The Influence of the Addition of Rubber Waste on the Properties of Polyurethane Coatings Subjected to Aging Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Aging Tests
2.2.2. Thermal Shock Process
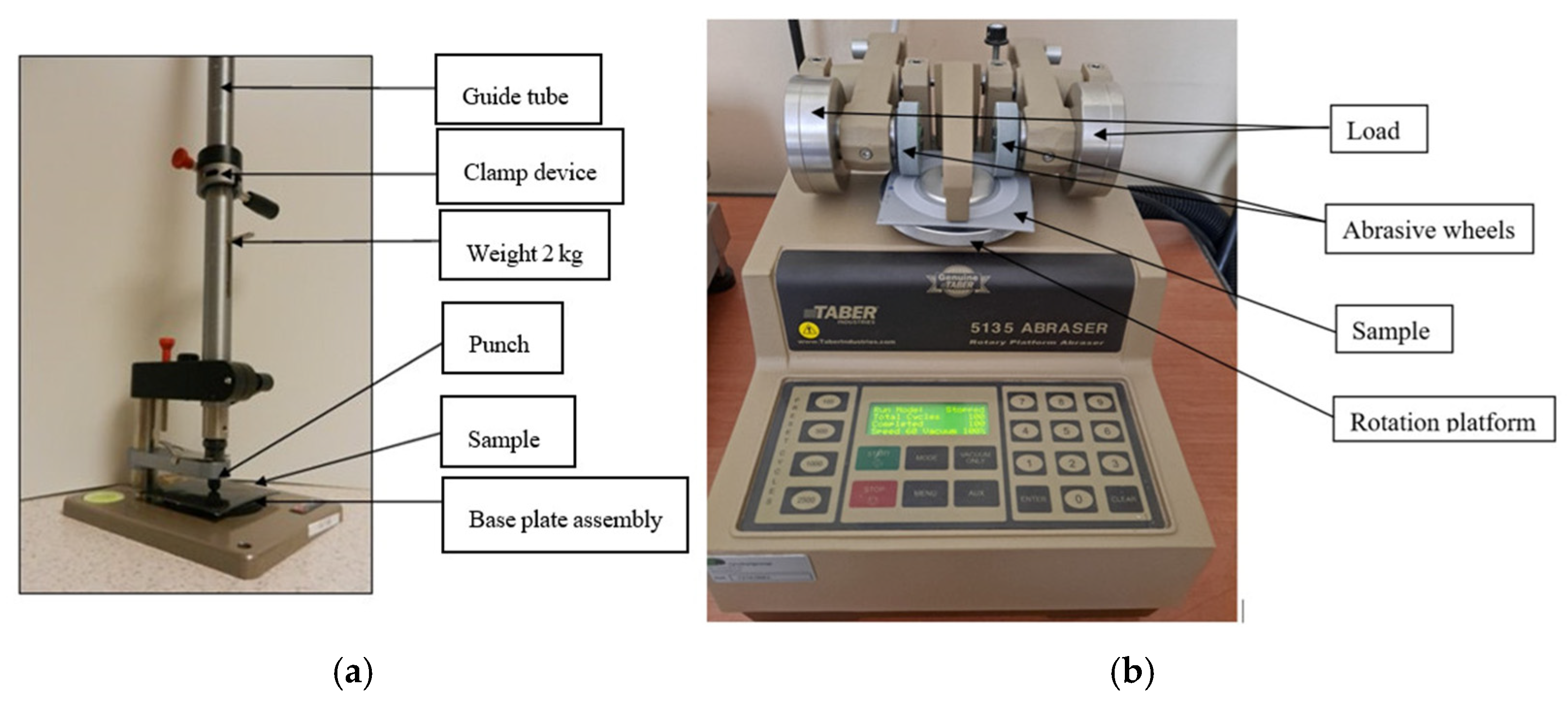
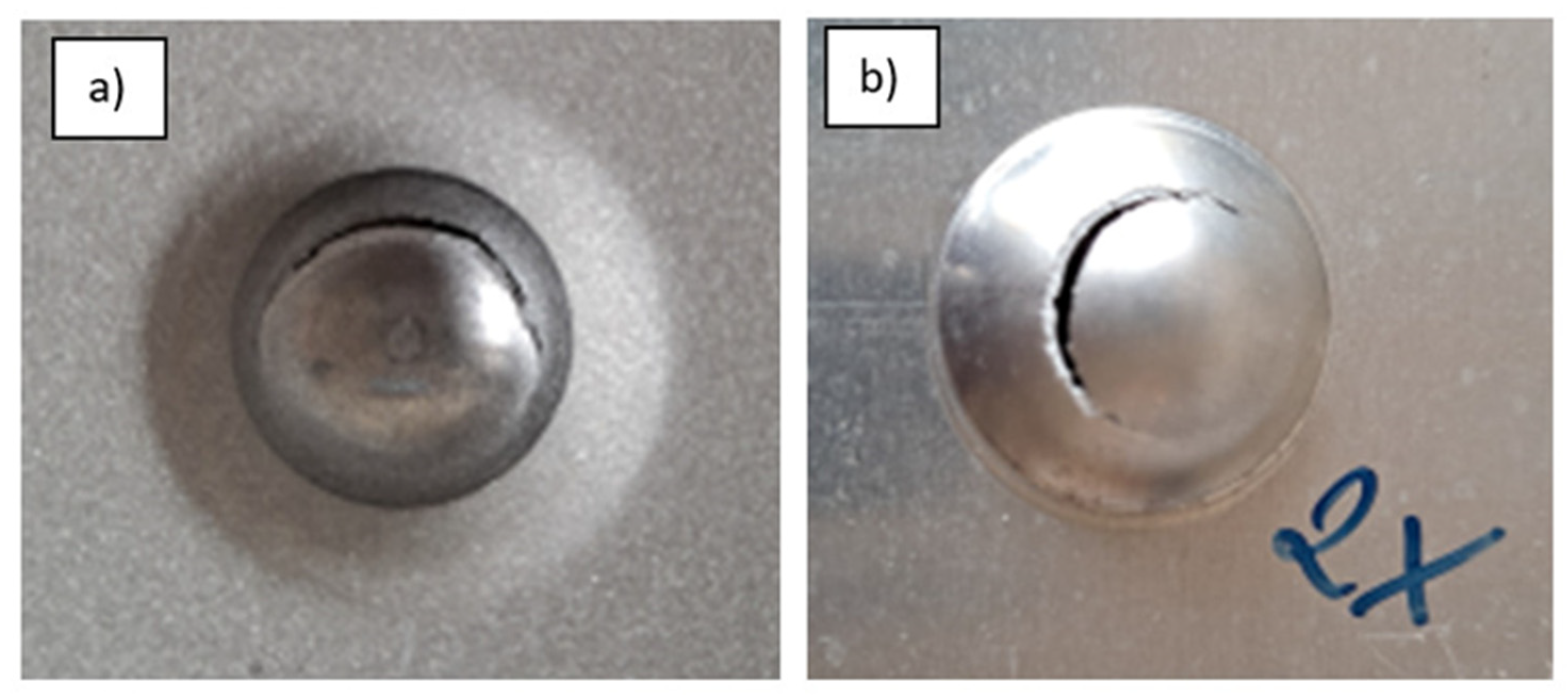
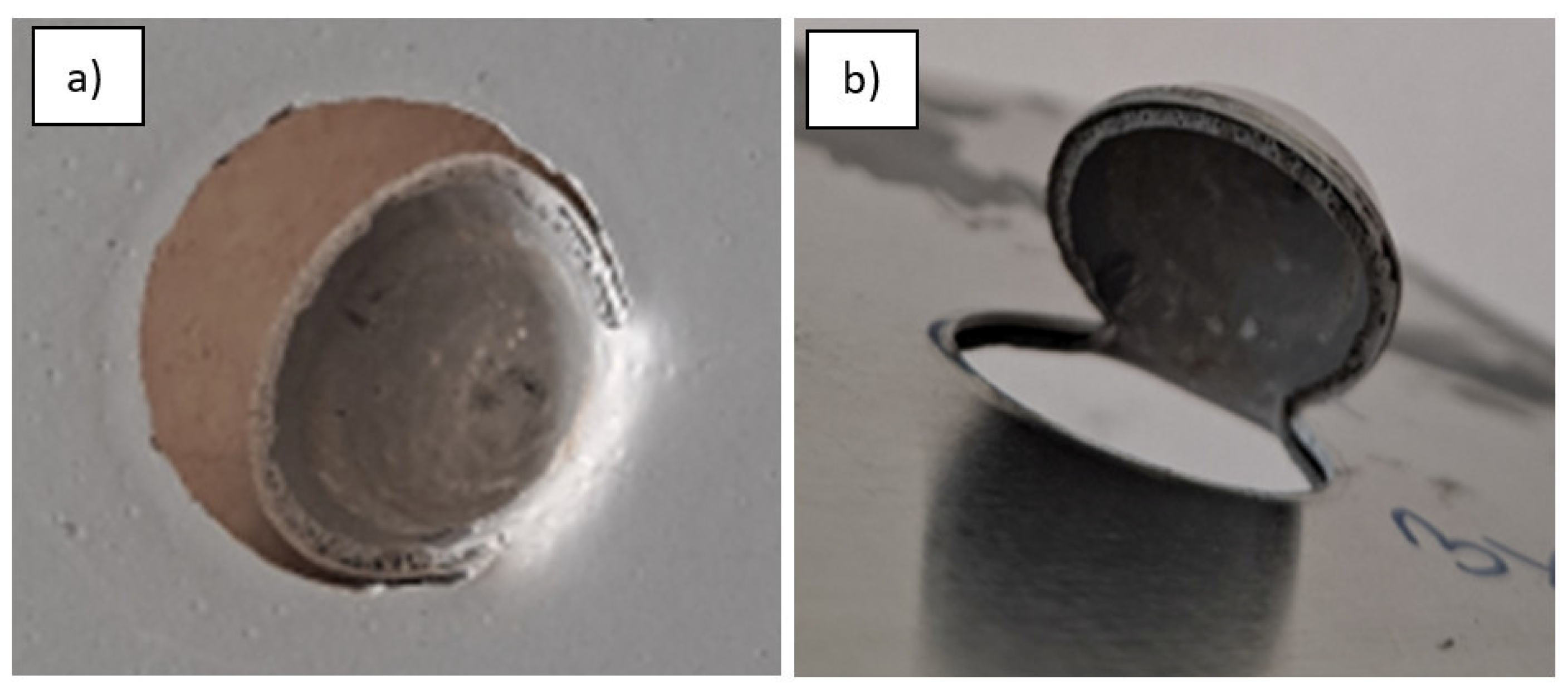
2.2.3. Impact Resistance
2.2.4. Abrasion Resistance Tests
- platform rotation speed: 60 rpm;
- load on the abrasive wheel arms: 1000 g;
- abrasive wheels used: Calibrase H 18;
- abrasive paper for renewing the surface of abrasive wheels: S-11.
2.2.5. Thermal Properties
2.2.6. FTIR Spectroscopy
2.2.7. Surface Wettability
2.2.8. SEM
3. Results
3.1. Impact Resistance
3.2. Abrasion Resistance
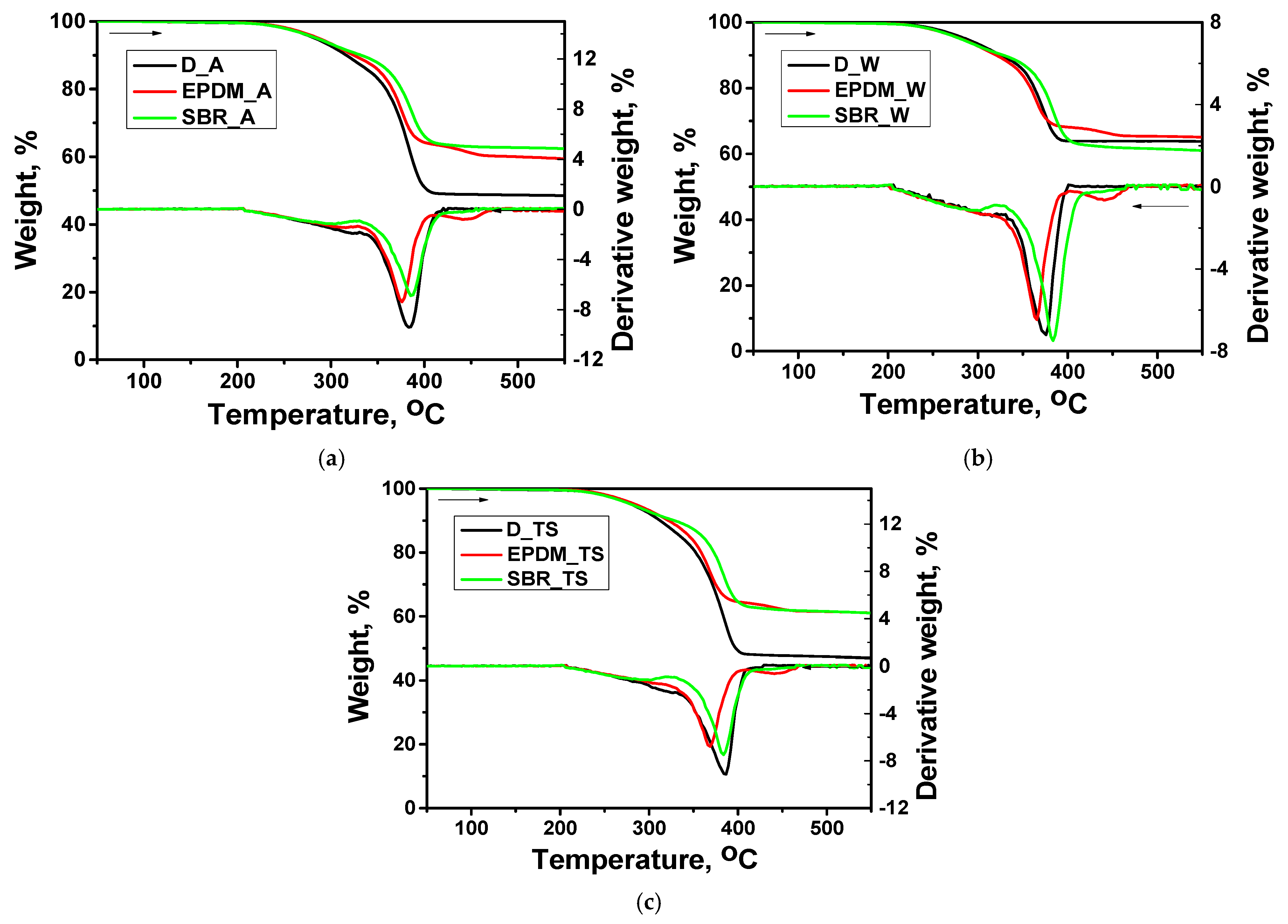
3.3. Thermal Analysis
3.4. Glass Transition Temperature
3.5. FTIR
3.6. Contact Angle
4. Conclusions
- Impact and Abrasion ResistancePolyurethane coatings without fillers maintained their impact and abrasion resistance after both UV ageing and thermal shock exposure.The addition of EPDM and SBR rubber fillers:
- ➢
- Improved impact resistance under thermal shock conditions.
- ➢
- Reduced abrasion resistance by about 40%, especially with SBR particles, which accelerated wear with surface material loss of about 25% in the case of EPDM filler.
The ageing process had a negative impact on impact resistance. Coatings with EPDM filler were characterized by a lower impact resistance by 20%, and those with SBR filler by 30%. - Chemical Structure and DegradationFTIR spectroscopy confirmed the presence of characteristic polyurethane functional groups across all samples. PUR coatings are significantly degraded by UV light. Fillers, especially EPDM contributed to chemical stabilization, slowing down UV-induced degradation. Thermal shocks led to formation of new groups on the surface containing oxygen and rearrangement of hydrogen bonds.
- Hydrophobicity and Surface PropertiesThe addition of rubber waste reduced the contact angle, indicating lower surface hydrophobicity. EPDM demonstrated better oxidation resistance and retained higher hydrophobicity after thermal shock compared to SBR.
- Thermal propertiesAgeing processes (UV and thermal shock) led to a decrease in Tg for SBR-containing coatings. This made the material more flexible but also more prone to degradation.The SBR-filled coatings exhibited higher thermal stability, decomposing at higher temperatures. EPDM-filled coatings showed lower thermal stability, decomposing at more rapidly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| D_W | sample without filler and without aging |
| SBR_W | sample with SBR filler and without aging |
| EPDM_W | sample with EPDM filler and without aging |
| D_A | sample without filler after UV aging |
| SBR_A | sample with SBR filler after UV aging |
| EPDM_A | sample with EPDM filler after UV aging |
| D_TS | sample without filler after thermal shock |
| SBR_TS | sample with SBR filler after thermal shock |
| EPDM_TS | sample with EPDM filler after thermal shock |
References
- Alfayez, S.A.; Suleiman, A.R.; Nehdi, M.L. Recycling Tire Rubber in Asphalt Pavements: State of the Art. Sustainability 2020, 12, 9076. [Google Scholar] [CrossRef]
- Hashamfirooz, M.; Dehghani, M.H.; Khanizadeh, M.; Aghaei, M.; Bashardoost, P.; Hassanvand, M.S.; Hassanabadi, M.; Momeniha, F. A systematic review of the environmental and health effects of waste tires recycling. Heliyon 2025, 11, e41909. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Balasch, A.; Bartrolí, R.; Eljarrat, E. A new look at rubber recycling and recreational surfaces: The inorganic and OPE chemistry of vulcanised elastomers used in playgrounds and sports facilities. Sci. Total Environ. 2023, 868, 161648. [Google Scholar] [CrossRef] [PubMed]
- Nuzaimah, M.; Sapuan, S.M.; Nadlene, R.; Jawaid, M. Recycling of waste rubber as fillers: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 12016. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef]
- Vermette, P.; Griesser, H.J.; Laroche, G.; Guidoin, R. Biomedical Applications of Polyurethanes: Tissue Engineering Intelligence Unit 6; Landes Bioscience: Georgetown, TX, USA, 2001; ISBN 1-58706-023-X. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for coatings and adhesives–chemistry and applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Pęczek, E.; Pamuła, R.; Białowiec, A. Recycled Waste as Polyurethane Additives or Fillers: Mini-Review. Materials 2024, 17, 1013. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Therias, S.; Rapp, G.; Masson, C.; Gardette, J.-L. Limits of UV-light acceleration on the photooxidation of low-density polyethylene. Polym. Degrad. Stab. 2021, 183, 109443. [Google Scholar] [CrossRef]
- Xu, X.; Deng, J.; Nie, S.; Lan, Z.; Xu, Z. Effect of Thermal Aging on Mechanical Properties and Morphology of GF/PBT Composites. Polymers 2023, 15, 3798. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, Z.; Zhao, J.; Zhang, S.; Li, P.; Meng, C.; Sui, J.; Zhang, X.; Hu, S.; Pan, J.; et al. Aging behavior of three-proof polyurethane coatings under UV radiation. Polimery 2022, 67, 433–437. [Google Scholar] [CrossRef]
- Mayer, P.; Lubecki, M.; Stosiak, M.; Robakowska, M. Effects of surface preparation on the adhesion of UV-aged polyurethane coatings. Int. J. Adhes. Adhes. 2022, 117, 103183. [Google Scholar] [CrossRef]
- Ishida, T.; Kitagaki, R. Mathematical Modeling of Outdoor Natural Weathering of Polycarbonate: Regional Characteristics of Degradation Behaviors. Polymers 2021, 13, 820. [Google Scholar] [CrossRef]
- Wang, X.; Andriani, Y.; Lim, P.C.; Lee, C.J.J.; Liu, S.; Lau, B.L.; Zhang, X. Effect of Thermal Cycling on the Thermal and Mechanical Properties of Dielectric Materials. IEEE Trans. Compon. Packag. Manufact. Technol. 2020, 10, 1166–1174. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Bhargava, S.; Kubota, M.; Lewis, R.D.; Advani, S.G.; Prasad, A.K.; Deitzel, J.M. Ultraviolet, water, and thermal aging studies of a waterborne polyurethane elastomer-based high reflectivity coating. Prog. Org. Coat. 2015, 79, 75–82. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Irigoyen, M.; Aragon, E.; Frizzi, M.A.; Perrin, F.X. Dynamic mechanical analysis and Vickers micro hardness correlation for polymer coating UV ageing characterisation. Polym. Degrad. Stab. 2001, 72, 63–68. [Google Scholar] [CrossRef]
- Frigione, M.; Rodríguez-Prieto, A. Can Accelerated Aging Procedures Predict the Long Term Behavior of Polymers Exposed to Different Environments? Polymers 2021, 13, 2688. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, J.; Tao, Y.; Zhao, S.; Zeng, W.; Shi, Y.; Lu, T.; Guo, L.; Wang, S.; Zhang, X.; et al. Sunlight tracking and concentrating accelerated weathering test applied in weatherability evaluation and service life prediction of polymeric materials: A review. Polym. Test. 2021, 93, 106940. [Google Scholar] [CrossRef]
- Xiong, G.; Al-Deen, S.; Guan, X.; Qin, Q.; Zhang, C. Economic and Environmental Benefit Analysis between Crumb Rubber Concrete and Ordinary Portland Cement Concrete. Sustainability 2024, 16, 4758. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, J.; Hou, G.; Yang, H.; Zhang, L. Study on structures, dynamics and mechanical properties of styrene butadiene rubber (SBR)/silica interfaces: A fully atomistic molecular dynamics. Polymer 2021, 218, 123523. [Google Scholar] [CrossRef]
- Tayefi, M.; Eesaee, M.; Hassanipour, M.; Elkoun, S.; David, E.; Nguyen-Tri, P. Recent progress in the accelerated aging and lifetime prediction of elastomers: A review. Polym. Degrad. Stab. 2023, 214, 110379. [Google Scholar] [CrossRef]
- PN-EN ISO 2808:2008; Paints and Varnishes-Coating Thickness Determination. ISO: Warsaw, Poland, 2008.
- Guha, R.D.; Danilov, E.O.; Berkowitz, K.; Oluwajire, O.; Grace, L.R. Consequences of Humidity Cycling on the Moisture Absorption Characteristics of Epoxy Resins with Different Network Architectures. ACS Appl. Polym. Mater. 2023, 5, 400–411. [Google Scholar] [CrossRef]
- Segerström, S.; Ruyter, I.E. Effect of thermal cycling on flexural properties of carbon-graphite fiber-reinforced polymers. Dent. Mater. 2009, 25, 845–851. [Google Scholar] [CrossRef]
- ISO 4892-1:2016; Methods of Exposure to Laboratory Light Sources, Part 1 and Part 2 The SUNTEST, Plastics. ISO: Warsaw, Poland, 2016.
- EN ISO 6272-1:2011; Paints and Varnishes-Rapid-Deformation (Impact Resistance) Tests—Part 1: Falling-Weight Test, Large-Area Indenter. ISO: Geneva, Switzerland, 2011.
- ASTM D4060-10; Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM International: West Conshohocken, PA, USA, 2015.
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Acierno, D.; Graziosi, L.; Patti, A. Puncture Resistance and UV aging of Nanoparticle-Loaded Waterborne Polyurethane-Coated Polyester Textiles. Materials 2023, 16, 6844. [Google Scholar] [CrossRef]
- Valášek, P.; Žarnovský, J.; Müller, M. Thermoset Composite on Basis of Recycled Rubber. Adv. Mater. Res. 2013, 801, 67–73. [Google Scholar] [CrossRef]
- Wiewióra, M.; Żaba, K.; Kuczek, Ł.; Balcerzak, M.; Madej, M. Analysis of Wear Resistance of Metallic-Reinforced Polyurethane Resin Composites for Sheet Metal Forming. Adv. Mater. Sci. 2024, 24, 18–29. [Google Scholar] [CrossRef]
- Nassar, A.; Younis, M.; Ismail, M.; Nassar, E. Improved Wear-Resistant Performance of Epoxy Resin Composites Using Ceramic Particles. Polymers 2022, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Mei Ding, Y.; Tao He, X.; Liu, Y.; An, Y.; Min Yang, W. Abrasion resistance of thermoplastic polyurethane materials blended with ethylene–propylene–diene monomer rubber. J Appl. Polym. Sci 2008, 110, 1851–1857. [Google Scholar] [CrossRef]
- Wolska, A.; Goździkiewicz, M.; Ryszkowska, J. Thermal and mechanical behaviour of flexible polyurethane foams modified with graphite and phosphorous fillers. J. Mater. Sci. 2012, 47, 5627–5634. [Google Scholar] [CrossRef]
- Zimmer, B.; Nies, C.; Schmitt, C.; Possart, W. Chemistry, polymer dynamics and mechanical properties of a two-part polyurethane elastomer during and after crosslinking. Part I: Dry conditions. Polymer 2017, 115, 77–95. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.; Jaszcz, K.; Chladek, G.; Grabowska, P.; Okseniuk, A.; Szpot, M.; Zawadzka, M.; Sokołowska, A.; Tarkiewicz, A. Characterization of changes in structural, physicochemical and mechanical properties of rigid polyurethane building insulation after thermal aging in air and seawater. Polym. Bull. 2022, 79, 3061–3083. [Google Scholar] [CrossRef]
- Mayer-Trzaskowska, P.; Robakowska, M.; Gierz, Ł.; Pach, J.; Mazur, E. Observation of the Effect of Aging on the Structural Changes of Polyurethane/Polyurea Coatings. Polymers 2023, 16, 23. [Google Scholar] [CrossRef]
- Beneš, H.; Černá, R.; Ďuračková, A.; Látalová, P. Utilization of Natural Oils for Decomposition of Polyurethanes. J. Polym. Environ. 2012, 20, 175–185. [Google Scholar] [CrossRef]
- Murali, A.; Gurusamy-Thangavelu, S.A.; Jaisankar, S.N.; Mandal, A.B. Enhancement of the physicochemical properties of polyurethane–perovskite nanocomposites via addition of nickel titanate nanoparticles. RSC Adv. 2015, 5, 102488–102494. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Xiang, S.; Yan, H.; Tian, H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers 2024, 16, 2807. [Google Scholar] [CrossRef]
- Chudzik, J.; Bieliński, D.M.; Bratychak, M.; Demchuk, Y.; Astakhova, O.; Jędrzejczyk, M.; Celichowski, G. Influence of Modified Epoxy Resins on Peroxide Curing, Mechanical Properties and Adhesion of SBR, NBR and XNBR to Silver Wires. Part I: Application of Monoperoxy Derivative of Epoxy Resin (PO). Materials 2021, 14, 1320. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]


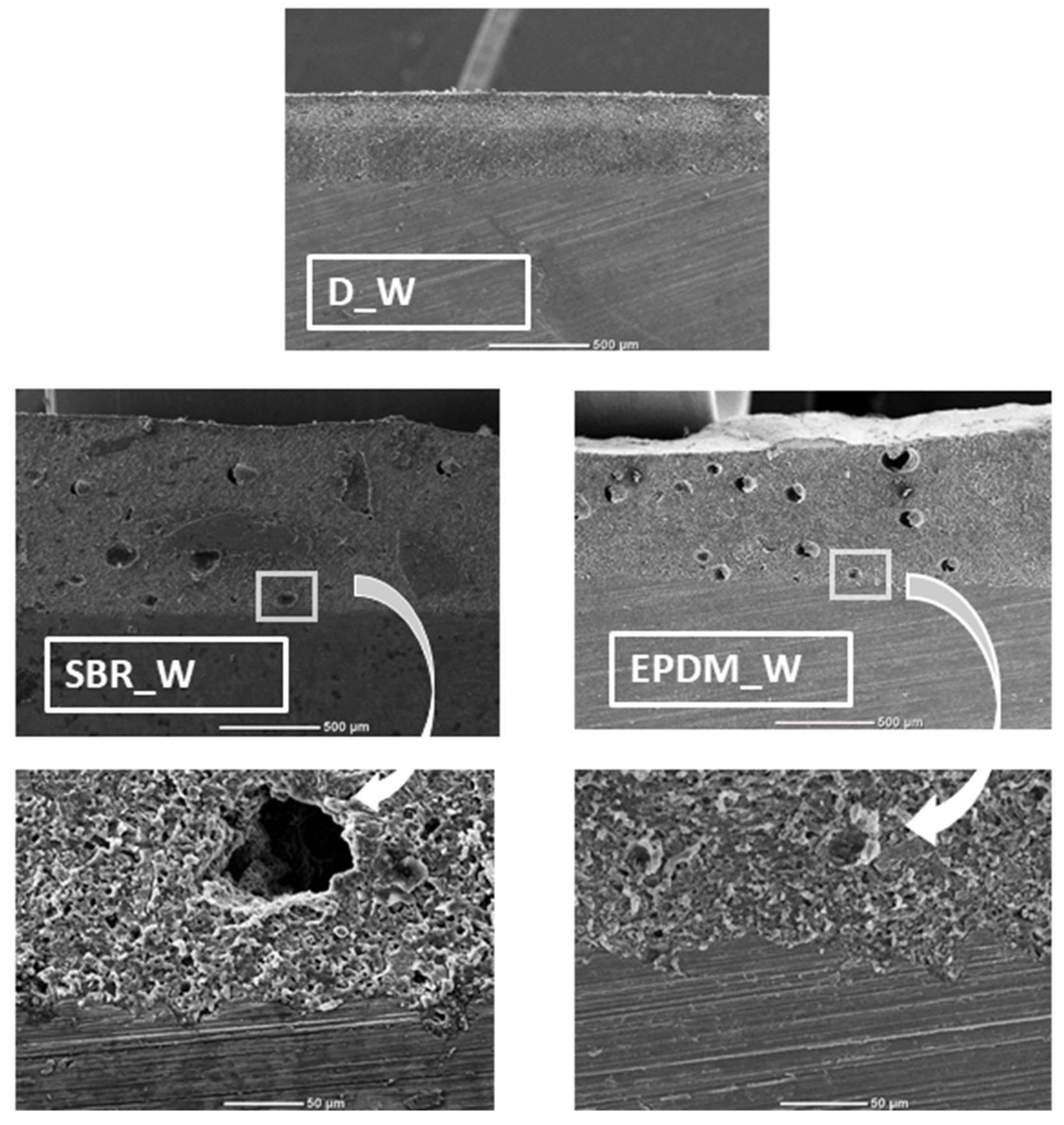




| No. | Determination | Type of Filler | Type of Aging Process |
|---|---|---|---|
| 1 | D_W | - | without |
| 2 | SBR_W | SBR | without |
| 3 | EPDM_W | EPDM | without |
| 4 | D_A | - | UV aging |
| 5 | SBR_A | SBR | UV aging |
| 6 | EPDM_A | EPDM | UV aging |
| 7 | D_TS | - | thermal shock |
| 8 | SBR_TS | SBR | thermal shock |
| 9 | EPDM_TS | EPDM | thermal shock |
| T5 [°C] | T10 [°C] | R [%] | V1 [°C] | V2 [°C] | |
|---|---|---|---|---|---|
| D_W | 268.8 | 325.8 | 63.68 | 375.4 | - |
| EPDM_W | 281.0 | 320.4 | 64.94 | 364.9 | 441.1 |
| SBR_W | 282.4 | 321.2 | 58.22 | 384.7 | - |
| D_A | 282.9 | 316.5 | 48.41 | 384.3 | - |
| EPDM_A | 287.0 | 324.4 | 59.18 | 375.9 | 441.4 |
| SBR_A | 284.8 | 332.4 | 62.28 | 386.2 | - |
| D_TS | 280.6 | 312.4 | 46.77 | 385.7 | - |
| EPDM_TS | 285.5 | 321.8 | 60.89 | 368.1 | 441.5 |
| SBR_TS | 280.5 | 328.6 | 60.82 | 383.6 | - |
| Sample | D_W | EPDM_W | SBR_W |
|---|---|---|---|
| Wetting angle image | 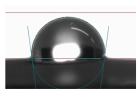 |  |  |
| Contact angle, Θ | 97.4° | 87.5° | 96.5° |
| SE [mN/m] | 80 | 65 | 79 |
| Sample | D_A | EPDM_A | SBR_A |
| Wetting angle image |  |  |  |
| Contact angle, Θ | 88.7° | 95.2° | 82° |
| SE [mN/m] | 69 | 86 | 58 |
| Sample | D_TS | EPDM_TS | SBR_TS |
| Wetting angle image |  |  |  |
| Contact angle, Θ | 82° | 89.7° | 89° |
| SE [mN/m] | 50 | 67 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer-Trzaskowska, P.; Ferraris, M.; Perero, S.; Robakowska, M. The Influence of the Addition of Rubber Waste on the Properties of Polyurethane Coatings Subjected to Aging Processes. Coatings 2025, 15, 677. https://doi.org/10.3390/coatings15060677
Mayer-Trzaskowska P, Ferraris M, Perero S, Robakowska M. The Influence of the Addition of Rubber Waste on the Properties of Polyurethane Coatings Subjected to Aging Processes. Coatings. 2025; 15(6):677. https://doi.org/10.3390/coatings15060677
Chicago/Turabian StyleMayer-Trzaskowska, Paulina, Monica Ferraris, Sergio Perero, and Mariola Robakowska. 2025. "The Influence of the Addition of Rubber Waste on the Properties of Polyurethane Coatings Subjected to Aging Processes" Coatings 15, no. 6: 677. https://doi.org/10.3390/coatings15060677
APA StyleMayer-Trzaskowska, P., Ferraris, M., Perero, S., & Robakowska, M. (2025). The Influence of the Addition of Rubber Waste on the Properties of Polyurethane Coatings Subjected to Aging Processes. Coatings, 15(6), 677. https://doi.org/10.3390/coatings15060677









