Abstract
Titanium and its alloys are widely used in orthopedics because of their excellent mechanical properties and biocompatibility; however, their bioinert surface results in sluggish osseointegration and renders implants susceptible to bacterial infection. This study innovatively constructed a “CHP-Ti-MAO” composite coating, which aims to simultaneously improve early osseointegration and antibacterial performance. CHP micron coatings coated with hydroxyapatite (HA) and curcumin (Cur) at different PLGA concentrations (50%, 100%, and 150%) were deposited on the basis of calcium–phosphorus ceramic coatings prepared by micro-arc oxidation (MAO) following the emulsification-solvent volatilization method. It was found that increasing the concentration of PLGA can increase the particle size of the coating, enhance the hydrophilicity, and significantly improve the sustained release performance of the drug. Among them, the 100% PLGA concentration group performed the best: the drug-release half-life reached 75 h, and the corrosion current density was the lowest (9.5 × 10−9 A/cm2), showing the best corrosion resistance. This group of coatings has a strong and long-term antibacterial effect on Escherichia coli, with an antibacterial rate of more than 95% at 24 h and more than 99% by day 17. The hemolysis rate of all coatings was lower than 5%, indicating good biocompatibility. This study confirmed that 100% CHP-Ti-MAO composite coating successfully solved the limitations of excessive pore size and insufficient antibacterial persistence of an MAO layer and also had excellent slow-release, corrosion resistance, and high-efficiency antibacterial capabilities, which provided an important basis for the development of a new generation of multifunctional titanium-based implants.
1. Introduction
Titanium and its alloys have become one of the most commonly used metallic implant materials in orthopedic, dental, and craniofacial reconstruction due to their excellent mechanical properties, good biocompatibility, and outstanding corrosion resistance [1,2]. However, the bioinert nature of titanium’s surface leads to slow bone integration (osseointegration) with host bone tissue. Clinically, it is observed that conventional titanium implants require 3–6 months to form a stable bone–implant interface [3]. Additionally, postoperative infection is a major cause of titanium implant failure. Escherichia coli on the implant surface can significantly inhibit osteoblast function and trigger inflammatory responses, eventually leading to implant loosening and even the need for revision surgery [4,5]. Therefore, simultaneously improving the early osteointegration ability and antibacterial performance of titanium implants has become a core challenge in current biomaterials research.
In recent years, micro-arc oxidation (MAO) technology has made systematic progress in surface modification of medical titanium and magnesium alloys. Through methods such as elemental doping and composite coating construction, MAO has successfully integrated multiple functions including antibacterial activity, osteogenesis promotion, and anti-inflammation [6]. The preparation strategies for MAO antibacterial coatings mainly fall into two categories [7]. In situ doping method: antibacterial elements (such as Ag+, Cu2+, and Zn2+ ions) or nanomaterials (such as graphene oxide (GO) and silver nanoparticles (AgNPs)) are directly introduced into the ceramic layer during MAO processing. Post-treatment modification method: an MAO layer is first generated on the substrate, and then, antibacterial agents are precisely loaded onto the coating via techniques like dipping, electrophoresis, or spin-coating. This two-step approach avoids the destruction of active ingredients under the high-temperature, high-electric-field conditions of MAO.
For example, studies have shown that cerium (Ce) doping combined with hydrophobic modification can increase the antibacterial rate to 98% [8]. Furthermore, optimization of a low-voltage (<50 V) unipolar MAO process has significantly reduced the density of surface cracks in the coating, ensuring strong bonding with the substrate while avoiding the increased brittleness associated with high-voltage MAO [9]. Despite these advances, MAO coatings have inherent limitations: their porous structure (with pore diameters of 2–10 µm) and lack of long-term capability to release bioactive molecules restrict their performance in long-term antibacterial applications. To address this issue, a key strategy has emerged: constructing a biodegradable, drug-loaded, bioactive polymer composite layer on top of the MAO coating [10]. This polymer layer can seal the MAO pores and impart sustained-release functionality, thereby enhancing the coating’s long-term antibacterial performance. For instance, researchers have combined polymer coatings with MAO to successfully create antibacterial composite coatings on magnesium alloys [11]. Shuangshuang Zhang [12] further integrated drug-loaded mesoporous silica nanoparticles into an MAO coating, achieving controlled drug release. These studies demonstrate that using a polymer composite layer to seal MAO pores and provide sustained release is an effective approach to improving the long-term antimicrobial properties of the coating.
Among various polymer coatings, poly (lactic-co-glycolic acid) (PLGA) has gained widespread use for functionalizing bone implant surfaces due to its good biodegradability, FDA-approved safety profile, and tunable drug release kinetics [13,14,15]. PLGA coatings offer advantages of controlled drug delivery and biocompatibility for bone implant modification; however, their relatively poor mechanical properties and interfacial adhesion require improvement via composite modification (e.g., combining with MAO) [16,17]. By optimizing the coating structure and interface design, it is possible to create a multifunctional implant surface with high mechanical stability, long-term drug release, and excellent osteointegration capability [18]. This underscores that polymer coatings combined with MAO technology to form composite coatings are a promising strategy to enhance the long-term performance of implants.
Breakthroughs in nanocarrier technology have further expanded the therapeutic functions of PLGA coatings [19]. Research indicates that PLGA-based nanocarriers can achieve drug encapsulation efficiencies exceeding 85%, with an initial burst release of less than 20% [20]. In a notable study, Hai J [21] used a combination of anodization and electrophoretic deposition to fabricate a uniform TiO2 nanotube array on a titanium alloy surface and then introduced a VA-PLGA composite drug delivery system. This approach resulted in a vancomycin loading capacity 2.3 times higher than traditional methods, and thanks to the sustained release by PLGA, zero-order release kinetics was maintained for up to 11 days [22]. These advancements have laid important theoretical and technological foundations for the clinical application of PLGA-based composite coatings in orthopedic implants [23,24].
Among natural bioactive agents, curcumin (Cur) has attracted significant attention for its broad-spectrum antibacterial, anti-inflammatory, antioxidant, and osteogenic differentiation-promoting properties [25]. However, curcumin’s poor water solubility, low chemical stability, and short in vivo half-life severely limit its clinical utility [26]. Encapsulating curcumin in biodegradable polymer nanoparticles can markedly improve its solubility and stability and enable long-term sustained drug release as the carrier material degrades [27]. PLGA, as an FDA-approved biodegradable polyester with an adjustable degradation rate, excellent biocompatibility, and ease of surface modification, is an ideal carrier for constructing curcumin nanodelivery systems [28,29]. Moreover, hydroxyapatite (HA), the major inorganic component of bone tissue, can not only enhance the mechanical strength and osteoconductivity of the nanoparticles [30,31,32] but also promote local calcium and phosphate deposition through the release of Ca2+ and PO43− ions, thereby accelerating the osteointegration process [33,34]. Therefore, integrating curcumin, HA, and PLGA into a “Cur-HA@PLGA” micron composite coating is expected to synergistically achieve osteogenesis promotion, infection resistance, and sustained drug release.
This study aims to construct a CHP-Ti-MAO composite implant (denoted as CHP-Ti-MAO) via a two-step process of micro-arc oxidation followed by application of a micron composite drug-loaded coating (Figure 1). This design is intended to achieve the following synergistic effects: (1) enhanced solubility of curcumin, in which the high specific surface area of PLGA nanospheres increases curcumin’s effective solubility; (2) improved mechanical properties, in which HA, as rigid microparticles dispersed in the PLGA matrix, can enhance the hardness and wear resistance of the coating, reducing micro-cracks at the titanium substrate–polymer interface. While existing studies have demonstrated the individual antibacterial efficacy of curcumin and the osteogenic activity of PLGA-HA composites, research on combining these with an MAO-treated titanium substrate remains relatively limited. Many prior investigations have focused on coatings containing only PLGA or HA, and there is a lack of systematic understanding regarding how PLGA concentration (ranging from 50% to 150%) influences drug release behavior, surface wettability, corrosion resistance, and antibacterial performance. This report presents a comprehensive study to fill these knowledge gaps, evaluating the physicochemical properties, in vitro drug release profile, antibacterial activity, and osteogenic capacity of the developed CHP composite coating on MAO-treated titanium. The findings provide valuable insights into optimizing such composite coatings for orthopedic implants with both long-term infection resistance and improved bone integration.
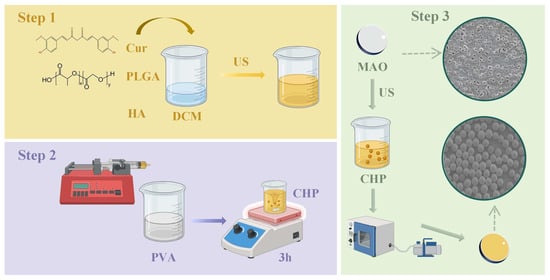
Figure 1.
Synthesis flowchart of CHP-Ti-MAO.
While existing studies have demonstrated individual functionalities of curcumin or PLGA-HA composites, no previous work has established how PLGA concentration governs the interdependent properties of drug release, corrosion resistance, and bacterial inhibition in MAO-based systems. This comprehensive study fills this knowledge gap by correlating structural variables (PLGA%) with functional outcomes—providing critical insights for developing orthopedic implants with combined infection resistance and accelerated osseointegration.
2. Materials and Methods
2.1. Preparation of CHP Microparticulate Drug-Loaded Coating
Curcumin (Cur; Komeo, Tianjin, China) and hydroxyapatite (HA) (HA; Zhongke Yannuo, Beijing, China) were accurately weighed (10 mg each) and combined with poly (lactic-co-glycolic acid) (PLGA; Daigang, Jinan, China; Mw ≈ 63 kDa) at three different mass ratios (50, 100, or 300 mg). These solid mixtures were transferred into individual beakers, and 2 mL of dichloromethane (DCM; Komeo, Tianjin, China) was added as the organic solvent. The suspensions were sonicated until both curcumin and PLGA were completely dissolved, yielding homogeneous, optically clear organic phases.
At room temperature (25 ± 2 °C), each organic phase was added dropwise (1 mL min−1) into 10 mL of an aqueous 3% (w/v) polyvinyl alcohol (PVA; Aladdin, Shanghai, China; Mw 17,600–26,400) solution under continuous magnetic stirring at 600 rpm. After complete addition, the resulting emulsions were sonicated for 6 min to achieve uniform dispersion of the organic phase within the aqueous phase, forming primary emulsions. The emulsions were then stirred at 600 rpm for 2 h to allow complete evaporation of DCM. Using this protocol, three micron coating formulations differing in PLGA content (50, 100, and 150 mg, corresponding to 50%, 100%, and 150% (w/w) relative to the initial Cur/HA mass), were prepared.
2.2. Morphological Characterization (SEM)
The prepared microspheres were frozen at −80 °C and then freeze-dried in a vacuum lyophilizer. The morphology of the samples was examined by scanning electron microscopy (SEM) (JSM-7800F, JEOL, Akishima, Tokyo, Japan). Prior to SEM observation, the samples were mounted on conductive adhesive stubs, sputter-coated with gold, and then imaged. The elemental composition of the microspheres was analyzed using an energy-dispersive spectrometer (EDS) (JSM-7800F, JEOL, Akishima, Tokyo, Japan).attached to the SEM.
2.3. Particle Size Distribution
The CHP micron coating was dispersed in deionized water, the particle size was measured using a laser particle size analyzer at 25 °C (Malvern Instruments Ltd Zetasizer ZS90, Malvern, UK), and a particle size distribution curve was plotted.
2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR (Bruker Optik GmbH VERTEX70, Ettlingen, Germany) spectroscopy was performed to determine the functional groups present in the samples and to confirm the composition of the composite microspheres. Approximately 20 mg of microspheres was analyzed using an FT-IR spectrometer, scanning in the wavenumber range of 4000–400 cm−1.
2.5. Phase Composition Analysis (XRD)
X-Ray diffraction (XRD) (D8 Advance, Bruker AXS, Karlsruhe, Germany) was used to analyze the phase composition of the samples. The XRD patterns were recorded with a diffractometer using Cu Kα radiation (λ = 1.5406 Å) at 30 kV and 10 mA, scanning from 3° to 90° at a rate of 10°/min. Samples were ground into a fine powder and packed into the sample holder for analysis.
2.6. Preparation of CHP-Ti-MAO-Coated Titanium
Titanium discs (99.9% purity, diameter 20 mm) (Hujin Titanium, Yancheng, China) were polished with 1200-grit silicon carbide sandpaper, then ultrasonically cleaned in absolute ethanol, and rinsed with deionized water. Micro-arc oxidation was carried out using a 40 V unipolar pulse power supply in a water-cooled electrolytic cell with a stainless-steel cathode and mechanical stirring. The electrolyte consisted of 0.06 mol/L calcium glycerophosphate (C3H7CaO6P·xH2O) (Komeo, Tianjin, China) and 0.3 mol/L calcium acetate (CH3COO)2Ca·xH2O) (Komeo, Tianjin, China). MAO treatment was performed at 600 Hz frequency and 20% duty cycle for 5 min, maintaining the electrolyte temperature below 50 °C using a cooling system. The resulting Ti-MAO substrates were then immersed in the CHP micron composite coating solution (prepared in Section 2.1) and sonicated for 10 min to obtain the CHP-Ti-MAO composite coating.
2.7. Surface Wettability of CHP-Ti-MAO
The surface wettability of the samples was evaluated by measuring the water contact angle using a contact angle goniometer (FCA 2000A3E, Shanghai Aifis, Shanghai, China). A 3 µL drop of deionized water was deposited onto the sample surface, and images were captured. At least three measurements were taken at different locations on each sample. The contact angles were calculated from the images using software, and the mean and standard deviation were determined for each sample group.
2.8. Corrosion Resistance of CHP-Ti-MAO
Electrochemical corrosion tests were conducted in 0.9% sodium chloride solution at 37 ± 1 °C using an electrochemical workstation (VersaSTAT 300, AMETEK, San Diego, CA, USA) with a standard three-electrode cell. A saturated calomel electrode (SCE, Shanghai INESA, Shanghai, China) served as the reference electrode, a platinum electrode (Shanghai INESA, Shanghai, China) as the counter electrode, and the test sample as the working electrode. The open-circuit potential was monitored for 3600 s to allow stabilization. Electrochemical impedance spectroscopy (EIS) was then performed over a frequency range of 0.01 Hz to 105 Hz. Following EIS, potentiodynamic polarization scans were carried out from –0.5 V to +0.5 V vs. open-circuit potential at a scan rate of 1 mV/s. At least three samples were tested for each condition.
2.9. In Vitro Drug Release Study of CHP-Ti-MAO
2.9.1. Chromatographic Conditions
The experiment was conducted on an Agilent 1100 high-performance liquid chromatography system. Stationary phase: chromatographic column Hypersil ODS2-C18 column (250 mm × 4.60 mm, 5 µm). After spectrum analysis, the mobile phase was screened out: acetonitrile-0.1% phosphoric acid solution (Komeo, Tianjin, China) = 80:20, detection wavelength: 425 nm, injection volume: 10 µL; column temperature: 30 °C ass the optimal detection condition. The chromatographic peaks obtained have good separation and no trailing phenomenon.
2.9.2. In Vitro Release Assay
The in vitro release of curcumin from the CHP-Ti-MAO coating was evaluated using a dialysis method in accordance with the Chinese Pharmacopoeia (2020 Edition) guidelines for dissolution and release testing. PBS solutions at pH 6 containing 0.5% Tween 80 were used as release media to simulate physiological and slightly acidic conditions. Approximately 10 mg of the CHP-Ti-MAO sample was placed in 10 mL of release medium and incubated at 37 °C with gentle shaking (100 rpm). At specified time points (30 min, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h, 96 h, 192 h, 240 h, 288 h, 336 h, 384 h, and 432 h), 1 mL aliquots of the release medium were withdrawn and replaced with an equal volume of fresh pre-warmed medium. Each sample was filtered through a 0.45 µm filter and analyzed by HPLC under the conditions described in Section 2.9.1. The curcumin concentration was determined from the peak area using a pre-established standard calibration curve. The cumulative release percentage was calculated for each time point and plotted over time. Release data were also fitted to various kinetic models (e.g., zero-order, first-order, Higuchi, and Korsmeyer–Peppas) to characterize the release mechanism. The cumulative release rate was calculated according to Equation (1) and fit the drug release kinetic equation.
Cₙ: drug concentration measured at the n-th sampling time point. Cᵢ (i = 1, …, n − 1): drug concentration measured at the i-th sampling time point. V0: initial total volume of the release medium. V: volume withdrawn at each sampling interval. Qₙ: total drug loading in the formulation.
2.10. Determination of Cur Solubility in CHP Micron-Scale Coating
An excess amount of curcumin (Cur) raw material was dispersed in the same release medium as described in Section 2.9.2 and incubated at 37 °C under continuous agitation at 100 rpm for 48 h with an additional 30 min of sonication. After equilibration, the suspension was filtered through a 0.45 µm membrane; the first 1 mL of filtrate was discarded to avoid adsorption artifacts. An appropriate volume of the subsequent filtrate was accurately diluted, and the curcumin concentration was quantified at 425 nm using the HPLC conditions specified in Section 2.9.1. Peak areas were recorded and converted to concentrations (µg mL−1) with the calibration curve established in Section 2.9.2.
2.11. Stability Evaluation of CHP Micron-Scale Coating
Sealed vials containing 50%, 100%, and 150% CHP microparticulate were stored at 25 °C for 30 days. On days 0, 10, and 30, the curcumin load was determined as follows: 2 mg lyophilized CHP was precisely weighed (W4) into a 20 mL volumetric flask, dissolved in acetonitrile (Kemiou, Tianjin, China), sonicated for 6 min, and vortex mixed for 3 min. The volume was adjusted to scale with acetonitrile, and the solution was cooled in an ice bath for 30 min in an ultrasonic bath. After filtration through a 0.45 µm membrane, the injection filtrate was used for HPLC analysis at 425 nm. According to the standard curve equation, the weight of curcumin (W3) was calculated, and the drug load (DL%) was calculated according to equation (2). W3: actual measured turmeric quality (µg); W4: total mass of precisely weighed CHP microparticulate (mg).
2.12. In Vitro Biocompatibility (Hemocompatibility) of CHP-Ti-MAO
The hemocompatibility of the CHP-Ti-MAO coating was assessed by a hemolysis test. Briefly, 3 mL of a 2% (v/v) suspension of red blood cells (RBCs) in saline was added to each CHP-Ti-MAO sample. Positive control samples (3 mL of RBC suspension in distilled water to induce complete hemolysis) and negative control samples (3 mL of RBC suspension in normal saline) were also prepared. All samples were incubated at 37 °C for 4 h and then centrifuged at 3000 rpm for 10 min. The supernatants were collected, and their absorbance at 540 nm was measured using a UV-visible spectrophotometer. The percentage hemolysis was calculated using Equation (3):
where Asample, Anegative, and Apositive are the absorbances of the test sample, negative control, and positive control supernatants, respectively. According to the Chinese standard YY/T 1819-2022 [35], a hemolysis rate of less than 5% is considered non-hemolytic (indicating no significant toxicity to red blood cells).
2.13. Antibacterial Activity of CHP-Ti-MAO
The antibacterial efficacy of CHP-Ti-MAO was evaluated against Escherichia coli (Gram-negative bacteria) using an agar diffusion method and colony counting. In brief, CHP-Ti-MAO samples were placed in 12-well plates, each containing 2 mL of bacterial suspension at a concentration of 1 × 105 CFU/mL. The plates were incubated at 37 °C for 3 h, 10 h, and 17 h. At each time point, 100 µL aliquots of the culture medium were removed and spread onto agar plates. After incubating these agar plates at 37 °C for 24 h, the number of viable bacterial colonies was counted using an automated colony counter (Shineso Technology Co., Ltd. Shineso V3, Hangzhou, China) following the guidelines of Chinese standard GB/T 4789.2-2020 [36]. The antibacterial rate (AR) was calculated using Equation (4):
where Ncontrol is the number of colonies in the control group and Nsample is the number in the test group (with the coating). According to the standard, an antibacterial rate ≥90% indicates effective antibacterial performance and ≥99% indicates strong antibacterial activity.
2.14. Statistical Analysis
All biological experiments were performed in triplicate. Data are presented as mean ± standard deviation. Statistical analysis was conducted using appropriate tests (e.g., one-way ANOVA or t-test) to determine significant differences between groups. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. SEM and EDS Analysis of CHP Composite
Figure 2 presents the micro-morphology of the CHP micron-scale coating prepared at different PLGA concentrations (50%, 100%, and 150% PLGA w/w). Scanning electron microscopy (SEM) images reveal that all three coatings exhibit uniformly distributed spherical microparticles, with no evident inter-particle agglomeration, indicating that the emulsion–solvent evaporation method effectively achieves monodisperse microparticulate loading. As the PLGA concentration increases, the packing density of surface particles rises slightly, and the particle boundaries become progressively less distinct, likely because the higher PLGA concentration forms a more continuous polymer film during solidification, resulting in a slight encapsulation effect on particle morphology.

Figure 2.
SEM micrographs of the CHP coatings (a) 50% CHP, (b) 100% CHP, and (c) 150% CHP.
Energy-dispersive X-ray spectroscopy (EDS) results (Table 1) further confirm the elemental composition and distribution characteristics of the CHP coatings. All samples contain C, O, Ca, and p. Carbon and oxygen originate primarily from PLGA and curcumin (Cur), whereas the presence of calcium and phosphorus unequivocally indicates the successful incorporation of hydroxyapatite (HA).

Table 1.
Elemental weight percentages of the 50% CHP, 100% CHP, and 150% CHP.
3.2. Determination of CHP Particle Size Distribution
Figure 3 provides a direct comparison of the macroscopic appearance and the corresponding particle size distribution profiles of CHP micron-scale coating prepared at different PLGA concentrations. As shown in Figure 3a, all three formulations present as homogeneous pale-yellow emulsions. The particle size data (Figure 3b–d) further elucidate the influence of PLGA concentration on dimensions.
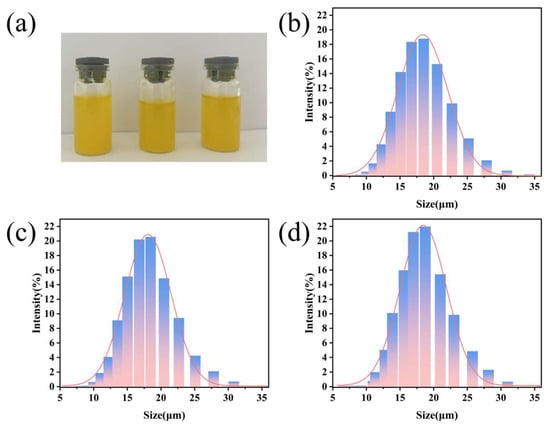
Figure 3.
(a) Macroscopic photograph of CHP micron-scale coating emulsions; (b) particle size distribution of 50% CHP; (c) particle size distribution of 100% CHP; (d) particle size distribution of 150% CHP.
For the 50% CHP group, the particle size is narrowly centered at 18.79 ± 1.3 µm (Figure 3b), exhibiting a unimodal distribution with a polydispersity index (PDI) < 0.20. This suggests that the lower PLGA concentration favors rapid microparticulate formation while minimizing aggregation. In the 100% CHP group, the mean diameter increases slightly to 20.57 ± 1.9 µm (Figure 3c), accompanied by a modest broadening of the distribution peak, presumably attributable to enhanced inter-chain interactions of the polymer segments promoting mild inter-particle adhesion. The 150% CHP group exhibits a further increment to 21.95 ± 2.1 µm (Figure 3d) and a markedly wider size range, indicating that the elevated PLGA concentration—via increased viscosity—induces localized agglomeration.
All three formulations fall within the ≈20 µm range, which is substantially smaller than conventional microspheres (50–200 µm). This submicron-to-micron transitional size not only increases the specific surface area, thereby affording improved control of the initial burst release, but also circumvents the rapid systemic clearance associated with nanoparticle <100 nm. Collectively, raising the PLGA concentration leads to a modest increase in particle size and a concomitant broadening of the distribution.
3.3. FTIR Analysis of CHP Micron-Scale Coating
Figure 4a presents the FTIR spectra of CHP micron coating prepared at different PLGA concentrations. A broad absorption band centered at approximately 3490 cm−1 is ascribed to the O–H stretching vibration, primarily originating from PLGA. The peaks observed at ≈1601 cm−1 and ≈1514 cm−1 are characteristic of aromatic ring stretching, confirming the presence of curcumin. Absorptions at ≈1352 cm−1 and ≈1036 cm−1 correspond to C–O–C and C–O stretching vibrations within PLGA and hydroxyapatite (HA), respectively. The distinctive peak at ≈565 cm−1 is attributed to the ν4 bending mode of PO43−, unequivocally demonstrating the incorporation of HA.
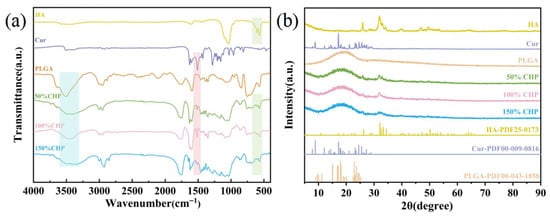
Figure 4.
(a) FTIR spectra of CHP micron-scale coating; (b) XRD patterns of CHP micron-scale coating.
The FTIR results demonstrate that the as-synthesized CHP micron-scale coating successfully encapsulates both curcumin and hydroxyapatite within the PLGA matrix. The appearance and positions of all characteristic peaks are fully consistent with the chemical structures of the individual components, thereby validating the successful fabrication and compositional integrity of the coatings [37,38].
3.4. XRD Analysis of CHP Micron-Scale Coating
Figure 4b presents the X-Ray diffraction (XRD) patterns of the CHP coatings, elucidating their crystalline phase composition with characteristic reflections attributable to both hydroxyapatite (HA) and PLGA. A broad, diffuse halo centered at approximately 22° confirms the amorphous nature of the PLGA matrix. Additional reflections corresponding to HA are observed; however, these peaks are markedly less intense and broader than those of pristine HA, indicating that HA is partially embedded within the PLGA matrix and that its crystallinity is consequently reduced. Notably, the diffraction peaks characteristic of curcumin are absent, suggesting that curcumin is dispersed within the PLGA matrix in an amorphous state [39].
The XRD data corroborate the successful synthesis of the CHP micron-scale coating. The coexistence of crystalline (HA) and amorphous (PLGA) signatures within the micron-scale coating demonstrates that HA is partially embedded in the PLGA matrix, while curcumin is likely present in a non-crystalline form. This structural configuration is anticipated to facilitate the controlled release of both HA and curcumin from the micron-scale coating [40].
3.5. Surface Wettability (Contact Angle) of CHP Micron-Scale Coating
Water-contact-angle measurements demonstrate that CHP micron-scale coating of varying PLGA concentrations markedly enhances the hydrophilicity of MAO-treated titanium surfaces (Figure 5f). The untreated KB-Ti-MAO control exhibits a contact angle of 36.59 ± 1.75°, indicating moderate hydrophilicity attributable to surface Ti–OH groups and the microporous architecture of the MAO ceramic layer. After CHP modification, all coated samples display significantly reduced contact angles (p < 0.05) that decrease monotonically with increasing PLGA concentration: 27.16 ± 1.96° for 50% CHP, 22.36 ± 2.19° for 100% CHP, and a minimum of 21.15 ± 2.01° for 150% CHP.
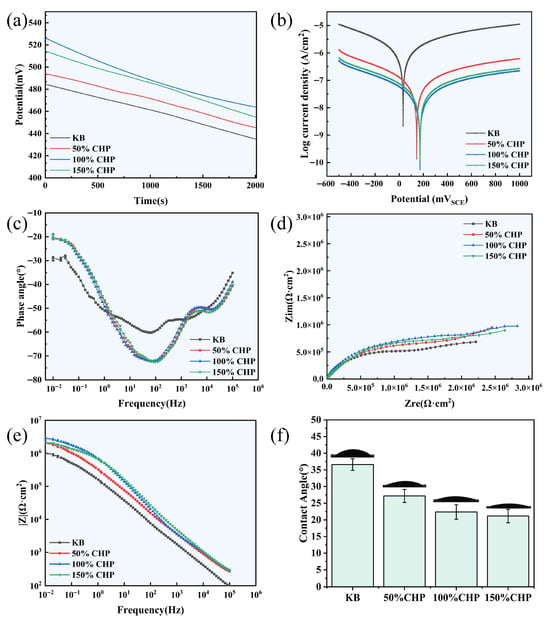
Figure 5.
Electrochemical analysis of CHP-Ti-MAO samples with varying PLGA content: (a) open-circuit potential–time curve (OCP-T); (b) Tafel curve; (c) Nyquist plot; (d,e) Bode plots; (f) surface water contact angle.
This progressive enhancement is ascribed to the formation of hydrogen bonds between water molecules and the ester linkages (–COO–) as well as terminal carboxyl groups (–COOH) of PLGA, which augment surface polarity. Additional contributions arise from hydrogen bonding between the phenolic hydroxyl groups (–OH) of curcumin and interfacial water. Collectively, the CHP micron-scale coating transforms the MAO titanium surface from “moderately hydrophilic” to “highly hydrophilic” (contact angle < 30°), thereby facilitating early cell adhesion, protein adsorption, and tissue fluid infiltration and establishing a favorable microenvironment for subsequent osseointegration.
3.6. Corrosion Resistance
The open-circuit potential (Eocp) curves depicted in Figure 5a demonstrate that CHP-treated Ti-MAO alloys exhibit markedly higher potentials than the KB-Ti-MAO control. According to the Eocp data, the maximum potential of 526.2 mV is achieved when the CHP content is 100%, representing an increase of 41.77 mV relative to the KB-Ti-MAO specimen. These results indicate that CHP treatment significantly reduces the corrosion propensity of the Ti-MAO alloy.
Tafel polarization curves (Figure 5b) further corroborate the diminished corrosion tendency, as evidenced by a positive shift in potential and a concurrent decrease in corrosion current density. After fitting the Tafel regions, the self-corrosion potential (Ecorr) and self-corrosion current density (icorr) were extracted for each sample. The polarization resistance (Rp0) was subsequently calculated using the Stern–Geary equation (Equation (5)).
where Rp0, βa, βc, and icorr denote the polarization resistance, anodic Tafel slope, cathodic Tafel slope, and corrosion current density, respectively. The calculated values are summarized in Table 2.

Table 2.
Electrochemical data of CHP-Ti-MAO samples in 0.9% NaCl solution obtained from Nyquist and Bode plots.
All CHP-treated samples display higher Ecorr values than the KB-Ti-MAO control (484.5 mV). Notably, the 100% CHP specimen attains the highest Ecorr of 165.9 mV and the lowest icorr of 10.5 × 10−9 A cm−2. These findings confirm that CHP treatment substantially enhances the polarization resistance of the Ti-MAO alloy, thereby improving its overall corrosion resistance.
Mechanistic discussion:
- (1)
- 100% CHP: The optimal PLGA loading produces a uniform, defect-free, and compact film (~18–20 µm, SEM) atop the MAO layer. This thickness is sufficient to establish an effective Cl− barrier while remaining below the critical level that would induce residual stresses. Consequently, the 100% CHP sample exhibits the highest open-circuit potential (Eocp) and largest polarization resistance (Rp0).
- (2)
- 150% CHP: Excess PLGA increases hydrophilic segment density, promoting pronounced water uptake and swelling in 0.9% NaCl. The resulting volumetric expansion generates tensile stresses that exceed the cohesive strength of the coating, producing micro-cracks and interconnected pores. These defects act as preferential pathways for electrolyte ingress, accelerating corrosion and manifesting as an elevated corrosion-current density (icorr) and diminished Rp0.
Hence, the 100% CHP system achieves the optimum balance between thickness and compactness, conferring superior long-term corrosion protection. For the 150% CHP system, the compromised integrity arising from swelling-induced cracking underscores that precise control of coating thickness and densification is essential for enhancing the durability of Ti-MAO substrates [41].
3.7. In Vitro Drug Release Study
As shown in Figure 6a and Table 3, the target peak retention time for the curcumin solution was 11.23 min. Figure 6b depicts the linear regression of peak area (A) versus curcumin concentration (C), yielding the regression equation A = 59.801C − 14.76. A good linear relationship (R2 = 0.999) was observed for curcumin within the concentration range of 2.006 to 12.03 µg·mL−1.
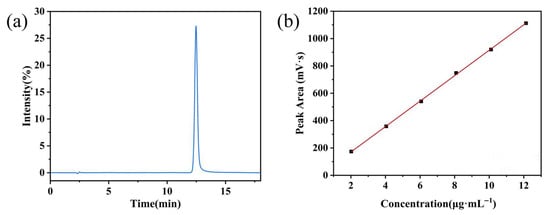
Figure 6.
(a) Representative chromatogram of curcumin; (b) calibration curve for curcumin quantification.

Table 3.
Linearity of curcumin in PBS (pH 6.0) containing 0.5% (v/v) Tween 20.
Figure 7 shows the cumulative drug release profiles of three coatings with different CHP contents (50%, 100%, and 150%) in phosphate-buffered saline (PBS, pH 6.0, 37 °C). Data are presented as “Cumulative Release Percentage (%)–Time (h)” over a period of 0–430 h. The 24 h cumulative release amounts for the 50% CHP, 100% CHP, and 150% CHP micron-scale coating were (26.74 ± 2.15)%, (30.72 ± 1.48)%, and (25.08 ± 0.75)%, respectively. This indicates a significant reduction (p < 0.05) in the initial burst release with increasing CHP content.
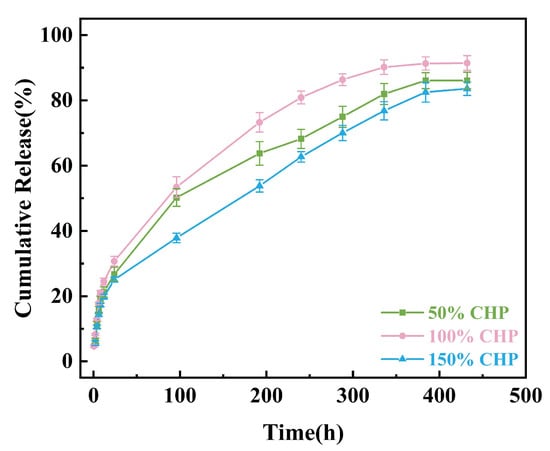
Figure 7.
Cumulative release percentage in vitro of 50% CHP, 100% CHP, and 150% CHP.
The release data were fitted to zero-order, first-order, Higuchi, Ritger–Peppas, and Weibull kinetic models; the results are summarized in Table 4a–e. For all three CHP formulations, the Ritger–Peppas (R2 = 0.982–0.995) and Weibull (R2 = 0.961–0.999) equations provided the best fit, followed by the Higuchi model (R2 = 0.927–0.984). The zero-order and first-order models showed lower correlation (R2 = 0.835–0.915).

Table 4.
Release kinetic equations for 50% CHP, 100% CHP, and 150% CHP.
- (1)
- 50% CHP: The Ritger–Peppas exponent (n = 0.423) suggests drug release is primarily governed by Fickian diffusion.
- (2)
- 100% CHP: The Weibull shape parameter (β = 0.836) indicates a release profile characterized by an initially rapid phase followed by a slower phase, achieving the most complete release.
- (3)
- 150% CHP: The exponent (n = 0.414) confirms diffusion-controlled release, but the significantly decreased rate constant is consistent with the observed release curve trend.
Analysis based on zero-order kinetics revealed that the 50% CHP micron-scale coating reached 50% cumulative release (t50) at approximately 50 h, while the t50 values for the 100% CHP and 150% CHP coatings were 75 h and 110 h, respectively. The final cumulative release percentages for the 50% CHP, 100% CHP, and 150% CHP coatings were (86.12 ± 2.5)%, (91.47 ± 2.2)%, and (83.57 ± 2.1)%, respectively. Increasing the CHP content resulted in approximately 10–15% drug residue.
Integrating the curve profiles and model fitting results, the 100% CHP formulation demonstrated rapid initial release while achieving near-complete drug release within 500 h, effectively balancing efficiency and completeness. It is therefore recommended as the optimal formulation for subsequent in vivo studies. The 150% CHP coating, however, exhibited structural densification due to high drug loading, leading to a reduced diffusion coefficient. Enhancing porosity or incorporating porogens is suggested to improve its later-stage release kinetics.
3.8. Determination of Cur Solubility from CHP Micron-Scale Coating
As can be seen from Table 5, the saturated solubility of the Cur drug substance in the release medium is 9.39 ± 0.30 µg·mL−1 (n = 3), indicating that the free drug solubility is extremely low and affects the bioavailability. The Cur solubility of the three CHP coatings (50%, 100%, and 150% PLGA w/w) was significantly higher than that of the API, which were 21.28 ± 0.9 µg/mL, 21.46 ± 0.9 µg/mL, and 21.67 ± 0.8 µg/mL, respectively. CHP particles increased the solubility of Cur by about 2.2-fold, which confirmed that PLGA microcarriers effectively overcome the hydrophobicity of drugs and significantly improve the solubility. PLGA ratio (50–150%) had no significant difference in solubilization effect (p > 0.05), suggesting that solubilization mainly depended on the encapsulation of microparticle, not the carrier concentration.

Table 5.
Determination of solubility of Cur drug substance, 50% CHP, 100% CHP, and 150% CHP.
3.9. Stability Evaluation Results from CHP Micron-Scale Coating
Three CHP coatings (50%, 100%, and 150% PLGA w/w) were stored in sealed amber vials at 25 °C in the dark for 30 days. Drug-loading (DL%) was determined in triplicate at 0, 10, and 30 days (Table 6). Within this period, the DL% of all formulations decreased by < 11%, indicating robust chemical stability under the specified conditions. Each coating retained ≥ 89% of its initial DL% after 30 days, fulfilling the requirements for routine transportation and short-term ambient storage.

Table 6.
Stability of 50% CHP, 100% CHP, and 150% CHP micron-scale coating.
Increasing the PLGA ratio did not further improve stability; conversely, the 150% CHP formulation exhibited a marginally greater DL% decline, tentatively attributed to interfacial drug solubilization and microphase separation.
3.10. In Vitro Safety (Hemocompatibility)
As shown in Figure 8, the solution in the positive control group (pure water) appeared clear red. Due to the osmotic pressure of pure water being lower than that of red blood cells (RBCs), the RBCs absorbed excess water and underwent rupture, resulting in hemolysis. In contrast, solutions containing different concentrations of CHP exhibited RBC sedimentation with clear, transparent supernatant. Furthermore, the hemolysis rates for all CHP groups were less than 5%, complying with pharmacopoeial requirements and indicating negligible hemolysis. These results demonstrate the favorable biocompatibility of the CHP micron-scale coating.
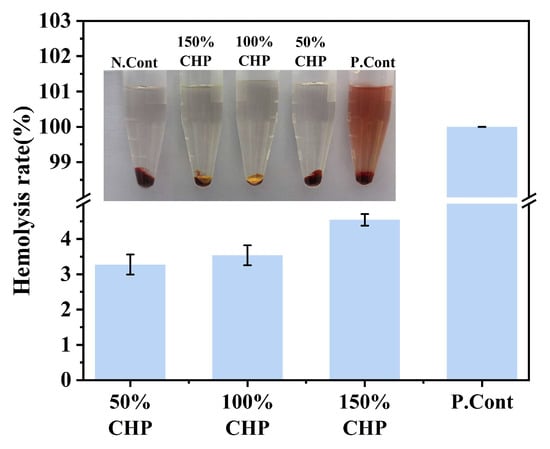
Figure 8.
Hemolysis test: representative visual samples and quantified hemolysis rate (n = 3).
3.11. Antibacterial Performance
Figure 9 and Figure 10 present the bacterial colonies and antibacterial rates of CHP-Ti-MAO samples with varying PLGA content, determined via the plate counting method. As shown in Figure 9 and Figure 10, a substantial number of Escherichia coli colonies proliferated on KB-Ti-MAO, which served as the blank control, confirming its lack of antibacterial activity. In contrast, the 50% CHP-Ti-MAO sample exhibited reduced bacterial colonization compared to KB-Ti-MAO. Its antibacterial rates were (76.75 ± 3.03)% at 24 h, (19.76 ± 0.52)% on day 3, (46.83 ± 1.39)% on day 10, and (51.37 ± 1.08)% on day 17.
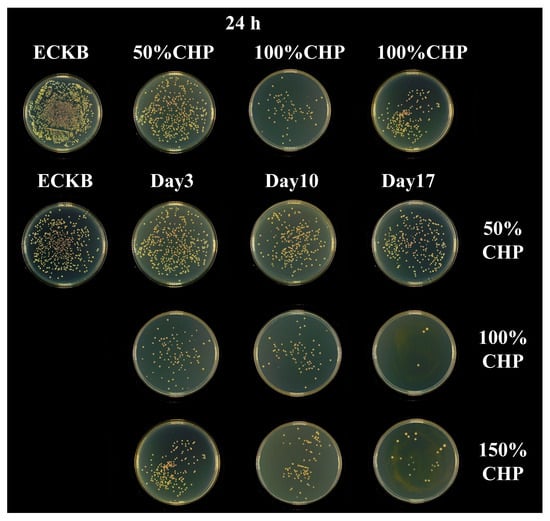
Figure 9.
Bacterial colony counts on 50% CHP-Ti-MAO, 100% CHP-Ti-MAO, and 150% CHP-Ti-MAO samples at 24 h on day 3, day 10, and day 17.
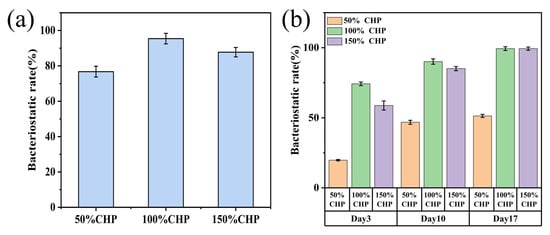
Figure 10.
Antibacterial rates of CHP-Ti-MAO samples: (a) 50% CHP-Ti-MAO, 100% CHP-Ti-MAO, and 150% CHP-Ti-MAO at 24 h; (b) corresponding samples at day 3, day 10, and day 17.
The 100% CHP-Ti-MAO sample also showed reduced colony counts with antibacterial rates of (95.41 ± 2.97)% at 24 h, (74.25 ± 1.30)% on day 3, (90.12 ± 1.93)% against E. coli on day 10, and (99.31 ± 0.93)% against E. coli on day 17.
Similarly, the 150% CHP-Ti-MAO sample demonstrated reduced colonization, achieving antibacterial rates of (87.74 ± 2.65)% at 24 h, (58.77 ± 1.30)% on day 3, (85.09 ± 1.49)% against E. coli on day 10, and (99.33 ± 0.72)% against E. coli on day 17.
4. Conclusions
This study successfully constructed a multifunctional composite implant (CHP-Ti-MAO) on titanium surfaces via a two-step approach combining micro-arc oxidation (MAO) and curcumin-hydroxyapatite@PLGA (CHP) micro-scale drug coating. The integrated system exhibits sustained antibacterial efficacy, controlled drug release, high hydrophilicity, and exceptional corrosion resistance.
The key findings demonstrate that Cur-HA@PLGA (CHP) microscale coatings with varying PLGA concentrations (50%, 100%, and 150%) uniformly adhere to MAO-treated titanium, forming stable spherical microparticle structures. Increased PLGA concentration enhanced coating densification and particle packing density. Fourier-transform infrared spectroscopy (FT-IR) and X-Ray diffraction (XRD) analyses confirmed successful encapsulation of curcumin (Cur) and hydroxyapatite (HA), with HA embedded in partially crystalline form within the PLGA matrix while Cur existed in an amorphous state—favoring sustained drug release.
Water contact angle measurements revealed significantly improved hydrophilicity (contact angle <30°) on CHP-coated surfaces. The 150% PLGA group exhibited optimal wettability (21.15 ± 2.01°), promoting cell adhesion and biological fluid infiltration. Electrochemical testing identified the 100% CHP-Ti-MAO formulation as having the highest corrosion resistance, evidenced by a noble open-circuit potential (Ecorr = 165.9 mV) and exceptional polarization resistance (Rp0 = 1.849 × 108 Ω·cm2), substantially reducing substrate corrosion susceptibility.
In vitro drug release studies established that higher PLGA concentrations effectively mitigated burst release. The 100% CHP group demonstrated near-zero-order release kinetics (k0 = 0.25% h), with only 37.6% initial burst at 24 h and a final cumulative release of 91.47%, surpassing other formulations. Antimicrobial assays demonstrated that the 100% CHP-Ti-MAO group elicited a significant reduction in viable colony counts, achieving 95.41 ± 2.97% inhibition after 24 h, 74.25 ± 1.30% on day 3, 90.12 ± 1.93% on day 10, and 99.31 ± 0.93% on day 17 against Escherichia coli, thereby exceeding the clinical efficacy threshold of >90%. Hemolysis assays revealed values below 5% across all experimental groups, confirming excellent hemocompatibility and conformity to established biocompatibility standards.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/coatings15080948/s1. Table S1. Abbreviations and their corresponding full names. Figure S1. Elemental weight percentages of the 50 % CHP, 100 % CHP, and 150 % CHP.
Author Contributions
L.M.: Methodology, Validation. Y.L.: Writing—original, Methodology, Formal analysis, Visualization. S.Z.: Visualization, Investigation. S.C.: Visualization, Investigation. X.L.: Visualization, Investigation. C.S.: Resources, Software, Data curation. H.Q.: Software, Conceptualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Heilongjiang Province Postdoctoral Fellowship Programme (LBH-Z23297); Heilongjiang Province Department of Education Basic Research Business Expense Foundation Research Project (2023-KYYWF-0585); Jiamusi University National Foundation Cultivation Project (JMSUGPZR2023-011); Heilongjiang Province New Drug Creation and Pharmacological Toxicology Evaluation Key Laboratory Open Project (kfkt2023-08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
All authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
References
- Ding, Y.; Tao, B.L.; Ma, R.C.; Zhao, X.; Liu, P.; Cai, K.Y. Surface modification of titanium implant for repairing/improving microenvironment of bone injury and promoting osseointegration. J. Mater. Sci. Technol. 2023, 143, 1–11. [Google Scholar] [CrossRef]
- Tang, H.; Xie, Y.S.; Xia, L.F.; Tang, Y.; Sun, Y.L. Review on the Fabrication of Surface Functional Structures for Enhancing Bioactivity of Titanium and Titanium Alloy Implants. Chin. J. Mech. Eng. 2024, 37, 87. [Google Scholar] [CrossRef]
- Dhein, J.; Haller, C.; Reichl, F.X.; Milz, S.; Hickel, R.; Kollmuss, M.; Högg, C. Intranuclear cell uptake and toxicity of titanium dioxide and zirconia particles as well as bacterial adhesion on dental titanium- and zirconia-implants. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2022, 38, 201–202. [Google Scholar] [CrossRef]
- Kuik, C.; de Boer, C.; van Hoogstraten, S.W.G.; Freulings, K.; Honing, M.; Arts, J.J.C.; Cillero-Pastor, B. Proteomic signatures of Staphylococcus aureus biofilm maturation on orthopaedic implants. Biofilm 2025, 9, 100287. [Google Scholar] [CrossRef]
- Bell, R.D.; Cann, E.A.; Mishra, B.; Valencia, M.; Zhang, Q.; Huang, M.; Yang, X.; Carli, A.; Bostrom, M.; Ivashkiv, L.B. Staphyloccocus aureus biofilm, in absence of planktonic bacteria, produces factors that activate counterbalancing inflammatory and immune-suppressive genes in human monocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2024, 42, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Liu, Y.; Xi, F.; Zhang, X.; Kang, Y. Micro-arc oxidation (MAO) and its potential for improving the performance of titanium implants in biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1282590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, R. Enhanced corrosion resistance and photothermal antibacterial properties of MAO/Fe/GO composite coating on Mg alloy. Mater. Lett. 2024, 369, 136755. [Google Scholar] [CrossRef]
- Yang, E.; Yang, R.; Wei, W.; Mo, Q.F.; Liang, F.Y.; Li, D.; Li, W.Z. Corrosion resistance and antibacterial properties of hydrophobic modified Ce-doped micro-arc oxidation coating. J. Mater. Res. Technol. 2024, 29, 3303–3316. [Google Scholar] [CrossRef]
- Lorena, K.; Luca, P.; Giorgio, A.S.; Franceschi Mattia, F.; Claudio, G.; Katya, B.; Chiara, R.; Manuele, D. Investigation of hydroxyapatite (HAP) containing coating on grade 2 titanium alloy prepared by plasma electrolytic oxidation (PEO) at low voltage. Surf. Interfaces 2022, 30, 101888. [Google Scholar]
- Roman, G.; Ladislav, C.; Martina, D.; Václav, N.; Josef, H.; Petr, U.; Matěj, B.; Adéla, P.; Jana, S.; Lucie, B. Hybrid coatings for orthopaedic implants formed by physical vapour deposition and microarc oxidation. Mater. Des. 2022, 219, 110811. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, B.; Xu, L.; Wan, S.; Guo, X.P. A novel anti-corrosion and antibacterial integrated MAO/PCNZ composite coating on AZ31B Mg alloy. Surf. Coat. Technol. 2024, 483, 130794. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, W.; Liu, F.; Xiang, S. Design of Spatial pore structures in Micro-Arc oxidation coatings of Ti implant for nanoparticle drug delivery. Mater. Des. 2025, 254, 113992. [Google Scholar] [CrossRef]
- Eskitoros-Togay, S.M.; Bulbul, Y.E.; Dilsiz, N. Combination of nano-hydroxyapatite and curcumin in a biopolymer blend matrix: Characteristics and drug release performance of fibrous composite material systems. Int. J. Pharm. 2020, 590, 119933. [Google Scholar] [CrossRef]
- Xu, W.; Huang, W.; Cai, X.; Dang, Z.; Hao, L.; Wang, L. Dexamethasone Long-Term Controlled Release from Injectable Dual-Network Hydrogels with Porous Microspheres Immunomodulation Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 40581–40601. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; Geng, K.; Shen, J.; Feng, X.; Xu, P.; Duan, Y.; Li, Y.; Wu, R.; Gou, Z.; et al. Large fuzzy biodegradable polyester microspheres with dopamine deposition enhance cell adhesion and bone regeneration in vivo. Biomaterials 2021, 272, 120783. [Google Scholar] [CrossRef]
- Gai, K.; Zhang, T.; Xu, Z.; Li, G.Z.; He, Z.H.; Meng, S.H.; Shi, Y.X.; Zhang, Y.H.; Zhu, Z.; Pei, X.B.; et al. Biomimetic management of bone healing stages: MOFs induce tunable degradability and enhanced angiogenesis-osteogenesis coupling. Chem. Eng. J. 2024, 493, 152296. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.; Zhang, Q.; Liu, C. Biomechanical and histomorphometric evaluation of biodegradable mini-implants for orthodontic anchorage in the mandible of beagle dogs. BMC Oral. Health 2025, 25, 516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Jia, Z.; Chen, G.L.; Song, X.J.; Yang, D.Y.; Zhu, K.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Highly Porous Microcarriers for Minimally Invasive In Situ Skeletal Muscle Cell Delivery. Small 2019, 15, e1901397. [Google Scholar]
- Jiang, X.; Lei, L.; Sun, W.; Wei, Y.M.; Han, J.Y.; Zhong, S.Q.; Yang, X.Y.; Gou, Z.G.; Chen, L.L. Bioceramic scaffolds with two-step internal/external modification of copper-containing polydopamine enhance antibacterial and alveolar bone regeneration capability. J. Zhejiang Univ. Sci. B 2024, 25, 65–82. [Google Scholar] [CrossRef]
- Yue, S.; Bolun, Z.; Ruowei, S.; Liu, W.F.; Zhu, Q.B.; Zhang, X.; Wang, R.R.; Chen, C.P. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Hai, J.; Zhang, X.; Lei, Y.; Shan, C.H.; Ma, X.H.; Jing, L.; Shi, W.H.; Yang, Q.W.; Li, Y.Y.; Xu, Q.Y.; et al. 3D printed TC4 titanium alloy bone scaffolds with TNTs-VA-PLGA composite coating on the surface: Improved infection resistance and biocompatibility. J. Drug Deliv. Sci. Technol. 2024, 102, 106386. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, W.; Li, J.H.; Shao, J.L.; Yi, B.C.; Zhang, C.F.; Liu, H.; Ma, B.J.; Ge, S.H. Multifunctional SDF-1-loaded hydroxyapatite/polylactic acid membranes promote cell recruitment, immunomodulation, angiogenesis, and osteogenesis for biomimetic bone regeneration. Appl. Mater. Today 2021, 22, 100942. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, C.; Wei, J.W.; Jin, S.; Zuo, Y.; Li, Y.B.; Liang, X.J.; Jidong, L. Electrospinning/3D printing-integrated porous scaffold guides oral tissue regeneration in beagles. Bio-Des. Manuf. 2024, 7, 1–18. [Google Scholar] [CrossRef]
- Wei, J.W.; Yan, Y.; Gao, J.; Li, Y.B.; Wang, R.L.; Wang, J.X.; Zou, Q.; Zuo, Y.; Zhu, M.F.; Li, J.D. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 133, 112618. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, Z.; Chen, Z.J.; Zhang, W.Z.; Liu, X.H.; Zhao, P.P.; Zhang, T.; Li, H.B. Curcumin-based antibacterial waterborne polyurethane/gelatin composite film for pork preservation and freshness indication. Chem. Eng. J. 2025, 520, 165793. [Google Scholar] [CrossRef]
- Bhalla, V.; Sahu, P.C.; Shashikala, R.A.; Singh, S.; Thangavelu, I.; Tadepalli, S.; Bhran, A.A. Synthesis and Multifunctional Evaluation of BaO2-Sodium Alginate-Curcumin Nanocomposite: Improved Antibacterial, Antioxidant, and Osteosarcoma Cell Inhibition. J. Inorg. Organomet. Polym. Mater. 2025; prepublish. [Google Scholar]
- Surya, M.; Kumar, A.S.; Sankar, M.; Moses, V.A.R.; Mahendran, K.; Muthupandian, S. One pot Hypnea valentiae mediated synthesis of silver doped curcumin nanoparticles and their evaluation of antibacterial, anti-inflammatory and anticancer activity. J. Ind. Eng. Chem. 2025, 149, 374–385. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, Q.; Zhang, M.; Zhang, S.; Zhao, W.; Song, X.; Wang, X.; Pan, L.; Zhao, Q.; Qiang, Q.; et al. Metal-Phenolic Network-Enhanced Curcumin Nanoparticles for Synergistic Antibacterial and Wound Healing Therapy. Nanotechnology 2025, 36, 265602. [Google Scholar] [CrossRef]
- Yeoh, S.G.; Liew, Y.K.; Lim, W.M.; Rahman, N.A.; Then, Y.Y. The Preparation and Characterization of Electrospun Chitosan-Gelatin Nanofibers Containing Copper-Metal-Organic Frameworks Loaded with Curcumin and Chrysin as Antibacterial Wound Dressings. ACS Omega 2025, 10, 21065–21076. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, N.; Bouhamed, A.; Ayadi, A.; Njeh, A.; Jeder, K.; Bouaziz, J.; Kanoun, O.; Yeoh, S.G.; Liew, Y.K.; Lim, W.M.; et al. Boosting α to β transformation of PVDF/HFP through natural hydroxyapatite derived from animal bones for eco-friendly energy harvesters. Ceram. Int. 2024, 50, 55598–55608. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Wang, L.; Yan, L.; Yan, Y.; Wang, C.; Peng, H.; Fan, X. Enhancing osteoblast proliferation and bone regeneration by poly (amino acid)/selenium-doped hydroxyapatite. Biomed. Mater. 2024, 19, 035025. [Google Scholar] [CrossRef]
- Boiko, A.A.; Malanchuk, A.V.; Myroshnychenko, S.M. Reparative osteogenesis in mandible in cases of filling a bone defect with hydroxyapatite-containing osteotropic material and injecting the surrounding soft tissues with thymalin: Experimental and morphological study. Wiad. Lek. 2024, 77, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Sun, X.; Bu, Y.; Yan, H.; Lin, Q. Chemical characterization and biological properties of titania/hydroxyapatite-promoted biomimetic alginate-chitosan-gelatin composite hydrogels. Ceram. Int. 2023, 49, 25744–25756. [Google Scholar] [CrossRef]
- Jamali, R.; Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M.; Kolahi, A. Effects of co-incorporated ternary elements on biocorrosion stability, antibacterial efficacy, and cytotoxicity of plasma electrolytic oxidized titanium for implant dentistry. Mater. Chem. Phys. 2022, 276, 125436. [Google Scholar] [CrossRef]
- YY/T 1819-2022; Dentistry—Films for Orthodontic Appliances. National Medical Products Administration: Beijing, China, 2022.
- GB 4789.2-2022; National Food Safety Standard – Microbiological Examination of Food: Determination of Aerobic Plate Count. National Health Commission: Beijing, China, 2022.
- Al Hawary, S.I.S.; Habash, R.T.; Abosaooda, M.; Hjazi, A.; Saleh, E.A.M.; Hassan, Z.F.; Bathaei, M.S. TiO2/PEG as smart anticorrosion and drug-eluting platforms in inflammatory conditions. Heliyon 2024, 10, e25605. [Google Scholar] [CrossRef] [PubMed]
- Al Zuhairy, S.A.K.S.; Elhabal, S.F.; Elrefai, M.F.M.; Hababeh, S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; Ewedah, T.M.; Mousa, I.S.; Hamdan, A.M.E. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a pH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals 2025, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Cursaru, L.M.; Iota, M.; Piticescu, R.M.; Tarnita, D.; Savu, S.V.; Savu, I.D.; Dumitrescu, G.; Popescu, D.; Hertzog, R.G.; Calin, M. Hydroxyapatite from Natural Sources for Medical Applications. Materials 2022, 15, 5091. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Ferrini, P.; Nguyen, D.; Zhang, Y.; Butler, J.M.; Hall, J.; Mistry, N. Integrating In Vitro Biopharmaceutics into Physiologically Based Biopharmaceutic Model (PBBM) to Predict Food Effect of BCS IV Zwitterionic Drug (GSK3640254). Pharmaceutics 2023, 15, 521. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Wang, J.; Zhang, Y.; Guo, P.; Lv, Y.; Ma, G.; Wei, W.; Wang, S. Versatile PLGA-Based Drug Delivery Systems for Tumor Immunotherapy. Small Methods 2025, 9, e2401623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).