Abstract
The polyurethane prepolymer terminated with a double bond was synthesized using isophorone diisocyanate (IPDI), hydroxyl terminated polybutadiene (HTPB), 1,4-butanediol (BDO), and 2-hydroxyethyl acrylate (HEA). Then, a series of innovative UV-curable polyurethane coatings were prepared by blending ene-terminated polyurethane, fluoroacrylate monomer, and multifunctional thiol crosslinker upon UV exposure. The incorporation of fluoroacrylate monomer and multifunctional thiols into polyurethane coatings significantly enhanced the hydrophobic property, mechanical property, pencil hardness, and glossiness of the polyurethane coatings. This method of preparing UV crosslinkable, hydrophobic polyurethane coatings based on thiol-ene chemistry exhibited numerous advantages over other UV photocuring systems.
1. Introduction
Polyurethane (PU) is one of the most versatile polymers. By changing the type and functionality of the polyol and isocyanate precursors, the properties of PU can be easily tailored, ranging from rigid solid to flexible elastomer [1,2,3]. Fluorinated polyurethane coatings not only maintain most of the outstanding mechanical properties of polyurethane, but also offer the basic advantages of improved solvent and chemical resistance, low surface energy, and low coefficient of friction by introducing a fluorine chain segment into the molecular chains of polyurethane. They thus have wide applications in the area of coatings, such as leather decoration, textiles, and medicine [4,5,6,7,8,9,10,11,12]. During the last few decades, much attention has been focused on introducing fluorinated polyether polyols into polyurethane to prepare fluorinated polyurethane. Liu et al. [13] synthesized fluorinated polyurethanes using novel fluorinated polyether diols and poly (butyl acrylate) as common soft segments, aiming at enlarging the molecular weight and improving the mechanical properties. However, the low reaction activity of the fluorinated polyol leads to the low molecular weight and poor mechanical property of the resulting products, which may restrict the wide applications of fluorinated polyurethane (FPU). Moreover, compared to other polyols, the fewer species and quantities of the fluorinated polyol further limited their use as modifying monomers for fluorinated polyurethane [14,15,16,17].
Ultraviolet (UV)-curing technology has gained increasing interests due to its high curing speed, environment safety, cost efficiency, low energy consumption, low temperature, and enhanced performance [18,19,20,21,22,23,24,25,26,27]. UV-curable polyurethane (PU) films have been extensively studied because of their structure versatility, environmental friendliness, and terrific mechanical properties [28,29,30]. The method of urethane-fluoroacrylate UV photopolymerization is another way to obtain fluorinated polyurethane. Çakmakçı et al. [31] prepared fluorine-containing photocurable hybrid coatings with thiol-ene click reaction and a sol-gel technique. 3-mercaptopropyltrimethoxysilane (MPTMS) was used as a coupling agent, and perfluorooctyltriethoxysilane (PFOTES) was used to introduce fluorine. The addition of fluorine and silica improved the mechanical property, flame retardancy, hydrophobicity, and oleophobicity of the coatings. A similar study was also conducted by Mackey et al. [32]. They prepared a series of organosilicate hydrolyzable monomers combined with trimethylolpropane tris (3-mercaptopropionate) (tri-thiol) in a thiol-ene “click” polymerization reaction to produce clear, colorless thiol-ene networks using radiation and thermal-cure techniques. Reinelt et al. synthesized oligomers based on innovative thiol derivative 4a for the production of low-odor and low-shrinkage thiol-ene dental composites, which showed good mechanical properties and reduced shrinkage stress [33].
In this work, the polyurethane prepolymer resins terminated with a double bond were synthesized. Then, a series of UV-curable polyurethane coatings were prepared. To improve the hydrophobicity of the coatings, (N-ethylperfluorooctylsulfonamido) methyl acrylate (EFCSA) was introduced by way of photo-initiated polymerization. Differing from the conventional method of preparing fluorinated polyurethane films, pentaerythritol tetra (3-mercaptopropionate) (PETMP) was further added to accelerate the polymerization rate and improve the reaction depth of the UV-curable polyurethane coatings based on a thiol-ene click reaction. The mechanical property, hydrophobicity, gel yield, pencil hardness, glossiness, and water absorption of the coatings were tailored by varying the contents of EFCSA and PETMP. The significance of this study lies in introducing a thiol-ene click crosslinking system for the fabrication of polyurethane coatings, which resulted in the advantages of high curing speed, low energy consumption, and improved yellowing resistance. This method could be easily achieved in the industrial application of UV-curable films.
2. Experimental
2.1. Materials
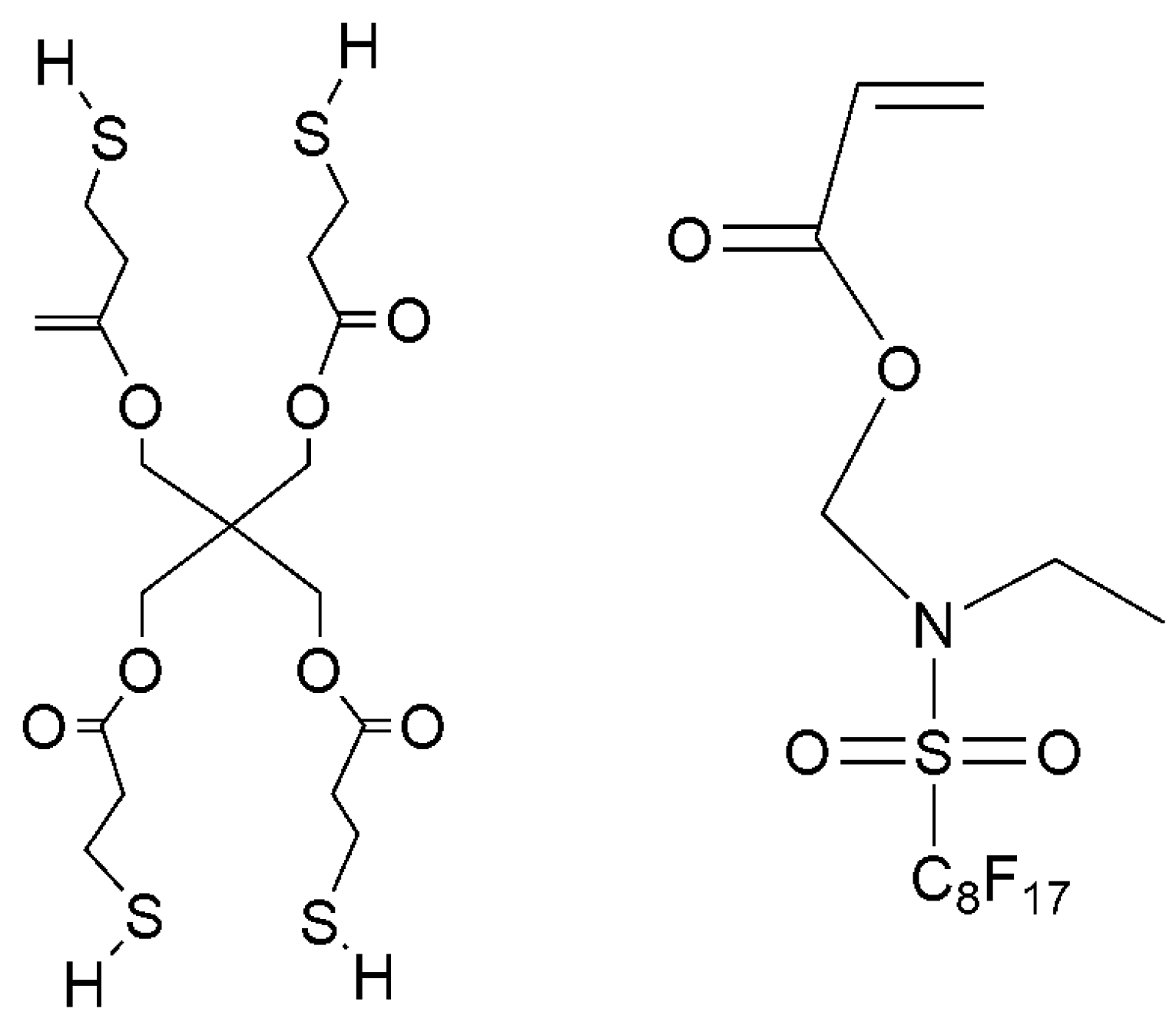
Hydroxyl terminated polybutadiene (HTPB, Mn = 3500–4500 g/mol) was received from Zibo Qilong Chemical Industry Co., Ltd, Zibo, China. Isophorone diisocyanate (IPDI), 1,4-butanediol (BDO), 2-hydroxyethyl acrylate (HEA), dibutyltin dilaurate (DBTDL), and 1-hydroxycyclohexyl acetophenone (Irgacure 184) were received from Wuxi Meihong Chemical Company, Wuxi, China. Pentaerythritol tetra (3-mercaptopropionate) (PETMP) was purchased from Aladdin Industry Corporation, Shanghai, China. (N-ethylperfluorooctylsulfonamido) methyl acrylate (EFCSA) was supplied by Beijing Zhongxi Science Technology Co., Ltd, Beijing, China. The structure of PETMP and EFCSA were illustrated in Scheme 1.
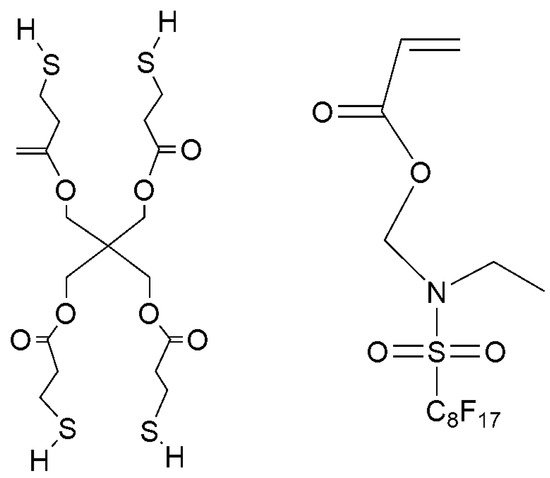
Scheme 1.
Chemical structures of PETMP and EFCSA.
Ethylacetate, toluene and chloroform were purchased from Sinopharm Chemical Reagent, Shanghai, China. All the solvents were purified with calcium hydride at room temperature before use. HTPB and BDO were dried in a vacuum oven at 60 °C overnight to remove residual water before reaction. The other chemicals were used as received without further purification.
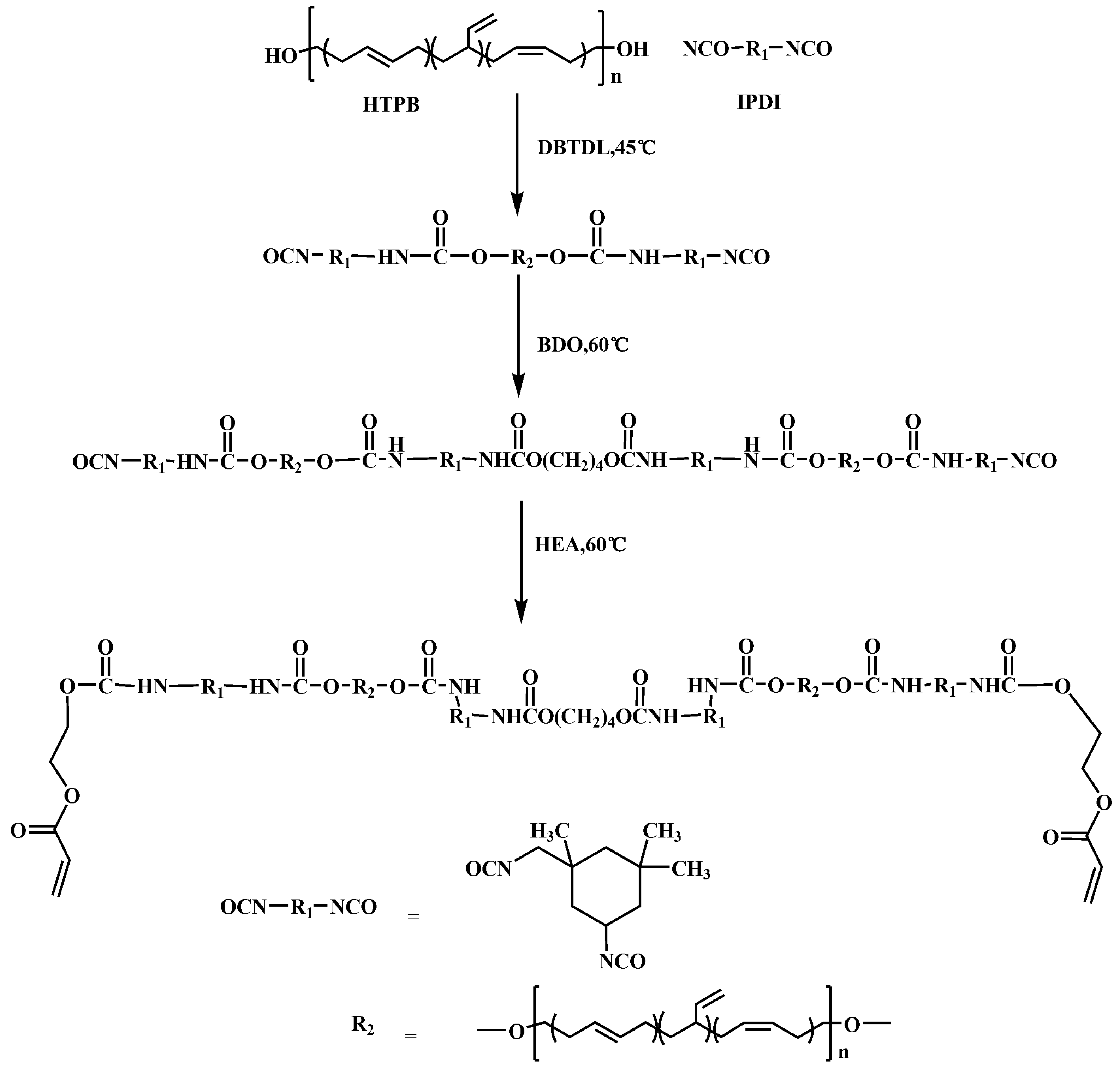
2.2. Synthesis of HEA-Terminated Polyurethane Resins
The HEA-terminated polyurethane resin (abbreviated as HTPU) was synthesized by a three-step polymerization. The overall preparation procedure is illustrated in Scheme 2. The stoichiometry of IPDI/HTPB/BDO/HEA was 4:2:1:1. In a typical example, HTPB (60 g, 30 mmol –OH) was dissolved with ethylacetate (20 mL) in a three-necked, round-bottom flask, and IPDI (6.67 g, 60 mmol –NCO) was added dropwise under magnetic stirring for 5 min, followed by the addition of three drops of DBTDL. The prepolymerization reaction was allowed to proceed for 2.5 h at 45 °C until the value of isocyanate reached the theoretical value by titration [34]. Afterward, BDO (0.68 g, 15 mmol –OH) dissolved in ethylacetate (40 mL) was added dropwise, and the reaction continued for another 3 h at 60 °C. Finally, HEA (1.74 g, 15 mmol –OH) was added into the flask, and the reaction mixture was stirred for about 3 h until the absorption peak of the –NCO group in the infrared spectra disappeared. The detailed information for synthesis of HTPU is summarized in Table 1.
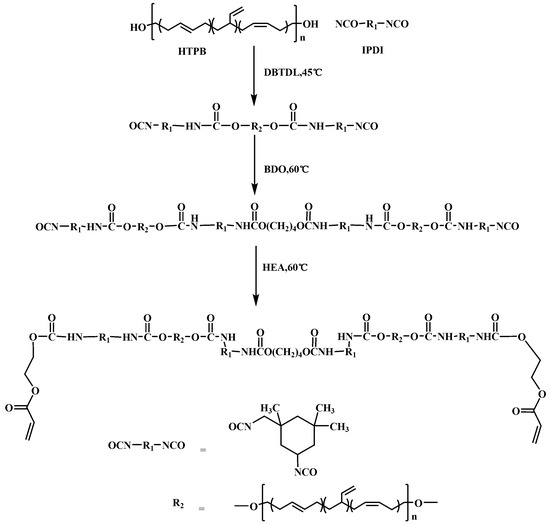
Scheme 2.
Preparation process of HTPU.

Table 1.
The molecular weight, molecular weight distribution and viscosity of HTPU.
2.3. Preparation of Complex Coating Solutions and Coatings
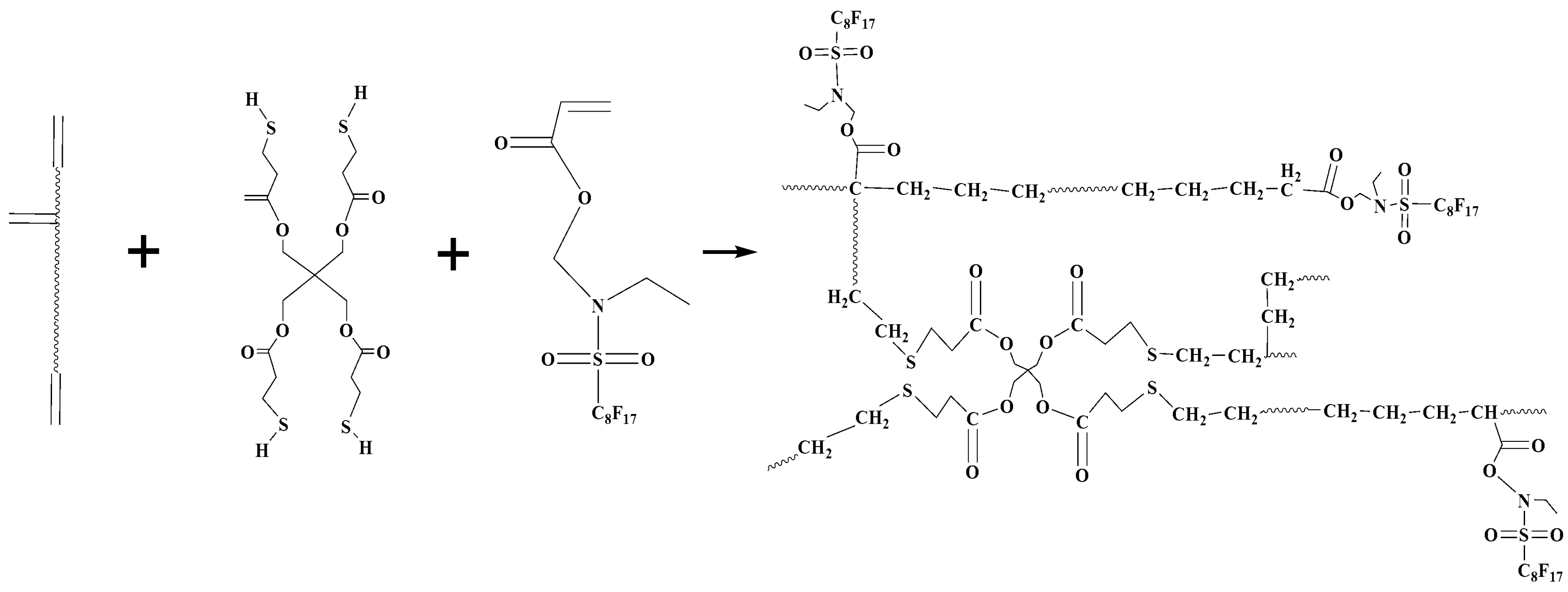
EFCSA (~5 wt %), crosslinker PETMP (~4 wt %), and photoinitiator Irgacure 184 (2 wt %) were added to the viscous urethane acrylate polyurethane resin at 45 °C for 3 h to form a series of UV-curable fluorinated urethane acrylates mixtures. The preparation procedure is illustrated in Scheme 3 and the resulted fluorinated urethane acrylates are abbreviated as HFTPU. Table 2 lists the composition of the UV-curable fluorinated urethane-acrylate. All the samples in this study were stored in the dark at room temperature before irradiation (curing). The UV-cured fluorinated urethane acrylates coatings (thickness: 0.3 mm) were prepared by casting the mixture onto a glass plate at room temperature followed by curing using a medium pressure mercury lamp (38 W/m2). The distance between the lamp and the sample was 20 cm. The UV-cured coatings were dried in a convection oven for at 50 °C for 24 h.
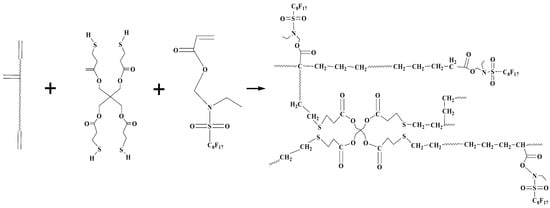
Scheme 3.
Preparation process of HFTPU.

Table 2.
Formulation of UV-curing coatings. HTPU (100 g), photoinitiator 184 (2 wt %).
2.4. Characterization
FTIR analysis was performed with an FTLA2000-104 spectrophotometer (Thermofisher Technology Co., Ltd., Waltham, MA, USA) between 4000 and 500 cm−1 at room temperature. 1H NMR analysis was carried out with a nuclear magnetic resonance spectrometer (AVANCE III HD 400 MHz, CDCl3, Bruker, Switzerland). The adhesions of the HTPU and HFTPU coatings were evaluated by means of a 3M tape test on aluminum sheets. A square of the samples were scratched (1 × 1 mm2) using a utility knife and, after 2 min, the tape was stripped away rapidly at a certain angle. There were five levels to differentiate the value of adhesion; 5 and 0 represented total detachment and no detachment, respectively. Gloss measurements were performed with a Byk-Gardner Multi-Gloss meter (Altana AG, Wesel, Germany). The static optical contact angle (OCA) on the surface hydrophobicity of the coatings were analyzed by evaluating the water contact angle (WCA) using a Drop Shape Analysis System (OCA40, Dataphysics, Beijing Eastern-Dataphy Instruments Co., Ltd., Beijing, China) with distilled water. Contact angle measurements were performed within 190 s. Water absorption was evaluated in terms of the difference of the wet mass (W1) and dry mass (W2) of the coating (20 × 20 × 0.12 mm3), and was calculated using the following equation:
Yellowing resistance was determined using a UV-Visible spectrophotometer (TU-1901, Beijing Purkinje General Instrument Co., Ltd., Beijing, China). To investigate the mechanical properties via uniaxial tensile testing, dumbbell-shaped strips (4 × 10 × 0.5 mm3, n = 5) were cut from the polymer films and their mechanical properties were studied on an Instron 5967 Dual Column Tabletop Testing Systems (Instron, Norwood, MA, USA) at room temperature with a tensile rate of 10 mm/min. The thickness of the samples was measured with a Gauge meter (Shanghai Liuling Instrument, Shanghai, China). Cyclic tensile testing (five cycles) at deformation (100%) was performed as previously described [35]. Before the measurement of their mechanical properties, the samples were kept at room temperature for 24 h to release any stress. The photopolymerization kinetics of the samples was investigated by real-time infrared spectroscopy (RT-IR) on a Nicolet 6700 instrument (Thermo Fisher Scientific Co., Ltd.) at room temperature. All the samples were spread on one side of a KBr disk, with spacers 15 ± 1 mm in diameter and 0.3 ± 0.1 mm thickness. A UV spot light source (OmniCure series 1000, EXFO Co. Ltd., Quebec City, QC, Canada) was irradiated directly onto the sample. The decrease of the C=C absorption peak area from 821.70 to 796.50 cm−1 in the near-IR range accurately reflected the extent of the polymerization, since the change of the absorption peak area was directly proportional to the number of the double bonds that had polymerized. After baseline correction, the conversion of the functional groups could be calculated by measuring the peak area at each time of the reaction. The rate of photopolymerization was calculated by the differential of the conversion of double bonds versus irradiation time.
3. Results and Discussion
3.1. FTIR Analysis
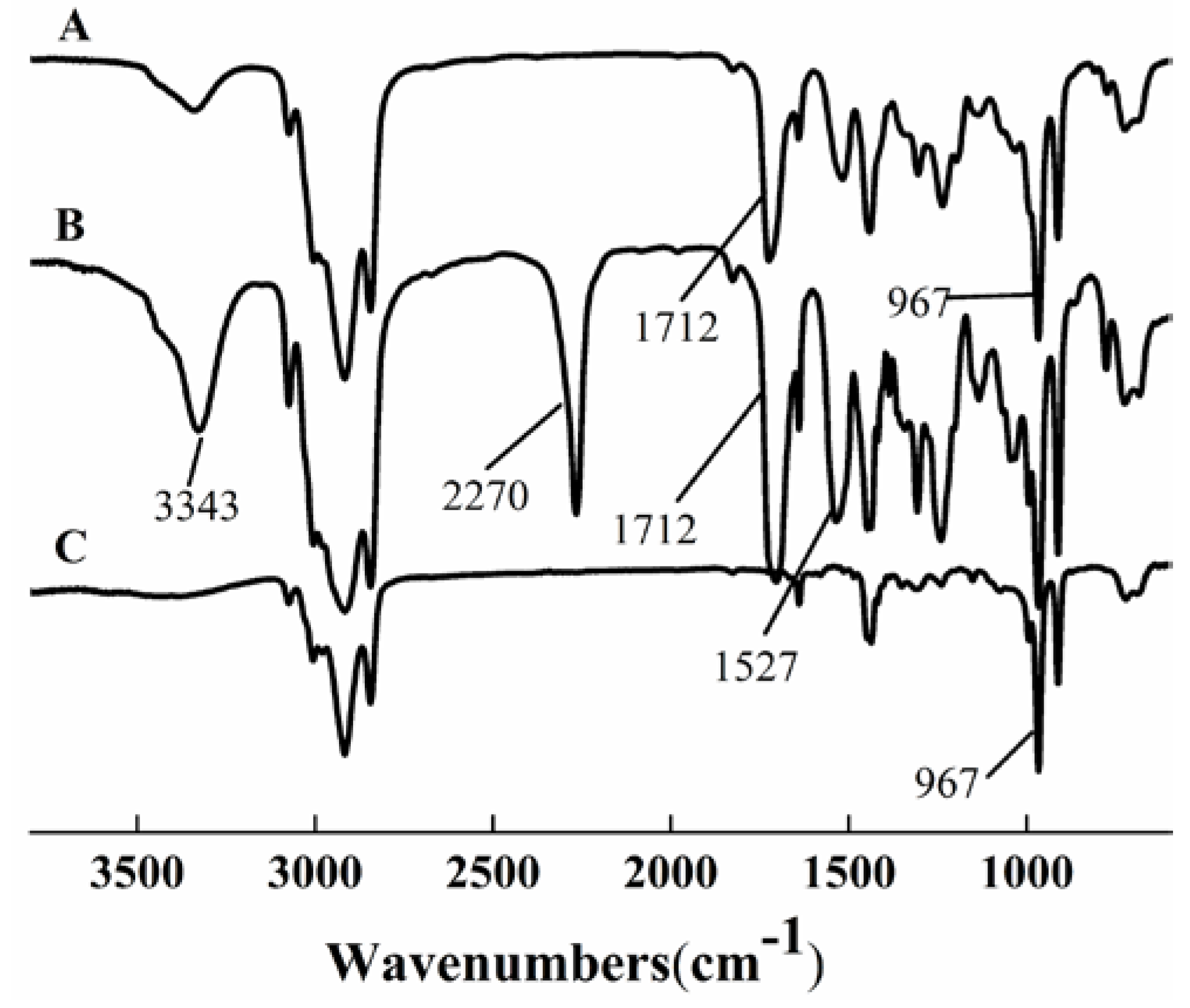
The FTIR spectra of HTPB, prepolymer, and HTPU are shown in Figure 1. The characteristic peak at 967 cm−1 of HTPB (curve C) corresponded to the trans olefin structure [36]. The band at 1712 cm−1 of prepolymer (curve B) corresponded to the stretching C=O vibration of the –NH–COO– group [37], and the appearance of a band at 967 cm−1 indicated the chemical reaction between –OH of HTPB and –NCO of IPDI. The bands at 3343 and 1527 cm−1 were the characteristic peaks of –NH– [38]. In curve A, the disappearance of the isocyanate band at 2270 cm−1 [37] and the appearance of the –NH– band of urethane linkage at 3343 cm−1 confirmed the formation of HTPU.
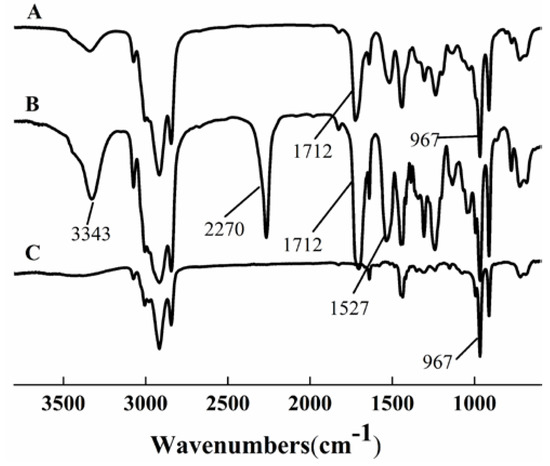
Figure 1.
FT-IR spectra of HTPU (A), prepolymer (B), and HTPB (C).
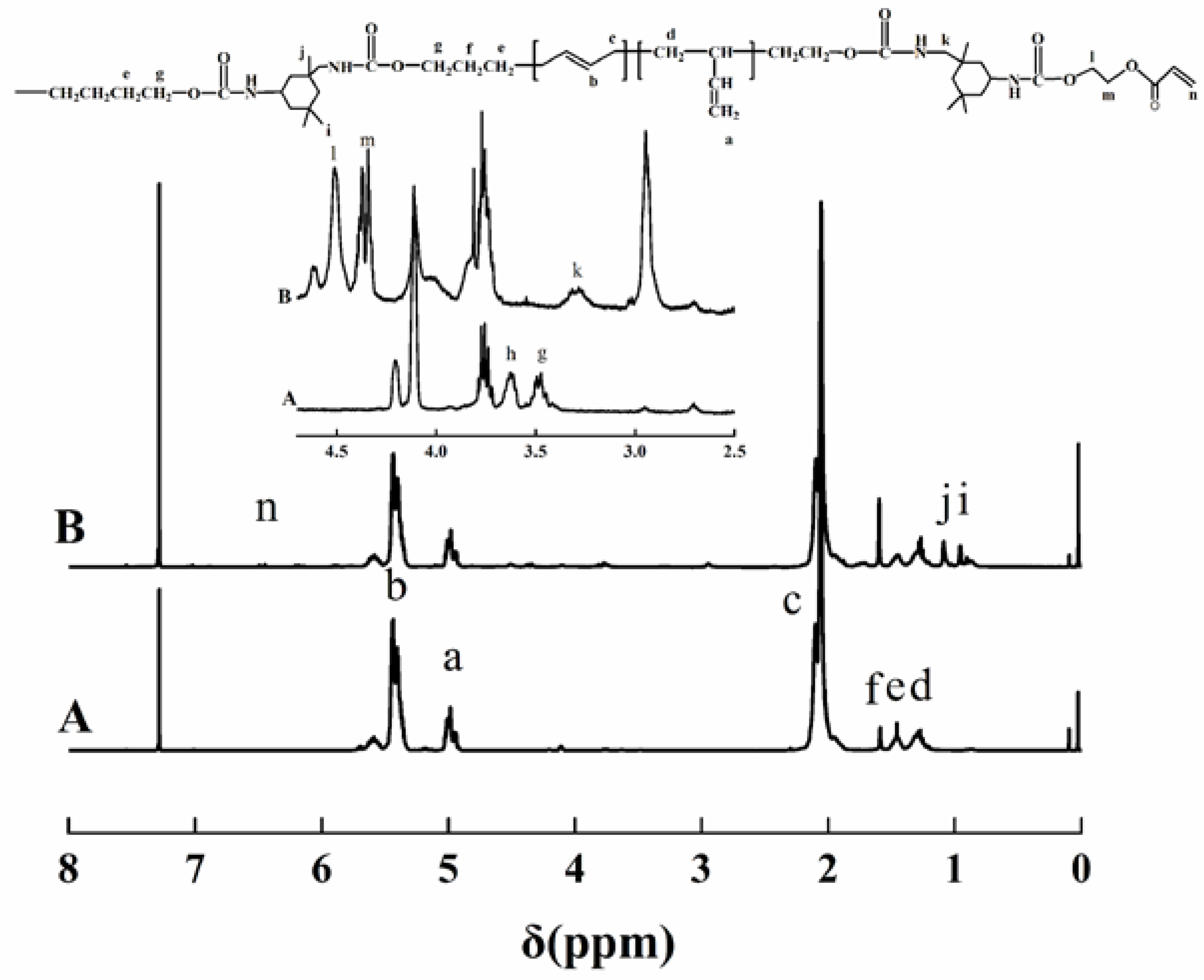
3.2. 1H NMR Analysis
Figure 2 shows the 1H NMR spectra of HTPB and HTPU represented by A and B, respectively. The chemical shifts at 5.0, 5.40, and 2.03 ppm attributed to the 1,2-structure –CH=CH2, 1,4-structure –CH=CH–, and –CH=CH–CH2– (indicated by a, b, and c, respectively) of HTPB appeared in HTPU [39,40]. Additionally, the disappearance of the signal at 3.62 ppm, assigned to the hydroxyl group proton, confirmed the introduction of HTPB into HTPU. The signals of 0.95 and 1.09 ppm (indicated by i, j, respectively) were attributed to the protons that attached to the methylene of IPDI. Moreover, the peaks at 1.46 and 3.47 ppm (indicated by e,g, respectively) belonged to the protons of –O–CH2– and –O–CH2–CH2–. These results indicated that the structure of HTPU was correct, which was also in accordance with the FTIR analysis.
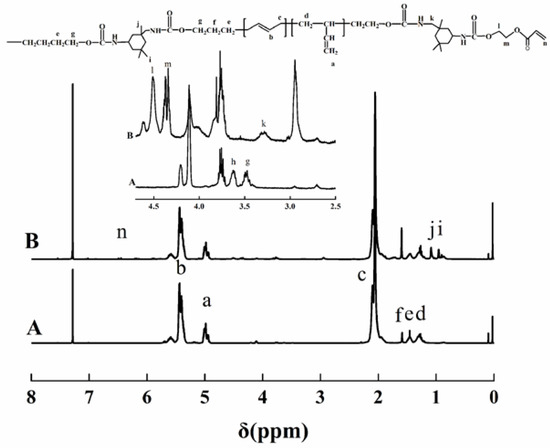
Figure 2.
1H NMR spectra of HTPB and HTPU in CDCl3.
3.3. Photo-Polymerization Kinetics Analysis
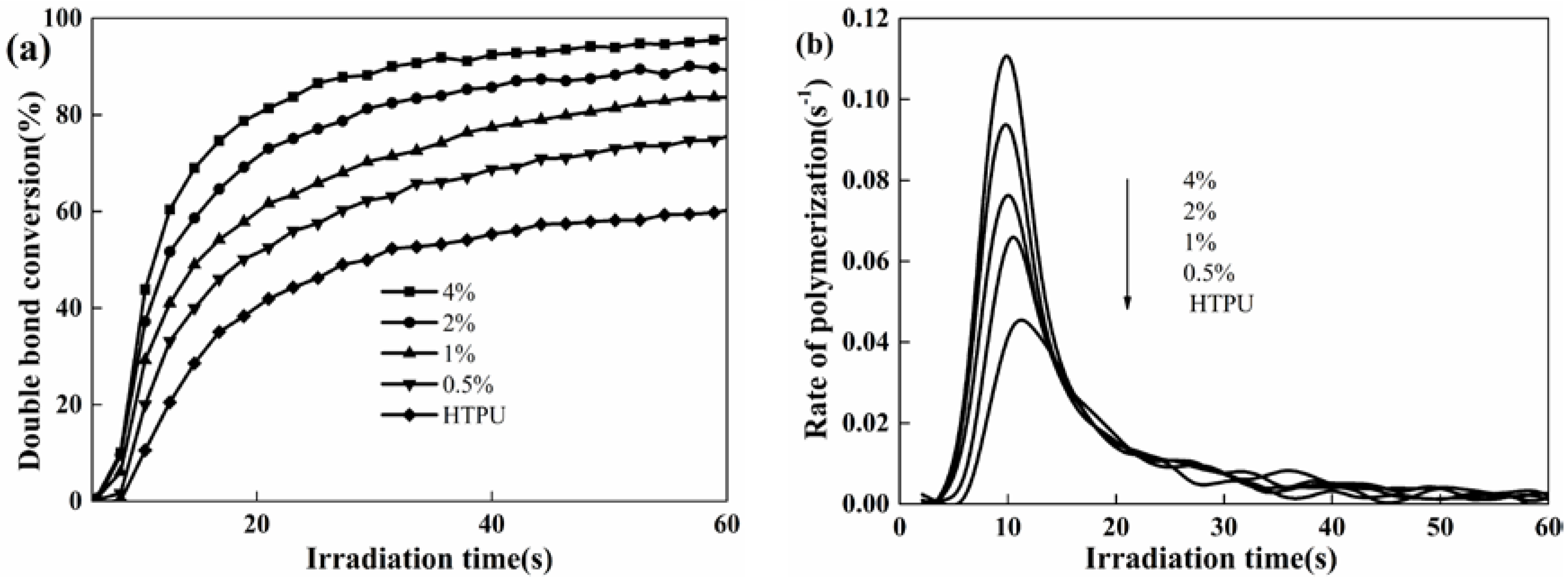
Herein, RT-IR analysis was used to investigate the photopolymerization kinetics of the polyurethane coatings. The final degree of double bond conversion (DC) and the rate of polymerization (Rp) [41], varying with the content levels of PETMP and photoinitiator 184, were analyzed. The effect of the photoinitiator 184 content on the DC and Rp of the polyurethane coatings are shown in Figure 3. The DC and Rp exhibited the same increasing tendency, and the DC achieved up to 90% when the photoinitiator dosage was 3 wt %. However, the excessive use of the photoinitiator in the UV-curing system might cause the coatings to yellow and affect the properties of the polyurethane coatings, such as their mechanical properties and yellowing resistance. As the photoinitiator 184 content increased from 2% to 3%, the DC and Rp had no significant changes, and maintained the same photoinitiation function. Thus, 2% photoinitiator 184 was appropriate for the UV-curing system.
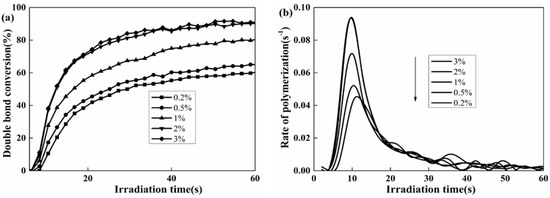
Figure 3.
Effect of photoinitiator 184 content on the double bond conversion (DC) (a) and the rate of polymerization (Rp), (b) Coating composition: HTPU, PETMP (2 wt %), EFCSA (2 wt %).
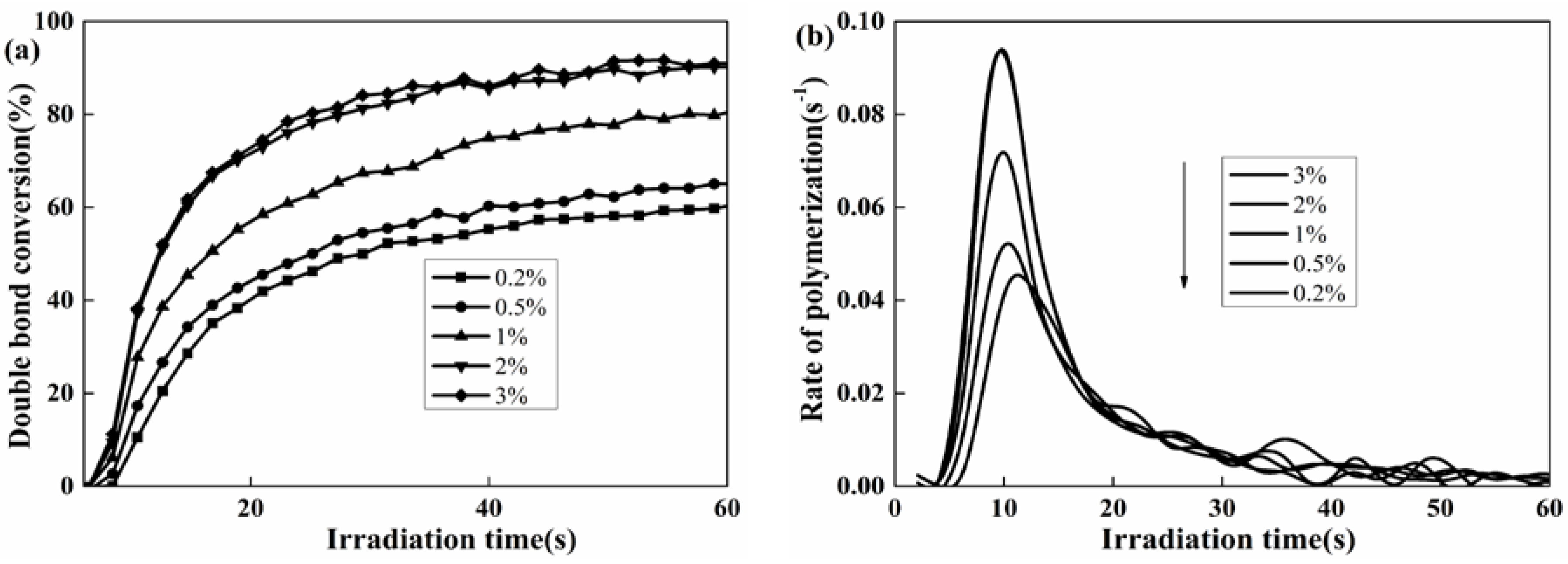
To verify that the thiol not only contributes to reduce the usage of photoinitiator, but also effectively promotes the DC and Rp, the effect of PETMP was further investigated. As shown in Figure 4, DC and Rp significantly increased in the system after the addition of PETMP. Also, both DC and Rp enhanced with increasing PETMP content. This may be because a small amount of 1,2-olefin structure in the side chain of HTPB does not completely participate in the photopolymerization reaction without thiol. After adding PETMP into the system, there was a deep reaction between the double bond in HTPB and thiol. Additionally, the reduced oxygen inhibition due to the addition of PETMP could be responsible for the increase in DC. The DC was almost 100% when the percentage of PETMP was above 2%.
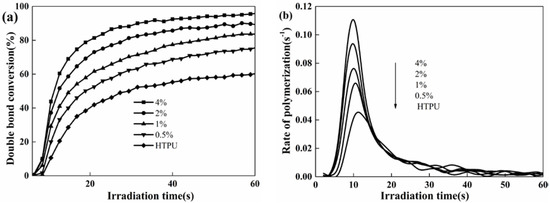
Figure 4.
Effect of PETMP content on the double bond conversion (DC) (a) and the rate of polymerization (Rp), (b) Coatings composition: HTPU, EFCSA (2 wt %), photoinitiator 184 (2 wt %).
3.4. Coating Properties Analysis
The effect of EFCSA content on coating properties is shown in Table 3. When the EFCSA content increased from 1% to 5%, the hardness of the polyurethane coatings enhanced from 5B to 2B, and the glossiness and tape adhesion notably increased. Meanwhile, the water absorption dropped from 5.2% to 1.8%, which might be due to the enrichment of the fluorine elements on the coatings surface of the cross-linked HFTPU.

Table 3.
The effect of EFCSA content on coating properties.
Table 4 shows the properties of coatings with the addition of PETMP in the UV-cured system. It was easily found that the hardness, glossiness, tape adhesion, and water absorption of the polyurethane coatings were further enhanced. This indicated that more and more double bonds could easily participate in the photo-curing reaction system due to the reaction between thiol and the double bonds. In addition, the lower viscosity of the system could promote a full crossing-linking reaction to form a more compact crossing-linking structure.

Table 4.
The effect of PETMP content on coating properties.
3.5. Yellowing Resistance
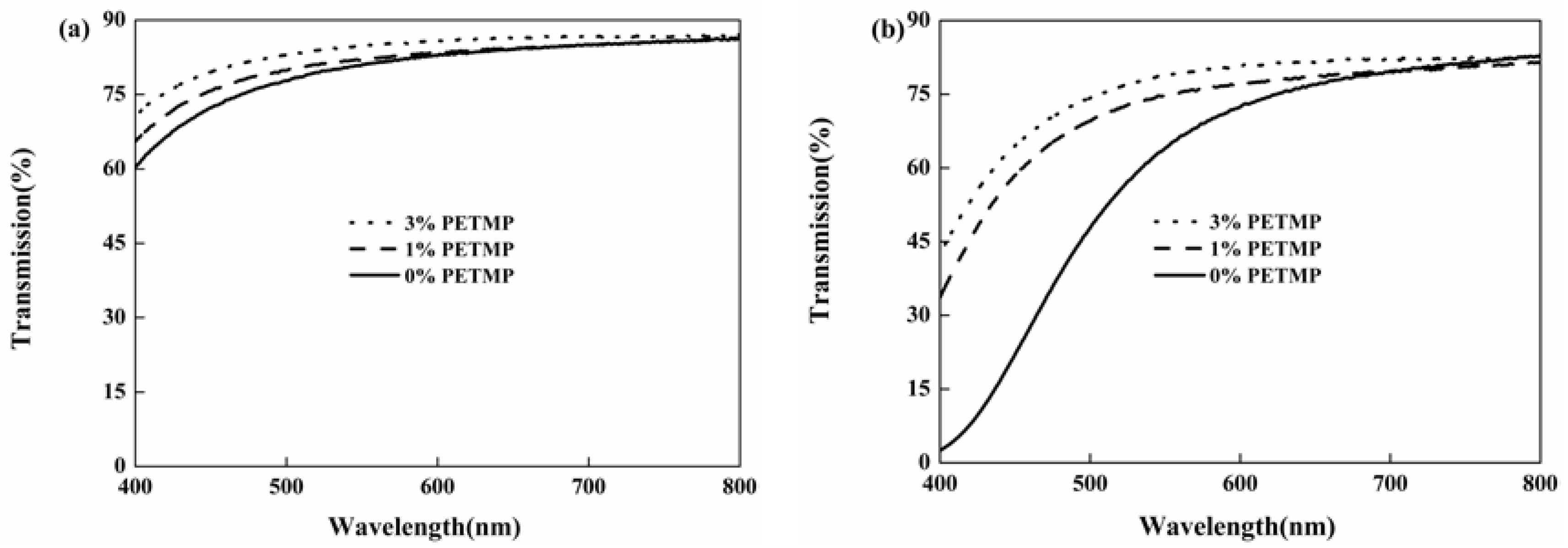
Figure 5 shows the effect of PETMP on the yellowing resistance of UV-cured polyurethane coatings (Formulation 1, 6, and 8). The transmittance of HTPU systems reduced by 50%–70% after placing at 120 °C for 4 h. However, when adding PETMP into the system, the decrease in the transmittance tendency of the polyurethane coating was obviously restrained. This indicated that PETMP could effectively promote the yellowing resistance of UV-cured polyurethane coatings. With the addition of PETMP, the antioxidative behavior of thioether groups resulted in the improved yellowing resistance of polyurethane coatings [42].
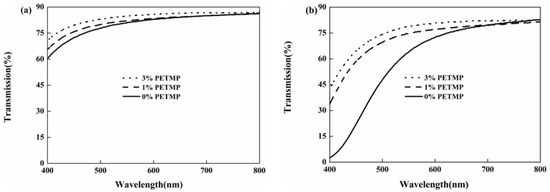
Figure 5.
Effect of PETMP on the yellowing resistance of UV-cured polyurethane coatings: the UV-cured coatings (a) and the UV-cured coatings which were treated at 120 °C for 4 h (b).
3.6. Contact Angle and Dispersion Surface Energy Analysis
The surface hydrophobicity of the polyurethane coatings was investigated by measuring the water contact angle on the coating surface and the dispersion surface energy () simultaneously, and was calculated using the following equations [43]:
Herein, rlv, rsl, rsv represented the surface tension at the liquid-vapor interface, solid-liquid interface, and solid-vapor interface, respectively; H2O (rlv = 72.7 mN/m, = 23.9 mN/m) was used as the testing liquid. The contact angles of water on polyurethane coatings are presented in Table 5. The contact angles of water on the polyurethane coatings increased with increasing EFCSA content, indicating an improved hydrophobic property. Many of the organic fluorine segments could generate motions toward the surface of coatings during the UV-curing process. The photopolymerization process between a double bond and thiol is illustrated in Scheme 3; PETMP acted as an effective crosslinker to incite more EFCSA and polyurethane prepolymer to participate in polymerization. With a higher loading of EFCSA, more organic fluorine segments gathered on the surface, and hence the hydrophobic property of the coatings improved. Meanwhile, the total amount of double bonds in the system increased, which enhanced the cross-linking density and caused the formation of a more compact surface, thus making it more difficult for water molecules to become immersed in the coating.

Table 5.
Contact angles and dispersion surface energies of UV-cured polyurethane coatings. Coatings composition: HTPU, PETMP (2 wt %), photoinitiator 184 (2 wt %).
3.7. Mechanical Properties
PETMP proved to be an effective photo-crosslinker. The effect of PETMP content on tensile strength and elongation at break is shown in Table 6. With the addition of 2% PEMPT, the tensile strength increased from 1.63 ± 0.17 to 2.59 ± 0.23 MPa and the elongation at break increased from 169.9% ± 8.8% to 240.4% ± 7.1%. However, with a higher loading of PEMPT, the elongation at break showed a slight decrease. This might be because moderate crosslinking promoted the improvement of the elongation at break, but excessive cross-linking density could limit the movement of molecular chains, resulting in the reduction of the elongation at break.

Table 6.
The effect of PETMP content on tensile strength and elongation at break.
To further investigate the effect of the PETMP content on the elasticity of polyurethane coatings, cyclic tensile loading with a maximum strain of 100% was performed (Table 7). All the samples exhibited large hysteresis loops in the first cycle, followed by much smaller hysteresis loops in the next four cycles. Upon increasing the PEMPT content, most of the samples showed small unrecoverable deformations (<5%). This effect could be explained by the previously proposed cross-linking structure in the polymer.

Table 7.
Cyclic tensile response curves for five cycles at deformation (100%) of polyurethane coatings.
4. Conclusions
A series of UV-curable polyurethane mixture solutions containing EFCSA and PETMP were prepared based on a reactive oligomer synthesized with IPDI/HTPB/BDO/HEA. The effect of EFCSA content on the hydrophobic property of the coatings was investigated. After optimization, the water contact angles of the polyurethane coatings improved from 82.5° to 108.6°, while the surface tensions decreased from 70.6 to 25.6 mN/m, indicating the dramatic hydrophobic property of the polyurethane coatings. The yellowing resistance of the polyurethane coatings increased with the addition of PETMP. In addition, the elongation at break of the polyurethane coatings increased significantly with increasing PETMP content, while the elastic recovery was also excellent. The mixed systems exhibited a high polymerization rate and a high degree of double bond conversion. Nevertheless, further improving the mechanical properties of coatings will be pursued. Overall, the fluorinated hydrophobic UV-crosslinkable thiol-ene polyurethane coatings exhibited good potential for industrial application.
Acknowledgments
This work is supported by the social development program of Taizhou (TS201516) and the second batch of excellent course (enzyme engineering) program of Nanjing Normal University Taizhou College.
Author Contributions
Nianqing Zhu and Mingqing Chen conceived and designed the experiments; Wenjing Xia, Rongjie Hou and Wengui Zhong performed the experiments; Wenjing Xia, Nianqing Zhu, Wengui Zhong and Mingqing Chen analyzed the data; Nianqing Zhu and Mingqing Chen contributed reagents/materials/analysis tools; Wenjing Xia and Nianqing Zhu wrote the paper.
Conflicts of Interest
The authors declare there is no conflict of interest.
References
- Bayer, O. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–288. (In German) [Google Scholar] [CrossRef]
- Valuev, I.L.; Valuev, L.I.; Obydennova, I.V.; Sytov, G.A.; Vanchugova, L.V. Modified polyurethanes as a new type of thromboresistant polymers. Polym. Sci. Ser. A 2010, 52, 824–827. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Goleneva, L.M.; Bychko, K.A.; Afonicheva, O.V. Synthesis and mechanical behavior of functionally gradient polyisocyanurate materials based on hydroxy-terminated butadiene rubber. Polym. Sci. Ser. A 2008, 50, 781–791. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Mays, J.W.; Hadjichristidis, N. Novel diblock copolymer-grafted multiwalled carbon nanotubes via a combination of living and controlled/living surface polymerizations. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1104–1112. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B.; Kostov, G.K. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Wu, W.L.; Zhu, Q.Z.; Qing, F.L.; Han, C.C. Water repellency on a fluorine-containing polyurethane surface: Toward understanding the surface self-cleaning effect. Langmuir 2008, 25, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.O.; Peterson, G.W.; Durke, E.M. Reduced chemical warfare agent sorption in polyurethane-painted surfaces via plasma-enhanced chemical vapor deposition of perfluoroalkanes. ACS Appl. Mater. Interfaces 2015, 7, 6402–6405. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Rogulska, M.; Podkosielny, W.; Kultys, A.; Stanislav, P.; Pozdzik, E. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. I. Synthesis and characterization of nonsegmented polyurethanes from HDI and MDI. Eur. Polym. J. 2006, 42, 1786–1797. [Google Scholar] [CrossRef]
- Yeganeh, H.; Barikani, M.; Khodabadi, N.F. Synthesis and properties of novel thermoplastic poly(urethane-imide)s. Eur. Polym. J. 2000, 36, 2207–2211. [Google Scholar] [CrossRef]
- Cao, Q.; Cai, Y.L.; Jing, B.; Liu, P.S. Structure and mechanical properties of thermoplastic polyurethane, based on hyperbranched polyesters. J. Appl. Polym. Sci. 2006, 102, 5266–5273. [Google Scholar] [CrossRef]
- Liu, P.F.; Ye, L.; Liu, Y.G.; Nie, F. Preparation and properties of the main-chain-fluorinated thermoplastic polyurethane elastomer. Polym. Bull. 2011, 66, 503–515. [Google Scholar] [CrossRef]
- Liu, T.; Ye, L. Synthesis and properties of fluorinated thermoplastic polyurethane elastomer. J. Fluorine Chem. 2010, 131, 36–41. [Google Scholar] [CrossRef]
- Turri, S.; Trombetta, T.; Levi, M. Catalyst effect on the crosslinking kinetics of a fluorine containing polyurethane network. Macromol. Mater. Eng. 2000, 283, 144–152. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.S.; Ji, Q.; McGrath, J.E. Surface properties of fluorinated oxetane polyol modified polyurethanes. Polymer 2002, 43, 7161–7170. [Google Scholar] [CrossRef]
- Trombetta, T.; Iengo, P.; Turri, S. Fluorinated segmented polyurethane anionomers for water–oil repellent surface treatments of cellulosic substrates. J. Appl. Polym. Sci. 2005, 98, 1364–1372. [Google Scholar] [CrossRef]
- Chen, K.Y.; Kuo, J.F. Synthesis and properties of novel fluorinated aliphatic polyurethanes with fluoro chain extenders. Macromol. Chem. Phys. 2000, 201, 2676–2686. [Google Scholar] [CrossRef]
- Asif, A.; Huang, C.Y.; Shi, W.F. Structure-property study of waterborne, polyurethane acrylate dispersions based on hyperbranched aliphatic polyester for UV-curable coatings. Colloid Polym. Sci. 2004, 283, 200–208. [Google Scholar] [CrossRef]
- Fu, Q.; Cheng, L.L.; Zhang, Y.; Shi, W.F. Preparation and reversible photo-crosslinking/photo-cleavage behavior of 4-methylcoumarin functionalized hyperbranched polyester. Polymer 2008, 49, 4981–4988. [Google Scholar] [CrossRef]
- Xu, G.; Shi, W.F. Synthesis and characterization of hyperbranched polyurethane acrylates used as UV curable oligomers for coatings. Prog. Org. Coat. 2005, 52, 110–117. [Google Scholar] [CrossRef]
- Asif, A.; Shi, W.F. UV curable waterborne polyurethane acrylate dispersions based on hyperbranched aliphatic polyester: Effect of molecular structure on physical and thermal properties. Polym. Adv. Technol. 2004, 15, 669–675. [Google Scholar] [CrossRef]
- Guenthner, A.J.; Hess, D.M.; Cash, J.J. Morphology development in photopolymerization-induced phase separated mixtures of UV-curable thiol-ene adhesive and low molecular weight solvents. Polymer 2008, 49, 5533–5540. [Google Scholar] [CrossRef]
- Wutticharoenwong, K.; Soucek, M.D. Influence of the thiol structure on the kinetics of thiol-ene photopolymerization with time-resolved infrared spectroscopy. Macromol. Mater. Eng. 2008, 293, 45–56. [Google Scholar] [CrossRef]
- Persson, J.C.; Josefsson, K.; Jannasch, P. Polysulfones tethered with benzimidazole. Polymer 2006, 47, 991–998. [Google Scholar] [CrossRef]
- Rydholm, A.E.; Reddy, S.K.; Anseth, K.S.; Bowman, C.N. Development and characterization of degradable thiol-allyl ether photopolymers. Polymer 2007, 48, 4589–4600. [Google Scholar] [CrossRef] [PubMed]
- Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Evaluation and control of thiol-ene/thiol-epoxy hybrid networks polymer. Polymer 2007, 48, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Bowman, C.N. The effect of functionalized nanoparticles on thiol–ene polymerization kinetics. Polymer 2006, 47, 6057–6065. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Nanostructured polyurethane/poss hybrid aqueous dispersions prepared by homogeneous solution polymerization. Macromolecules 2006, 39, 7037–7043. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A. The influence of the ionic concentration, concentration of the polymer, degree of neutralization and chain extension on aqueous polyurethane dispersions prepared by the acetone process. Polymer 2006, 47, 1805–1811. [Google Scholar] [CrossRef]
- Wicks, Z.W.; Wicks, D.A.; Rosthauser, J.W. Two package waterborne urethane systems. Prog. Org. Coat. 2002, 44, 161–183. [Google Scholar] [CrossRef]
- Çakmakçı, E.; Mülazim, Y.; Kahraman, M.V. UV-curable fluorine-containing hybrid coatings via thiol-ene “click” reaction and an in situ sol-gel method. Polym. Bull. 2013, 70, 1037–1048. [Google Scholar] [CrossRef]
- Mackey, N.M.; Confait, B.S.; Wynne, J.H.; Buchanan, J.P. Preparation of novel hydrolyzing urethane modified thiol-ene networks. Polymers 2011, 4, 1849–1865. [Google Scholar] [CrossRef]
- Reinelt, S.; Tabatabai, M.; Moszner, N.; Fischer, U.K.; Utterodt, A.; Ritter, H. Synthesis and photopolymerization of thiol-modified triazine-based monomers and oligomers for the use in thiol-ene-based dental composites. Macromol. Chem. Phys. 2014, 215, 1415–1425. [Google Scholar] [CrossRef]
- Thomas, R.; Sinturel, C.; Pionteck, J.; Puliyalil, H.; Thomas, S. In-situ cure and cure kinetic analysis of a liquid rubber modified epoxy resin. Ind. Eng. Chem. Res. 2012, 51, 12178–12191. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, Y.-H.; Park, H.; Kim, H.-D. Preparation and properties of UV-curable fluorinated polyurethane acrylates. J. Appl. Polym. Sci. 2014, 131, 42168. [Google Scholar] [CrossRef]
- Nazare, A.N.; Deuskar, V.D.; Asthana, S.N.; Shrotri, P.G. Influence of characteristics of HTPB on mechanical properties and ballistic behavior of composite propellant. J. Polym. Mater. 1993, 10, 213–216. [Google Scholar]
- Malkappa, K.; Jana, T. Simultaneous improvement of tensile strength and elongation: An unprecedented observation in the case of hydroxyl terminated polybutadiene polyurethanes. Ind. Eng. Chem. Res. 2013, 52, 12887–12896. [Google Scholar] [CrossRef]
- Nagle, D.J.; Celina, M.; Rintoul, L.; Fredericks, P.M. Infrared microespectroscoic study of the thermo-oxidative degradation of hydroxyl-terminated polybutadiene/isophorone diisocyanate polyurethane rubber. Polym. Degrad. Stab. 2007, 92, 1446–1454. [Google Scholar] [CrossRef]
- Quack, G.; Fetters, L.J. The nuclear magnetic resonance characterization of a cyclic structure in anionically prepared polybutadiene. Macromolecules 1978, 11, 369–373. [Google Scholar] [CrossRef]
- Shankar, R.M.; Roy, T.K.; Jana, T. Terminal functionalized hydroxyl-terminated polybutadiene: An energetic binder for propellant. J. Appl. Polym. Sci. 2009, 114, 732–741. [Google Scholar] [CrossRef]
- Temel, G.; Karaca, N.; Arsu, N. Synthesis of main chain polymeric benzophenone photoinitiator via thiol-ene click chemistry and Its use in free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5306–5312. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. Res. 1964, 56, 40–52. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).