Research on the Preparation and Performance of Wood with High Negative Oxygen Ion Release Induced by Moisture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hexagonal Stone Dispersion by Chemical Dispersion Treatment
2.3. Preparation of Hexagonal Stone Dispersion by Physical Dispersion Treatment
2.4. Preparation of Hexagonal Stone Dispersion by Composite Dispersion Treatment
2.5. Drying of Poplar Samples
2.6. Preparation of Healthy Wood
2.7. Particle Size Test
2.8. SEM Characterization
2.9. Monitoring of Negative Oxygen Ion Release from Hexagonal Stone
2.10. FT-IR Characterization of Hexagonal Stone Powder
2.11. XRD Characterization of Hexagonal Stone Powder
2.12. Weight Gain Rate and Fluid Absorption Rate of Healthy Wood
2.13. Dimensional Stability
2.14. SEM Characterization
2.15. Monitoring of Negative Oxygen Ion Release from Healthy Wood
2.16. FT-IR Characterization of Healthy Wood
2.17. XRD Characterization of Healthy Wood
3. Results
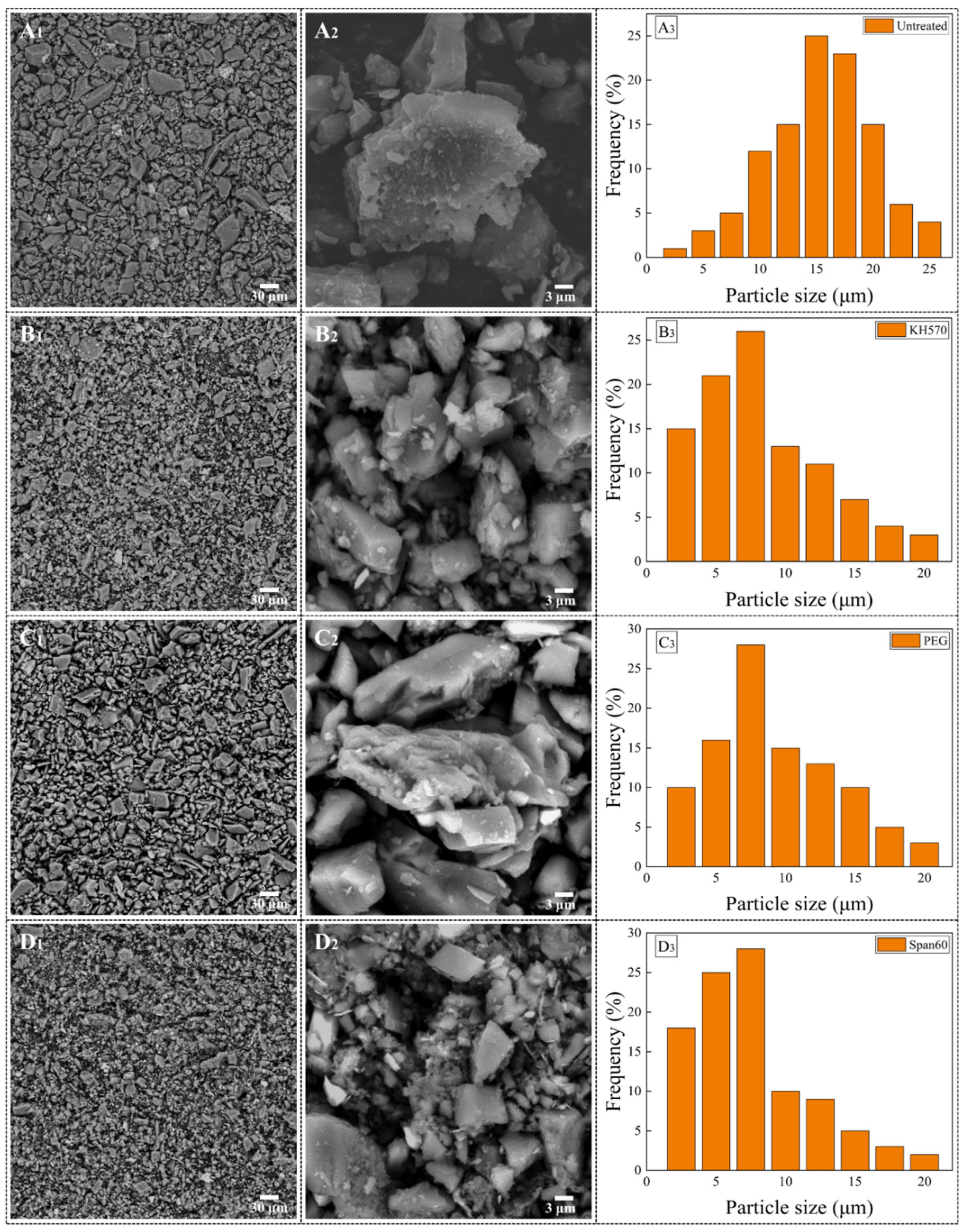
3.1. Study on the Dispersion Process of Hexagonal Stone Powder
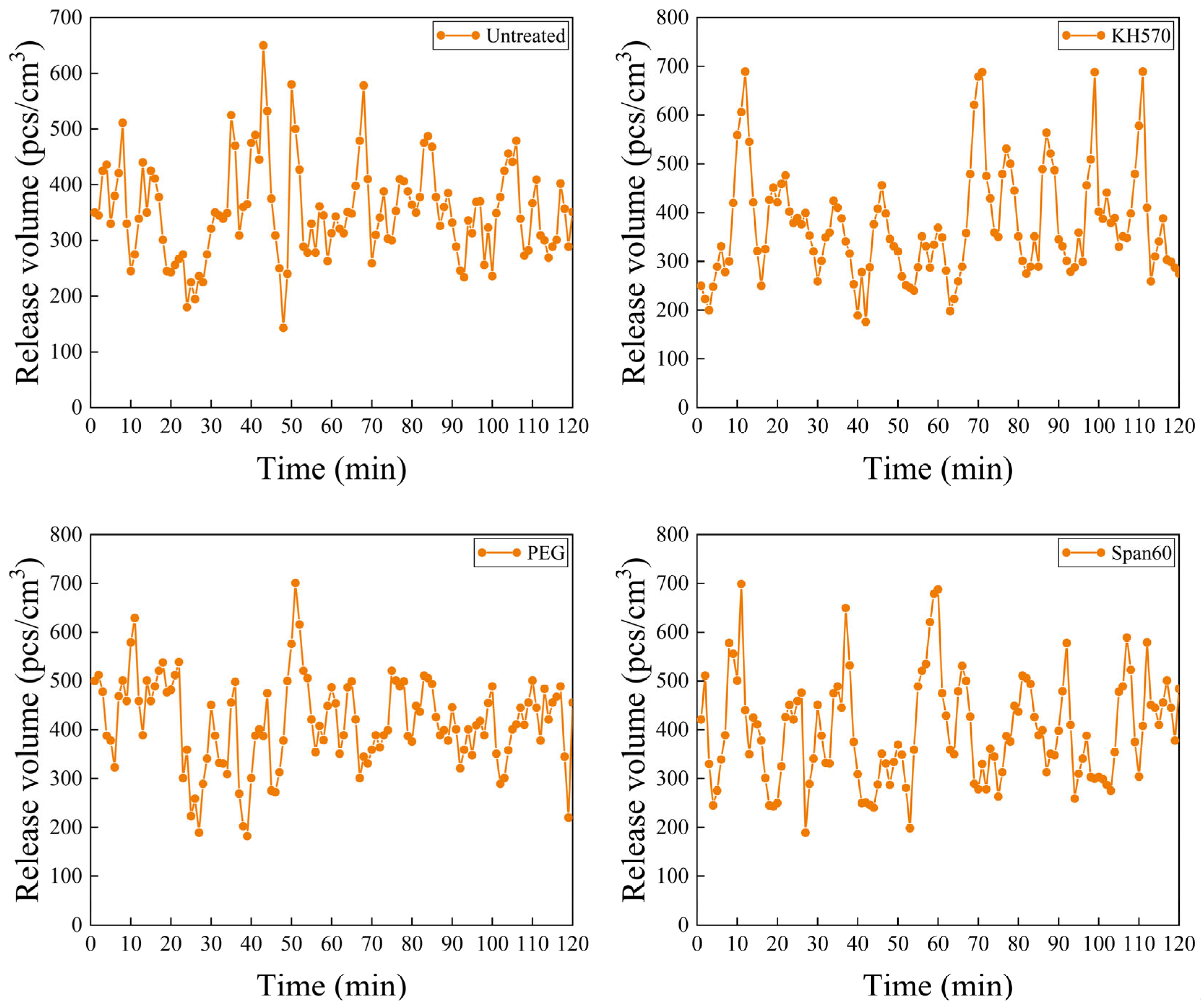
3.2. The Effect of Dispersion Treatment on the Microstructure and Negative Oxygen Ion Release Capability of Hexagonal Stone Powder
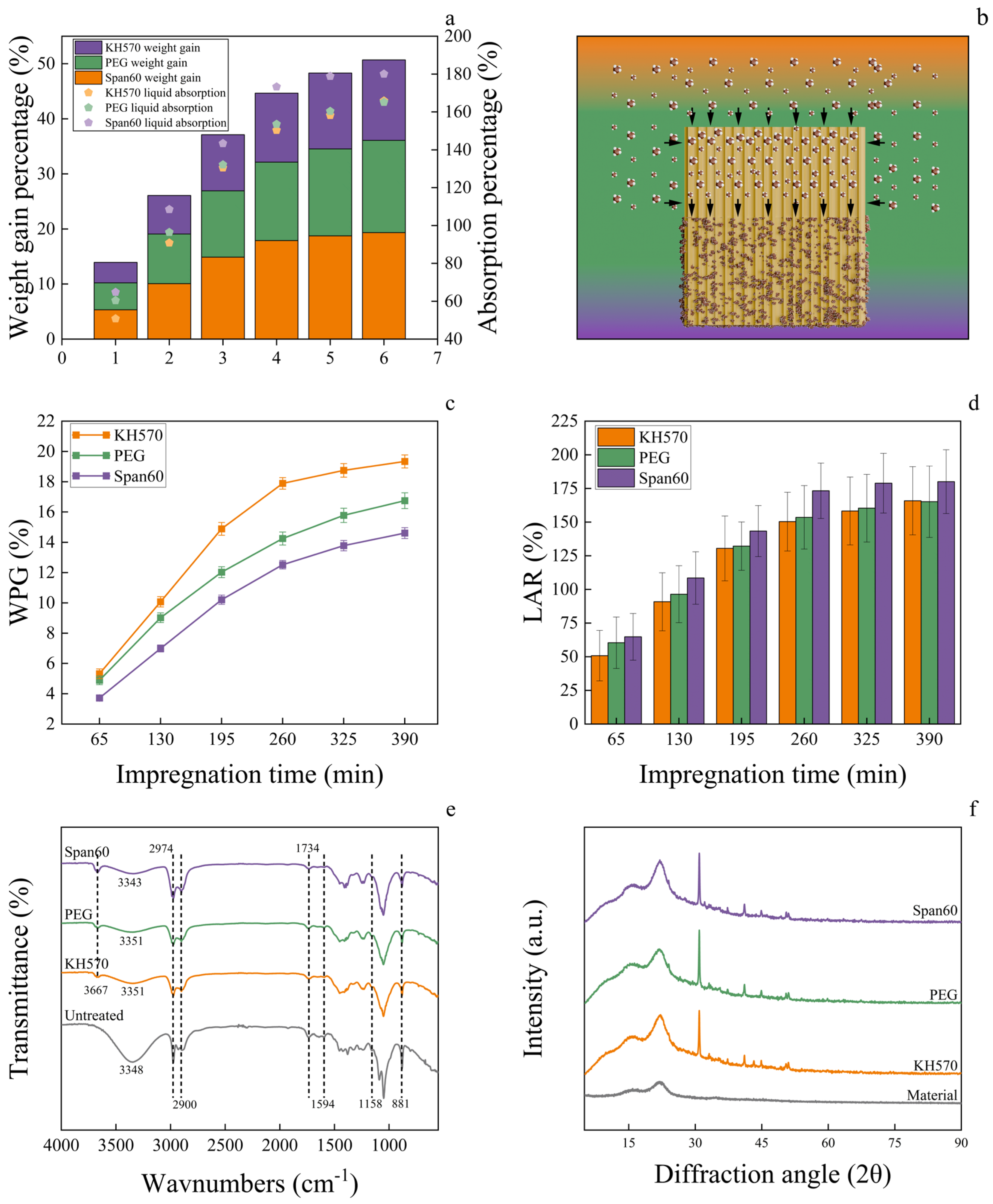
3.3. Preparation and Characterization of Healthy Wood
3.4. Microscopic Structure of Healthy Wood
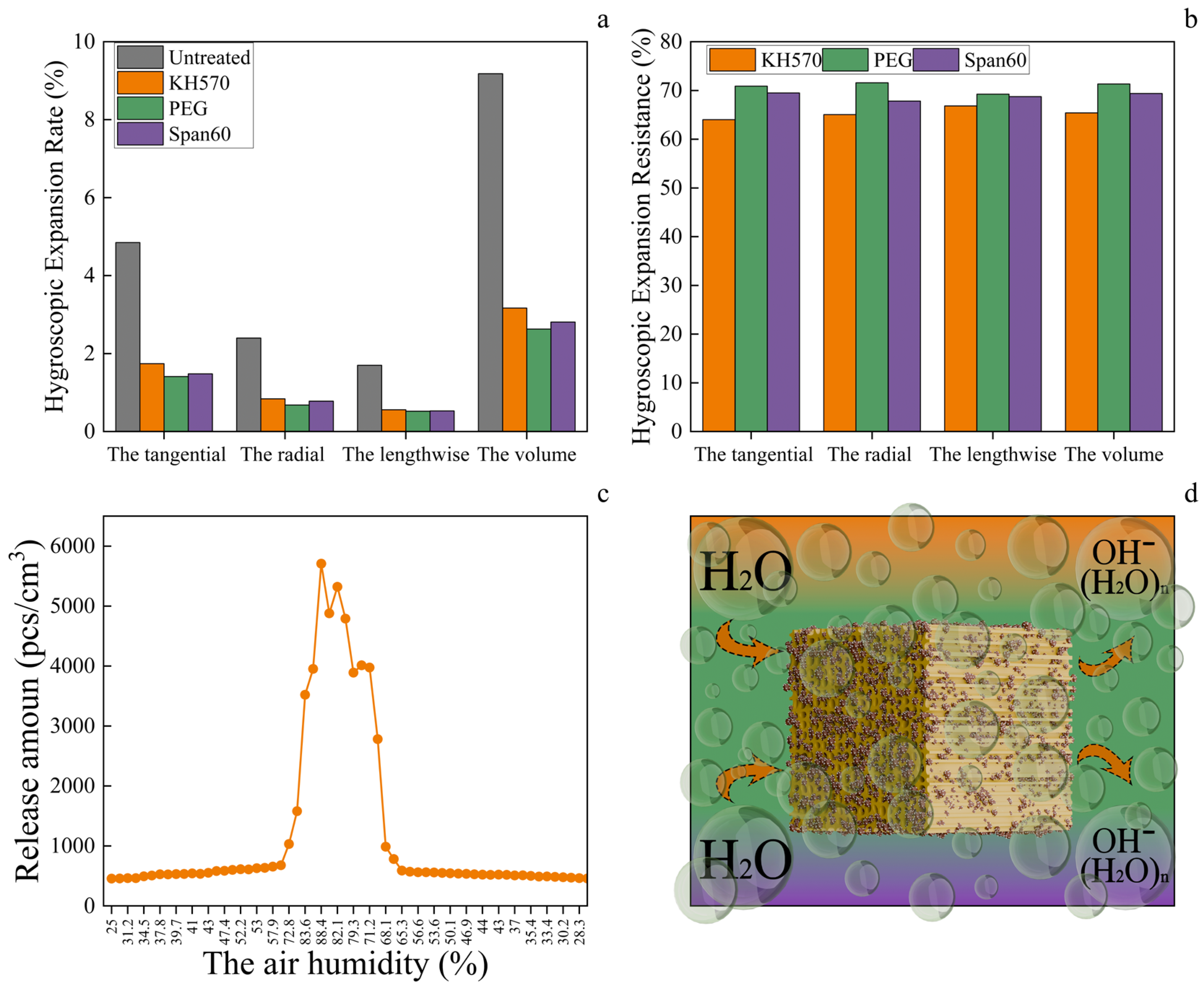
3.5. Changes in the Properties of Modified Healthy Wood and the Effect of Moisture on Negative Oxygen Ion Release Capacity
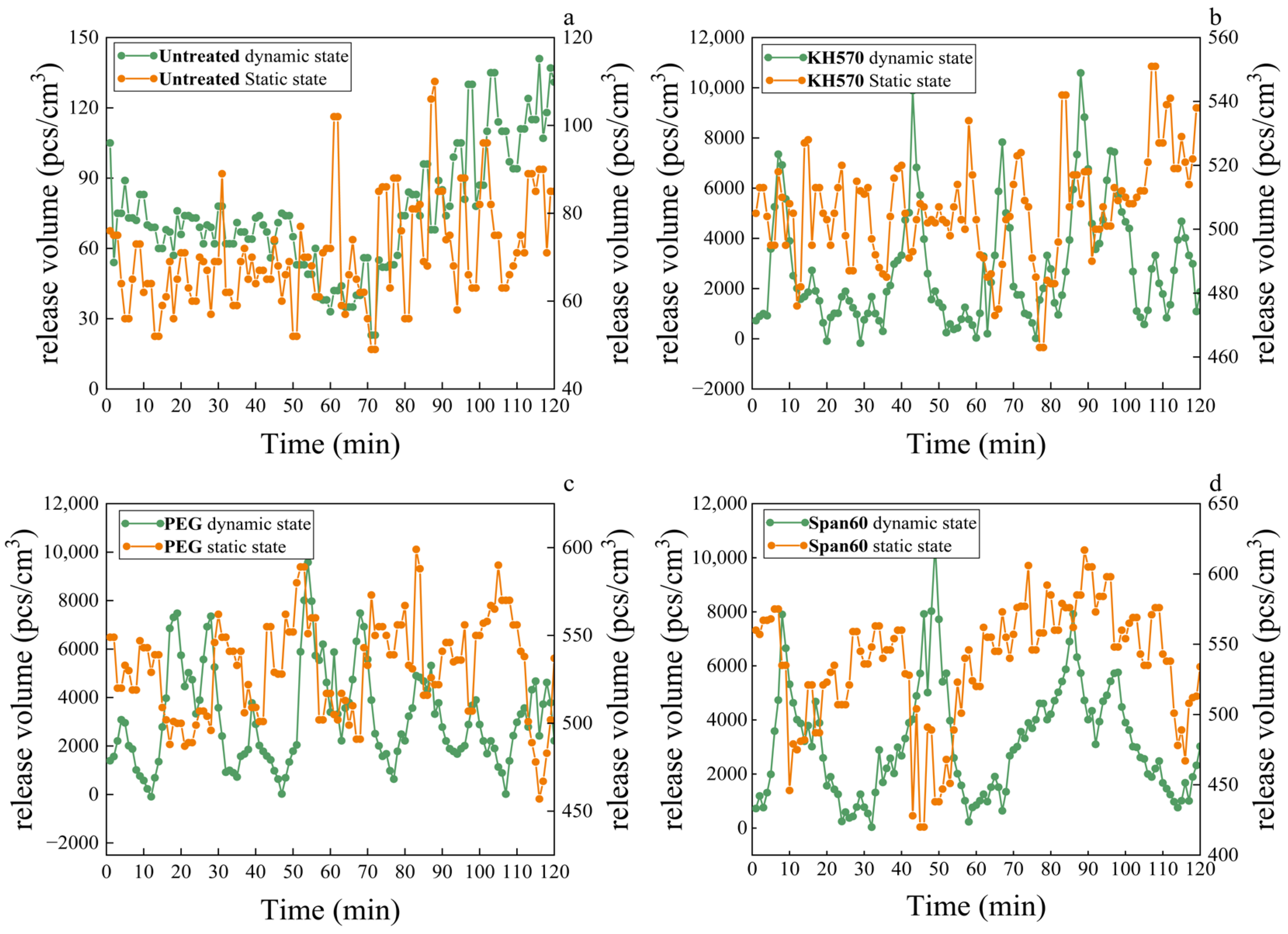
3.6. Release Amount of Negative Oxygen Ions from Healthy Wood
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wu, J.; Lam, F.; Zhang, C.; Kang, J.; Xu, H. Effect of the degree of wood use on the visual psychological response of wooden indoor spaces. Wood Sci. Technol. 2021, 55, 1485–1508. [Google Scholar] [CrossRef]
- Kotradyova, V.; Vavrinsky, E.; Kalinakova, B.; Petro, D.; Jansakova, K.; Boles, M.; Svobodova, H. Wood and Its Impact on Humans and Environment Quality in Health Care Facilities. Int. J. Environ. Res. Public Health 2019, 16, 3496. [Google Scholar] [CrossRef]
- Muilu-Mäkelä, R.; Aapola, U.; Tienaho, J.; Uusitalo, H.; Sarjala, T. Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood. Molecules 2022, 27, 3891. [Google Scholar] [CrossRef]
- Guo, S.; De Wolf, S.; Sitti, M.; Serre, C.; Tan, S.C. Hygroscopic Materials. Adv. Mater. 2023, 36, e2311445. [Google Scholar] [CrossRef]
- Hietikko, J.; Tuominen, E.; Valovirta, I.; Vinha, J. Timber-framed exterior walls insulated with wood shavings: A field study in a nordic climate. Build. Environ. 2024, 254, 111371. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.W. High acoustic absorption properties of hackberry compared to nine different hardwood species: A novel finding for acoustical engineers. Appl. Acoust. 2020, 169, 107475. [Google Scholar] [CrossRef]
- Mi, H.N.; Yu, J.; Wang, Z.; Zhang, T.; Guo, J.R.; Wang, X.M. Advances in the preparation and properties of wood with health benefits. Mater. Rep. 2021, 35, 11215–11221. [Google Scholar]
- Qi, Y.; Dai, X.; Wei, L.; Luo, H.; Liu, Y.; Dong, X.; Yang, D.; Li, Y. Nano-AgCu Alloy on Wood Surface for Mold Resistance. Nanomaterials 2022, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wei, L.; Dong, Y.; Gong, R.; Wang, X.; Yao, F.; Liu, Y.; Kong, L.; Dong, X.; Li, Y. AgCu Nanoparticles as an Antibacterial Coating for Wood. ACS Appl. Nano Mater. 2024, 7, 5339–5347. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Cheng, S.; Qin, Z.; Fu, Y. Photocatalytic Performance and Kinetic Studies of a Wood Surface Loaded with Bi2O3-Doped Silicon–Titanium Composite Film. Polymers 2022, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Ba, Z.; Liang, D.; Xiao, Z.; Wang, Y.; Wang, H.; Xie, Y. Electromagnetic shielding and fire-retardant wood obtained by in situ aniline polymerization. Wood Sci. Technol. 2023, 57, 1467–1483. [Google Scholar] [CrossRef]
- Wei, Y.; Dai, Z.; Zhang, Y.; Zhang, W.; Gu, J.; Hu, C.; Lin, X. Multifunctional waterproof MXene-coated wood with high electromagnetic shielding performance. Cellulose 2022, 29, 5883–5893. [Google Scholar] [CrossRef]
- Shi, L.; Yuan, S.F.; Wang, H.Y.; Zhang, J.; Chen, J.; Li, Q. Study on Dispersion Technology of Hexacyclic Stone Powder and Negative Ion Release of Blockboard. J. For. Eng. 2022, 7, 78–86. [Google Scholar] [CrossRef]
- Liu, L.L.; Geng, M.N. The Development of A Mask Releasing Negative Ions. Guangzhou Chem. Ind. 2010, 38, 123–125+137. [Google Scholar]
- Gui, S.L. A Nano New Material with Far-Infrared Energy-Saving Radiation Resonance Wave Frequency Function. CN Patent 109364379A, 22 February 2019. [Google Scholar]
- Qin, Y.T. Surface Modification of Hexacyclic Stone and Its Application in Antibacterial Wood Coatings. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.; Qiu, L.; Zhang, J.; Yu, J.; Wang, X. Preparation and drying characteristics of poplar wood modified by hexacyclite. Dry. Technol. 2024, 42, 1567–1577. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Ma, A.; Ramachandran, S. Negative Air Ions and Their Effects on Human Health and Air Quality Improvement. Int. J. Mol. Sci. 2018, 19, 2966. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, J.; Wu, Q.; Chen, Z.; Wang, G. Seasonal dynamics of VOCs released from Cinnamomun camphora forests and the associated adjuvant therapy for geriatric hypertension. Ind. Crop. Prod. 2021, 174, 114131. [Google Scholar] [CrossRef]
- Krueger, A.P.; Smith, R.F. An Enzymatic Basis for the Acceleration of Ciliary Activity by Negative Air Ions. Nature 1959, 183, 1332–1333. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.H.; Barker, T. Air Ions and Human Performance. Ergonomics 1978, 21, 273–278. [Google Scholar] [CrossRef]
- Krueger, A.P.; Reed, E.J. Biological Impact of Small Air Ions. Science 1976, 193, 1209–1213. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, T.; Petersen, J.D.; Zhou, J.; Lu, X. Biological effects of negative air ions on human health and integrated multiomics to identify biomarkers: A literature review. Environ. Sci. Pollut. Res. 2023, 30, 69824–69836. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, W.; Cao, G.; Zhan, X.; Qiang, T.; Li, J. Visible-light activate Ag/WO 3 films based on wood with enhanced negative oxygen ions production properties. Appl. Surf. Sci. 2017, 425, 889–895. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Zhang, J.; Zeng, P.; Liang, H.; Wang, Y.; Sun, Q.; Zhou, G.; Li, H. Personal Microenvironment Management by Smart Textiles with Negative Oxygen Ions Releasing and Radiative Cooling Performance. ACS Nano 2023, 17, 13269–13277. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; Pu, J. TiO2/graphene oxide and SiO2 nanocomposites based on poplar wood substrate under UV irradiation and negative oxygen ions generation. BioResources 2019, 14, 1781–1793. [Google Scholar] [CrossRef]
- Yang, M.; Sun, C.; Chang, L.; Liu, S.; Zheng, D.; Chen, Y.; Sun, X.; Tan, H.; Zhang, Y. A novel sustainable wood-based negative air anion generator utilizing in-situ polymerization of polylactic acid to reinforce the cellulose framework. Int. J. Biol. Macromol. 2024, 282, 137166. [Google Scholar] [CrossRef]
- Han, M.; Liu, Z.; Zhang, T.; Liu, M.; Li, C. Preparation and formation mechanism study of tourmaline@nano-alumina composite filler. Ceram. Int. 2025, 51, 13959–13967. [Google Scholar] [CrossRef]
- Augustina, S.; Dwianto, W.; Wahyudi, I.; Syafii, W.; Gérardin, P.; Marbun, S.D. Wood impregnation in relation to its mechanisms and properties enhancement. BioResources 2023, 18, 4332–4372. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, H.; Hou, H.; Li, C.; Liu, M. Research on the Impregnation Process and Mechanism of Silica Sol/Phenolic Resin Modified Poplar Wood. Forests 2023, 14, 2176. [Google Scholar] [CrossRef]
- Ureña, J.; Mendoza, C.; Ferrari, B.; Castro, Y.; Tsipas, S.A.; Jiménez-Morales, A.; Gordo, E. Surface Modification of Powder Metallurgy Titanium by Colloidal Techniques and Diffusion Processes for Biomedical Applications. Adv. Eng. Mater. 2016, 19, 1600207. [Google Scholar] [CrossRef]
- GB/T 28628-2012; Test Method for Air Ion Concentration of Materials. Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Miao, X.W. Research on The Preparation of Composite Wood Modifiers and Their Strengthening Mechanism. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Chai, Y.B. Research on The Process and Mechanism of Wood Acetylation. Ph.D. Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2015. [Google Scholar]
- LY/T2490-2015; Test Method for Dimension Stability of Modified Wood. National Forestry and Grassland Administration: Beijing, China, 2015.
- Pasupathy, M.; Martín, J.M.; Rivas, A.; Iturriza, I.; Castro, F. Effect of the solidification time on the median particle size of powders produced by water atomisation. Powder Met. 2016, 59, 128–141. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, G.H.; Jiang, F.H. Influence of powder dispersion method on grit size change during production of fine WC. Sichuan Nonferrous Met. 2021, 1, 31–35. [Google Scholar]
- Si, H.; Che, M.; Chen, Z.; Qiu, S.; Cui, M.; Huang, R.; Qi, W.; He, Z.; Su, R. Efficient removal of chloroform in groundwater by polyethylene glycol-stabilized Fe/Ni nanoparticles. Environ. Chem. Lett. 2021, 19, 3511–3515. [Google Scholar] [CrossRef]
- Xu, G.D.; Zhang, X.Y.; Liu, J.K.; Sun, N.; Zhang, J.R. Preparation of antimony doped tin oxide conductive nanoslurry and its antistatic application. Shanghai Coat. 2014, 52, 13–17. [Google Scholar]
- Zhang, G.L.; Zhao, Y.H.; Song, P.; Li, G.J. In-Site surface modification of Mg-Al composite flame retardant with silane coupling agent KH-570. J. Salt Chem. Ind. 2016, 45, 25–28. [Google Scholar]
- Zhang, S.P.; Tie, S.G. Surface modification of silica fume dispersivity with silane coupling agent KH-570. J. Synth. Cryst. 2018, 47, 1396–1401. [Google Scholar]
- He, L.H.; Li, L.; Zhou, C.; Li, W.H. Hydrophobic surface modification of diatomit with silane coupling agent KH-570. Mod. Chem. Ind. 2014, 34, 93–97. [Google Scholar]
- Yi, D.L.; Ouyang, Z.H.; Wu, L.; Qin, X.R. Surface modification of nano-sio2 and its application in butyl rubber. J. Wuhan Univ. Sci. Technol. (Nat. Sci. Ed.) 2007, 30, 640–658. [Google Scholar]
- Zhang, Q.; Bi, C.; Li, Y.G.; Zhu, M.F.; Wang, H.Z. Study on surface modification of the sio2 nanoparticles and dispersion. New Chem. Materials. 2008, 36, 41–42. [Google Scholar]
- Su, R.C.; Li, W.F.; Peng, J.H.; Du, J. Surface modification of nano-sized sio2 with silane coupling agent and its dispersion. Chem. Ind. Eng. Prog. 2009, 28, 1596–1599. [Google Scholar]
- Xue, R.J. Surface Modification and Physical Properties of Inorganic Nanostructured Units. Ph.D. Thesis, Hefei University of Technology, Hefei, China, 2008. [Google Scholar]
- Zhang, Y.H.; Zhai, L.L.; Wang, Y.; Liu, R.W.; Yuan, J.X. Surface modification of nano-sio2 by silane coupling agent 3-(methacryloyloxy) propyltrimethoxysilane. J. Mater. Sci. Eng. 2012, 30, 752–756. [Google Scholar]
- Zhang, C.; Yang, H.; Guo, X.Z. Surface modification of ATO nanopowders with KH560. China Ceram. Ind. 2014, 21, 1–4. [Google Scholar]
- Guo, Y.; Wang, Y.-Q.; Wang, Z.-M.; Shen, C.-J. Study on the preparation and characterization of high-dispersibility nanosilica. Sci. Eng. Compos. Mater. 2016, 23, 401–406. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Feng, Y.; Chen, H.; Zheng, H.; Sun, X.; Duan, J. Dispersion of silica nanoparticles in water/ethanol/PEG mixtures for stimuli-responsive aggregation to prepare improved fused silica glass. Ceram. Int. 2023, 50, 2340–2349. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.; Xing, P.; Zhuang, Y.; Wang, L. Influence of dispersant on the microstructure and performance of the hot-pressed B4C-YB4 ceramics. J. Aust. Ceram. Soc. 2023, 59, 1065–1077. [Google Scholar] [CrossRef]
- Feng, Z.; Qi, J.; Huang, Z.; Xie, X.; Wei, N.; Lu, T. Optimization of the Amount and Molecular Weight of Dispersing Agent PEG During the Co-Precipitation Preparation of Nano-Crystalline C-YSZ Powder. J. Nanosci. Nanotechnol. 2017, 17, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chang, Q.; Wang, B. The dispersion and tribological performances of magnesium silicate hydroxide nanoparticles enhanced by Span60 oleogel. J. Sol-Gel Sci. Technol. 2019, 94, 165–173. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X. The surface organic modification of tourmaline powder by span-60 and its composite. Appl. Surf. Sci. 2012, 258, 7540–7545. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.G.; Lan, X.Y.; Pu, J.W. Research on application of PEG400 with epsilon-caprolactone in waterlogged planted scotch pine wood for dehydration and reinforcement. Chem. Ind. For. Products. 2017, 37, 28–38. [Google Scholar]
- Chen, L.; Li, J.; Lu, M.; Guo, X.; Zhang, H.; Han, L. Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover. Carbohydr. Polym. 2016, 141, 1–9. [Google Scholar] [CrossRef]
- Xue, Z.H.; Zhao, G.J. Influence of different treatments of on wood crystal properties. J. Northwest For. Univ. 2007, 22, 169–175. [Google Scholar]
- Balan, A.K.; Parambil, S.M.; Vakyath, S.; Velayudhan, J.T.; Naduparambath, S.; Etathil, P. Coconut shell powder reinforced thermoplastic polyurethane/natural rubber blend-composites: Effect of silane coupling agents on the mechanical and thermal properties of the composites. J. Mater. Sci. 2017, 52, 6712–6725. [Google Scholar] [CrossRef]
- He, L.; Li, W.; Chen, D.; Zhou, D.; Lu, G.; Yuan, J. Effects of amino silicone oil modification on properties of ramie fiber and ramie fiber/polypropylene composites. Mater. Des. 2015, 77, 142–148. [Google Scholar] [CrossRef]
- Lei, S.S.; Shi, Y.; Zhi, Y.M.; Yin, H.; Yao, L. Optimization of fenton oxidation pretreatment of poplar by response surface methodology. Trans. China Pulp Pap. 2020, 35, 27–33. [Google Scholar]
- Wang, J.W.; Liu, J.; He, Z.B.; Yi, S.L. Effects of different pretreatment on the physical characteristics of negative ions impreg-nated wood. Furniture 2017, 38, 17–20. [Google Scholar]
- Wang, Z.; Wang, X.M. Research progress of multi-scale pore structure and characterization methods of wood. Sci. Silvae Sin. 2014, 50, 123–133. [Google Scholar]
- Sun, W.L.; Li, J. Analysis and characterization of dimensional stability and crystallinity of heat-treated Larix spp. Sci. Silvae Sin. 2010, 46, 114–118. [Google Scholar]
- He, L.; Zhang, T.; Zhao, X.; Zhao, Y.; Xu, K.; He, Z.; Yi, S. Synergistic effect of tung oil and heat treatment on surface characteristics and dimensional stability of wood. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131233. [Google Scholar] [CrossRef]
- Zhang, T. Study on the Properties of Poplar Wood Modified with Litsea cuheba Oil. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2020. [Google Scholar]
- Xu, J.; Yang, T.; Xu, X.; Guo, X.; Cao, J. Processing Solid Wood into a Composite Phase Change Material for Thermal Energy Storage by Introducing Silica-Stabilized Polyethylene Glycol. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106098. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, P.; Ma, X.; Li, P.; Sun, Z.; Li, X.; Zuo, Y. Study on the Effect of Acrylic Acid Emulsion on the Properties of Poplar Wood Modified by Sodium Silicate Impregnation. Forests 2023, 14, 1221. [Google Scholar] [CrossRef]
- Yang, T.; Wang, J.; Xu, J.; Ma, E.; Cao, J. Hygroscopicity and dimensional stability of Populus euramericana Cv. modified by furfurylation combined with low hemicellulose pretreatment. J. Mater. Sci. 2019, 54, 13445–13456. [Google Scholar] [CrossRef]
- Fredriksson, M. On Wood–Water Interactions in the Over-Hygroscopic Moisture Range—Mechanisms, Methods, and Influence of Wood Modification. Forests 2019, 10, 779. [Google Scholar] [CrossRef]
- QX/T 380-2017; Grade of Air Negative (Oxygen) Ion Concentration. China Meteorological Administration: Beijing, China, 2017.
- Weng, H.; Zhang, Y.; Huang, X.; Liu, X.; Tang, Y.; Yuan, H.; Xu, Y.; Li, K.; Zhang, Y. Pilot Study on the Production of Negative Oxygen Ions Based on Lower Voltage Ionization Method and Application in Air Purification. Atmosphere 2024, 15, 860. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Y.J.; Jin, Q.Y.; Liu, Y.G.; Li, L.B.; Zhang, P.; Zhang, S.; Zhao, H.W.; Sun, L.T. A compact radio-frequency ion source for high brightness and low energy spread negative oxygen ion beam production. Rev. Sci. Instrum. 2023, 94, 093301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rui, Y.; Yu, B.; Fu, L.; Lu, G.; Liu, J. Study on the negative oxygen ion release behavior and mechanism of tourmaline composites. Mater. Chem. Phys. 2023, 313, 128779. [Google Scholar] [CrossRef]



| Name | Specification | Manufacturer |
|---|---|---|
| Hexagonal stone | 8000-mesh | Lingshou Yonghui Mineral Processing Factory (Shijiazhuang, China). |
| Sodium metasilicate | Na2SiO3·9H2O AR | Tianjin Fengchuan Chemical Reagent Technology Co., Ltd. (Tianjin, China). |
| 3-(Trimethoxysilyl)propyl methacrylate | KH570 AR | Shandong Youso Chemical Technology Co., Ltd. (Linyi, China). |
| Sopropoxy tris(dioctyl phosphonate) titanate | KR-12 AR | Nanjing Chuangshi Chemical Auxiliary Co., Ltd. (Nanjing, China). |
| Aluminate coupling agent | UP-801 AR | Nanjing Youpu Chemical Co., Ltd. (Nanjing, China). |
| Polyethylene glycol | PEG AR | Fuchen (Tianjin) Chemical Reagent Co., Ltd. (Tianjin, China). |
| Cocoamidopropyl betaine | CAB-35 AR | Shandong Yousuo Chemical Technology Co., Ltd. (Linyi, China). |
| Sorbitan monostearate | Span60 AR | Wuxi Yatai United Chemical Co., Ltd. (Yixing, China). |
| Absolute alcohol 400 | C2H6O AR | Tianjin Fengchuan Chemical Reagent Technology Co., Ltd. (Tianjin, China). |
| Deionized water | H2O AR | Self-made by Inner Mongolia Agricultural University |
| Populus bolleana Lauche | Free of skin defects, straight trunk, and a diameter of 35 cm at the chest; 20 mm × 20 mm × 20 mm | Saihan District, Hohhot City, Inner Mongolia Autonomous Region |
| Instrument Name | Model | Manufacturer |
|---|---|---|
| Blast drying oven | 101A-3B | Tianjin Hongnuo Instrument Co., Ltd. (Tianjin, China). |
| Ultrasonic cell disruptor | SM-1800D | Nanjing Shunma Instrument Equipment Co., Ltd. (Nanjing, China). |
| Scanning image grit size analyzer | BT-1700 | Dandong Baite Instrument Co., Ltd. (Dandong, China). |
| Atmospheric negative (oxygen) ion detector | XDB-6400 | Shenzhen New Landmark Environmental Technology Development Co., Ltd. (Shenzhen, China). |
| Fourier-transform infrared spectrometer | Nicolet Magna-IR 750 | Thermo Nicolet Corporation (Madison City, State of Wisconsin, United States). |
| X-ray diffractometer | ESCA-14 | Bruker Spectrum Instruments, Karlsruhe, Germany |
| X-ray fluorescence analyzer | EA1000VX | Hitachi, Tokyo, Japan |
| Scanning electron microscope | S-3400N | Hitachi, Tokyo, Japan |
| Miniature plant grinder | RS-FS1401 | Hefei Rongshida Small Appliance Co., Ltd. (Hefei, China). |
| Type blast drying oven | 101A-3B | Tianjin Hongnuo Instrument Co., Ltd. (Tianjin, China). |
| Vacuum drying oven | DZF-ZASB | Beijing Kewei Yongxing Instrument Co., Ltd. (Beijing, China). |
| Constant temperature and humidity oven | BPS-100CA | Tianjin Weiss Experimental Instrument Technology Co., Ltd. (Tianjin, China). |
| Type horizontal slicer | SM2010R | Leica Biosystems Nussloch GmbH (Nussloch, Germany). |
| Dispersant | Maximum (μm) | Minimum (μm) | Average (μm) |
|---|---|---|---|
| Untreated | 25.04 | 2.12 | 18.37 |
| KH570 | 19.32 | 0.24 | 8.93 |
| PEG | 20.05 | 0.35 | 9.21 |
| Span60 | 18.97 | 0.19 | 9.89 |
| Dispersant | Average Values/ (pcs/cm3) | Highest Values/ (pcs/cm3) | Lowest Values/ (pcs/cm3) |
|---|---|---|---|
| Untreated | 352 | 650 | 143 |
| KH570 | 374 | 689 | 176 |
| PEG | 415 | 701 | 182 |
| Span60 | 398 | 699 | 189 |
| KH570 (pcs/cm3) | PEG (pcs/cm3) | Span60 (pcs/cm3) | |
|---|---|---|---|
| Average static | 507 | 531 | 537 |
| Highest values | 551 | 563 | 617 |
| Lowest values | 463 | 462 | 420 |
| Average dynamic | 2780 | 3170 | 3171 |
| Highest values | 10,590 | 9580 | 10,510 |
| Lowest values | 831 | 909 | 1037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Zhang, Y.; Lu, Y.; Fu, Z.; Mi, H.; Yu, J.; Wang, X. Research on the Preparation and Performance of Wood with High Negative Oxygen Ion Release Induced by Moisture. Coatings 2025, 15, 905. https://doi.org/10.3390/coatings15080905
Yin M, Zhang Y, Lu Y, Fu Z, Mi H, Yu J, Wang X. Research on the Preparation and Performance of Wood with High Negative Oxygen Ion Release Induced by Moisture. Coatings. 2025; 15(8):905. https://doi.org/10.3390/coatings15080905
Chicago/Turabian StyleYin, Min, Yuqi Zhang, Yun Lu, Zongying Fu, Haina Mi, Jianfang Yu, and Ximing Wang. 2025. "Research on the Preparation and Performance of Wood with High Negative Oxygen Ion Release Induced by Moisture" Coatings 15, no. 8: 905. https://doi.org/10.3390/coatings15080905
APA StyleYin, M., Zhang, Y., Lu, Y., Fu, Z., Mi, H., Yu, J., & Wang, X. (2025). Research on the Preparation and Performance of Wood with High Negative Oxygen Ion Release Induced by Moisture. Coatings, 15(8), 905. https://doi.org/10.3390/coatings15080905







