Microstructural Evolution and Wear Resistance of Silicon-Containing FeNiCrAl0.7Cu0.3Six High-Entropy Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
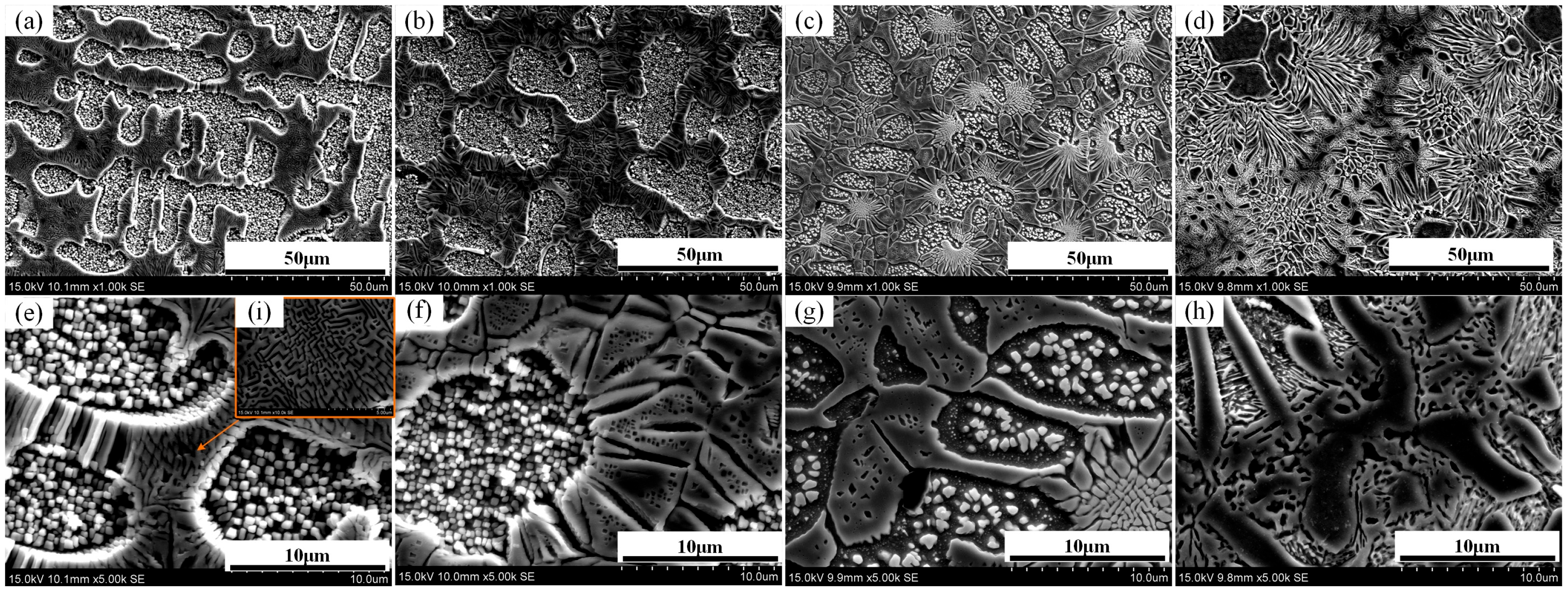
3.1. Microstructure Analysis
3.2. Microhardness Analysis
3.3. Wear Analysis
4. Conclusions
- With increasing Si content, the microstructure changes from dendritic (Si0) to a transitional structure (Si1), then to chrysanthemum-like (Si3), and finally to island-like grains (Si5). Al and Ni are enriched in dendrites, while Cr and Fe are in interdendritic areas. Si and Cu tend to co-segregate with Al and Ni.
- The microhardness of FeCrNiAl0.7Cu0.3Six alloys increases with rising Si content, from 484 HV for the Si0 alloy to 864 HV for the Si5 alloy. This enhancement is attributed to solid solution strengthening and the formation of the σ phase. The presence of secondary phase particles in Si3 and Si5 further contributes to mechanical strengthening.
- The wear resistance is significantly improved with increasing Si. The wear mass decreases from 1.31 mg for the Si0 alloy to 0.78 mg for the Si5 alloy. Si0 and Si1 alloys primarily experience abrasive wear, whereas Si3 and Si5 exhibit smoother worn surfaces with layered wear debris, indicating a shift toward adhesive wear as the dominant mechanism. This transformation reflects the influence of Si on the alloy’s tribological behavior and provides insight into tailoring wear properties through composition design.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, C.J.; Chen, Y.L.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Tsau, C.H.; Lin, S.J.; Chang, S.Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2005, 36A, 881–893. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, D.X.; Liu, C.; Tao, S.S.; Cai, J.M.; Zhong, B.; Li, J.; Deng, W.T.; Hou, H.S.; Zou, G.Q.; Ji, X.B. High-Entropy Electrode Materials: Synthesis, Properties and Outlook. Nano-Micro Lett. 2025, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.T.; Song, Z.; Wang, K.L.; Li, H.Q.; Pan, Y.; Hou, H.; Zhao, Y.H. Cryo-rolling and annealing-mediated phase transformation in Al5Ti2.5Fe25Cr25Ni42.5 high-entropy alloy: Experimental, phase-field and CALPHAD investigation. J. Mater. Sci. Technol. 2025, 219, 307–325. [Google Scholar] [CrossRef]
- Wang, J.M.; Jiang, H.; Xie, W.L.; Kong, X.; Qin, S.X.; Yao, H.W.; Li, Y. Effect of Mo addition on microstructural evolution and corrosion behaviors of AlCrFeNi3 eutectic high-entropy alloy. Corros. Sci. 2024, 229, 111879. [Google Scholar] [CrossRef]
- Charkhchian, J.; Zarei-Hanzaki, A.; Schwarz, T.M.; Lawitzki, R.; Schmitz, G.; Schell, N.; Shen, J.J.; Oliveira, J.P.; Waryoba, D.; Abedi, H.R. Unleashing the microstructural evolutions during hot deformation of as-cast AlCoCrFeNi2.1 eutectic high entropy alloy. Intermetallics 2024, 168, 108253. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Hua, K.; Cao, Y.; Song, Y.Q.; Li, X.L.; Zhou, Q.; Wang, H.F. Microstructures and properties of FeCrAlMoSix high entropy alloy coatings prepared by laser cladding on a titanium alloy substrate. Surf. Coat. Technol. 2024, 478, 130437. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, X.J.; Lu, X.Q.; Sun, W.; Liu, K.P.; Fei, Y.F. Predictions of friction and wear in ball bearings based on a 3D point contact mixed EHL model. Surf. Coat. Technol. 2025, 502, 131939. [Google Scholar] [CrossRef]
- Shen, J.J.; Choi, Y.T.; Yang, J.; He, J.J.; Zeng, Z.; Zhou, N.; Baptista, A.C.; Kim, H.S.; Oliveira, J.P. Fabrication of spatially-variable heterostructured CoCrFeMnNi high entropy alloy by laser processing. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2024, 896, 146272. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Yu, K.D.; Guo, N.; Xiao, G.C.; Wang, Z.M.; Zhang, H. Effect of Y2O3 on microstructure and properties of CoCrFeNiTiNb high entropy alloy coating on Ti-6Al-4V surface by laser cladding. J. Rare Earths 2024, 42, 586–599. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Z.; Zhang, Y.; Yu, Z.; Shi, J.; Xiong, R. Low temperature fabrication of the giant dielectric material CaCu3Ti4O12 by oxalate coprecipitation m ethod. Mater. Chem. Phys. 2009, 113, 746–748. [Google Scholar] [CrossRef]
- Zhu, B.-P.; Tang, Z.; Zhao, L.-H.; Wang, L.-L.; Li, C.-Z.; Yin, D.; Yu, Z.-X.; Tang, W.-F.; Xiong, R.; Shi, J.; et al. Synthesis of Mg[Ti2]O4 by spark plasma sintering. Mater. Lett. 2007, 61, 578–581. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, X.H.; Huang, Y.Y.; Xu, Z.X.; Deng, Y.L.; Jiang, X.Y.; Yang, X.H. Microstructure, Mechanical Property, and Wear Behavior of NiAl-Based High-Entropy Alloy. Coatings 2023, 13, 1737. [Google Scholar] [CrossRef]
- Zheng, W.J.; Lu, S.L.; Wu, S.S.; Chen, X.H.; Guo, W. Development of MoNbVTx refractory high entropy alloy with high strength at elevated temperature. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2022, 850, 143554. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhu, Z.B.; Huang, H.F.; He, T.; Yan, H.W.; Zhang, Y.A.; Lu, Y.P.; Wang, T.M.; Li, T.N.G. Microstructures, mechanical properties, and irradiation tolerance of the Ti-Zr-Nb-V-Mo refractory high-entropy alloys. Intermetallics 2023, 157, 107873. [Google Scholar] [CrossRef]
- Gong, P.; Wang, D.L.; Zhang, C.; Wang, Y.; Jamili-Shirvan, Z.; Yao, K.F.; Wang, X.Y. Corrosion behavior of TiZrHfBeCu(Ni) high-entropy bulk metallic glasses in 3.5 wt. % NaCl. NPJ Mater. Degrad. 2022, 6, 77. [Google Scholar] [CrossRef]
- Yu, K.D.; Zhao, W.; Li, Z.; Guo, N.; Xiao, G.C.; Zhang, H. High-temperature oxidation behavior and corrosion resistance of in-situ TiC and Mo reinforced AlCoCrFeNi-based high entropy alloy coatings by laser cladding. Ceram. Int. 2023, 49, 10151–10164. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.F.; Jin, G.; Liu, Y.F.; Zhang, Y.; Zhang, X.R.; Liu, E.B.; Tian, H.L.; Fang, Y.C. Corrosion and tribo-corrosion behaviors of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings: The role of dual-phase microstructure. Corros. Sci. 2022, 201, 110305. [Google Scholar] [CrossRef]
- Ju, Y.; Konoplianchenko, I.; Pu, J.F.; Zhang, Z.C.; Dong, Q.; Dumanchuk, M. Optimization of structure and properties of WC-reinforced FeCoNiCr high-entropy alloy composite coating by laser melting. Results Eng. 2024, 21, 101985. [Google Scholar] [CrossRef]
- Lin, T.; Feng, M.; Lian, G.; Lu, H.; Chen, C.; Huang, X. Effects of Si content on the microstructure and properties of CoCrFeMnNiSix high-entropy alloy coatings by laser cladding. Mater. Charact. 2024, 216, 114246. [Google Scholar] [CrossRef]
- Liu, G.L.; Xu, D.; Yang, H.Y.; Liu, F.X.; Wang, B.P.; Wang, L.; Guo, X.; Sui, M.B.; Wang, L.; Xue, Y.F. Tailoring the non-coherent interfacial precipitation to overcome the trade-off between strength-ductility and impact energy release in Ti-Zr-Hf-Nb-Ta high entropy alloy. Intermetallics 2024, 166, 108194. [Google Scholar] [CrossRef]
- Ward, T.Z.; Wilkerson, R.P.; Musicó, B.L.; Foley, A.; Brahlek, M.; Weber, W.J.; Sickafus, K.E.; Mazza, A.R. High entropy ceramics for applications in extreme environments. J. Phys.-Mater. 2024, 7, 021001. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.T.; Zhao, S.C.; Chen, L.; Li, Y.T.; Jiang, X.; Leng, Y.X. Enhancing wear resistance: In-situ ceramic phase precipitation for strengthening and toughening FeCoNiCrNx high-entropy alloy films. Surf. Coat. Technol. 2024, 478, 130466. [Google Scholar] [CrossRef]
- Ali, S.; Ahmed, M.; Liu, B.Y.; Balal, A.H.; Jia, Y.F.; Tariq, N.H.; Sun, K.; Mu, Y.K.; Jia, Y.D.; Wang, G. Microstructural stability and mechanical property of AlCrFeMoTi high-entropy amorphous alloy thin films under He plus ions irradiation. Surf. Coat. Technol. 2024, 487, 130952. [Google Scholar] [CrossRef]
- Wang, J.C.; Man, J.; Wang, Q.T.; Liu, G.G.; Xiao, S.C.; Dong, N. Effect of Nano WC on Wear and Corrosion Resistances of CoCrFeNiTi High Entropy Alloy Coating. J. Mater. Eng. Perform. 2025, 34, 1078–1087. [Google Scholar] [CrossRef]
- Valiev, R.R.; Oleinik, A.V.; Asfandiyarov, R.N.; Nazarov, A.Y.; Ramazanov, K.N.; Savina, Y.N.; Kilmametov, A.R. Mathematical Modeling of Complex-Shape Forming of Ultrafine-Grained Ti Alloy and Subsequent Deposition of Protective High-Entropy Coatings. Phys. Mesomech. 2024, 27, 725–735. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.G.; Wang, G.F.; Zhu, J.; Zhan, R.; Liu, Y.K.; Guan, W.; Liang, H.; Cui, L.; Liu, Y.C. Deformation-induced grain boundary segregation in a powder-metallurgy ultrafine-grained MoNbTaTiV refractory high-entropy alloy. Heliyon 2024, 10, e37392. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Tojibaev, A.; Novikov, V.; Salishchev, G.; Zherebtsov, S.; Stepanov, N. Tuning the grain and domain sizes to achieve superior room-temperature tensile ductility in a B2-ordered refractory Al15Nb40Ti40V5 medium-entropy alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2023, 874, 145073. [Google Scholar] [CrossRef]
- Wang, W.Q.; Qu, L.D.; Lu, Y.Z. Effect of heat treatment on microstructure and mechanical properties of lightweight high-entropy alloy. J. Alloys Compd. 2024, 1008, 176219. [Google Scholar] [CrossRef]
- Chen, Y.W.; Zheng, J.Y.; Xu, Z.Q.; Cheng, B.; Li, Y.K.; Zhang, J. A lightweight TiZrV0.5Nb0.3Al0.2 refractory high-entropy alloys with enhanced strength and ductility at low and high strains loading. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2025, 922, 147653. [Google Scholar] [CrossRef]
- Shen, J.J.; Gonçalves, R.; Choi, Y.T.; Lopes, J.G.; Yang, J.; Schell, N.; Kim, H.S.; Oliveira, J.P. Microstructure and mechanical properties of gas metal arc welded CoCrFeMnNi joints using a 410 stainless steel filler metal. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2022, 857, 144025. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Q.; Dai, J.B.; Chen, P.J.; Gao, W.P.; Hao, J.B.; Yang, H.F. Microstructure and high-temperature wear behavior of CoCrFeNiWx high-entropy alloy coatings fabricated by laser cladding. Tribol. Int. 2022, 172, 107574. [Google Scholar] [CrossRef]
- Yuan, G.; Pestana, L.R. The Effect of Surface Oxygen Coverage on the Oxygen Evolution Reaction over a CoFeNiCr High-Entropy Alloy. Nanomaterials 2024, 14, 1058. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Hong, L.; Wu, S.Y.; Qin, Y.; Yang, S.; Huang, M. Effect of transition-metals (Fe, Co and Ni) on the L12-phase strengthening in the Al0.3CrFeCoNi high-entropy alloy. Intermetallics 2024, 166, 108206. [Google Scholar] [CrossRef]
- Song, J.F.; Chai, Z.S.; Zheng, J.; Wu, Q.F.; He, F.; Yang, Z.N.; Li, J.J.; Wang, J.C.; Yang, H.O.; Wang, Z.J. Design Fe-based Eutectic Medium-Entropy Alloys Fe2NiCrNbx. ACTA Metall. Sin.-Engl. Lett. 2021, 34, 1103–1108. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.; Wang, X.; Leng, Y. Integration of hardness and toughness in (CuNiTiNbCr)Nx high entropy films through nitrogen-induced nanocomposite structure. Scr. Mater. 2024, 238, 115763. [Google Scholar] [CrossRef]
- Zhu, B.P.; Guo, W.K.; Shen, G.Z.; Zhou, Q.; Shung, K.K. Structure and electrical properties of (111)-oriented Pb(Mg1/3Nb2/3)O3-PbZrO3-PbTiO3 thin film for ultra-high-frequency transducer applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011, 58, 1962–1967. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Yang, X.C.; Li, X.M. Research on the synergistic damage behavior of tribocorrosion of CoCrFeNi-X (Ti, Mn, Mo, Al) high-entropy alloys. Tribol. Int. 2025, 201, 110185. [Google Scholar] [CrossRef]
- Wu, M.; Setiawan, R.C.; Li, D.Y. Benefits of passive element Ti to the resistance of AlCrFeCoNi high-entropy alloy to corrosion and corrosive wear. Wear 2022, 492–493, 204231. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Song, M.; Zhou, R.; Gu, J.; Wang, Z.; Ni, S.; Liu, Y. Nitrogen induced heterogeneous structures overcome strength-ductility trade-off in an additively manufactured high-entropy alloy. Appl. Mater. Today 2020, 18, 100498. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Fu, R.; Li, Y.; Xu, B.; Qi, X. Influences of nitrogen alloying on microstructural evolution and tensile properties of CoCrFeMnNi high-entropy alloy treated by cold-rolling and subsequent annealing. Mater. Sci. Eng. A 2020, 787, 139472. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Mater. Sci. Eng. A 2010, 527, 7210–7214. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.; Qi, M.; Xu, Y.; Ezatpour, H.R. Effects of heat treatment on the microstructure, wear behavior and corrosion resistance of AlCoCrFeNiSi high-entropy alloy. Intermetallics 2021, 138, 107324. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y.; Chen, R.N.; Wang, H.X.; Zhang, P.; Zhou, B.J.; Li, D.F.; Lin, H.; Ding, L. Influence of Al content on the elastic properties and various anisotropies of AlxCrFeCoNiCu high entropy alloys base on the first-principles calculation. Mater. Res. Express 2023, 10, 066505. [Google Scholar] [CrossRef]
- Xi, R.; Li, Y.Z. Influence of Si Content on the Microstructure, Wear Resistance, and Corrosion Resistance of FeCoNiCrAl0.7Cu0.3Six High Entropy Alloy. Coatings 2024, 14, 1309. [Google Scholar] [CrossRef]
- Shi, X.; Liang, H.; Li, Y. Effect of Si Content on Phase Structure, Microstructure, and Corrosion Resistance of FeCrNiAl0.7Cu0.3Six High-Entropy Alloys in 3.5% NaCl Solution. Coatings 2025, 15, 342. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Wang, Z.Y.; Zhang, C.H.; Chen, H.T.; Chen, J. New studies on wear and corrosion behavior of laser cladding FeNiCoCrMox high entropy alloy coating: The role of Mo. Int. J. Refract. Met. Hard Mater. 2022, 102, 105721. [Google Scholar] [CrossRef]
- Hajilou, N.; Javaheri, M.; Ebadzadeh, T.; Farvizi, M. Investigation of the electrochemical behavior of AlCoCrFeNi-ZrO2 high entropy alloy composites prepared with mechanical alloying and spark plasma sintering. J. Appl. Electrochem. 2024, 54, 457–466. [Google Scholar] [CrossRef]
- Sekhar, R.A.; Shifin, A.S.; Firoz, N. Microstructure and mechanical properties of AlCoCrNiTi-C High Entropy Alloy processed through Spark Plasma Sintering. Mater. Chem. Phys. 2021, 270, 124846. [Google Scholar] [CrossRef]
- Li, M.; Gazquez, J.; Borisevich, A.; Mishra, R.; Flores, K.M. Evaluation of microstructure and mechanical property variations in AlxCoCrFeNi high entropy alloys produced by a high-throughput laser deposition method. Intermetallics 2018, 95, 110–118. [Google Scholar] [CrossRef]
- Chaudhary, V.; Soni, V.; Gwalani, B.; Ramanujan, R.V.; Banerjee, R. Influence of non-magnetic Cu on enhancing the low temperature magnetic properties and Curie temperature of FeCoNiCrCu(x) high entropy alloys. Scr. Mater. 2020, 182, 99–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Cao, D.D.; Zhang, J.Y.; Zhang, P.; Tao, Q.; Yang, X.Q. Concurrence of spinodal decomposition and nano-phase precipitation in a multi-component AlCoCrCuFeNi high-entropy alloy. J. Mater. Res. Technol.-JMRT 2019, 8, 726–736. [Google Scholar] [CrossRef]
- Hussain, M.; Najib, A.S.M.; Fadil, N.A.; Bakar, T.A.A. Microstructure and Phase Chemistry of Vacuum Induction Melting Fabricated-Equimolar AlCoCrFeNi HEA During Spinodal Dissolution Annealing. Int. J. Integr. Eng. 2024, 16, 145–153. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, T.; Sun, S.; Zhang, G.; Tian, X.; Chen, P. Microstructure and dislocation density of AlCoCrFeNiSix high entropy alloy coatings by laser cladding. Mater. Lett. 2021, 283, 128746. [Google Scholar] [CrossRef]
- Zhu, B.P.; Wu, D.W.; Zhou, Q.F.; Shi, J.; Shung, K.K. Lead zirconate titanate thick film with enhanced electrical properties for high frequency transducer applications. Appl. Phys. Lett. 2008, 93, 012905. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Y.; Zhao, Y.; Ma, K.; Cui, J. Corrosion behaviors and mechanism of AlxCrFeMnCu high-entropy alloys in a 3.5 wt% NaCl solution. Corros. Sci. 2024, 233, 112087. [Google Scholar] [CrossRef]







| Alloy | Abbreviation | Fe | Ni | Cr | Al | Cu | Si |
|---|---|---|---|---|---|---|---|
| FeNiCrAl0.7Cu0.3 | Si0 | 25.00 | 25.00 | 25.00 | 17.50 | 7.50 | 0.00 |
| FeNiCrAl0.7Cu0.3Si0.1 | Si1 | 24.39 | 24.39 | 24.39 | 17.07 | 7.32 | 2.44 |
| FeNiCrAl0.7Cu0.3Si0.3 | Si3 | 23.26 | 23.26 | 23.26 | 16.28 | 6.98 | 6.98 |
| FeNiCrAl0.7Cu0.3Si0.5 | Si5 | 22.22 | 22.22 | 22.22 | 15.56 | 6.67 | 11.11 |
| Alloy | Abbreviation | Fe | Ni | Cr | Al | Cu | Si |
|---|---|---|---|---|---|---|---|
| FeNiCrAl0.7Cu0.3 | Si0 | 12.63 | 40.46 | 8.18 | 25.65 | 13.08 | 0.00 |
| FeNiCrAl0.7Cu0.3Si0.1 | Si1 | 16.18 | 35.99 | 13.46 | 22.34 | 11.23 | 0.80 |
| FeNiCrAl0.7Cu0.3Si0.3 | Si3 | 16.79 | 26.47 | 22.38 | 16.79 | 7.64 | 4.91 |
| FeNiCrAl0.7Cu0.3Si0.5 | Si5 | 24.80 | 27.32 | 17.01 | 17.01 | 8.18 | 5.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Han, X.; Liu, J.; Wang, X.; Li, Y. Microstructural Evolution and Wear Resistance of Silicon-Containing FeNiCrAl0.7Cu0.3Six High-Entropy Alloys. Coatings 2025, 15, 676. https://doi.org/10.3390/coatings15060676
Li J, Han X, Liu J, Wang X, Li Y. Microstructural Evolution and Wear Resistance of Silicon-Containing FeNiCrAl0.7Cu0.3Six High-Entropy Alloys. Coatings. 2025; 15(6):676. https://doi.org/10.3390/coatings15060676
Chicago/Turabian StyleLi, Junhong, Xuebing Han, Jiaxin Liu, Xu Wang, and Yanzhou Li. 2025. "Microstructural Evolution and Wear Resistance of Silicon-Containing FeNiCrAl0.7Cu0.3Six High-Entropy Alloys" Coatings 15, no. 6: 676. https://doi.org/10.3390/coatings15060676
APA StyleLi, J., Han, X., Liu, J., Wang, X., & Li, Y. (2025). Microstructural Evolution and Wear Resistance of Silicon-Containing FeNiCrAl0.7Cu0.3Six High-Entropy Alloys. Coatings, 15(6), 676. https://doi.org/10.3390/coatings15060676






