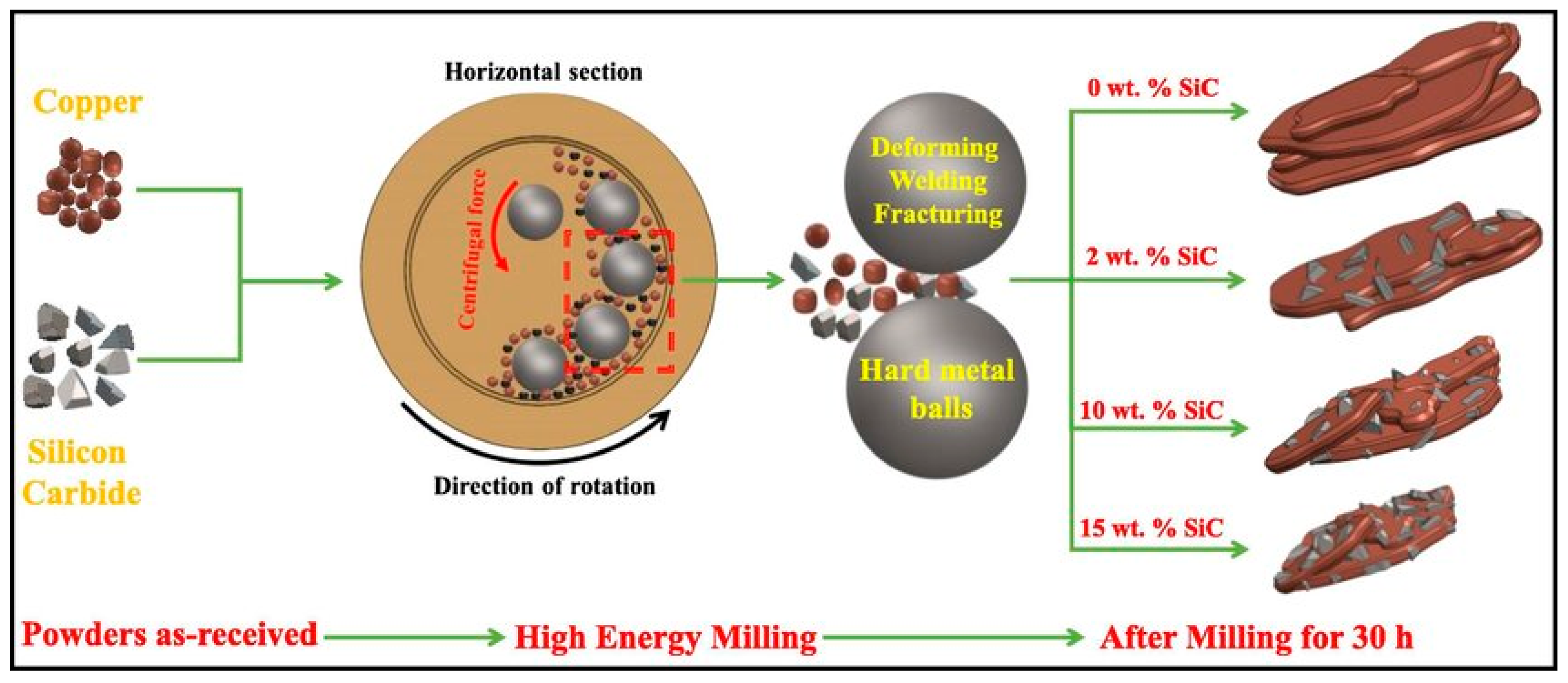
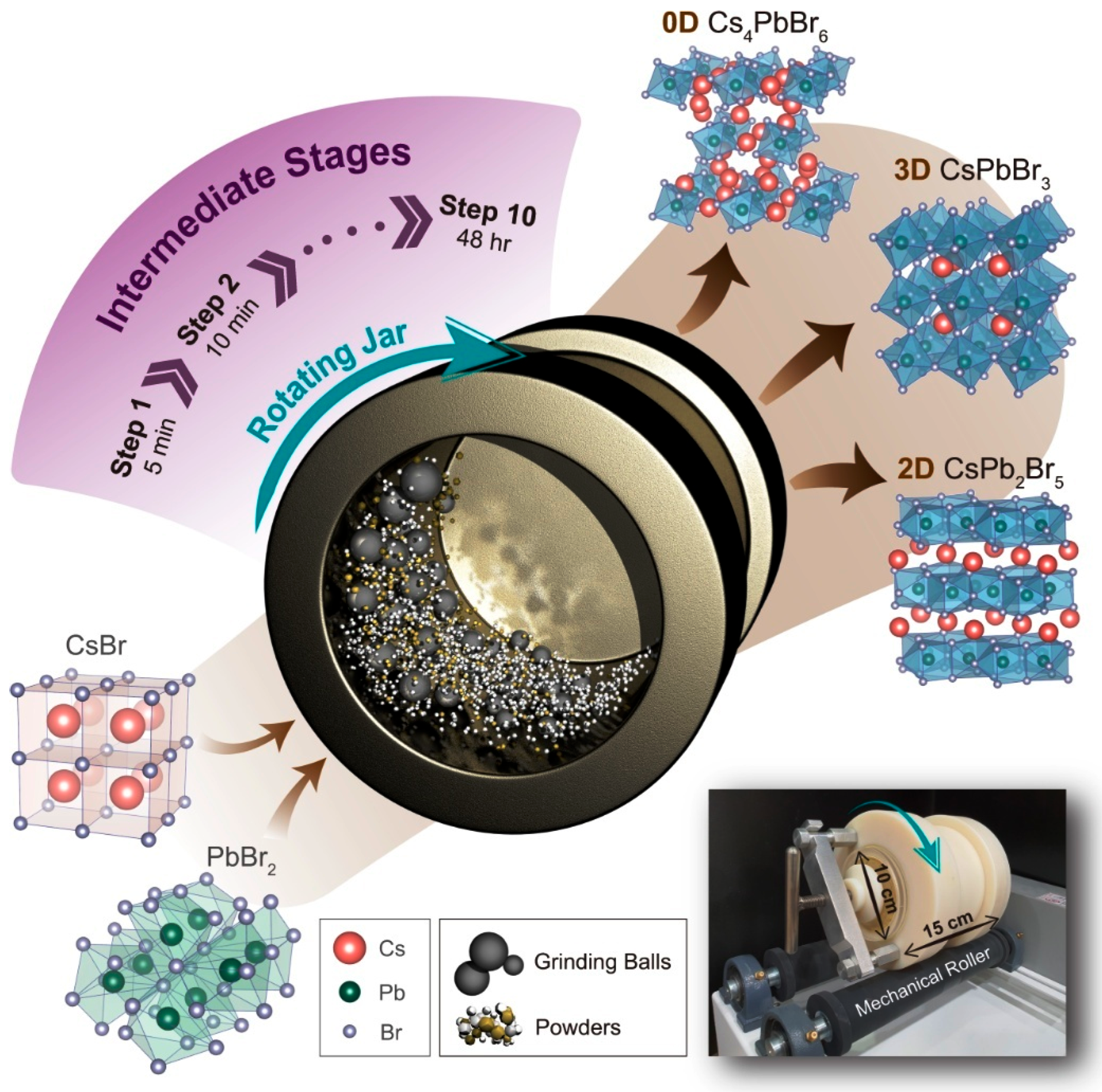
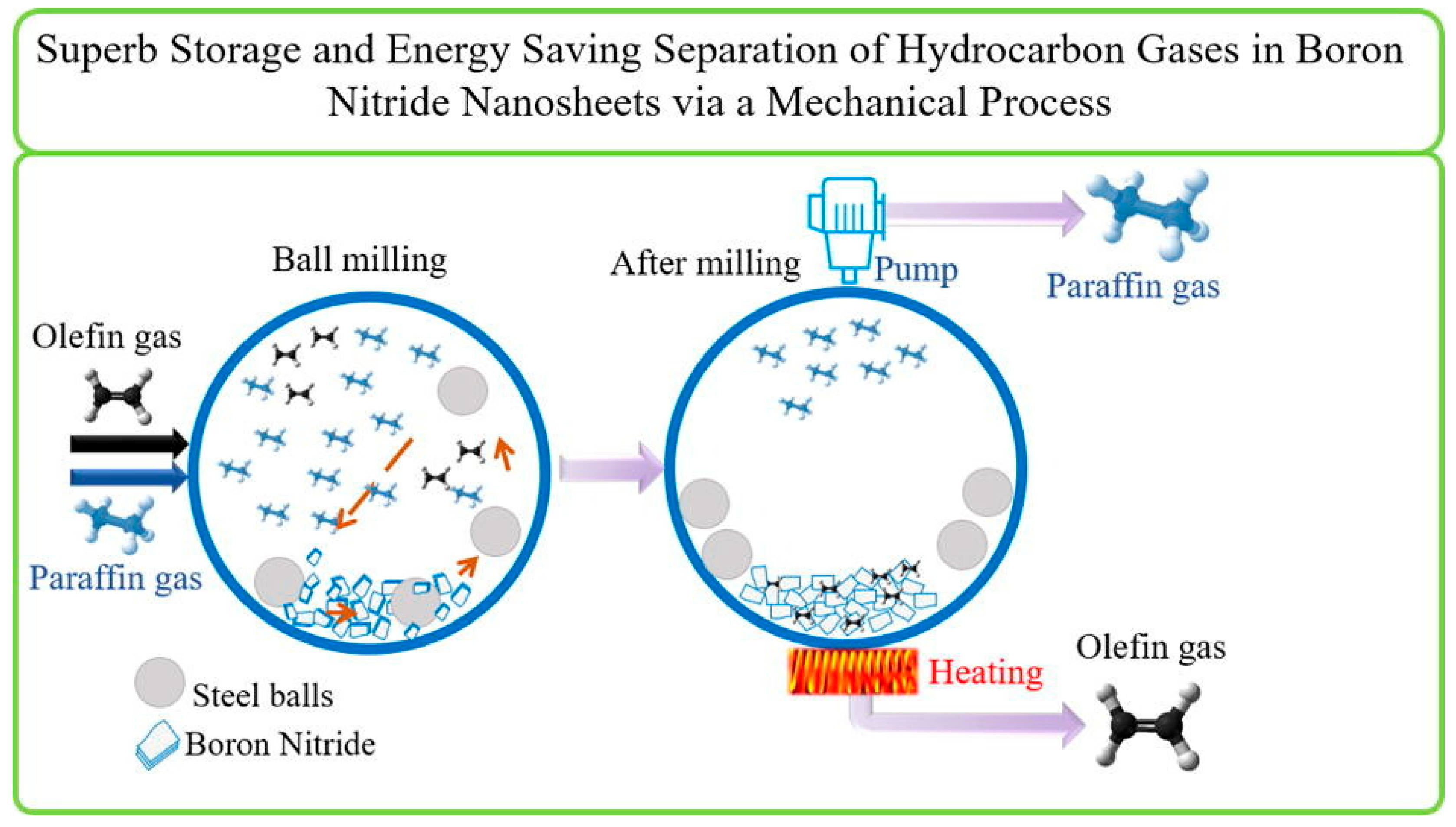
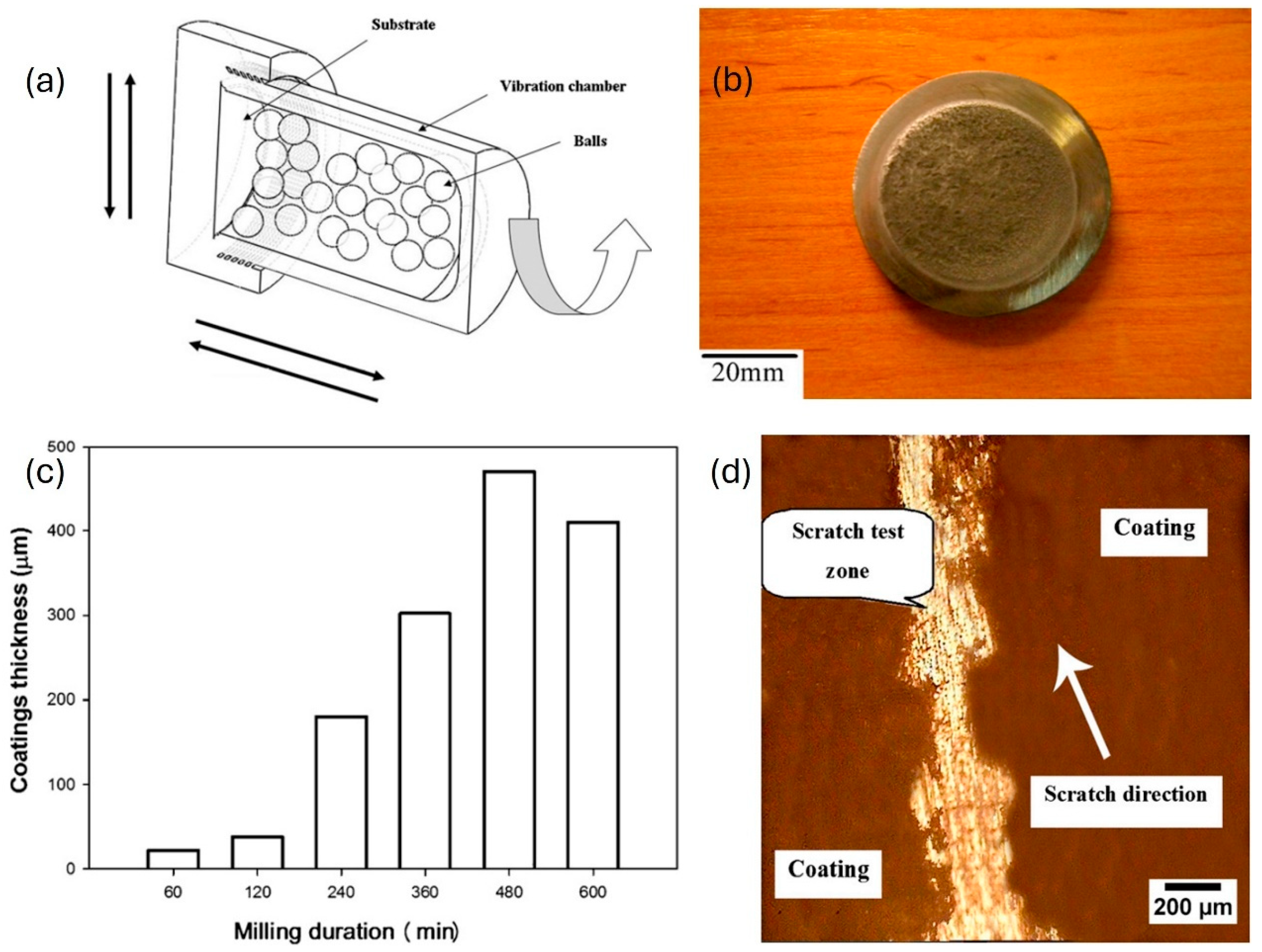
Abstract
This review explores various mechanical coating techniques, with a specific emphasis on ball milling for coating metal balls. Mechanical coatings play a crucial role in enhancing material properties such as wear resistance, durability, and functionality across various industries. Among these methods, ball milling stands out for its cost-effectiveness, simplicity, and ability to produce uniform coatings. This paper comprehensively discusses the principles, mechanisms, and process parameters influencing the efficiency of ball milling, including milling time, speed, and material properties. Additionally, advancements in nanostructured coatings, in situ coating techniques, and the challenges of contamination and scalability are examined. Future directions involve automation, real-time monitoring, and AI-driven optimization for improved performance. The findings aim to provide valuable insights for researchers and industries seeking to optimize mechanical coating processes.
1. Introduction
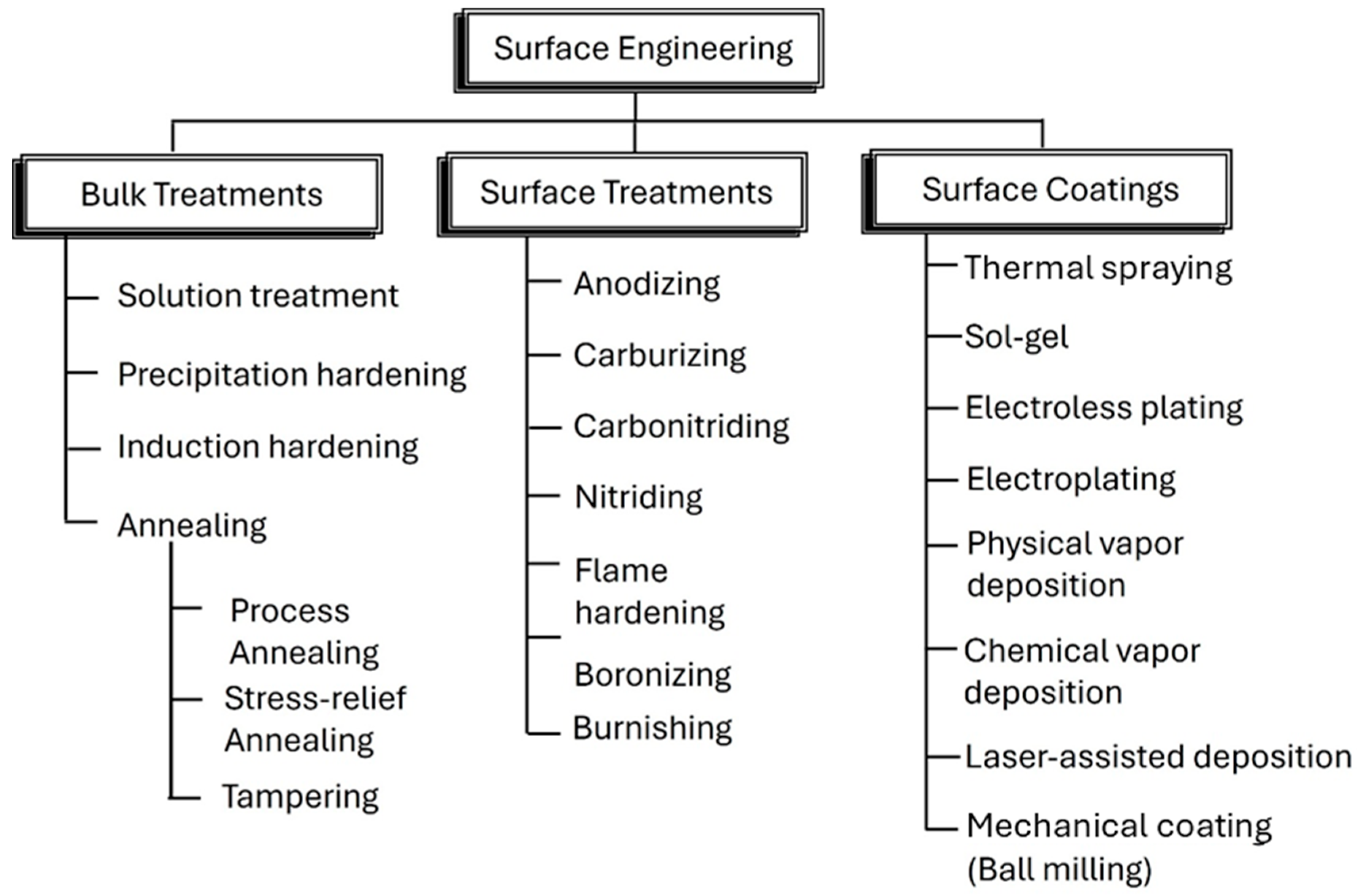
Surface engineering and coatings of powders are methods used to coat particles and enhance their surface properties. This is performed to improve the functional properties of materials, including wear resistance, corrosion resistance, and mechanical strength. The particle’s surface is significantly modified to reach these goals, which is why a huge proportion of the total cost of the production process is dedicated to the coating step. Since “simple mixing or blending” is insufficient to produce appropriate coating layer characteristics for relevant applications, many techniques have been developed to control the surface’s properties through the coating steps, including mechanical operations, with the lowest functional cost [1,2,3,4]. Traditional coating methods such as chemical vapor deposition (CVD), physical vapor deposition (PVD), and electroplating have limitations related to cost, complexity, and environmental concerns. Mechanical coating methods, particularly ball milling, offer a more sustainable and cost-effective alternative.
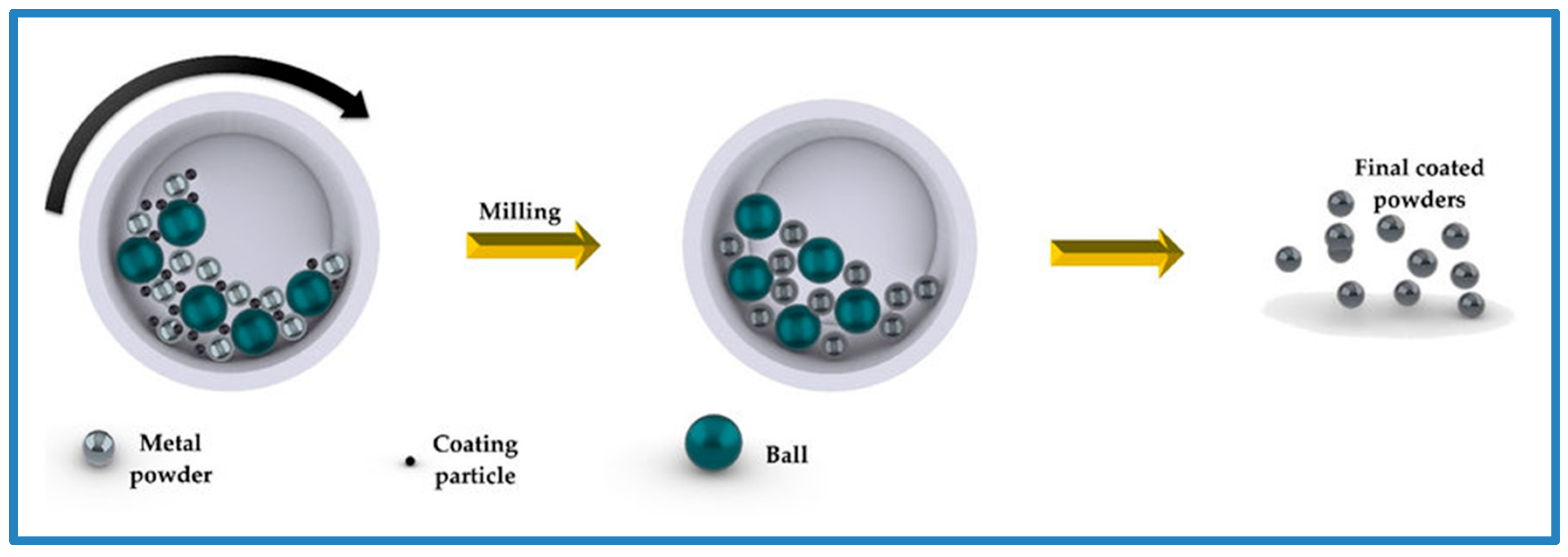
Mechanical coating is emerging as an efficient and cost-effective process for coating surfaces, especially for powder particles (Figure 1). Optimization is based on the conditions of mechanical coating, the intensity and the energy of the mechanical forces applied to the coating particles, as well as the balls used for the operation. When the coating operations are directly applied to the product’s surface, the operation is called external mechanical coating (EMC). Correspondingly, “Internal Using Mechanical Coating” is applied if the operation is performed inside the product. The mechanical coating can handle various shapes of the body, such as particulate or flat bodies [3,4,5,6,7,8,9,10].
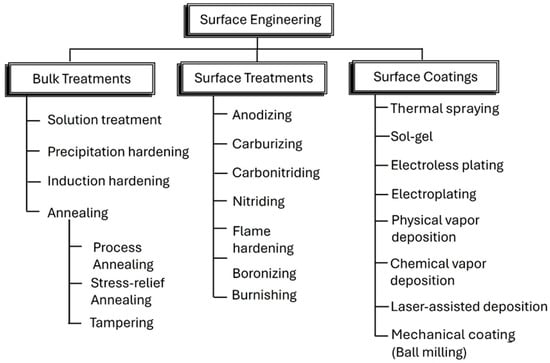
Figure 1.
List of various surface engineering treatments.
The novelty of this review lies in explicitly addressing several underexplored gaps in the literature. While prior reviews have broadly introduced mechanical coating techniques (MCTs), limited attention has been given to a comparative analysis of these techniques, especially highlighting the distinct advantages and limitations of ball milling. Furthermore, although challenges such as contamination control and scalability are well recognized, they have not been systematically analyzed within a unified framework. Another critical gap addressed here is the integration of AI-driven process optimization and real-time monitoring approaches, which remain largely absent in discussions of mechanical coating. By framing these aspects within the Energy–Structure–Property relationship, this review not only synthesizes existing knowledge but also provides a forward-looking perspective that connects process parameters to coating performance and industrial scalability.
Therefore, this review aims to provide a comprehensive understanding of the mechanical coating method, with emphasis on ball milling, and its applications across various industrial fields.
1.1. Overview of Mechanical Coating Techniques (MCTs)
Mechanical coating can be applied using various techniques, each offering distinct advantages depending on the application. These methods include powder immersion coating, where the substrate is submerged inside the fluidized powder particles [9]. This method has several advantages, such as producing uniform coating layers, especially on complex-shaped objects, and is often a quicker and more effective substitute for conventional liquid coating techniques. In addition to the powder immersion method, other alternative MCTs are available and can be utilized to attain diverse results. These methods include fluidized bed coating (solid particles are suspended in a fluidization chamber with a controlled gas flow), centrifugal coating (uses centrifugal forces to distribute coating material evenly), air-jet coating (a high-speed air stream carries the coating particles to the substrate), and ball milling (utilizes mechanical energy from milling balls to apply coatings in a controlled manner) [3,4,7,9,10,11]. Each method has distinct benefits and can be customized to fulfill certain coating requirements.
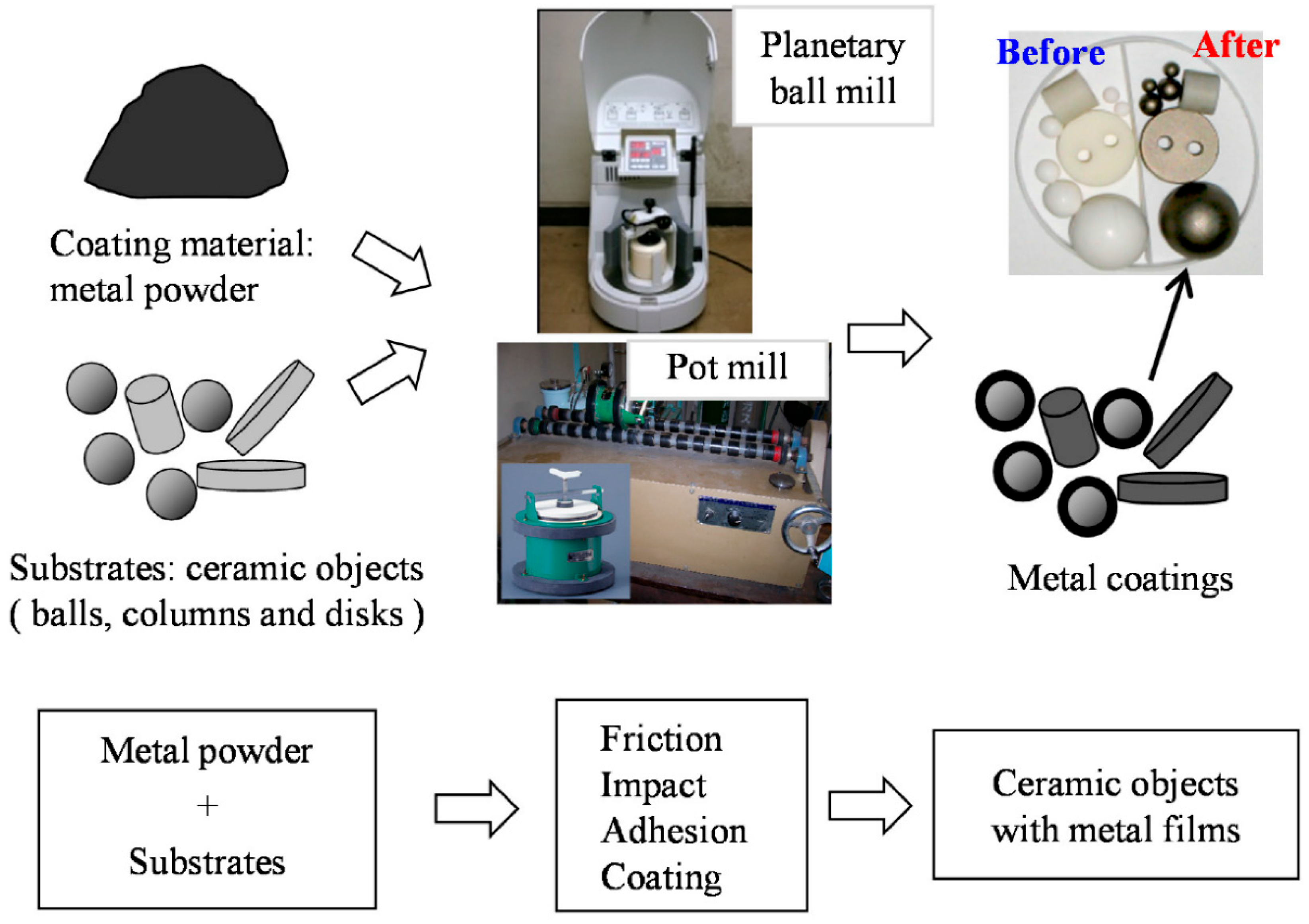
Of the different kinds of mechanical coating methods, the ball milling method (Figure 2) is the most useful and widely used in industrial applications [12]. The effectiveness of the ball milling method is highly dependent on several critical parameters, which determine the energy input, coating quality, and reproducibility. Among these, milling speed, ball-to-powder ratio (BPR), milling time, and the size and material of the milling media are the most influential [13,14,15]. For example, variations in rotational speed directly affect the collision energy and frequency, thereby influencing coating adhesion and particle refinement [16,17]. Similarly, an optimized BPR is essential to balance sufficient energy transfer while avoiding excessive cold welding or contamination [18,19]. Milling time determines the extent of particle deformation and alloying, while the selection of milling media and process control agents (PCA) significantly impacts contamination levels and surface purity [13,14,20,21,22].
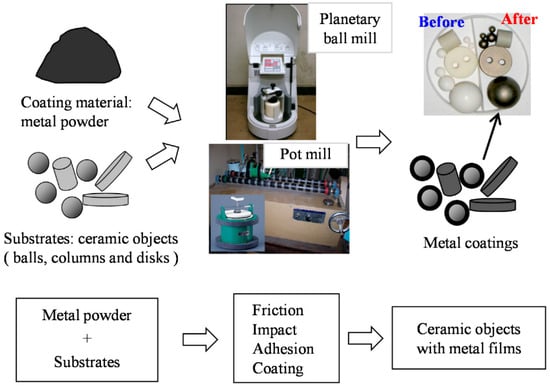
Figure 2.
Schematic illustration of the MCTs for various substrates of different shapes using the ball milling method. Adapted with permission from ref. [12] Copyright 2015 Coatings.
The importance of process parameters in ball milling is further underscored by experimental evidence. For instance, in the synthesis of nano-sized amorphous colemanite, systematic variations in milling time were shown to directly alter both the crystal structure and thermal properties of the resulting material [22]. Prolonged milling progressively transformed crystalline colemanite into an amorphous phase, which was accompanied by a significant reduction in crystallite size and an increase in specific surface area. These structural changes not only modified the thermal stability of the product but also demonstrated how milling time can act as a critical lever to tune phase transitions and functional properties [15,20]. Such findings emphasize that what may initially appear to be a simple parameter, such as milling duration, actually governs fundamental material transformations and ultimately dictates performance in downstream applications.
A parallel insight comes from nanosized ulexite, where the role of process control agents was investigated in detail [23]. Here, the type and concentration of PCA were found to profoundly influence particle morphology, degree of agglomeration, and contamination levels. Specifically, insufficient PCA addition led to severe cold welding and irregular particle growth, whereas excessive PCA inhibited effective energy transfer, resulting in incomplete refinement [19,22,24]. The optimal PCA balance minimized contamination while maintaining high milling efficiency, thereby demonstrating that surface chemistry and coating integrity can be directly controlled through careful additive selection [19,22,24]. These results highlight the dual role of PCAs not only as mechanical aids to reduce cold welding, but also as chemical agents that can subtly alter the interfacial characteristics of milled powders. Consequently, both milling time and PCA composition emerge as decisive parameters whose interplay determines whether mechanical coating produces uniform, stable, and application-ready surfaces.
Therefore, precise control and systematic optimization of these parameters are indispensable for achieving consistent coating performance across different applications [14,25].
This is due to its advantages, which include simple operation, low cost, uniformly coating complex surfaces, and the absence of heating cracks (unlike other coating techniques like CVD). The ball mill is the most utilized blending tool and can be categorized as an effective solution [3,7,10,26]. Ball milling for mechanical coating includes balls that directly improve both the particles’ textures and blend powders with effective shape change [3,4,7,10,26]. The ball mill contains grinding vessels and a PCA (balls/impeller), and both components affect the product of the ball mill. The working handle of the ball milling system for performing the process is mostly achieved by changing the balls. The duration of the ball milling method varies depending on the thickness and composition of the coated layers, as well as the extent of surface coverage. These factors can impact the efficiency and effectiveness of the ball milling process [9,26,27,28].
The interior of the grinding vessels and the substance being crushed usually comprise a softer layer, while the grinding balls, made from tougher materials, are frequently coated by employing a variety of MCTs. This safeguards them against wear and tear, prolonging their lifespan and ensuring optimal efficiency. Such MCTs include, but are not limited to, PVD, CVD, electroplating, and thermal spraying. Each technique presents distinct advantages and characteristics, enabling manufacturers to select the most suitable method for coating the grinding balls based on their specific requirements. With these advanced techniques at their disposal, manufacturers can enhance the performance and longevity of grinding balls, thus improving the overall efficiency and effectiveness of the crushing process [29,30]. The success of mechanical coating heavily relies on the wheel or container that facilitates the movement of balls/rollers. These components are crucial in ensuring uniform and effective coverage throughout the coating process. By carefully designing and optimizing these supporting components, the efficiency and effectiveness of the coating process can be significantly improved [9,27,29].
1.2. Significance of Mechanical Coating Using Ball Milling Processes
In industrial sectors, there is a tremendous demand for fabricating innovative coatings, which can be a very effective modification method to improve the functionality and performance of materials. The majority of modern industrial coatings are applied using CVD, plasma-enhanced chemical vapor deposition (PECVD), PVD, electroplating, anodizing, sol–gel coating, etc. [11,31,32,33]. However, these methods have several deficiencies in practice. Most of these techniques use a vacuum, which may incur high costs and require a very clean environment. Furthermore, there are inherent material limitations when dealing with corrosive environments. The bonding between the coating and the substrate in these conditions tends to be significantly weaker compared to the mechanical bonding achieved through other methods [11,31,32,34,35].
Therefore, understanding the advantages of utilizing mechanical coating in the industry is essential for achieving success and maintaining a competitive edge. MCTs, such as ball milling, are some of the most effective methods for creating a stronger mechanical bond [26]. This mechanical treatment provides coating products with very high adhesion strength, due to increased energy between the interface coating and substrate as a result of defects and increasing the surface area, while decreasing the strain energy of the system and interfacial energy between the two phases [3,4,7]. Consequently, the properties depend mainly on the type and composition of the intermetallic compounds formed as a function of the material system of the coating and substrate, which can be developed through the design of the coating method. Based on the aforementioned factors, MCTs lead to significant enhancements in material properties and extend the lifespan of components by applying protective coatings, thus enhancing wear resistance. In addition to substantial improvements in mechanical properties (hardness, tensile strength, and durability), the thermal and electrical conductivity properties of materials are also improved [6,9,29,36]. Moreover, this technique is considered one of the most cost-effective and efficient methods with the ability to tailor the coating thickness, composition, and properties to meet specific industrial requirements.
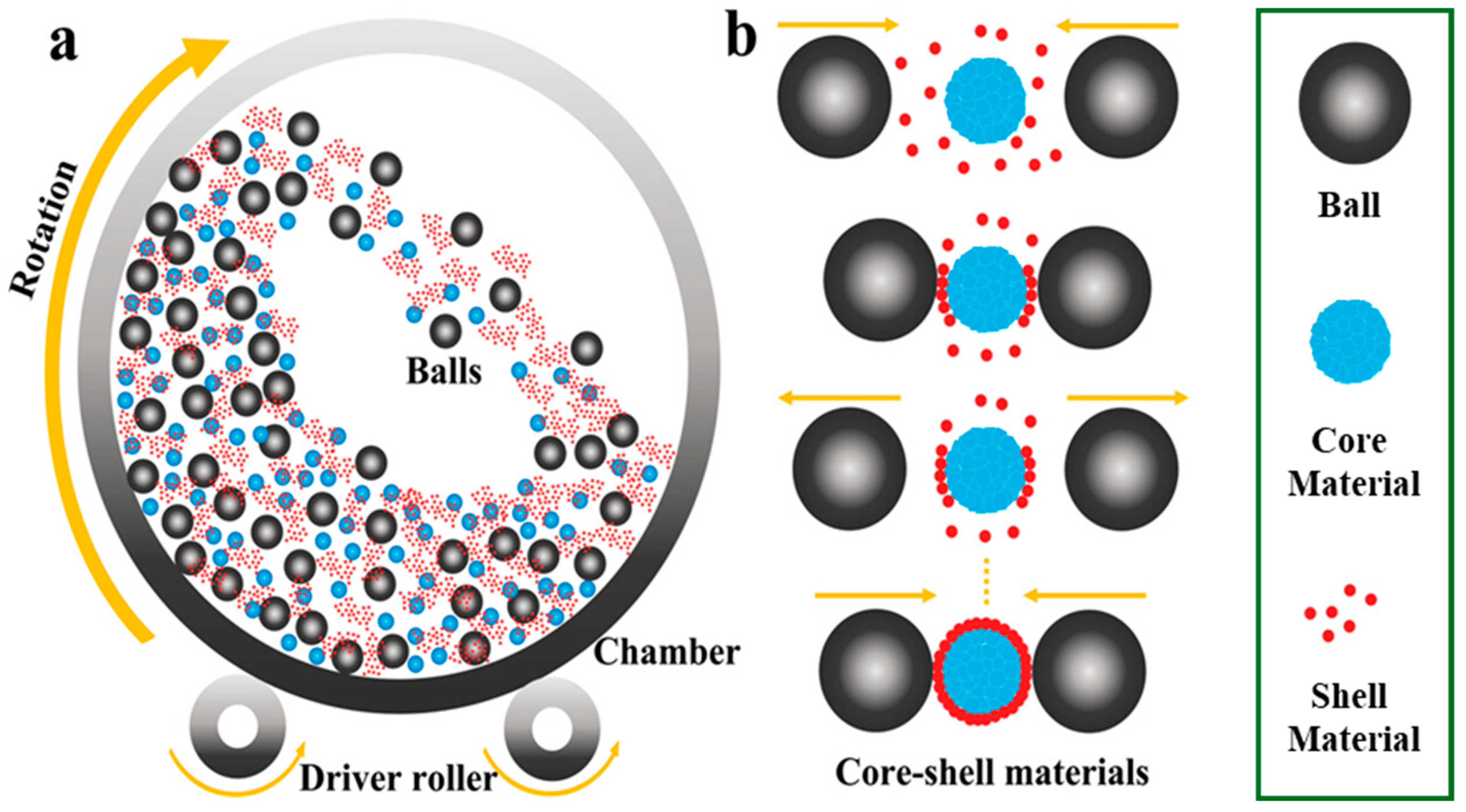
The complexity, inefficiency, and high costs often hinder the mass production of core–shell materials. However, ball-milling presents a low-cost, high-yield, and scalable solution for the efficient fabrication of core–shell structures in cathode materials and silicon (Si) anode materials, making it suitable for pilot plant manufacturing (Figure 3) [37]. The synthesis of core–shell structures in ball mill equipment is driven by the energy generated from the impacts of the balls colliding with the shell and core materials. These collisions occur between either two balls or a ball striking the wall of the chamber, which results in the shell material adhering to the core material due to the impact energy produced during the rotational movement. This continuous coating process ensures that all particles are subjected to the balls’ impact energy thousands of times, thereby facilitating the mechanical ball-milling method to create a non-destructive coating layer on the host material, which is commercially viable. Additionally, the literature indicates that high temperatures applied during the calcination process in air contribute to achieving a smooth and compact surface for the core–shell structure following the ball-milling process.
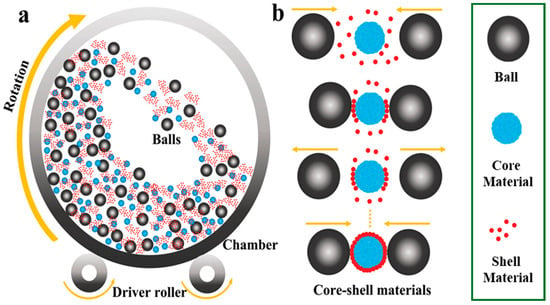
Figure 3.
Schematic illustration of (a) Ball milling process and (b) scalable synthesis of core–shell materials using the ball milling process. Modified from ref. [37] Copyright 2023 Small Methods.
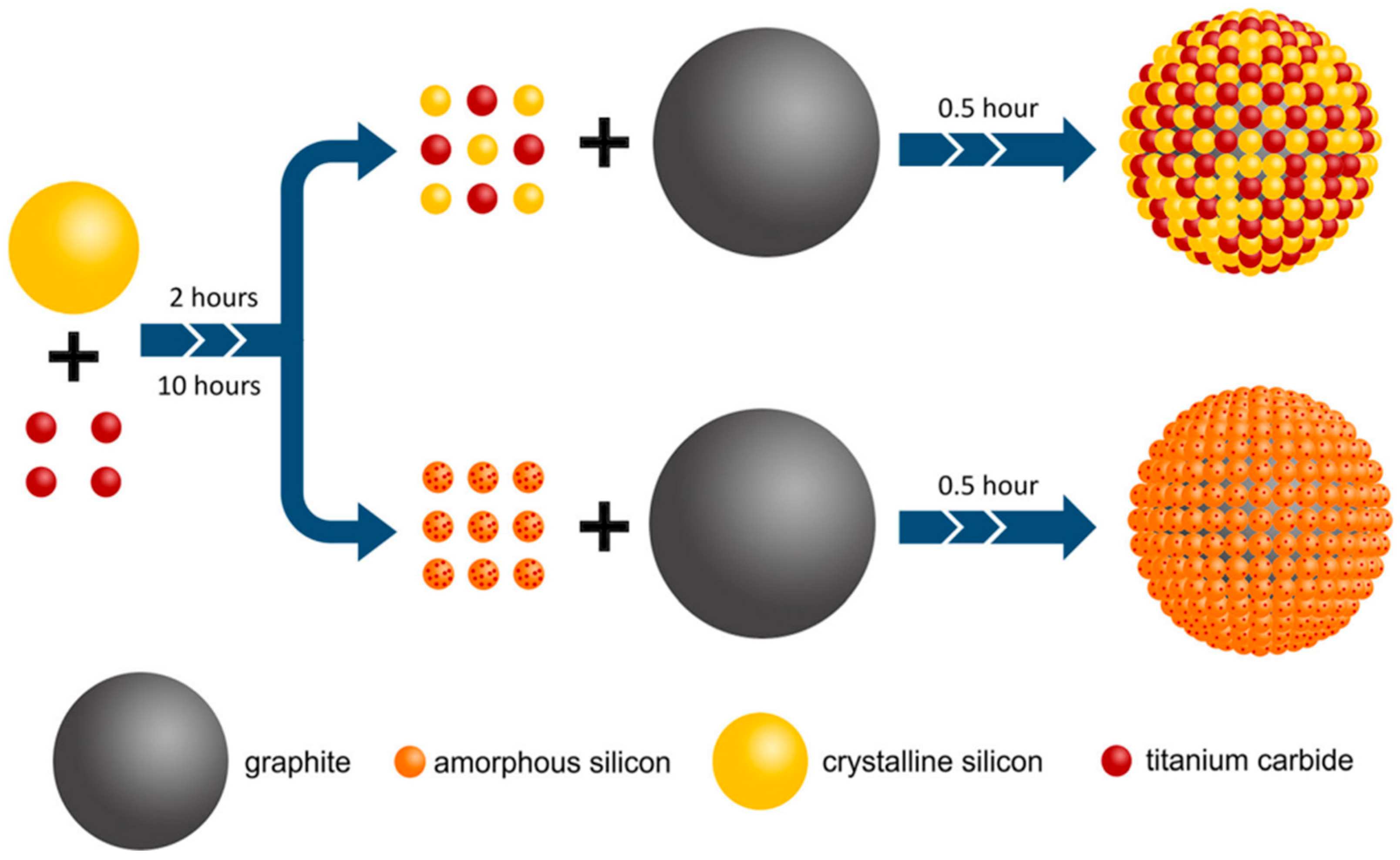
Recently, Pan et al. demonstrated that a straightforward multi-step high-energy ball-milling method provides an efficient and scalable approach to coat amorphous silicon/titanium carbide (Si/TiC) onto graphite (G), intended for use as anode materials in lithium-ion batteries (Figure 4) [38]. In this unique preparation technique, Si is entirely transformed into an amorphous phase along with TiC following an extended milling time. TiC is gradually converted into nanocrystalline grains that are distributed across the surface of the amorphous Si, forming a network of nanosized conductive channels. It is also shown how the duration of ball-milling influences the degree of amorphization of Si and the structure of the Si/TiC composite, as well as the impact of the composite’s unique structure on the electrochemical performance of the Si/TiC/G electrode.
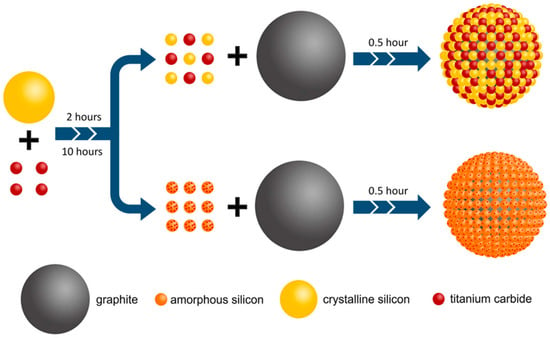
Figure 4.
Schematic illustration of Si/TiC-coated G using the ball milling process. Adapted with permission from ref. [38] Copyright 2021 Journal of Electronic Materials.
This study not only demonstrates the flexibility of mechanical coating techniques for producing advanced electrode materials, but also underscores the broader perspective developed in this review. We position mechanical coating and ball milling as not merely surface-engineering tools, but as enabling technologies for functional and scalable applications such as energy storage. This framing distinguishes our review from prior work by highlighting cross-disciplinary applications, where coating science directly supports emerging technologies like lithium-ion batteries.
This review aims to provide a comprehensive analysis of MCTs with a special focus on ball milling. The emphasis on ball milling is particularly timely given recent developments in materials science and industrial processing. The rise in nanostructured core–shell and multilayered systems in biomedical, energy storage, and catalytic applications has created a strong demand for coating methods that can reliably produce fine, uniform structures [38,39]. At the same time, industries are increasingly prioritizing scalable, cost-effective, and environmentally friendly processes, making mechanical coating by ball milling highly attractive compared to vapor-phase or chemical methods [25,40]. Moreover, the growing interest in data-driven optimization and sustainable manufacturing further enhances the relevance of re-examining ball milling, not only as a traditional method but as a platform adaptable to next-generation applications [41,42]. Key topics include the principles of ball milling, different types of mills, process parameters, coating mechanisms, and industrial applications. Recent advancements, challenges, and future research directions are also discussed to offer a holistic understanding of mechanical coatings.
2. Fundamentals of Ball Milling
Ball milling falls under the broader category of mechanical alloying, which refers to a process enabling the synthesis of new materials starting from elemental powders. This technique coincides with the conceptual onset of mechanical coating in one context: the coating of balls that subsequently proceed to coating (or milling) powders via either the formation of ball-powder-ball collisions or other methods such as plowing or shearing. Some studies on mechanical coating using other coating devices, which we will not further cover in this review, were present in the literature just before the inception of mechanical coating in the ball mill [3,4,7,9,26,43,44].
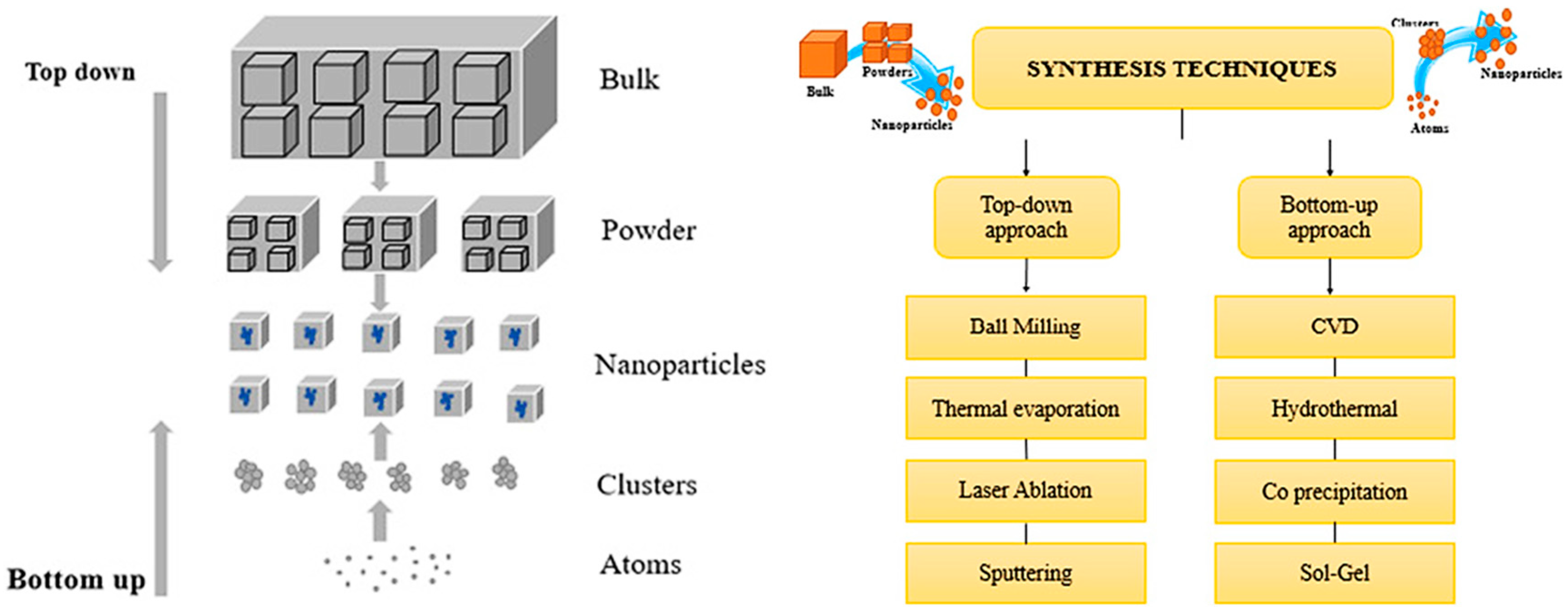
Ball milling is a top-down technique that grinds bulk materials into fine powders using a rotating chamber filled with hard grinding media, such as stainless steel or ceramic balls (Figure 5) [45]. The rotation causes collisions that reduce particle size, and by adjusting factors like speed and media size, the final characteristics of the particles can be controlled. This method is commonly used in materials science and manufacturing to create and enhance composite materials. The effectiveness of ball milling depends on the mill’s rotational speed, the size and weight of the grinding media, and the ratio and distribution of the materials being processed [3,4,7,26,46,47]. These factors will be further discussed in this review.
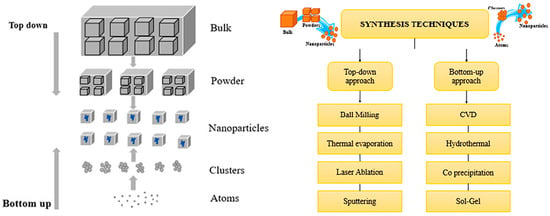
Figure 5.
Synthesis of nanomaterials using various top-down and bottom-up approaches. Adapted with permission from ref. [45] Copyright 2022 Advances in Colloid and Interface Science.
Moreover, different types of ball milling equipment are available to suit specific material pulverization needs. These include traditional ball mills, planetary ball mills, and vibratory mills, each designed with unique mechanisms to enhance grinding efficiency and achieve desired specific purposes [46]. This review will also explore the applications and advantages of each type, providing a comprehensive understanding of their roles in material processing. By examining these aspects, we aim to offer insights into the selection and optimization of ball milling techniques for various industrial applications.
2.1. Principles of Ball Milling
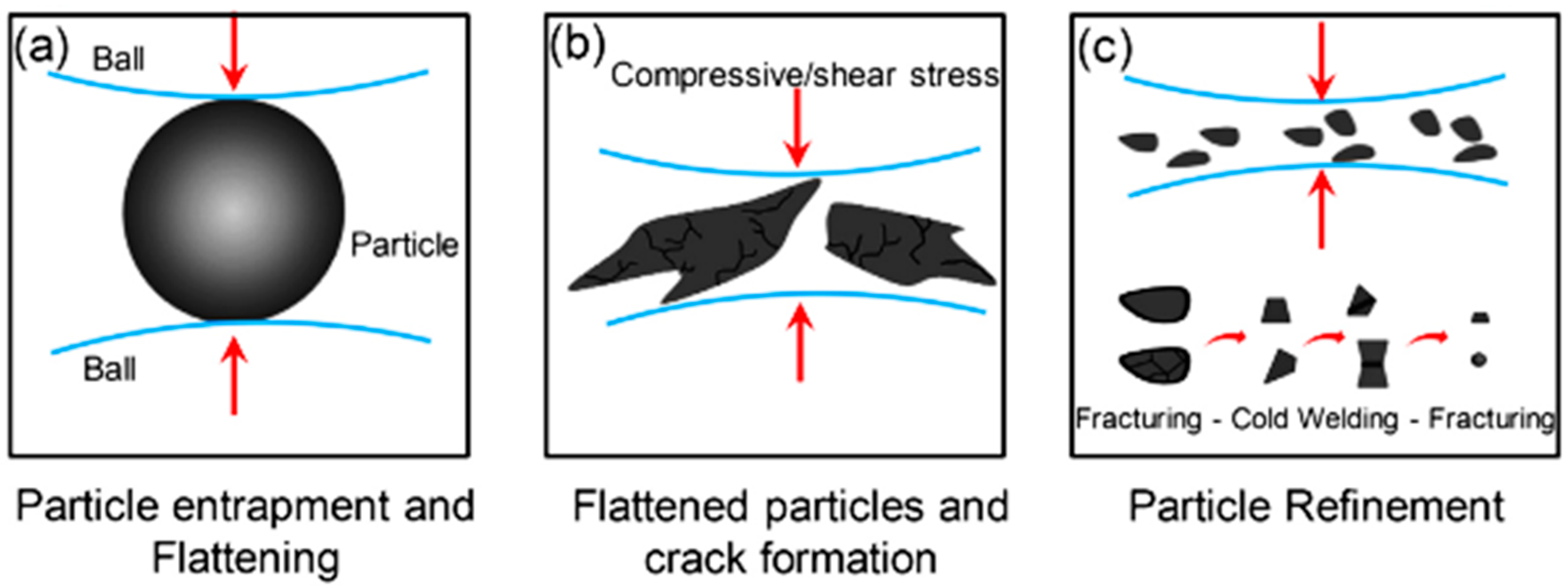
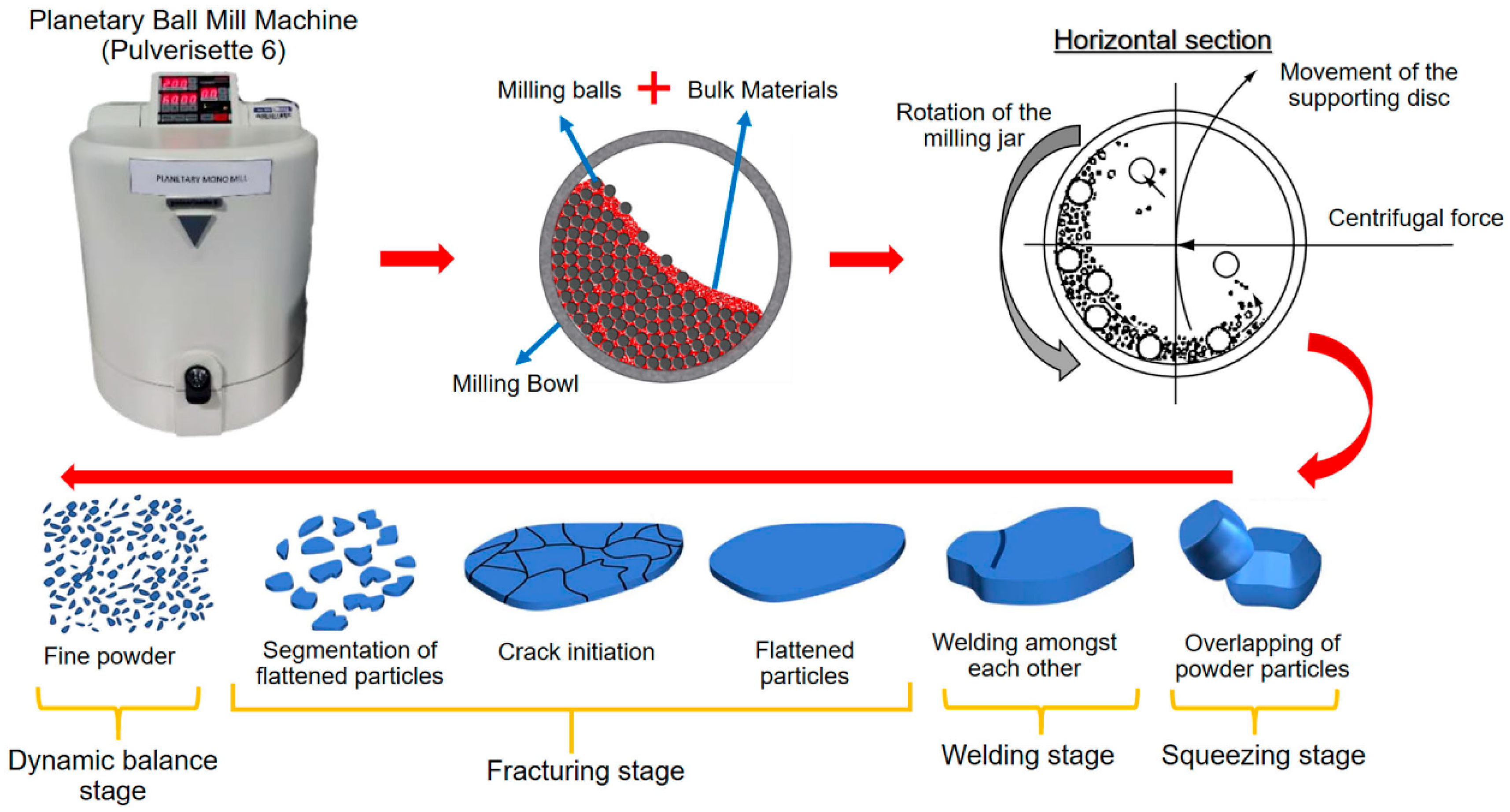
The process of ball milling utilizes a grinding vessel (container) filled with material to grind into fine powders (Figure 6). These containers are designed to produce frictional stresses that lead to creating fracture planes and removing surface layers. The efficiency of this method depends on the complex interaction and collaboration between the various components in the grinding medium and the material being worked on [26,27,45,48,49].
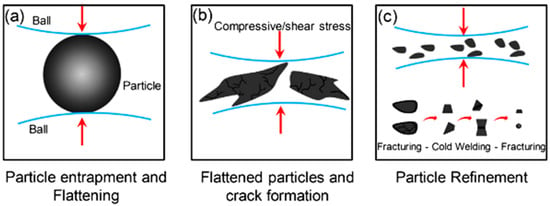
Figure 6.
Principle of mechanical alloying by the HEBM process. (a) Particle entrapment and flattening during ball-particle collision, (b) Deformation of particles by flattening and propagation of cracks, (c) Particle refinement undergoes cold-welding and agglomeration that increase particle size. Modified with permission from ref. [49] Copyright 2016 Journal of Environmental Management.
2.2. Types of Ball Mills
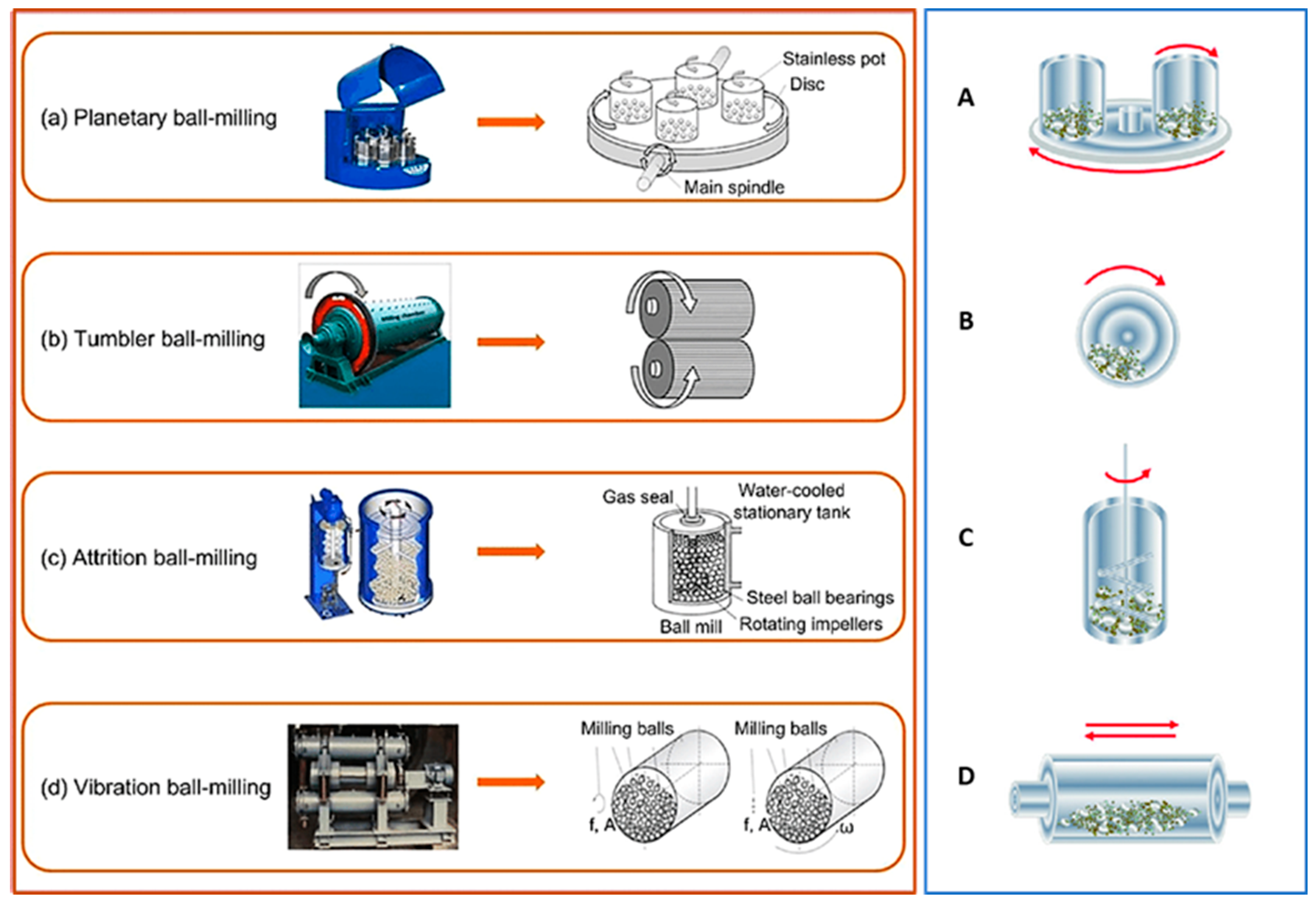
Ball mills are classified according to their design features as tumbling (cone or ball) mills, vibratory ball mills, planetary ball mills, and attrition ball mills as shown in Figure 7 [1,5,8,48,50,51]. Each type of ball mill has its own distinct set of characteristics and benefits, that make it appropriate for certain mechanical coating processes and materials. The design, size, speed, and energy input of each type of ball mill are critical in determining its suitability for a variety of industrial and research purposes.
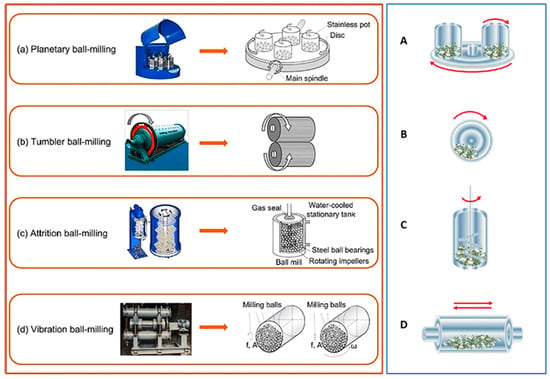
Figure 7.
Different types of ball milling and its working principles: (a) Planetary ball milling and (A) its cylinder rotation direction, (b) Tumbler ball milling and (B) its cylinder rotation direction, (c) Attrition ball milling and (C) its cylinder rotation direction, and (d) Vibration ball milling (where f is vibration frequency, A is vibration amplitude, and ω is angular velocity) and (D) its cylinder rotation direction. Modified from ref. [24,25].
2.2.1. Tumbling Ball Mills (TBM)
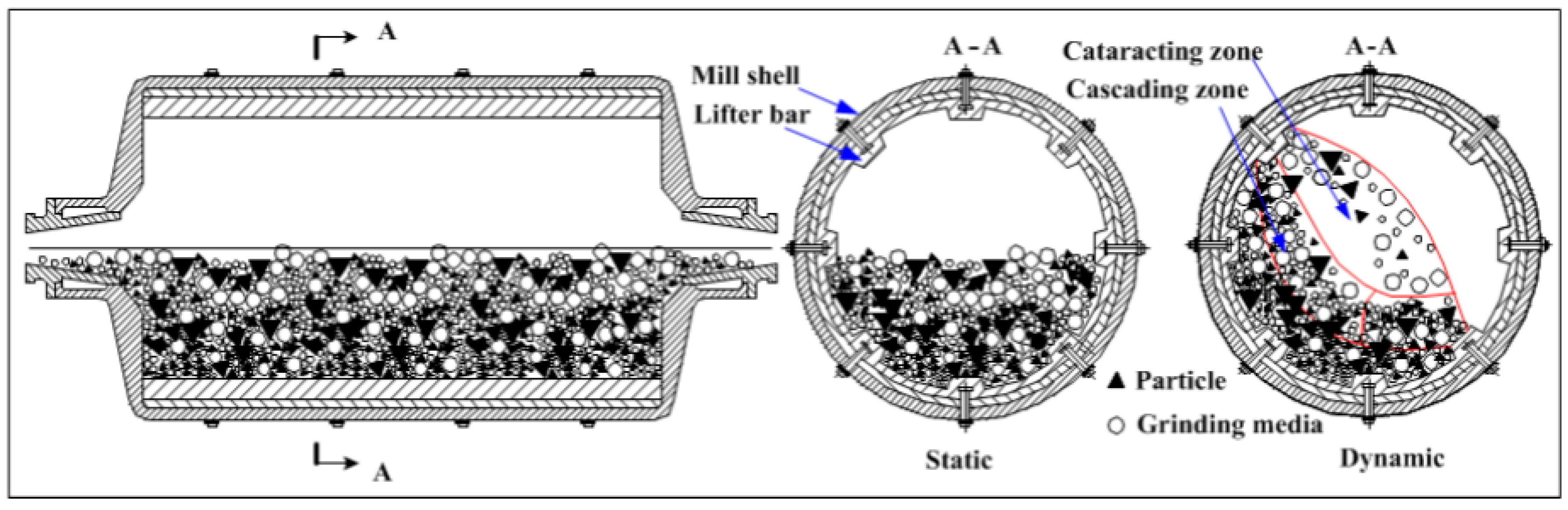
Tumbling Mills, also known as ball mills or tube mills, operate by rotating a cylinder bowl filled with grinding media such as balls and the material to be coated (Figure 8). The dynamic movement of the media generates powerful impact and shear forces, allowing coating and grinding procedures to be carried out successfully. Tumbling mills are ideal for large-scale applications because of their simple design, ease of operation, and cost-effectiveness [46,48,52]. Nonetheless, they give less exact control over particle size distribution and coating homogeneity than other types of mills. They also provide less precise control over particle size distribution and coating uniformity compared to other types of mills [9,47].
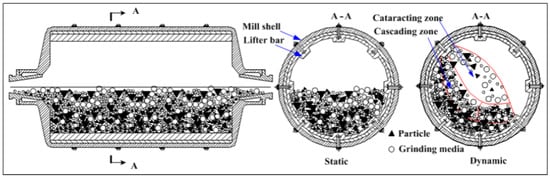
Figure 8.
TBM working principle. Adapted with permission from ref. [52] Copyright 2017 Materials.
Coating Mechanism: In a TBM (a horizontal rotating cylinder), coatings form through repeated impact and friction as the balls cascade and tumble. The kinetic energy is transferred to powder particles via collisions between balls and powder, pressure loading of particles trapped between balls or against the mill wall, and shear/abrasion as the media roll over the material [9]. These repetitive forces cause ductile coating materials to cold-weld onto substrate particles. Over extended milling, the coating material first adheres in patches then coalesces into a continuous layer on the particle surface. This low-energy process proceeds gradually, but it yields a fairly uniform and homogenous coating given sufficient time. TBMs operate at relatively low speeds (typically 10–50 rpm) and impart lower impact energy, resulting in slower coating kinetics than high-energy mills [9]. However, the gentle action minimizes excessive fracturing of particles and tends to produce coatings of consistent thickness across the powder batch.
One real-world use of a TBM for coatings is in mechanical plating (also known as peen plating). In this industrial process, a rotating drum is filled with the target parts (e.g., steel nails), metal powder, additive agents, and hard media (often glass or steel beads). As the drum tumbles, fine metal particles are cold-welded onto the workpiece surfaces, building up a coating [53]. Common plating metals include zinc, tin, or cadmium for corrosion protection. For instance, small steel components can be zinc-coated by tumbling in a barrel with zinc powder and media, avoiding the hydrogen embrittlement associated with electroplating [54]. In academic studies, TBM have been used to coat metal powders with soft phases: Kim et al. demonstrated dry coating of wax onto copper (Cu) powders using a simple tumbling mill [55]. Such coatings improved the powder’s handling and thermal properties. Although hours or days of milling may be required for full coverage in a tumbling mill, the technique’s scalability and simplicity make it valuable for bulk powder coating and large-batch processes.
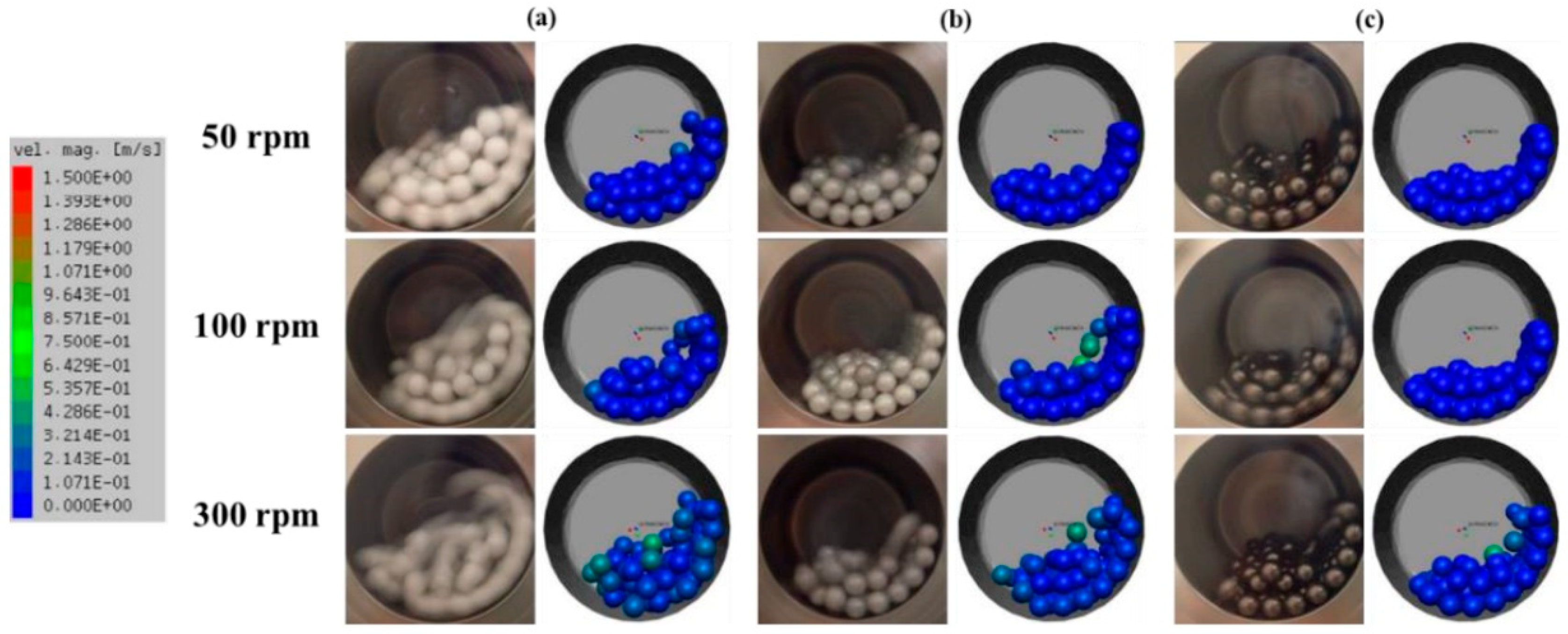
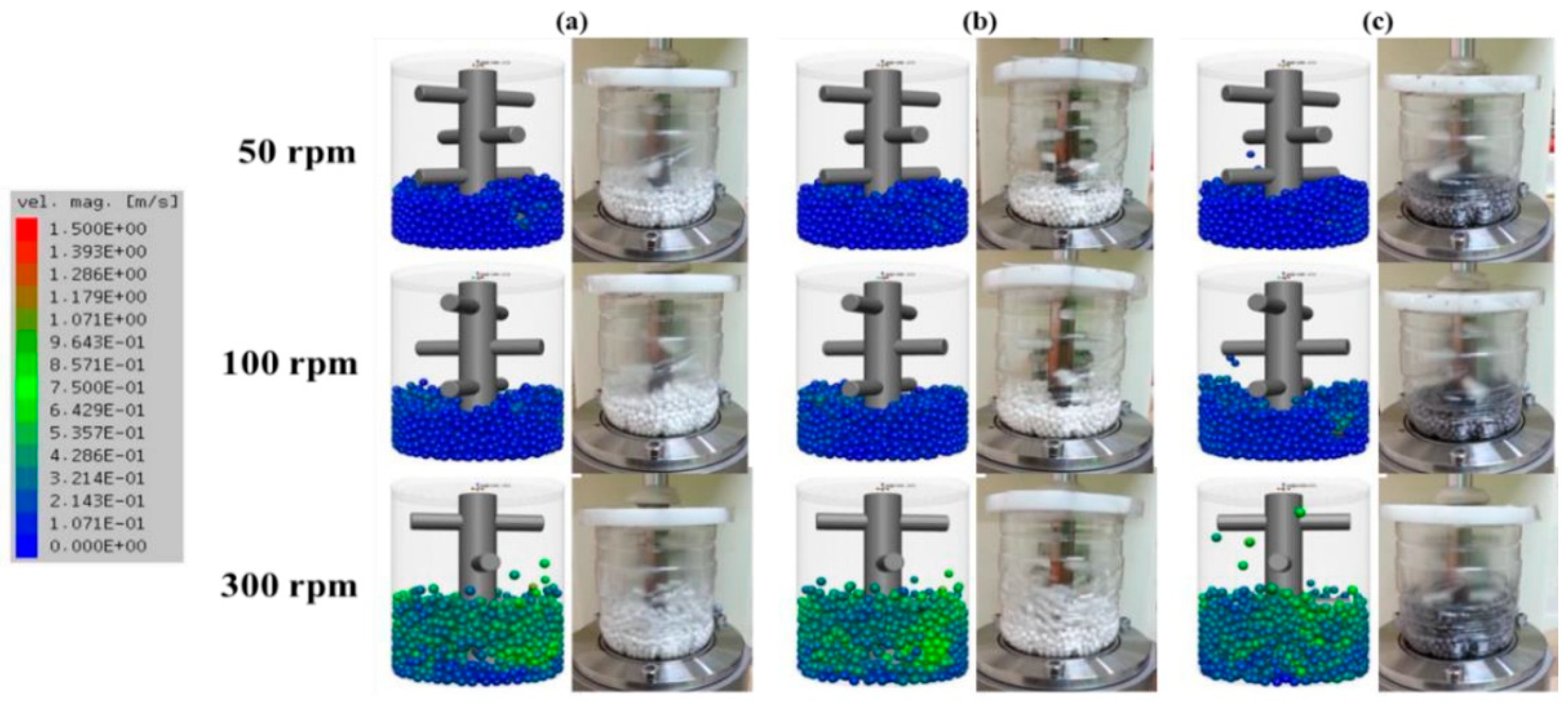
Another example is the work of Bor et al., who examined the surface coating of Cu powder using TBM with different milling media ZrO2 and Al2O3 [56]. The TBM demonstrated a more vigorous and less controlled impact-based milling environment, which led to irregular particle morphologies and weaker coating uniformity. Simulations via the Discrete Element Method (DEM) revealed that TBM generated higher collision energies, particularly near the vessel walls, causing more fragmentation and agglomeration of Cu particles (Figure 9). The analysis suggested that the choice of harder media like ZrO2 in TBM resulted in more pronounced particle deformation and surface coating inconsistencies due to the random motion and lower media-powder contact frequency.
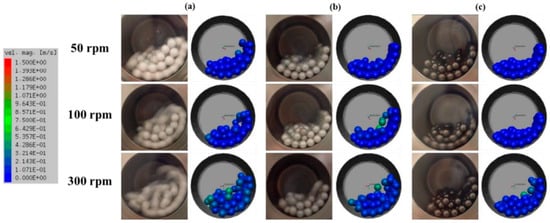
Figure 9.
Simulation and actual snapshot photograph of the media motion by DEM simulation (a) alumina ball, (b) zirconia ball, (c) stainless steel ball with different revolution speeds in a TBM. Adapted with permission from ref. [56] Copyright 2020 Coating.
2.2.2. Vibratory Ball Mills (VBM)
Vibratory mills utilize high-frequency vibrations to agitate the grinding media and material (Figure 10). This unique method creates high friction and impact forces, resulting in the production of fine and homogeneous particle sizes. These mills excel in fine and ultra-fine grinding, as well as in coating processes requiring precision and uniformity. Their advantages include precise particle size control, high energy efficiency, and the ability to process heat-sensitive materials with minimal heat generation. However, they have higher operational costs and more complex maintenance compared to tumbling mills [1,5,7,8,9,48,57,58].
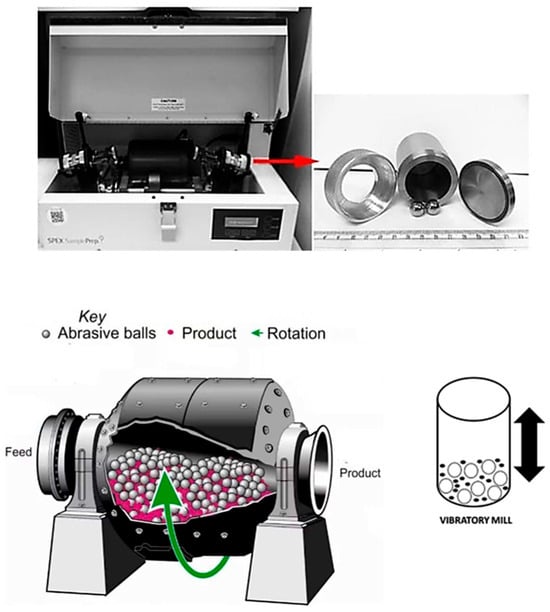
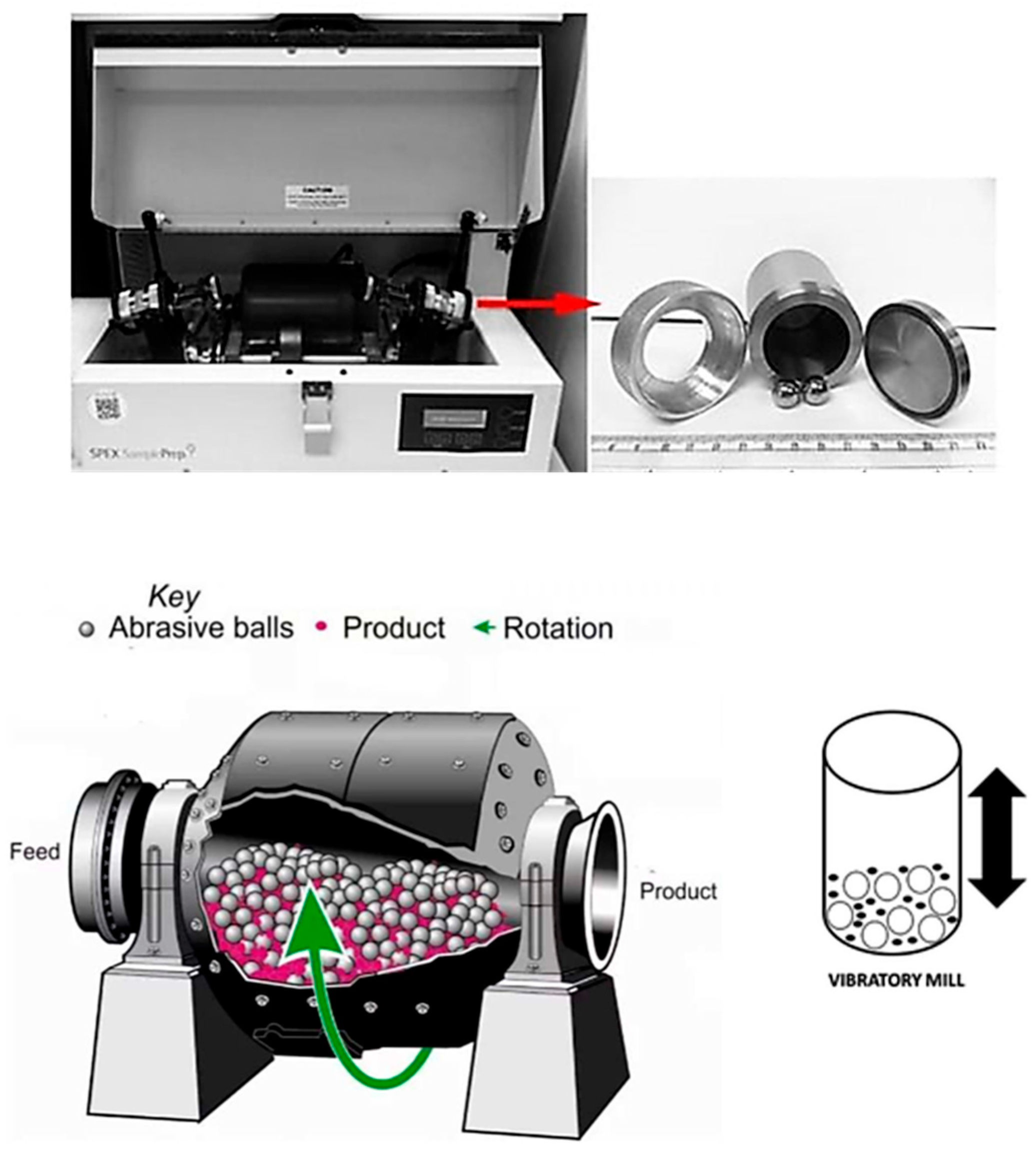
Figure 10.
(Top) photo of SPEX8000D high-energy ball mill with its milling tools (vials and balls) (Spex Sample Prep Mixer Mill 8000M, Metuchen, NJ, USA) (Bottom) principle of VBM. Modified from refs. [9] Copyright 2021 Nanomaterials and 2018 Materials Science and Engineering [57] and 2019 Nanoscale Advances [58].
Coating Mechanism: A VBM (also called a shaker mill) applies high-frequency, low-amplitude oscillation to a milling vial, agitating the ball media with intense kinetic energy. For example, the SPEX mill oscillates a sealed vial ~1200 times per minute in three dimensions [9]. This rapid vibration produces very fast impacts between the balls and particles, leading to high localized pressures and shear forces. The coating material is repeatedly hammered and smeared onto the substrate particle surfaces in a short time. Because ball velocities in a VBM can exceed those in a planetary mill [5], the powder experiences energetic collisions that promote cold-welding of coatings quickly. The mechanism is efficient: brittle phases are pulverized and embedded onto ductile surfaces, and ductile phases flatten into films. However, violent milling can also fragment coated particles if not controlled, so optimizing parameters (impact frequency, time, ball-to-powder ratio) is vital to achieve a uniform coating without significant particle damage. VBM typically handles small powder batches (on the order of tens of grams) due to their intensive energy input and heat generation, but they excel in speed of coating and particle size reduction.
VBM is widely used in laboratory research for the rapid synthesis of coated composite powders and nanostructured materials. For instance, one study used a vibratory milling approach to deposit bioactive hydroxyapatite (HAp) ceramic coatings onto titanium alloy substrates [59]. Using a ZrO2-lined vibrating chamber with zirconia balls, researchers achieved a high-density HAp coating that covered over 95% of the Ti surface after milling [59].
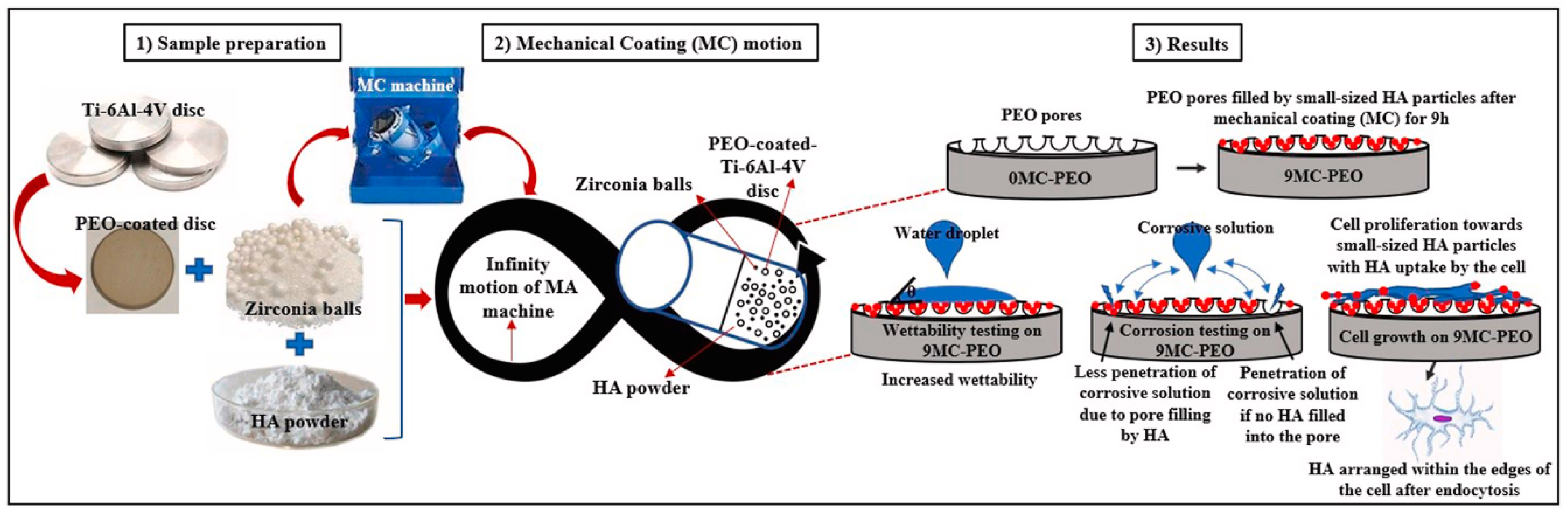
Another study conducted by Nisar et al., where he applied a porous oxide layer on Ti–6Al–4V alloy using Plasma Electrolytic Oxidation (PEO), created a rough, bioactive surface. Subsequently, HAp particles were mechanically deposited onto the PEO-treated surface via a high-energy ball impact process, enhancing the coating’s adhesion and coverage (Figure 11). The dual treatment synergistically improved surface roughness, wettability, and apatite-forming ability in simulated body fluid (SBF), indicating a significant enhancement in the implant’s potential for bone integration [60].
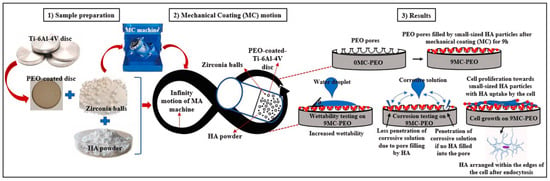
Figure 11.
Mechanical HAp coatings on PEO-treated Ti–6Al–4V alloy for enhancing implant’s surface bioactivity. Adapted with permission from ref. [60] Copyright 2024 Ceramics International.
This demonstrates how vibratory milling can effectively peen a brittle ceramic onto a metal substrate, producing a uniform biomedical coating at room temperature. In another example, a SPEX shaker mill was employed to embed carbon nanotubes into aluminum (Al) matrix powder, rapidly producing a nano-reinforced composite; the high-energy oscillating impacts enabled a homogeneous dispersion of CNTs on Al particles in a fraction of the time required by conventional milling [5]. Such high-energy mills have also been used to amorphize alloy powders and to coat catalytic materials on supports. The VBM’s ability to induce quick, aggressive mixing makes it ideal for small-scale applications like preparing novel composites or coatings for research, where speed is more critical than volume.
2.2.3. Planetary Ball Mills (PBM)
Planetary mills are equipped with one or more grinding jars that are carefully mounted on a rotating sun wheel. The grinding jars rotate around their axes in the opposite direction of the sun wheel’s rotation, creating intense impact and shear forces (Figure 12). This unique milling process is ideal for mechanical alloying, fine grinding, and for producing nano-sized particles. This makes PBM particularly effective for materials that demand thorough mixing and grinding. These mills are known for providing high energy input, precise control over milling parameters, and the ability to produce very fine and uniform particle sizes. Despite these benefits, PBM have limited scalability for industrial applications and higher costs for equipment and maintenance [1,5,8,48,61,62].
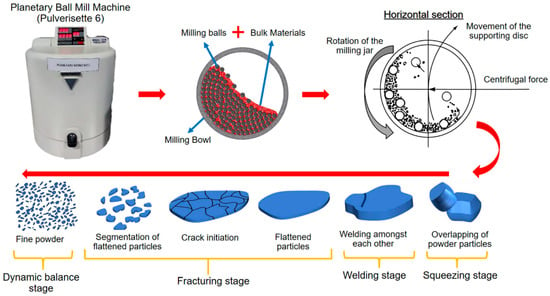
Figure 12.
Principles of the PBM method for reducing powder particle size. Adapted with permission from ref. [41] Copyright 2023 Materials.
Coating Mechanism: A PBM consists of a rotating support disk on which milling jars spin around their own axes, creating complex motion of the balls. The jars and supporting disk rotate in opposite directions, so the balls experience alternating centrifugal forces that can reach ~10–20 times gravity in standard configurations [9]. This produces powerful collisions as the balls are lifted and then thrown across the jar, as well as vigorous friction as they roll along the walls [9]. Under these high-energy impacts and shear forces, coating materials are repeatedly fractured and cold-welded onto base particles. The mechanism typically involves a sequence of stages: initially, tiny fragments of the coating material adhere (nucleation by adhesion); continued milling causes these fragments to flatten and coalesce into discrete island patches; further collisions join the islands into a continuous coating film that thickens over time; if over-milled, the coating may eventually spall in places (exfoliation) due to strain buildup [12]. Because of the intense mechanical action, PBMs can coat particles much faster than a tumbling mill. They effectively embed reinforcing phases or surface layers onto host particles, often reaching nanoscale structural refinement. Careful control (e.g., periodic milling with cooling pauses) is used to prevent overheating due to the high energy input. Overall, the PBM’s combination of impact and shear provides an aggressive coating mechanism well-suited for producing mechanically alloyed coatings and nano-composites.
Example Application: PBMs are a staple in academic research for creating advanced coated powders and novel material phases. A notable example is the MCT to produce photocatalytic coatings: Lu et al. used a PBM to coat Ti metal onto alumina spheres, then oxidized it to form a TiO2 photocatalyst layer (Figure 13) [12]. Thanks to the high impact forces, the PBM achieved the desired coating thickness (about 10–12 µm) in only ~26 h of milling, whereas a traditional low-energy “pot” mill required on the order of 1000 h for a similar result. This dramatic reduction in processing time highlights the efficiency of PBM for coating formation [12].
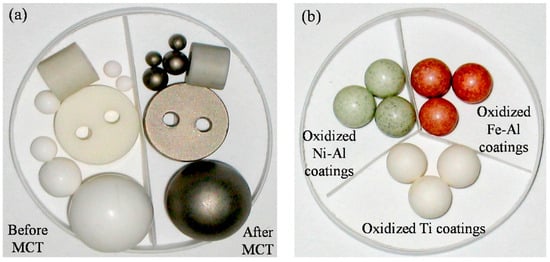
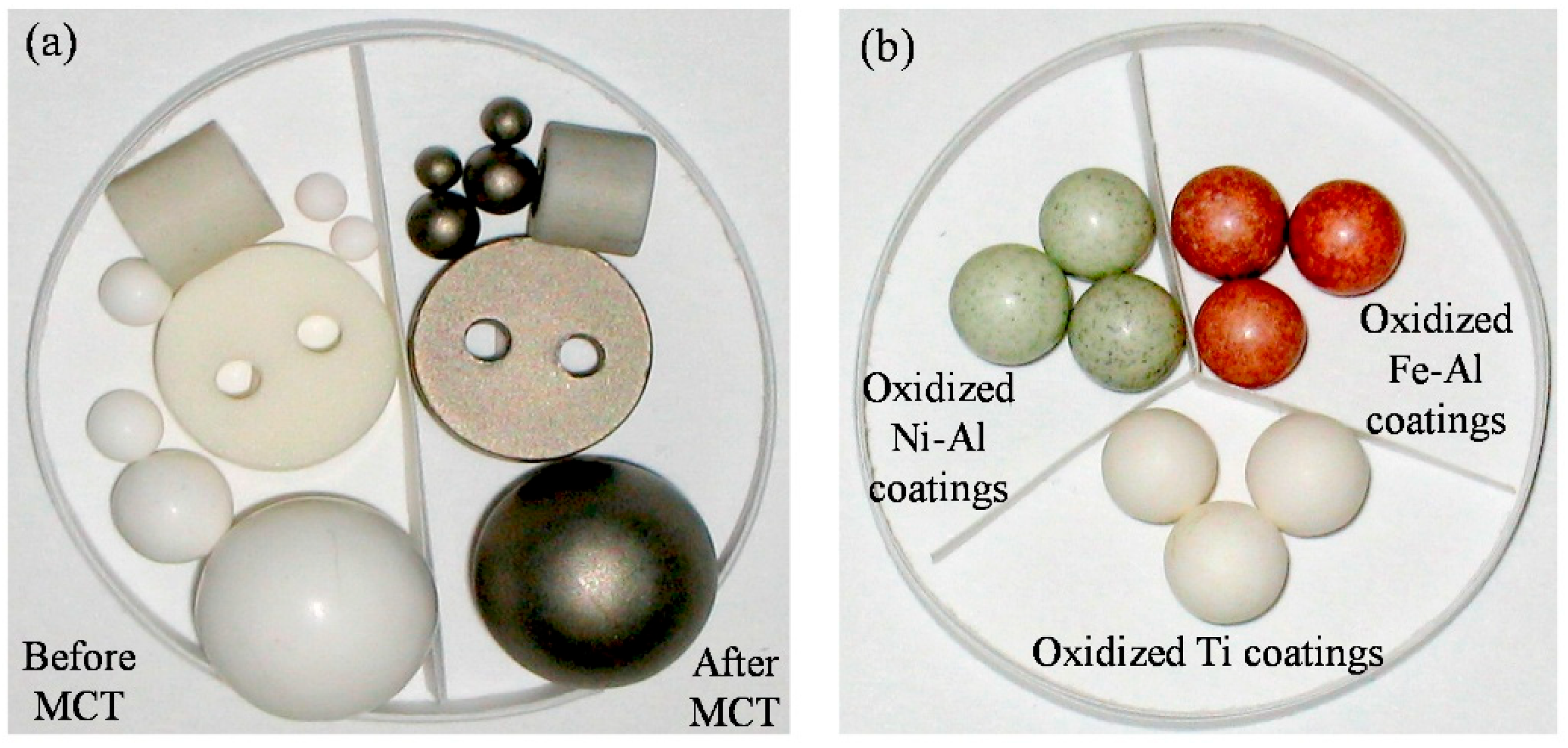
Figure 13.
Appearance of samples by MCT and oxidized metal coatings. (a) Al2O3 objects before and after MCT; (b) The oxidized metal coatings. Adapted with permission from ref. [12] Copyright 2015 Coatings.
PBMs have also been employed to synthesize nanocomposite powders; for example, preparing alloyed metal-ceramic composite particles or core–shell structures for thermal barrier coatings. In one study, a PBM was used to uniformly coat silicon carbide (SiC) nanoparticles onto Al powder, creating a dispersion-strengthened Al/SiC composite suitable for powder metallurgy [9]. Additionally, battery and fuel cell materials often utilize PBM to coat active material particles with carbon or metal oxide layers, improving conductivity and stability. While PBMs are generally limited to laboratory or pilot-scale quantities, they provide unparalleled control over coating microstructure and are invaluable for investigating coating processes in a research setting.
2.2.4. Attrition Ball Mills (ABM)
Attrition mills, also called stirred ball mills, are equipped with a high-speed rotor that effectively agitates the grinding media and material. The movement of the rotor causes the media to grind and coat the material through shear and impact forces (Figure 14). These mills are perfect for producing fine and ultrafine powders as well as for coating processes demanding uniform particle sizes and high surface areas. The advantages of ABMs include high energy efficiency, good control over particle size and distribution, and the capability to create homogeneous coatings. However, they have greater operational costs, a more sophisticated design, and require more intensive maintenance compared to tumbling mills [1,5,8,48].
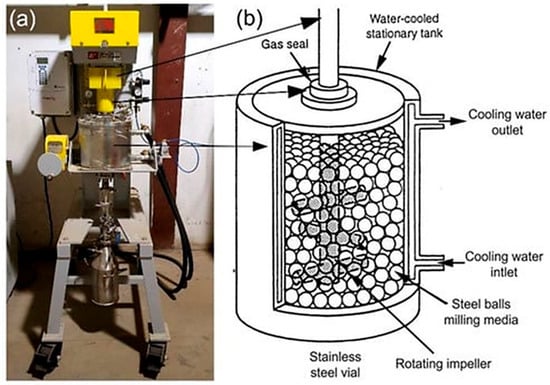
Figure 14.
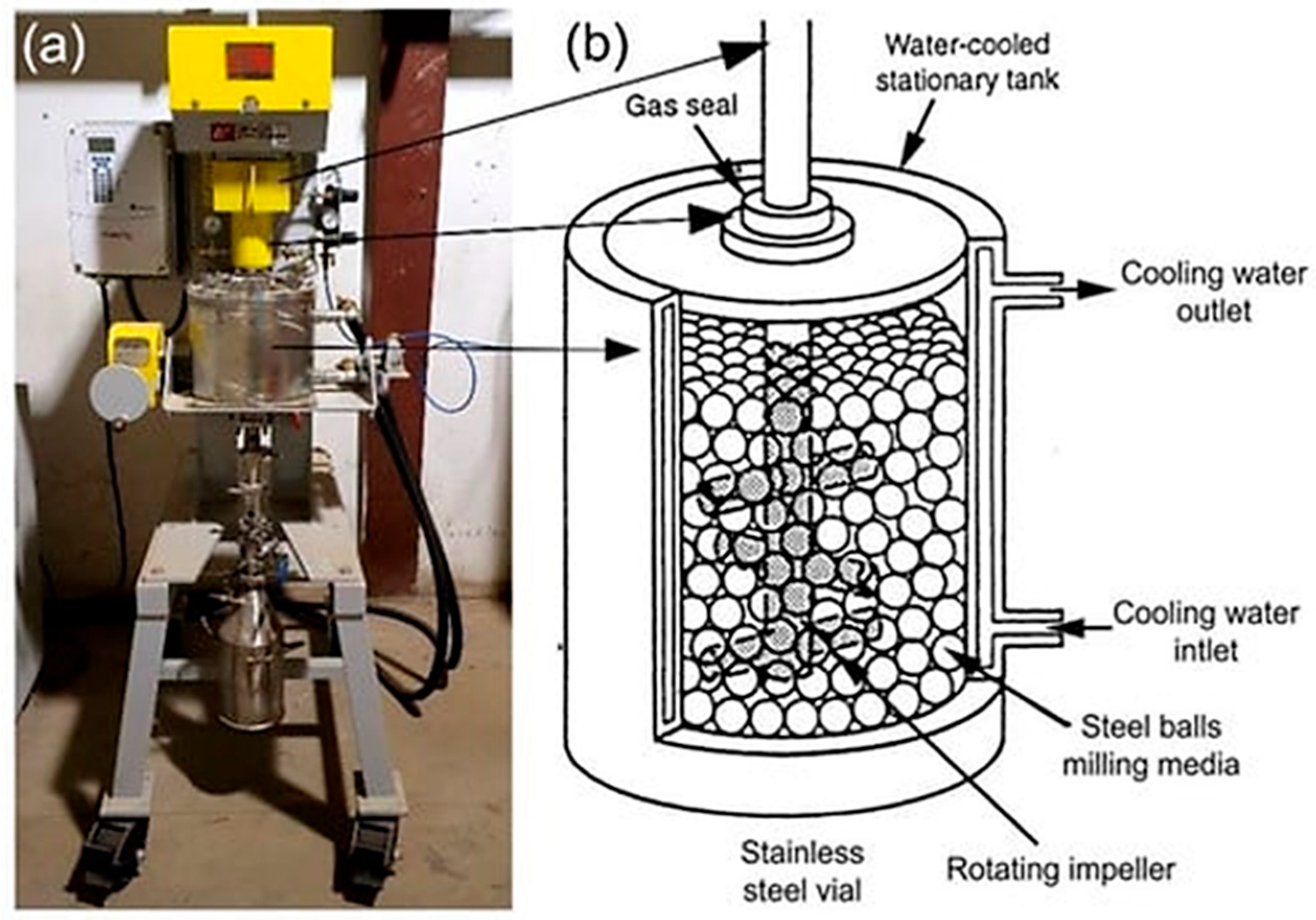
(a) A photo of an ABM; sketches illustrate (b) ball movement inside attritor ball mill. The equipment is housed in the Nanotechnology and Applications Program (NAP), Energy and Building Research Center (EBRC), Kuwait Institute for Scientific Research (KISR). Adapted with permission from ref. [9] Copyright 2021 Nanomaterials.
Coating Mechanism: An ABM (also known as a stirred media mill or attritor) uses a stirring shaft with impeller arms to agitate a dense bed of milling balls. The stationary milling tank and the rapidly rotating agitator (typically 75–500 rpm, with some high-speed attritors up to ~2000 rpm) impose continuous impact and shear on the particles [9]. The milling media in an attritor are much smaller and more numerous than in a tumbling mill, creating a large number of simultaneous collision sites. Coating in this environment occurs via repeated micro-impacts and intense rubbing: powder particles are circulated through the milling chamber and collide with the moving beads, which press coating material onto their surfaces via impact loading and shearing action [9]. The process promotes very intimate blending of dissimilar materials in fact, attritors are known for achieving “the highest intensity intimate blending” and can disperse or mechanically coat additives onto particles during grinding [63]. Compared to other mills, the attritor’s efficient energy transfer and continuous agitation lead to quicker coverage of particles with the coating material, albeit sometimes at the expense of generating smaller particle sizes. The closed, stirred configuration also ensures uniform treatment of all particles (minimizing dead zones), which tends to produce consistent coating thickness and composition throughout the batch. In summary, the ABM’s mechanism is characterized by a combination of rapid impact collisions and vigorous shear flow, making it highly effective for coating formation in both dry and wet milling modes.
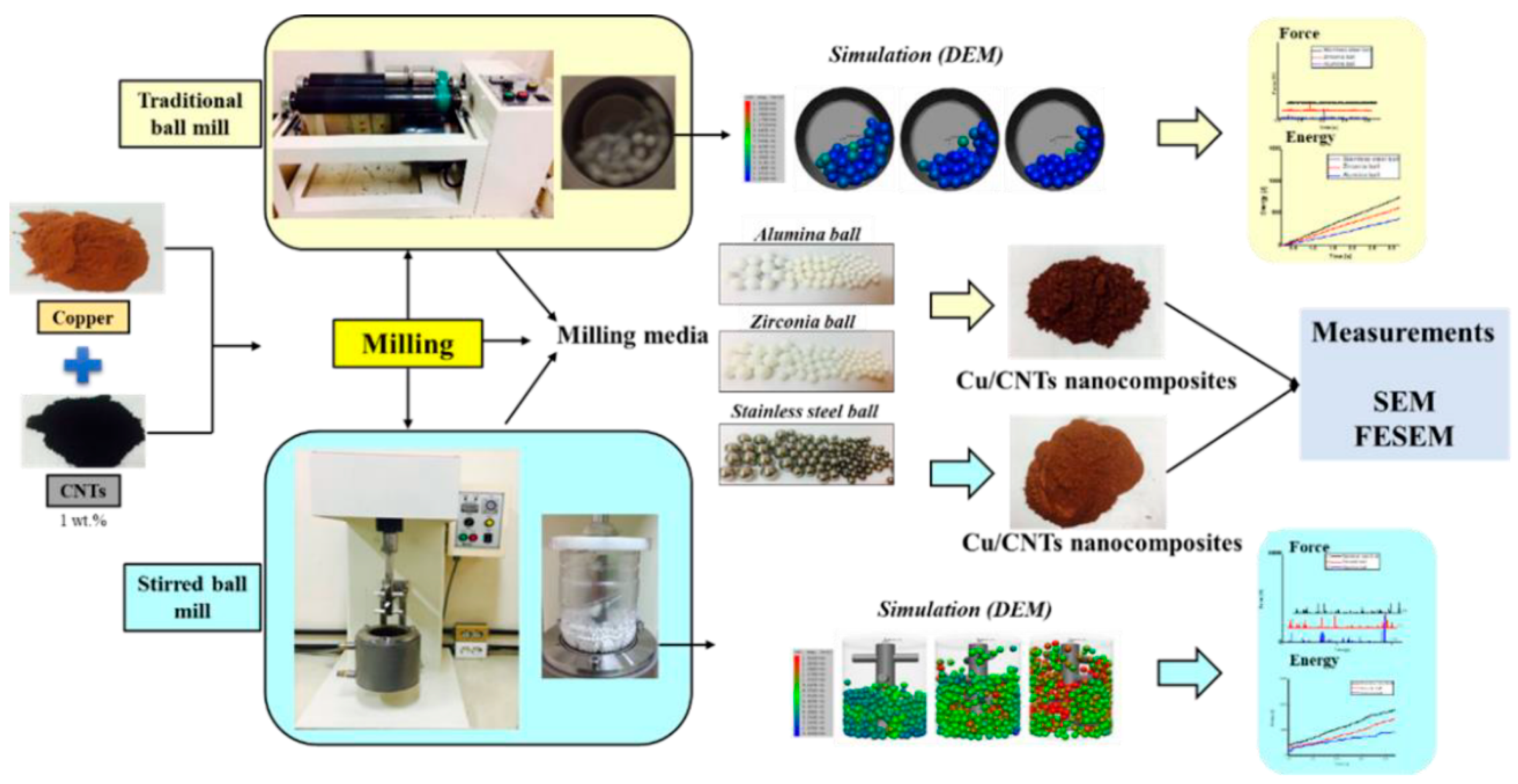
ABMs are frequently used for large-scale or rapid production of coated powders in both research and industry. For example, Bor et al. compared a traditional tumbler to a stirred ABM for coating Cu powder with carbon nanotubes (CNTs) (Figure 15) [56]. The attritor (vertical stirred mill) exhibited stronger impact energy and achieved a uniform CNT film on Cu particles after just 12 h of milling at relatively low speed, whereas the tumbler required longer durations for comparable results [56].
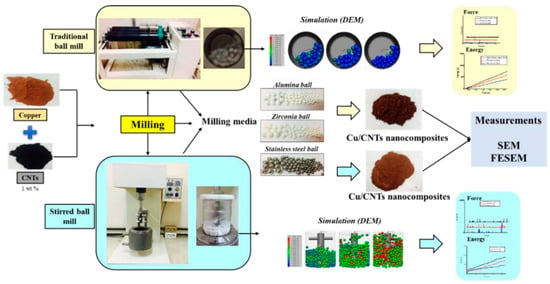
Figure 15.
Schematic view of the CNT coating on the metal surface powders by two kinds of ball milling processes. Adapted with permission from ref. [56] Copyright 2020 Coating.
The SBM offered a more refined and efficient coating mechanism. The continuous stirring motion resulted in higher contact frequency and a more homogeneous energy distribution among particles and milling media [64,65,66]. DEM simulations showed that the SBM produced lower collision energy but significantly improved the uniformity of coating on Cu powder, especially when using ZrO2 media (Figure 16) [56]. The spherical morphology and better dispersion of particles achieved in the SBM highlighted its superiority in promoting even coating and minimizing particle agglomeration. Overall, the SBM was found to be more effective than TBM for precise and uniform surface coating in powder processing applications.
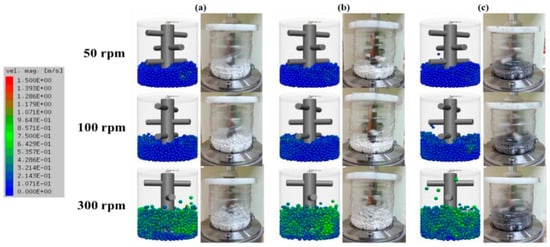
Figure 16.
Simulation and actual snapshot photographs of the media motion by DEM simulation (a) alumina ball, (b) zirconia ball, (c) stainless steel ball with different revolution speeds in an SBM. Adapted with permission from ref. [56] Copyright 2020 Coating.
This indicates the attritor’s advantage in accelerating the coating process. In industrial contexts, ABMs are used to produce pigment-coated particles and composite powders for paints, inks, and ceramics; for instance, difficult-to-disperse pigments (iron oxides, carbon black, etc.) can be mechanically coated or finely dispersed onto extender particles using an attritor [63]. Attritors have also been employed to make dispersion-strengthened metals via mechanical alloying, where powders of different metals are co-milled to coat and embed hard phases within a ductile matrix [63]. This technique has been applied to produce oxide-dispersion strengthened alloys and composite metal powders for aerospace materials. A key benefit of ABMs is their scalability: laboratory attritors with ~100 mL vessels are available for experiment development, and the same process can be scaled up to production attritors holding hundreds of liters, all while maintaining similar shear/impact conditions. This scalability, combined with efficient energy use and uniform mixing, makes the ABM a workhorse for coating processes that require both high throughput and consistent quality of the coated product.
In summary, as shown in Table 1, planetary and vibratory mills provide superior control over particle size and their distribution, making them ideal for fine and ultrafine grinding. Furthermore, VBM and ABMs demonstrate greater energy efficiency compared to TBMs. TBMs are easier to scale for industrial applications, whereas planetary mills are more suited for laboratory and small-scale applications. In terms of cost-effectiveness and maintenance, TBMs come out on top, while vibratory and ABMs entail higher operational and maintenance expenses. Understanding these differences allows researchers and industry experts to select the most suitable milling method for their specific coating and material processing requirements.

Table 1.
Comparison of different types of ball mills based on grinding mechanism, energy efficiency, particle size control, scale, operational speed, contamination risk, and application suitability.
3. Mechanism of Metal Coating on Spherical Substrates Using Ball-Milling Process
3.1. Zinc (Zn) Coating on Alumina (Al2O3) Balls
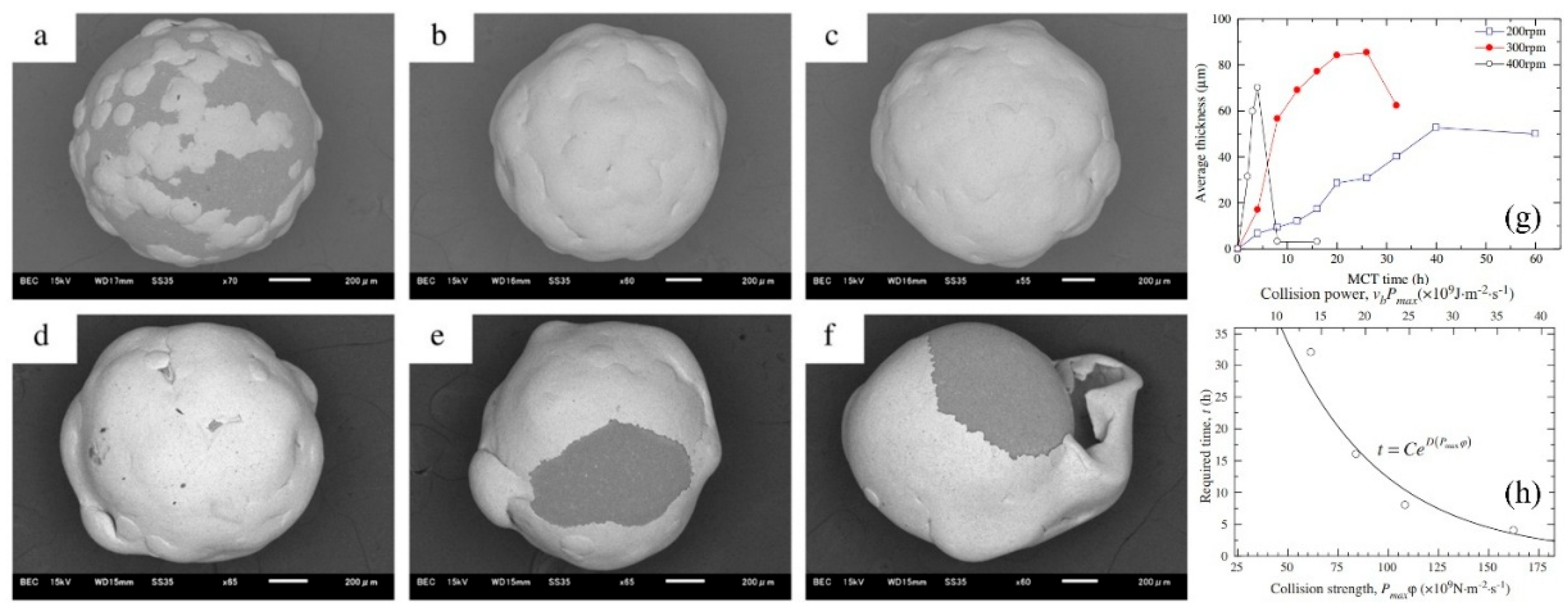
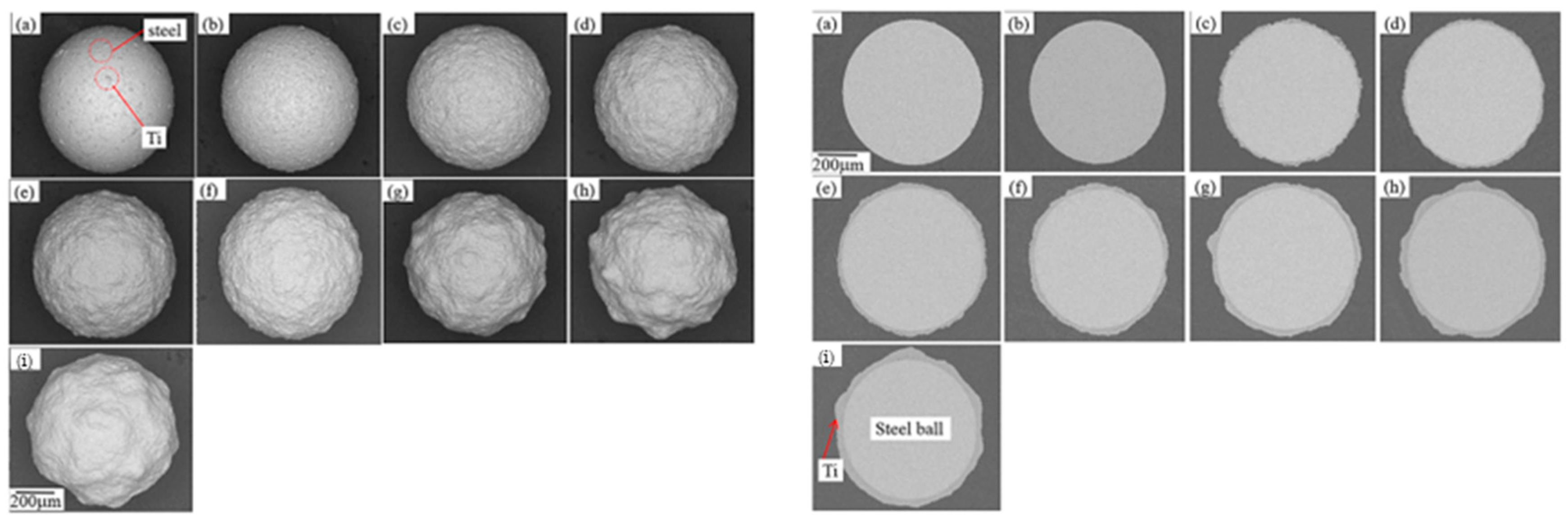
Hao et al. reported the effect of processing parameters on the detailed evolution of coating for the fabrication of Zn coatings on Al2O3 balls (Figure 17) [67]. The effect of the rotation speed of the planetary ball mill on the evolution of coatings was specifically investigated. The findings revealed that continuous and highly dense zinc coatings, averaging about 100 μm in thickness, were formed during the milling process at a speed of 300 rpm. To achieve these continuous Zn coatings, critical collision pressure, which corresponds to a critical minimum rotation speed, was essential to induce a critical plastic strain in the zinc particles. Exponential relationships between the time required to form continuous zinc coatings and both collision strength and collision power were established. These relationships indicated that the necessary time is influenced by the collision stress, or the work done on the zinc particles per unit area in unit time. The evolution of the coatings encompasses the nucleation, formation and coalescence of discrete islands, the formation and thickening of continuous coatings, and the exfoliation of those continuous coatings.
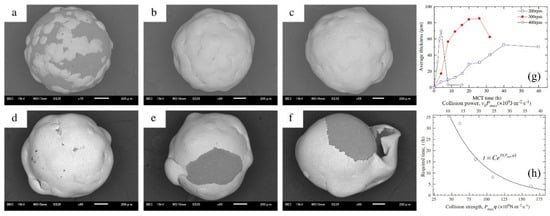
Figure 17.
The SEM images of the surfaces of the Zn-coated Al2O3 balls following the ball milling at 300 rpm: (a) 4 h, (b) 8 h, (c) 12 h, (d) 16 h, (e) 20 h and (f) 26 h, (g) illustrates the average thickness of the coatings as a function of milling time at different rotational speeds, (h) presents the required time to achieve continuous Zn coatings based on collision strength or collision power. Adapted with permission from ref. [67] Copyright 2012 Powder Technology.
The SEM images of the surfaces of Zn-coated Al2O3 balls after the milling operation at 300 rpm are presented in Figure 17. In these images, the dark areas represent the surfaces of the Al2O3 balls, while the gray areas indicate the Zn coatings. The progression of the Zn coatings during the milling process is clearly observable. As milling time increases, a greater number of Zn powder particles adhered to the surfaces of the Al2O3 balls. By the 8 h mark, continuous Zn coatings had formed (Figure 17b). However, with an increase in milling time beyond 16 h, some portions of the Zn coatings began to peel away, exposing the surfaces of the Al2O3 balls once again (Figure 17e,f).
As illustrated in Figure 17g, it can be observed that the average thickness initially increased, reached its peak, and then subsequently decreased, regardless of the rotation speeds of 200, 300, or 400 rpm. Higher rotation speeds expedited the evolution of the average thickness. These findings align well with the previously discussed analysis on the evolution of the coatings. Figure 17h illustrates the time required to form continuous Zn coatings, denoted as t, as a function of the collision strength or collision power. The constant C (h) is influenced by the characteristics of the metal powder particles and the grinding balls. D and F are negative constants determined by the geometry of the bowl and planetary ball mill, as well as the size of the grinding balls. The dimensional units for D and F are m2·s·N−1 and m2·s·J−1, respectively. In this study, C was calculated to be e4.52, while D and F were found to be −2.01 × 10−2 and −9.12 × 10−2, respectively. The variable ωp represents the rotation speed of the planetary ball mill (rad·s−1), while ωb denotes the rotation speed of the bowl, which is equal to ωp/Tr. K is a constant based on the ball diameter and is estimated to be approximately 1.5 (r−1) for balls with diameters ranging from 8 to 10 mm. Due to the lack of data for balls with a diameter of 1 mm, the influence of the ball diameter in this case is considered negligible. Therefore, the values for the 8–10 mm diameter balls will be utilized to evaluate the collision frequency for an Al2O3 ball.
In the initial phase of the milling process, several zinc clusters transfer from Zn powder particles to the surfaces of Al2O3 balls due to friction and abrasion. The adhesive strength of metals varies, which is mainly influenced by their electronic structure and the extent of charge transfer between the adsorbed metal and the surface. The adhesion of Zn clusters to the Al2O3 ball surfaces is referred to as “nucleation.” It is important to note that this type of nucleation differs from the transition of metal from a liquid to a solid phase; it specifically involves the adhesion of solid metal clusters to the surface of solid ceramics. The size of these “nuclei” typically ranges from several nanometers to several hundred nanometers.
Subsequently, additional zinc clusters transferred and adhered to the surfaces of Al2O3 balls or to the existing Zn nuclei. The increase in size of the Zn nuclei is due to cold welding between the Zn powder particles and the nuclei, leading to the formation of discrete Zn islands. This mode of film growth is referred to as island-type or Volmer–Weber growth [68]. Following this, Zn particles attached themselves to the discrete islands and the nuclei, resulting in the coalescence of these islands; this phase is known as the formation and coalescence of discrete islands. The mechanical interlocking of Zn particles with the surface irregularities of the Al2O3 balls likely promoted the formation of these discrete islands [69]. During this stage, the surface coverage of Al2O3 balls with Zn increased rapidly. Cold welding was the predominant mechanism at this point. In the third stage, the surfaces of the Al2O3 balls became coated with Zn, culminating in nearly 100% surface coverage. This indicates the formation of continuous Zn coatings. Then, the cold welding between the zinc particles and the coatings continued, resulting in an increase in the thickness of the coatings. This phase is referred to as the formation and thickening of continuous coatings. In the final stage, the continuous Zn coatings began to peel off. During the development of these continuous coatings, internal stress was induced due to work hardening and the discrepancies in the thermal expansion coefficients of the Al2O3 ball coatings. As the thickness of the coatings increased, so did the internal stress. This internal stress subsequently weakened the adhesion of the coatings to the surfaces of the Al2O3 balls.
The process of forging fracture also plays a significant role in the exfoliation of coatings. After undergoing repetitive plastic deformation, the zinc powder particles and the continuous coatings become brittle due to work hardening. When Zn-coated Al2O3 balls collide with the inner wall of the bowl, a crack may initiate once a critical tensile strain is achieved. Following this, crack propagation occurs when the rate of plastic energy release surpasses a threshold characteristic of the material. Maurice and Courtney have outlined the fragmentation mechanisms of metal powder particles [70]. In summary, the exfoliation of continuous coatings arises from the combined effects of internal stress and external collision stress. This stage is referred to as the exfoliation of continuous coatings.
3.2. Iron (Fe) Coating on Alumina (Al2O3) Balls
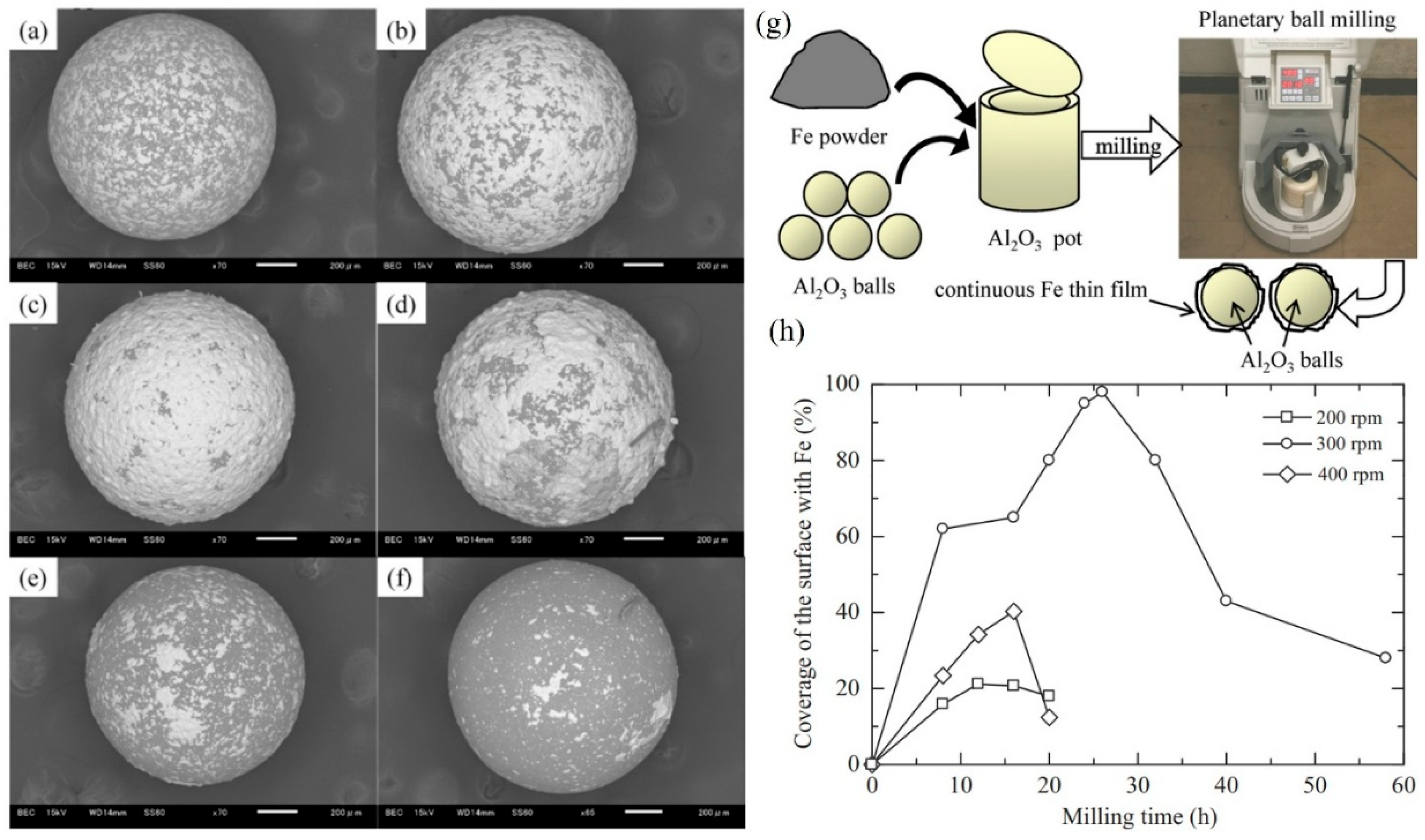
Hao et al. also reported and discussed the influence of the processing parameters on the formation of iron thin films on Al2O3 balls by the ball milling coating technique (Figure 18a–g) [71]. The Fe particles reacted with oxygen in the atmospheric air, leading to the formation of ferro-ferric oxide, which impeded the creation of thin films. The rotation speed also significantly influenced the outcome. Continuous Fe thin films, with an average thickness of approximately 10 µm, were produced during the milling operation at 300 rpm; however, they could not be achieved at 200 and 400 rpm (Figure 18h) [71]. This apparent anomaly can be rationalized by considering the balance between impact energy and oxidation kinetics. At 200 rpm, the collision energy is too low to fracture the oxide barrier, preventing metallic bonding. At 400 rpm, the excessive energy leads to severe particle fragmentation and localized heating, which accelerates re-oxidation and hinders stable film growth. At 300 rpm, however, the collision energy lies within a critical window: it is sufficient to disrupt surface oxides and expose fresh metal, but not so high as to favor rapid re-oxidation. The plastic deformation that follows enables metallic contact and cold welding, allowing a continuous film to form despite the oxygen-present environment. Thus, 300 rpm represents an optimal impact-energy regime where oxide breakdown and film formation outweigh oxidation.
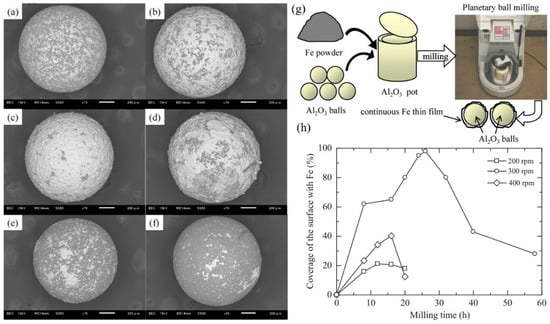
Figure 18.
The SEM images of the surfaces of the Fe-coated Al2O3 balls following the ball milling at 300 rpm: (a) 8 h, (b) 20 h, (c) 26 h, (d) 32 h, (e) 40 h and (f) 58 h, (g) illustrates the schematic Fe-coating onto Al2O3 balls, and (h) surface coverage as function of milling time at different rpms. Adapted with permission from ref. [71] Copyright 2012 Journal of Materials Processing Technology.
Additionally, the development of thin films was studied and analyzed. This progression was observed to involve nucleation, growth of nuclei, thin film formation, and exfoliation. It was determined that mechanical interlocking played a crucial role in the formation of these thin films.
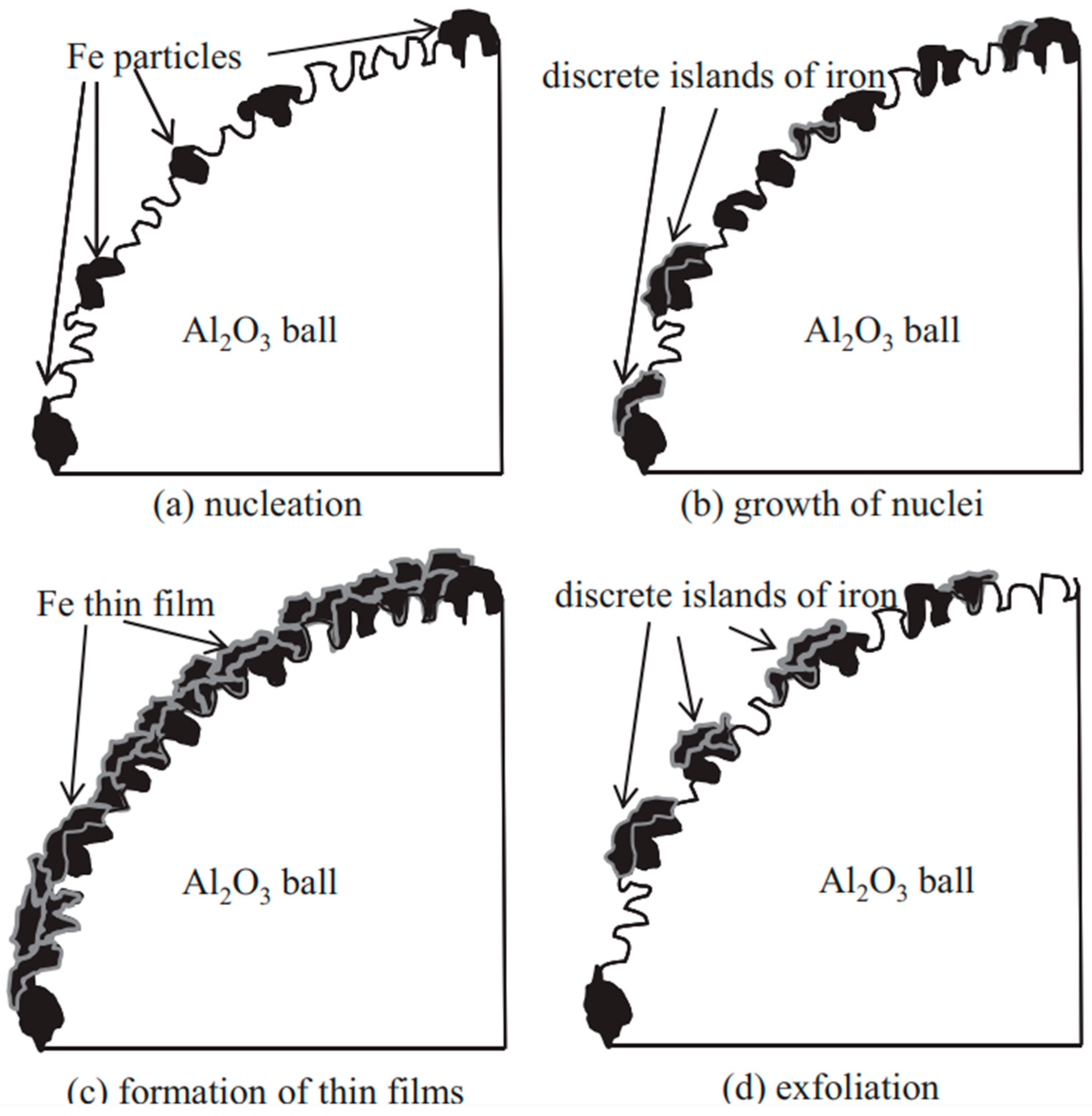
At the beginning of the milling operation, Fe particles were immobilized either between Al2O3 balls or between the Al2O3 balls and the surface of the milling pot. Under the action of impact force, numerous small Fe particles became embedded in the pores, cavities, and other irregularities of the surfaces of the Al2O3 balls (“nucleation” Figure 19a) [71]. The air trapped at these surfaces was displaced by the Fe particles. In the initial stages of milling, the Fe particles were relatively soft and underwent plastic deformation due to repeated impact forces. This deformation led to the creation of new particle surfaces before they adhered to the Al2O3 balls. The generation of these new surfaces facilitated adhesion between the impacted Fe particles and the Fe coatings previously formed on the Al2O3 balls. This adhesion aligns with the weld theory of metal particles discussed in earlier studies [5]. This phenomenon is referred to as the “growth of nuclei” (Figure 19b) [71]. and occurs as a result of direct or angled impact forces rather than through friction. During milling at 200 rpm, the formation of thin films was unsuccessful, likely due to the insufficient impact force.
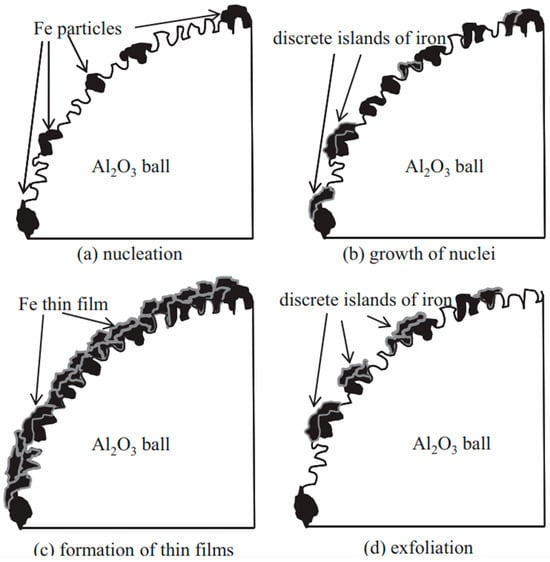
Figure 19.
Coating of Fe thin films onto Al2O3 balls using the ball milling process. Adapted with permission from ref. [71] Copyright 2012 Journal of Materials Processing Technology.
As discrete islands of iron grew, more iron particles were deposited on and around them, leading to an increased coverage of the Al2O3 ball surfaces with iron. At milling times of 16 and 26 h during operation at 300 rpm, the surface coverage reached 65% and 98%, respectively (Figure 19) [71]. Continuous thin films began to form after 26 h, and the thickness of these films continued to increase. This process is referred to as the “formation of thin films” (Figure 19c) [71]. However, as the continuous Fe thin films underwent further plastic deformation, they became work-hardened and consequently brittle. Under repeated impacts, portions of these thin films, which were mechanically interlocked with the Al2O3 balls, could fracture. The mechanical interlocking between the thin films and Al2O3 balls may have weakened over time. The mechanical interlocking force was considered the primary adhesive force. Moreover, the internal stress within the thin films increased with their thickness [68]. Once the internal stress exceeded the adhesive force between the thin films and the Al2O3 balls, the films began to peel off. This phenomenon is known as “exfoliation” (Figure 19d) [71].
3.3. Mechanism of Formation of Nanostructured Coatings on Disk Metal Substrates Using Ball-Milling Process
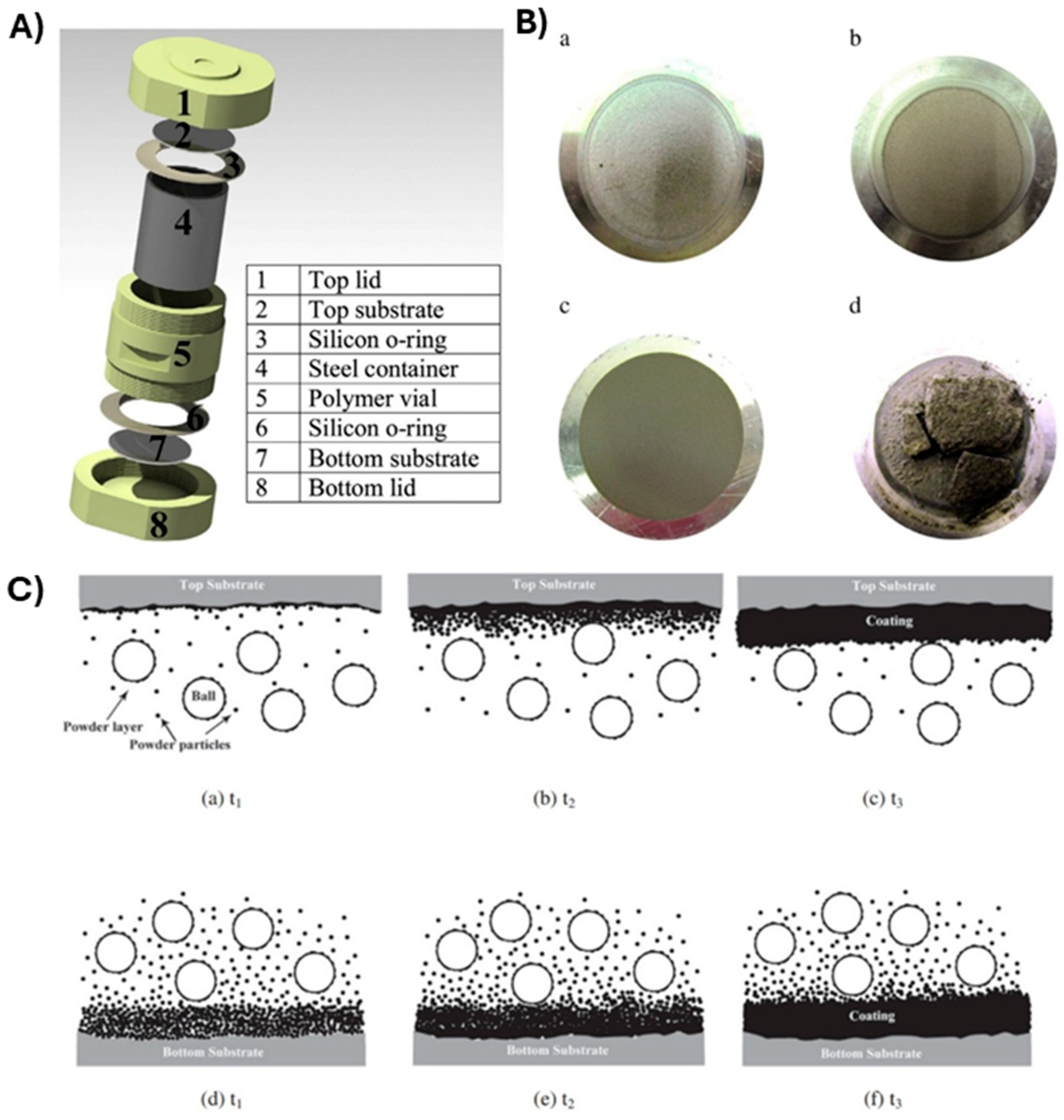
Yazdani et al. studied Ni and SiC powders coating on Al disk substrates using the planetary ball-milling process [72]. Ni and SiC were individually used as coating materials that were mechanically deposited onto an Al substrate fixed at either the top or bottom of the milling vial (Figure 20A). Under the milling conditions applied in this study, the SiC coatings exhibited low microhardness and wear resistance, regardless of the substrate’s position, primarily due to inadequate consolidation (refer to Figure 20B). In contrast, the Ni coatings produced under similar milling conditions yielded excellent results (as shown in Figure 20C). It was observed that positioning the substrate at the top significantly enhanced microhardness, wear resistance, and adhesion strength. The Ni coating on the top substrate, with an average crystallite size of approximately 58 nm, demonstrated a microhardness of 422 ± 12 HV, which is at least 30% higher than that of a typical electroplated Ni coating. The variation in coating characteristics between the two substrate positions was attributed to differing deposition mechanisms.
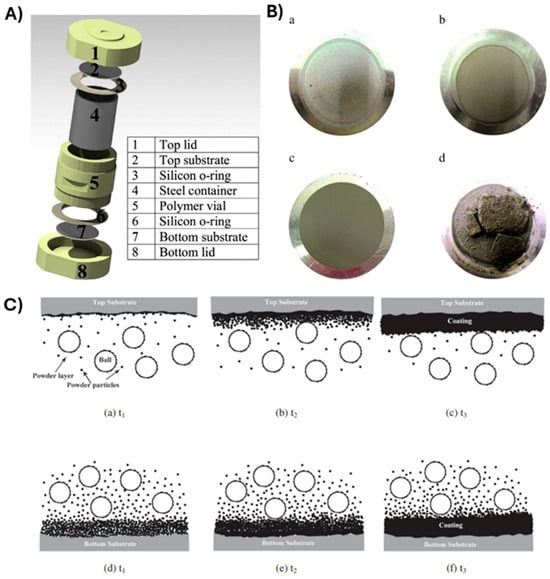
Figure 20.
(A). Ni and SiC coating setup on Al disk substrates. (B). Photographs of (a) Ni-Top, (b) Ni-Bottom, (c) SiC-Top, and (d) SiC-Bottom coatings onto Al substrates. (C). Coating evolution on (a–c) the top and (d–f) the bottom of the Al substrate at three continued milling times (t1, t2, and t3). Adapted with permission from ref. [72] Copyright 2015 Powder Technology.
The different mechanisms for coating formation on the top and bottom substrates are illustrated in Figure 20C over three time sequences. For the top substrate, it is believed that most of the powder is transferred from the surface of the ball to the substrate. Upon impact, loosely bonded particles on the colliding ball’s surface adhere to the substrate. Subsequent ball collisions increase the compaction of the coating and reduce micro voids. As the kinetic energy of the colliding balls is imparted to a small amount of powder on the substrate (shown in Figure 20C(a)), the powder gains the chance to be compressed into the substrate, potentially leading to cold welding. This process can create a metallurgical bond between the coating and the substrate. As milling continues, the sequential deposition and compaction mechanism leads to the accumulation of coating thickness with effective adhesion and cohesion (illustrated in Figure 20C(b,c)).
The formation of the coating on the bottom substrate is thought to occur through a different mechanism. As demonstrated in Figure 20C(d–f), a significant amount of starting powder accumulates on the bottom substrate due to gravity settling. Unlike the top substrate, the impact energy of the flying balls is distributed over a larger volume of powder on the bottom substrate, resulting in a lower degree of adhesion to the substrate. However, as milling continues, the compaction of the coating improves. Extended milling times enhance the cohesion of the coating and reduce the presence of microvoids. Thus, the formation mechanism can be primarily attributed to an initial lump sum deposition followed by successive compactions. In this context, the impact energy from the colliding balls is insufficient for the effective compaction and cold welding of a relatively thick layer of nickel deposit on the bottom substrate. This leads to non-uniform densification throughout the coating thickness, resulting in lower microhardness and a higher wear rate.
Understanding the coating mechanisms in ball milling techniques properly is crucial for researchers and industry experts looking to improve coating quality and efficiency for particular applications. Every mechanism plays a vital role in enhancing the ball milling process, which in turn results in the creation of superior materials and coatings. The movement of the ball (or particles) inside ball mills is depicted in Figure 21, which sheds light on how coating balls travel inside these mills.
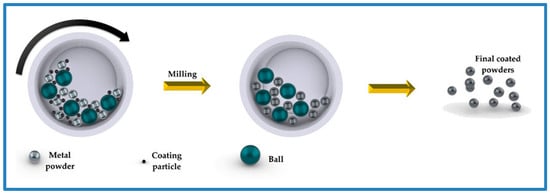
Figure 21.
Schematic diagram of ball milling process for coating powders. Adapted with permission from ref. [73]. Copyright 2021 Metals.
During the use of ball milling for coating, various forces, impacts, and mechanisms take part in creating the final coated surfaces or newly produced particles with each collision. These include impact and attrition forces, as well as important coating mechanisms, governed by inertial forces—primarily centrifugal force and, in planetary or vibratory mills, the Coriolis force, which are one to two orders of magnitude greater than gravity. Consequently, gravitational effects can be disregarded, and the true mechanism of coating formation is collision energy transfer, assisted by mechanical alloying [44,56].
Impact forces are created when milling balls collide with each other and with the coating material, generating high energy. This dispersion of the coating material onto the substrate results in the formation of a uniform and densely layered coating, effectively improving the coatings’ adhesion and durability.
Meanwhile, attrition forces are created when the grinding balls hit each other and against the container walls. This collision produces frictional forces that shear the particles, which helps distribute the coating substance evenly throughout the milled particles.
The gravitational effect involves movement of the milling balls within the coating material, resulting in the gradual formation of layers due to the impact of gravity.
In comparison, the rotation effect includes the rolling motion of the milling balls caused by the rotation movement of the milling container, which facilitates the uniform distribution of the coating material across the particles.
Conversely, the mechanical alloying process is used predominantly beside coatings to produce composite materials. In this process, particles are forced together by energy transfer and mechanical collisions and friction. This mechanical activity results in the formation of new alloys, which is achieved through cold welding [3,4,5,9,10,44,74].
Cold-welding phenomena contribute to the formation of agglomerates and the consolidation of particles, leading to the production of unique structures and properties. The interplay of these forces, impacts, and mechanisms not only influences the morphology and composition of the coated surfaces but also affects their functional properties, such as hardness, wear resistance, and corrosion resistance [5,8,28,29,30,44,75]. Therefore, understanding and controlling these processes are essential for achieving desired coating characteristics and optimizing the performance of coated materials in various applications.
4. Parameters Affecting Coating Efficiency
Coating parameters play a critical role in determining the efficiency and quality of the coat produced by various coating methods. Therefore, it is crucial to thoroughly examine these parameters to enhance and optimize the coating process. There are two sets of parameters to consider: process parameters (e.g., the ratio of balls to powder, milling time and speed, ball size) and the intrinsic material properties of the coating material and the coated particle (such as hardness, structure, and chemistry) [11,76,77].
4.1. Applied Ball Milling Process Parameters
4.1.1. Ball to Powder Ratio (BPR)
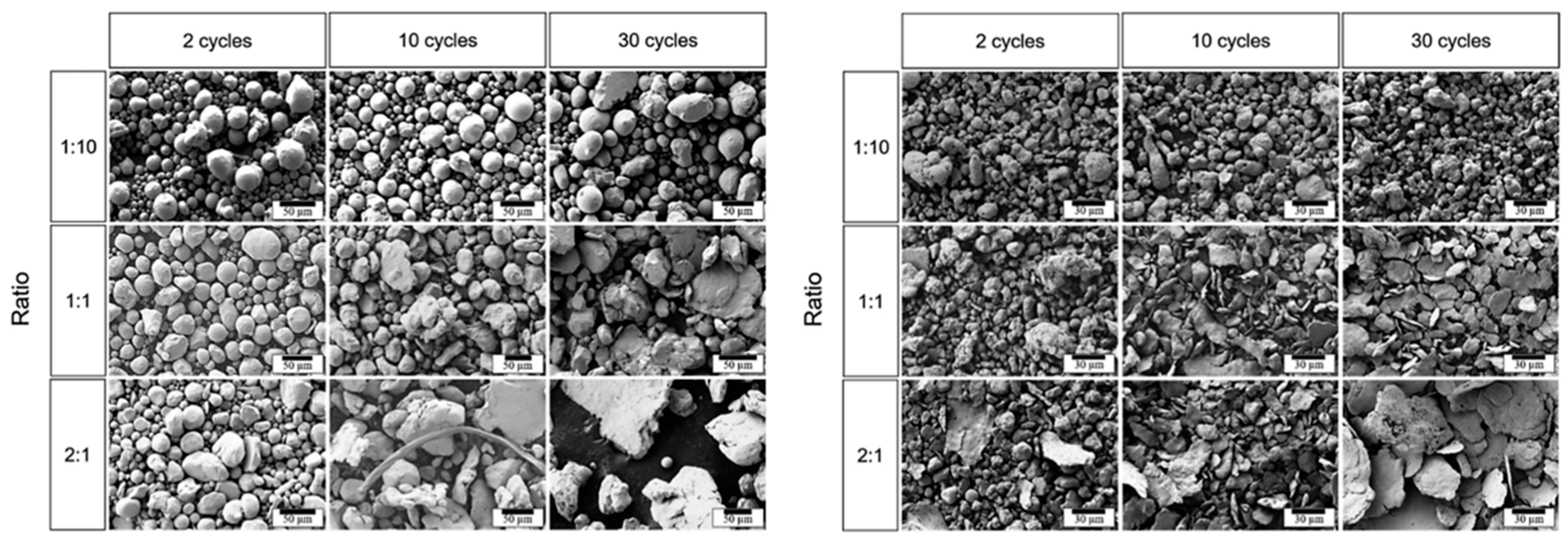
The BPR is considered a key parameter in determining the coating efficiency of ball milling, measured by coating coverage, adhesion, and minimal contamination. Increasing the BPR results in a more efficient coating process. This happens because a higher ratio provides additional energy and creates more contact surfaces, which ultimately leads to improved coating performance. Studies have shown that key powder properties (morphology, hardness) can change more with BPR than with milling duration. For example, Ansell et al. conducted a study on the impact of high-energy ball milling (HEBM) on spherical Cu and stainless steel powders. Their research focused on how varying the BPR influences several factors, including the morphology (Figure 22), particle size, and hardness of the powders. These factors are significant as they directly affect the flow characteristics and adhesion properties of the powders in additive manufacturing applications [14,25,78]. For instance, increasing BPR leads to faster work-hardening of powders: high BPR can raise powder hardness in minutes, whereas at low BPR, similar hardening may take hours. An optimal BPR promotes efficient coating by providing sufficient impacts for particle adhesion without causing excessive fracturing of either the powder or the nascent coating [14,25,78].
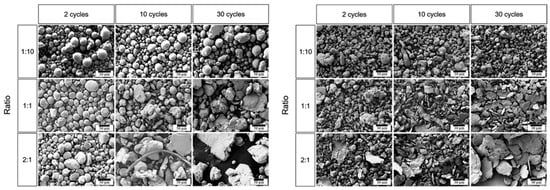
Figure 22.
SEM images of (Left) milled 316L stainless steel powders and (Right) milled Cu powders processed at different BPRs and under varying cycles. Modified and adapted with permission from ref. [78] Copyright 2021 Particulate Science and Technology.
It is noteworthy that the ratio of ball weight to powder weight is an important economic factor in milling processes. Increasing this ratio results in higher output, improving efficiency in terms of coating coverage and energy use, while also supporting industrial scalability. These improvements are closely connected to the properties of the final product and the specific requirements of the milling process [11,14,28,78,79].
4.1.2. Milling Time and Speed
The duration and speed of the milling process are other crucial factors in defining the final coating properties. The duration of milling significantly impacts the thickness and uniformity of the coating. Meanwhile, the speed of the milling process influences the quality of the coating.
Milling Time
Longer milling times result in finer particle sizes and thicker, more uniform coatings due to the increased frequency of powder impacts. Initially, this process enhances both coating thickness and coverage. However, excessive milling may lead to complications such as particle agglomeration, contamination, and phase transformations caused by heat generation (Figure 23). Furthermore, beyond a certain duration, the coating may become saturated or even experience delamination, entering an exfoliation stage [25,30,80].
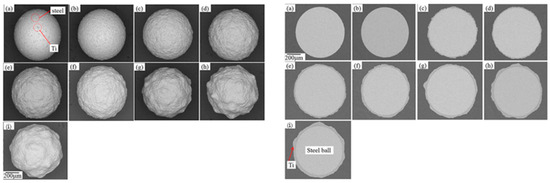
Figure 23.
SEM images for the (Left) surface morphologies and (Right) cross-section of the samples prepared by mechanical coating at 300 rpm after different durations: (a) 4 h; (b) 8 h; (c) 10 h; (d) 12 h; (e) 16 h; (f) 20 h; (g) 26 h; (h) 32 h; and (i) 50 h. Modified and adapted with permission from ref. [80] Copyright 2017 Coatings.
Rotational Speed
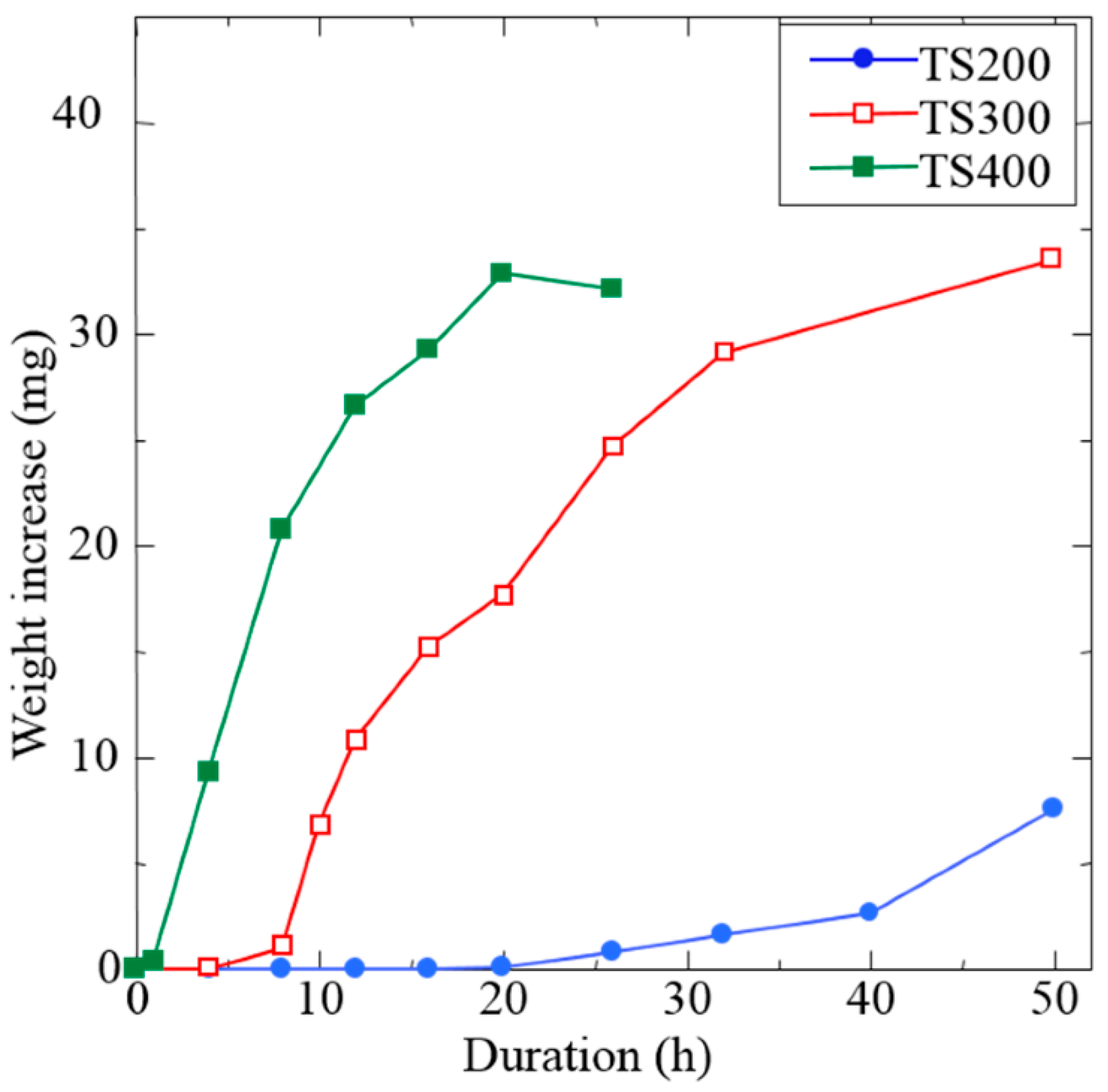
The rotation speed of the milling process strongly influences the quality of the coating. Higher speeds impart greater kinetic energy, which can accelerate coating formation; e.g., faster revolutions produce continuous coatings sooner. In one study, increasing the rotation speed from 200 to 400 rpm dramatically increased the metal deposition rate on balls [80]. Yet, excessive speed can be detrimental: very high speeds lead to early coating peeling as the intense impacts cause the formed layers to spall off. There is thus an optimal intermediate speed for maximum coating efficiency, defined by strong adhesion and minimized contamination, while remaining practical for large-scale production. For instance, Ni coating on ZrO2 balls grew fastest at ~240 rpm for 15 h, whereas at 270 rpm the coating began to exfoliate earlier [25,30]. This balance is illustrated in Figure 24, which shows the weight gain of substrate balls over time at different milling speeds. Higher speeds yield a steeper initial weight increase (faster coating) but can reach a plateau or decline once the coating starts to detach at long times [25,80].
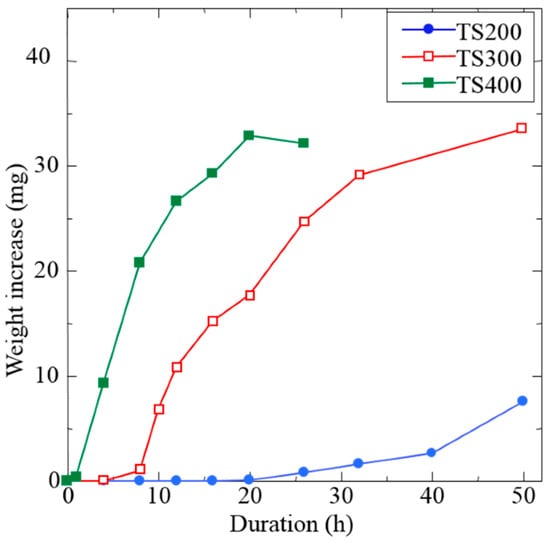
Figure 24.
Weight increase in substrate balls versus milling time at three rotation speeds (200 rpm in blue, 300 rpm in red, 400 rpm in green). Higher rotation speed accelerates coating growth, but an excessively high speed (green) leads to an earlier saturation and slight decrease in net coating mass due to exfoliation of the over-thickened coating. Adapted with permission from ref. [80] Copyright 2017 Coatings.
Conversely, shorter milling times may lead to a thinner coating but may also result in incomplete substrate coverage [62].
Overall, a higher milling speed may result in a thinner and more uniform coating distribution, while a lower speed could lead to a thicker and less uniform coating. It is vital to note that each material system has an ideal speed range for efficient milling. Exceeding this range can cause overheating and material degradation, while speeds that are too low may not provide enough energy for effective milling [5,9,25,27,44,61,77].
4.1.3. Influence of Ball Size
The size of milling balls is a crucial factor that affects the impact energy and contact area during collisions in milling processes. Larger balls provide higher impact forces, which can enhance the deformation of powder particles and the substrate surface, making them advantageous for applications like cold welding and coating deposition [56,76]. However, excessively large impacts can also result in particle pulverization or the dislodgment of coatings if the energy is too high.
On the other hand, smaller balls generate lower force impacts but do so in larger quantities, leading to increased collision frequency and more uniform dispersion of powder impacts [56,76]. This can facilitate the formation of finer and more homogeneous coatings. Nevertheless, if the balls are too small, the impact energy may fall short of achieving the necessary plastic deformation for harder particles, which can reducing coating efficiency as measured by adhesion quality and scalability to industrial milling
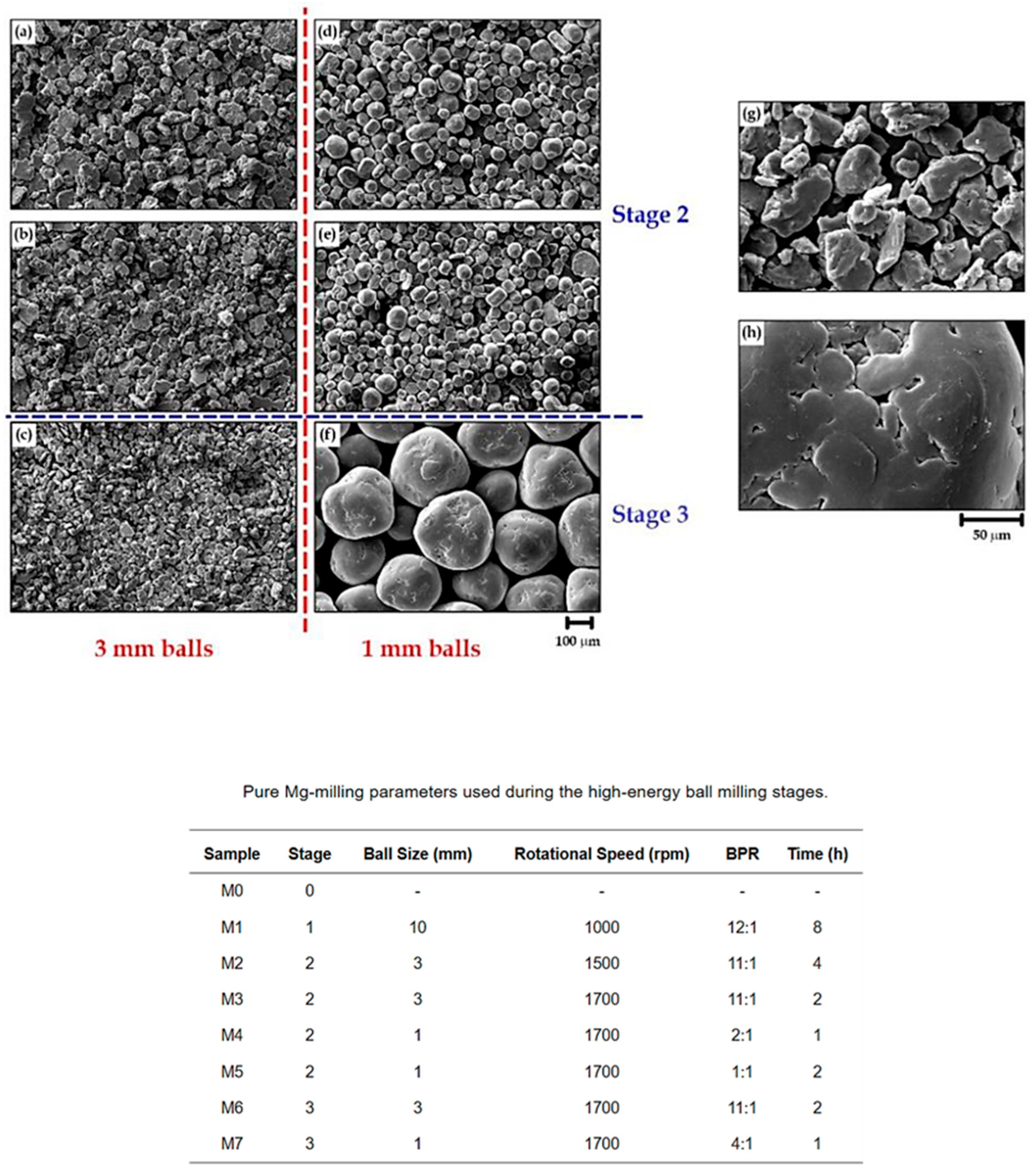
Overall, the impact energy produced during milling is closely tied to ball size, significantly influencing the final quality of the mechanical coatings. Larger balls excel at breaking down coarse particles, whereas smaller balls are more effective for grinding fine particles and achieving narrow size distributions and uniform coatings (Figure 25).
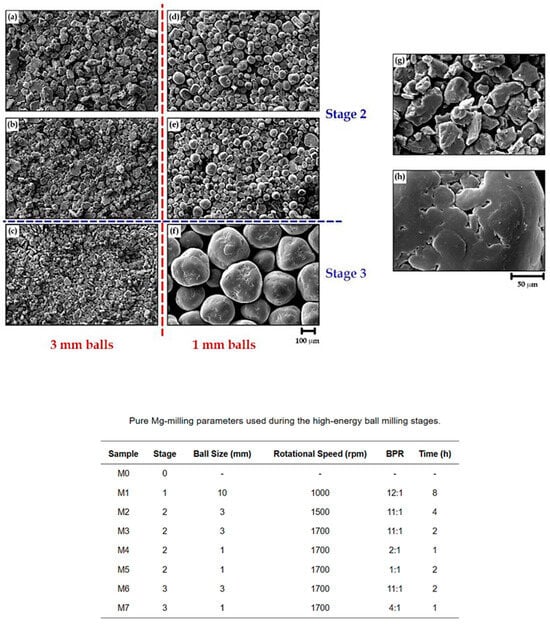
Figure 25.
SEM images show the resulting morphologies following the second and third milling stages with 3 and 1 mm balls. (a) M2, (b) M3, (c) M6, (d) M4, (e) M5 and (f) M7. (g,h) are enlargements of those in (c,f), respectively. Modified and adapted with permission from ref. [76] Copyright 2021 Metals.
In the milling process, achieving an optimal balance in ball size distribution is crucial for effective results. Fine balls are beneficial for adhering and burnishing coating particles onto surfaces, while larger balls provide the necessary impact energy to deform and weld those particles. The selection of ball size depends on several factors, including the material being milled, the desired final particle size, the duration of milling, and the intended application. By carefully tuning the ball size along with other parameters like ball-to-powder ratio, milling time, and speed, one can ensure that powder particles adhere effectively instead of being reduced to dust. Experimental studies show that utilizing a mixed particle size distribution leads to improved efficiency, reflected in more uniform coatings and reproducible performance at scale [5,30,44,48,76].
4.2. Intrinsic Material Properties
4.2.1. Material Properties
The efficiency of coated balls, understood as their ability to achieve durable coatings and scalable production, relies heavily on the properties of the materials being milled. Yet, surprisingly, there is a lack of comprehensive information on this topic in the existing literature. Key parameters related to material properties have been notably overlooked in the study of mechanical processing techniques for ball coating. The physical and chemical properties of the coating material, such as hardness, ductility, and reactivity, directly influence its ability to adhere to the balls. As an illustration, hard materials need more energy and longer milling times, resulting in increased equipment wear, while soft materials are easier to grind and require less energy. Furthermore, heat-sensitive materials require careful temperature control during milling to prevent degradation and melting, while thermally stable materials can endure higher temperatures and more aggressive milling conditions [5].
It is imperative to thoroughly understand the various properties involved in the milling operation, such as hardness, density, and particle size distribution, and optimize them accordingly. This can significantly enhance efficiency, both in terms of coating performance (adhesion, uniformity) and industrial effectiveness (cost, throughput, reproducibility). This, in turn, contributes to the improvement of key material properties, such as strength and durability, ultimately leading to enhanced performance of the milled materials in their intended applications. Furthermore, the complex and labyrinthine nature of the material’s structure and morphology significantly impacts the effective participation of balls in the coating process [28,44]. This knowledge gap underscores the urgent need for further research and data collection in this field.
4.2.2. Hardness
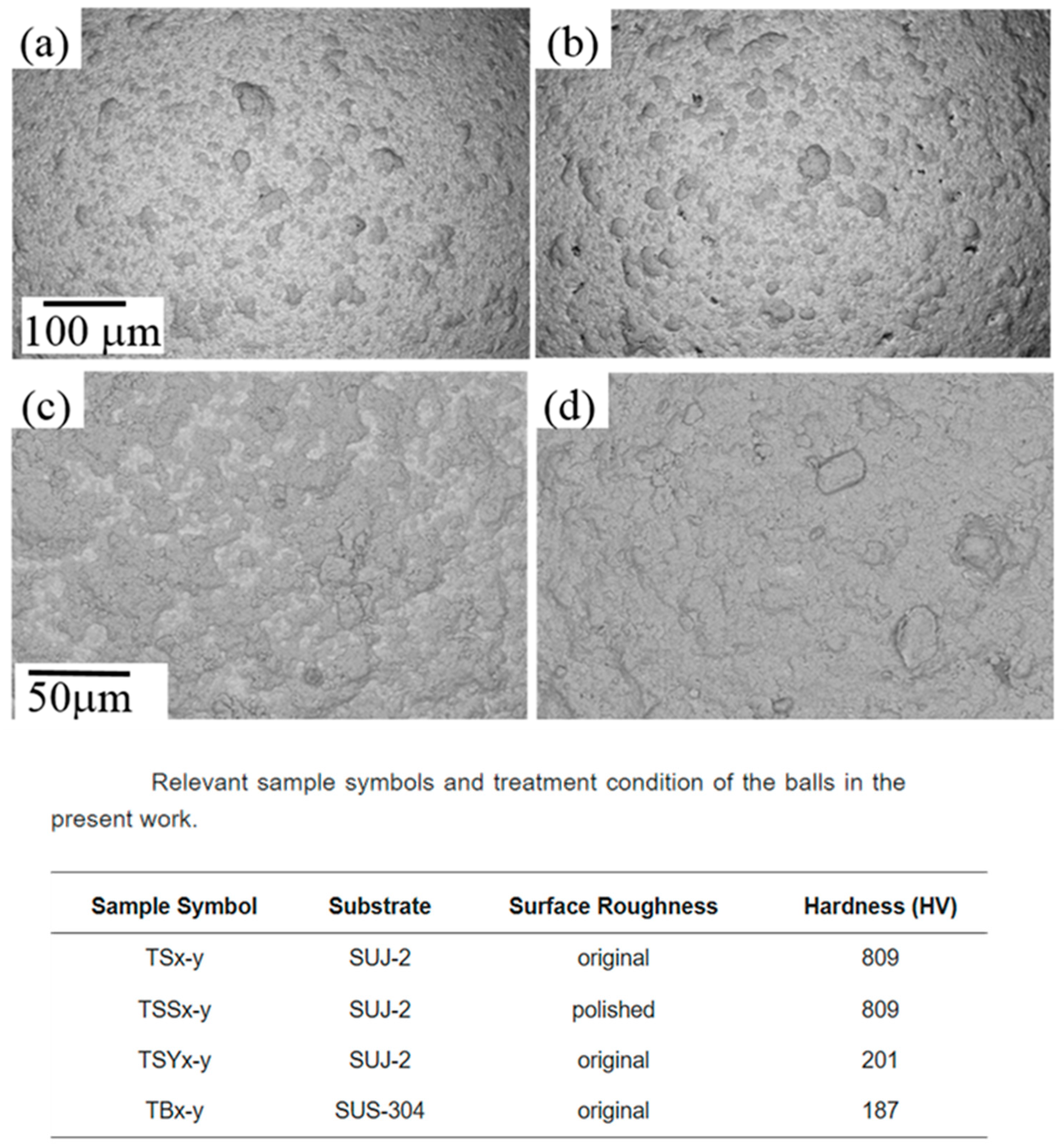
The hardness of both the substrate and coating material critically determines coating efficiency. A softer substrate surface is more amenable to coating deposition as it can undergo local plastic deformation to “grab” and hold incoming particles [80]. For example, steel balls (softer than ceramic) accept metal coatings more readily than extremely hard ceramic balls, which offer little yielding or mechanical interlocking [80]. If the powder to be coated is much harder than the substrate, it may peen and indent the substrate, embedding itself more firmly. Conversely, a very hard substrate tends to cause rebounding or shattering of incoming particles rather than bonding. Thus, lower substrate hardness generally promotes coating formation in mechanical milling (Figure 26) [80]. Hardness of the coating material itself also matters: extremely hard brittle powders might not deform enough to weld onto the substrate, whereas a moderately hard but still deformable powder can flatten and adhere under impact.
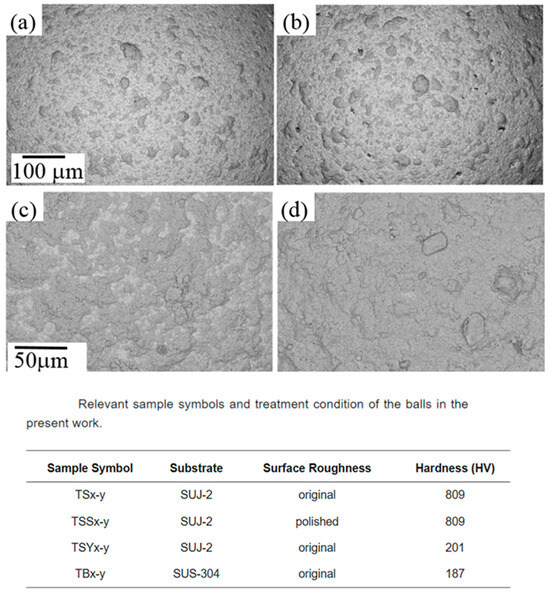
Figure 26.
SEM images of morphologies of the samples: (a) TS300-4; (b) TSS300-4; (c) TS300-8; and (d) TSY300-8. Modified and adapted with permission from ref. [80] Copyright 2017 Coatings.
Ductility
Ductility (plastic deformability) of the coating metal is a key enabler of cold welding. High-ductility metals (e.g., Au, Cu, soft Al) can endure repeated collisions by flattening and smearing onto surfaces, which fosters continuous coating build-up [81,82]. In contrast, brittle or low-ductility materials (e.g., ceramics, hard intermetallics) tend to fracture instead of plastically flowing, hindering the formation of a uniform coat. As one study noted, the better the plastic deformability of a metal powder, the easier it is for particles to cold-weld together and form thicker continuous coatings [81]. Ductile particles effectively “stick” to each other and the substrate upon impact, whereas brittle ones may pulverize into debris that does not adhere. Notably, insufficient ductility was a factor for Ni failing to form a continuous coating in a comparative experiment, where Ni’s work-hardening and lower plasticity (relative to Cu, Zn, etc.) limited its cold-weld behavior [81]. Thus, high ductility in the coating material correlates with higher coating efficiency, here expressed as greater adhesion quality and reliability under scalable milling conditions.
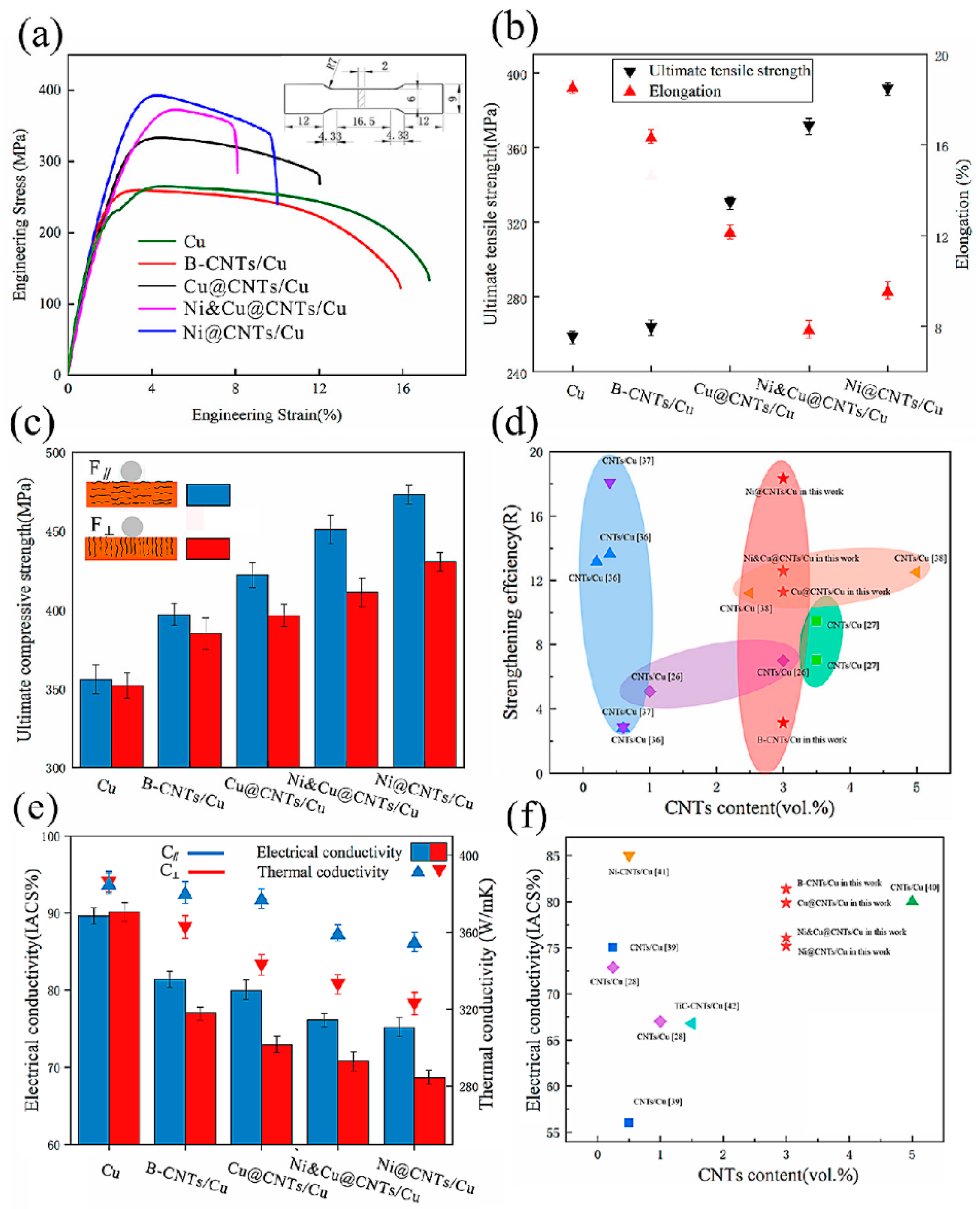
For example, Luo et al. showed that combining ball milling with Cu coating on CNTs and high-temperature sintering enhances the dispersion of CNTs and their interfacial bonding. This improves the ductility of the Cu matrix, addressing coating efficiency by optimizing the intrinsic material property of ductility through interfacial and processing control [83]. Figure 27 illustrates the enhanced ductility achieved through interfacial engineering. Specifically, the application of a Cu coating on CNTs, combined with ball milling and high-temperature sintering, leads to improved dispersion and stronger interfacial bonding between CNTs and the Cu matrix. This results in composites exhibiting higher elongation at fracture compared to those with uncoated CNTs, indicating improved ductility [83].
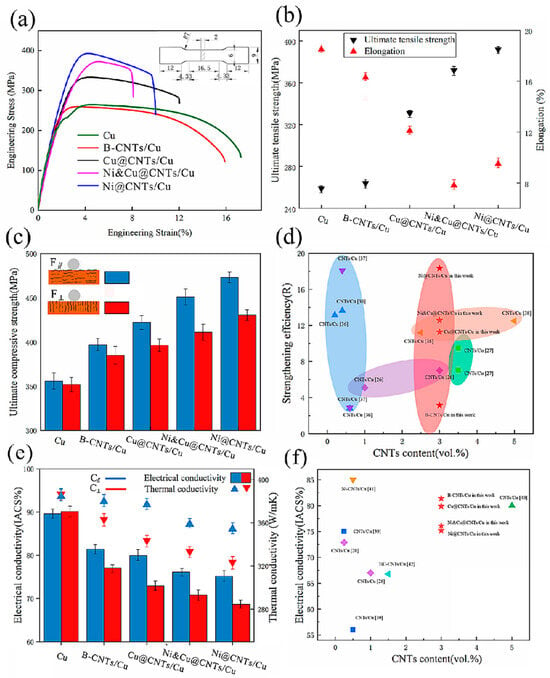
Figure 27.
(a) Stress–strain curves, (b) ultimate tensile strength and elongation, (c) compressive strength of samples. (d) Rvalue of CNTs in this work compared with data in the literature (e) Electrical conductivity and thermal conductivity of samples. F∥, C∥ test plane parallel to the drawing direction, F⊥, C⊥ test plane perpendicular to the drawing direction. (f) Electrical conductivity of samples compared with the published data. Adapted with permission from ref. [83] Copyright 2022 Nanomaterials.
Surface Energy
Materials with high surface energy (and clean, oxide-free surfaces) have a greater driving force for bonding upon contact. In mechanical coating, high surface-energy metals tend to cold-weld more readily, since clean metal–metal contacts can form metallic bonds under pressure. Conversely, metals that rapidly form stable surface oxides or possess lower surface energy present a barrier to cold welding. For example, Al powders quickly develop an oxide layer that impedes direct metal contact, making mechanical coating more difficult unless that oxide is disrupted. In general, a high surface energy and chemically “clean” surface helps particles adhere during collisions [81]. This is closely related to chemical affinity: if the coating metal can form a strong interfacial bond with the substrate (either metallic bonding or some reactive adhesion), the coating will nucleate and grow more effectively. Surface treatments or process control (e.g., milling under inert gas or adding a PCA) are sometimes used to manage surface oxide films, essentially to raise the effective surface energy of particles so that cold welding can occur. For example, Hao and his colleagues discovered that metals with higher surface energy can improve adhesion between the coating material and the substrate. This enhanced adhesion facilitates better bonding during the mechanical coating process. As a result, it contributes to the formation of uniform and durable coatings. This finding underscores the importance of surface energy as a key material property that influences coating efficiency [81].
Thermal Conductivity
The thermal conductivity of the materials influences local heating and cooling during impact events. Each collision in high-energy milling generates heat at the contact interface. Low thermal conductivity materials (e.g., ceramics, polymers, or metals like titanium with relatively lower conductivity) will retain that heat longer at the impact site, potentially softening the particle or substrate locally and promoting bonding. High thermal conductivity materials (e.g., Cu) dissipate heat quickly into the bulk, so the interface cools rapidly [84]. While milling is predominantly a room-temperature process, these rapid thermal exchanges can affect coating efficiency: a brief localized softening can assist particle bonding. In practice, the effect is subtle, but it has been suggested that harder balls with high thermal conductivity (like steel) may remove heat from the collision zone, whereas lower conductivity media (like ceramic balls) allow higher interface temperatures leading to better particle adhesion [85]. Indeed, one report identified the ball material’s thermal conductivity (along with hardness) as a key factor in coating effectiveness [85]. Overall, low conductivity of either the powder or the substrate tends to favor the “mechanical alloying” process by prolonging contact time at elevated temperature, thereby aiding consolidation of the coating.
Density
The density of the powder and substrate influences momentum transfer during milling. Higher-density particles (whether coating powder or milling media) carry more momentum at a given velocity, resulting in more forceful impacts. In mechanical coating, a dense coating powder can better penetrate any surface films and embed into the substrate upon impact. For instance, lead or tungsten-based particles (very dense) would hit with greater inertia than lighter Al particles, possibly improving their adhesion if the substrate can accommodate the impact. Similarly, dense milling balls (e.g., steel) impart larger impact forces than lighter ones (e.g., Al2O3) under the same speed [56]. This can enhance coating efficacy up to a point. However, if the density disparity is too high, lighter powder particles might simply rebound off heavy balls rather than deforming. An optimal matching of densities helps efficient energy transfer. Experiments comparing milling media found that stainless steel balls (~7.9 g/cc) generated higher collision forces and coating yields than lighter ceramic balls under identical conditions [56]. Thus, material density plays a role in how effectively kinetic energy is converted into plastic deformation and bonding in the coating process.
Chemical Affinity
Chemical compatibility or affinity between the coating material and the substrate strongly affects adhesion. If the two metals have mutual solubility or can form compounds, a chemical driving force may enhance bonding. For example, metals that tend to alloy or have low electronegativity can adhere more readily to ceramic or dissimilar substrates [81]. A study on coating Al2O3 balls with various metals showed that metals with lower electronegativity (more electropositive) covered the ceramic surface more completely in the initial stages [81]. This was attributed to their higher chemical reactivity leading to stronger interfacial adhesion (possibly via an oxide bridge or direct bonding). In contrast, metals like Ni (with higher electronegativity) had poorer initial attachment on ceramic, failing to form a continuous coat [81]. Chemical affinity also encompasses the tendency to avoid interfacial brittle phases: if the coating and substrate react to form a brittle intermetallic, the coating may spall. Ideally, the coating material should either inertly adhere or form a thin diffusion bond without adverse phases. In summary, coatings form more efficiently when there is a favorable chemical or metallurgical interaction at the interface (short of any deleterious reaction). This can be seen as an analog to plating materials that “wet” or bond each other, which will mechanically coat more easily than those that are chemically repellent.
Crystal Structure and Deformation Behavior
The crystal structure (FCC, BCC, HCP, etc.) of the coating metal influences its deformation mode under milling. FCC metals (like Cu, Ni, Au) have multiple slip systems and generally deform readily at room temperature, which helps in forming coatings via severe plastic deformation. HCP metals (such as Zn, Ti, and Mg) have fewer slip systems at room temperature, which can sometimes limit their ductility. Consequently, HCP powders may require higher energies to coat or may crack during milling. BCC metals (like Fe at room temperature) often have a ductile–brittle transition; they can deform, but may also work-harden significantly. Materials that work-harden rapidly (e.g., Ni or some BCC alloys) can lose ductility during milling, eventually behaving in a brittle manner that impedes coating growth [81]. Additionally, differences in crystal structure between coating and substrate can affect the ease of epitaxial bonding or the strain at the interface. During the mechanical coating process, if the coating metal accommodates strain by dislocation motion (plastic flow), it will form a better film. If instead it fractures (as a brittle crystal would), coating efficiency drops. For instance, Cu (FCC) cold-welds into a coherent layer relatively easily, whereas a brittle intermetallic compound with complex structure would not. Moreover, structure-related behavior such as twinning or phase transformations under stress (as in some metastable alloys) can either aid or hinder the process. In essence, metals with crystal structures that allow sustained plastic deformation (many active slip systems, moderate stacking fault energy) are ideal for mechanical coating [81]. Those with restrictive deformation mechanisms require either special conditions (e.g., elevated temperature milling) or tend to form only discontinuous coatings.
In summary, achieving high coating efficiency via mechanical ball milling requires optimizing the process parameters (like BPR, milling intensity, and ball size) while selecting material combinations with favorable intrinsic properties (sufficient ductility, chemical compatibility, appropriate hardness, etc.). Process parameters dictate the energy and shear environment that drive particles to adhere as a coating, whereas material properties govern how the particles respond to that environment (deforming vs. shattering, bonding vs. rebounding). A softer and ductile powder with high surface energy will readily flatten and bond under a well-chosen milling regimen, yielding a thick, continuous coating [80]. By contrast, a hard brittle powder or an overly aggressive milling schedule can result in poor coatings or even erosion of the substrate. Thus, effective mechanical coating is a multivariate interplay one must tune the milling conditions to the specific powder/substrate characteristics to promote steady coating growth without reaching a point of degradation (where coatings shear off). This understanding is supported by extensive peer-reviewed studies examining how each parameter influences coating formation, as discussed above [80,81]. The consensus in the literature is that an optimal combination of medium-high collision energy, adequate time (up to the point of coating saturation), and compatible material properties leads to the highest coating efficiency in ball-milled metal-coated systems [80,85].
5. Ball Milling Techniques for Coating Applications
5.1. In Situ Coating
The in situ coating principle emerged as an innovative milling operations technique with an eco-friendly method for coating powder. The principle is based on producing a composite milling media with an in situ coated surface created during the milling of the coated media and metal/ceramic media [86]. This method can produce coated balls in one step only, and allows for increased productivity and immediate cost savings [9]. Lim et al. reported in situ coating of SiOx integrated with N-doped carbon containing Fe2O3 (Fe2O3/N-C@SiOx) through mechanical milling (see Figure 28) [86]. Characterization results showed a uniform distribution of Si, O2, C, N2, and Fe within the particles, which comprise amorphous SiO and N-doped amorphous carbon with Fe2O3. The combination of Fe2O3 and N-doped C functions synergistically as a reinforcing matrix, effectively mitigating internal breakdown between particles caused by the volume expansion of the SiOx active materials and enhancing electrical conductivity.
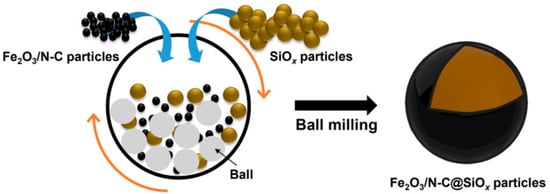
Figure 28.
Schematic illustration of the in situ coating process of Fe2O3/N-C onto SiOx particles using the ball milling process. Adapted with permission from ref. [86] Copyright 2022 Journal of Applied Electrochemistry.
Depending on the state of the coating powder, in situ coatings are divided into subcategories: in situ fusion techniques, in situ reactive techniques, in situ composite techniques, and mechanochemical in situ coating. In in situ fusion techniques, the coating powder is melted in the milling process, and then the melted coating material is coated on the balls. In in situ reactive techniques, the coating powder reacts chemically with the balls. In in situ composite techniques, the coating powder is embedded within the balls to form a composite layer [9,28,47,74,77]. In mechanochemical in situ coating, the mechanical energy is used to induce chemical reactions to produce a uniform and durable mechanochemical in situ coating. This process is used in the manufacturing industry for a range of applications [74] (see Table 2).

Table 2.
Summary of Ball-milling techniques for Core–Shell structured materials synthesis.
5.1.1. In Situ Fusion Method
Recently, Bage et al. reported the synthesis of a TiFeSi2 composite material using the in situ fusion coating method with ball milling technique, with the aim of obtaining a potential anode material for a Li-ion battery [96]. In this method, porous Si was created through the acid etching of Al65Si35, followed by milling with elemental titanium, iron, and carbon powders (Figure 29) [96]. The resulting composite featuring porous Si demonstrated an impressive initial coulombic efficiency of 99.8%. This remarkable performance can be attributed to the voids within the Si particles, which effectively mitigate their expansion and stabilize the structure of the electrode. Additionally, the formation of an inactive matrix along with a carbon conductive network further enhances the high efficiency and overall performance of the cell. Moreover, the ball milling of the acid-etched porous Si decreased the particle sizes of Si, significantly improving the electrochemical performance of the composite and prolonging its cycle life.
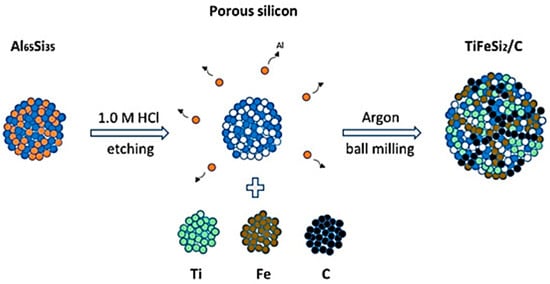
Figure 29.
Schematic illustration of the in situ fusion synthesis of TiFeSi2/C using the ball milling process. Adapted with permission from ref. [96] Copyright 2024 Electrochem.
Similarly, Hu et al. reported a facile method for synthesizing silicon/iron oxide/carbon (Si/Fe3O4/C) composites by ball-milling using supercritical carbon dioxide (scCO2) as a medium (Figure 30) [97]. This method uses the diffusion characteristics, extremely low viscosity, and superior mass transfer properties of supercritical fluids. When exposed to scCO2 fluid, mesophase carbon microspheres (MCMB) are exfoliated into graphite flakes, achieving effective interfacial fusion with Si and Prussian blue during ball-milling. The Prussian blue is subsequently transformed into Fe3O4 through heat treatment in a nitrogen atmosphere. This introduction of Fe3O4 significantly enhances the lithium-storage capacity, cycling stability, and rate performance of Si/C anodes. As a result, the reversible capacity of the Si/Fe3O4/C composite reaches 1363 mA h g–1 after 600 cycles at a rate of 1 A g–1. This study offers valuable insights for the design and fabrication of Si-based anode materials with high capacity and prolonged cycle life.
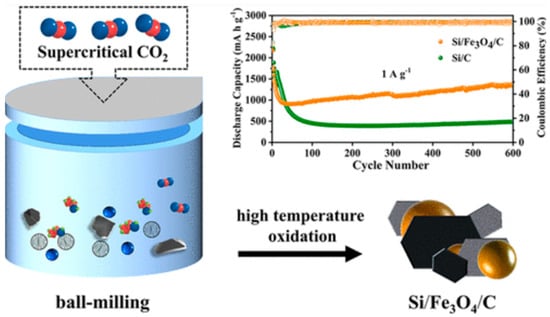
Figure 30.
Schematic illustration of the preparation of Si/Fe3O4/C using the ball milling process. Adapted with permission from ref. [97] Copyright 2023 Energy & Fuels.
5.1.2. In Situ Reactive Method
Scalable wet HEM was employed to refine commercial micro-Si using water and ethanol as PCA (Figure 31) [98]. Following the grinding process, the original particle size of 15 microns was reduced to approximately 1 micron for both types of PCA. Notably, the sample processed with water as the PCA exhibited a more significant formation of amorphous silicon oxide (SiO2) on the surface of Si particles than the one processed with ethanol, leading to relatively poorer electrochemical performance. This difference is attributed to the higher proportion of high-valence Si in the water-induced amorphous layer, as opposed to the ethanol-milled surface layer.
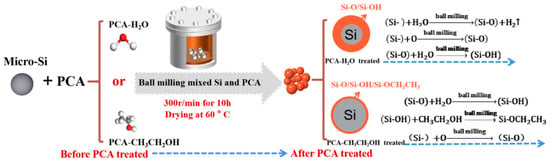
Figure 31.
Schematic illustration of surface refinement of micro-Si particles with the wet HEM process. Adapted with permission from ref. [98] Copyright 2024 Journal of Materials Engineering and Performance.
Yang et al. reported a facile reactive coating method to enhance cycling lifetime by creating a strengthened surface coating layer on Si microparticles in situ. This coating layer is formed by mechanically milling partially pre-lithiated Si microparticles in a CO2 atmosphere at room temperature, utilizing amorphous SiOx, C, SiC, and Li2SiO3, as illustrated in Figure 32 [99]. The in situ formation of amorphous SiC is crucial for reinforcing the amorphous SiOx/C buffer coating. At the same time, Li2SiO3 acts as a Li-ion conductor, facilitating the transport of Li ions within the surface coating layer. Notably, the thickness of the surface coating can be easily adjusted by varying the milling duration. Si microparticles milled for 12 h, with an average particle size of 0.993 μm, demonstrated significantly improved electrochemical properties.
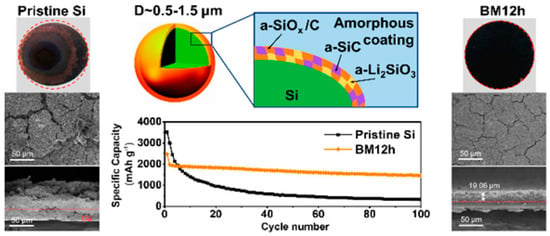
Figure 32.
Reaction ball-milling driven surface coating of pristine Si. Adapted with permission from ref. [99] Copyright 2018 ACS Applied Materials & Interfaces.
5.1.3. In Situ Composite Method
HEM is an effective method for particle engineering, wherein the milling medium transmits energy to the particles, leading to deformation, shape control, uniform dispersion of elements, and amorphization and refinement within short time frames. This technique is extensively utilized in processing metals, ceramics, and nanostructured composites. The microstructural properties of powders processed by HEM are significantly impacted by factors such as milling time, rotational speed, and the nature of each component within the mixture. In ductile–brittle systems, the ductile components (such as Cu, Ni, Fe, Co) transform into plate-like structures. Conversely, the brittle particles (like Al2O3 and WC) are refined and integrated into the surfaces of these deformed plates. This behavior arises from successive milling cycles that induce plastic deformation, cold welding, fragmentation, and strain hardening. Numerous studies have documented the morphological, structural, and magnetic changes in metal-ceramic composite materials produced by HEM. Notably, Câmara et al. investigated the morphological, structural, mechanical, and magnetic characteristics of Cu matrix powders reinforced with SiC particles prepared through HEM (Figure 33) [100].
Figure 33.
Schematic illustration of Cu-SiC composite preparation using HEM methods. Adapted with permission from ref. [100] Copyright 2021 Advanced Powder Technology.
Yan et al. introduced an innovative strategy to convert CO2 into Si/C/SiC composites through a one-step ball-milling reaction with Mg2Si at room temperature [101]. Additionally, they incorporated mesophase carbon microspheres (MCMB) into the reactants to produce Si/C/SiC@MCMB composites, which hold potential for commercial applications. This method enables the simultaneous formation of Si, C, and SiC with strong interfacial contact at room temperature, thereby enhancing the electronic conductivity and structural stability of the Si/C composites. This room-temperature synthesis approach offers advantages such as low cost, a straightforward operational process, and easy scalability, presenting a novel pathway for converting CO2 into a valuable resource and a new avenue for designing and synthesizing high-capacity Si/C anodes.
5.1.4. Mechanochemical Method
Baek et al. reported the synthesis of Cs4PbBr6 powder through guided heterostructural configuration engineering enabled by solid-state mechanochemical synthesis (Figure 34) [102]. Realized by an in-depth study on time-dependent evaluation of optical and structural properties during the synthesis of Cs4PbBr6, our target-designed synthesis protocol to promote the endotaxial formation of Cs4PbBr6/CsPbBr3 heterostructures provides key insights for understanding and designing kinetics-guided syntheses of highly luminescent perovskite emitters for light-emitting applications.
Figure 34.
Mechanochemical synthesis of different Cs–Pb–Br perovskite phases of 0D Cs4PbBr6, 3D CsPbBr3, and 2D CsPb2Br5 using the ball milling process. Adapted with permission from ref. [102] Copyright 2022 Nature Communications.
Mateti et al. have developed a low-energy mechanochemical separation process in which boron nitride (BN) powders are ball milled at room temperature within an atmosphere of alkyne or olefin mixed with paraffin gas (Figure 35) [103]. BN exhibits a significantly higher capacity for selectively adsorbing alkyne and olefin gases compared to paraffin gases, resulting in the purification of paraffin gas after the ball milling process. The adsorbed olefin gas can subsequently be recovered from the BN through a low-temperature heating process. This mechanochemical method achieves exceptionally high uptake capacities for alkyne and olefin gases in BN, with values of 708 cm3/g for acetylene (C2H2) and 1048 cm3/g for ethylene (C2H4). With the potential use of the ball milling process, BN nanosheets have reached the highest uptake capacities for alkyne and olefin gases reported to date, surpassing all other materials. This scalable approach of mechanochemical process holds great promise as an industrial separation method and offers considerable energy savings.
Figure 35.
Efficient separation process of olefin gas from paraffin gas using mechanochemistry with boron nitride nanosheets. Adapted with permission from ref. [103] Copyright 2022 Materials Today.
5.2. Nanostructured Coating
Ball milling is one of the various fabrication methods used in synthesizing nanostructured coatings. In ball milling, the powder particles are milled into nanoscale materials or structures. The produced materials can be further used to coat the substrate surface using various methods or to coat the balls themselves. This technique is very suitable for producing a large quantity of nanostructured material for different purposes. Due to the unique properties of nanomaterials, nanostructured coatings using ball milling can significantly enhance both the surface characteristics and the mechanical properties of coated balls, such as hardness, corrosion resistance, wear resistance, and thermal stability [29,36,75].
Carbon steels are widely utilized in various applications; however, they exhibit weak wear, corrosion, and oxidation resistance. To enhance these properties, surface engineering techniques can be employed. Among the most prevalent coatings for steel is the NiAl intermetallic compound. This intermetallic form of NiAl demonstrates remarkable characteristics, including a high melting point (1911 K), low density (5890 kg m−3), high thermal conductivity (76 W m−1 K−1), and excellent corrosion resistance at elevated temperatures, making it an ideal coating material for high-temperature and corrosive environments. The mechanical alloying (MA) process can be utilized to apply NiAl intermetallic coatings at room temperature in an ambient atmosphere. Through repeated collisions of the balls with the substrate, the powder particles are cold-welded to the substrate, resulting in the formation of a coating on its surface (Figure 36) [104], Notably, thicker coatings can be developed on the substrate. As the milling duration increases, the coating thickness rises significantly, reaching a maximum of approximately 470 µm after a treatment time of 480 min. The mechanical alloying method effectively produces nanostructured coatings on carbon steel substrates.
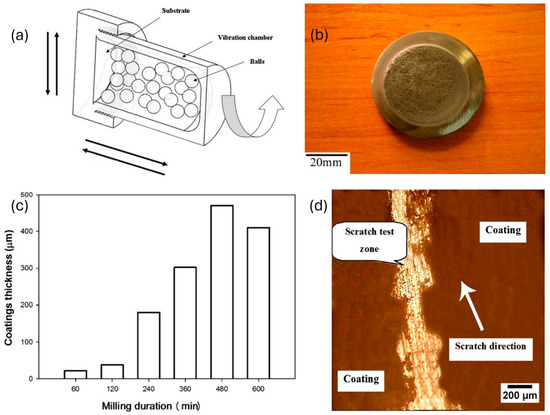
Figure 36.
(a). Schematic illustration of the ball milling process for nanostructured coating. (b). Nanostructured coating of NiAl on carbon steel substrates. (c). Coating thickness as a function of milling time. (d). Scratch tests of the coated sample. Adapted with permission from ref. [104] Copyright 2012 Applied Surface Science.
Yan et al. reported a sustainable low-temperature synthesis strategy (≤200 °C) that combines ball milling and solvent-recrystallization of lithium iodide to prepare the LATP (Li1.3Al0.3Ti1.7(PO4)3)/C coated LiNi1/3Co1/3Mn1/3O2 (LNCMO) material (Figure 37) [105]. Characterization of the structures and morphology reveals that LATP and a porous carbon powder are blended with ethanol-dissolved lithium iodide through a straightforward ball milling process. Upon vaporization of the ethanol at low temperatures, the lithium iodide recrystallizes, acting as a binder that forms a uniform coating and ensures a homogeneous distribution of LATP/C on the surface of the LNCMO cathode. The charge–discharge results demonstrate that the cycling performance and rate-discharge capability of the active materials coated with LATP/C significantly outperform those of the uncoated LNCMO.
Figure 37.
Sustainable Coating of LATP/C solid electrolyte composites on LiNi1/3Co1/3Mn1/3O2 cathode. Adapted with permission from ref. [105] Copyright 2022 Journal of The Electrochemical Society.
6. Application of Coated Balls
Ball milling is used in various industries for materials synthesis as illustrated in Figure 38 (e.g., producing nanomaterials such as nanoparticles and nanocomposites), chemical processing (such as increasing the surface area of catalysts, which in turn significantly enhances their performance in various chemical reactions), pharmaceuticals (e.g., improving the solubility and bioavailability of poorly soluble drugs by reducing particle size) and metallurgy (e.g., producing fine metal powders for making metal parts) [8,9,11,41,56], Due to their improved properties, coated balls produced using ball milling techniques have a wide range of industrial applications.
Figure 38.
An overview of the applications of the mechanical alloying process. Adapted with permission from ref. [41] Copyright 2023 Materials.
These properties include increased hardness and wear resistance, improved thermal and chemical properties, corrosion resistance, and durability while maintaining the mechanical strength of the base materials [106,107,108]. Unlike uncoated balls, coated balls exhibit exceptional qualities tailored to specific application areas as a result of different coating techniques [5,8,11,29,44,56].
To further illustrate the positive technical effects of ball milling, additional figures are provided. Figure 39 shows significant improvements in hardness, where both MAP and SMAP samples demonstrated increased microhardness, with SMAP reaching 753 HV (11.42% higher than UP). Microhardness decreased with depth before stabilizing, confirming the coating’s influence [106]. Figure 40 highlights corrosion resistance, where the CuAl/BN coating outperformed CuAlLaF3/BN and CuAlY/BN coatings, as reflected in its more positive self-corrosion potential and lower corrosion current density [108]. Figure 41 compares the wear and thermal/oxidation stability of coated versus uncoated samples, revealing that SMAP exhibited lower friction coefficients, faster stabilization, and substantially less wear volume, confirming superior durability [106]. Collectively, these figures emphasize the capability of ball milling to tailor material performance in terms of mechanical strength, corrosion protection, and long-term stability.
Figure 39.
(a) The sectional microhardness as function of the distance from the surface for all the samples (“UP” refers to steel balls treated only with heat, “SMAP” are those with Al2O3 powders, and “MAP” are without); (b) The sectional residual stress as a function of the distance from the surface for all the samples. Adapted with permission from ref. [106] Copyright 2024 Wear.
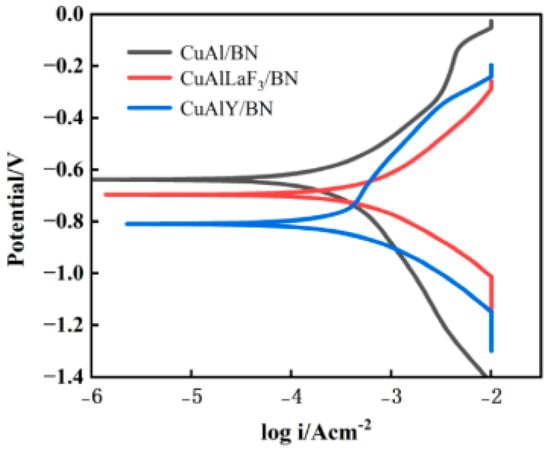
Figure 40.
Results of the coating polarization curve test. Adapted with permission from ref. [108] Copyright 2024 Crystals.
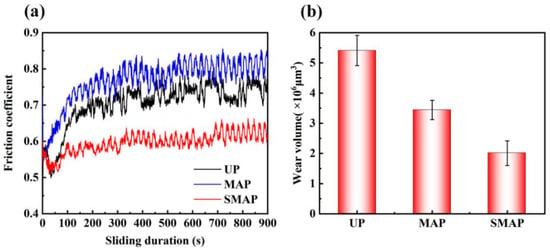
Figure 41.
(a) The friction coefficient of the UP, MAP, and SMPA samples versus sliding time; (b) the measured wear volume for each sample after the friction and wear test. Adapted with permission from ref. [106] Copyright 2024 Wear.
Owing to the previously mentioned distinguishing characteristics of coated balls, they have been used widely in a diverse range of industries, including but not limited to the automotive (e.g., engine components use coated balls to minimize friction, wear, and improve performance), aerospace (e.g., coated balls improve turbine blades’ resistance to high temperatures and corrosion), electronics and electrical (e.g., coated balls are used in electronic components to add a conductive layer, improving their electrical performance), and biomedical (e.g., coated balls enhance biocompatibility, wear resistance, and longevity in biomedical implants and prosthetics) industries. These coated balls enhance material properties and extend component lifespans across different sectors [5,8,9,29,56,75].
7. Conclusions and Future Perspectives
This review offers comprehensive insights into the potential mechanisms and categories of mechanical coating, focusing on providing a detailed overview of ball milling for coating balls. Selecting an appropriate method based on desired outcomes and material characteristics is crucial for achieving optimal coating results efficiently and effectively in MCTs. Mechanical coating presents a versatile and cost-effective solution for various applications, including the automotive, aerospace, and industrial sectors. In conclusion, mechanical coating, with a special focus on the ball milling method, is a versatile process with numerous benefits for achieving desired coatings. It provides opportunities to tailor the process to specific requirements. Careful consideration of supporting components and process parameter selection can lead to efficient and effective coating results. Overall, mechanical coating is a valuable technique for creating economical, composite, and high-performance materials that meet the diverse needs of various industries. Ball milling is the most suitable method among state-of-the-art and conventional techniques due to its eco-friendly nature. By addressing the listed challenges below and focusing on future directions, the ball milling process can continue to evolve, meeting the growing demands of various industries and contributing to advancements in material science.
The future development of mechanical coating techniques, particularly ball milling, requires deeper integration of advanced process control, sustainability, and industrial scalability. Several research directions emerge:
- Scalability and Industrial Translation
While laboratory-scale ball milling has been extensively demonstrated, challenges remain in scaling the process for consistent, large-batch production. Developing standardized protocols for process parameters and contamination control is essential. Industry-driven pilot plants should emphasize reproducibility, uniformity of coatings, and cost efficiency [40,109].
- 2.
- Contamination Control and Purity
One unresolved limitation of ball milling lies in contamination from milling media and PCA. Future work must focus on developing inert or self-lubricating media, surface-treated balls, and protective coatings to minimize contamination without compromising coating efficiency [109].
- 3.
- AI-Driven Optimization and Real-Time Monitoring
Artificial intelligence and machine learning can play a transformative role in predicting coating outcomes, optimizing parameters such as ball-to-powder ratio, milling time, and rotational speed. Coupling AI models with in situ sensors (acoustic emission, infrared thermal imaging, or real-time XRD) could enable adaptive control of the process. This would shift mechanical coating from empirical trial-and-error to predictive, data-driven optimization [41,42,110,111].
- 4.
- Energy–Structure–Property Framework
The future challenges associated with mechanical coatings can be systematically interpreted through the Energy–Structure–Property (ESP) framework, which connects process parameters (energy input) to structural evolution and ultimately to functional performance. Mapping process parameters such as milling speed, ball-to-powder ratio, milling time, and ball size onto this framework allows researchers to directly correlate specific milling energies with resulting microstructures (grain refinement, amorphization, coating adhesion) and their functional properties (hardness, wear resistance, corrosion resistance). This approach not only provides a predictive basis for coating design but also integrates the other challenges discussed—scalability, contamination control, AI-driven optimization, sustainability, and emerging applications—into a unified theoretical logic [112].
- 5.
- Sustainability and Green Manufacturing
While mechanical coating inherently avoids many environmental issues of vapor-phase methods, future research should aim to further minimize energy consumption and waste. Hybrid approaches combining ball milling with eco-friendly binders or solvents, as well as renewable energy-powered milling, could strengthen its role as a sustainable technology.
- 6.
- Emerging Applications
Beyond traditional wear- and corrosion-resistant coatings, future work should explore biomedical, energy-storage, and electronic applications. For example, ball milling can create bioactive coatings for implants, nanostructured electrodes for batteries, and conductive layers for flexible electronics. Each application requires process parameters tailored to specific functional requirements.
In summary, the convergence of scalable process design, contamination control, AI-driven optimization, and sustainability considerations positions ball milling as a versatile and future-ready mechanical coating method. Its integration with industrial automation and digital manufacturing frameworks will determine its success in next-generation applications.
Funding
This research is part of a research project agreement no. AFU-05-2023 in collaboration with EXPEC Advanced Research Center, Saudi Aramco. The authors gratefully acknowledge the continued support from Alfaisal University and its Office of Research & Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koch, C.C. Nanostructured Materials: Processing, Properties and Applications; William Andrew: Norwich, NY, USA, 2006. [Google Scholar]
- Takadoum, J. Nanomaterials and Surface Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Liu, Z.; Luo, L.; Zhang, Z.; Song, S. Preparation of Ti–MoS2 coating and gradient structure via mechanical ball milling to improve the wear resistance of AISI 440C stainless steel bearing balls. Vacuum 2024, 227, 113426. [Google Scholar] [CrossRef]
- Hamedi, H.; Isfahani, T. Wear and corrosion properties of mechanically coated 316 stainless Steel-TiC nanocomposites. Results Eng. 2024, 24, 102966. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Benjamin, J.S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Trudeau, M.L. Nanostructured Materials Produced by High-Energy Mechanical Milling and Electrodeposition. In Nanostructured Materials: Science & Technology; Chow, G.-M., Noskova, N.I., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 47–70. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin, Germany, 2008; pp. 1–413. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical milling: A superior nanotechnological tool for fabrication of nanocrystalline and nanocomposite materials. Nanomaterials 2021, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, M.; Gao, J.; He, Z.; Tian, S.; Li, K.; Zhao, Y.; Miao, Z. Super-hydrophobic coating prepared by mechanical milling method. J. Coat. Technol. Res. 2022, 19, 587–595. [Google Scholar] [CrossRef]
- Savrai, R.A.; Morozova, A.N. A review of studies in the field of production of coatings on metals by means of mechanical alloying. Surf. Interfaces 2021, 27, 101451. [Google Scholar] [CrossRef]
- Lu, Y.; Guan, S.; Hao, L.; Yoshida, H. Review on the photocatalyst coatings of TiO2: Fabrication by mechanical coating technique and its application. Coatings 2015, 5, 425–464. [Google Scholar] [CrossRef]
- Akçay, S.B.; Çuvalcı, O.; Varol, T.; Çuvalcı, H.; Kocaman, M.; Alver, Ü.; Beder, M.; Güler, O.; Aksa, H.C.; Gasha, S.B.; et al. Effect of mechanical milling parameters on the properties of electrolytic pure copper powders and hot pressed billets fabricated from recycled copper wastes. Mater. Chem. Phys. 2025, 346, 131367. [Google Scholar] [CrossRef]
- Alavizadeh, S.A.R.; Shahbaz, M.; Kavanlouei, M.; Kim, S.S. The effect of mechanical milling for enhanced recycling Ti6Al4V powder from machining chips. Sci. Rep. 2025, 15, 444. [Google Scholar] [CrossRef]
- Guaglianoni, W.C.; Garcia, A.P.; Basegio, T.M.; Felisberto, R.; Bergmann, C.P. Influence of Milling parameters in the morphology of nanostructured CrC-30wt%NiCr. Tecno-Logica 2017, 21, 37–40. [Google Scholar] [CrossRef]
- Zhao, L.; Zwick, J.; Lugscheider, E. The influence of milling parameters on the properties of the milled powders and the resultant coatings. Surface Coat. Technol. 2003, 168, 179–185. [Google Scholar] [CrossRef]
- Varol, T.; Ömür, F.D.; Akçay, S.B.; Güler, O.; Erdemir, F. Evolution of Morphology, Particle Size and Oxidation resistance of recycled Ti-6Al-4V powders prepared by planetary ball Milling. Arab. J. Sci. Eng. 2025, 50, 9123–9140. [Google Scholar] [CrossRef]
- Sripada, J.V.S.N.; Gallagan, F.M.; Saha, C.G. Study the Effect of Milling Parameters on HE-MA Nanostructured Al-Graphene Cermet Particles. In Proceedings of the 1st Coatings and Interfaces Web Conference, Basel, Switzerland, 15–29 March 2019; p. 6160. [Google Scholar]
- Biyik, S.; Aydin, M. The effect of the amount of process control agent on the properties of Cu25W composite powders. WIT Trans. Built Environ 2014, 137, 189–200. [Google Scholar] [CrossRef]
- Matula, I.; Zubko, M.; Dercz, G. Role of Sn as a Process Control Agent on Mechanical Alloying Behavior of Nanocrystalline Titanium Based Powders. Materials 2020, 13, 2110. [Google Scholar] [CrossRef]
- Yazdani, N.; Toroghinejad, M.R.; Shabani, A.; Cavaliere, P. Effects of Process Control Agent Amount, Milling Time, and Annealing Heat Treatment on the Microstructure of AlCrCuFeNi High-Entropy Alloy Synthesized through Mechanical Alloying. Metals 2021, 11, 1493. [Google Scholar] [CrossRef]
- Kutuk, S. Morphology, Crystal Structure and Thermal Properties of Nano-Sized Amorphous Colemanite Synthesis. Arab. J. Sci. Eng. 2024, 49, 11699–11716. [Google Scholar] [CrossRef]
- Kutuk, S.; Kutuk-Sert, T. Effect of PCA on Nanosized Ulexite Material Prepared by Mechanical Milling. Arab. J. Sci. Eng. 2017, 42, 4801–4809. [Google Scholar] [CrossRef]
- Kurama, H.; Erkuş, S.; Gaşan, H. The effect of process control agent usage on the structural properties of MgB2 synthesized by high energy ball mill. Ceram. Int. 2017, 43, S391–S396. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Wang, D.; Ren, B.; Song, P.; Zhang, G.; Cai, B.; Liu, Z. Effect of ball-milling process parameters on the mechanical and thermal properties of the nanodiamond/2024Al composites. Micron 2021, 148, 103104. [Google Scholar] [CrossRef] [PubMed]
- Dudina, D.V.; Bokhonov, B.B. Materials development using high-energy ball milling: A review dedicated to the memory of MA Korchagin. J. Compos. Sci. 2022, 6, 188. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent developments on the synthesis of nanocomposite materials via ball milling approach for energy storage applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Bor, A.; Jargalsaikhan, B.; Uranchimeg, K.; Lee, J.; Choi, H. Particle morphology control of metal powder with various experimental conditions using ball milling. Powder Technol. 2021, 394, 181–190. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A.; Galiana, B. Functionalizing organic powder coatings with nanoparticles through ball milling for wear applications. Appl. Surf. Sci. 2020, 513, 145834. [Google Scholar] [CrossRef]
- Lijia, C.; Haibin, C.; Rongyong, L.; Weifeng, H.; Yuhui, Y. Fabrication of Nickel Coatings on Zirconia Balls by Mechanical Ball Milling and the Process Analysis. Mater. Sci. 2022, 28, 35–40. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Choy, K.-L. Chemical Vapour Deposition (CVD): Advances, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Droes, S.R.; Kodas, T.T.; Hampden-Smith, M.J. Plasma-enhanced chemical vapor deposition (PECVD). In Carbide, Nitride and Boride Materials Synthesis and Processing; Springer: Dordrecht, The Netherlands, 1997; pp. 579–603. [Google Scholar]
- Schlesinger, M.; Paunovic, M. Modern Electroplating; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Gulf Professional Publishing: Santa Rosa, CA, USA, 1990. [Google Scholar]
- Wu, Q.; Miao, W.-s.; Zhang, Y.-d.; Gao, H.-j.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Tubtimkuna, S.; Danilov, D.L.; Sawangphruk, M.; Notten, P.H. Review of the Scalable Core–Shell Synthesis Methods: The Improvements of Li-Ion Battery Electrochemistry and Cycling Stability. Small Methods 2023, 7, 2300345. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Cai, X.; Yang, C.; Zhou, L. Amorphous Si/TiC/graphite composite fabricated by high-energy ball-milling as an anode for lithium-ion batteries. J. Electron. Mater. 2021, 50, 2584–2593. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Wei, L.K.; Abd Rahim, S.Z.; Al Bakri Abdullah, M.M.; Yin, A.T.M.; Ghazali, M.F.; Omar, M.F.; Nemeș, O.; Sandu, A.V.; Vizureanu, P.; Abdellah, A.E.-h. Producing metal powder from machining chips using ball milling process: A review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef]
- Willis-Fox, N. In situ monitoring of polymer mechanochemistry: What can be learned from small molecule systems. Frontiers in Chemistry 2024, 12, 1490847. [Google Scholar] [CrossRef]
- Cvrtila, I.; Štrukil, V.; Alešković, M.; Kulcsár, I.; Mrla, T.; Colacino, E.; Halasz, I. Real-time in situ Raman monitoring of photomechanochemical reactions. Chem. Methods 2025, 5, e202400089. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Joubori, A.A.; Wang, Z. Nanostructured materials and nanocomposites by mechanical alloying: An overview. Met. Mater. Int. 2022, 28, 41–53. [Google Scholar] [CrossRef]
- Weissgaerber, T.; Kieback, B. Dispersion Strengthened Materials Obtained by Mechanical Alloying—An Overview. Mater. Sci. Forum 2000, 343–346, 275–283. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, W.; Han, Y.; Li, Y.; Ren, W. Enhancing the capacity of large-scale ball mill through process and equipment optimization: An industrial test verification. Adv. Powder Technol. 2020, 31, 2079–2091. [Google Scholar] [CrossRef]
- Trautmann, M.; Ahmad, H.; Wagner, G. Influencing the size and shape of high-energy ball milled particle reinforced aluminum alloy powder. Materials 2022, 15, 3022. [Google Scholar] [CrossRef]
- Chaira, D. Milling devices and mechanisms of mechanical alloying. In Mechanical Alloying of Ferrous and Non-Ferrous Alloys; Elsevier: Amsterdam, The Netherlands, 2024; pp. 39–57. [Google Scholar] [CrossRef]
- Ambika, S.; Devasena, M.; Nambi, I.M. Synthesis, characterization and performance of high energy ball milled meso-scale zero valent iron in Fenton reaction. J. Environ. Manag. 2016, 181, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, F.; Rong, M.; Wang, Z.; Yang, S.; Wang, J.; Zhou, H. Recent advances on preparation method of Ti-based hydrogen storage alloy. J. Mater. Sci. Chem. Eng. 2020, 8, 18–38. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef]
- Yin, Z.; Peng, Y.; Zhu, Z.; Yu, Z.; Li, T. Impact Load Behavior between Different Charge and Lifter in a Laboratory-Scale Mill. Materials 2017, 10, 882. [Google Scholar] [CrossRef]
- Mechanical Plating: The Forté of Plating Systems & Technologies; Plating Systems & Technologies, Inc.: Minneapolis, MN, USA, 2007.
- Chatterjee, B. Mechanical Plating. Galvanotechnik 2005, 96, 1589–1599. [Google Scholar]
- Kim, J.; Satoh, M.; Iwasaki, T. Mechanical-dry coating of wax onto copper powder by ball milling. Mater. Sci. Eng. A 2003, 342, 258–263. [Google Scholar] [CrossRef]
- Bor, A.; Jargalsaikhan, B.; Lee, J.; Choi, H. Effect of Different Milling Media for Surface Coating on the Copper Powder Using Two Kinds of Ball Mills with Discrete Element Method Simulation. Coatings 2020, 10, 898. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Ibragimov, R.A.; Lesovik, V.S.; Pak, A.A.; Krylov, V.V.; Poleschuk, M.M.; Stoyushko, N.Y.; Gladkova, N.A. Processing equipment for grinding of building powders. IOP Conf. Ser. Mater. Sci. Eng. 2018, 327, 042029. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef]
- Hayashi, N.; Ueno, S.; Komarov, S.V.; Kasai, E.; Oki, T. Fabrication of hydroxyapatite coatings by the ball impact process. Surf. Coat. Technol. 2012, 206, 3949–3954. [Google Scholar] [CrossRef]
- Nisar, S.S.; Choe, H.-C. Mechanical hydroxyapatite coatings on PEO-treated Ti–6Al–4V alloy for enhancing implant’s surface bioactivity. Ceram. Int. 2024, 50, 17703–17719. [Google Scholar] [CrossRef]
- Sapkota, R. Nanostructured Thin Films Prepared by Planetary Ball Milling: Fabrication, Characterization and Applications. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2022. [Google Scholar]
- Guzzo, P.L.; de Barros, F.B.M.; Soares, B.R.; Santos, J.B. Evaluation of particle size reduction and agglomeration in dry grinding of natural quartz in a planetary ball mill. Powder Technol. 2020, 368, 149–159. [Google Scholar] [CrossRef]
- Attritors and Ball Mills: How They Work; Union Process Inc.: Akron, OH, PA, USA, 2000.
- Jayasundara, C.; Zhu, H.P. Impact energy of particles in ball mills based on DEM simulation and data-driven approach. Powder Technol. 2022, 395, 226–234. [Google Scholar] [CrossRef]
- Rodríguez, V.A.; Ribas, L.; Kwade, A.; Tavares, L.M. Mechanistic modeling and simulation of wet planetary ball mills. Powder Technol. 2023, 429, 118901. [Google Scholar] [CrossRef]
- Kondo, A.; Ishihara, S.; Kushimoto, K.; Kozawa, T.; Kano, J.; Naito, M. Relationship between grinding results in a planetary ball mill with liquid media and the distribution of ball impact energy calculated by DEM simulation. J. Soc. Powder Technol. Japan. 2020, 57, 176–183. [Google Scholar] [CrossRef]
- Hao, L.; Lu, Y.; Sato, H.; Asanuma, H. Fabrication of zinc coatings on alumina balls from zinc powder by mechanical coating technique and the process analysis. Powder Technol. 2012, 228, 377–384. [Google Scholar] [CrossRef]
- Wasa, K.; Kitabatake, M.; Adachi, H. Thin Film Materials Technology: Sputtering of Compound Materials; Springer William Andrew Inc Publishing: New York, NY, USA, 2004. [Google Scholar]
- Ebnesajjad, S.; Ebnesajjad, C. Surface Treatment of Materials for Adhesive Bonding; William Andrew: Norwich, NY, USA, 2013. [Google Scholar]
- Maurice, D.; Courtney, T. Modeling of mechanical alloying: Part I. deformation, coalescence, bdand fragmentation mechanisms. Metall. Mater. Trans. A 1994, 25, 147–158. [Google Scholar] [CrossRef]
- Hao, L.; Lu, Y.; Asanuma, H.; Guo, J. The influence of the processing parameters on the formation of iron thin films on alumina balls by mechanical coating technique. J. Mater. Process. Technol. 2012, 212, 1169–1176. [Google Scholar] [CrossRef]
- Yazdani, A.; Zakeri, A. An insight into formation of nanostructured coatings on metallic substrates by planetary ball milling. Powder Technol. 2015, 278, 196–203. [Google Scholar] [CrossRef]
- Bidulsky, R.; Gobber, F.S.; Bidulska, J.; Ceroni, M.; Kvackaj, T.; Grande, M.A. Coated metal powders for laser powder bed fusion (L-PBF) processing: A review. Metals 2021, 11, 1831. [Google Scholar] [CrossRef]
- Lapshin, O.; Boldyreva, E.; Boldyrev, V. Role of mixing and milling in mechanochemical synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Farooq, S.A.; Raina, A.; Mohan, S.; Arvind Singh, R.; Jayalakshmi, S.; Irfan Ul Haq, M. Nanostructured coatings: Review on processing techniques, corrosion behaviour and tribological performance. Nanomaterials 2022, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.; Restrepo, A.; Zuleta, A.; Bolívar, F.; Castaño, J.; Correa, E.; Echeverria, F. Effect of ball size on the microstructure and morphology of Mg powders processed by high-energy ball milling. Metals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Witharamage, C.; Christudasjustus, J.; Gupta, R. The effect of milling time and speed on solid solubility, grain size, and hardness of Al-V alloys. J. Mater. Eng. Perform. 2021, 30, 3144–3158. [Google Scholar] [CrossRef]
- Ansell, T.Y.; Hanneman, T.; Gonzalez-Perez, A.; Park, C.; Nieto, A. Effect of high energy ball milling on spherical metallic powder particulates for additive manufacturing. Part. Sci. Technol. 2021, 39, 981–989. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Shao, X.-Y.; Wang, K.; Wang, Q.; Xing, Y.-Z. Effect of ball-to-powder ratio on morphology, structure, and flowability of ball-milled gray cast iron powder. J. Therm. Spray Technol. 2021, 30, 1679–1691. [Google Scholar] [CrossRef]
- Hao, L.; Yoshida, H.; Itoi, T.; Lu, Y. Preparation of Metal Coatings on Steel Balls Using Mechanical Coating Technique and Its Process Analysis. Coatings 2017, 7, 53. [Google Scholar] [CrossRef]
- Hao, L.; Lu, Y.; Sato, H.; Asanuma, H.; Guo, J. Influence of metal properties on the formation and evolution of metal coatings during mechanical coating. Metall. Mater. Trans. A 2013, 44, 2717–2724. [Google Scholar] [CrossRef]
- Luo, S.; Chen, B.; Song, M.; Zhang, Z.; Yi, J.; Zhou, S.; Guo, B.; Yu, Z.; Li, W. Improving the strength-ductility synergy of carbon nanotubes reinforced Cu matrix composites through interfacial regulation. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107787. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, A.; Tao, J.; Li, J.; Zhang, W.; Li, X.; Xue, H. Mechanical and Conductive Properties of Cu Matrix Composites Reinforced by Oriented Carbon Nanotubes with Different Coatings. Nanomaterials 2022, 12, 266. [Google Scholar] [CrossRef]
- Mariño-Salguero, J.; Jorge, J.; Menéndez-Aguado, J.M.; Álvarez-Rodriguez, B.; De Felipe, J.J.M.; Processing, M. Heat generation model in the ball-milling process of a tantalum ore. Min. Metall. Explor. 2017, 34, 10–19. [Google Scholar] [CrossRef]
- Komarov, S.V.; Romankov, S.E.; Son, S.H.; Hayashi, N.; Kaloshkin, S.D.; Ueno, S.; Kasai, E. Production of LaPO4 coatings using a novel ultrasonically-assisted plating technique. Surf. Coat. Technol. 2008, 202, 5180–5184. [Google Scholar] [CrossRef]
- Lim, A.S.; Kim, J.; Hwa, Y.; Cho, K.Y.; Yoon, S. Fe2O3/N-doped carbon-modified SiOx particles via ionic liquid as anode materials for Li-ion batteries. J. Appl. Electrochem. 2022, 52, 1163–1171. [Google Scholar] [CrossRef]
- Li, T.; Wu, C.; Pan, N.; Hua, Q.; Sun, A. Design, synthesis and improved sintering behavior of core-shell Mo–Cu composite powders. Solid State Sci. 2024, 147, 107405. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Wu, Y.; Lv, W.; Zhou, S. Theoretical prediction and experimental study on catalytic mechanism of incorporated Ni for hydrogen absorption of Mg. Int. J. Hydrogen Energy 2019, 44, 27885–27895. [Google Scholar] [CrossRef]
- Geng, K.; Xie, Y.; Yan, L.; Yan, B. Fe-Si/ZrO2 composites with core-shell structure and excellent magnetic properties prepared by mechanical milling and spark plasma sintering. J. Alloys Compd. 2017, 718, 53–62. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Chen, Z.; Yan, Y.; Zhao, J.; Li, J.; Jiang, Z. In Situ synthesis of SiC-graphene core-shell nanoparticles using wet ball milling. Ceram. Int. 2018, 44, 8283–8289. [Google Scholar] [CrossRef]
- Xia, M.; Yao, Q.; Yang, H.; Guo, T.; Du, X.; Zhang, Y.; Li, G.; Luo, Y. Preparation of Bi2O3/Al core-shell energetic composite by two-step ball milling method and its application in solid propellant. Materials 2019, 12, 1879. [Google Scholar] [CrossRef]
- Kuzovnikova, L.; Komogortsev, S.; Denisova, E.; Iskhakov, R.; Nemtsev, I.; Volochaev, M.; Shepeta, N. Structure and magnetism in ball-milled core-shell Al2O3@ Co particles. Mater. Today Proc. 2019, 12, 159–162. [Google Scholar] [CrossRef]
- Yan, L.; Yan, B. Fe-Si/MnZn (Fe2O4)2 core-shell composites with excellent magnetic properties by mechanical milling and spark plasma sintering (SPS). Metals 2018, 8, 553. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, K.-J.; Yoon, Y.-J.; Sinn, D.-S.; Kim, Y.-T.; Hur, K.-H. Effects of milling condition on the formation of core–shell structure in BaTiO3 grains. J. Eur. Ceram. Soc. 2008, 28, 2589–2596. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, M.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. In situ generation of few-layer graphene coatings on SnO2-SiC core-shell nanoparticles for high-performance lithium-ion storage. Adv. Energy Mater. 2012, 2, 95–102. [Google Scholar] [CrossRef]
- Bage, A.N.; Bamisile, O.; Adun, H.; Takyi-Aninakwa, P.; Ekekeh, D.G.; Tu, Q.H. Enhanced Performance with Nano-Porous Silicon in TiFeSi2/C Composite Anode for Lithium-Ion Batteries. Electrochem 2024, 5, 560–573. [Google Scholar] [CrossRef]
- Hu, L.; Lu, Z.; Chen, F.; Zhang, J.; Xia, Y.; Zhang, W.; Gan, Y.; He, X.; Song, W.; Huang, H. Supercritical-assisted ball-milling synthesis of multicomponent Si/Fe3O4/C composites for outstanding lithium-storage capability. Energy Fuels 2023, 37, 8042–8050. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Z.; Wu, Q.; Li, Q.; Ahsan, Z.; Ma, Y.; Jing, H.; Song, G.; Yang, W.; Wen, C. Microstructural Characteristics and Electrochemical Performance of Core-Shell-Structured Si Powders Fabricated by Ball Milling with Different Process Control Agents. J. Mater. Eng. Perform. 2024, 33, 9084–9094. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, X.; Zhang, L.; Gao, M.; Liu, Y.; Pan, H. Reaction-ball-milling-driven surface coating strategy to suppress pulverization of microparticle Si anodes. ACS Appl. Mater. Interfaces 2018, 10, 20591–20598. [Google Scholar] [CrossRef] [PubMed]
- Câmara, N.T.; Raimundo, R.A.; Lourenço, C.S.; Morais, L.M.F.; Silva, D.D.S.; Gomes, R.M.; Morales, M.A.; Macedo, D.A.; Gomes, U.U.; Costa, F.A. Impact of the SiC addition on the morphological, structural and mechanical properties of Cu-SiC composite powders prepared by high energy milling. Adv. Powder Technol. 2021, 32, 2950–2961. [Google Scholar] [CrossRef]
- Yan, X.; Hu, L.; He, X.; Zhang, J.; Zhang, W.; Gan, Y.; Xia, Y.; Huang, H. Ball-milling-triggered synthesis of Si/C/SiC@ MCMB composites from carbon dioxide for improved lithium storage capability. Energy Fuels 2023, 37, 746–753. [Google Scholar] [CrossRef]
- Baek, K.-Y.; Lee, W.; Lee, J.; Kim, J.; Ahn, H.; Kim, J.I.; Kim, J.; Lim, H.; Shin, J.; Ko, Y.-J. Mechanochemistry-driven engineering of 0D/3D heterostructure for designing highly luminescent Cs–Pb–Br perovskites. Nat. Commun. 2022, 13, 4263. [Google Scholar] [CrossRef]
- Mateti, S.; Zhang, C.; Du, A.; Periasamy, S.; Chen, Y.I. Superb storage and energy saving separation of hydrocarbon gases in boron nitride nanosheets via a mechanochemical process. Mater. Today 2022, 57, 26–34. [Google Scholar] [CrossRef]
- Mohammadnezhad, M.; Shamanian, M.; Enayati, M.H. Formation of nanostructured NiAl coating on carbon steel by using mechanical alloying. Appl. Surf. Sci. 2012, 263, 730–736. [Google Scholar] [CrossRef]
- Yang, T.; Chin, C.-T.; Li, Y.; Cheng, C.-H. Sustainable Low-Temperature Coating of LATP/C Solid Electrolyte Composites on LiNi1/3Co1/3Mn1/3O2 Cathode Based on Lithium Iodide Solvent-Recrystallization for Li-Ion Batteries. J. Electrochem. Soc. 2022, 169, 113505. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Z.; Zhao, Z. Improving wear resistance of 440C bearing steel balls through mechanical ball milling induced gradient structure. Tribol. Int. 2024, 191, 109115. [Google Scholar] [CrossRef]
- Özdemir, F.; Gupta, R.K. Influence of vanadium on corrosion behavior of high-energy ball milled aluminum alloy 2024. Mater. Corros. 2023, 74, 285–292. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.; Yang, H.; Sun, X.; Zhang, H.; Sun, Y.; Zhang, W.; Lan, H.; Yu, S. Enhanced Corrosion Resistance of CuAl/BN coatings through the addition of rare-earth and high-temperature oxidation treatment. Crystals 2024, 14, 808. [Google Scholar] [CrossRef]
- Gao, C.; Fan, X.; Sun, D.; Zeng, G.; Wang, Q.; Wang, Q. Recent advances in ball-milled materials and their applications for adsorptive removal of aqueous pollutant. Water 2024, 16, 1639. [Google Scholar] [CrossRef]
- Vashishtha, G.; Chauhan, S.; Zimroz, R.; Yadav, N.; Kumar, R.; Gupta, M.K. Current applications of machine learning in additive manufacturing: A review on challenges and future trends. Arch. Comput. Methods Eng. 2025, 32, 2635–2668. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhang, S.; Wang, J.; Bai, Z.; Du, Z.; Huang, K.; Zhang, Q.; Su, Y.; Wen, G.; et al. In-situ monitoring in laser powder bed fusion based on acoustic-signal time-frequency synchrosqueezing transform and multi-scale spatially interactive fusion convolutional neural network. J. Manuf. Process. 2024, 126, 471–486. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A critical review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.