Abstract
Based on the principle of a micropore-filling electrolyte, a graphene composite conductive coating combined with impressed current cathodic protection (ICCP) technology was constructed and applied in a marine atmospheric environment. To further explore the optimal protection parameters of the graphene composite conductive coating combined with ICCP technology in a marine atmospheric environment, the effects of the coating damage area (A), impressed voltage (B), and distance from the contact point (C) on the protective performance of the coating were investigated via orthogonal experiments. The optimal protection voltage and effective protection distance were verified by super-depth-of-field morphology observations and electrochemical tests. The orthogonal experimental results show that the primary and secondary orders affecting the protective performance of the conductive graphene composite coating are as follows: applied voltage (B) > coating damage area (A) > distance from the point of contact (C). The optimal protective parameters of the coating in the marine atmospheric environment are an applied voltage of 0.7 V, a damage rate of ≤1%, and a distance from the point of contact of 190 mm. The experimental results show that the corrosion potential of the sample is the highest under an applied voltage of 0.7 V, and the corrosion products do not diffuse to the surface of the coating. When the polarization resistance (Rp) values at 110 mm and 190 mm from the negative electrode at the point of contact are greater, the corrosion rate is lower, and the coating protection performance is better.
1. Introduction
With advancements in marine science and technology, marine engineering equipment is being developed in the direction of improved localization accuracy and higher quality. Corrosion has always affected the development of marine engineering equipment [,]. Taking the South China Sea environment as an example, marine engineering equipment has been affected by a dry–wet alternate marine monsoon climate characterized by high winds, salt fog, and high ultraviolet radiation for a long period of service. Metals are prone to corrosion when in contact with corrosive media [,]. Currently, coating protection technology is widely used in marine engineering equipment. A uniform thickness of an anti-corrosion coating on the surface of a metal substrate can effectively isolate H2O, O2, Cl−, and other corrosive media from contact with the metal substrate to prevent corrosion [,,,]. In addition, technologies such as sacrificial anode protection and impressed current cathodic protection are also commonly used in the protection of marine engineering equipment [,]. For impressed current cathodic protection systems, although international engineering standards (NACE RP-0176) state that metal substrates in marine atmospheric environments can be effectively protected if their potential is greater than −800 mV Ag/AgCl [], single anti-corrosion techniques have limited protection capabilities in complex corrosive environments. Therefore, many researchers have carried out joint anti-corrosion technological research. Jeong et al. [] investigated the effect of sacrificial anodes combined with impressed current cathodic protection technology on reinforced-concrete cylindrical samples and noted that the combined technology can provide effective protection for marine engineering facilities. Deshpande et al. [] combined impressed current cathodic protection technology with zinc-based coatings, which can significantly reduce the corrosion rate of low-carbon steel in a 3.5% NaCl solution.
For successful application of impressed current cathodic protection technology, a macroscopic continuous electrolyte is needed to provide an ion circuit. The atmospheric environment cannot use impressed current cathodic protection because of the lack of macroscopic continuous electrolytes as ion circuits. Therefore, the key to realizing cathodic protection in the atmospheric environment is to create ion conductive conditions. Usually, the ability of a coating to act as a barrier to a corrosive medium is limited. In high-humidity and high-salt spray environments, water and salt ions can enter the interior of the coating through permeation [,,]. Owing to the cracks and micropores generated by the curing effect of the coating, the corrosive medium is easily stored in these microscopic defects to form a microscopic local electrolytic cell [,,,]. The principle of coating with micropore-filling electrolytes provides the necessary conditions for the application of impressed current cathodic protection in marine atmospheric environments. In the early stage, we prepared a graphene conductive composite coating using an epoxy universal primer, graphene, and a urethane antifouling topcoat. The coating was an anode, the metal substrate was a cathode, and the applied current cathodic protection technology was successfully applied to the atmospheric environment based on the principle of a micropore-filling electrolyte. For samples with ‘X’-shaped scratches, the application of an applied voltage of 0.6 V can more effectively inhibit the corrosion of carbon steel with coating cracks []. In this study, we employed a three-factor, four-level orthogonal experimental design to expose coating samples to the marine atmospheric environment of Zhanjiang, China, for 20 days. The effects of the coating damage area (A), applied voltage (B), and distance from the contact point (C) on the corrosion resistance of the coating were investigated. Combining observations from high-depth-of-field morphology and electrochemical tests, the optimal protective voltage and effective protection distance were validated, the combined coating was optimized, and impressed current cathodic protection (ICCP) corrosion prevention technology was utilized under atmospheric conditions.
2. Experimental Part
2.1. Preparation of Coating Samples
The experimental materials included graphene powder, waterborne epoxy resin, deionized water, an epoxy universal primer, a polyurethane antifouling topcoat, and four Q235A (GB/T 700-2006) [] carbon steel plates. Table 1 is the chemical composition table of Q235 low-carbon steel. The size of each metal sample was 300 × 200 × 3 mm. The Q235 carbon steel was polished step by step with #180, #400, #800, and #1000 water-grinding sandpaper via the cross-grinding method. The polished samples were placed in acetone solution and then anhydrous ethanol for ultrasonic cleaning. After being dried by cold air, the samples were placed in a drying oven for subsequent use.

Table 1.
Chemical composition table of Q235 low-carbon steel.
Q235 steel served as the cathode, and an epoxy primer, a graphene composite coating, and a polyurethane antifouling topcoat were uniformly coated on the surface. The graphene composite coating was prepared by mixing waterborne epoxy resin, graphene powder, and deionized water at a ratio of 1:1:2 for ultrasonic dispersion. As the graphene composite coating contained water-based epoxy resin, it had certain adhesion in the undried state, and 1.0 mm wide carbon-fiber strips could be adhered to the surface of the graphene composite coating. After the graphene composite coating was dried, the polyurethane antifouling topcoat was applied to further fix the carbon fiber strips in the coating, which were used as anodes. After each coating, the sample was placed in a backlit, dry environment for 6 h.
2.2. The Design of a Coating Combined with an Impressed Current Cathodic Protection System
Figure 1 is a schematic diagram of the structural design of the composite conductive coating and the principle of a micropore-filling electrolyte. It can be seen from Figure 1a that the protected metal is used as the cathode and the graphene composite coating is the anode. Using the excellent insulation of the epoxy primer, the matrix and graphene composite coating are effectively isolated. At this time, the metal substrate is mainly protected by the shielding property of the coating. When the coating produces microdefects, it can be seen from Figure 1b that based on the principle of coating with a micropore-filling electrolyte, the microlocal electrolytic cell formed by the corrosion medium penetrating into the surface of the metal substrate through micropores provides ion circuit conditions. In the closed circuit, an external power supply continuously transports electrons to the surface of the substrate, effectively inhibiting the formation of corrosion products on the substrate and delaying the failure of the coating.
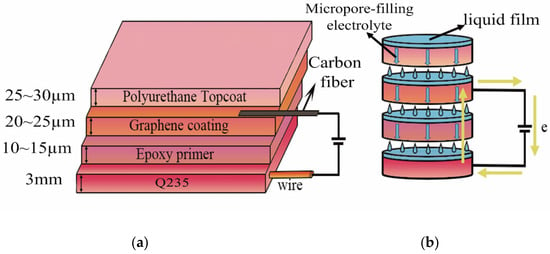
Figure 1.
The structure of the composite conductive coating and the principle of electrolyte-filled coating micropores: (a) the structure of the composite conductive coating; (b) the principle of filling micropores in coatings with electrolytes.
2.3. Orthogonal Experimental Design
Orthogonal experimental design, which has the advantages of rapidity and efficiency, is a mathematical statistical method commonly used to analyze multifactor-problem experiments. An orthogonal table can be used to select representative-level combinations for experiments, which can fully reflect the influence of each factor level on the test index [,]. In this study, the effects of different factors on the composite coating were investigated via orthogonal experiments. The coating damage area (A), the impressed voltage (B), and the distance from the point of contact (C) were selected as the influencing factors, and four levels were set for each factor. The level values of each factor are shown in Table 2. Therefore, the orthogonal table L16(43) was selected for the orthogonal experiment to optimize the experimental process and the experimental efficiency.

Table 2.
Level setting of each influencing factor.
2.4. Marine Atmospheric Corrosion Exposure Test
The location of the marine atmospheric exposure experiment was the atmospheric corrosion exposure site on Donghai Island, Zhanjiang, China. Zhanjiang city is regulated by a marine climate, with an annual average temperature of 23.2 °C, an annual average humidity of 88%, and an average Cl− deposition rate of 58.2 mg·m−2·d−1, which is typical of a high-temperature, high-humidity, high-salt, and high-radiation marine atmospheric corrosion environment. The coating samples were surrounded by insulating tape, and silicone rubber was applied to the soldered wires. To ensure that the requirements of cathodic protection are met, the coating damage rate needs to be set as small as possible. Therefore, the coating damage rate is defined as the percentage of the coating’s damaged area relative to the effective area (19.6 cm2) of the electrochemical test on the coating. According to the orthogonal experimental design, circular defects with areas of 19.6 mm2, 39.2 mm2, 58.8 mm2, and 78.4 mm2 were created on the coating surface using a puncher, simulating coating damage rates of 1%, 2%, 3%, and 4%, respectively. Four test points were set to 30 mm, 110 mm, 190 mm, and 270 mm from the negative position of the grounding point and labeled test points #1, #2, #3, and #4, respectively. A DC-regulated power supply was used to apply 0.6 V, 0.7 V, 0.8 V, and 0.9 V to the four coating samples, and then, the samples were placed in the Donghai Island atmospheric corrosion exposure field to conduct outdoor exposure experiments for 20 days. The hanging samples are shown in Figure 2.
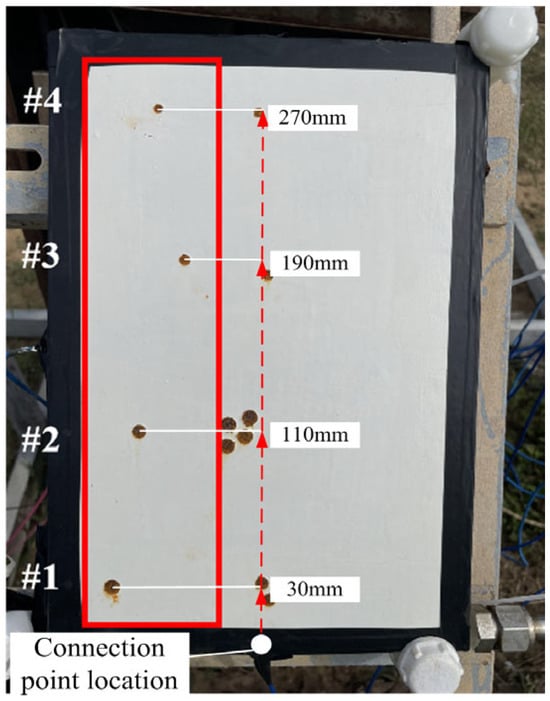
Figure 2.
Hanging samples.
2.5. Test and Characterization Methods
Electrochemical tests were performed using the CS350 electrochemical workstation provided by Wuhan Corrtest Instrument Co., Ltd., Wuhan, China. Figure 3 is the schematic diagram of the electrochemical test system for the coating. The traditional three-electrode system is adopted. In the electrolytic cell, Ag/AgCl is used as the reference electrode, the sample is the working electrode, and the platinum sheet is the auxiliary electrode. The effective area of the test is 19.6 cm2. All electrochemical tests were performed in 3.5 wt% NaCl solution at 25 °C. The samples underwent a series of electrochemical tests, including an open-circuit potential (OCP) test for 300 s, an electrochemical impedance spectroscopy (EIS) test, and a potentiodynamic polarization curve test. The EIS test parameters include a test frequency range of 105–10−2 Hz and an AC excitation signal amplitude of 10 mV. The parameters of the potentiodynamic polarization curve test include a scanning range of 0.05 V and a scanning rate of 0.1 mV/s.
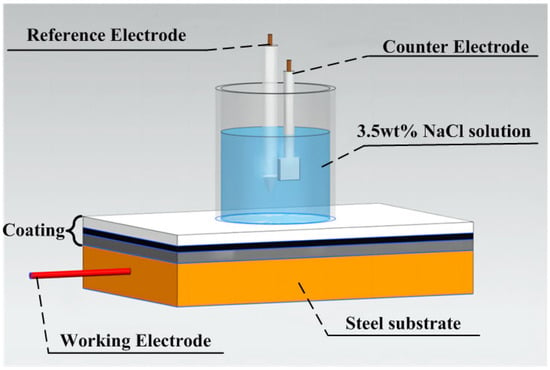
Figure 3.
The schematic diagram of the electrochemical test system for the coating. The test environment: 25 °C; the electrolyte solution is 3.5 wt% NaCl solution.
The morphology of the corrosion product area on the surface of the coating was observed using the Leica DVM6A, a super-depth-of-field microscope provided by Leica Instruments GmbH in Wetzlar, Germany, with a magnification of 500 times.
3. Results and Discussion
3.1. Orthogonal Experimental Results
The orthogonal experiment was carried out via an L16 (43) orthogonal table, and the open-circuit potential was selected as the protective performance index of the conductive graphene composite coating. The size of the open-circuit potential can be used to evaluate the degree of metal corrosion; the lower the open-circuit potential is, the more likely the metal is to corrode. Table 3 shows the open-circuit potential test results of 16 groups of different experimental combinations. The orthogonal experimental results show that when the damage rate is 1%, the impressed voltage is 0.7 V, the open-circuit potential is the highest at 110 mm from the point of contact, and the metal matrix is effectively protected.

Table 3.
Orthogonal experimental results.
3.2. Range Analysis
To analyze the effect of each factor on the graphene composite conductive coating, the experimental results were analyzed using range analysis, and the results are shown in Table 4, where Ki (i = 1, 2, 3, and 4) is the average sum of all of the experimental results of the ith level of the corresponding factor, i (i = 1, 2, 3, and 4) is the average value of the corresponding level of each factor, and R is the difference between the maximum level value of i and the minimum level value of , i.e., the extreme variance. A greater R indicates a greater influence on the protective performance of the graphene composite conductive coating []. As shown in Table 4 of the extreme variance analysis results, the order of the three factors for the protective performance of the composite conductive coating is as follows: impressed voltage (B) > coating damage area (A) > position from the point of contact (C).

Table 4.
Range analysis.
Figure 4 shows the change in the open-circuit potential under different influencing factors. Figure 4a shows that when the damage rate was 1%, the coating’s open-circuit potential was the highest, and the coating’s barrier performance was the best. When the coating’s damage rate of 1% increased to 2% and the open-circuit potential decreased by 0.013 V, the tendency toward corrosion began to increase. The damage rate of 3% was no longer consistent with the principle of a microporous-filling electrolyte, the loss of applied current cathodic protection, and the bare substrate coming into contact with the corrosive medium, resulting in the lowest open-circuit potential and severe corrosion. Figure 4b shows that an applied voltage of 0.6 V when the metal substrate was in a state of under-protection resulted in the lowest open-circuit potential. The open-circuit potential was greater at 0.7 V, which resulted in the best protection performance for the broken coating samples. Figure 4c shows that the change in the position from the grounding point had the smallest influence on the open-circuit potential, and the fluctuation interval was 0.03 V. The cathodic protection effect was the best at a distance of 190 mm from the grounding point, and the different positions from the grounding point affected the migration and diffusion of the charged ions, which influenced the effect of cathodic protection. A comprehensive analysis revealed that the optimal protection parameters of the graphene composite conductive coating in the marine atmospheric environment were an applied voltage of 0.7 V, a damage rate of ≤1%, and a distance of 190 mm from the junction point.
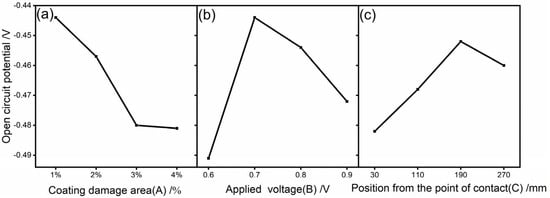
Figure 4.
Changes in the open-circuit potential under different influencing factors: (a) coating damage area; (b) applied voltage; and (c) distance from the point of contact.
4. Determination of the Optimal Protection Voltage and Effective Protection Distance of the Coating
4.1. Optimal Protection Voltage
Figure 5 shows the potentiodynamic scanning curves of each test point under different applied voltages. Overall, the corrosion potential of the sample under the applied voltage of 0.7 V was the highest, the surface coating had good protective performance [], and the metal substrate had the lowest corrosion tendency.
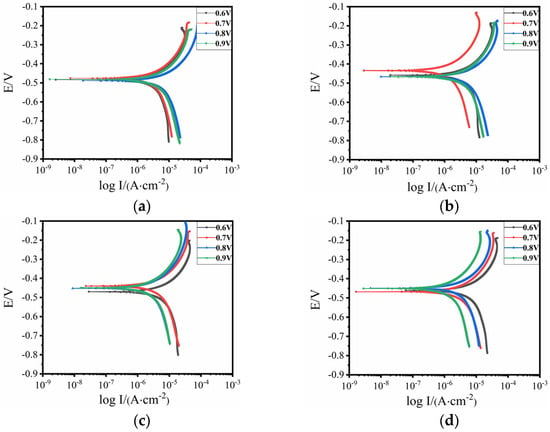
Figure 5.
Polarization curves of each test point under different voltages. (a) Test point #1, (b) test point #2; (c) test point #3, and (d) test point #4.
A super-depth-of-field microscope was selected to observe the morphology of each test point of the coating when different voltages were applied. Figure 6 shows the shooting position map of the damaged area. The shooting position selects the area at the junction of the damaged circular hole and the coating. The left side of the red arc line is the coating area, and the right side of the red arc line is the corrosion product area. When the corrosion products diffuse to the surface of the coating through rainwater, chemical reactions may occur between the corrosion products and the coating materials, which changes the composition of the coating or causes the formation of new compounds, affecting the stability and durability of the coating []. Figure 7 shows the damage morphology of each test point under different voltages. By comparison, the diffusion of corrosion products was most obvious in the sample with 0.6 V, followed by the sample with 0.9 V. When 0.7 V was applied, there was no obvious rust on the surface of the coating. Only a small amount of reddish-brown corrosion products appeared at the junction of the damaged round hole and the coating, and the overall durability of the coating was better.
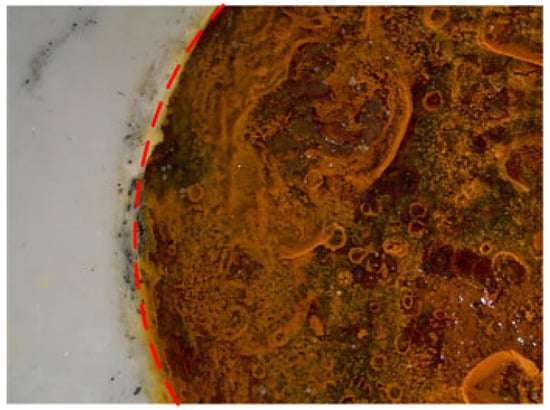
Figure 6.
Shooting position of damage.
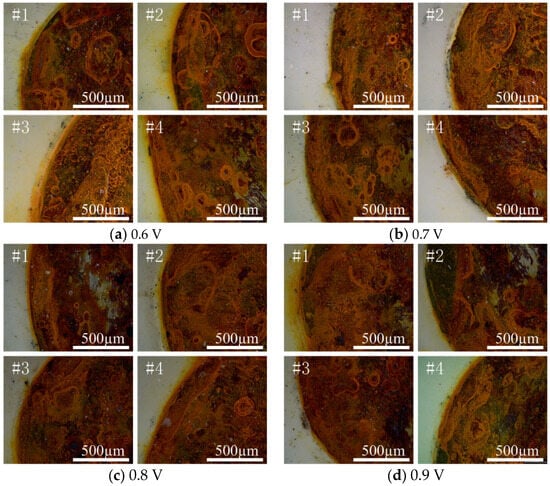
Figure 7.
Morphology of damage at each test point under different voltages.
Figure 8 shows the corrosion morphology of the coating, and the corrosion product structure is an important factor for the speed of metal corrosion. When a 0.7 V coating was used, the corrosion product size was smaller, the distribution was more uniform, the metal substrate protection effect was stronger, the penetration of environmental media to the substrate was slower [,], and the protection performance of the coating was better. The corrosion product film can block the transport of H2O, O2, and Cl− from the atmospheric environment to the surface of the substrate, effectively slowing the occurrence of corrosion. A total of 0.7 V for the graphene composite conductive coating can be obtained, which is the optimal cathodic protection voltage.
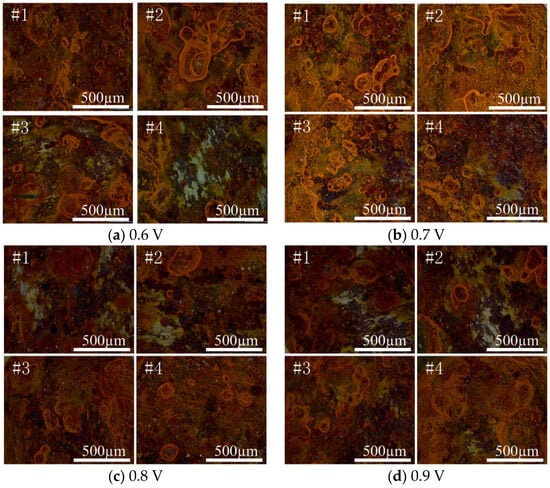
Figure 8.
Corrosion morphology of each test point of coating sample under different voltages.
4.2. Optimal Protection Distance
Figure 9 shows the Nyquist diagram and Bode diagram of the coating sample at different voltages. The radius of the capacitive arc reflects the impedance of the material in the corrosive medium. The larger the radius of the capacitive arc, the higher the impedance, and the better the corrosion resistance of the material [,]. It can be seen from Figure 9b that when the optimal cathodic protection voltage is 0.7 V, the radius of the impedance arc increases first and then decreases with the increase in the distance between the test points. The radius of the impedance arc at test points #2 and #3 is larger than that at both ends, indicating that the charge transfer resistance of test points #2 and #3 in the middle area of the coating is large and the corrosion rate is low [].
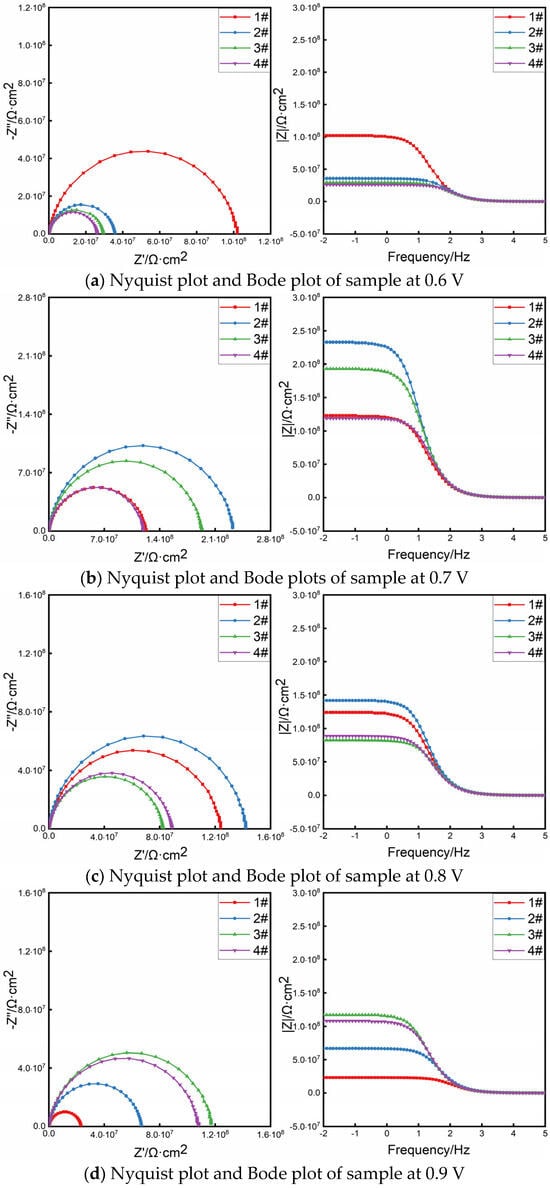
Figure 9.
Nyquist and Bode plots of coating sample under different voltages.
The EIS curve of Figure 9 is fitted by the equivalent circuit shown in Figure 10, where Rs is the solution resistance, Rc is the coating resistance, and CPEc is the constant phase angle element. Table 5 is the equivalent circuit fitting results. Rp is the polarization resistance, which is one of the important indexes to evaluate the protective performance of the coating. The larger the Rp value is, the better the protective performance of the coating is. Figure 11 is the polarization resistance comparison diagram of each test point of the coating under different applied voltages. From Figure 11, it can be seen that the polarization resistance of the composite conductive coating at test points #1–#4 is ordered as follows: 0.7 V > 0.8 V > 0.9 V > 0.6 V. When a 0.6 V voltage is applied, the migration ability of charge ions gradually weakens with the distance of the test point, and the coating is in an under-protected state in the area of test points #2–#4, resulting in the decrease in the Rp value with the increase in the distance of the test point. The Rp value of the sample with a 0.9 V voltage is the smallest at test point #1, because it is the closest to the output end, and the current density is too large to affect the cathodic protection effect. Under the applied voltage of 0.7 V, the Rp values of the coating samples at the four test points are all greater than 108·cm2, and the overall anti-corrosion effect is good, which can effectively protect the substrate for a long time []. The Rp values at test points #2 and #3 are greater than 2 × 108·cm2 and greater than the polarization resistance values of test points #1 and #4, indicating that the coating at test points #2 and #3 (i.e., 110–190 mm) is more conducive to protecting the metal substrate from external material erosion.
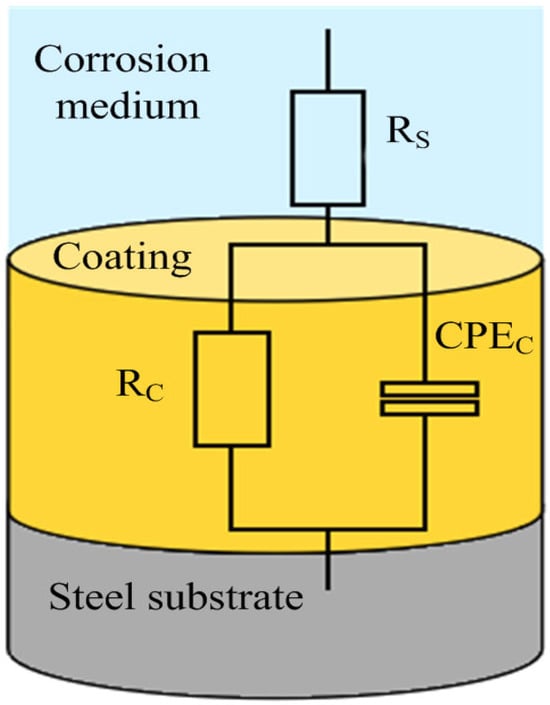
Figure 10.
Equivalent circuit diagram.

Table 5.
The fitting results of the equivalent circuit.
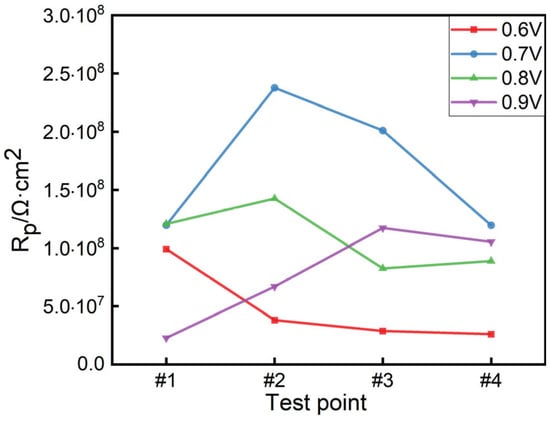
Figure 11.
The polarization resistance of the coating at each test point under different applied voltages.
Figure 12 shows the polarization curves of the coating samples at different test points under an applied voltage of 0.7 V, and Table 6 presents the fitting results of these curves. As the distance from the test point increases, the corrosion potential at test points #2 and #3 in the middle region is higher than at test points #1 and #4. The corrosion potential difference between #2 and #3 is only 0.0055 V, with corrosion rates below 0.1 mm/a for both. Compared to the ends of the coating, the barrier performance is better at distances of 110–190 mm from the connection point. Thus, under the optimal cathodic protection voltage of 0.7 V, the ideal protection distance for the sample is between 110 and 190 mm.
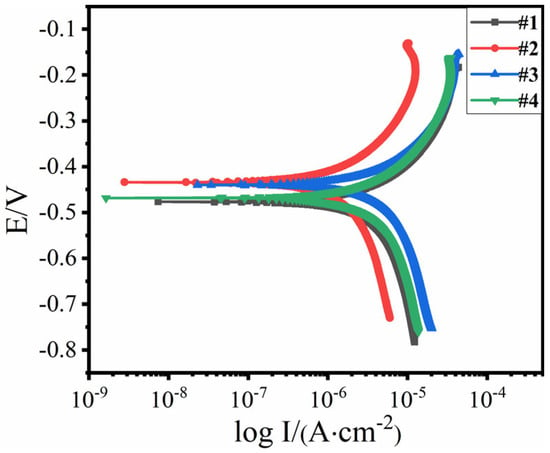
Figure 12.
Polarization curves of different test points of coated specimen after applying 0.7 V voltage.

Table 6.
Fitting results of polarization curves at different test points of coated specimen after applying 0.7 V voltage.
5. Conclusions
(1) The order of influence of the three factors on the protective performance of the graphene composite conductive coating is as follows: applied voltage B > coating damage area A > distance from the point of contact C. The results of the extreme variance analysis revealed that the composite conductive coating achieves the optimal protection parameters in the marine atmospheric environment at an applied voltage of 0.7 V, a coating damage area of ≤1%, and a distance of 190 mm from the location of the point of contact.
(2) Dynamic potential polarization curve tests and microscopic morphology observations of the coating sample revealed that the corrosion potential of the coating sample was the highest when a voltage of 0.7 V was applied, and the degree of corrosion was lower. In addition, the corrosion products did not diffuse to the surface of the coating, the coating stability and durability were better, the substrate was uniformly covered by the corrosion product film, and the corrosion rate was lower.
(3) The polarization resistance (Rp) values of the four test points of the sample at a 0.7 V voltage were all above 108 Ω·cm2, indicating a good anti-corrosion effect. Compared with the two ends of the coating sample, the polarization resistance (Rp) value of test points 2 # and 3 # was greater, the corrosion potential was greater, and the corrosion rate was lower. The coating has the best barrier property in this area to protect the metal substrate from external material erosion. Therefore, for the optimum cathodic protection of the sample at 0.7 V, the optimum protection distance was at test points #2–#3 (110–190 mm).
Author Contributions
Conceptualization, P.D. and J.H.; methodology, P.D.; software, D.L. and J.S.; validation, J.S., G.Z. and J.Y.; formal analysis, G.Z.; investigation, D.L.; resources, J.H.; data curation, D.L. and J.S.; writing—original draft preparation, D.L.; writing—review and editing, D.L.; visualization, P.D.; supervision, J.Y.; project administration, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Natural Science Foundation of Guangdong Province (No. 2021A1515012129), the Science & Technology Development Foundation of Zhanjiang (2022A01029), and the Zhanjiang Power Company Limited of Guangdong.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations. They will be available on request.
Conflicts of Interest
Guo Zheng is employed by Zhanjiang Power Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hu, Q.; Yang, S.; Zhang, W.; Da, G.; Xu, X.; Wang, X. Corrosion failure analysis of engineering structural steels in tropical marine atmospheres: A comparative study of ordinary and new weathering steels. Eng. Fail. Anal. 2024, 156, 107830. [Google Scholar] [CrossRef]
- Qian, R.; Li, Q.; Fu, C.; Zhang, Y.; Wang, Y.; Jin, X. Atmospheric chloride-induced corrosion of steel-reinforced concrete beam exposed to real marine-environment for 7 years. Ocean Eng. 2023, 286, 115675. [Google Scholar] [CrossRef]
- Bhat, S.I.; Mobin, M.; Islam, S.; Zehra, S.; Islam, S.U. Recent advances in anticorrosive coatings based on sustainable polymers: Challenges and perspectives. Surf. Coat. Technol. 2024, 480, 130596. [Google Scholar] [CrossRef]
- Lin, S.; Li, D.; Zhou, Q.; Chu, M.; Sun, Y.; Liu, M.; Zheng, K.; Qiao, S.; Zhao, L.; Zhao, L.; et al. Study on corrosion perforation behavior of copper nickel alloy pipe during service in marine environment. Eng. Fail. Anal. 2023, 153, 107628. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, X.; Zhang, L.; Lu, G.; Liu, M.; Liu, S. Anticorrosion mechanism of Al-modified phosphate ceramic coating in the high-temperature marine atmosphere. Surf. Coat. Technol. 2022, 441, 128572. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Wang, L.; Wang, J.; Wang, S.; Liu, G. A Mechanically and Chemically Stable Superhydrophobic Coating for Preventing Marine Atmospheric Corrosion. Surf. Interfaces 2021, 27, 101537. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.-W.; Zhao, H.-T.; Pan, C.; Wang, Z.-Y. Corrosion behavior of copper in extremely harsh marine atmosphere in Nansha Islands, China. Trans. Nonferrous Met. Soc. China 2021, 31, 703–714. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xing, S.; Zhang, L.; Lu, Z.; Zhang, P. Failure Behavior and Damage Mechanism of Acrylic Polyurethane Coating in Tropical Marine Atmospheric Environment. Int. J. Electrochem. Sci. 2020, 15, 2511–2527. [Google Scholar] [CrossRef]
- Erdogan, C.; Swain, G. Conceptual Sacrificial Anode Cathodic Protection Design for offshore wind monopiles. Ocean Eng. 2021, 235, 109339. [Google Scholar] [CrossRef]
- Law, D.W.; Nicholls, P.; Christodoulou, C. Residual protection of steel following suspension of Impressed Current Cathodic Protection system on a wharf structure. Constr. Build. Mater. 2019, 210, 48–55. [Google Scholar] [CrossRef]
- NACE Standard RP0176-2003; Standard Recommended Practice RP0176. Item No. 21018. NACE International: Houston, TX, USA, 2003.
- Jeong, J.-A.; Jin, C.-K.; Chung, W.-S. Tidal Water Effect on the Hybrid Cathodic Protection Systems for Marine Concrete Structures. J. Adv. Concr. Technol. 2012, 10, 389–394. [Google Scholar] [CrossRef]
- Deshpande, P.; Kolekar, A.; Bhopale, A.; Kalendova, A.; Kohl, M. Impressed current cathodic protection (ICCP) of mild steel in association with zinc based paint coating. Mater. Today Proc. 2022, 50, 1660–1665. [Google Scholar] [CrossRef]
- Sun, J.; Sun, W.; Wang, L.; Xu, K.; Yang, Z.; Ma, Y.; Zhao, L.; Ma, S.; Xing, W.; Liu, G. Boosting the acid penetration barrier of epoxy-silica organic-inorganic hybrid coating via adjusting its molecular structures: Experimental and molecular dynamics simulation study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133634. [Google Scholar] [CrossRef]
- Donkers, P.; Huinink, H.; Erich, S.; Reuvers, N.; Adan, O. Water permeability of pigmented waterborne coatings. Prog. Org. Coat. 2013, 76, 60–69. [Google Scholar] [CrossRef]
- Quelennec, B.; Duan, Z.; Delannoy, R.; Gay, N.; Briffaut, M.; Tognetti, V.; Delpouve, N.; Delbreilh, L.; Bredif, L.; Duthoit, A.; et al. Effect of physical and chemical ageing on barrier properties of epoxy coating. Constr. Build. Mater. 2023, 409, 133908. [Google Scholar] [CrossRef]
- Shi, C.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Influence of submicron-sheet zinc phosphate synthesised by sol–gel method on anticorrosion of epoxy coating. Prog. Org. Coat. 2018, 117, 102–117. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Effect of ZnO pore-sealing layer on anti-corrosion and in-vitro bioactivity behavior of plasma electrolytic oxidized AZ91 magnesium alloy. Mater. Lett. 2020, 258, 126779. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Wu, J.; Xiang, J.; Dai, X.; Wu, T.; Yuan, Y.; Wang, J.; Jiang, B.; et al. Development of metal-organic framework (MOF) decorated graphene oxide/MgAl-layered double hydroxide coating via microstructural optimization for anti-corrosion micro-arc oxidation coatings of magnesium alloy. J. Mater. Sci. Technol. 2022, 130, 12–26. [Google Scholar] [CrossRef]
- Kumar, S.S.A.; Bashir, S.; Ramesh, K.; Ramesh, S. New perspectives on Graphene/Graphene oxide based polymer nanocomposites for corrosion applications: The relevance of the Graphene/Polymer barrier coatings. Prog. Org. Coat. 2021, 154, 106215. [Google Scholar] [CrossRef]
- Deng, P.; Shangguan, J.; Hu, J.; Huang, H.; Zhou, L. Anticorrosion Method Combining Impressed Current Cathodic Protection and Coatings in Marine Atmospheric Environment. Coatings 2024, 14, 524. [Google Scholar] [CrossRef]
- GB/T 700-2006; Carbon Structural Steels. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- He, Y.; Lin, C.D.; Sun, F. A new and flexible design construction for orthogonal arrays for modern applications. Ann. Stat. 2022, 50, 1473–1489. [Google Scholar] [CrossRef]
- Chae, K.-S.; Choi, H.-K.; Ahn, J.-H.; Song, Y.-S.; Lee, D.Y. Application of Taguchi method and orthogonal arrays for characterization of corrosion rate of IrO2–RuO2 film. J. Mater. Sci. 2002, 37, 3515–3520. [Google Scholar] [CrossRef]
- Wang, K.; Kan, Y.; Zhu, Y.; Zhong, K.; Hu, J. Optimization of synthesis parameters for C-S-H/PCE nanocomposites: A comprehensive study using orthogonal experiments and multi-analysis approaches. Constr. Build. Mater. 2024, 417, 135332. [Google Scholar] [CrossRef]
- An, D.; Wang, Z.; Qin, L.; Wu, Y.; Lu, S.; Yang, H.; Ma, Z.; Mawignon, F.J.; Liu, J.; Hao, L.; et al. Preparation of MXene/EP coating for promising anticorrosion and superlow friction properties. Prog. Org. Coat. 2023, 183, 107779. [Google Scholar] [CrossRef]
- Araneda, A.A.B.; Kappes, M.A.; Rodríguez, M.A.; Carranza, R.M. Pitting corrosion of Ni-Cr-Fe alloys at open circuit potential in chloride plus thiosulfate solutions. Corros. Sci. 2022, 198, 110121. [Google Scholar] [CrossRef]
- Dan, N.; Yang, Y.; Ying, T.; Zeng, X. Corrosion Inhibition Effects of Corrosion Products on High-purity Mg and AZ91D under Thin Electrolyte Layers. J. Electrochem. Soc. 2022, 169, 091502. [Google Scholar] [CrossRef]
- Zheng, L.; Matin, N.S.; Landon, J.; Liu, K. CO2 loading-dependent corrosion of carbon steel and formation of corrosion products in anoxic 30wt.% monoethanolamine-based solutions. Corros. Sci. 2016, 102, 44–54. [Google Scholar] [CrossRef]
- Li, G.; Fang, H.; Hu, Y.; Chen, X.; Chu, Z.; Yang, Z. Construction of vinyl ester resins composite coatings via introducing silane-functionalized gra-phene oxide for enhancing comprehensive performance. Compos. Sci. Technol. 2022, 228, 109670. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, F.; Jin, F.; Zhang, R.; Luo, B.; Zhang, T. The Evaluation of Coating Performance by Analyzing the Intersection of Bode Plots. Int. J. Electrochem. Sci. 2014, 9, 5116–5125. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Bi, J.; Huo, D.; Liu, J.; Dong, J.; Zhan, C.; Nan, D. Paraphenylenediamine modified graphene oxide for enhancement protection performance of epoxy coating on Q235 steel. Diam. Relat. Mater. 2024, 148, 111425. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zheng, H.; Deng, S.; Hou, Y.; Wu, Y.; Liu, M. A waterborne coating system for constructing inorganic-organic composite anti-corrosion and wear-resistant coating. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).