1. Introduction
Strawberries (
Fragaria ananassa) are a seasonal fruit known for their vibrant red hue and juicy texture, balancing sweetness with a hint of tartness. Belonging to the Rosaceae family, this fruit is grown globally, making it widely accessible [
1]. However, due to their high water content, strawberries are very perishable and need to be consumed or processed quickly [
2]. Over 20 species of strawberries and various commercial cultivars exist [
3]. Interestingly, while they are commonly termed fruits, strawberries are not true fruits in a botanical sense. The edible part is an enlarged receptacle, featuring numerous small fruits called achenes embedded within. This unique structure sets them apart from other berries morphologically [
4].
Fruit quality and shelf life are vital for consumers and producers alike. Despite their delicious taste and nutritional value, strawberries are especially vulnerable to spoilage. To combat this, mild preservation methods such as applying natural biopolymer coatings have emerged [
5]. A UNEP report (2024) indicates that in 2022, fruits and vegetables accounted for up to 20% of global food waste [
6].
Strawberries’ delicate structure makes them particularly prone to post-harvest losses [
7]. Edible protective coatings present an efficient solution, forming a barrier that reduces evaporation, oxidation, and microbial growth [
8,
9]. Coatings, including films, serve as modern methods for protecting fresh fruits [
10]. These coatings create a thin layer over the fruit, which can enhance shelf life and potentially impart new functional properties [
11]. The key features of effective coatings include no impact on flavor, proper color, resilience to external factors, safety for human consumption, cost-effectiveness, and eco-friendliness [
12].
According to Hassan et al. [
13], coatings can be classified as edible coatings, applied directly to the product, and edible films, placed on or between products.
Various application techniques exist, including dipping, spraying, manual application, vacuum impregnation, and electrospinning [
14]. The dipping method is the most commonly used, resulting in a thin, often undetectable layer [
15]. When combined with refrigeration, edible coatings can help minimize mass loss and preserve the flavor and texture of strawberries [
16].
Pectin is a noteworthy coating material; it is a natural, biodegradable polysaccharide primarily found in citrus fruits and apples, playing a key role in plant cell structure. Its antimicrobial properties and ability to form flexible barriers make pectin a popular choice for extending the shelf life of food products [
17,
18]. The effectiveness of pectin-based coatings hinges on the type of pectin (low- or high-methoxyl) and the incorporation of plasticizers like sorbitol or glycerol, which can help protect fruits during storage [
19].
Innovatively, pectin coatings enhanced with active compounds, such as gamma-decalactone, a peach-scented antimicrobial compound, show great promise [
20]. This study focuses on assessing the impact of such coatings on the quality and shelf life of strawberries (
Fragaria ananassa) during refrigerated storage [
21].
Originally, coatings focused primarily on moisture loss reduction. Today, they also aim to provide selective gas permeability, improving overall fruit protection [
22]. Active packaging, which may include films with antibacterial properties, can significantly enhance shelf life and product quality [
23]. Furthermore, bioactive packaging can incorporate probiotics, enhancing gut health and suppressing pathogen growth [
24,
25]. Edible coatings can also protect microorganisms from acidic and temperature effects, thereby increasing their stability in food products [
26].
Given their high water content and sensitivity to external conditions, strawberries are highly susceptible to spoilage. Edible coatings effectively limit evaporation, manage gas exchange, and inhibit microbial growth, thereby significantly prolonging shelf life while maintaining sensory qualities [
27,
28].
Recent studies have explored various coating materials to safeguard strawberries. For instance, Popescu et al. [
29] developed chitosan-based coatings enriched with sea buckthorn and grape seed essential oils, achieving effective microbial protection and preserving high vitamin C levels. Similarly, Rahimi et al. [
30] utilized guar gum coatings to minimize water loss and sustain fruit firmness during storage. Other research highlighted the success of gum arabic enriched with bergamot extract in protecting against oxidation and extending shelf life [
31]. Chitosan–aloe vera coatings were shown to effectively prevent moisture loss, retaining the sensory attributes of strawberries for longer durations [
32]. Melikoğlu et al. [
33] combined carboxymethyl cellulose with pomegranate seed oil to improve firmness while providing additional antioxidant and antimicrobial properties. Muñoz-Almagro et al. [
18] created sunflower pectin coatings enriched with stevia and saccharin, which successfully extended strawberries’ shelf life while preserving their firmness.
Scientists are also increasingly focusing on the issue of introducing bioactive substances into coatings, which, without changing the appearance of the coating and its physicochemical parameters, contribute to extending the shelf life of fruits covered with it. Lactones are very popular cyclic esters with proven antibacterial properties, mainly bacteriostatic ones [
34]. Kim et al. [
35] demonstrated the antimicrobial activity of artemisinin against periodontopathic microorganisms—
Gram-negative anaerobic bacteria such as
Aggregatibacter actinomycetemcomitans,
Fusobacterium species, and
Prevotella intermedia. Appalasamy et al. [
36] proved that this lactone can be an effective inhibitor of the
Gram-negative bacteria
Salmonella sp. and Gram-positive bacteria
Staphylococcus aureus and
Bacillus subtilis. In another study by Boulanger et al. [
37], helenalin was shown to be a potent in vivo antimicrobial agent against
S. aureus.
In addition to sesquiterpene lactones, simple lactones such as γ-lactones, including gamma-decalactone (GDL), a naturally occurring lactone in peaches and strawberries, also exhibit antimicrobial properties [
38]. GDL is an intramolecular ester of 10-hydroxdecanoic acid, which, in addition to its pleasantly fruity, creamy scent, has potent inhibitory activity against bacteria and fungi [
39]. Due to the above properties, this compound has excellent potential as an additive to coatings, especially those applied to fruits, where GDL occurs naturally and can act synergistically to enhance the natural aroma and limit the growth of undesirable microorganisms. This study aims to evaluate the impact of pectin coatings enriched with gamma-decalactone on the quality and shelf life of strawberries (
Fragaria ananassa) during refrigerated storage. The assessment focused on quality attributes like pH, mass changes, soluble solids content, texture, spoilage rate, and color change.
2. Materials and Methods
2.1. Materials
The research material consisted of edible protective coatings used in the strawberry coating process, made from a solution of apple pectin (ZPOW “PEKTOWIN” S.A., Jasło) with a degree of esterification of 30-35% and a degree of amidation of 15-20%, supplemented with (+)-γ-decalactone (natural, ≥97%, Sigma-Aldrich, Poznań, Poland) that served to extend the shelf life of the fruit. Glycerol (Avantor Performance Materials Poland, Gliwice) was used as a plasticizer, and Tween 80 (Sigma-Aldrich, Poznań) served as an emulsifier. The microbiological media used to determine the level of strawberry spoilage were DRBC—Dichloran Rose-Bengal Chloramphenicol Agar Base (BTL, Łódź, Poland)—and TSA—Tryptic Soy Agar (BTL, Łódź, Poland). Sterile plastic stomacher bags were used to homogenize strawberry fruits (Bionovo, Wrocław, Poland).
2.2. Preparation of Film-Forming Solutions
Aqueous film-forming solutions were prepared using apple pectin at a concentration of 5% (5 g/100 g) and heated to 50 °C, maintaining this temperature for 15 min. The solution was stirred with a magnetic stirrer (RCT Basic IKAMAG, IKA Poland Sp. z o.o., Warsaw, Poland) at 500 rpm to achieve a clear consistency. Glycerol was then incorporated as a plasticizer, constituting 30% of the pectin content (1.5 g/100 g). Gamma-decalactone was added in volumes of 2.5, 5, and 10 mL per 100 mL of the solution, alongside Tween 80 (Sigma-Aldrich, Poznań, Poland) used as an emulsifier at 0.5 mL per 100 mL of the film-forming solution. The mixtures first underwent preliminary homogenization with a homogenizer (IKA T25 digital ULTRA TURRAX, IKA Poland Sp. z o. o., Warsaw, Poland) at a speed of 10,000 rpm for 2 min, followed by ultrasonic homogenization with an ultrasonic homogenizer (VC 505, Labo Plus Sp. z oo, Warsaw, Poland) operating at a power of 20 Hz for 2 min.
2.3. Coating of Strawberries
Strawberries (Fragaria × ananassa) were purchased from a local greengrocer, selected for similar ripeness, weight of 15 ± 2 g, and dimension of 40 ± 5 mm. The fruits were rinsed with water and then gently dried using a paper towel at a temperature of 23 ± 1 °C. The strawberries were immersed in coating solutions for 30 s, then removed and left to air dry for 1 min. This dipping and removal procedure was repeated twice to ensure complete surface coverage. After this step, the samples were left to dry for 30 min to allow full adsorption of the coatings. Sets of seven strawberries were then placed into ventilated PET (polyethylene terephthalate) fruit containers (size 142 mm × 96 mm × 29 mm) and stored in a refrigerated chamber (Pol-EKO APARATURA sp.j., Wodzisław Śląski) at a constant temperature of 4 °C and 90% humidity. There was no airflow in the storage chamber. The process was monitored every 24 h, with measurements taken on specific storage days: 0, 5, 7, and 9. Three types of samples were prepared: uncoated strawberries (control—C), strawberries coated with apple pectin (AP), and strawberries coated with apple pectin enriched with gamma-decalactone (AP + GDL).
2.4. Characterization of Coating Solutions
2.4.1. Emulsion Density
The emulsion (film-forming solutions) density was measured using a Densito 30PX densimeter from Mettler Toledo to understand the effect of GDL concentration on the pectin emulsion. Each measurement was performed with at least three replicates for each sample. The measurement was performed at a temperature of 23 °C.
2.4.2. Emulsion pH
The pH of the film-forming solutions was determined using a SevenCompactTM S220-basic pH meter from Mettler Toledo. The measurement was carried out at a temperature of 23 °C, with at least three repetitions.
2.4.3. Rheological Properties
A Haake MARS 40 rheometer (Thermo Fisher Scientific Inc., Waltham, MA, USA ) was used to observe the flow behavior of the solutions at 25 °C in a system of coaxial cylinders (CC25DIN/Ti) with a linearly increasing shear rate up to 100 s
−1. The flow curves were fitted using the Ostwald de Waele model [
40,
41]:
where
τ is the shear stress (Pa);
is the shear rate (s
−1);
K is the consistency index (Pa⋅sn); and
n is the dimensionless flow behavior index.
2.4.4. Emulsion Stability
The emulsion stability measurement was performed using the Turbiscan Lab
® Expert apparatus by Formulaction SA in accordance with the manufacturer’s operating instructions. The intensity of backscattered monochromatic light with a wavelength of 880 nm was measured for emulsions with added gamma-decalactone. Emulsions (20 mL) were scanned across the entire height of the measuring vial at least 5 times over 7 days. The emulsions were stored in a refrigerator at 4 °C between measurements. The Turbiscan Stability Index (TSI) was determined based on the TurbiSoftLab 2.3.1.125 software version 2.3., Turbiscan Lab® Expert (Formulaction, Toulouse, France). The measurement was performed in triplicate for each emulsion.
where
h is the height of the material in the vial (mm) and
H is the total height of the vial (mm).
2.4.5. Viscosity of Coating Emulsions
The study was conducted using a Haake MARS 40 rheometer from Thermo Scientific. The emulsions were tested at a temperature of 20 °C in a coaxial cylinder system at a shear rate increasing linearly to 100 s−1. The Rheowin Job Manager program was used to record and process the obtained results.
2.5. Characterization of Coated Strawberries
2.5.1. Weight Loss
The weight loss of strawberries during storage was assessed in duplicate using an electronic balance (WLC 20/A2 RADWAG, Radom, Poland), with an accuracy of 0.0001 g. The percentage of weight loss was calculated using the following formula [
33]:
where
M1 is the initial mass (g) and
M2 is the final mass (g).
2.5.2. Total Soluble Solids
The total soluble solids from strawberries were measured using a digital refractometer PAL-1 (ATAGO) with three repetitions, and the obtained results were expressed in Brix degrees.
2.5.3. pH
The pH measurement was conducted with three repetitions using the pH meter Lab 850 (SCHOTT Instruments, Warsaw, Poland). The pH meter electrode was immersed in the juice of mashed strawberries, and the results were read from the digital display.
2.5.4. Color
The color measurement was conducted using the CIELAB (
L*
a*
b*) system. A portable colorimeter from Konica Minolta CR5 was used for the measurement, which was calibrated against a white standard (
L* = 37.41,
a* = 36.82, and
b* = 22.58) before testing. The color was measured ten times for each sample, and then the average color components (
L*, a*, and
b*) were determined from the measurements. Additionally, the saturation (
C*) and hue angle (
h*) values were automatically generated by the colorimeter software and included in the analysis. The
L* component, representing brightness, ranges from 0 to 100, the
a* component indicates the degree of green (−120) to red (+120), while the
b* component indicates the degree of blue (−120) to yellow (+120). The obtained color components were used to calculate the absolute color difference (Δ
E) using the formula [
42]:
where
L*, a*, and b* are the color parameters of the standard with constant color parameters (
L* = 37.41,
a* = (36.82),
b* = 22.58) and L, a, and b are the color parameters of the tested films.
2.5.5. Texture
The texture measurement was carried out using the TA-XT2i texture analyzer (Stable Micro Systems Products, Surrey, UK). The results of the measurements were recorded using the TextureExpert software (version 2.3). The penetration test involved applying mechanical force using a 5 kg calibrated load cell and a cylindrical probe with a flat head, 5 mm in diameter (P/5), which penetrated the fruit to a depth of 5 mm. The fruits were placed on a plate with a calyx indentation facing the pressure probe to assess the firmness of the fruit. The mechanical profiles were recorded at a data acquisition frequency of 300 points per second (pps), with the following instrumental settings: pre-test speed, 10.00 mm/s; test speed, 5 mm/s; post-test speed, 10.00 mm/s; and trigger force, 2.0 g [
31]. Seven repetitions were performed for each sample.
2.5.6. Decay Analysis
Strawberries were visually assessed and considered spoiled when visible damage, such as brown spots, softening, or mold presence, was observed. The percentage of decay (DC) was calculated using the following equation [
31]:
where
NIF is the number of infected fruit samples and
INF is the initial number of all fruit samples.
2.5.7. Determination of the Microorganism Community in Fruit After Storage
On the third day after the fruit was removed from the cold store (twelfth day of total storage), microorganisms were determined in the individual strawberry varieties. For this purpose, 10 g of each strawberry variant (C; AP; AP + GDL) was weighed and suspended in 100 mL sterile saline solution in a sterile filter bag stomacher. Three replicates were performed for each variant. This matrix was crushed and shaken to standardize.
Specific culture media were used to determine the content of microorganisms in individual fruits—DRBC (to determine the total number of yeasts and molds) and TSA (to determine bacteria). To determine the number of microorganisms, 1 mL of the initial solution was taken and, after appropriate serial dilution, was plated on Petri dishes. The plates were incubated for 24 h at 37 °C (TSA medium) and 48 h at 28 °C (DRBC). Characteristic colonies grown on individual media were counted, giving the result as the log number of colony-forming units per gram of sample (log10 CFUs/g).
2.6. Statistical Analysis
Statistical analysis was performed using Statistica 13 software (StatSoft Inc., Tulsa, OK, USA). One-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed to detect significant differences in the properties of the samples at the 0.05 significance level. Principal Component Analysis (PCA) was applied to classify and discriminate between different samples of control and coated fruits.
3. Results and Discussion
3.1. The Effect of Gamma-Decalactone on the Stability of Pectin Emulsions
The density of the emulsion depends on the content of the dispersed phase, the type of emulsifier, and the viscosity of the dispersing phase [
43]. The addition of gamma-decalactone affected the density of polysaccharide emulsions (
Table 1). With the increase in the share of gamma-decalactone, a decrease in the density of the emulsion was observed. The reason for these changes is that gamma-decalactone is an oily substance that has a lower density (0.952 g/cm
3) than water. The higher the addition of this substance, the lower the density of the entire system [
44]. Various additives affect the microstructure of the emulsion, which entails consequences concerning the impact on viscosity, stability, or density.
On the other hand, the addition of gamma-decalactone to the apple-pectin-based emulsion formulation revealed a certain tendency to lower the pH of the emulsion with the use of gamma-decalactone (
Table 1). Gamma-decalactone, which comes from the lactone group, can undergo hydrolysis in an aqueous environment, which leads to its transformation into the corresponding carboxylic acids [
45]. The described hydrolysis process explains the noticeable decrease in the pH value of the emulsions tested.
Emulsion viscosity is a measure of the internal resistance of the fluid to flow. Emulsions can exhibit different rheological behaviors, depending on composition, temperature, concentration, or the size of dispersed drops. Flow and viscosity curves allow for the prediction of how the emulsion will behave during processing, storage, and dosing. These issues are crucial for ensuring the appropriate quality, functionality, and safety of food industry products, as well as in other industries such as cosmetology or pharmacy. For food emulsions, the desired sensory properties may require appropriate viscosity values, which will allow, for example, adjusting the appropriate lubricity while maintaining the ingredients in suspension and preventing their separation [
46]. Flow curves (
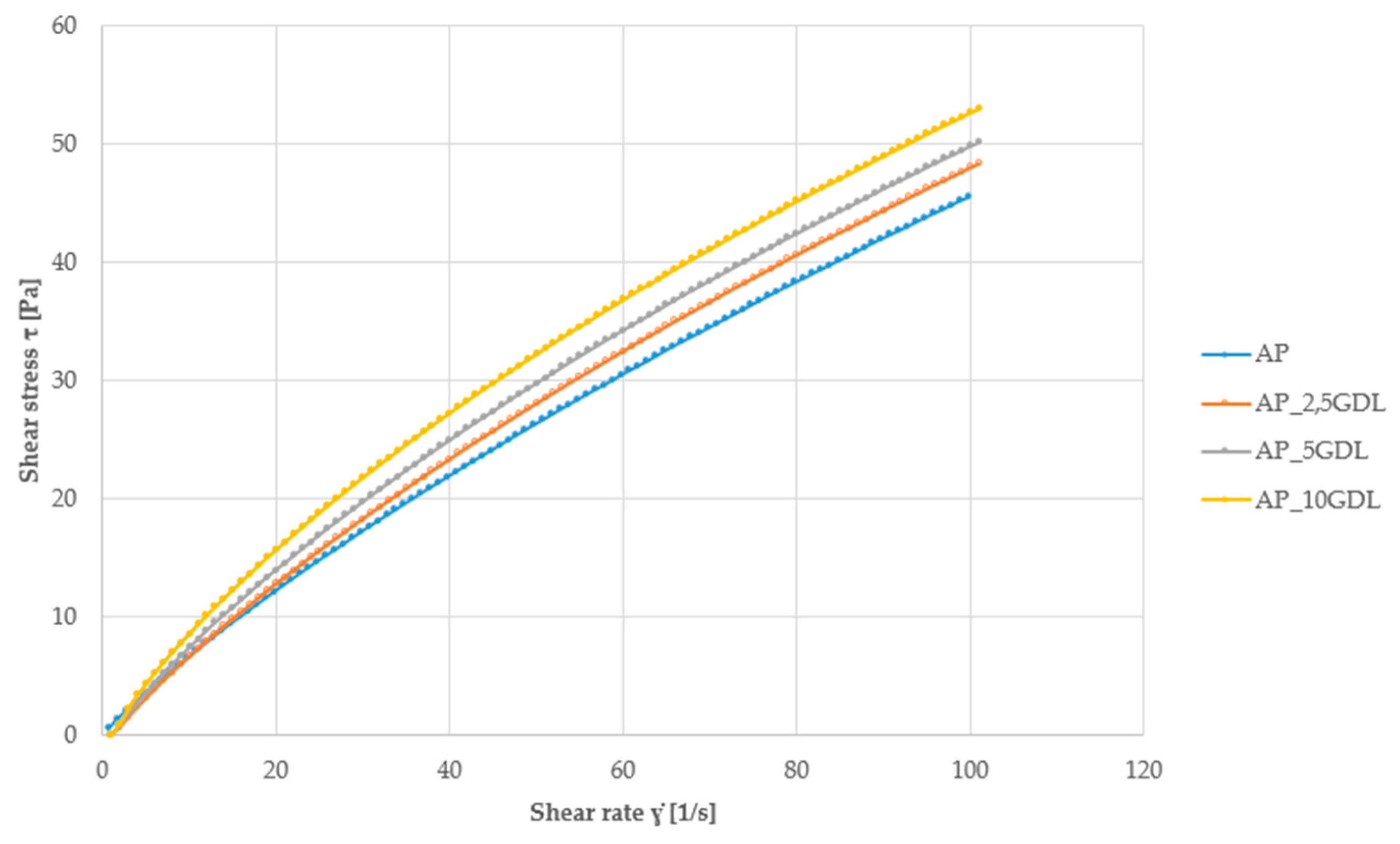
Figure 1) are a graphical method for presenting the relationship between shear stress and the shear rate. Viscosity curves (
Figure 2) present the dependence of viscosity on the shear rate. In the case of Newtonian fluids, it is a straight line that is parallel to the shear rate axis [
47].
The flow curves shown in
Figure 1 indicate that the apple pectin emulsions studied are not Newtonian fluids, because the graphs do not have the form of a straight line passing through the origin of the coordinate system. Instead, they exhibit a nonlinear course, which indicates the pseudoplastic nature of these emulsions—i.e., the viscosity decreases with an increasing shear rate.
The curves for samples containing different concentrations of gamma-decalactone are similar but slightly different—which indicates a certain effect of the lactone addition on the flow properties of the emulsion. The curvature of the graphs confirms that we are dealing with shear-thinning fluids.
Figure 2 shows viscosity curves that show the dependence of viscosity on the shear rate. All analyzed samples show a decrease in viscosity with an increasing shear rate, which confirms the non-Newtonian and pseudoplastic nature of the emulsions studied. This is typical for systems containing polysaccharides, such as pectin, which is also confirmed in the literature [
48]. Thixotropic features were also observed, i.e., a decrease in viscosity over time under the influence of constant shear stress. In practice, this means that the emulsion can easily flow during processing or dosing and then regain its structure after the force has ceased to act. It is worth noting that despite the different concentrations of gamma-decalactone, the viscosity curves for emulsions with pectin assume different viscosity values at low shear rates but approach each other at higher speeds. This may mean that the effect of pectin concentration on viscosity becomes less significant with intensive mixing or flow.
The physical stability of the emulsion was tested using the Turbiscan Lab
® Expert apparatus, which allows for the testing of the backscattering of light throughout the entire height of the vial. The Turbiscan Stability Index (TSI) describes the stability of the system; the higher the value of this index, the less stable the emulsion. The selected method for testing the stability of systems allows for very early detection of the occurrence of the phenomena of creaming, sedimentation, coalescence, or flocculation. It is these physical phenomena that cause a decrease in the stability of the emulsion [
49].
Figure 3 shows the emulsions tested, in appropriate vials, prepared for stability measurement. The photo shows that the tested systems are continuous and there is no noticeable separation of the individual emulsion phases.
However, after a 7-day storage period, no significant changes in emulsion stability were observed in the case of samples with apple pectin. It can therefore be assumed that these emulsions retained their stability. It was also observed that samples with pectin gelled during storage. This process contributed to the improvement in the stability of the system, preventing its delamination.
The physical stability of emulsions refers to the ability of the system to resist changes occurring at the liquid–liquid interface during storage [
43].
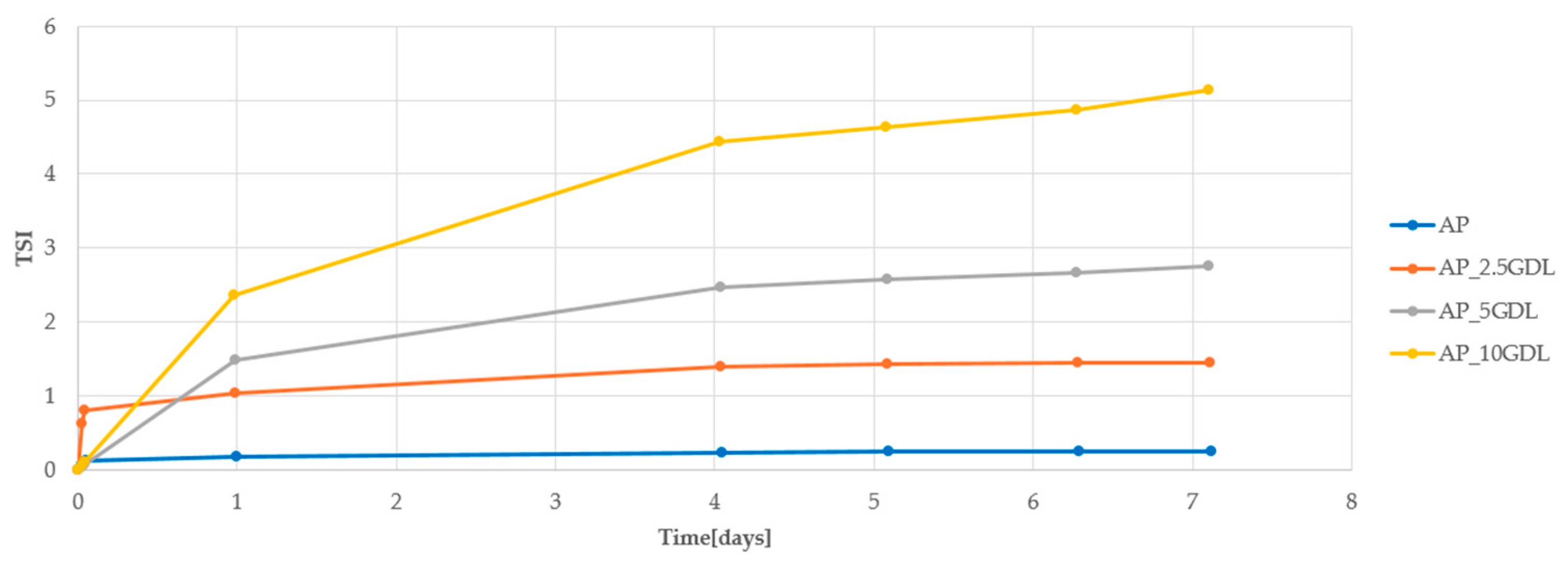
Figure 4 shows the changes in the Turbiscan Stability Index (TSI) during 7-day storage of the emulsion. The TSI value takes into account all destabilization processes; therefore, lower values indicate greater stability of the system. Emulsions based on apple pectin showed high stability.
These emulsions gelled, which is a physical change that helps maintain the stability of the system. As a result, all emulsions with apple pectin remained stable for 7 days of storage at 4 °C. Among them, the best stability was observed in the sample containing 2.5% of gamma-decalactone.
3.2. Characteristics of Uncoated and Coated Strawberries
The visual analysis of strawberries stored under refrigerated conditions, presented in
Figure 5, revealed significant differences in external appearance and pulp structure, depending on the applied protective coating. The appearance of the product plays a crucial role in its acceptance by consumers. The coatings imparted a slight yellowish tint to the fruits but did not significantly affect their overall appearance after application [
50].
In the case of the control sample (C), the fruits were initially glossy and maintained a good appearance; however, after 7 days, they began to show clear signs of quality deterioration, including the loss of freshness and the first symptoms of degradation. Over the following days, these changes became increasingly evident, and on day 9, it was observed that the fruits had begun to lose their color, indicating further progression of the spoilage process. The pulp in the control sample became progressively softer, resulting in a deterioration in the fruit structure. Strawberries are a delicate fruit, where the ripening and aging processes cause rapid softening of the pulp [
51]. This is probably attributed to the nature of the fruit, especially the soft structure and high water content. Strawberries are not a climacteric fruit; thus, the aging process starts at the time of harvesting.
Strawberries coated with apple pectin (AP) showed a similar trend in changes, although they looked better for a longer time compared to the control sample. The surface of the fruits maintained a slight gloss, and the quality deterioration process was slower. When cut, the pulp in this group was still in good condition, but by day 9, the first signs of moisture loss began to appear, suggesting initial changes in the pulp structure. Only on the last day, subtle effects similar to those seen with gamma-decalactone began to appear, though they were less pronounced.
The outer layer of the fruit showed signs of drying, which may indicate an evaporation process. Additionally, a small amount of liquid was observed in the storage containers, suggesting changes in the fruit’s structure, even though there was no visible damage to the surface. Cross-sections of strawberries from this group revealed an interesting effect—despite the loss of gloss on the surface, the flesh inside remained moist. This suggests that the gamma-decalactone coating influenced uneven moisture retention, limiting moisture loss from the inside of the fruit but simultaneously contributing to surface drying. By the fifth day, the beginnings of this effect were noticeable, unlike in the samples coated with apple pectin (AP), where similar changes were visible only on day nine.
3.3. Effect of Coatings on Mass Changes in Strawberries During Refrigerated Storage
The loss of fruit mass is an important indicator of quality, closely related to the processes of transpiration and respiration. During storage, water is lost, which can lead to a deterioration in quality, with respiration playing a particularly important role under conditions of high humidity when transpiration becomes less significant [
52]. Strawberries are highly susceptible to water loss, which results in fruit shrinkage and weakening of their tissue, mainly due to their exceptionally thin skin [
53]. Therefore, mass changes in strawberries were monitored during refrigerated storage for 0, 5, 7, and 9 days to assess the effect of protective coatings on their quality. The results are presented in
Table 2.
On the fifth day of refrigerated storage, the first mass changes in strawberries were observed. The highest increase was recorded in the group of strawberries coated with pectin enriched with gamma-decalactone (14.81%), indicating its low effectiveness in moisture retention. Control samples had the lowest value (11.78%), while strawberries coated with pectin (12.87%) showed better moisture retention than the enriched version but less effective retention than the uncoated fruits. Similar observations were made by Muñoz-Almagro et al. [
18], who noted that the pectin coating applied to strawberries, without additives, limits water loss through transpiration compared to other edible coatings, such as chitosan-based coatings and agar gels.
After seven days of refrigerated storage, the highest mass change still occurred in the group of strawberries coated with the gamma-decalactone-containing coating (AP + GDL) (18.81%), exceeding both strawberries coated with pectin (17.10%) and control samples (15.44%). The largest differences were noted after nine days, when the mass of fruits coated with pectin enriched with gamma-decalactone (AP + GDL) increased to 22.80%, and the pectin-coated group reached 21.79%. Control samples had a value of 19.05%, suggesting that uncoated fruits lost less moisture than those coated with gamma-decalactone.
Comparing the changes within each group, mass loss increased with the length of refrigerated storage but at different rates. In the control group, the rate of mass loss was relatively uniform—the values differed significantly (p ≤ 0.05) between days 5 and 9, but the changes were not as drastic as in the other variants. In the pectin-coated samples, the initial mass loss was similar to that of the control samples, but later on, this rate was somewhat higher, suggesting that the coating did not provide long-term protection against dehydration. The gamma-decalactone-enriched variant, from the very beginning, exhibited the highest mass loss, and the differences between days 5, 7, and 9 were more pronounced than in the other groups.
In many studies, it has been observed that strawberries coated with various materials, regardless of type, show better water retention compared to uncoated fruits. However, the results presented in the above studies differ from this general trend. On the other hand, Estrada-Girón et al. [
19] observed that on days 7 and 14 after applying pectin coatings obtained from by-products of
Hibiscus sabdariffa L., the coated strawberries exhibited faster mass loss compared to control samples. However, by the end of the storage period, these fruits showed significantly less mass loss than uncoated strawberries. The authors suggest that the initial mass loss may result from the evaporation of water near the fruit surface, but over time, the coating acts as a barrier, preventing further water loss from deeper layers of the fruit. Some researchers indicate that such properties of coatings, especially pectin coatings, may result from their ability to limit water diffusion, especially when combined with lipids [
54].
In contrast, in the study by Guerreiro et al. [
55], mass loss increased in all strawberry samples, including the coated ones. Edible coatings had no effect on reducing mass loss, and it can be stated that, compared to control samples, they did not result in less water loss. Additionally, in the case of sodium alginate with citral at various concentrations, as well as pectin with citral (0.3 and 0.15%) and eugenol (0.1%), significant mass loss was observed, greater than in the control samples.
Despite the visible differences, statistical analysis showed that the differences between the types of coatings were not statistically significant, which means that the mass loss processes did not differ significantly from a statistical point of view. However, the differences between the individual storage times within a given protective coating were statistically significant (p ≤ 0.05), especially in the case of the coating enriched with gamma-decalactone. The obtained results allow us to conclude that the pectin coating enriched with gamma-decalactone was the least effective in reducing the mass loss of strawberries, while the pectin coating slightly improved water retention, though it was not significantly better than no protective coating.
3.4. Effect of Coatings on Total Soluble Solids in Strawberries During Storage
Changes in the extract content in fruits are the result of the degree of ripeness and metabolic processes, which during storage lead to an increase in the sugar content and intensification of the sweetness of the fruit [
56]. The study concerns the changes in the extract content (°Brix) depending on the cold storage time and the type of material used. The extract values are presented in
Table 3.
Analysis of the results showed that the extract values increased with the extension of storage time: from 9.77 to 11.37 °Brix in the case of control samples, from 9.80 to 10.80 °Brix in the case of apple pectin coating, and from 9.92 to 12.00 °Brix in the case of apple pectin coating enriched with gamma-decalactone. After 5 days, an increase in the extract was noted in all groups compared to the initial values. The highest values were noted after 9 days, with the largest increase (up to 12.00 °Brix) noted in the group with gamma-decalactone. The values in this group were significantly higher (p ≤ 0.05) than in the others, which may suggest the influence of gamma-decalactone on the change in the chemical composition and concentration of soluble components.
An important observation is the fact that the lowest extract values throughout the entire cold storage period were recorded in the group of strawberries covered with a pectin coating (AP), where, after 9 days, they amounted to 10.80 °Brix. This indicates the stability of this sample and its lower susceptibility to metabolic changes that lead to an increase in sugar content [
57]. Statistical analysis showed significant differences (
p ≤ 0.05) between samples, with the smallest differences observed in the case of fruits coated with apple pectin (AP), which is consistent with the results of De Bruno et al. [
31], who noted that the extract content in strawberries increased statistically significantly (
p ≤ 0.05) with the cold storage time.
The lower dynamics of the increase in extract content may be due to the protective properties of the pectin coating (AP), resembling the barrier mechanism of edible coatings. Such selective properties were described by Adhikari et al. [
58], who showed that edible coatings limit gas exchange, which can slow down metabolism and reduce changes in the extract content in the fruit. This type of mechanism may confirm the observed stability of the extract value in the group of fruits treated with the apple pectin (AP) coating.
The extract values obtained in this study (9.77–12.00 °Brix) are within the range given by Treviño-Garza et al. [
21], who determined that the extract content in strawberries ranges from 8.27 to 13.13 °Brix. The increase in extract is the result of the decomposition of carbohydrates into simple sugars, which are used in respiration [
59]. Additionally, differences in values may result from the variety, cultivation conditions, or sunlight, as well as the degree of fruit ripeness. The decomposition of polysaccharides in fruits during ripening leads to an increase in the extract content, and water loss additionally contributes to its increase by increasing the concentration of sugars due to the reduction in fruit mass [
60].
In the control group (C), the extract values increased from 9.77° to 11.37 °Brix after 9 days of cold storage. However, the difference between days 7 and 9 was not statistically significant. In the group of fruits coated with apple pectin (AP), the increase was less dynamic (from 9.80 to 10.80 °Brix), which suggests that the chemical composition of this sample was more stable. In the group of fruits covered with pectin coating with the addition of gamma-decalactone (AP + GDL), the highest extract values were recorded, and the difference between days 7 and 9 was statistically significant (p ≤ 0.05), which indicates further development of biochemical processes leading to an increase in the content of soluble substances.
The results of the current study confirm the observations of Shafique et al. [
61], who showed that the use of edible coatings containing moringa leaf extract, aloe gel, oxalic acid, and ascorbic acid contributed to reducing the losses of extract and organic acids in strawberry fruits, positively affecting their quality during storage. Similar results were obtained by Silva et al. [
16], showing that edible coatings based on apple pectin, cellulose nanocrystals, and lemongrass essential oil contribute to maintaining higher values of water-soluble components, limiting gas exchange between fruits and the environment, which slows down degradation processes and favors the preservation of soluble components such as sugars and organic acids.
3.5. Effect of Coatings on the pH of Strawberries During Storage
Strawberries, due to their properties, are classified as sour fruits, with pH ranging from 3.2 to 3.4 [
62]. During fruit ripening, intensive biochemical processes occur, which lead to an increase in the production of organic acids. These acids are used in cellular respiration, which in turn contributes to an increase in the pH of the fruit [
63]. Changes in pH can also be attributed to a decrease in available organic acids, which are consumed in the ripening process, which affects the metabolic activity of the fruit [
64].
The increase in pH during the shelf-life study of the fruit may be the result of enzymatic activity and aging, which ultimately led to a decrease in the acidity level. Some components of edible coatings, such as chitosan or essential oils, may also have an impact on these processes, as noted in the studies by Perdones et al. [
65], who showed that a coating of chitosan and lemon oil effectively delayed the spoilage processes of strawberries, and Martínez et al. [
66], who found that the addition of thyme oil to chitosan coatings helped maintain the quality of fruit during cold storage.
The results of the conducted studies show a general trend of increasing pH in the tested samples, as illustrated in
Table 4. Statistical analysis confirmed statistically significant differences (
p ≤ 0.05) between individual measurements, suggesting a significant effect of cold storage time on changes in fruit pH.
In the control samples (C), the pH increased from 3.67 to 3.72, while in the apple pectin (AP)-coated fruit, a slight change from 3.68 to 3.67 was noted. In the samples coated with apple pectin enriched with gamma-decalactone (AP + GDL), the pH value increased from 3.71 to 3.73. The rate of these changes and the final pH values varied depending on the coating used. In the control samples (C) and those coated with apple pectin (AP), the pH increased until the 7th day of cold storage, reaching values of 3.74 and 3.79, respectively. After this period, on the 9th day, a slight decrease in pH was noted (to 3.72 and 3.67).
In samples coated with apple pectin enriched with gamma-decalactone (AP + GDL), the maximum pH value (3.80) was reached earlier, on day 5, followed by a gradual decrease to 3.73 on day 9. The greatest increase in pH was observed in the group with added gamma-decalactone, which may suggest that gamma-decalactone accelerates certain biochemical processes, leading to faster neutralization of organic acids. Zheng et al. [
67] reported that the increase in pH of strawberry juices during refrigerated storage can be attributed to a decrease in fruit acidity. According to the results of the study conducted by De Bruno et al. [
31], a similar effect could result from the action of enzymes and the ripening process of strawberries. However, in a later period (after day 5), a decrease in pH was noted in this group, which indicates a gradual stabilization of these changes. It is also worth noting that pH changes in the group of strawberries coated with pectin coating with added gamma-decalactone (AP + GDL) were more even compared to the control sample, which suggests that the addition of gamma-decalactone slowed down the further increase in pH.
Statistical analysis showed significant differences (
p ≤ 0.05) between days of cold storage; however, a particularly interesting result is the fact that on day 7 all samples did not show significant statistical differences, suggesting a relative stabilization of pH at this time point. Importantly, the smallest statistical difference between storage times (
p ≤ 0.05) was noted in samples coated with apple pectin (AP), indicating their greatest pH stability. The buffering properties of pectin could limit the consumption of organic acids in metabolic processes, stabilizing the pH of the fruit [
68]. In addition, apple pectin slows down degradation processes associated with the metabolism of organic acids, which delays fruit ripening and thus extends its shelf life. Such pectin action supports the extension of the shelf life of strawberries, which was confirmed by [
66].
Strawberries coated with the pectin coating enriched with gamma-decalactone (AP + GDL) were initially characterized by gloss, which disappeared over time (
Figure 5). The outer surface of the fruit showed signs of drying, and liquid leakage was observed in the storage containers. This may suggest the loss of water and soluble components, resulting in a decrease in the amount of available solvent. This phenomenon could have affected the results, leading to changes in the pH value, especially in the final days of refrigerated storage.
3.6. Effect of Coatings on the Color of Strawberries During Storage
The color of strawberries is a key criterion for assessing their quality and degree of ripeness.
Table 5 presents the results of the analysis of strawberry color parameters:
L*—lightness;
a*—color component from green (negative values) to red (positive values);
b*—color component from blue (negative values) to yellow (positive values);
C*—color saturation;
h*—color angle; and Δ
E—total color difference.
Statistical analysis showed significant differences (p ≤ 0.05) in the color values of strawberries during the cold storage period. In particular, for the parameters b* (color from green to yellow), h* (color angle), and ΔE (total color difference), changes were observed between days of storage in cold conditions and types of coatings, which suggests the influence of both the storage time and the protective coatings used on the color of the fruit.
The value of the
L* parameter, defining the lightness of the fruit, did not change significantly under the influence of the coatings and remained at a level close to the reference value (
L* = 37.41). According to the data presented in the table, the lightness of the control samples (C) at the beginning of cold storage was higher on day 0 in comparison to day 5 and then increased. In the case of the apple pectin (AP) coating, the initial lightness was slightly lower than for the control films and increased on day 5 and decreased on the following days (7 and 9), indicating maintained stability. The apple pectin coating enriched with gamma-decalactone (AP + GDL) had a similar trend, which also indicates insignificant changes. A statistically significant difference (
p ≤ 0.05) was observed only on the 5th day of cold storage between the coating types and in the case of the storage time of uncoated strawberries, and the difference was marginal. However, a slight decreasing trend was observed during cold storage, which may be related to the natural aging process of the fruit and enzymatic oxidative browning occurring in its tissues [
33]. Additionally, during the later period of cold storage, mold could appear on the fruit surface, which could additionally reduce its brightness. Despite these changes, the coatings did not have a significant effect on the preservation of fruit brightness compared to the control samples [
64].
Statistical analysis showed significant differences (p ≤ 0.05) in the color values of strawberries during the cold storage period. In particular, for the parameters b* (color from green to yellow), h* (color angle), and ΔE (total color difference), changes were observed between days of storage in cold conditions and types of coatings, which suggests the influence of both the storage time and the protective coatings used on the color of the fruit.
The color parameter
a*, which is responsible for the intensity of the red color, remained stable and close to the initial value, which suggests that the coatings had an effect on the preservation of the red color of the fruit. In the first 7 days of cold storage, a slight increase in the values of the parameter
a* was noted. These values showed slight differences in both the control and coated samples. Then, a decrease was observed on the 9th day of cold storage. It should be noted that a statistical difference (
p ≤ 0.05) can be observed between coating types after 7 days of refrigerated storage. Similar observations were noted by Barikloo et al. [
69], who found that the
a* parameter increased until the 4th day of storage for fruits in nanocomposite- and chitosan-coated packaging, after which it showed a decreasing trend until the 10th day.
The b* parameter value, which determines the yellow–blue shade, remained at a level close to the reference value, and the slight fluctuations observed can be attributed to the natural ripening process of the fruits. In the samples with the pectin coating (AP), the b* value decreased. In the case of coatings, statistically significant changes (p ≤ 0.05) were observed, especially for the apple pectin coating, between the times of cold storage. These differences were also noticeable in relation to specific days of cold storage between different types of coatings. However, in the control sample and on day 0 of storage, no significant statistical changes were observed.
The
C* parameter, defining the color saturation, remained stable, showing only a slight downward trend, which suggests that the coatings used did not have a significant effect on the color intensity of the strawberries. The
C* value in control samples was initially 43.04, and after 9 days, it dropped to 42.93. In pectin samples (AP), the decrease was from 45.18 to 42.62, and in samples enriched with gamma-decalactone (AP + GDL), it was from 41.63 to 39.12. Statistical analysis showed only significant differences (
p ≤ 0.05) related to the cold storage time between different types of coatings, while no significant statistical changes were noted between storage times with the same coating. As shown by Estrada-Girón et al. [
19], the decrease in the
C* value occurred both in coated fruit and in the control sample, which confirms the relationship that a higher color saturation is associated with a higher anthocyanin content and greater color purity.
According to the study by Moreno et al. [
70], the change in fruit color is considered an important indicator of its ripeness, and the stability of color parameters indicates the maintenance of fruit quality during storage. The study by De Bruno et al. [
31] showed that a gum arabic-based coating enriched with natural antioxidants (2.5%), such as citrus fruit extract, and bergamot essential oil (0.1%) effectively stabilized the parameters
b* (color component from blue to yellow) and
C* (color saturation). The observed changes in these parameters were statistically insignificant, even after 14 days of refrigerated storage.
The hue angle (
h*) is another important indicator of color quality [
71]. Similarly to the
b* value, a statistical difference (
p ≤ 0.05) was visible, except for in the control sample, and the hue angle showed a general decreasing trend. The
h* values in control samples decreased from 31.81 (day 0) to 31.21 after 9 days. In samples with the pectin coating (AP), the decrease was from 31.41 to 28.66, and in samples with the coating enriched with gamma-decalactone (AP + GDL), it was from 31.15 to 24.63. Similar relationships were observed in the study by Méndez et al. [
72], where the
h* value was also lower compared to the initial measurements.
In the case of the h* and b* values, statistical differences (p ≤ 0.05) in fruit samples coated with apple pectin enriched with gamma-decalactone (AP + GDL) were noticeable only between day 0 and the following days, indicating a clear decrease compared to the initial day. This may suggest that gamma-decalactone affects both the decrease in the hue angle and the decrease in the intensity of the yellow color, which may be important in the context of changes in the color and quality of products.
The total color difference (ΔE) value allows us to assess the degree of color change in strawberries during cold storage. In all samples, the value of this parameter gradually increased from 3.20 (day 0) to 4.29 (day 9) in control samples, from 2.41 (day 0) to 3.88 (day 9) in samples with the pectin coating, and from 1.65 (day 0) to 6.90 (day 9) in samples enriched with gamma-decalactone. These differences were statistically significant (p ≤ 0.05) but remained insignificant in the control sample. Nevertheless, the extent of these differences did not indicate radical visual changes, suggesting that the coatings used did not have a significant effect on the color stability of the strawberries.
The total color difference (Δ
E) values in the gamma-decalactone-enriched samples were higher, suggesting slightly greater color changes in these fruits compared to the control and pectin-coated samples. However, these changes were relatively small and could result from natural ripening and aging processes. Similar results were obtained by Mizielińska et al. [
23] for coatings containing zinc oxide nanoparticles and Verbascum L. and Formitopsis betulina extracts, who found that after the storage period, there were no significant differences in the total color difference (Δ
E) values and the
L* parameter in all strawberry samples coated with these coatings.
The obtained results for optical properties indicate that the biological changes during fruit storage had the most significant impact on the quality attributes of the strawberries. This is also connected with water movement during storage, and thus fruit deterioration, which was observed at different levels.
3.7. Effect of Coatings on Strawberry Texture During Storage
Fruit softening is a natural physiological process that occurs during fruit ripening. As a result, changes occur in the structure of the cell wall and the middle lamella disintegrates, which results in the weakening of intercellular connections. Strawberries are characterized by particularly low durability, which makes them more susceptible to mechanical damage, pathogen infections, and loss of quality during storage [
21,
31]. It is also worth adding that strawberries are not climacteric fruits, which means that they do not ripen after harvesting. This means that their degradation process occurs almost immediately or shortly after harvesting, which further affects their faster deterioration in quality during storage [
73].
Analysis of the results showed that the firmness of the fruit gradually decreased during cold storage, which is also confirmed by the research in [
61]. In the control samples (C), the firmness value decreased from 3.07 to 2.09 N, in the samples coated with apple pectin (AP), it decreased from 3.18 to 2.45 N, while in the samples coated with apple pectin enriched with gamma-decalactone (AP + GDL), it decreased from 2.75 to 1.44 N. The rate of these changes depended on the variant used. Detailed results are presented in
Table 6.
Aging, cell degradation, and pectin hydrolysis or depolymerization could cause fruit softening, which affects the firmness stability during storage [
64]. These processes could also explain the decrease in firmness in the control samples and in fruits coated with apple pectin enriched with gamma-decalactone (AP + GDL). However, in the case of the apple pectin (AP) coating, the rate of this process was slower than in the other samples.
Statistical analysis showed significant differences (p ≤ 0.05) for samples coated with apple pectin enriched with gamma-decalactone (AP + GDL) compared to control samples, indicating that the addition of this ingredient negatively affected firmness stability. On the other hand, the apple pectin (AP) coating showed smaller, although still significant, statistical differences (p ≤ 0.05). Firmness values in this group remained at a similar level, although there was a gradual decrease, which was slower than in the case of the control samples and those with added gamma-decalactone.
Similar relationships were observed in a study conducted by Treviño-Garza et al. [
21], which showed that strawberries coated with pectin, without additional substances, were characterized by higher firmness compared to control samples. In turn, Khammayom et al. [
73] noticed that during storage, strawberries not covered with an edible coating showed a greater decrease in firmness compared to fruits protected with a coating containing bay oil.
In relation to the effect of the cold storage time, statistically significant differences (p ≤ 0.05) were noted on each day of storage, although the largest difference occurred on day 7, when the highest firmness value was achieved by samples coated with apple pectin (AP) (2.76 N), and the lowest was achieved by samples with added gamma-decalactone (AP + GDL) (1.72 N). This indicates that at this point of storage the effect of the additives used on the stability of the material structure was most noticeable.
Fruit softening during cold storage was mainly due to changes in the cell walls of strawberries, associated with a decrease in the level of pectins [
74]. High respiration accelerated the ripening and aging process, increasing the amount of soluble pectin in the fruit, which additionally affected its softness [
75]. Reducing respiration could therefore help to better preserve fruit firmness.
The results of the study clearly indicate that the apple pectin (AP) coating had the most positive effect on maintaining the firmness and structure of the material compared to the other groups. The worst effect was observed in the group with the addition of gamma-decalactone (AP + GDL), where the decrease in firmness was the fastest, which may suggest an acceleration of the degradation processes of the product structure. It is also worth noting that the presented observations are confirmed not only by the results of fruit firmness measurements but also by the analysis of appearance (
Figure 5), which shows a significant difference in the condition of the fruits from the individual groups. Especially in the samples with apple pectin with the addition of gamma-decalactone (AP + GDL), leakage and deterioration in firmness were visible, which additionally emphasizes the effect of the additive on the structural quality of the fruits.
3.8. Effect of Coatings on the Spoilage of Strawberries During Storage
Strawberries are fruits with a short shelf life, which is due to their rapid ripening, high respiration rate, and intensive fruit-softening process. These features promote the development of microorganisms such as bacteria and fungi, which accelerate their spoilage [
76]. For this reason, there is a need to develop effective methods that will extend the shelf life of these fruits. One of the solutions may be edible coatings, which are an alternative to traditional storage methods, contributing to delaying the spoilage processes of strawberries [
50]. The aim of the conducted research was to assess the effectiveness of edible coatings based on apple pectin and enriched with gamma-decalactone in protecting strawberries against spoilage in refrigerated conditions.
The results presented in
Table 7 confirm the effectiveness of edible coatings in extending the shelf life of strawberries. After 7 days, the spoilage level in the control group was 14.29%, which was the same as in the case of fruit coated with apple pectin (AP). This means that at this stage there were no statistically significant differences between these groups. However, after 9 days, the situation changed significantly—in the control sample, the percentage of spoiled fruit increased by 57.14%, while in the case of fruit coated with apple pectin (AP), it was 42.86%, which was already a statistically significant difference (
p ≤ 0.05).
The best protection was provided by the coating enriched with gamma-decalactone (AP + GDL), in which the spoilage level reached only 14.29% after 9 days, which was the lowest value among all the groups tested. This indicates that the addition of gamma-decalactone significantly increases the effectiveness of the coating, more effectively limiting the development of microorganisms and slowing down the processes of fruit degradation. This mechanism is based on a reduction in the respiration rate and limiting the contact of fruits with microorganisms, which significantly extends their shelf life [
64].
Statistical analysis clearly showed significant differences (p ≤ 0.05) between the control group and the fruit covered with edible coatings. In particular, strawberries without a coating deteriorated quickly—after 7 days, the level of deterioration was 14.29%, and after 9 days, it increased to 57.14%. In the case of strawberries protected with a pectin coating (AP), the rate of degradation was slower, and this process was inhibited to an even greater extent by the addition of gamma-decalactone (AP+ GDL).
The obtained results clearly indicate that edible coatings, especially those enriched with bioactive substances such as gamma-decalactone, can effectively slow down the development of mold on fruit. The apple pectin coating, especially in combination with gamma-decalactone, has exceptional protective properties. This is also confirmed by the studies by Martínez et al. [
66] where coatings constitute a barrier that limits the contact of fruits with external microorganisms and slows down their metabolism, which results in longer storage time and better quality of strawberries. Additionally, in the study by Treviño-Garza et al. [
21], it was found that coatings based on pectin, pullulan, and chitosan reduce the rate of fruit decomposition compared to that in uncoated fruits. The above results confirm this trend, especially in the case of the coating of apple pectin enriched with gamma-decalactone, which showed the highest protective efficacy.
In the study by Bermúdez-Oria et al. [
77], it was shown that strawberries covered with an edible coating based on pectin and fish skin protein had less mold growth after 157 h of storage, compared to uncoated strawberries. These results indicate that these coatings can effectively protect fruits from microbial attack, as well as extend their shelf life. Similar results were presented by Benhabiles et al. [
56], indicating that the application of coatings effectively delayed the development of fungal infection compared to that in uncoated strawberries, which showed signs of rotting after only three days of storage.
Edible coatings, especially due to their micropore structure, also act as a gas barrier, slowing down the rate of fruit respiration, which helps to delay their ripening and decomposition [
78]. This property, especially in the context of refrigerated storage, results in an extended shelf life of strawberries, which is of great importance from the point of view of the food industry.
3.9. Principal Component Analysis (PCA) of Coated and Uncoated Strawberries During Storage
In order to determine the relationship between the quality parameters of strawberries coated with edible coatings during cold storage, Principal Component Analysis (PCA) was performed. As shown in
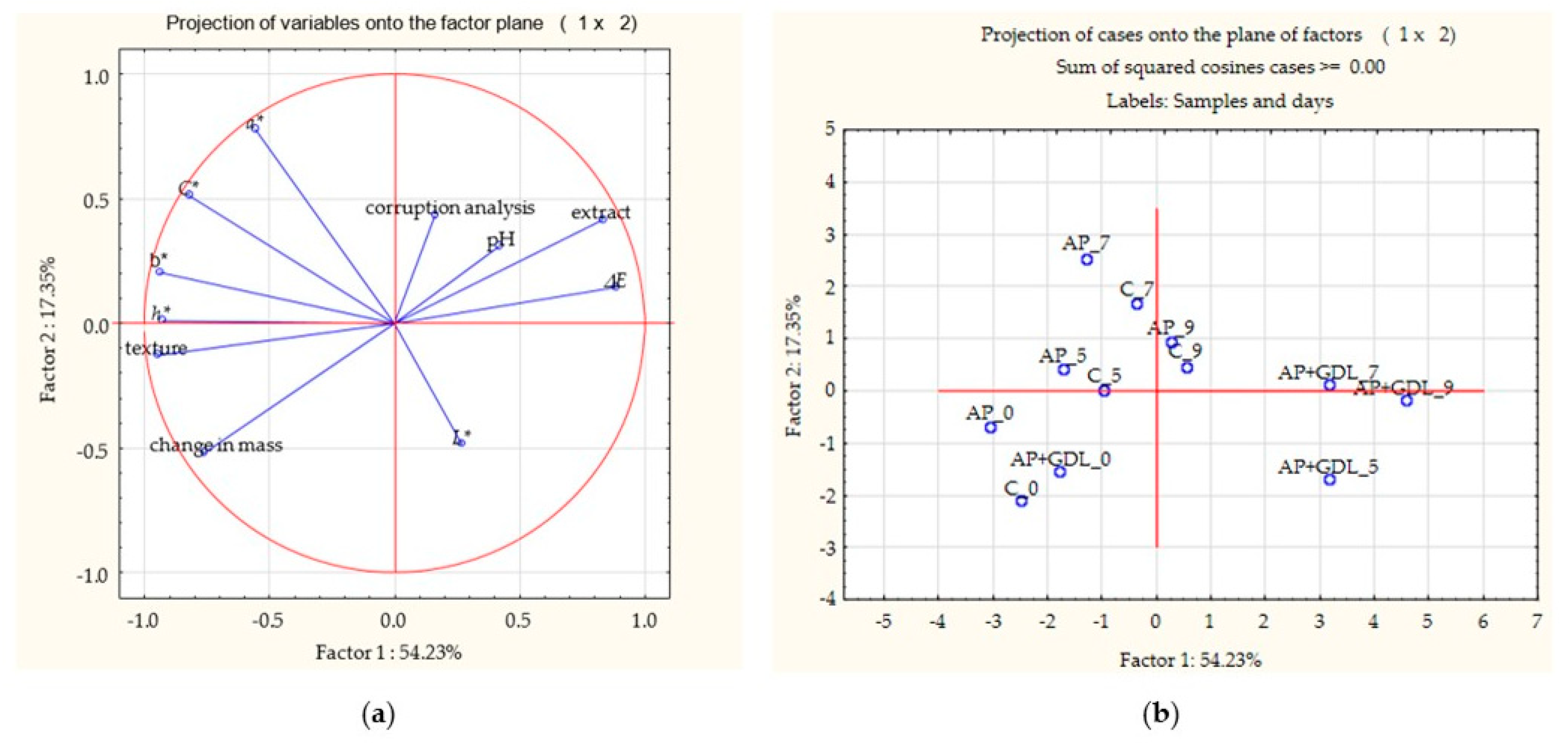
Figure 6, the first two components (Factor 1 and Factor 2) together explain 71.58% of the total variability in the data, which allows for their reliable interpretation.
Based on the graph of variables on the factor plane (1 × 2), it can be observed that parameters such as the ΔE (total color difference) and extract are strongly positively correlated with the first factor—their long vectors and position on the right side of the graph indicate a significant effect on sample differentiation. In turn, the pH variables and spoilage analysis, although also directed towards positive values of the first factor, have shorter vectors, which indicates their smaller contribution to shaping the variability in the space of the first two components.
The L* variable is located on the right side of the graph, in the lower part of the system, which indicates a positive correlation with the first factor and a negative correlation with the second. However, the short vector indicates its small impact on the variability in the data. The color parameters a*, b*, C*, and h* are located in the upper left quadrant of the graph, which suggests their negative correlation with Factor 1 and a positive correlation with Factor 2. The vectors of these variables are longer, which indicates their greater importance in differentiating samples in these dimensions. On the other hand, the variables describing texture and mass change are located in the lower left quadrant of the graph, which indicates negative correlations with both the first and the second factors. Their location suggests that they behave in the opposite way to variables such as the ΔE (total color difference), extract, pH, and spoilage analysis.
Samples AP + GDL_5, AP + GDL_7, and AP + GDL_9 (strawberries with gamma-decalactone coating after 5, 7, and 9 days of refrigerated storage) are located on the right side of the graph, in the area of positive Factor 1 values. Their location correlates with higher values of extract, pH, ΔE (total color difference), and spoilage level, suggesting intensification of qualitative changes in these samples with the elapsed storage time.
In contrast, samples C_0, AP_0, and AP + GDL_0 (uncoated strawberries, strawberries coated with apple pectin, and strawberries coated with apple pectin with added gamma-decalactone after 0 days of cold storage) were placed in the area of negative values of Factor 1. Their location indicates better texture retention, lower mass loss, and a lower level of spoilage, as well as an inverse correlation with parameters such as the extract, pH, and ΔE (total color difference).
The conducted studies confirmed that the use of pectin coatings, especially those enriched with gamma-decalactone (AP + GDL), affects the quality of strawberries stored in refrigerated conditions. These coatings significantly affected the appearance and texture of the fruits, slowing down the processes of their spoilage and reducing the development of microorganisms. The appearance of the fruits, however, did not meet expectations when compared to other samples.
The use of apple pectin allowed for more effective preservation of firmness, pH stability, and limitation of extract changes, which suggests a beneficial effect of this biopolymer on slowing down metabolic processes. In turn, the coating with the addition of gamma-decalactone, despite a greater loss of fruit mass, showed high antimicrobial efficacy and a positive effect on color stability and overall durability of the products.
PCA analysis confirmed that edible coatings, especially those containing gamma-decalactone, differentiate the physicochemical properties of strawberries during refrigerated storage, which can be used in the development of advanced fruit protection systems.
The use of bioactive coatings based on apple pectin with added gamma-decalactone may be a promising solution for the food industry, serving to extend the shelf life of fresh fruits and reduce food losses. Further research should focus on optimizing the composition of coatings and controlling the rate of release of active substances.
3.10. Effect of Coatings on the Level of Development of Microorganisms in Strawberries
Strawberries are a highly perishable fruit, and their shelf life usually ends due to microbial infection. This is due to, among other things, the soft texture of the fruit and its susceptibility to mechanical damage and, thus, to the proliferation of various pathogens [
79,
80]. Various microorganisms such as
Rhizopus stolonifera, Botrytis cinerea, Mucor spp.,
Colletotrichum spp., and
Penicillium spp. are the most commonly reported pathogens affecting strawberry fruit [
81].
To date, various strategies have been used to prevent post-harvest fruit rot, including using fungicides or breeding plant varieties that are more resistant to the development of microorganisms. In recent years, there has been an increase in interest in more environmentally friendly solutions, an example of which is protective coating for fruits [
82].
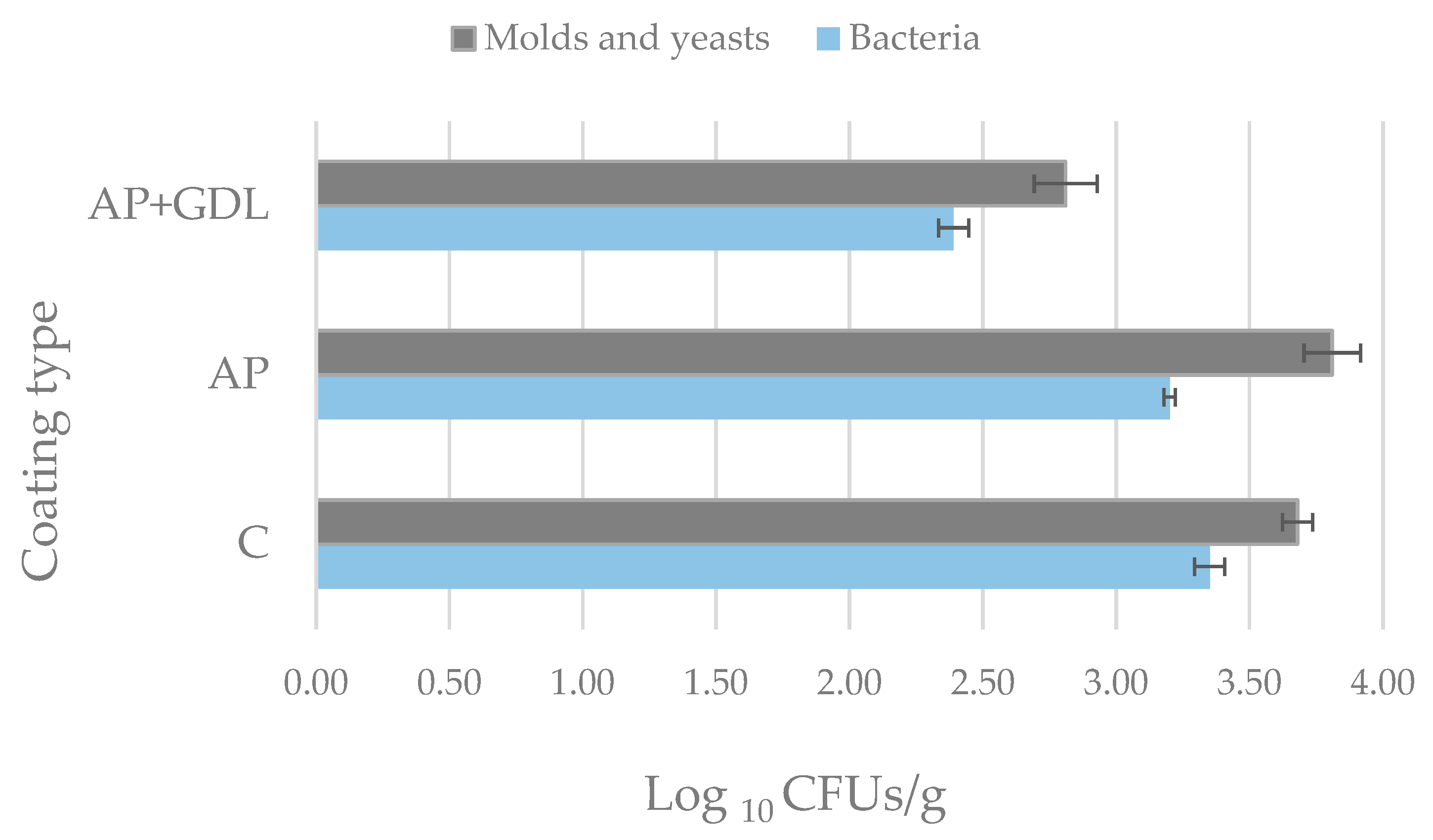
Figure 7 shows the effect of coating on the growth of bacteria, yeasts, and molds on strawberries.
Studies have shown that coating strawberries affects the level of microbial development, which is tantamount to the quality of the fruit. The inhibitory effect on bacteria, yeast, and molds is most clearly visible when using a coating enriched with GDL (AP + GDL). The concentration of microorganisms in fruit covered with such a coating after 12 days of storage was about one logarithmic unit lower than that in the control fruit (C) (not covered with a coating). The level of bacteria for coated fruit was 2.39 ± 0.06 log10 CFUs/g, while for control fruit (without a coating), it was about 3.35 ± 0.06 log10 CFUs/g. The level of yeasts and molds was 2.81 ± 0.12 log10 CFUs/g and 3.68 ± 0.07 log10 CFUs/g for the fruit with the GDL coating and the control sample, respectively.
Mentions of gamma-decalactone toxicity towards microbial cells date back to 1996. Feron et al. [
38], working with
Sporidiobolus species, proved that lactone inhibits, among others, the activity of H
+-ATPase in microbial cells, which results in a change in the integrity of the cell membrane. In addition, lactone can create hydrophobic interactions with the acyl chains of phospholipids of the cell membrane, leading to increased permeability disorders of the membrane and a decrease in the intracellular pH of cells.
Analyzing the results presented in
Figure 7, it can also be seen that the fruits covered with the pectin coating (AP) were more infected with yeasts and molds compared to the control fruits (C)—3.81 ± 0.11 log
10 CFUs/g and 3.68 ± 0.06 log
10 CFUs/g, respectively. This may be due to the fact that microorganisms growing on strawberries produce enzymes (pectinases) that break down polysaccharides, which, when transformed into simple sugar, become a nutrient for microorganisms. In future studies on the effect of pectin coatings on fruits, it would be worthwhile to attempt to identify the strains developing on their surface.