Enhanced Energy Storage Properties of Ba0.96Ca0.04TiO3 Ceramics Through Doping Bi(Li1/3Zr2/3)O3
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization Techniques
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Guo, B.; Jin, F.; Li, L.; Pan, Z.Z.; Xu, X.W.; Wang, H. Design strategies of high-performance lead-free electroceramics for energy storage applications. Rare Met. 2024, 43, 853–878. [Google Scholar] [CrossRef]
- Wang, T.; Jin, L.; Tian, Y.; Shu, L.; Hu, Q.; Wei, X. Microstructure and ferroelectric properties of Nb2O5-modified BiFeO3-BaTiO3 lead-free ceramics for energy storage. Mater. Lett. 2014, 137, 79–81. [Google Scholar] [CrossRef]
- Lee, M.H.; Choi, H.I.; Kim, D.J.; Kim, J.S.; Song, T.K. Hard piezoelectric properties of lead-free BiFeO3–BaTiO3 ceramics. J. Am. Ceram. Soc. 2024, 107, 244–252. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Wang, Y.; Qin, H.; Zhao, J.; Lu, Z.; Mao, Z.; Wang, D. Microstructure, dielectric, and piezoelectric properties of BiFeO3–SrTiO3 lead-free ceramics. J. Am. Ceram. Soc. 2024, 107, 205–213. [Google Scholar] [CrossRef]
- Yan, F.; Qian, J.; Lin, J.; Ge, G.; Shi, C.; Zhai, J. Ultrahigh Energy Storage Density and Efficiency of Lead-Free Dielectrics with Sandwich Structure. Small 2024, 20, 2306803. [Google Scholar] [CrossRef]
- Lin, J.; Lv, F.; Hong, Z.; Liu, B.; Wu, Y.; Huang, Y. Ultrahigh Piezoelectric Response Obtained by Artificially Generating a Large Internal Bias Field in BiFeO3–BaTiO3 Lead-Free Ceramics. Adv. Funct. Mater. 2024, 34, 2313879. [Google Scholar] [CrossRef]
- Xu, L.; Lin, J.; Yang, Y.; Zhao, Z.; Shi, X.; Ge, G.; Qian, J.; Shi, C.; Li, G.; Wang, S.; et al. Ultrahigh thermal stability and piezoelectric of lead-free KNN-based texture piezoceramics. Nat. Commun. 2024, 15, 9018. [Google Scholar] [CrossRef]
- Jain, A.; Wang, Y.G.; Guo, H. Microstructural properties and ultrahigh energy storage density in Ba0.9Ca0.1TiO3-NaNb0.85Ta0.15O3 relaxor ceramics. Ceram. Int. 2020, 46, 24333–24346. [Google Scholar] [CrossRef]
- Zhang, L.; Thakur, O.P.; Feteira, A. Comment on the use of calcium as a dopant in X8R BaTiO3-based ceramics. Appl. Phys. Lett. 2007, 90, 142914. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Riggs, B.C.; Chrisey, D.B.; Katiyar, R.S. Investigations on structure; ferroelectric, piezoelectric and energy storage properties of barium calcium titanate (BCT) ceramics. J. Alloys Compd. 2014, 584, 369–373. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Shi, M.Q.; Yu, L.J.; Chen, P.; Yu, K.; Yan, Y.; Jin, L.; Wang, D.W.; Gao, J.H. An investigation of the dielectric energy storage performance of Bi(Mg2/3Nb1/3)O3-modifed BaTiO3 Pb-free bulk ceramics with improved temperature/frequency stability. Ceram. Int. 2019, 45, 19189–19196. [Google Scholar] [CrossRef]
- Jiang, X.W.; Hao, H.; Zhang, S.J.; Lv, J.H.; Cao, M.H.; Yao, Z.H.; Liu, H.X. Enhanced energy storage and fast discharge properties of BaTiO3 based ceramics modified by Bi(Mg1/2Zr1/2)O3. J. Eur. Ceram. Soc. 2019, 39, 1103–1109. [Google Scholar] [CrossRef]
- Hou, D.; Usher, T.M.; Zhou, H.; Raengthon, N.; Triamnak, N.; Cann, D.P.; Forrester, J.S.; Jones, J.L. Temperature-induced local and average structural changes in BaTiO3-xBi(Zn1/2Ti1/2)O3 solid solutions: The origin of high temperature dielectric permittivity. J. Appl. Phys. 2017, 122, 064103. [Google Scholar] [CrossRef]
- Zeb, A.; Milne, S.J. Stability of High temperature dielectric properties for (1-x)Ba0.8Ca0.2TiO3-xBi(Mg0.5Ti0.5)O3 ceramics. J. Am. Ceram. Soc. 2013, 96, 2887–2892. [Google Scholar] [CrossRef]
- Shamim, M.K.; Sharma, S.; Singh, A.; Rai, R.; Rani, R. Study of the structural and electrical behavior of Bi(Mg,Ti)O3 modified (Ba,Ca)TiO3 ceramics. J. Adv. Dielectr. 2016, 6, 1650035. [Google Scholar] [CrossRef]
- Xu, M.X.; Peng, B.L.; Zhu, J.N.; Liu, L.J.; Sun, W.H.; Leighton, G.J.T.; Shaw, C.; Luo, N.N.; Zhang, Q. Enhanced energy storage performance of (1-x)(BCT-BMT)-xBFO lead-free relaxor ferroelectric ceramics in a broad temperature range. J. Alloys Compd. 2019, 789, 303–312. [Google Scholar] [CrossRef]
- Yu, S.H.; Zhang, C.M.; Wu, M.Y.; Dong, H.L.; Li, L.X. Ultra-high energy density thin-film capacitors with high power density using BaSn0.15Ti0.85O3/Ba0.6Sr0.4TiO3 heterostructure thin films. J. Power Sources 2019, 412, 648–654. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Jin, L.; Hu, Q.Y.; Tian, Y.; Hou, L.; Yu, K.; Zhang, L.; Wei, X.Y.; Yan, Y.; et al. Ultra-low hysteresis electro-strictive strain with high thermal stability in Bi(Li0.5Nb0.5)O3-modified BaTiO3 lead-free ferroelectrics. J. Alloys Compd. 2018, 753, 558–565. [Google Scholar] [CrossRef]
- Gong, Y.Y.; He, X.; Chen, C.; Yi, Z.G. Composition-dependent phase evolution and enhanced electro-strain properties of (Bi0.5Na0.5)TiO3-BaTiO3-Bi(Li0.5Ta0.5)O3 lead-free ceramic. J. Alloys Compd. 2020, 818, 152822. [Google Scholar] [CrossRef]
- Noma, T.; Wada, S.; Yano, M.; Suzuki, T. Analysis of lattice vibration in fine particles of barium titanate single crystal including the lattice hydroxyl group. J. Appl. Phys. 1996, 80, 5223–5233. [Google Scholar] [CrossRef]
- Peng, B.; Xie, Z.K.; Yue, Z.X.; Li, L.T. Improvement of the recoverable energy storage density and efficiency by utilizing the linear dielectric response in ferroelectric capacitors. Appl. Phys. Lett. 2014, 105, 052904. [Google Scholar] [CrossRef]
- Phani, K.K. A New Modified Weibull Distribution Function. J. Am. Ceram. Soc. 1987, 70, 182–184. [Google Scholar] [CrossRef]
- Si, F.; Tang, B.; Fang, Z.X.; Li, H.; Zhang, S. A new type of BaTiO3-based ceramics with Bi(Mg1/2Sn1/2)O3 modification showing improved energy storage properties and pulsed discharging performances. J. Alloys Compd. 2020, 819, 153004. [Google Scholar] [CrossRef]
- Huang, N.; Liu, H.X.; Hao, H.; Yao, Z.H.; Cao, M.H.; Xie, J. Enengy storage properties of MgO-doped 0.5Bi0.5Na0.5TiO3-0.5SrTiO3 ceramics. Ceram. Int. 2019, 45, 14921–14927. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, L.X.; Li, W.B.; Zhou, D. Extreme high energy efficiency in perovskite structure (1-x)(Ba0.8Sr0.2)TiO3-xBi(Zn2/3Nb1/3)O3 (0.04 ≤ x ≤ 0.16) ceramics. J. Eur. Ceram. Soc. 2020, 40, 3343–3347. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Guo, B.; Tang, M.Y.; Li, Q.; Dong, J.; Yu, L.J.; Yu, K.; Yan, Y.; Wang, D.W.; et al. Ultrahigh dielectric breakdown strength and excellent energy storage performance in lead-free barium titanate-based relaxor ferroelectric ceramics via a combined strategy of composition modification viscous polymer processing, and liquid-phase sintering. Chem. Eng. J. 2020, 398, 125625. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Cao, W.J.; Cheng, C.; Huang, S.G.; Wang, C.C. A-/B-site co-doping towards outstanding energy-storage performance in BaTiO3-based low-entropy ceramics. J. Energy Storage 2025, 114, 115865. [Google Scholar] [CrossRef]
- Gong, Y.Q.; Tang, Z.L.; Tang, Z.Y.; Zhao, C.X.; Zu, D.; Liu, Y.Y.; Zhang, D. Significantly improved energy storage performance of Na0.5Bi0.5TiO3–BaTiO3 ferroelectric ceramics with a wide temperature range via high-entropy doping. Ceram. Int. 2024, 50, 42904–42912. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Xie, S.; Leng, J.; Zhu, Q.; Li, K.; Gong, W.; Zhu, J.; Wang, Q. High entropy strategy for improved mechanical and energy storage properties in BaTiO3-based ceramics. ACS Appl. Mater. Interfaces 2024, 16, 12521–12533. [Google Scholar] [CrossRef]
- Zhang, J.B.; Pu, Y.P.; Hao, Y.X.; Han, Y.; Ning, Y.T.; Zhang, L.; Wang, B. Achieving superior energy storage density in BiFeO3-BaTiO3 based relaxor ceramics via configurational entropy tunning and phase structure evolution. Ceram. Int. 2025, 51, 4048–4057. [Google Scholar] [CrossRef]
- Lin, W.C.; Ren, P.R.; Wan, Y.H.; Yang, S. Dielectric properties, electrical and thermal conductivity of BaTiO3 and SrTiO3-based high-entropy ceramics. J. Alloys Compd. 2024, 1004, 175915. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Cai, Z.M.; Li, L.T.; Wang, X.H. High energy density, high efficiency and excellent temperature stability of lead free Mn-doped BaTiO3-Bi(Mg1/2Zr1/2)O3 ceramics sintered in a reducing atmosphere. J. Alloys Compd. 2023, 816, 152498. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, H.; Peng, F.; Zhang, W.; Su, C.; Guo, Q. Defect evolution and effect on structure and electric properties of A/B site Sm doped BaTiO3 sintered in different atmospheres. J. Alloys Compd. 2023, 945, 169211. [Google Scholar] [CrossRef]
- Bi, Z.W.; Zhou, S.M.; Ye, J.P.; Wang, N.; Shang, F.; Xu, J.W.; Wang, H. BaTiO3-based high-entropy ceramics for enhanced capacitive energy storage performance. Ceram. Int. 2025, 51, 16052–16060. [Google Scholar] [CrossRef]

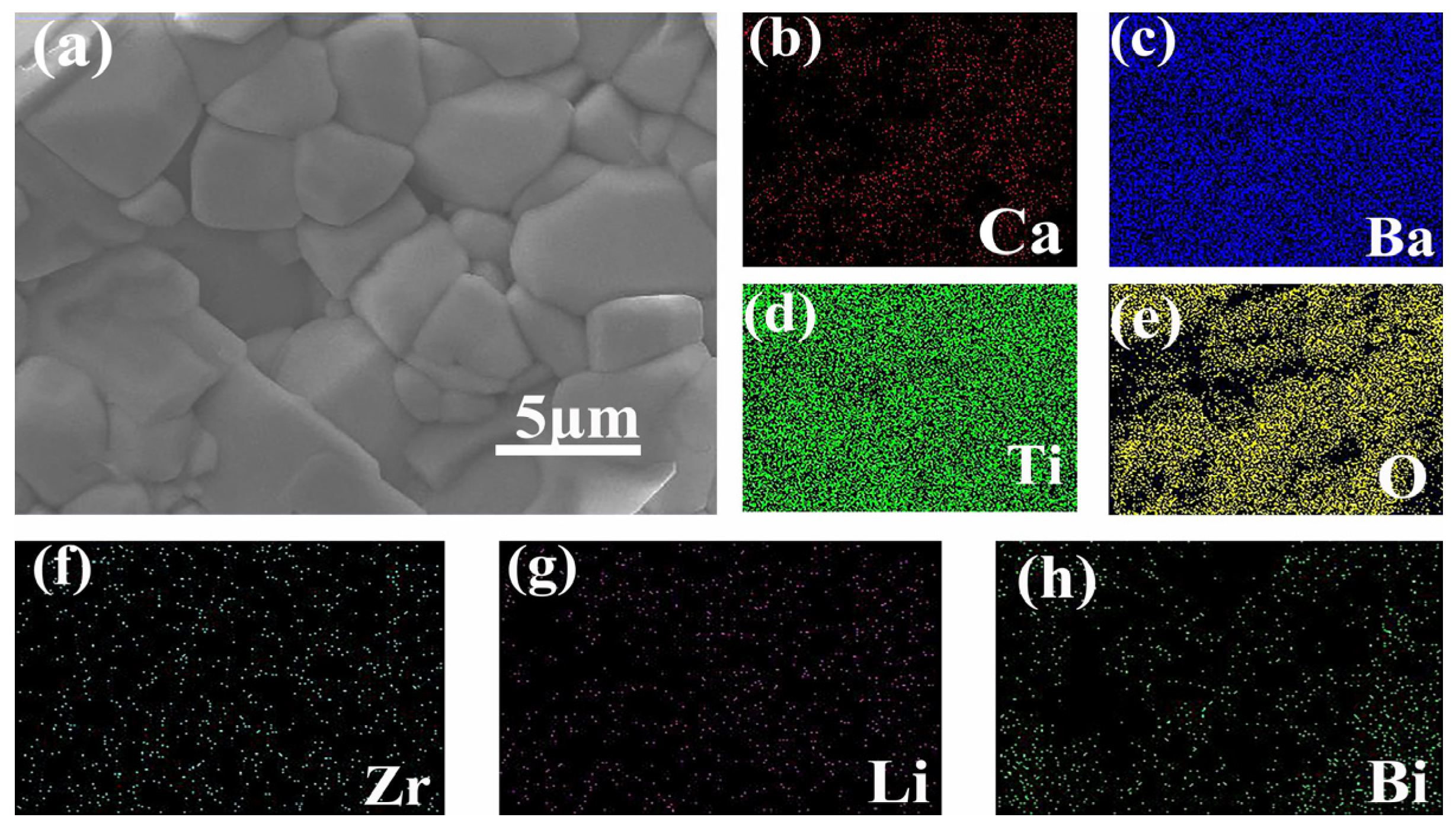
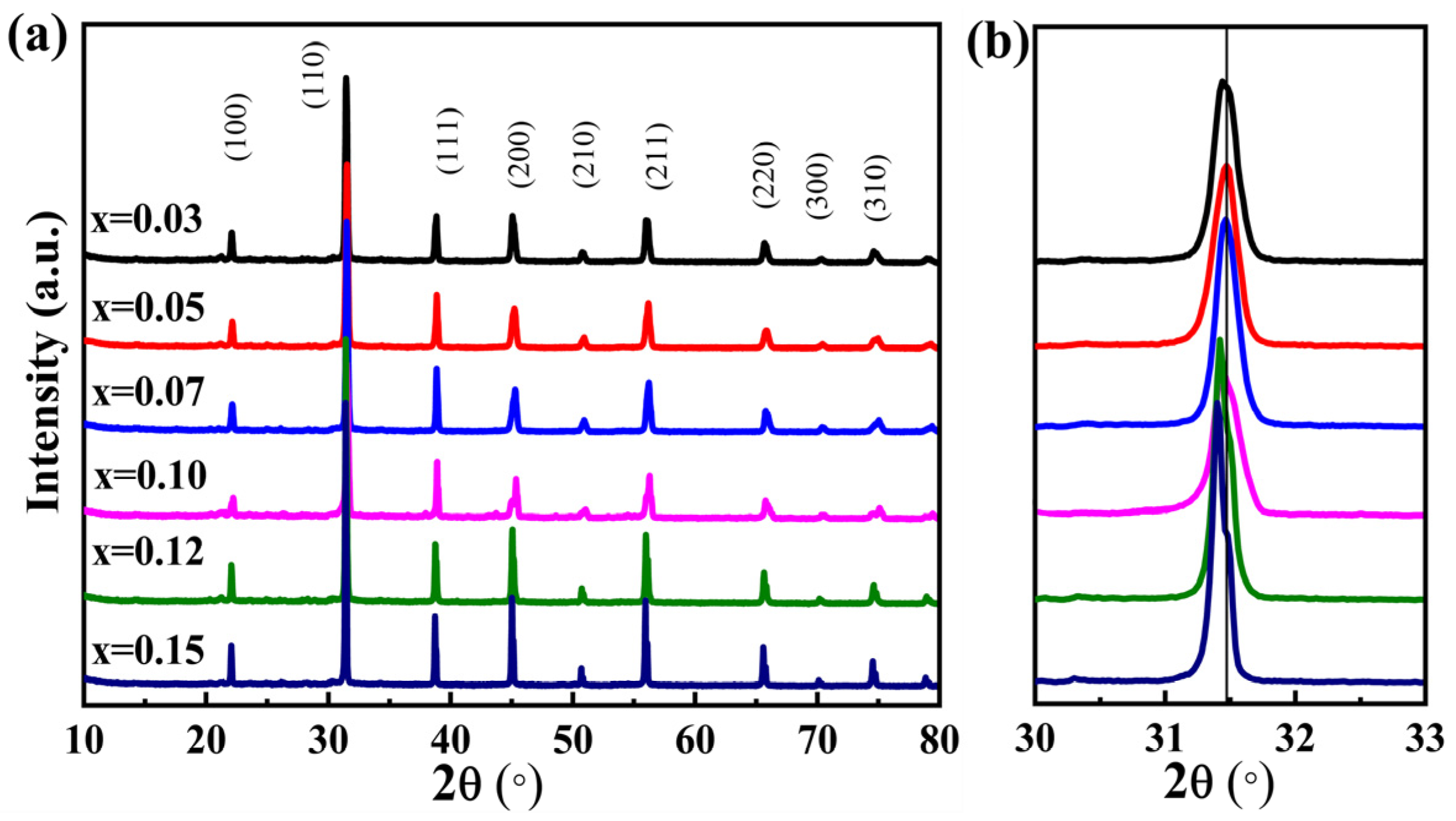
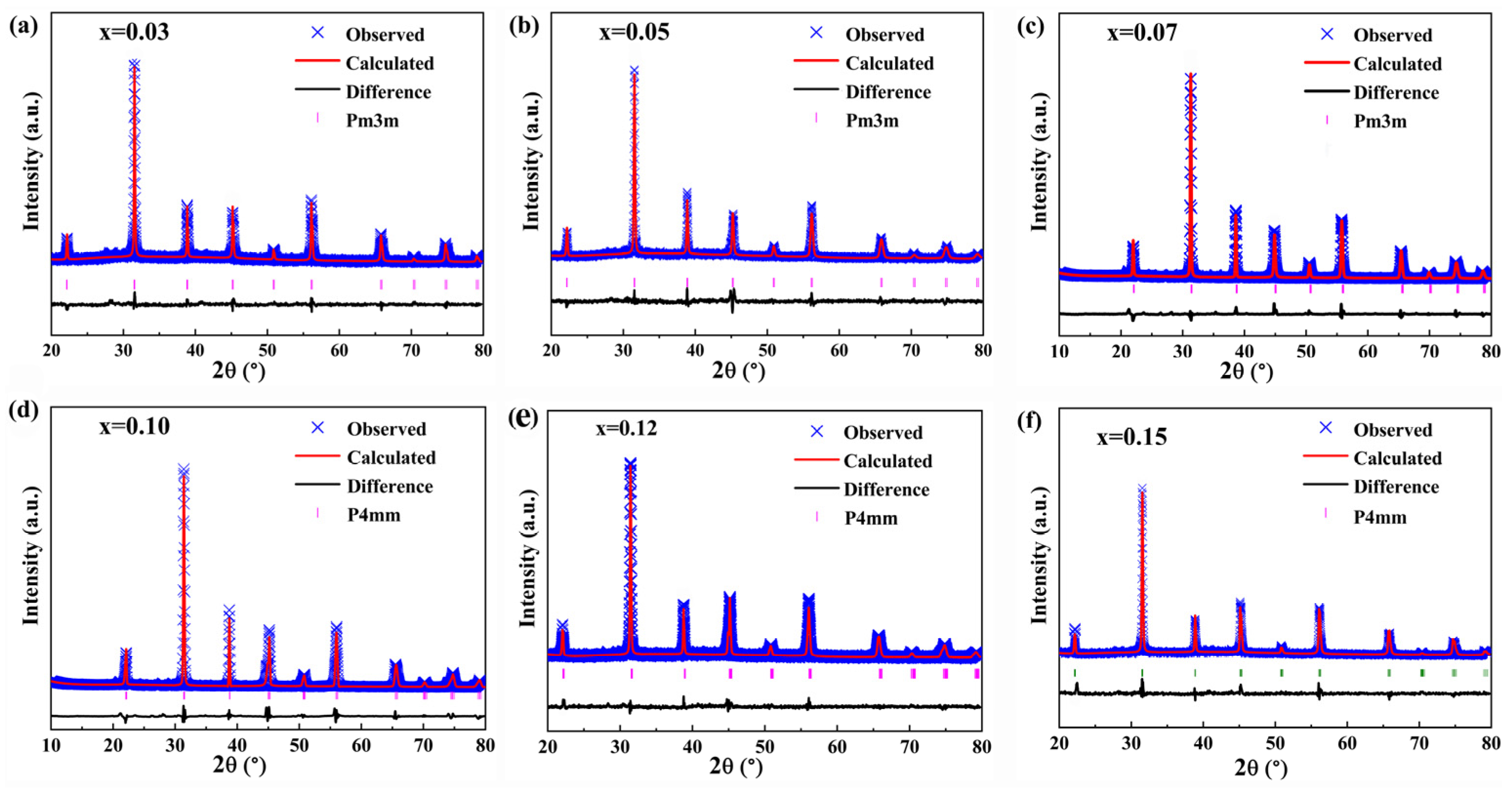
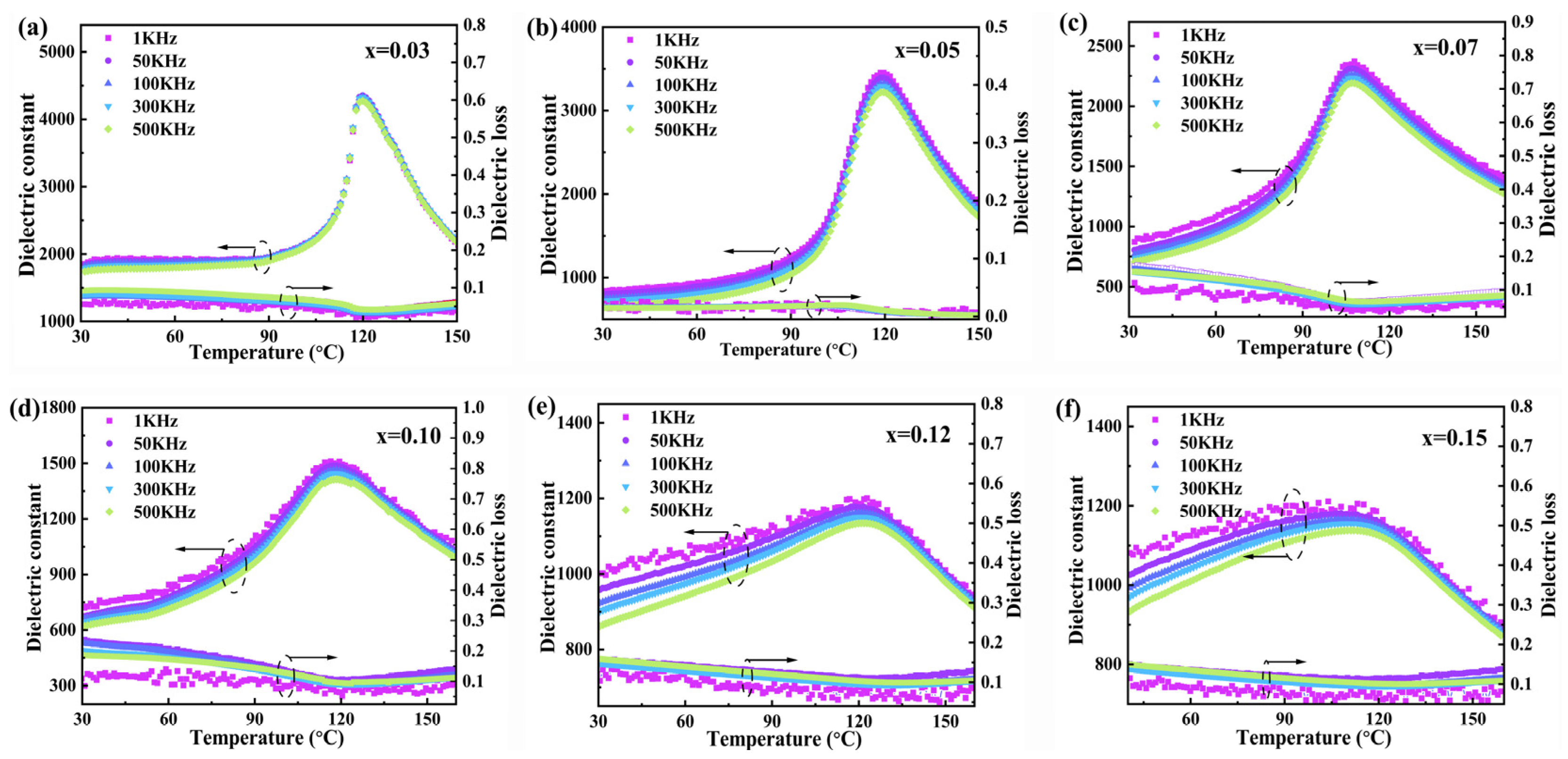
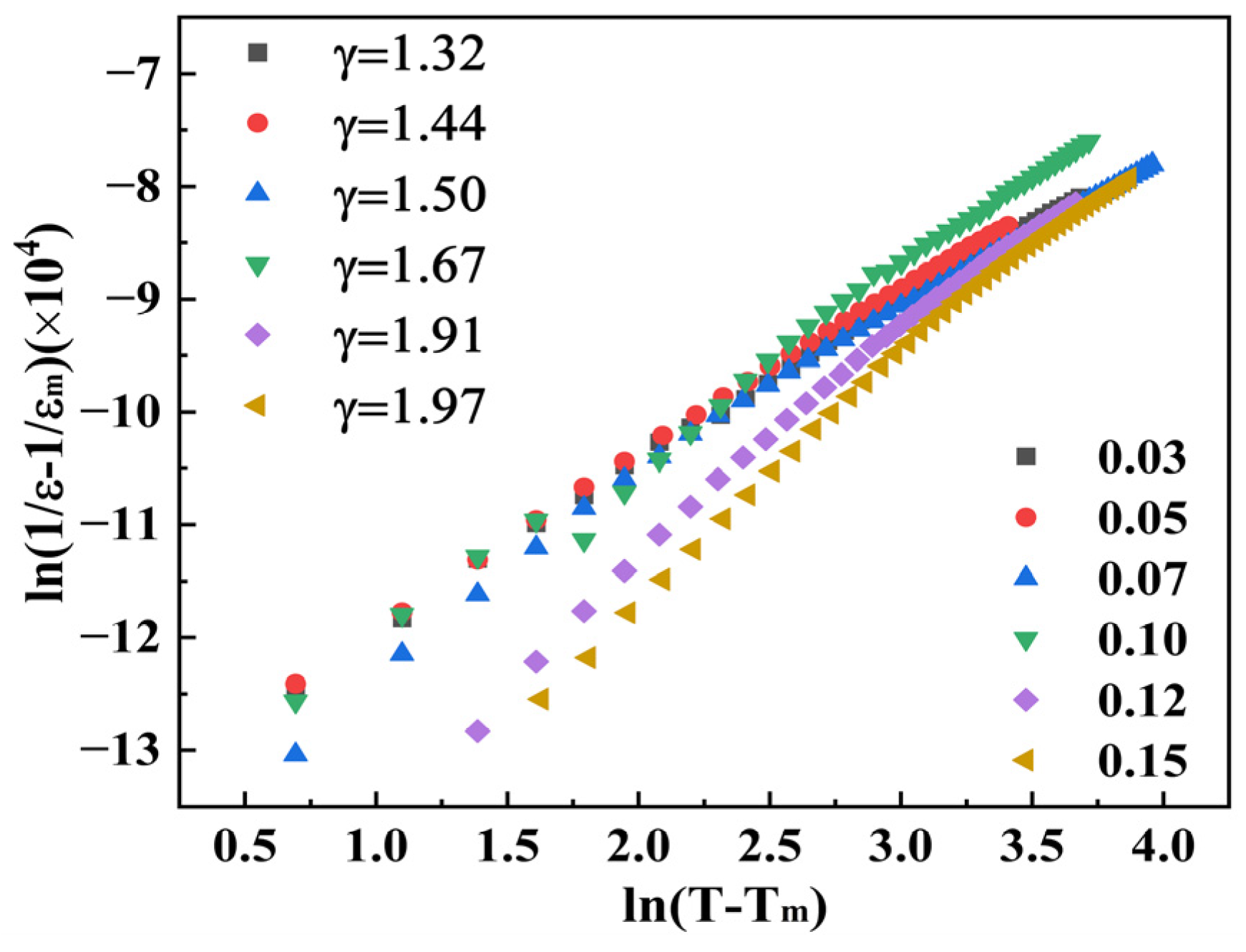
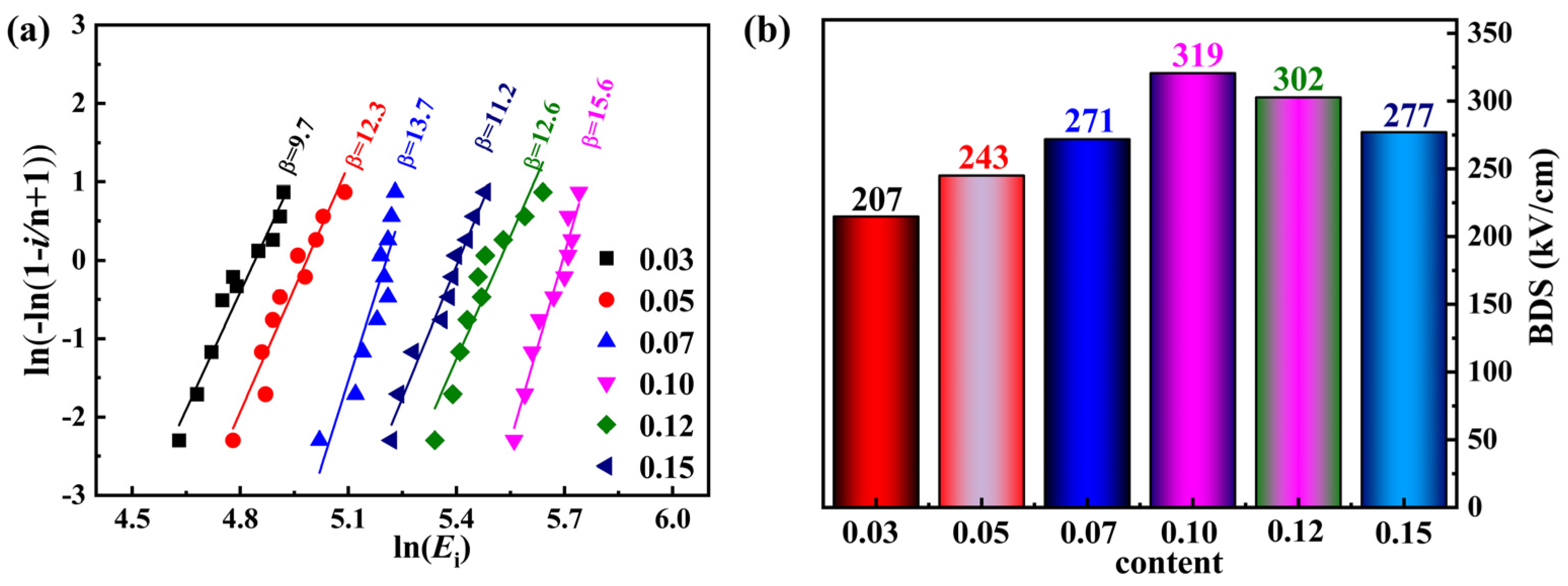
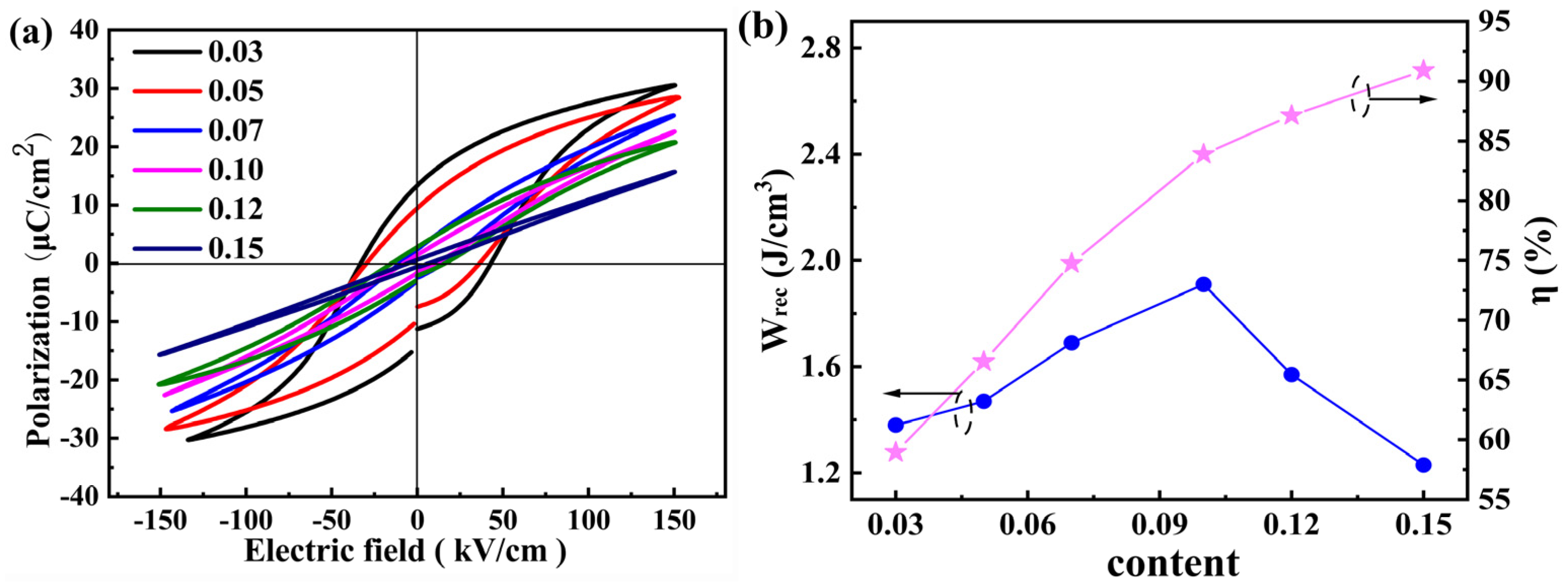
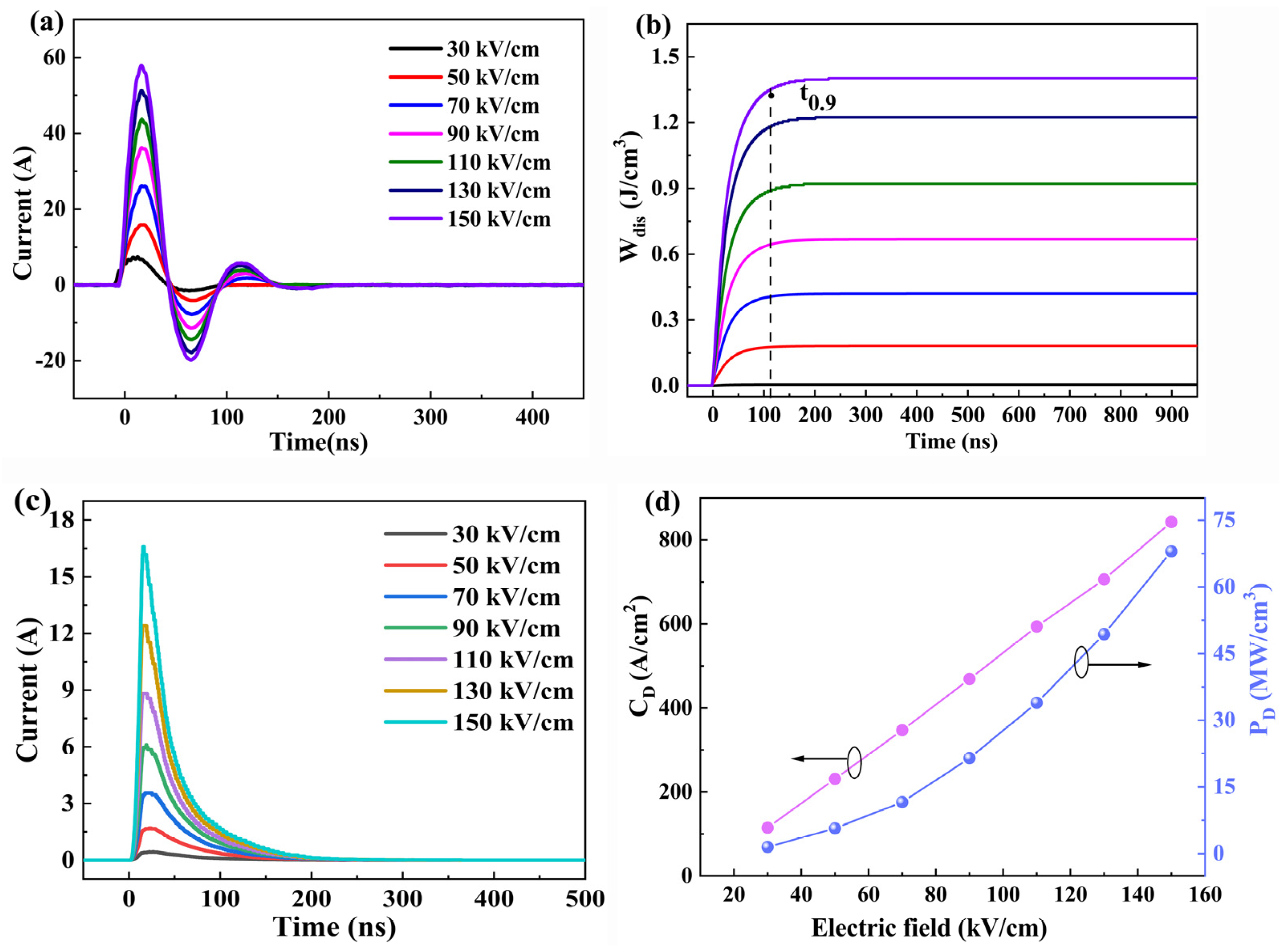
| Sample | Lattice Parameters (Å) | Volume (Å3) | Theoretical Densities (g/cm3) | C Phase (%) | T Phase (%) | R Factors (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | a | b | c | Rwp | Rp | χ2 | ||||
| 0.03 | 4.0106 | 4.0106 | 4.0106 | 64.35 | 6.002 | 93.76 | 6.34 | 6.34 | 5.79 | 5.35 |
| 0.05 | 4.0078 | 4.0078 | 4.0078 | 64.43 | 5.997 | 82.33 | 17.67 | 5.62 | 6.21 | 4.91 |
| 0.07 | 4.0041 | 4.0041 | 4.0041 | 64.51 | 5.994 | 63.74 | 36.26 | 6.71 | 5.94 | 6.12 |
| 0.10 | 4.0012 | 4.0012 | 4.0218 | 64.58 | 5.989 | 26.85 | 73.15 | 5.86 | 5.76 | 4.76 |
| 0.12 | 3.9991 | 3.9991 | 4.0249 | 64.63 | 5.986 | 14.34 | 85.66 | 6.27 | 5.69 | 6.97 |
| 0.15 | 3.9989 | 3.9989 | 4.0301 | 64.68 | 5.983 | 4.75 | 95.25 | 5.41 | 6.17 | 5.46 |
| Composition | W (J/cm3) | η (%) | E (kV/cm) | References |
|---|---|---|---|---|
| BCT-BLZ | 1.91 | 90.87% | 150 | This work |
| BT-based | 1.5 | 91.7% | 160 | [27] |
| BNT-BT | 1.26 | 84.3% | 120 | [28] |
| BT-based high entropy | 1.57 | 72% | 170 | [29] |
| BF-BT | 1.27 | 95.4% | 185 | [30] |
| BT-ST | 0.59 | 95.5% | 120 | [31] |
| 0.85BT-0.15BMZ | 0.8 | 89% | 150 | [32] |
| BST | 1.76 | 77.6% | 120 | [33] |
| BBTS | 1.52 | 72.3% | 100 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhu, D.; Ding, X.; Cui, L.; Wang, J. Enhanced Energy Storage Properties of Ba0.96Ca0.04TiO3 Ceramics Through Doping Bi(Li1/3Zr2/3)O3. Coatings 2025, 15, 906. https://doi.org/10.3390/coatings15080906
Li Z, Zhu D, Ding X, Cui L, Wang J. Enhanced Energy Storage Properties of Ba0.96Ca0.04TiO3 Ceramics Through Doping Bi(Li1/3Zr2/3)O3. Coatings. 2025; 15(8):906. https://doi.org/10.3390/coatings15080906
Chicago/Turabian StyleLi, Zhiwei, Dandan Zhu, Xuqiang Ding, Lingling Cui, and Junlong Wang. 2025. "Enhanced Energy Storage Properties of Ba0.96Ca0.04TiO3 Ceramics Through Doping Bi(Li1/3Zr2/3)O3" Coatings 15, no. 8: 906. https://doi.org/10.3390/coatings15080906
APA StyleLi, Z., Zhu, D., Ding, X., Cui, L., & Wang, J. (2025). Enhanced Energy Storage Properties of Ba0.96Ca0.04TiO3 Ceramics Through Doping Bi(Li1/3Zr2/3)O3. Coatings, 15(8), 906. https://doi.org/10.3390/coatings15080906




