, including the bonding strength, lubrication, and antiwear [18]. Therefore, the film formation process and the film performance still need to be investigated for optimizing the preparing process and achieving high-quality tribological films prepared with UVO assistance.
In this paper, UVO pretreatment of glass surface was used for forming lubrication and antiwear SAM rapidly. Due to its low friction, water repellence, and chemical resistance, -CF3 group-tailed self-assembled films have found great application in lubrication, hydrophobicity improvement, and anti-corrosion [19,20,21,22], with perfluorosilane being used for the assembling in the present study. The effect of UVO exposure time on the performances of glass surface was investigated through the detection of surface wettability and topography. Then, the film deposition condition was investigated and optimized towards the formation of high-quality tribological SAM on glass surface. The quality of the SAM was finally evaluated by elemental composition analysis and nanotribology tests.
2. Materials and Methods
Soda–lime–silica glass slides were employed as SAM substrates in the present study. 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDS, 97%; Nanjing Capatue Chemical Co., Ltd., Nanjing, China) was used as the film-forming agent for preparing SAM on glass surface. Ethanol purity was ≥99.7%, purchased from Chengdu Chron Chemicals Co., Ltd., Chengdu, China. In order to remove surface contaminants preliminarily, all glass substrates were ultrasonically cleaned by acetone and alcohol in turn, and then rinsed by deionized water. The cleaned glass substrates were placed into the UV/ozone cleaner (PSDP UV-8T, Novascan, Ames, IA, USA) for the pretreatment at different times, and then were put into the mixture of PFDS and ethanol with a volume ratio of 1:50, which corresponded to 0.043 mol/L of PFDS. The temperature was set at 25 °C for all of the self-assembly process, and the film deposition time was from 0 to 5 h. After the deposition, the film-coated glass was washed with acetone and alcohol in ultrasonic cleaner in sequence for 10 min. Then, the glass was rinsed with continuous deionized water and dried by high-pressure nitrogen flow for further tests. For comparison, cleaned glass surface without any treatment and the untreated glass with PFDS deposition were also investigated in this study.
Following the UVO treatment, water contact angles of the glass substrates were measured by the goniometer (Krüss GmbH DSA 100 Mk 2, Hamburg, Germany) at room temperature. The contact angles were measured three times for each sample at different places. Surface topography and wear tests on glass surface were conducted by atomic force microscope (AFM; E-Sweep, Hitachi Instruments Inc., Tokyo, Japan) in contact mode. All the roughness of glass surface was calculated from AFM images of a 2 × 2 µm2 square. Chemical compositions of different sample surfaces were characterized by X-ray photoelectron spectroscopy (XPS; ThermoFisher Co., Waltham, USA) using Al K-Alpha. Lubricating performance was investigated by a Si3N4 AFM tip with a tip radius (R) of ~20 nm in atmosphere, and the friction force between tip and sample surface was measured at least five times to obtain reliable data. For investigating the wear resistance of the SAM prepared, nanowear tests under area-scanning mode were carried out by a diamond AFM tip (R = ~50 nm). The scan size was 2 × 2 µm2, consisting of 2048 line scanning. The stiffness of the cantilever was 0.1 N/m, 0.6 N/m, and 100 N/m for topography detection, friction tests, and wear tests, respectively.
3. Results and Discussion
3.1. Pretreatment with UV/Ozone Exposure
UVO treatment can further remove contaminants from the glass surface, making the glass surface more hydrophilic [10]. Figure 1 shows the RMS roughness of original glass treated by UVO treatment with various periods (0, 5, 10, 15, 20, 25, and 30 min), where the roughness was obtained from AFM images of glass surface. The roughness of glass decreased and reached a minimum value of ~0.37 nm at 10 min UVO treatment, which can be attributed to the decomposition of contaminants on the glass surface under UVO etching [10]. Although it was not fully clear, the increase of the roughness with the treatment time could be partly attributed to further oxidation and photocorrosion of glass surface when it occurred more than 10 min [23]. It has been reported that hydrophilic hydroxyl groups can be generated on substrate surface during UVO treatment, which lead to the reduction of water contact angle of substrate surface [11,24]. On the other hand, the water contact angles of glass decreased drastically from 54° to 9° with the UVO treatment from 0 to 10 min, and then the contact angles continued to decrease (Figure 1). After 25 min UVO treatment, the water contact angles of glass remained stable and tended to be 0°.
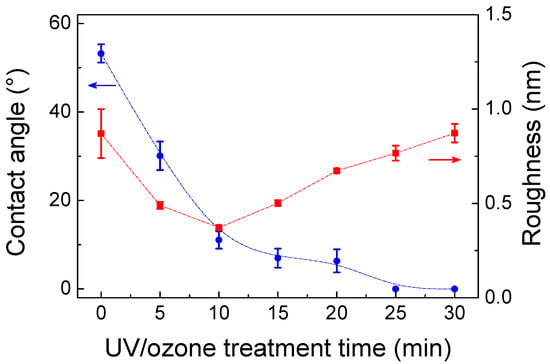
Figure 1.
Effect of UV/ozone (UVO) pretreatment time on root-mean-square (RMS) roughness and hydrophilicity (contact angle) of glass surface. The change of surface roughness (red line) and water contact angle (blue line) was plotted as a function of UVO treatment time.
During the molecular self-assembly process, the roughness of substrate surface influences the packing and organization of molecules, and the presence of conformational defects is inevitable on rough substrates [25]. Smooth substrate was hence expected to facilitate the formation of a high-quality monolayer. As a result, after 10 min UVO treatment, the hydrophilic glass substrates with the smallest surface roughness were used as substrates for SAM.
3.2. UV/Ozone-Assisted Rapid Formation of PFDS SAM
Deposition time plays an important role in the surface coverage of molecules on the substrate [26]. As depicted in Figure 2, original glass without pretreatment and 10 min UVO-pretreated glass surfaces were immersed into the film-forming solution for different deposition times (0, 10, 30 min, 1, 2, and 5 h). Two-stage process of SAM was revealed by kinetic study of the SAM formation [27]. At the first stage, there was a fast growth process of SAM formation within a few minutes, and the second stage took several hours to rearrange and re-orientate the adsorbed molecules. As can be clearly seen from the topography in Figure 2, there was fast growth of PFDS SAM on original glass surface and UVO-pretreated glass surface at an early period. When deposition time was 2 h, the smoothest PFDS SAM (with a thickness of ~1.6 nm) was formed on the 10 min UVO-pretreated glass surface [28], and water contact angle reached 104° [17], suggesting that a high-quality SAM was achieved. As the deposition time increased to 5 h, some molecule accumulation could be detected on the 10 min UVO-pretreated surface. In comparison, relatively smooth PFDS SAM was formed on the original glass surface after deposition for 5 h.
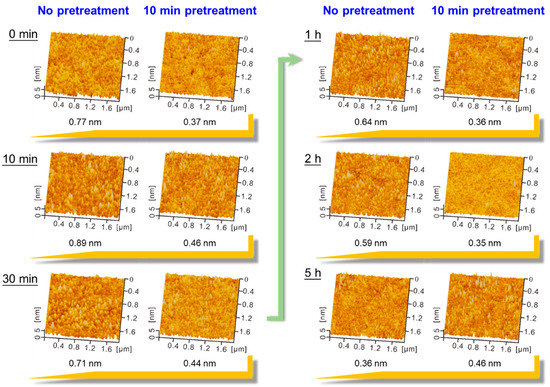
Figure 2.
The comparison of atomic force microscope (AFM) topography after depositing 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDS) film for different times on unpretreated glass surface and 10 min UVO-pretreated surface. The corresponding RMS roughness value is shown below the AFM images.
To verify the quality of PFDS SAM, widen scan and high-resolution XPS characterization was conducted on original glass, 10 min UVO-pretreated glass, SAM-coated glass without UVO pretreatment (SAM-glass (0)), and SAM-coated glass with 10 min UVO-pretreatment (SAM-glass (10 min pretreatment)). In Figure 3a, the wide-scan spectrum of SAM-glass (0) and SAM-glass (10 min pretreatment) was mainly composed of F1s, O1s, C1s, and Si2p signals. In Figure 3b, compared to original glass and 10 min UVO-pretreated glass, an evident peak at 688.5 eV was detected on SAM-glass (0) and SAM-glass (10 min pretreatment). As shown in Figure 3c, only one peak at 284.8 eV was found on the original glass surface, which can be ascribed to the absorbed C-based surface contaminants. In comparison, C1s core-level spectrums of SAM-glass (0) and SAM-glass (10 min pretreatment) presented peaks at 284.8, 286.5, 289.4, 291.5, and 293.8 eV, corresponding to C–C/C–H, C–O, CF–CFX, –CF2, and –CF3, respectively [29,30]. It was also noted that an obvious -CF2 peak at 291.5 eV was detected on SAM-glass (10 min pretreatment) resulting from well adsorption of PFDS molecules, but a weak -CF2 peak was also found on the SAM-glass (0) surface. In addition, relative stronger intensity of –CF2 and –CF3 peaks in contrast with C–C/C–H peak at 284.8 eV were detected on SAM-glass (10 min pretreatment) rather than that on SAM-glass (0). Therefore, it is demonstrated that UVO pretreatment for 10 min can induce rapid formation of high-quality PFDS film on glass surface [17].
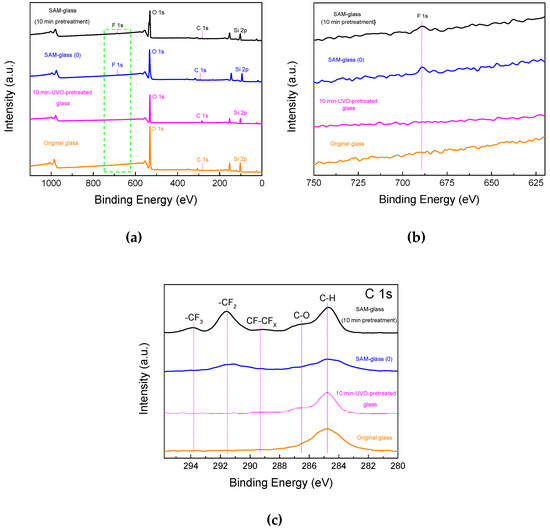
Figure 3.
(a) Widen scan of glass surfaces. (b) Zoomed widen scan of F 1s (green dashed frame, Figure 3a) of glass surfaces. (c) High-resolution C1s core-level spectra of glass surfaces.
The mechanism of UVO-assisted rapid formation of high-quality PFDS SAM on glass surface is shown in Figure 4. The photo-oxidation reaction of UVO treatment is indispensable for rapid formation of high-quality PFDS SAM on a glass surface [11]. As illustrated in Figure 4a, the photo-oxidation reaction of UVO treatment can be summarized as surface contaminant decomposition and surface hydroxylation. Firstly, UV rays’ radiation causes the generation of atomic oxygen, and the ozone is synthesized when atomic oxygen reacts with oxygen. Simultaneously, UV rays and the newly formed ozone lead the carbon and nitrogen element-based contaminants absorbed on a glass surface to form H2O, NOx, and CO2, which can leave the glass surface during the treatment [10]. At the second stage, ozone photolysis was induced by UV/ozone treatment to produce hydroxyl radical (·OH) in the atmosphere. Then, ·OH, which is one of the most highly reactive chemical species, reacts with glass surface to form OH groups, providing a chemically active surface for adsorbing PFDS molecules [31]. Figure 4b shows schematically the formation process of PFDS film on glass surface. After physically adsorption of PFDS molecules on the hydroxylated surface, the hydrolyzation and polymerization occur, resulting in the final chemisorption and formation of PFDS SAM on glass [32].
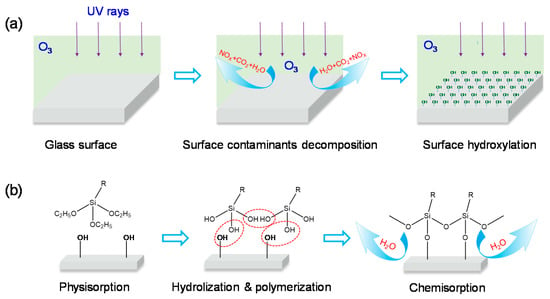
Figure 4.
The illustration of UVO-assisted rapid formation of PFDS self-assembled monolayer (SAM) on a glass surface. (a) The photo-oxidation reaction. (b) The formation mechanism of PFDS SAM on UVO-treated glass surface, where R represents the group CH2CH2(CF2)7CF3.
3.3. Friction and Wear Tests
Lubrication and wear resistance are crucial indicators for PFDS SAM to be used as a protective layer. To investigate the tribological property of SAM-coated glass surfaces, different normal loads were applied on glass surfaces during the scanning under friction force microscope (FFM) mode. For the friction tests, the friction force is calibrated using a silicon grating with a wedge angle of 54.77° to obtain calibration coefficient [33,34]. Under different applied normal loads, the friction force is gained when the output voltage signal is multiplied by the calibration coefficient [35]. Figure 5 shows the friction force measured from original glass, SAM-glass (0), and SAM-glass (10 min pretreatment) as the function of applied normal load during nanoscratch tests. SAM-glass (0) exhibits better lubricating property than original glass, and the lowest friction was obtained on SAM-glass (10 min pretreatment). The low friction force can be attributed to the energy dissipation of a compact-structured SAM [4], showing that a high-quality lubrication PFDS SAM was formed on 10 min UVO-pretreated glass surface.
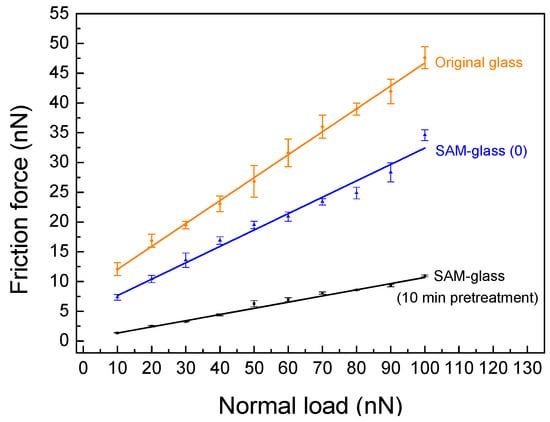
Figure 5.
Friction force plotted as a function of applied normal load measured from original glass, SAM-glass (0), and SAM-glass (10 min pretreatment), respectively.
In order to study the wear resistance of SAM-coated glass, a diamond tip with R of ~50 nm was used for nanowear tests with the area of 2 × 2 μm2 on the surface of original glass, SAM-glass (0), and SAM-glass (10 min pretreatment) under different normal loads. As can be seen from Figure 6a,b, hole-shaped scratches were produced on original glass and SAM-glass (0) under 20 and 40 nN, whereas no scratch could be detected on SAM-glass (10 min pretreatment) at the same loading conditions. In Figure 6c, a slight scratch appeared on SAM-glass (10 min pretreatment) surface under the applied normal load of 100 nN, corresponding to a Hertz contact pressure of 3.5 GPa [36]. In comparison, deeper holes were created on original glass and SAM-glass (0) at 100 nN. It was demonstrated that the SAM-glass (10 min pretreatment) presented the best antiwear performance in the nanowear tests, which was further supported by cross-section profiles in Figure 6d. In addition, as 3.5 GPa for wear occurrence (Figure 6) is much higher than the typical contact stress of ~330 MPa in dynamic MEMS [37], the SAM-glass (10 min pretreatment) surface is expected to withstand typical contact and sliding in dynamic devices. As a result, UVO assistance can facilitate rapid formation of high-quality SAM with excellent tribological performances.
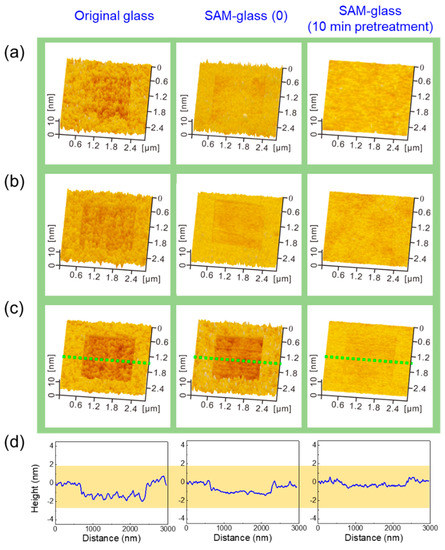
Figure 6.
The comparison of wear resistance of original glass and SAM-coated glass surfaces under different normal loads of (a) 20, (b) 40, and (c) 100 nN, respectively. (d) Cross-section profiles of the scratched area obtained from (c) AFM images along the dotted lines.
In summary, a high-quality PFDS SAM with excellent tribological performances can be produced rapidly with the assistance of UVO pretreatment. Investigations towards revealing optimal conditions for UVO pretreatment for high-quality SAM formation were conducted. It was noted that the chemical activation by UVO pretreatment is efficient and environmentally friendly for film assembling comparing to traditional immersion in a chemical solution [4]. For the rapid formation of different silane films, a similar mechanism, such as surface contamination removal and exposure of fresh surface, can facilitate the film adsorption, as depicted in Figure 4. Expanding the UVO emission scale can make it feasible to form a high-quality self-assembled film with good tribological performances on the surface of large-size engineering materials, such as stainless steel, to prolong the material’s serviceable life.
4. Conclusions
In this paper, UV/ozone-treated glass surface was employed for the formation of high-quality -CF3-tailed silane SAM. On the basis of investigations of surface topography, hydrophobicity, chemical composition, and tribological performances of the film, conclusions can be drawn, as shown below.
- The hydrophilicity and roughness changes of the glass surface were detected with UVO treatment time, and hydrophilic glass with the smallest surface roughness was prepared at 10 min UVO treatment.
- After deposition in PFDS solution for 2 h, high-quality PFDS SAM was rapidly formed on a 10 min UVO-treated glass surface.
- Compared with original glass surface and film-deposited glass without pretreatment, SAM-coated glass with 10 min pretreatment possessed excellent lubrication and antiwear properties.
Author Contributions
Methodology, Z.F. and B.Y.; Investigation, Z.F. and P.Z.; Data curation, L.W. and L.D.; Writing—original draft preparation, Z.F. and C.F.; Writing—review and editing, B.Y., C.Z. and L.Q.; Funding acquisition, C.Z. and B.Y.
Funding
This research was funded by the Sichuan Science and Technology Program (2018HH0151), National Natural Science Foundation of China (51775462), and Science and Technology Innovative Fund from Institute of Electronic Engineering, China Academy of Engineering Physics (S201901).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhushan, B.; Kasai, T.; Kulik, G.; Barbieri, L.; Hoffmann, P. AFM study of perfluoroalkylsilane and alkylsilane self-assembled monolayers for anti-stiction in MEMS/NEMS. Ultramicroscopy 2005, 105, 176–188. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Influence of chain ordering on frictional properties of self-assembled monolayers (SAMs) in nano-lubrication. Adv. Colloid Interface 2012, 171–172, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, D.; Song, Y.; Han, E. Effect of assembled time on the corrosion behaviors of SAMs film on the AM60B alloy and its assembled mechanism. Mater. Chem. Phys. 2015, 149–150, 559–565. [Google Scholar] [CrossRef]
- Yu, B.; Qian, L.; Yu, J.; Zhou, Z. Effects of tail group and chain length on the tribological behaviors of self-assembled dual-layer films in atmosphere and in vacuum. Tribol. Lett. 2009, 34, 1–10. [Google Scholar] [CrossRef]
- Huo, L.; Du, P.; Zhang, K.; Liu, P.; Zhou, H. Self-assembled monolayer of multiply-alkylated cyclopentenes on silicon via thiol-ene “click” reaction and its self-lubricating properties. Appl. Surf. Sci. 2019, 477, 96–103. [Google Scholar] [CrossRef]
- Li, G.; Zheng, S.; Bai, C.; Li, X.; Cheng, C. Self-assembled monolayer of mica coating using organobisphosphonic acid. Appl. Surf. Sci. 2018, 457, 449–455. [Google Scholar]
- Hosseinpour, S.; Forslund, M.; Johnson, C.M.; Pan, J.; Leygraf, C. Atmospheric corrosion of Cu, Zn, and Cu–Zn alloys protected by self-assembled monolayers of alkanethiols. Surf. Sci. 2016, 648, 170–176. [Google Scholar] [CrossRef]
- Kondo, R.; Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Superhydrophilic and superhydrophobic aluminum alloys fabricated via pyrophosphoric acid anodizing and fluorinated SAM modification. J. Alloys Compd. 2017, 725, 379–387. [Google Scholar] [CrossRef]
- Lee, H.C.; Jo, M.; Lim, H.; Yoo, M.S.; Lee, E.; Nguyen, N.N.; Han, S.Y.; Cho, K. Toward near-bulk resistivity of Cu for next-generation nano-interconnects: Graphene-coated Cu. Carbon 2019, 149, 656–663. [Google Scholar] [CrossRef]
- Kohli, R.; Mittal, K.L. Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wang, H.; Yu, B.; Jiang, S.; Jiang, L.; Qian, L. UV/ozone-assisted tribochemistry-induced nanofabrication on Si(100) surface. RSC Adv. 2017, 7, 39651–39656. [Google Scholar] [CrossRef]
- Wu, L.; Fan, Z.; Peng, Y.; Zhou, H.; Wang, H.; Yu, B.; Qian, L. Rapid nanofabrication via UV-assisted selective etching on GaAs without templates. Chem. Phys. Lett. 2019, 717, 152–157. [Google Scholar] [CrossRef]
- Kimura, Y.; Kasai, K.; Miyata, S. Feeder-free culture for mouse induced pluripotent stem cells by using UV/ozone surface-modified substrates. Mat. Sci. Eng. C-Mater. 2018, 92, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Li, Y.; Yang, L.; Fan, Z.; Liu, C.; Zhang, X. Improved self-assembly through UV/ozone surface-modification of colloidal spheres. Thin Solid Films 2011, 519, 5203–5207. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Domantovskiy, A.G.; Emelyanenko, A.M.; Pashinin, A.S.; Ionin, A.A.; Kudryashov, S.I.; Saltuganov, P.N. Femtosecond laser treatment for the design of electro-insulating superhydrophobic coatings with enhanced wear resistance on glass. ACS Appl. Mater. Int. 2014, 6, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.C.O.; Schmuki, P.; Killian, M.S. Tuning Anatase Surface Reactivity toward Carboxylic Acid Anchor Groups. Langmuir 2017, 33, 13913–13922. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Yan, H.; Ji, S.; Zhang, G. Designing superhydrophobic surfaces with SAM modification on hierarchical ZIF-8/polymer hybrid membranes for efficient bioalcohol pervaporation. RSC Adv. 2014, 4, 59750–59753. [Google Scholar] [CrossRef]
- Cichomski, M. Tribological investigation of perfluoroalkysilanes monolayers deposited on titanium surface. Mater. Chem. Phys. 2012, 136, 498–504. [Google Scholar] [CrossRef]
- Wang, B.; Hua, Y.; Ye, Y.; Chen, R.; Li, Z. Transparent superhydrophobic solar glass prepared by fabricating groove-shaped arrays on the surface. Appl. Surf. Sci. 2017, 426, 957–964. [Google Scholar] [CrossRef]
- Cichomski, M.; Kośla, K.; Pawlak, W.; Kozłowski, W.; Szmaja, W. Stability and tribological investigations of 1H,1H,2H,2H-perfluoroalkyltrichlorosilane on titania surface. Tribol. Int. 2014, 77, 1–6. [Google Scholar] [CrossRef]
- Wu, L.; Cai, L.; Liu, A.; Wang, W.; Yuan, Y.; Li, Z. Self-assembled monolayers of perfluoroalkylsilane on plasma-hydroxylated silicon substrates. Appl. Surf. Sci. 2015, 349, 683–694. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology and Nanomechanics: Self-Assembled Monolayers (SAMs) for Nanotribology and Surface Protection; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Fu, Y.; Qui, H.; Liao, K.; Lue, S.; Hu, C.; Lee, K.R.; Lai, J. Effect of UV-ozone treatment on poly(dimethylsiloxane) membranes: Surface characterization and gas separation performance. Langmuir 2010, 26, 4392–4399. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yan, W.; Liu, Z.; Li, J. Hydrophilic surface modification of polydimethylsiloxane with UV/ozone treatment. Spectrosc. Spect. Anal. 2016, 36, 1033–1037. (In Chinese) [Google Scholar]
- Uehara, T.M.; de Aguiar, H.B.; Bergamaski, K.; Miranda, P.B. Adsorption of alkylthiol self-assembled monolayers on gold and the effect of substrate roughness: A comparative study using scanning tunneling microscopy, cyclic voltammetry, second-harmonic generation, and sum-frequency generation. J. Phys. Chem. C 2014, 118, 20374–20382. [Google Scholar] [CrossRef]
- Aswal, D.K.; Lenfant, S.; Guerin, D.; Yakhmi, J.V.; Vuillaume, D. Self assembled monolayers on silicon for molecular electronics. Anal. Chim. Acta 2006, 568, 84–108. [Google Scholar] [CrossRef]
- Hong, H.; Park, W. A study of adsorption kinetics and thermodynamics of ω- mercaptoalkylhydroquinone self-assembled monolayer on a gold electrode. Electrochim. Acta 2005, 51, 579–587. [Google Scholar] [CrossRef]
- Choi, J.; Kawaguchi, M.; Kato, T. Self-assembled monolayer formation on magnetic hard disk surface and friction measurements. J. Appl. Phys. 2002, 91, 7574–7576. [Google Scholar] [CrossRef]
- Kang, C.; Lu, H.; Yuan, S.; Hong, D.; Yan, K.; Liang, B. Superhydrophilicity/superhydrophobicity of nickel micro-arrays fabricated by electroless deposition on an etched porous aluminum template. Chem. Eng. J. 2012, 203, 1–8. [Google Scholar]
- Yuan, S.; Pehkonen, S.O.; Liang, B.; Ting, Y.; Neoh, K.G.; Kang, E. Superhydrophobic fluoropolymer-modified copper surface via surface graft polymerisation for corrosion protection. Corros. Sci. 2011, 53, 2738–2747. [Google Scholar] [CrossRef]
- Yue, P. Modelling of kinetics and reactor for water purification by photo-oxidation. Chem. Eng. Sci. 1993, 48, 1–11. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, X.; Jiang, B.; Weng, C. Fabrication of high aspect ratio nanopillars and micro/nano combined structures with hydrophobic surface characteristics by injection molding. Appl. Surf. Sci. 2018, 427, 854–860. [Google Scholar] [CrossRef]
- Varenberg, M.; Etsion, I.; Halperin, G. An improved wedge calibration method for lateral force in atomic force microscopy. Rev. Sci. Instrum. 2003, 74, 3362–3367. [Google Scholar] [CrossRef]
- Yu, B.; Qian, L.; Dong, H.; Yu, J.; Zhou, Z. Friction-induced hillocks on monocrystalline silicon in atmosphere and in vacuum. Wear 2010, 268, 1095–1102. [Google Scholar] [CrossRef]
- Chen, L.; Li, N.; Yang, B.; Zhang, J. A comparative study of the tribological behaviors of CH3- and CF3-terminated bilayer films. J. Adhes. Sci. Technol. 2016, 30, 677–689. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Satyanarayana, N.; Sinha, S.K. Tribology of PFPE over coated self-assembled monolayers deposited on Si surface. J. Phys. D Appl. Phys. 2005, 38, 3512–3522. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).