Abstract
The chemical, electrochemical and microbiological corrosive degradation of metals is a versatile harmful problem that causes significant economic loss all over the world. The mitigation of these undesired processes needs basic knowledge on the mechanisms of processes in order to control these reactions with environmentally acceptable chemicals and techniques. This paper focuses on the up-to-date possibilities that help in the mitigation of chemical/electrochemical corrosion and, at the same time, in decrease the deposition of corrosion relevant microorganisms, as the microbes in biofilms are more dangerous than the planktonic cells. Some chemicals or coatings due to their specific properties can fulfill multiple functions; they are able to control the corrosion caused by aggressive materials (that could be the metabolites of a corrosion relevant microorganism) and, at the same time, reduce the microbial adhesion. These additives that have important application possibilities in the chemical industry, marine environment, medical field, nanoelectronics, etc., can save energy, materials consumption and cost, and, at the same time, the efficiency is improved. All resolutions will be brought into prominence when the same chemicals (either in dissolved form or in coatings/nanolayers) can effectively control the different appearance of corrosion and, additionally, the microbial adhesion and microbiologically influenced corrosion.
1. Introduction
All over the world, researchers and industrial specialists have made a lot of effort not to overcome but to decrease these undesired corrosion processes, i.e., the metal dissolution. It is well known that the yearly cost of corrosion is extremely high. The expense of all types of corrosion is estimated to be about 3% of the global GDP. According to a general estimation, the microbiologically influenced corrosion makes up about 20%–30% of the total corrosion cost. In the USA, this is equivalent to USD 300–500 billion annually. In the UK, the untimely replacement of biologically corroded tubes costs EUR 250 million per year. This horrible high expense caused by corrosion relevant microorganisms is due to direct costs (change in equipment, in machinery) and indirect expenses (loss of products, insurance, technical support, etc.). To reduce this very high sum, the only thing a society can do is to study the basic degrading processes caused either by chemicals or by microorganisms and find the best resolutions (application of proper materials, especially the most resistant metals, reduction of aggressive environment), and finding the clue of diminishes of degrading processes. It explains the importance of intensive research and development as well as the application of the best inhibitors and biocides. This paper summarizes the efforts taken to understand the mechanisms and inhibition possibilities of different types of corrosion [1]. This explains why it is important to apply the most proper quality of alloys at an industrial scale (as well as in households) and try to use special additives to help in decreasesing corrosive reactions.
The aim of this review is to summarize the most important knowledge on the undesired deteriorating electrochemical/chemical or microbial corrosion processes of metals and to demonstrate the possibilities for corrosion rate reduction either by chemicals (inhibitors) used in dissolved form or applied in nanocoatings. The paper gives information on those chemicals that alone can control both the corrosive metal dissolution and the microbial activities (biofilm formation, growth, aggressive metabolite productions) at the same time.
2. Corrosion
Corrosion is a very complex, well-known, natural, undesired, destructive process when solid materials are degraded. The production of pure metals needs a large amount of energy; at this high energy level the metals become unstable and under the influence of the environment, they get rid of the additional energy and change into chemically stable forms of salts, oxides, etc., via electrochemical or chemical reactions. These processes, which are termed corrosion, need the presence of water (in liquid or in the condensed phase) when on anodic parts of metal surface electrons are released (oxidation); these electrons are used at the cathodic site where the predominant reaction is oxygen reduction, hydrogen or hydroxonium ion evolution (depending on the pH of the aqueous solution). These conversions result in formation of oxides, oxihydroxides and hydroxides as well as of different salts. The rate of corrosion is determined by the electrochemical potential and electron activity of the metals.
Corrosion can degrade not only metals but other solid materials like polymers, stones or ceramics.
These redox processes are the oxidation of metals on the anode by electron donation and the parallel reduction of an oxidant by electron acceptors:
Me → Me+ + ne−
Ox + ne− → Red
At the cathode, one of the following reactions may take place:
O2 (g) + 4H+ + 4e− → 2H2O (at acidic pH)
O2 (g) + 2H2O + 4e− → 4OH− (at basic pH)
On iron-based metals, the negatively charged OH− ions react with the positively charged Fe2− ions:
4Fe2+ + 8OH− → 4Fe(OH)2 (white color)
In the presence of water and oxygen:
4Fe(OH)2 + O2 → 2Fe2O3 + 4H2O (brown rust)
There are two categories of corrosion theories:
Homogeneous theory: anodic and cathodic reactions go parallel at the metal–electrolyte interface;
Heterogeneous theory: surface heterogeneities on metal surfaces allow the formation of local cells where electrochemical reactions convert the metal into an unstable state.
2.1. Types of Corrosion
The form of corrosion could be the following: uniform (the homogeneous metal surface is uniformly dissolved in the aggressive environment); pitting (on the metal surface the protective passive film is damaged; pits are formed, their depth is much deeper than their diameter); galvanic (when two different metals are in contact); stress corrosion (corrosion fatigue); selective leaching (caused by the dissolution of the noble metal; the structure becomes porous); crevice (in crevices); intergranular (at grain boundaries); hydrogen damage (caused by the diffusion of hydrogen); high temperature induced; and biocorrosion (special type of corrosion when corrosion relevant microorganisms are present).
Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 illustrate the visible differences that appear in different types of corrosion.
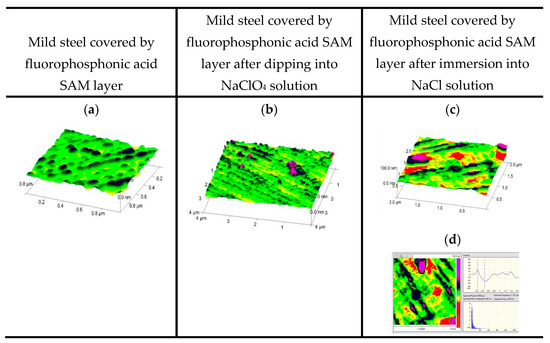
Figure 1.
Demonstration of general and pitting corrosion by atomic force microscope: mild steel with fluorophosphonic acid self-assembled layer (SAM) (a); after immersion into NaClO4 solution for 1 h (b); after immersion into NaCl solution for 1 h (c) and its section; (a–c): 3D; section imaging (d); (0.1 M NaClO4; 3% NaCl; room temperature).
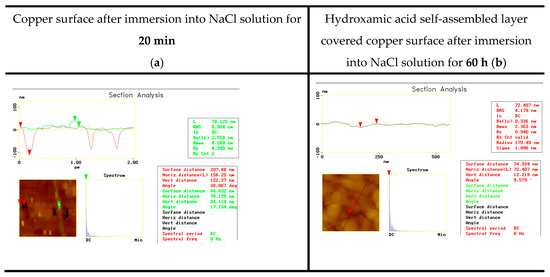
Figure 2.
Demonstration of pitting corrosion and the importance of surface coating: copper surface without nanolayer (a) and after surface covering by self-assembled layer (b) (3% NaCl; room temperature).

Figure 3.
Scanning electron microscopic image of galvanic corrosion of galvanized screw after immersion into 3% NaCl solution.
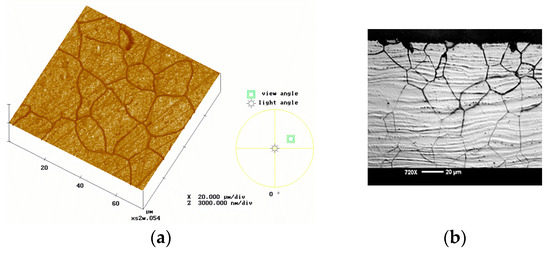
Figure 4.
View of a polished material attacked by intergranular corrosion; (a) surface visualized by AFM in 3D; (b) microscopic visualization of cross-section [2].

Figure 5.
Microscopic image of selective corrosion on cast iron (magnification 500×) [3].
Figure 1 and Figure 2 demonstrate the differences in the types of corrosion. It is clear that in the presence of the NaClO4 electrolyte, the dissolved oxygen but not the perchlorate ions attack the metal surface and causes general corrosion. The NaCl solution is an aggressive electrolyte that immediately initiates pit formation. Figure 1 demonstrates the importance of a nanolayer coating that can save the metal surface against the neutral electrolyte but not against the chloride attack. The deep pits prove this phenomenon. In Figure 2, not only the susceptibility of copper to pit formation is demonstrated (the depth of the pits proves it) but also the importance of the surface nanolayer built up of proper amphiphilic molecules is clearly visible; the surface is smooth and the chloride ions could not attack the metal surface.
Figure 3 demonstrates the influence of NaCl solution on the Zn-covered iron screw.
Figure 4 demonstrates the intergranular corrosion visualized by two different techniques. After the corrosive attack, the AFM image shows the surface and the other one shows the cross-section of the metal, while Figure 5 displays the importance of selective leaching in the case of alloys.
The special effect of microbiologically influenced corrosion is demonstrated in Figure 6; Acidithiobacillus ferrooxidans, acid producer microbes embedded in biofilm, etched the mild steel surface. Measuring the length and width of holes by section analysis enabled to prove that the microorganisms are responsible for the metal dissolution.
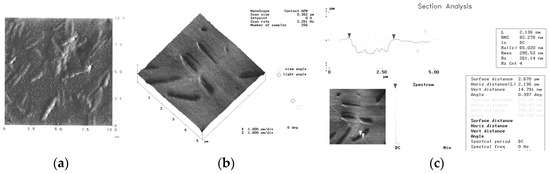
Figure 6.
Acid producer microorganisms on mild steel surface; (a): biofilm formed in 24 h, microorganisms are embedded in biofilm; (b): the surface with the “fingerprint” of the microbes visualized after removal of the biofilm; (c): section analysis of the metal surface after removal of the biofilm.
Parameters that influence corrosion: the pH (the pH value is crucial in the corrosion process; at lower pH values, generally the corrosion rate increases and the rate of metal dissolution is high; in neutral and alkali environment, only some metals are dissolved (Al, Pb, Zn); noble metals are resistant to corrosion), temperature (the rate of corrosion is accelerated by a higher temperature, and in most cases there is an exponential increase; the temperature influences other factors that affect the corrosion rate), purity of metals, nature of surface films, defects on the metal surface, corrosive chemicals, surface roughness, etc. The corrosion rate is generally expressed in mm/year (mpy).
2.2. Inhibition of Corrosion
The decrease in the corrosion rate depends on the change in the anodic and cathodic polarization, on the decrease in ion diffusion to the metal surface, as well as on the increase in the electrical resistance of metal surfaces.
To decrease the rate of corrosion, there are several possibilities:
A change in the environment: (decrease in oxygen, chloride and sulfide ion content);
the application of the most proper metals and the best surface conditions that fit the environmental conditions;
Coatings: coverage of the metal surface in order to prevent metal surfaces from the contact with an aggressive environment; these coatings could be organic or inorganic (e.g., metallic) and they are either decorative or functional when the substrate surface properties are altered (wettability, adhesion, corrosion resistance, etc.);
Cathodic protection: alteration of the anodic sites of the metal surface by an opposite current;
Anodic protection: by an electrochemical reaction that keeps the metals in a passive state by electrode potential control;
The application of inhibitors: chemicals used at a very low concentration are adapted to the whole system (metal, electrolyte, pH, temperature, etc.)
The categories of inhibitors that can control the chemical/electrochemical and microbiologically influenced deterioration are as follows:
- Anodic type (passivators, film formation on the metal surface; it prevents the oxidation reaction, nitrates, molybdates, etc.);
- Cathodic inhibitors: they reduce the cathodic reactions; zinc, aluminum and their alloys;
- Volatile corrosion inhibitors: they release a vapor that protects the metal surface temporarily; organic acids, amines, carboxylates;
- Film-former inhibitors: a protective barrier film is formed on the metal surface that keeps the corrosive environment far from the solid metal; surfactants, amines, phosphates;
- Biocide type: in the presence of corrosion relevant microbes, these chemicals can control the microbial life (growth, multiplication, metabolite secretion); hypochlorite, chlorine dioxide, formaldehyde, glutaraldehyde, quaternary ammonium compounds, etc.
3. Corrosion Control by Inhibitors
In the case of aqueous environment special chemicals, the so-called inhibitors can save the metals from the dissolution. They could be organic or inorganic molecules. The inorganic ones help in the passivation of the metal surfaces on the anodic part by their anions. The organic inhibitors can form a film or precipitation via a reaction between the functional part of the inhibitor molecule and the metal ions; this is how electrochemical reactions on the anodic and cathodic sites are confined. These molecules generally have special hetero atoms (e.g., S, N, P) that help in the adsorption (physical and chemical interaction) to a metal surface and formation of a monomolecular protective film, which increases the energy of corrosion reactions, i.e., decrease the corrosion rate. The corrosion inhibitors should be very effective in corrosion control as they contribute to the use of less expensive metals. The development of inhibitor molecules is summarized in Table 1.

Table 1.
Development of corrosion inhibitors.
The change in the anodic or cathodic polarization, the decrease in the ion diffusion to metals’ surfaces and the increase in the electrical resistance of surfaces can slow down the corrosion processes too.
The following important factors should be taken into account in the selection of proper corrosion inhibitors:
- The environment of the instrument where they will be used: temperature, pH, humidity, aggressive ions and molecules—they should be compatible with the inhibitors;
- The compatibility of the structural material with the inhibitor used for protection;
- The corrosion mechanisms: this is a crucial factor as it determines the proper selection of inhibitors to structural metals; it is influenced by the type of corrosion (general, pitting, crevice, etc.) that can deteriorate metals;
- The cost of inhibitors and their availability;
- The environmental impact: the health restrictions should always be considered.
The type of inhibitors depends on which reactions are influenced: anodic reaction, cathodic reaction or mixed type when both reactions are influenced.
Types of Inhibitors [4]
The inhibitors could be interface (2D) and interphase (3D) types. The importance is to control the interaction between the aggressive environment and the metal surface.
Anodic inhibitors (carbonates, molybdates, phosphates): a protective layer is formed between the metal oxides/ hydroxides/salts and inhibitors.
Cathodic inhibitors: the inhibitors interact with the cathodic reaction products (e.g., OH−).
Organic inhibitors: their adsorption to the metal surface happens through their polar functional groups (-CS, -CO, -CN, -C-P); the unpaired electron pairs of N, O, S and P atoms are responsible for the adsorption. In case of double or triple bonds as well as aromatic rings, these molecules can interact with the metal surfaces too.
The selection of inhibitors depends on the inhibition mechanisms, as well as on the electron donating–accepting capability of the inhibitors.
In the selection of inhibitors, all the following information should be taken into account: the metal quality, environment, temperature, pH and presence of gases (especially oxygen). It is important that the inhibitors used in dissolved form are active at very low concentrations and their concentration does not change over time. These types of inhibitors are used in cooling/heating/boiler/washing water systems, in the oil and gas industry (water injection, oil transportation), and in different chemical industries [5,6,7].
In the last two decades, the importance of the so-called “green corrosion inhibitors” has increased enormously. They should be biodegradable, eco-friendly, cost-effective and non-toxic; it is not recommended to apply them together with heavy metal ions; they are naturally occurring substances that could be active either in acidic or in neutral as well as in an alkali environment. These types of molecules could be pigments, alkaloids, amino acids and plant extracts.
A new trend among corrosion inhibitors is the use of nanomaterials. Their efficiency is due to the increased surface/volume ratio and unique electronic, physical, chemical and mechanical characteristics. They generally adsorb to metal surfaces by physisorption or chemisorption and so increase the coating’s lifetime [8]. These nanomaterials are metal nanoparticles (silver, gold, TiO2) [9,10,11]. Nanocontainers can also effectively control corrosion [12,13].
4. Corrosion Control by Coatings
An important technique for corrosion control is the application of coatings. The coatings could be applied by spraying or dipping or in very thin molecular layers, such as Langmuir–Blodgett film or self-assembled molecular layers. Paints help to protect metal surfaces by coatings in some ten micrometer thickness. The organic coatings that control the corrosion rate could be alkyd, epoxy, urethane, acrylic and styrene polymers as well as powder coatings. In all cases, the coatings do not allow close contact between the metal surface and the aggressive environment [14,15,16,17,18].
5. Corrosion Monitoring
There are several techniques that help in corrosion monitoring. One of the first very good summaries was given by Marcus and Mansfeld [19] who not only collected the instruments applicable for corrosion monitoring but also explained the principles of all measuring techniques: X-ray photoelectron spectroscopy (XPS): a surface-sensitive quantitative spectroscopy that identifies the elements that are within a material or in the surface coverage, such as the chemical state or electronic structure; Electron Spectroscopy for Chemical Analysis (ESCA): provides elemental and chemical binding information about a material; scanning electron microscopy (SEM): provides visual images of surfaces that are of high quality with spatial resolution; nanoprobes: provide information regarding the morphology and chemical composition; infrared and Raman spectroscopy: provides a chemical fingerprint on the corrosion inhibitors and corrosion products that cover the solid surfaces; glow discharge optical emission spectroscopy: a method for the quantitative analysis of metals; radiotracer method: nanoindentation for checking the hardness of the surface layer; Auger–Mössbauer spectroscopy: gives information about the surface compositions; and electrochemical impedance spectroscopy: provides important information about the surface layers and shows how the surface layer can control the corrosive dissolutions [20]. Some other electrochemical methods that are very useful to follow corrosion processes are the linear polarization resistance, electrochemical noise analysis and potentiostatic/dynamic techniques, and Tafel extrapolation, to mention only the most important ones [21,22,23].
Table 2 summarizes the most often used corrosion monitoring techniques and it indicates the advantages and disadvantages of the methods. Generally, researchers and specialists use different complementary methods in parallel in order to get more detailed information about the corrosion process.

Table 2.
Techniques used for monitoring corrosion processes.
The corrosion processes that regulate the material degradation could be categorized as follows:
- Electrochemical corrosion of metals: electrons from the surface metal atoms are formed and then transferred to electron acceptors (oxygen, acids, etc.) in the presence of water. This is a hybrid process when the chemical state of metal atoms is transformed to metal ions and then the metal valence electrons are transferred to electrochemically active ions/molecules [24].
- Chemical corrosion: in this case, the presence of an external environment causes a gradual destruction of metals, e.g., the oxidation/dissolution of metals via acids. electron transfer is not necessary.
- Microbiologically influenced corrosion (MIC) [25]; the reactions caused by microbes that deteriorate metals are due to chemical or electrochemical processes. The presence of microorganisms such as bacteria, fungi and algae in the environment can especially enhance the corrosion when they are embedded in biofilms.
6. Microbiologically Influenced Corrosion
The MIC, which was first mentioned by Garrett in 1891 [26], happens only in the presence of corrosion relevant microorganisms, they increase the metal dissolution by their presence, by their excreted metabolites (e.g., acids), by macromolecules (exopolymeric substances) with complexing ability, as well as by other molecules, ions that form insoluble precipitates (e.g., FeS) on the metal surface. All these processes increase the metal dissolution.
6.1. Corrosion Relevant Microorganisms
The presence of microorganisms can lead to biofilm formation, biofouling, biodeterioration and biocorrosion (MIC). Several dozens of microorganisms can cause the dissolution of different metals (Table 3). MIC could be seriously influenced by anaerobic microorganisms (sulfate reducers species (Desulfovibrio, Desulfomonas, Desulfotomaculum, Desulfocurvus, etc.)) that cannot or only partly tolerate the presence of oxygen. They gain energy from the conversion of sulfate to sulfide ions (which forms FeS black precipitate) and hydrogen sulfide (that causes stress cracking). The other classes of microbes need the presence of oxygen, and some of them can oxidize metals (e.g., iron alloys), like Thiobacillus ferrooxidans, Pseudomonas, Gallionella or Spherotilus, or convert sulfur (e.g., Thiobacillus thiooxidans). There are some acid producers, to mention only some of the corrosion relevant microorganisms. The biofilms formed in natural waters will involve not only bacteria and fungi but also algae, plants and small animals, and then the name of the slimy film is biofouling

Table 3.
The most important corrosion relevant microorganisms [27].
6.2. Biofilms
Planktonic microorganisms, after coming in contact with solid surfaces, start to adhere to them. First, the accumulation of organic molecules will cover the wetted surface; this is a conditional layer that consists of polysaccharides, nucleic acids, proteins, (excreted by the planktonic microbes, EPS), organic and inorganic molecules and ions (which originate from the aqueous environment). Then, the free-floating planktonic cells start to adhere to the conditional layer, first reversible and then irreversible. For the adhesion of cells, which is enhanced by the further excretion of EPS, electrostatic, van der Waals forces and hydrogen bonds are responsible. These forces keep the biofilm together. Within the biofilm, the sessile microbes are permanently bound and they continue their normal life; they metabolize, grow and multiply. The biofilm that consists of 75%–90% water could be containing different microorganisms that grow in time, and after a while, when the biofilm is already too thick, its upper part comes loose/unstuck. Within the biofilm, the amount of nutrients determines the life of the microbial community. The microbes are less dangerous in planktonic form than involved in biofilms. A biofilm is generally a consortium of different microorganisms; these microbes can form a synergistic layer that can be more aggressive in metal degradation and, additionally, more actively resist biocides.
There are several factors that influence the biofilm formation: the types of microorganisms, surface roughness, chemicals in the biofilm, diffusive transport of nutrients, etc. [28]. The structure of the biofilm is determined by several weak interactions like van der Waals forces, electrostatic interactions and hydrogen bonds. The anaerobic microorganisms are close to the metal surface and the aerobic microbes are near to the water–air interface. The aggressive metabolites (especially sulfide ions) initiate pit formation and this leads to localized corrosion.
It is important to emphasize that the planktonic microbes are much less dangerous than those embedded in the biofilms.
In the biofilm formation, i.e., in the adhesion of microorganisms and colonization, the follow factors play an important role:
- Metal/solution interface: chemical composition, concentration of components (organic, inorganic ions, pH), thickness of the passive film, roughness, polarization;
- Characteristics of bacteria and the media: most of the microbial cells are negatively charged at a neutral pH value. The adhesion of corrosion relevant bacteria to metal surfaces is the strongest near to the bacterial isoelectronic points [22,29,30].
6.3. Inhibition of MIC
There are two important tasks in controlling the microbiologically influenced corrosion: one is to influence the microbial life (inhibition of growth and proliferation as well as the metabolite production) and the other is to inhibit the microbial adhesion and biofilm formation. In the case of planktonic cells, the influence on the microbial life is much easier than on those embedded in the microbial slime.
The biofilms influence the chemical transport at the metal/biofilm interface as they can remove the passive films from the metal surface by the excreted metabolites as well as by changing the oxidation–reduction reactions.
After pointing out the undesired effect of MIC, it is clear that several important techniques and methods were elaborated to save metals from the undesired effects of microorganisms.
Some simple techniques for controlling MIC are the following:
- Physical treatment: removal of already developed biofilms;
- Electrochemical methods, e.g., cathodic protection;
- Chemical treatment: application of several chemicals (oxidizers, non-oxidizers, membrane active, chelate formers, etc.);
- Biological resolution: in this technique, microorganisms are used to control the biofilm formation, and this is called microbially controlled corrosion inhibition (MICI).
The removal of a well-developed biofilm is not easy. It needs either a harsh mechanical treatment or the application of aggressive (mainly oxidizing) biocide at a very high concentration [30,31].
6.4. MIC Inhibition by Chemicals
The histories of the chemicals that have been used for controlling MIC are summarized in Table 4.

Table 4.
History of the antifouling techniques.
The requirements that the chemicals should solve in order to reduce the undesired influence of the corrosion relevant microorganisms are as follows:
They should control the microbial life, either as biostatics (reduction in metabolic life, the microbial growth) or as biocides (they fully destroy the microorganisms) (Figure 1);
They are supposed to have a broad spectrum for controlling the anaerobic as well as the aerobic microbial activity;
Additionally, they must be compatible with the other additives used in the same system. In other words, e.g., the activity of the corrosion inhibitors should be unaffected by biocides;
They should control the life both of the planktonic cells and of those ones that are built in a biofilm.
The often-used biostatics/biocides are listed in Table 5.

Table 5.
The most often applied antimicrobial chemicals.
Several biocides, e.g., tin compounds, are today forbidden because of their toxicity.
The biocides are supposed to control the microbial metabolisms or destroy the viable cells. They cause oxidative stress, enzyme inhibition, cell membrane disruption, cell death, etc.
The effects of the biocides used in different industries (water treatment in cooling systems, in gas- and oil/pulp- and paper/metal working industries, etc.) are summarized in Table 6.

Table 6.
Summary of biocides with examples and explanations to demonstrate what they cause.
Figure 7 show some examples of how biocides can inhibit the microbial life of corrosion relevant microorganisms.
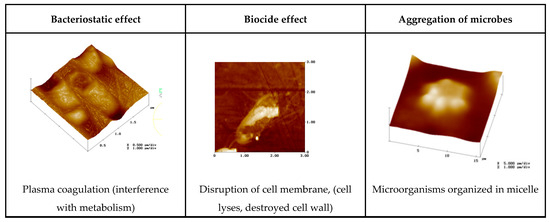
Figure 7.
Cells affected by biocides in different ways.
Some possibilities to decrease the use of harmful biocides
Physical methods: UV light can control the microbial growth and biofilm formation without biocides;
Non-toxic biocides: enzymes, bio-based additives and natural agents that can control the microbial life and biofilm formation;
Surface modification: by this method, not only the corrosion but the microbial adhesion can be diminished; these are special surface coatings alone (they change the surface wettability or pH) or are used in combination with special, environmentally acceptable chemicals;
Destruction of biofilms: special enzymes and some corrosion inhibitors can eliminate biofilms with corrosion relevant microorganisms;
Biomimetics: a limitation of living organisms, applied mainly in marine environments.
7. Multifunctional Chemicals with Anticorrosion and Antimicrobial Activity
In the previous sections not only the corrosion processes and the corrosion inhibition but also the corrosion relevant microorganisms and their undesired deteriorating effects as well as the chemicals methods were demonstrated that are able to diminish the MIC.
- Chemicals efficient mainly in affecting microbial life as well as they have anticorrosion activity, too: quaternary ammonium compounds (control of microbial growth and corrosion, applied especially on copper and iron alloys as they form protective films on metal surfaces; reduction in microbial adhesion and corrosion); cetyl pyridinium bromid; izothiazolinon derivatives (with antimicrobial activity of broad-spectrum, mainly applied in the cooling industry); benzalkonium chloride (it forms a protective layer that reduces the corrosion and controls the microbial growth);
- Multifunctional organic inhibitors: imidazolines (applied mainly in the gas and oil industry), amines: cyclohexyl amine, morpholine and other amines;
- Polymeric biocides: poly-quaternary ammonium compounds (they control both corrosion and microbial attachment as well as the formation of biofilms) and chitosan, a renewable marine polymer (film-forming, protection against corrosion and microbial adhesion) [32];
- Combination of biocides: the parallel application of different biocides often results in a synergistic effect (e.g., thymol and benzyldimethyl-dodecylammonium chloride) that could control a microbial diversity in marine environments [33].
8. MIC Inhibition by Coatings
The MIC could be controlled not only by dissolved chemicals but also by the following special coatings:
- Traditional biocidal antifouling and fouling-release coating: antibacterial coatings involve three categories: contact killing coatings [34], antifouling coatings [34,35,36,37] and biocide-releasing coatings [38,39,40];
- Slippery liquid-infused porous surfaces show anticorrosion and anti-fouling activity. They consist of porous solid substrates and lubricant oil. This system can cover a metal surface and repel not only the corrosive chemical species but also microorganisms, because of the surface lubricant layer [41]. The explanation of the antibacterial activity is that the fatty acid in the oil will destroy the cell membrane;
- Superhydrophobic or superamphiphobic coatings: they are inspired by nature and their activity depends on special wettability properties [42];
- Silver-based antimicrobial coatings: the silver nanoparticles incorporated into coatings can regulate the microbial adhesion and biofilm formation; these special coatings have anticorrosion activity too.
9. Anticorrosion, Antimicrobial, Antifouling Coatings
MIC, which is a special type of corrosion that starts in the presence of corrosion relevant microorganisms, is enhanced by the biofouling that forms on slimy biofilms when macroorganisms, plants and animals adhere to the biofilm. All these processes start with the biofilm formation and continue with the adhesion of marine organisms (mussels, algae, barnacles, etc.). It is clear that the initial adhesion of microorganisms should be avoided in order to decrease the possibility of solid surface deterioration.
9.1. Biocide-Based Antifouling Paint
Coatings with anti-adhesion and antifouling activity applied on a solid surface can control the adhesion of microorganisms and of other organs. These special chemicals, i.e., biocides either by leaching out from the coatings or covering homogeneously the coated surface, are able to repel the micro- or macro-organisms, plants and other living creatures, and, at the same time, decrease the corrosion processes.
The active components in coatings could be the following:
Tributyltin: it was very effective but because of its toxicity, its use is banned;
Copper, copper oxide: they are effective but nowadays they are not recommended because of the accumulation in natural water.
Organic additives: benzoic acid, sodium benzoate, Zosteric acid, chlorothalonil (2,3,5,6-tetrachloroisophtalonitril), dichlofluanid (N-dichloro-fluoromethylthio-N’,N’-dimethyl-N-phenylsulfamide), DCOIT (4,5-dichloro-2-n-octyl-4-isothiazolin-3-one), Diuron (1-(3,4-dichlorophenyl)-3,3-dimethylurea, Irgarol (2-methylthio-4-tercbutylamino-6-cyclopropylamino-s-triazine), TCMS pyridine (2,3,5,6-tetrachloro-4-methylsulfonyl pyridine), Zinc-pyrithione-bis (2-pyridylthio)zinc-1,1′-dioxide, capsaicin, etc.;
Polymers: izothiazolinone derivatives with quaternary ammonium groups are embedded in polymers; silicon elastomer polymers with 3-(trimethoxysilyl) propyloctadecyl-dimethyl ammonium chloride; phosphorylcholine-substituted methacrylate, siloxane in polyurethane, to mention some of them.
9.2. Nanolayers for Controlling MIC and Corrosion Parallel [31,43]
These chemicals listed previously when used either alone or embedded in coatings are generally very active under different conditions. Their use depends on the solid materials (i.e., on the metals), on the environment (aqueous, oily or atmospheric), on the temperature, pH, presence of gases, flow conditions, etc. In these cases, the antimicrobial chemicals can leach out from the coating, the concentration of the active material decreases in time and they pollute the environment.
There are some other possibilities to diminish the undesired, negative environmental impact. When a metal surface is covered by effective nanolayers (e.g. in the form of Langmuir–Blodgett film (LB) or self-assembled molecular layers (SAM)), the materials used for the nanocoating will alter the surface characteristics; their solubility is negligible during the utilization because of their adsorbed state on the metals’ surface. The chemicals applicable for these types of layer productions are so-called amphiphilic molecules with a small ionic or ionizable head groups and hydrophobic (minimum C11 long) alkyl/alkenyl/aralkyl chains. The head group decides the adsorption to the metal surface; alkyl thiols can form a compact nanolayer on copper, gold and silver. The carboxylic and phosphonic acid groups can adhere only to metal oxides either by mono- or bi- as well as tridentate bounding. The adsorption is followed by water formation. On two examples will be demonstrated how these nanolayers can actively decrease the corrosion and inhibit the microbial adhesion of the mixed population present in cooling water. The metals are iron alloy and copper, and the amphiphiles are the octadecyl phosphonic acid (C18P) and the octadecyl hydroxamic acids (C18N). Both chemicals could form a molecular deposition on the metal surface, either as Langmuir–Blodgett film or as self-assembled molecular layers. The layers changed the water wettability and the coated surfaces were much more hydrophobic than the metals without coatings. After placing these coupons into cooling water for five days, both the anticorrosion and the microbial adhesion inhibiting properties are demonstrated. On copper coupons (uncovered and covered by LB layers), the dark brownish color demonstrates the presence of corrosion. Where both amphiphiles’ types of LB layers were present, the coupons stayed bright, keeping the original copper color that proved the anticorrosion activity of both molecular layers. The presence of microorganisms on the same coupons was visualized by a fluorescence microscope after acridine orange dying. Although the copper has some antimicrobial effect, the uncoated coupons were invaded by microbes, but both nanolayers could keep the metal surfaces almost intact from microorganisms (Figure 8).
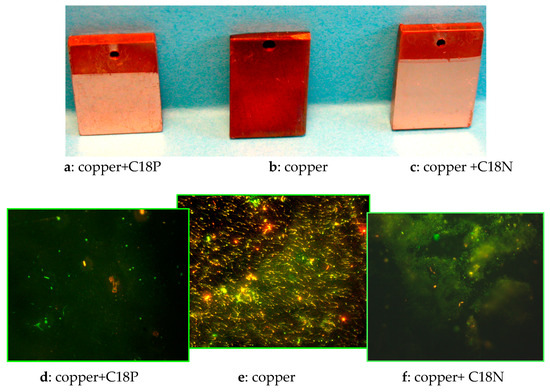
Figure 8.
The influence of LB molecular film on corrosion and on microbial adhesion; copper surfaces dipped into cooling water of mixed microbial population for five days; photos on coupons after corrosion test: (a–c); microbial adhesion visualized by fluorescence microscopy: (d–f); (a,d): copper surface covered by Langmuir–Blodgett molecular film of octadecyl phosphonic acid (C18P); (b,e) copper surface; (c,f) copper surface covered by Langmuir–Blodgett molecular film of octadecyl hydroxamic acid (C18N).
The anticorrosion activity of the C18P and C18N amphiphilic LB and SAM layers on an iron surface were proven. Now, in the presence of corrosion relevant microorganisms, the anti-adhesion properties are demonstrated. Figure 9 unequivocally shows that the C18P LB layer cannot hinder the adhesion of microorganisms; there are a lot of small yellow particles (that are the microorganisms) on the surface. On the other hand, the C18N with nitrogen atom in the molecule either in the LB or SAM layers could inhibit the microbial adhesion very effectively. The explanation for this interesting behavior in the case of the C18P LB layer on iron could be attributed to the differences in the structure of the iron and copper oxide layer, which is much less compact on iron than on copper.
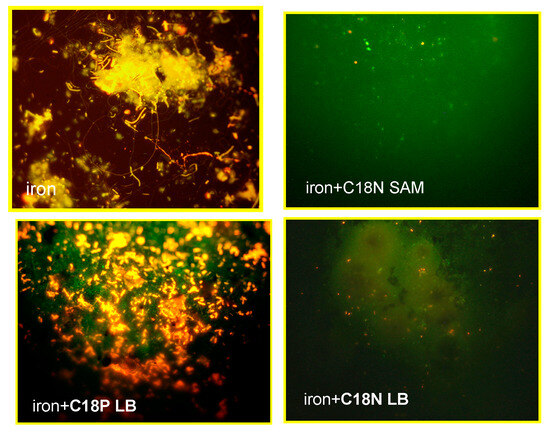
Figure 9.
Fluorescence images taken on adhered microorganisms developed on iron surfaces in cooling water of mixed microbial population after five days; upper left: iron without nanocoating; down left: iron surface covered by LB film of octadecyl phosphonic acid (C18P); upper right: iron surface covered by self-assembled layer of octadecyl hydroxamic acid (C18N); lower right: iron surface covered by Langmuir–Blodgett layer of octadecyl hydroxamic acid (C18N).
It is important to mention that in some cases the more hydrophobic and in other cases the more hydrophilic surfaces can better control the microbial adhesion. It depends on the type of microbes. The corrosion relevant microorganisms do not prefer hydrophobic surfaces; in other words, they do not like to adsorb and start colonization on hydrophobic surfaces.
10. Some Techniques Used for Monitoring MIC [44]
The first is to identify the microbes involved in MIC by microbiological methods. Then, their activities should be followed by special electrochemical methods. There are some techniques that can explain what happens when the microbes attack a metal surface, including the following: anodic/cathodic polarization, cyclic voltammetry and electrochemical noise analysis, to mention some of them. These measurements should be combined with surface analysis. About the composition of the original and the altered metal surface, scanning electron microscope, X-ray photoelectron spectroscope and Auger electron microscope can inform. It is necessary to take into account that the biofilm will alter the electrode surface and can disturb the electrochemical measurements. The morphology of microorganisms, biofilms and the corrosively destroyed metal surface can be easily followed by surface visualizing techniques (stereo microscope, atomic force microscope, SEM, etc.). The Fourier transform infrared spectroscope can show the chemical composition of the surface. These techniques will supply the most important information about MIC. But always it should be remembered that the presence of microbes and the activity of vial cells and the biofilm continuously change the layer thickness on the metal surface. This is the reason that different techniques are applied parallel to get a more reliable, precise result [45,46,47,48].
11. Summary
The review demonstrated the corrosive metal dissolutions and their external appearance, pinpointed the generating conditions and summarized the possibilities that help in diminishing the corrosion rate. The aim was to show the influence of different environmental conditions, especially the effect of corrosion relevant microorganisms, and to introduce the chemicals (inhibitors, biocides) that can control the microbial adhesion/biofilm formation (which leads to enhanced corrosion), and also introduce those methods that are able to decrease the corrosion and the microbiological adhesion. The effect of different chemicals was shown and some advice was suggested concerning when and how to choose the proper condition. It is important to emphasize that the decision to use this or the other inhibitor and/or biocide depends always on the given conditions (composition of alloys, environmental conditions: pH, ions—e.g., chloride ions—responsible for pitting corrosion, oxygen content, etc.). The most important is to point out that there are several possibilities for the reduction of the corrosion rate caused by an aggressive environment and, at the same time, by corrosion relevant microorganisms when the same chemicals are used either in dissolved form or in nanolayers.
Funding
This research no received external finding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Maji, K.; Lavanya, M. Microbiologically Influenced Corrosion in Stainless Steel by Pseudomonas aeruginosa: An Overview. J. Bio- Tribo-Corros. 2024, 10, 2024. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Intergranular_corrosion (accessed on 30 July 2023).
- Available online: https://en.wikipedia.org/wiki/Selective_leaching (accessed on 30 July 2021).
- Dariva, C.G.; Galio, A.F.; Dariva, C.G.; Galio, A.F. Corrosion Inhibitors–Principles, Mechanisms and Applications. In Developments in Corrosion Protection; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Shaban, A.; Vastag, G.; Telegdi, J. Chapter 2. Metal Corrosion and Its Inhibition Mechanisms: An Overview. In Corrosion Inhibitors: An Overview, 2021st ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; Chapter 2. [Google Scholar] [CrossRef]
- Balangao, J.K.B. Corrosion of Metals: Factors, Types and Prevention Strategies. J. Chem. Health Risks JCHR 2024, 14, 79–87. [Google Scholar]
- Telegdi, J. Chapter 3-History of phosphorus-containing corrosion inhibitors: From the beginning till the present time. In Water-Formed Deposits; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–68. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W. Formulation of Corrosion Inhibitors. In Water Chemistry; Eyvaz, M., Yüksel, E., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Palanisamy, K.; Devabharathi, V.; Sundaram, N.M. Corrosion inhibition studies of mild steel with carrier oil stabilized of iron oxide nanoparticles incorporated into a paint. Int. J. ChemTech Res. 2015, 7, 1661–1664. [Google Scholar]
- Obot, I.; Umoren, S.; Johnson, A. Sunlight-mediated synthesis of silver nanoparticles using honey and its promising anticorrosion potentials for mild steel in acidic environments. J. Mater. Environ. Sci. 2013, 4, 1013–1018. [Google Scholar]
- Migahed, M.A.; Azzam, E.M.S.; Morsy, S.M.I. Electrochemical behaviour of carbon steel in acid chloride solution in the presence of dodecyl cysteine hydrochloride self-assembled on gold nanoparticles. Corros. Sci. 2009, 51, 1636–1644. [Google Scholar] [CrossRef]
- Atta, A.; EL-Mahdy, G.; Al-Lohedan, H.; Al-Hussain, S. Application of Eco-friendly Magnetite Nanoparticles Coated with Rosin Amidoxime as Corrosion Inhibitor for Mild Steel in 1 M Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2015, 10, 2621–2633. [Google Scholar] [CrossRef]
- Zheng, Z.; Schenderlein, M.; Huang, X.; Brownbill, N.J.; Blanc, F.; Shchukin, D. Influence of Functionalization of Nanocontainers on Self-Healing Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2015, 7, 22756–22766. [Google Scholar] [CrossRef] [PubMed]
- Keyvani, A.; Yeganeh, M.; Rezaeyan, H. Application of mesoporous silica nanocontainers as an intelligent host of molybdate corrosion inhibitor embedded in the epoxy coated steel. Prog. Nat. Sci. Mater. Int. 2017, 27, 261–267. [Google Scholar] [CrossRef]
- Rigó, T.; Mikó, A.; Telegdi, J.; Lakatos-Varsányi, M.; Shaban, A.; Kálmán, E. Inhibition Effect of Hydroxamic and Phosphonic Acids Langmuir-Blodgett Films on Iron Corrosion in Sodium Perchlorate Solution. Electrochem. Solid-State Lett. 2005, 8, B51–B54. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Xuan, C.N. Nanocomposite Coatings: Preparation, Characterization, Properties, and Applications. Int. J. Corros. 2018, 2018, 4749501. [Google Scholar] [CrossRef]
- Mansfeld, F. Analytical Methods in Corrosion Science and Engineering; Marcus, P., Mansfeld, F., Eds.; CRC Publisher: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Collazo, A.; Nóvoa, X.R.; Pérez, C.; Puga, B. The corrosion protection mechanism of rust converters: An electrochemical impedance spectroscopy study. Electrochim. Acta 2010, 55, 6156–6162. [Google Scholar] [CrossRef]
- Telegdi, J. Formation of Self-Assembled Anticorrosion Films on Different Metals. Materials 2020, 13, 5089. [Google Scholar] [CrossRef] [PubMed]
- Telegdi, J.; Shaban, A.; Trif, L. Review on the microbiologically influenced corrosion and the function of biofilms. Int. J. Corros. Scale Inhib. 2020, 9, 1–33. [Google Scholar] [CrossRef]
- Telegdi, J.; Abdul, S.; Gyöngyi, V. Micro/nanocapsules for anticorrosion coatings. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 521–551. [Google Scholar] [CrossRef]
- Kutz, M. Handbook of Environmental Degradation of Materials; William Andrew Applied Science Publisher: Chennai, India, 2018. [Google Scholar]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Garrett, J.H. The Action of Water on Lead; H.K. Lewis: London, UK, 1981. [Google Scholar]
- Dexter, S.C. Localized Biological Corrosion. In Metal Handbook: Corrosion, 9th ed.; American Society for Metals: Detroit, MI, USA, 1987; Volume 13, p. 118. [Google Scholar]
- Beyenal, H.; Lewandowski, Z. Internal and External Mass Transfer in Biofilms Grown at Various Flow Velocities. Biotechnol. Prog. 2002, 18, 55–61. [Google Scholar] [CrossRef]
- Flemming, H.; Wingender, J.; Griebe, T.; Mayer, C. Physico-Chemical Properties of Biofilms. 2000. [Online]. Available online: https://www.semanticscholar.org/paper/Physico-chemical-properties-of-biofilms.-Flemming-Wingender/66170a2774ac27af7e73f150d30b205fb07a9f8c (accessed on 7 April 2024).
- Little, B.; Lee, J. Microbiologically Influenced Corrosion. In International Materials Reviews; Wiley: Hoboken, NJ, USA, 2009; Volume 59. [Google Scholar] [CrossRef]
- Telegdi, J.; Shaban, A.; Trif, L. Microbiologically influenced corrosion (MIC). In Trends in Oil and Gas Corrosion Research and Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–214. [Google Scholar] [CrossRef]
- Abd El-Fattah, M.; El Saeed, A.M.; Azzam, A.M.; Abdul-Raheim, A.R.M.; Hefni, H.H. Improvement of corrosion resistance, antimicrobial activity, mechanical and chemical properties of epoxy coating by loading chitosan as a natural renewable resource. Prog. Org. Coat. 2016, 101, 288–296. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Huang, S.-Y.; Chu, Y.-R.; Jian, S.-Y.; Lo, K.-Y.; Lee, Y.-L. Antimicrobial and Anticorrosion Activity of a Novel Composite Biocide against Mixed Bacterial Strains in Taiwanese Marine Environments. Materials 2021, 14, 6156. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, S.; Wu, X.; She, Y.; Liu, R.; Lin, H. In vitro and in vivo evaluation of implantable bacterial-killing coatings based on host defense peptides and their synthetic mimics. J. Mater. Sci. Technol. 2021, 91, 90–104. [Google Scholar] [CrossRef]
- Pourhashem, S.; Seif, A.; Saba, F.; Nezhad, E.G.; Ji, X.; Zhou, Z.; Zhai, X.; Mirzaee, M.; Duan, J.; Rashidi, A.; et al. Antifouling nanocomposite polymer coatings for marine applications: A review on experiments, mechanisms, and theoretical studies. J. Mater. Sci. Technol. 2022, 118, 73–113. [Google Scholar] [CrossRef]
- Tian, J.; Xu, K.; Hu, J.; Zhang, S.; Cao, G.; Shao, G. Durable self-polishing antifouling Cu-Ti coating by a micron-scale Cu/Ti laminated microstructure design. J. Mater. Sci. Technol. 2021, 79, 62–74. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Feng, D.Q.; Zhu, P.Y.; Song, W.L.; Yasir, M.; Zhang, C.; Liu, L. Hydrogel-Anchored Fe-Based Amorphous Coatings with Integrated Antifouling and Anticorrosion Functionality. ACS Appl. Mater. Interfaces 2023, 15, 13644–13655. [Google Scholar] [CrossRef]
- Egghe, T.; Morent, R.; Hoogenboom, R.; De Geyter, N. Substrate-independent and widely applicable deposition of antibacterial coatings. Trends Biotechnol. 2023, 41, 63–76. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Zheng, H.; Meng, F.; Wang, F. Superhydrophobic, corrosion resistance, and antibacterial coating with delayed release of Ag ions. Compos. Commun. 2022, 31, 101134. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Zheng, H.; Meng, F.; Wang, F. Thermoresponsive PNIPAm on anti-corrosion antibacterial coating for controlled Ag ions release. Compos. Commun. 2022, 35, 101327. [Google Scholar] [CrossRef]
- Jing, Y.; Meng, F.; Wang, F.; Liu, L. Design of an anticorrosion/bactericidal dual functional organic coating based on the slippery liquid-infused porous surface. Appl. Surf. Sci. 2023, 639, 158214. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W. Superhydrophobic, superamphiphobic and SLIPS materials as anti-corrosion and anti-biofouling barriers. New J. Chem. 2021, 45, 15170–15179. [Google Scholar] [CrossRef]
- Telegdi, J.; Rigó, T.; Pfeifer, É.; Keszthelyi, T.; Kálmán, E. Nanolayer Coatings. In Colloids for Nano- and Biotechnology; Hórvölgyi, Z.D., Kiss, É., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 77–86. [Google Scholar] [CrossRef]
- Trif, L.; Shaban, A.; Telegdi, J. Electrochemical and surface analytical techniques applied to microbiologically influenced corrosion investigation. Corros. Rev. 2018, 36, 349–363. [Google Scholar] [CrossRef]
- Dexter, S.C.; Duquette, D.J.; Siebert, O.W.; Videla, H.A. Use and Limitations of Electrochemical Techniques for Investigating Microbiological Corrosion. Corrosion 1991, 47, 308–318. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Xu, H.; Li, X. Electrochemical techniques used in MIC studies. Mater. Corros. 2006, 57, 800–804. [Google Scholar] [CrossRef]
- Little, B.J.; Wagner, P.A. Application of Electrochemical Techniques to the Study of Microbiologically Influenced Corrosion. In Modern Aspects of Electrochemistry; Bockris, J.O., Conway, B.E., White, R.E., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2002; Volume 34, pp. 205–246. [Google Scholar] [CrossRef]
- Little, B.J.; Mansfeld, F.B.; Arps, P.J.; Earthman, J.C. Microbiologically Influenced Corrosion. In Encyclopedia of Electrochemistry, 1st ed.; Bard, A.J., Stratmann, M., Gileadi, E., Urbakh, M., Calvo, E.J., Unwin, P.R., Frankel, G.S., Macdonald, D., Licht, S., Schäfer, H.J., et al., Eds.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).