Abstract
Epoxy asphalt is a superior polymer-modified asphalt material; however, significant differences in physicochemical properties, such as solubility parameters and dielectric constants, between epoxy resin and asphalt have led to compatibility issues that hinder its development. This study employed molecular dynamics simulations to investigate the effect of maleic anhydride-grafted styrene-butadiene-styrene (MAH-g-SBS) on the compatibility of epoxy asphalt. By analyzing parameters such as cohesive energy density, solubility parameters, energy distribution, interaction energy, radial distribution function, free volume fraction, and mean square displacement, the molecular mechanism underlying the enhanced compatibility was elucidated. The results indicate that the amphiphilic molecular structure of MAH-g-SBS significantly improves the thermodynamic compatibility between asphalt and epoxy resin, enhances interfacial affinity and stability, reduces the system’s total interaction and nonbonded energies, facilitates the dispersion and permeation of epoxy molecules into asphalt, and increases molecular mobility, thereby comprehensively enhancing the compatibility of the epoxy asphalt blend. Segregation tests and fluorescence microscopy further verified the simulation results, demonstrating that MAH-g-SBS improves the storage stability and phase uniformity of the epoxy asphalt system.
1. Introduction
Epoxy asphalt (EA) is a high-performance polymer-modified binder synthesized by blending epoxy resin, a curing agent, and base asphalt in defined proportions. Due to its excellent thermal stability and superior fatigue resistance, EA has been extensively applied in the construction of roads and bridges [1,2,3]. However, due to significant differences in physicochemical properties such as solubility parameters and dielectric constants between epoxy resin and asphalt [4], compatibility issues have become a major constraint in the development of epoxy asphalt. In recent years, researchers have attempted to improve compatibility through asphalt modification [5,6,7], curing agent modification [8,9], or the addition of compatibilizers. In recent years, long-chain fatty acids or their anhydrides, due to their good compatibility with asphalt, have been commonly used as curing agents for epoxy asphalt to address the compatibility issue between epoxy resin and asphalt [10]. The non-polar long-chain aliphatic end of such curing agents exhibits good compatibility with asphalt, while the polar carboxyl or anhydride end can react with the epoxy resin [11]. This amphiphilic molecular structure helps enhance compatibility between asphalt and epoxy resin, allowing asphalt to be more uniformly dispersed within the epoxy network. Chen et al. [12] reported that in a system utilizing an anhydride curing agent and epoxy soybean oil (ESO) as a compatibilizer, both the resin and ESO participated in the reaction, forming a polyester network through their epoxy functional groups. On the other hand, polymer modifiers have also proven effective in enhancing compatibility. Xu et al. [13] incorporated SBS as a modifier into epoxy asphalt to improve compatibility. The study demonstrated that SBS significantly enhances the phase-separated structure and interfacial compatibility of epoxy asphalt, thereby comprehensively improving its mechanical properties. Liu et al. [14] incorporated SBS-grafted carbon nanotubes (SBS-CNTs) into epoxy asphalt to explore the influence of varying epoxy resin contents on both the microstructure and overall performance of the material. Their findings revealed that at an epoxy resin content of 30%, SBS-CNTs were uniformly dispersed within the asphalt matrix and facilitated the formation of a three-dimensional, bi-continuous cross-linked network, thereby significantly enhancing the compatibility of the system. Notably, Cong et al. [15] demonstrated that maleic anhydride-grafted styrene-butadiene-styrene copolymer (SBS-g-MAH) significantly enhances the compatibility of SBS-modified asphalt, attributing this to the MAH groups strengthening polar interactions while the SBS backbone maintains non-polar compatibility.
Building upon this research background, this study hypothesizes that SBS-g-MAH combines the polar reactivity of anhydride-like substances (via MAH groups) with the non-polar compatibility and network-forming capability of SBS (via the SBS backbone), potentially serving as an efficient compatibilizer to synergistically enhance the interfacial interactions between epoxy resin and asphalt. However, the mechanism of action of SBS-g-MAH within the complex epoxy asphalt system, particularly how it influences interfacial compatibility at the molecular scale, requires in-depth investigation.
With the continuous advancement of computer technology, materials simulation methods have become increasingly diverse. Molecular dynamics simulation (MS) is a computational technique that models the motion of atoms and molecules by solving Newton’s equations of motion under defined force fields. It has been widely applied in materials science to investigate the microstructural evolution, interaction mechanisms, and thermomechanical properties of complex systems [16,17,18,19]. At present, molecular simulation methods are increasingly applied in the study of road materials, and have been utilized to investigate the self-healing behavior [20], regeneration capacity [21], and rheological viscosity characteristics [22] of asphalt. However, research on epoxy asphalt remains limited, mainly focusing on component transformation and compatibility. Li et al. [23] prepared epoxy asphalt using a binary acid/anhydride composite curing agent and investigated the effect of curing agent formulation on the performance of epoxy asphalt. The results demonstrated that optimizing the curing agent formulation significantly improves the low-temperature toughness of epoxy asphalt. The incorporation of anhydride-based curing agents can effectively disrupt the aggregated structure of asphaltenes while reducing the required dosage of epoxy resin. Liu et al. [23] integrated experimental and molecular simulation techniques to investigate the interfacial interactions between epoxy resin and asphalt in cold-mixed epoxy asphalt. Their simulation results showed that the interaction between asphalt and epoxy resin is mainly driven by hydrogen bonding and van der Waals forces, with the interfacial distance correlating positively with the reaction degree. Yi et al. [24] proposed an optimization approach for liquid asphalt mixtures using modified epoxy resin, and assessed the most suitable types of epoxy resin and curing agents through an enhanced Flory–Huggins model combined with quantum chemical methods.
Therefore, building upon previous research, this study employs molecular dynamics (MD) simulations to validate the hypothesis that SBS-g-MAH acts as an efficient compatibilizer with synergistic effects. Through quantitative analysis of key parameters—including cohesive energy density, solubility parameters, energy distribution profiles, interaction energy, radial distribution functions, fractional free volume, and mean square displacement—the mechanism by which SBS-g-MAH enhances epoxy asphalt compatibility is elucidated at the molecular scale. This work provides a theoretical basis for understanding its synergistic effects and guiding subsequent experimental design.
2. Experimental and Simulation Methods
2.1. Experimental
2.1.1. Materials
The base asphalt used in this study was a 70# penetration grade petroleum asphalt, obtained from Yueyang Chemical Co., Ltd., Yueyang, China. Its main physical properties are listed in Table 1. Epoxy resin E-51 was purchased from Nantong Xingchen Co., Ltd., Nantong, China, and the curing agent oleylamine was supplied by Jinan Jinyu Chemical Co., Ltd., Jinan, China. The SBS-g-MAH was purchased from Arkema Co., Ltd., Paris, France, with its key properties presented in Table 2.

Table 1.
Physical properties of base asphalt.

Table 2.
Properties of SBS-g-MAH.
2.1.2. Preparation of Epoxy Asphalt Samples
To compare the effect of MAH-g-SBS on the compatibility of epoxy asphalt, two types of epoxy asphalt materials were prepared: MAH-g-SBS modified epoxy asphalt (EA-30-MAH-g-SBS) and unmodified epoxy asphalt (EA-30). The mass ratio of epoxy resin to base asphalt was fixed at 3:7, while the curing agent and epoxy resin were mixed at a molar ratio of 1:1 between epoxy groups and active hydrogen atoms. The dosage of MAH-g-SBS was set at 5% of the asphalt. Prior to blending, each component was individually preheated: the epoxy resin and curing agent were heated to 60 °C, while the base asphalt was heated to 150 °C to ensure adequate fluidity. Subsequently, the curing agent, epoxy resin, and MAH-g-SBS modifier were sequentially added into the preheated base asphalt and mixed thoroughly at 150 °C using a high-shear mixer operating at 2000 rpm for 10 min to ensure homogeneous dispersion. The resulting blend was then subjected to a two-stage curing process: initial curing at 150 °C for 2 h, followed by post-curing at 60 °C for 48 h. Upon completion of the curing process, stable and uniform epoxy asphalt samples were obtained. These samples were prepared primarily to provide auxiliary experimental validation of the molecular dynamics simulation results. The preparation process is illustrated in Figure 1.
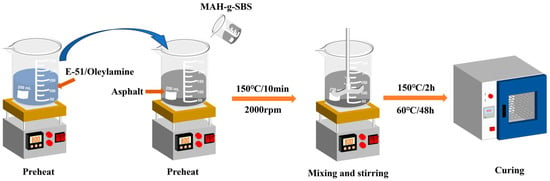
Figure 1.
Preparation process of epoxy asphalt samples.
2.1.3. Test Methods
Segregation softening point test: The prepared epoxy asphalt was heated until it reached a fully flowable state, after which approximately 50 g was poured into a standard segregation tube and placed in an oven at 163 °C for 48 h. After heating, the sample was stored in a refrigerator for 4 h and then removed for analysis. The sample was divided equally into three parts, and the top and bottom segments were subjected to softening point tests. The softening point difference between the upper and lower segments was then calculated.
Phase observation: The microstructure of the epoxy asphalt was observed using a fluorescence microscope. After shearing, an appropriate amount of epoxy asphalt was picked up using a glass rod and placed on a microscope slide. The prepared slide was then cured in an oven. Once cured, the sample was taken out and its microscopic morphology was examined under a fluorescence microscope.
2.2. Molecular Dynamics Simulation
2.2.1. Molecular Model Selection
Asphalt primarily consists of carbon and hydrogen, with trace amounts of sulfur, nitrogen, and oxygen. Based on its composition, asphalt is typically categorized into four fractions: saturates, aromatics, asphaltenes, and resins. The AAA-1 model, proposed by Li, has been shown to closely match the real asphalt system [25]. Consequently, this study utilizes the AAA-1 molecular model for simulations. The number of molecules for each component is listed in Table 3, and the molecular structures of the individual components are depicted in Figure 2.

Table 3.
Molecular model of asphalt and component proportions.
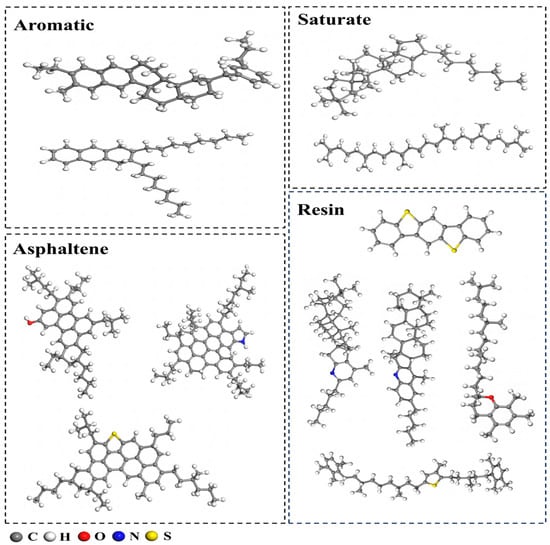
Figure 2.
Molecular models of asphalt components.
The epoxy resin used in this study is bisphenol A-type diglycidyl ether (E-51), the curing agent is oleylamine, and the modifier is maleic anhydride-grafted styrene-butadiene-styrene block copolymer (MAH-g-SBS). The molecular models of E-51, the curing agent, and the modifier are shown in Figure 3a, Figure 3b, and Figure 3c, respectively.
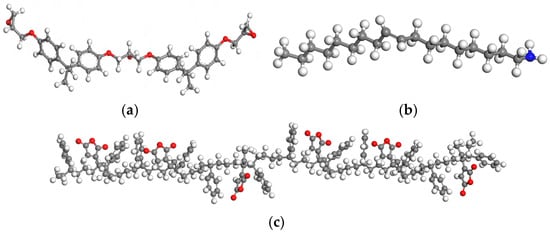
Figure 3.
Molecular models: (a) epoxy resin; (b) curing agent; (c) modifier.
2.2.2. Simulation Process
In this study, molecular dynamics (MD) simulations were conducted using Materials Studio 2017 software. Initially, molecular models of the asphalt, E-51, curing agent, and MAH-g-SBS were constructed based on their chemical structural formulas and subsequently optimized. Then, amorphous molecular systems of the polymers were generated using the Amorphous Cell module in Materials Studio. Four different models were built, including three MAH-g-SBS-modified epoxy asphalt systems with varying epoxy content (EA-10-MAH-g-SBS, EA-20-MAH-g-SBS, and EA-30-MAH-g-SBS), as well as one unmodified epoxy asphalt system (EA-30) used for comparison.
In this study, the initial density of each molecular model was set to 0.5 g/cm3 to ensure sufficient mobility of molecular chains at the early stage of simulation. This value balances avoiding excessive molecular crowding and maintaining computational efficiency. Structural optimization was performed using the Smart Minimizer method, a hybrid energy minimization algorithm that combines the steepest descent and conjugate gradient techniques to efficiently reduce the system’s potential energy and obtain stable molecular conformations [26]. The lowest-energy conformation obtained was selected for subsequent simulations. The simulation time consisted of 3000 ps NVT (constant volume and temperature) at 298 K and 3000 ps NPT (constant pressure and temperature) runs, respectively, to eliminate internal stresses and allow the system to reach thermodynamic equilibrium. Convergence was assessed by monitoring key thermodynamic and structural parameters, including total energy, temperature, density, and cohesive energy density, to ensure that equilibrium was achieved within this timescale. To regulate temperature and pressure, the Andersen and Berendsen thermostats were employed, respectively. The van der Waals interactions were treated using an atom-based method, while electrostatic forces were calculated using the Ewald summation approach. The Ewald summation is a classical technique that decomposes long-range electrostatic interactions into short-range real-space and long-range reciprocal-space components, enabling efficient and accurate calculation of Coulombic forces in periodic systems [27]. All molecular dynamics simulations were carried out utilizing the COMPASS II force field. Since the epoxy asphalt structure consists of a cross-linked network formed by the reaction between epoxy resin and curing agents, simulating this cross-linking process is crucial for accurately representing the molecular architecture. To achieve this, a Perl script was independently developed based on the well-established chemical reaction mechanism between epoxy resin and anhydride curing agents. The script dynamically identifies reactive sites and forms bonds when atomic distances fall below a gradually increased cutoff radius-from an initial 3 Å (typical bond length) up to 15 Å to realistically simulate the stepwise curing reaction. The simulation was performed at 398 K to reflect experimental curing conditions, facilitating molecular mobility and reaction kinetics. This approach is based on three key assumptions [28]: (1) crosslinking occurs primarily between epoxy groups and anhydride groups; (2) by-products from side reactions such as hydrocarbons and water are negligible under high-temperature curing; and (3) the reaction process is mainly diffusion-controlled. System stability and convergence were monitored throughout to ensure a reliable cross-linked molecular model.
Following the cross-linking process, further geometry optimization and MD simulations were performed to relieve the internal stresses generated during the reaction and to bring the system to thermodynamic equilibrium. A stable structural configuration was thus obtained. A schematic diagram of the EA-30-MAH-g-SBS model before and after cross-linking is shown in Figure 4, where newly formed bonds during the cross-linking reaction are highlighted in green using a ball-and-stick representation.
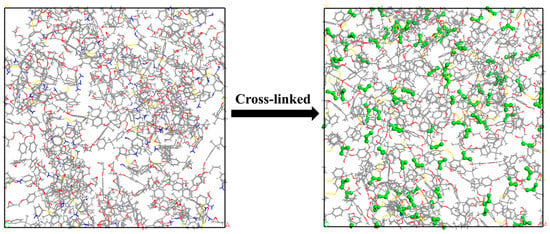
Figure 4.
Crosslinking process of epoxy asphalt molecular model.
3. Results and Discussion
3.1. Cohesive Energy Density and Solubility Parameter Analysis
Cohesive energy density (CED) quantifies the energy required to overcome intermolecular forces and vaporize one mole of condensed matter per unit volume during the evaporation process. CED serves as an indicator of the strength of intermolecular interactions and can be expressed by Equation (1) [29]:
where CED denotes the cohesive energy density, Ecoh is the cohesive energy, and V is the system volume.
The solubility parameter, which is the square root of the cohesive energy density, is widely used to assess the compatibility between different materials. A smaller discrepancy in solubility parameters suggests improved compatibility. Consequently, the compatibility of epoxy asphalt can be evaluated by comparing the differences in CED values between asphalt and epoxy resin. In this study, the CED values and corresponding solubility parameters for both the EA-30 and EA-30-MAH-g-SBS systems were calculated, as summarized in Table 4. The results indicate that the incorporation of MAH-g-SBS significantly reduces the solubility parameter disparity between asphalt and epoxy resin, thereby improving their compatibility. This enhancement is primarily attributed to the amphiphilic nature of MAH-g-SBS: the nonpolar SBS backbone exhibits strong affinity with the nonpolar components of asphalt, such as saturates and aromatics, through favorable van der Waals interactions and hydrophobic effects. Meanwhile, the polar maleic anhydride grafts on MAH-g-SBS are capable of forming hydrogen bonds, dipole–dipole interactions, and possibly covalent interactions with polar functional groups present in the epoxy resin. These dual affinities allow MAH-g-SBS to act as a molecular bridge at the interface between the two phases, effectively reducing interfacial tension and promoting molecular interpenetration. Moreover, the flexible polymer chains of SBS help improve the dispersion of epoxy resin molecules within the asphalt matrix by mitigating phase separation and promoting a more uniform microstructure [30]. Consequently, the introduction of MAH-g-SBS significantly enhances the thermodynamic compatibility and structural stability of the epoxy asphalt system.

Table 4.
CEDs and solubility parameters of EA-30 and EA-30-MAH-g-SBS.
3.2. Energy Distribution Analysis
In order to further examine the impact of MAH-g-SBS on the epoxy asphalt system’s compatibility, a comparative analysis of the total energy and its components for the EA-30 and EA-30-MAH-g-SBS systems was carried out. The results are presented in Table 5 and Figure 5. As shown in the energy comparison results in Table 5, the incorporation of MAH-g-SBS notably altered the internal energy profile of the epoxy asphalt system. In contrast to the unmodified EA-30 system, the total energy of the EA-30-MAH-g-SBS system decreased from 16,479.44 kcal/mol to 13,516.89 kcal/mol, a reduction of approximately 3000 kcal/mol, indicating a substantial enhancement in the thermodynamic stability of the system. In terms of energy composition, the incorporation of MAH-g-SBS notably reduced the valence energy (including bond stretching, angle bending, and torsional energies), suggesting that the presence of MAH-g-SBS strengthened the interfacial bonding between the epoxy resin and asphalt, thereby reducing interfacial shear stress and material separation. Simultaneously, the non-bonding energy decreased from −795.21 kcal/mol to −1127.12 kcal/mol, further indicating enhanced intermolecular interactions such as van der Waals forces and electrostatic interactions. These enhanced physical interactions stem from the amphiphilic MAH-g-SBS: the polar maleic anhydride grafts enhance hydrogen bonding and dipole interactions with epoxy resin, while the SBS backbone stabilizes nonpolar asphalt components. The simultaneous reduction in both valence and non-bonding energies demonstrates that MAH-g-SBS not only improves the chemical compatibility of the system but also promotes tighter physical integration, thereby effectively enhancing the overall compatibility and structural stability of the epoxy asphalt system [31].

Table 5.
Results of orthogonal experiment.
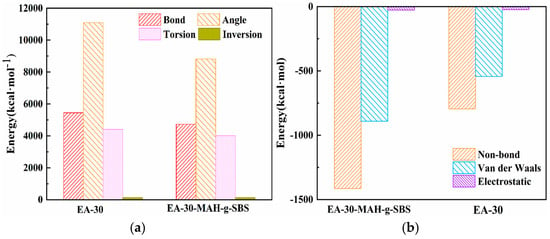
Figure 5.
The valence energy (a) and non-bond energy (b) of EA-30 and EA-30-MAH-g-SBS.
3.3. Interaction Energy Analysis
The interaction energy serves as a critical indicator for assessing the strength of intermolecular forces between different components within a molecular simulation system. A negative interaction energy indicates the presence of attractive forces within the system, which reflects favorable thermodynamic compatibility. The interaction energy of the EA-30 and EA-30-MAH-g-SBS epoxy asphalt systems was calculated using Equation (2) [32], and the results are presented in Table 6.
where Etotal denotes the total energy of the system, and Easphalt, Eepoxy, and EMAH-g-SBS represent the individual energies of asphalt, epoxy resin, and MAH-g-SBS, respectively.

Table 6.
The energy of EA-30 and EA-30-MAH-g-SBS.
As shown in Table 6, the energy of asphalt in the EA-30-MAH-g-SBS system exhibits minimal change compared to EA-30, indicating that the introduction of MAH-g-SBS exerts little influence on the intrinsic chemical properties of asphalt. However, the energy values of epoxy resin and MAH-g-SBS increase significantly, suggesting enhanced intermolecular interactions between these two components. This improvement can be primarily ascribed to the strong intermolecular forces—such as hydrogen bonding and dipole–dipole interactions—formed between the polar groups of maleic anhydrides and the functional groups present in the epoxy resin. Notably, the modified system EA-30-MAH-g-SBS exhibits a significantly negative interaction energy (−4161.32 kcal/mol), this dramatic shift evidences the formation of strong attractive forces due to MAH-g-SBS, which acts as an amphiphilic compatibilizer. Specifically, the polar maleic anhydride groups form hydrogen bonds and dipole–dipole interactions with epoxy resin, while the SBS backbone interacts hydrophobically with asphalt. This dual interaction network forms molecular “bridges” that reduce interfacial tension and enhance interphase adhesion. Consequently, the thermodynamic driving force for phase mixing is increased, inhibiting phase separation and promoting a more homogeneous epoxy asphalt microstructure [33].
Furthermore, the total energy of EA-30-MAH-g-SBS is markedly lower than that of EA-30, further confirming that the incorporation of MAH-g-SBS significantly enhances the overall stability of the epoxy asphalt system. This synergistic reduction in system energy not only strengthens chemical compatibility but also facilitates tighter physical interactions among components, thereby improving the overall performance and structural integrity of the epoxy/asphalt composite.
3.4. Radial Distribution Function Analysis
The radial distribution function (RDF) characterizes the likelihood of locating neighboring particles at a certain distance from a reference particle, thereby providing insight into the spatial arrangement and distribution of surrounding molecules. RDF is widely utilized to analyze molecular clustering, short-range order, and intermolecular interactions [34]. In this study, to elucidate how MAH-g-SBS influences the interaction strength and spatial homogeneity within the epoxy asphalt matrix, RDF profiles between key components and epoxy resin were computed for both the EA-30 and EA-30-MAH-g-SBS systems, as illustrated in Figure 6.
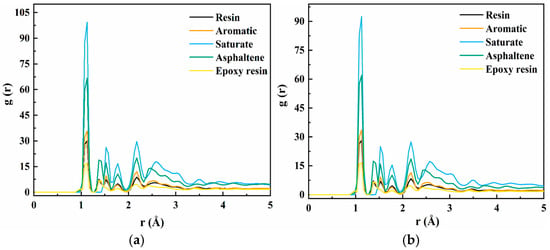
Figure 6.
RDF curves of EA-30 (a) and EA-30-MAH-g-SBS (b).
As illustrated in Figure 6, both systems exhibit comparable RDF curve patterns, suggesting that the incorporation of MAH-g-SBS has minimal impact on the fundamental molecular arrangement and structural organization of the asphalt matrix. However, at a more localized scale, the RDF intensities of aromatics, saturates, and resins are notably enhanced after the addition of MAH-g-SBS. This enhancement suggests stronger interactions between MAH-g-SBS and the primary components of asphalt, thereby improving internal compatibility within the system. This phenomenon can be attributed to the amphiphilic structure of the MAH-g-SBS molecule: the nonpolar SBS backbone interacts well with the nonpolar components in asphalt, while the polar maleic anhydride grafts can form hydrogen bonds or dipole–dipole interactions with polar functional groups in the epoxy resin [35].
In the region below 0.9 Å, the RDF value approaches zero, which is mainly due to strong van der Waals repulsion between atoms, preventing overlap. A prominent peak around 1.1 Å corresponds to covalent bonds between hydrogen and carbon atoms, while the peaks in the range of 1.41–1.6 Å represent covalent interactions between carbon atoms and other non-hydrogen atoms. Peaks beyond 1.8 Å arise from nonbonded interactions, such as van der Waals forces and dipole–dipole interactions, which dominate the system. Notably, at radial distances greater than 4 Å, the RDF value tends toward 1, indicating the absence of long-range order and thus confirming the amorphous nature of the epoxy asphalt system [36]. Additionally, the RDF peak intensity of saturates is generally higher than that of resins, suggesting that during the curing process of epoxy resin, molecular segments tend to migrate toward the saturates phase and interact preferentially. This may be due to the lower polarity and steric hindrance of saturates compared to resins, allowing easier contact and interaction with epoxy molecules.
3.5. Fractional Free Volume Analysis
In order to gain a clearer understanding of the molecular packing and spatial structure within epoxy asphalt systems, free volume theory was applied to investigate the impact of incorporating MAH-g-SBS on factors such as molecular packing density, chain mobility, and structural relaxation. This analysis seeks to uncover the mechanism by which MAH-g-SBS improves compatibility and modifies the system’s structural properties. According to free volume theory, a polymer’s total volume can be divided into two components: the occupied volume and the unoccupied (or free) volume. The free volume fraction (FFV), which quantifies the ratio of unoccupied space within the system, is calculated using Equation (3) [37].
where FFV represents the free volume fraction of epoxy asphalt, V0 denotes the occupied volume, and Vf is the free volume.
Figure 7a shows the visualized free volume of the EA-30-MAH-g-SBS systems. The gray cloud-like regions represent the surfaces of free volumes, the blue areas represent the free volumes themselves, and the remaining atoms are displayed using a ball-and-stick model. The variations in free volume for EA-30 and EA-MAH-g-SBS systems with different epoxy contents are shown in Figure 7b. As illustrated, the EA-30 system exhibits the lowest FFV at 21.16%, indicating a densely packed molecular structure with restricted chain segment mobility [38]. After the introduction of MAH-g-SBS, the FFV of EA-30-MAH-g-SBS increases to 22.21%, suggesting that the incorporation of MAH-g-SBS leads to partial relaxation of the network structure, thereby providing more space for chain segment motion. Furthermore, with increasing epoxy content, the FFV of the EA-MAH-g-SBS system shows a clear upward trend. This enhancement is attributed to the flexible SBS segments and the reactive MAH functional groups, which disrupt the original densely cross-linked structure, making the system more disordered and favorable for the formation of free volume [39].
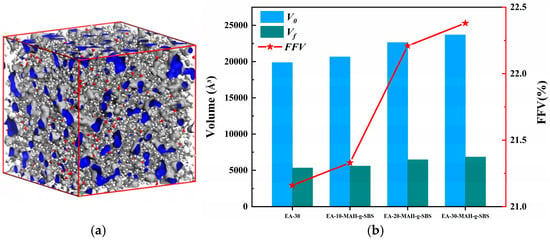
Figure 7.
Visualization of free volume for EA-30-MAH-g-SBS (a) and the trend of free volume variation for epoxy asphalts (b).
3.6. Mean Square Displacement Analysis
Mean square displacement (MSD) is used to evaluate the extent of molecular movement within a system and serves as a key parameter for characterizing the mobility of particles. The MSD is calculated using Equation (4) [40].
where Ri(0) and Ri(t) represent the position vectors of any atom i in the system at the initial time and after elapsed time t, respectively; N is the total number of atoms included in the calculation; and the angle brackets denote the average value.
To investigate the influence of MAH-g-SBS incorporation and variations in epoxy content on the molecular chain mobility within the system, MSD values were calculated for EA-30 and EA-MAH-g-SBS with different epoxy contents under the NVT ensemble at 300 ps. The results are shown in Figure 8. As observed from the figure, the MSD curve of the EA-30 system exhibits the lowest slope, indicating the strongest restriction on chain mobility. This is mainly attributed to its dense cross-linked network structure, which effectively suppresses molecular chain displacement [41]. After introducing MAH-g-SBS, the slope of the MSD curve for EA-30-MAH-g-SBS increases slightly, suggesting that the addition of the compatibilizer partially disrupts the rigid network and provides more degrees of freedom for local chain movement. As the epoxy content decreases, the MSD slopes of EA-20-MAH-g-SBS and EA-10-MAH-g-SBS increase sequentially, indicating enhanced molecular mobility. However, the slope for EA-20-MAH-g-SBS remains slightly lower than that of EA-10-MAH-g-SBS, suggesting that although chain mobility is improved, it is still partially constrained by the residual cross-linked network. This may be due to the relatively higher epoxy content in EA-20-MAH-g-SBS, which helps maintain a certain degree of structural integrity while enhancing chain flexibility.
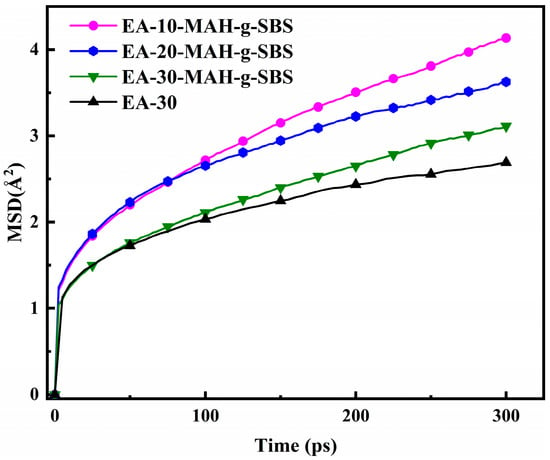
Figure 8.
MSD curves of EA-30 and EA-MAH-g-SBS systems with different epoxy contents.
3.7. Segregation Resistance Performance Analysis
To verify the effect of MAH-g-SBS incorporation on the compatibility of epoxy asphalt, segregation tests were conducted, and the softening point differences of EA-30 and EA-30-MAH-g-SBS epoxy asphalt samples were calculated. The results are shown in Figure 9. As illustrated in the figure, the unmodified EA-30 system exhibited a large softening point difference of 5.4 °C between the upper and lower parts, indicating a pronounced phase separation between the epoxy resin and asphalt, and thus poor compatibility. However, with the incorporation of MAH-g-SBS, the softening point difference significantly decreased to 0.8 °C, demonstrating a marked improvement in the compatibility and storage stability of the epoxy asphalt system.
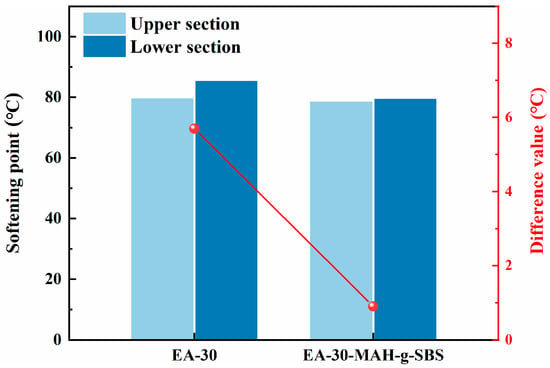
Figure 9.
Softening point test results of segregation experiment.
3.8. Microscopic Analysis
The dispersion of the epoxy phase in EA-30 and EA-30-MAH-g-SBS was observed using fluorescence microscopy, as shown in Figure 10. It is evident from the images that the epoxy phase in EA-30 exhibits uneven particle sizes and poor dispersion. However, after the addition of MAH-g-SBS, the epoxy phase in EA-30-MAH-g-SBS becomes more uniform and is evenly distributed within the asphalt phase. This indicates that the incorporation of MAH-g-SBS enhances the dispersion of the epoxy resin and promotes better compatibility between the epoxy resin and asphalt.
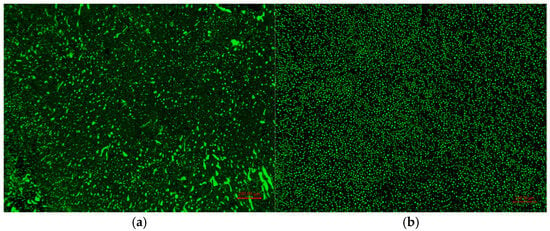
Figure 10.
Fluorescence images of EA-30 (a) and EA-30-MAH-g-SBS (b).
4. Conclusions
In this study, MD simulations were employed to systematically investigate the effect of MAH-g-SBS on the compatibility between epoxy resin and base asphalt. By analyzing a series of thermodynamic and structural parameters—including cohesive energy density, solubility parameter, energy distribution, interaction energy, RDF, FFV, and MSD, the enhancement mechanisms of compatibility at the molecular level were revealed. The main conclusions are summarized as follows:
- (1)
- The amphiphilic molecular structure of MAH-g-SBS significantly improves the thermodynamic compatibility between asphalt and epoxy resin, as evidenced by the reduced solubility parameter differences and interfacial energy, thereby enhancing the interfacial affinity and stability of the system;
- (2)
- The introduction of MAH-g-SBS reduces the total interaction energy and non-bonded energy of the epoxy asphalt system, indicating stronger intermolecular interactions and a more tightly bonded interface, which contributes to the formation of a more stable and compatible blend at the molecular level;
- (3)
- RDF and FFV analyses reveal that MAH-g-SBS promotes better dispersion and interpenetration between epoxy and asphalt molecules. It enhances the interactions between epoxy resin and polar components in asphalt such as asphaltenes and aromatics, thereby improving phase homogeneity and increasing free volume;
- (4)
- The presence of MAH-g-SBS also facilitates molecular mobility, as demonstrated by an increase in MSD, which helps improve the flexibility and diffusion capacity of the system and promotes stress relaxation at the interface, thus further enhancing the overall compatibility of the epoxy asphalt blend;
- (5)
- Segregation tests and fluorescence microscopy confirmed that MAH-g-SBS significantly improves the storage stability and phase uniformity of the epoxy asphalt system, which is consistent with the molecular-level predictions.
Compared with existing studies on compatibilizers in epoxy asphalt systems, this work provides a systematic and comprehensive molecular-level elucidation of the synergistic compatibilization mechanism of MAH-g-SBS, offering a theoretical basis for subsequent formulation optimization and performance evaluation. This study advances the understanding of compatibility mechanisms in epoxy asphalt materials. The next phase of research will focus on experimental validation of the simulation results and clarification of the actual enhancement effects of MAH-g-SBS on the mechanical properties, thermal stability, and phase behavior of epoxy asphalt systems.
Author Contributions
Conceptualization, P.L. and B.S.; methodology, B.S.; software, J.W.; validation, J.W. and B.T.; formal analysis, B.W.; investigation, J.W. and B.T.; resources, B.S.; data curation, P.L.; writing—original draft preparation, P.L., B.W. and B.T.; writing—review and editing, B.S.; visualization, J.W.; supervision, K.N.; project administration, K.L.; funding acquisition, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program Project (No. 2022YFB2602605), the National Natural Science Foundation of China General Project (No. 52178440), the Science and Technology Plan of Xizang Autonomous Region (No. XZ.202401jD0019), and the Chongqing Municipal Natural Science Foundation General Project (CSTB2024NSCQ-MSX0097).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, S.; Zhang, H.L.; Zhou, M.Y.; Ye, J. Investigation on the influence of gradation design on the road performance of cold mixed epoxy asphalt mixture based on microstructure evaluation. J. Mater. Res. Technol. 2024, 30, 2018–2028. [Google Scholar] [CrossRef]
- Wang, Q.C.; Min, Z.H.; Huang, W.; Chen, F. Effects of thermal-oxidative aging on mechanical behavior and microstructure of epoxy asphalt binder containing different asphalt contents. Constr. Build. Mater. 2024, 442, 137569. [Google Scholar] [CrossRef]
- Shan, B.L.; Cao, X.J.; Hao, Z.H.; Wei, K.L.; Tang, B.M. Synthesis of a novel curing agent containing dynamic disulfide bonds and the preparation of repairable epoxy vitrimer modified asphalt. Constr. Build. Mater. 2025, 463, 140053. [Google Scholar] [CrossRef]
- Abbas, H.A.; Manasrah, A.D.; Carbognani, L.; Sebakhy, K.O.; El Nokab, M.E.; Hacini, M.; Nassar, N.N. A study on the characteristics of Algerian Hassi-Messaoud asphaltenes: Solubility and precipitation. Pet. Sci. Technol. 2022, 40, 1279–1301. [Google Scholar] [CrossRef]
- Wang, J.Y.; Si, J.J.; Yu, X.; Jiang, Z.Q.; Zhang, M.Z.; Ding, G.Y.; Huang, J.L. Enhancing the compatibility of cold-mixed epoxy asphalt binder via graphene oxide grafted plant oil-based materials. J. Clean. Prod. 2023, 418, 138209. [Google Scholar] [CrossRef]
- Lu, Q.; Bors, J. Alternate uses of epoxy asphalt on bridge decks and roadways. Constr. Build. Mater. 2015, 78, 18–25. [Google Scholar] [CrossRef]
- Li, M.Y.; Min, Z.H.; Wang, Q.C.; Huang, W.; Shi, Z.Y. Influence of curing agent ratio, asphalt content and crosslinking degree on the compatibility and component distribution of epoxy asphalt in compound curing agent system. Int. J. Pavement Eng. 2023, 24, 2136375. [Google Scholar] [CrossRef]
- Gong, J.; Jing, F.; Zhao, R.K.; Li, C.X.; Cai, J.; Wang, Q.J.; Xie, H.F. Waste Cooking Oil-Modified Epoxy Asphalt Rubber Binders with Improved Compatibility and Extended Allowable Construction Time. Molecules 2022, 27, 7061. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yu, X.; Si, J.J.; Shao, X.Y.; Zhao, S.; Ding, G.Y. Tailoring compatibility and mechanical properties of cold-mixed epoxy asphalt via external epoxy group content manipulation. Constr. Build. Mater. 2024, 414, 134948. [Google Scholar] [CrossRef]
- Xiang, Q.; Xiao, F.P. Applications of epoxy materials in pavement engineering. Constr. Build. Mater. 2020, 235, 117529. [Google Scholar] [CrossRef]
- Wang, Q.C.; Min, Z.H.; Wong, Y.D.; Shi, Z.Y.; Huang, W. Aging degradation of anhydride-cured epoxy asphalt binder subjected to ultraviolet exposure. Int. J. Pavement Eng. 2023, 24, 2171037. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.T.; Wu, J.H.; Ma, L.J.; Finlow, D.E.; Lin, S.Q.; Song, K.M. Thermal and mechanical properties of epoxy resin toughened with epoxidized soybean oil. J. Therm. Anal. Calorim. 2013, 113, 939–945. [Google Scholar] [CrossRef]
- Xu, P.J.; Zhu, Z.; Wang, Y.D.; Cong, P.L.; Li, D.G.; Hui, J.Z.; Ye, M. Phase structure characterization and compatibilization mechanism of epoxy asphalt modified by thermoplastic elastomer (SBS). Constr. Build. Mater. 2022, 320, 126262. [Google Scholar] [CrossRef]
- Liu, P.; Niu, K.M.; Tian, B.; Wang, M.; Wan, J.X.; Gong, Y.; Wang, B.B. Improving the Compatibility of Epoxy Asphalt Based on Poly(styrene-butadiene-styrene)-Grafted Carbon Nanotubes. Coatings 2025, 15, 314. [Google Scholar] [CrossRef]
- Cong, P.L.; Chen, S.F.; Chen, H.X. Preparation and Properties of Bitumen Modified with the Maleic Anhydride Grafted Styrene-Butadiene-Styrene Triblock Copolymer. Polym. Eng. Sci. 2011, 51, 1273–1279. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.F.; Xu, M.; Ji, J.; Dai, Q.L.; You, Z.P. Discussion on molecular dynamics (MD) simulations of the asphalt materials. Adv. Colloid Interface Sci. 2022, 299, 102565. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Q.; Qiu, X.; Xu, W.Y.; Xiao, S.L.; Gu, Y.; Ye, Y.C. An investigation toward adhesion characteristics of emulsified asphalt residue-aggregate interface through MD simulation. Constr. Build. Mater. 2024, 438, 137251. [Google Scholar] [CrossRef]
- Hu, M.J.; Sun, D.Q.; Hofko, B.; Sun, Y.R.; Mirwald, J.; Xu, L. Multiscale optimization on polymer-based rejuvenators for the efficient recycling of aged high-viscosity modified asphalt: Molecular dynamics simulation and experimental analysis. J. Clean. Prod. 2024, 449, 141736. [Google Scholar] [CrossRef]
- Tang, Y.J.; Fu, Z.; Raos, G.; Ma, F.; Zhao, P.; Hou, Y.J. Molecular dynamics simulation of adhesion at the asphalt-aggregate interface: A review. Surf. Interfaces 2024, 44, 103706. [Google Scholar] [CrossRef]
- He, L.; Zheng, Y.F.; Alexiadis, A.; Falchetto, A.C.; Li, G.N.; Valentin, J.; Van den Bergh, W.; Vasiliev, Y.E.; Kowalski, K.J.; Grenfell, J. Research on the self-healing behavior of asphalt mixed with healing agents based on molecular dynamics method. Constr. Build. Mater. 2021, 295, 103706. [Google Scholar] [CrossRef]
- Yan, S.; Dong, Q.; Chen, X.Q.; Wang, X.; Shi, B.; Yao, K. Study on inherent characteristics of asphalt regenerating agents: Insights from density functional theory calculations and molecular dynamics simulations. Constr. Build. Mater. 2024, 411, 134390. [Google Scholar] [CrossRef]
- Li, G.N.; Tan, Y.Q.; Fu, Y.K.; Liu, P.F.; Fu, C.L.; Oeser, M. Density, zero shear viscosity and microstructure analysis of asphalt binder using molecular dynamics simulation. Constr. Build. Mater. 2022, 345, 128332. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, G.Y.; Zhang, Z.Y.; Fu, C.L.; Oeser, M. Investigation on bitumen-epoxy interface in cold mixed epoxy bitumen using experimental observation and molecular dynamics simulation. Constr. Build. Mater. 2021, 303, 124490. [Google Scholar] [CrossRef]
- Abdukadir, A.; Pei, Z.S.; Yu, W.; Wang, J.M.; Chen, A.L.; Tang, K.; Yi, J.Y. Performance optimization of epoxy resin-based modified liquid asphalt mixtures. Case Stud. Constr. Mater. 2022, 17, e01598. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nourian, P.; Wasiuddin, N.; Peters, A. Investigation of Polymer-Asphalt Compatibility Using Molecular Dynamics Simulation. J. Phys. Chem. B 2024, 128, 4821–4829. [Google Scholar] [CrossRef]
- Wei, K.L.; Su, Y.; Cao, X.J.; Jiang, T.Q.; Deng, M.; Wu, Y. Molecular Dynamics Simulation of Interaction between Polymer Modifier and Asphalt. J. Test. Eval. 2022, 50, 2175–2189. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tanaka, K. Molecular Dynamics Simulation of Cross-linked Epoxy Resins: Past and Future. Macromol. Rapid Commun. 2025, 46, e2400978. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.T.; Feng, Y.P.; Hang, Y.T. Effect of diffusion on interfacial properties of polyurethane-modified asphalt-aggregate using molecular dynamic simulation. Rev. Adv. Mater. Sci. 2022, 61, 778–794. [Google Scholar] [CrossRef]
- Li, N.; Liu, Z.Z.; Yin, J.Q.; Zhang, H.; Dou, H.; Li, B. Investigation of Compatibility Mechanisms and Diffusion Behavior of Polymer SBS-Modified Asphalt Compatibilizer Using Molecular Dynamics Simulation. Materials 2025, 18, 2238. [Google Scholar] [CrossRef]
- Li, M.Y.; Min, Z.H.; Wang, Q.C.; Huang, W.; Shi, Z.Y. Effect of epoxy resin content and conversion rate on the compatibility and component distribution of epoxy asphalt: A MD simulation study. Constr. Build. Mater. 2022, 319, 126050. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Liang, M. Molecular simulation and experimental analysis of interaction and compatibility between asphalt binder and Styrene-Butadiene-Styrene. Constr. Build. Mater. 2022, 342, 128028. [Google Scholar] [CrossRef]
- Shan, B.; Cao, X.; Hao, Z.; Wu, Y.; Yang, X.; Li, X. Experimental and Molecular Dynamics Simulations Investigating the Flexibility and Compatibility of Epoxidized Soybean Oil-Reinforced Epoxy Asphalt. J. Mater. Civ. Eng. 2024, 36, 04024327. [Google Scholar] [CrossRef]
- Xu, Z.H.; Xiong, Z.J.; Gong, M.H.; Zeng, Q.; Hong, J.X.; Fan, J. Molecular dynamics-based study of the modification mechanism of asphalt by graphene oxide. J. Mol. Model. 2023, 29, 368. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, Z.P.; Wang, L.; Sun, J.; Wang, Z.F.; Liu, H.; Chen, L.Q. Study on the compatibility between polyurethane and asphalt based on experiment and molecular dynamics simulation. Case Stud. Constr. Mater. 2022, 17, e01424. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Wang, Z.F.; Zhang, S.Y.; Liu, H.; Guo, Y.X.; Liu, X.S.; Tian, P.J.; Yang, Y.; Xia, J.J. Experimental studies and molecular dynamics simulation of the compatibility between thermoplastic polyurethane elastomer (TPU) and asphalt. Constr. Build. Mater. 2024, 411, 134316. [Google Scholar] [CrossRef]
- Gao, L.; Ji, X.; Tan, Y.W.; Wang, Z.Q.; Zhang, Y.; Kong, H.M. Molecular dynamics simulation of interfacial adhesion behavior between waterborne epoxy resin emulsified asphalt and aggregate. Compos. Interfaces 2023, 30, 749–770. [Google Scholar] [CrossRef]
- Jiao, B.Z.; Pan, B.F.; Che, T.K. Evaluating impacts of desulfurization and depolymerization on thermodynamics properties of crumb rubber modified asphalt through molecular dynamics simulation. Constr. Build. Mater. 2022, 323, 126360. [Google Scholar] [CrossRef]
- Chen, F.; Min, Z.H.; Li, M.Y.; Huang, W.; Wang, Q.C. Molecular dynamics study of cross-linking degrees effect on the aging resistance of epoxy asphalt: Insights from oxygen diffusion. J. Appl. Polym. Sci. 2024, 141, e55908. [Google Scholar] [CrossRef]
- Li, M.Y.; Min, Z.H.; Ouyang, H.; Chen, F.; Huang, W. The effect of composite curing agent ratio on the photooxidative aging behavior of epoxy asphalt based on molecular dynamics simulation. Constr. Build. Mater. 2023, 409, 134157. [Google Scholar] [CrossRef]
- Wang, Z.K.; Lv, Q.; Chen, S.H.; Li, C.L.; Sun, S.Q.; Hu, S.Q. Effect of Interfacial Bonding on Interphase Properties in SiO2/Epoxy Nanocomposite: A Molecular Dynamics Simulation Study. Acs Appl. Mater. Interfaces 2016, 8, 7499–7508. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).