Abstract
Biomass materials with representative morphologies and compositions were employed to study the activation effect of KHCO3. As the activation time increased from 1 to 3 h, the products derived from puffed rice and pleurotus eryngii achieved a hierarchical porous structure, while the products derived from cotton still presented a microporous structure. In the electrochemical test of a three-electrode system, the specific capacitance of these products was 352, 319, and 216 F g−1, respectively. In the two-electrode system, the PR-2-based symmetric supercapacitor presented with a specific capacitance of 280.7 F g−1 at 0.5 A g−1, and the energy density of 14.03 Wh kg−1 at 150.04 W kg−1 and an energy density retention of 73.7% was at an even higher power density of 8380.4 W kg−1. After 10,000 cycles of charging and discharging at 5 A g−1, the specific capacitance retention of the supercapacitor reached 108.8%. Based on the experimental analysis, a likely mechanism for the formation of pores was proposed. The results indicate that biomass materials with soft layered or a network structure are the best candidates to obtain a hierarchical porous structure by KHCO3 activation.
1. Introduction
To face the energy challenges of modern society, it is urgent to vigorously develop renewable energy sources and storage devices. As a promising candidate technology, supercapacitors have received wide attention because of their great application value in the fields of electric vehicles, power systems, and consumer electronics [1,2].
Based on different energy storage mechanisms, supercapacitors are generally divided into two categories: electrochemical double-layer capacitors and pseudocapacitors. The former mainly uses carbon materials as electrodes, while the latter mainly uses transition metal oxides and conductive polymers as electrodes. In general, metal oxides are often hampered by the serious aggregation of nanoparticles and low electron transport, resulting in their poor specific capacitance in practical applications [3]. The conductivity of these polymers is excellent; however, significant expansion and contraction during charging and discharging can reduce the mechanical properties and cycling stability of the conductive polymer [4]. By comparison, carbon materials have always been the most widely studied and applied electrode materials, with the advantages of abundant raw materials, stable chemical properties, high specific surface area, and environmental friendliness. Carbon materials that are commonly used for the electrode mainly include activated carbon, graphene, and carbon nanotubes. Compared to graphene and carbon nanotubes, biomass material is one of the most popular carbon precursors due to its high porosity, low cost, and renewability.
As shown by vast amounts of research, the three-dimensional (3D) hierarchical porous structure of biomass-derived carbon electrode materials can be beneficial in improving the electrochemical properties of supercapacitors [5,6,7]. Generally, macropores (>50 nm) are usually distributed on the surface of the carbon material, which acts as ion reservoirs for storing huge amounts of electrolyte ions. The mesopores (2~50 nm) provide expressways for the transport of electrolyte ions. In addition, the existence of micropores (<2 nm) can significantly improve the adsorption capacity of carbon materials and form more electrical double layers [8].
Chemical activation is one of the common methods to prepare 3D hierarchical porous carbon materials [9,10,11]. Compared to obtaining microporous structures through KOH activation, the decomposition of KHCO3 during the activation process leaves abundant macropores in carbon materials, which facilitate the formation of hierarchically porous structures that are rich in macropores, mesopores, and micropores, resulting in an improvement in the electrochemical properties of supercapacitors. In addition, from a safety perspective, KHCO3 is not as corrosive as KOH and is more environmentally friendly. Some scholars have employed KHCO3 [12,13,14] as a substitute for KOH and obtained an excellent activation effect. The activation process of KHCO3 can be divided into two aspects. On the one hand, the CO2 and water vapor released by the thermal decomposition of KHCO3 forms a honeycomb structure in the carbon material. On the other hand, decomposition products further produce abundant mesopores and micropores during high-temperature activation, resulting in 3D hierarchical porous carbon materials [15,16].
Among the thousands of biomass materials, how to quickly select the optimum carbon precursor and achieve the desired performance is a cumbersome task. In this work, to study the impact of KHCO3 activation on different biomass materials, various representative biomass materials with typical morphology and composition (Table 1) were adopted to prepare porous carbon materials through a one-step activation method by using KHCO3 as the activation agent (Figure 1). The sample derived from puffed rice (PR-2) exhibited a maximum specific capacitance of 352 F g−1 in the three-electrode system due to its high specific surface area and hierarchical porous structure. In the two-electrode system, the PR-2-based symmetric supercapacitor presented a specific capacitance of 280.7 F g−1 at 0.5 A g−1 and an energy density of 14.03 Wh kg−1 at 150.04 W kg−1, with an energy density retention of 73.7% even at the higher power density of 8380.4 W kg−1. After 10,000 cycles of charging and discharging at 5 A g−1, the specific capacitance retention of the supercapacitor reached 108.8%. Based on the experimental analysis, a likely mechanism for the formation of pores was established for these biomass materials. The results indicate that biomass materials with a soft layered or network structure are the best candidates to obtain a 3D hierarchical porous structure.

Table 1.
Various biomass materials with different morphologies and compositions.
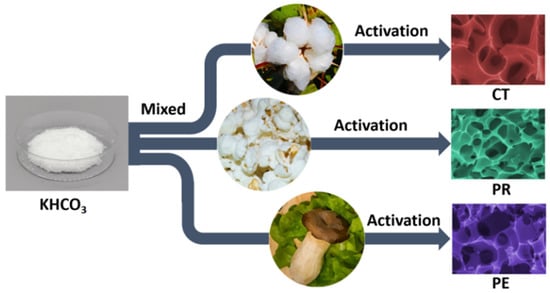
Figure 1.
A schematic diagram illustrating the preparation of porous activated carbon.
2. Materials and Methods
2.1. Material Synthesis
The porous carbon materials were prepared by a one-step activation method. Typically, the washed biomass materials were mixed with KHCO3 in a mass ratio of 1:3 before they were stirred fully with moderate deionized water. Typically, 5 g of biomass, 15 g of KHCO3, and 100 mL of deionized water were mixed. After the mixture dried at 80 °C, it was ground to fine powder by a high-speed ball crusher. Subsequently, the powder was put into a tubular furnace at 900 °C in a nitrogen atmosphere for x (x = 1, 2, 3) hours and was then naturally cooled to room temperature. Afterward, the product was neutralized with a sufficient 1 M HCl solution and filtered until neutral with excessive deionized water. Finally, the product was placed into a vacuum oven to dry at 80 °C for 48 h. In this work, cotton, puffed rice and pleurotus eryngii were used as the carbon precursor, and corresponding samples were named CT-x, PR-x, and PE-x, respectively, where x (x = 1, 2, 3) is the activation hours.
2.2. Material Characterizations
The morphologies of the samples were acquired on a Hitachi SU8010 scanning electron microscopy (SEM) system. A Bruker D8 ADVANCE diffractometer was employed to analyze X-ray diffraction (XRD) patterns. The specific surface areas (SSA) and pore size distribution (PSD) were analyzed on a Micromeritics ASAP 2460 instrument.
2.3. Electrochemical Testing
To fabricate the working electrode, the as-prepared carbon materials were mixed with carbon black and polyvinylidene fluoride according to a mass ratio of 8:1:1. Then, the appropriate N-methyl-2-pyrrolidone solvent was injected into the mixture and stirred sufficiently with a magnetic stirrer for 6~8 h until uniform was obtained. Afterward, the mixed slurry was evenly coated on foam nickel with a brush and thoroughly dried at 80 °C under vacuum conditions. Then, the electrode was compressed with a tablet press machine to ensure close contact between the electrode material and the foam nickel. A platinum sheet was employed as a counter electrode, whereas an Hg/HgO electrode served as a reference electrode. Furthermore, in the two-electrode system, two pieces of PR-2 electrodes were used to assemble the symmetric supercapacitor. All the electrochemical measurements of the as-prepared materials were conducted on a Metrohm Autolab PGSTAT302N electrochemical workstation with 6 M KOH aqueous as the electrolyte.
The specific capacitance could be calculated from CV curves [18,19]:
In this equation, m represents the mass of active materials on the electrode, is the voltage scan rate, V represents the potential variation region, and could be obtained from the area enclosed by CV curves.
The specific capacitance calculated from GCD curves used the following equation [20,21]:
where k is a constant in a three-electrode system, and the value of k is set to 1, while in a two-electrode system, k is taken as 2. I and represent the discharge current and time, respectively, while m is the active mass on each electrode and is the potential change during the discharge process.
The energy density (E) and power density (P) for the symmetric two-electrode system were calculated by [17,22]:
where C is the specific capacitance obtained from a two-electrode system, V is the discharge voltage range, and is the discharge time.
3. Results
SEM images of cotton, puffed rice, pleurotus eryngii, and their activated samples are shown in Figure 2. The cotton exhibited a slender strip structure, while the puffed rice and pleurotus eryngii presented a scaly layer and 3D network structure, respectively. Moreover, all the activated samples (Figure 2d–l) presented an interlaced 3D honeycomb structure, which could be attributed to the escaping gases from the KHCO3 decomposition and volatile components in raw materials during the activation process [23]. These macropores acted as ion reservoirs to shorten the diffusion distance of the electrolyte ions [24,25].
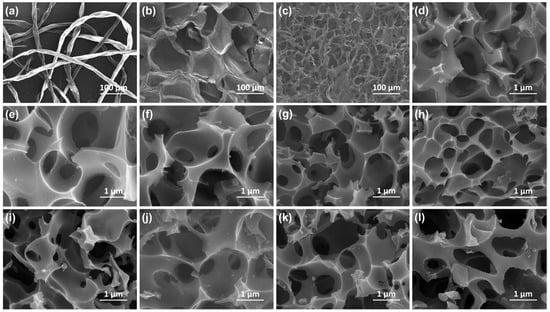
Figure 2.
SEM images of (a) Cotton, (b) Puffed rice, (c) Pleurotus eryngii, (d) CT-1, (e) CT-2, (f) CT-3 (g) PR-1, (h) PR-2, (i) PR-3 (j) PE-1, (k) PE-2 and (l) PE-3.
The XRD patterns of all the samples are displayed in Figure 3. It can be seen that all patterns exhibited similar shapes with two board peaks (~25° and 43°), which indicated their amorphous structure and low degree of graphitization [26]. In addition, as the activation time increased, slight fluctuations could be observed in the strength of the two diffraction peaks. In general, the lower the intensity of the diffraction peak, the stronger the etching effect of KHCO3, resulting in a reduction in the degree of graphitization [27].
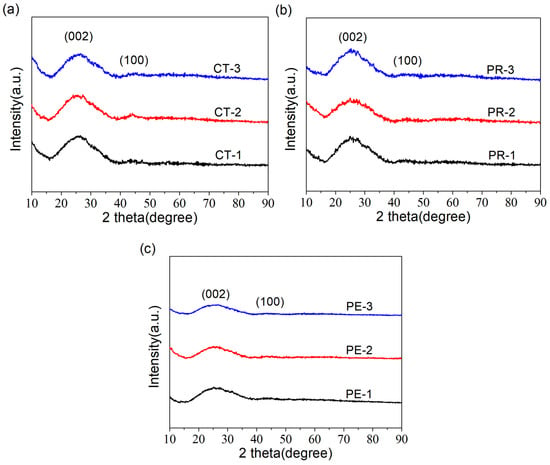
Figure 3.
XRD patterns of (a) CTs, (b) PRs and (c) PEs.
The nitrogen adsorption–desorption measurements were applied to investigate the SSA and PSD in all the samples. The isotherms of CTs are shown in Figure 4a. The three isotherms presented characteristic type I isotherms [28] with conspicuous adsorption in the low-pressure region. Moreover, more and more adsorptions could be observed when the activation time increased from 1 h to 3 h, which indicated that more micropores were generated over time [29,30]. However, in the high-pressure region, tiny hysteresis loops implied the existence of a small number of mesopores in these three samples. In addition, the PSD curves of CTs (Figure 4b) also exhibited that the micropores and mesopores increased with the extension of the activation time. Thus, increasing the activation time was beneficial in improving the porosity of carbon materials derived from cotton.
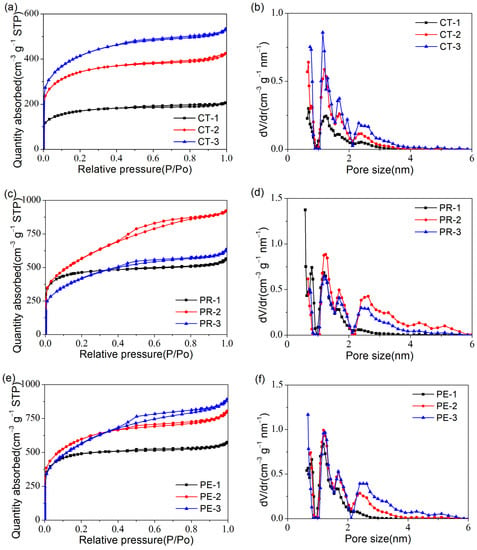
Figure 4.
(a) The adsorption–desorption isotherms and (b) The PSD curves of CTs. (c) The adsorption–desorption isotherms and (d) The PSD curves of PRs. (e) The adsorption–desorption isotherms and (f) The PSD curves of PEs.
The adsorption–desorption isotherms and PSD curves of PRs are shown in Figure 4c,d, respectively. The typical type I isotherm of PR-1 illustrates its microporous structure, while the isotherm of PR-2 possesses the characteristics of type IV with an obvious hysteresis loop [31]. The results show that more micropores and mesopores were produced when the activation time increased to 2 h [32]. Nevertheless, when increasing the activation time to 3 h, the isotherm of PR-3 decreased, as well as the hysteresis loop. This could be caused by the collapse of the pore structure due to an excessive activation time. A similar conclusion could be obtained from the PSD curves in Figure 4d.
Figure 4e shows the adsorption–desorption isotherms of PEs. In the high relative pressure zone, with the activation time increasing from 1 h to 3 h, the isotherms increased gradually, and the hysteresis loops became larger and larger. Combining the traits of the PSD curves (Figure 4f), it was easy to find out that the content of mesopores in these three samples was: PE-1< PE-2 < PE-3.
The pore structural parameters of the CTs, PRs, and PEs samples are summarized in Table 2. To facilitate the comparison and analysis, the histogram of a specific surface area and line chart for the proportion of micropores in all the samples is given in Figure 5. The CTs prepared from cotton were mainly microporous, and the proportion of the micropore was basically unchanged. Unlike CTs, PRs, and PEs obtained the lowest proportion of micropores when activated for 2 h and 3 h, respectively, suggesting the generation of massive mesopores and the hierarchical porous structure of PR-2 and PE-3.

Table 2.
The pore structural parameters of all samples.
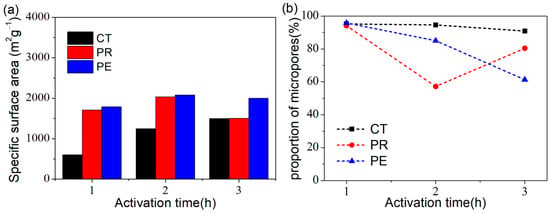
Figure 5.
(a) The histogram of specific surface area and (b) Line chart for the proportion of micropores in all the samples.
To sum up, the highly specific surface area could be attributed to the activation effect of KHCO3. According to a relevant report [23], KHCO3 would completely decompose at around 200 °C, releasing CO2 and water vapor (Equation (5)) while leaving a large number of macropores in the carbon materials when these gases escaped. As the temperature further rises, the carbon material is etched in the redox reactions shown in Equations (6)–(9) and then removes K and K compounds by neutralization with hydrochloric acid and excess deionized water, thus forming a rich porous structure.
2KHCO3 → K2CO3 + CO2 + H2O
K2CO3→ K2O + CO2
K2CO3 + 2C → 2K + 3CO
K2O + C → 2K + CO
CO2 + C → 2CO
However, the activation effect of KHCO3 varies significantly among different carbon precursors. Based on the above observations, the disparate activation performance could be due to the structure and components of the biomass carbon precursors. Firstly, the main component of cotton was cellulose (~94%). Its solid structure of cellulose might not be easily etched onto the mesopores by KHCO3 activation. In contrast, the pores in the carbon materials derived from puffed rice and pleurotus eryngii, which had a soft layered or network structure, were easier to expand the micropores to mesopores with a prolonged activation time. Secondly, as mentioned above, the activation time of PR-2 was shorter than that of PE-3 when the carbon materials obtained a generous amount of mesopores. One possible reason was that the layered structure of PR-2 was conducive to the lateral expansion of micropores to form mesopores, while pores in the 3D network structure of PE-3 expanded horizontally and vertically at the same time. A diagrammatic sketch of the likely mechanism for the formation of pores is shown in Figure 6.
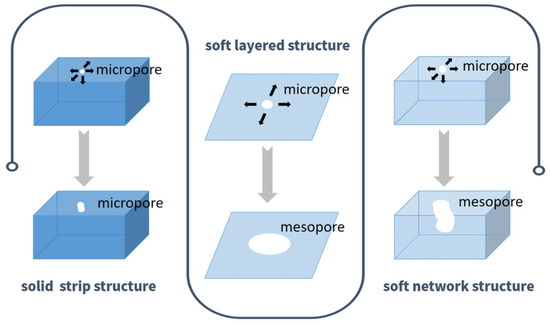
Figure 6.
Diagrammatic sketch of the likely mechanism for the formation of pores.
To explore the electrochemical behavior of all the samples, the CV test was carried out at 50 mV s−1. All the CV curves presented in Figure 7a demonstrated their double-layer signatures [33,34]. According to the area surrounded by CV curves, CT-3, PR-2, and PE-3 obtained the highest specific capacity among their respective carbon precursors, which could be related to their high specific surface area. By comparison, it was evident that the specific capacity of these three samples was: PR-2 > PE-3 > CT-3. Therefore, in the following study, the electrochemical properties of CT-3, PR-2, and PE-3 are discussed.
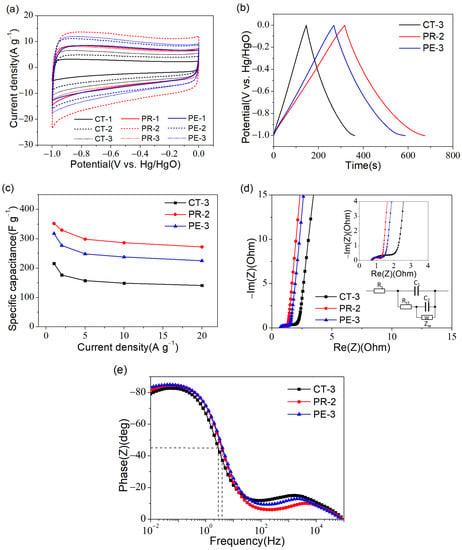
Figure 7.
The electrochemical properties of samples: (a) CV curves of CT, PR and PE at 50 mV∙s−1; (b) GCD curves of CT-3, PR-2 and PE-3 at 1 A g−1; (c) Specific capacitances of CT-3, PR-2 and PE-3 at 1–20 A g−1; (d) Nyquist diagrams of CT-3, PR-2 and PE-3 where the inset window displays the equivalent circuit as well as a magnified drawing at high frequency; (e) The phase diagram of CT-3, PR-2 and PE-3.
Figure 7b shows the GCD curves of CT-3, PR-2, and PE-3. The approximate symmetrical profiles once again indicated their double-layer capacitive behavior [35,36]. Figure 7c displays the specific capacitance of three samples at 1–20 A g−1. The specific capacitance of CT-3, PR-2, and PE-3 at 1 A g−1 was 216, 352, and 319 F g−1, respectively. When the current density increased by 20 times, the specific capacitance remained at 65.3%, 77.3%, and 70.5%, respectively. The differences in the specific capacitance and rate performance of these three samples were mainly attributed to their specific surface area and pore structure. Compared with CT-3, PR-2, and PE-3 possessed not only a highly specific surface area but also plentiful mesopores and macropores; thus, the electrolyte ions could shuttle freely during the charge–discharge course, even at a high current density. This resulted in more excellent capacitance performance. Moreover, the electrochemical property of PR-2 was compared with reported biomass-derived porous carbon materials. As seen from Table 3, PR-2 exhibited a performance that was comparable to or better than others, as well as a quite efficient and competitive preparation method.

Table 3.
Electrochemical properties of reported biomass-derived porous carbon materials.
The Nyquist plots of CT-3, PR-2, and PE-3 are shown in Figure 7d. Additionally, the corresponding equivalent circuit [43] and a locally enlarged view are inserted in Figure 7d. Due to the absence of frequency effects in an ideal capacitor, the corresponding Nyquist curve is a vertical straight line. Therefore, as shown in Figure 7d, all samples displayed nearly perfect capacitive properties with a steep and straight shape in the low-frequency region. At high frequency, the intersection of the curve and the horizontal axis denoted that the three samples revealed roughly equal equivalent serial resistance (~0.7 Ω) [44]. In addition, semicircles could be observed in the high-frequency region, which was linked to the charge transfer impedance. The maximum semicircle diameter of CT-3 presented the largest charge transfer resistance due to its microporous structure. In the phase diagram (Figure 7e), the frequency corresponding to the curve at a phase angle of −45° was called characteristic frequency, which reflected the transport speed of the ions [45]. From the Bode plot, the characteristic frequency of CT-3 was 2.8 Hz, while PR-2 and PE-3 had almost the same characteristic frequency (3.9 Hz). Therefore, compared with CT-3, PR-2 and PE-3 demonstrated a much stronger charge–discharge capability and more excellent frequency response, further confirming the superiority of the 3D hierarchical structure [46,47].
As the optimal as-prepared sample, the PR-2-based symmetric supercapacitor was tested in a two-electrode system to evaluate the electrochemical behaviors practically. Figure 8a shows the CV curves of the supercapacitor at 50 mV s−1. When the voltage window increased from 1 V to 1.2 V, the shape of the CV curves remained basically unchanged. However, a significant deformation occurred at the point of voltage conversion when the voltage window increased to 1.3 V. Therefore, 0–1.2 V was used as the voltage window for the symmetric supercapacitor. As shown in Figure 8b, the quasi-rectangular shape of all CV curves revealed the emblematic features of double-layer capacitors, the specific capacitance was calculated as 248 F g−1 for a scan rate of 5 mV s−1. Meanwhile, the quasi-symmetric triangle shape of all the GCD curves (Figure 8c) represented good electrochemical behavior with high reversibility. The specific capacitance from GCD curves decreased from 280.7 to 206.9 F g−1 when the current density increased from 0.5 to 20 A g−1 (Figure 8d), indicating excellent rate performance.
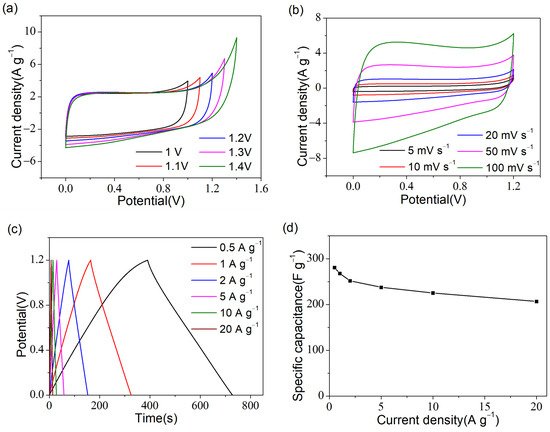
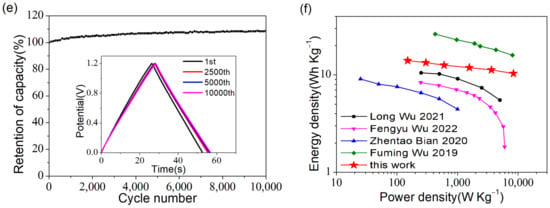
Figure 8.
The electrochemical properties of a PR-2-based symmetric supercapacitor: (a) CV curves with different potential windows; (b) CV curves at 5–100 mV s−1; (c) GCD curves at 0.5–20 A g−1; (d) The specific capacitance at 0.5–20 A g−1; (e) Cyclability test at 5 A g−1, where the illustration shows the GCD curves of the 1st, 2500th, 5000th and 10,000th cycle; (f) Ragone plots of PR-2 compared with previous reported symmetric supercapacitors [37,38,40,41].
The cycle life test was applied to study the cyclability of the supercapacitor. As shown in Figure 8e, after 10,000 cycles at 5 A g−1, the high-capacity retention of the supercapacitor reached 108.8%. From the illustration in Figure 8e, it can be seen that there was a significant improvement between the 1st and 2500th cycles, while GCD curves basically overlapped in the 2500th, 5000th, and 10,000th cycles, demonstrating excellent cycle stability. It was not difficult to speculate that the cycling of charge and discharge improved the wettability of the electrode material, making it easier for electrolyte ions to penetrate deeper into the electrode material, thus forming more double-layer capacitors.
In addition, as shown by the Ragone plot in Figure 8f, the PR-2-based symmetric supercapacitor exhibited an energy density of 14.03 Wh kg−1 at 150.04 W kg−1 and an energy density retention of 73.7% even at the power density, which was as high as 8380.4 W kg−1 and presented a much better performance than some previous examples in the literature [37,40,41] that also used the 6 M KOH aqueous solution as an electrolyte. The gap between the PR-2-based and albizia flowers-based [38] supercapacitor was principal because the latter was assembled in a 1 M Na2SO4 aqueous solution with a potential window of 1.8 V.
4. Conclusions
In summary, various biomass materials were used to study the activation effect of KHCO3 by a one-step activation method. As the activation time increased from 1 to 3 h, PR-2, PE-3, and CT-3 obtained the highest specific capacity (352, 319, and 216 F g−1, respectively) among their respective carbon precursors due to their high specific surface area. The PR-2-based symmetric supercapacitor presented a specific capacitance of 280.7 F g−1 at a current density of 0.5 A g−1 and an energy density of 14.03 Wh kg−1 at 150.04 W kg−1, with the energy density still retaining 73.7% even at a higher power density of 8380.4 W kg−1. After 10,000 cycles of charging and discharging at 5 A g−1, the specific capacitance retention of the supercapacitor reached 108.8%. Based on the experimental analysis, it could be clearly seen that the biomass with a soft layered or network structure had the best prospect of obtaining a 3D hierarchical porous structure. The mechanism for the formation of these pores was proposed for different kinds of biomass materials. It provided guidance for the selection of biomass materials to obtain a better electrochemical performance and other 3D hierarchical porous structure-related device applications.
Author Contributions
Conceptualization, Y.Y.; Data curation, Y.S.; Formal analysis, Y.Y.; Investigation, Y.S.; Methodology, Y.Y.; Project administration, L.Y. and C.Z.; Supervision, L.Y. and C.Z.; Validation, C.L.; Writing—original draft, Y.Y.; Writing—review and editing, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Starting Research Fund from the Suzhou Vocational Institute of Industrial Technology (2023kyqd001), the Suzhou Science and Technology Development Planning Programme (SYC2022101), the BAOSHENG (Suzhou) industrial cooperative fund (RDS10120220070), and the XJTLU Research Development Funding (RDF-21-01-040).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A review of supercapacitor modeling, estimation, and applications: A control/management perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.B.; Lee, S.Y.; Park, S.J. Recent Advanced Supercapacitor: A Review of Storage Mechanisms, Electrode Materials, Modification, and Perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Nagarajarao, S.H.; Nandagudi, A.; Viswanatha, R.; Basavaraja, B.M.; Santosh, M.S.; Praveen, B.M.; Pandith, A. Recent Developments in Supercapacitor Electrodes: A Mini Review. ChemEngineering 2022, 6, 5. [Google Scholar] [CrossRef]
- Qi, X.; Xu, F.; Sun, P.; Lin, T.; Huang, F. Constructing Hierarchical Porous Carbon of High-Performance Capacitance through a Two-Step Nitrogen-Fixation Method. Energy Technol. 2020, 8, 2000107. [Google Scholar] [CrossRef]
- Qin, C.; Wang, S.; Wang, Z.; Ji, K.; Wang, S.; Zeng, X.; Jiang, X.; Liu, G. Hierarchical porous carbon derived from Gardenia jasminoides Ellis flowers for high performance supercapacitor. J. Energy Storage 2021, 33, 102061. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, X.; Du, Y.; Jiang, Y.; Wan, J.; Ma, F. In-situ template cooperated with urea to construct pectin-derived hierarchical porous carbon with optimized pore structure for supercapacitor. Electrochim. Acta 2020, 355, 136801. [Google Scholar] [CrossRef]
- Yao, L.; Yang, G.; Han, P.; Tang, Z.; Yang, J. Three-dimensional beehive-like hierarchical porous polyacrylonitrile-based carbons as a high performance supercapacitor electrodes. J. Power Sources 2016, 315, 209–217. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Feizbakhshan, M.; Liu, C.; Hong, S.; Yang, P.; Zhou, X. Preparation of lignin-based porous carbon with hierarchical oxygen-enriched structure for high-performance supercapacitors. J. Colloid Interface Sci. 2019, 540, 524–534. [Google Scholar] [CrossRef]
- Yan, X.H.; Xu, H.; Fang, G.; Xue, T.; Meng, Z.; Lee, J.M. A Reactive Template Synthesis of Hierarchical Porous Carbon and Its Application to Supercapacitor Electrodes. Macromol. Mater. Eng. 2020, 305, 2000168. [Google Scholar] [CrossRef]
- Cai, P.; Zou, K.; Deng, X.; Wang, B.; Zou, G.; Hou, H.; Ji, X. Defect Rich Hierarchical Porous Carbon for High Power Supercapacitors. Front. Chem. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xiong, T.; Xu, F.; Li, M.; Han, C.; Gong, Y.; Wang, H.; Wang, Y. Inspired by bread leavening: One-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chem. 2015, 17, 4053–4060. [Google Scholar] [CrossRef]
- Hong, P.; Liu, X.; Zhang, X.; Peng, S.; Wang, Z.; Yang, Y.; Zhao, R.; Wang, Y. Hierarchically porous carbon derived from the activation of waste chestnut shells by potassium bicarbonate (KHCO3) for high-performance supercapacitor electrode. Int. J. Energy Res. 2019, 44, 988–999. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Liu, G.; Li, M. Tobacco-Stem-Derived Nitrogen-Enriched Hierarchical Porous Carbon for High-Energy Supercapacitor. ChemistrySelect 2021, 6, 532–537. [Google Scholar] [CrossRef]
- Momodu, D.; Sylla, N.F.; Mutuma, B.; Bello, A.; Masikhwa, T.; Lindberg, S.; Matic, A.; Manyala, N. Stable ionic-liquid-based symmetric supercapacitors from Capsicum seed-porous carbons. J. Electroanal. Chem. 2019, 838, 119–128. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Yang, X.; Yu, Y.; Lin, H.; Sheng, K. Synthesis of Fe/N Co-doped Porous Carbon Spheres Derived from Corncob for Supercapacitors with High Performances. Energy Fuels 2021, 35, 14157–14168. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Feng, Z.; Li, X.; Yi, R.; Sun, W.; Zhao, C.; Yang, L. Nitrogen-Doped Hierarchical Porous Activated Carbon Derived from Paddy for High-Performance Supercapacitors. Materials 2021, 14, 318. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, Y.; Geng, X.; Han, C.; Lu, S.; Mitrovic, I.; Yang, L.; Song, P.; Zhao, C. Biochar-derived material decorated by MXene/reduced graphene oxide using one-step hydrothermal treatment as high-performance supercapacitor electrodes. Carbon 2022, 199, 224–232. [Google Scholar] [CrossRef]
- Pour, G.B.; Aval, L.F.; Mirzaee, M. CNTs Supercapacitor Based on the PVDF/PVA Gel Electrolytes. Recent Pat. Nanotechnol. 2020, 14, 163–170. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Tao, Y.; Zhou, J.; Zhang, H. Hierarchical porous carbon materials produced from heavy bio-oil for high-performance supercapacitor electrodes. J. Energy Storage 2022, 47, 103624. [Google Scholar] [CrossRef]
- Young, C.; Chen, H.-T. Supercapacitor application of a three-dimensional carbon sphere–intercalated porous carbon fabricated using a hard template and a biomass material. Diam. Relat. Mater. 2022, 130, 109528. [Google Scholar] [CrossRef]
- Feng, H.; Hu, H.; Dong, H.; Xiao, Y.; Cai, Y.; Lei, B.; Liu, Y.; Zheng, M. Hierarchical structured carbon derived from bagasse wastes: A simple and efficient synthesis route and its improved electrochemical properties for high-performance supercapacitors. J. Power Sources 2016, 302, 164–173. [Google Scholar] [CrossRef]
- Shen, F.; Zhu, L.; Qi, X. Nitrogen Self-Doped Hierarchical Porous Carbon from Myriophyllum Aquaticum for Supercapacitor Electrode. ChemistrySelect 2018, 3, 11350–11356. [Google Scholar] [CrossRef]
- Julnaidi; Saputra, E.; Nofrizal; Taer, E. Renewable palm oil sticks biomass-derived unique hierarchical porous carbon nanostructure as sustainability electrode for symmetrical supercapacitor. J. Chem. Technol. Biotechnol. 2022, 98, 45–56. [Google Scholar]
- Liu, L.; An, X.; Tian, Z.; Yang, G.; Nie, S.; Shang, Z.; Cao, H.; Cheng, Z.; Wang, S.; Liu, H.; et al. Biomass derived carbonaceous materials with tailored superstructures designed for advanced supercapacitor electrodes. Ind. Crops Prod. 2022, 187, 115457. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.C.; Mascorro-Gutiérrez, I.; Arreola-Ramos, C.E.; Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Ramos-Sánchez, V.H.; Cuentas-Gallegos, A.K. A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon 2019, 148, 403–412. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Sun, L.; Li, N.; Wang, X.; Wang, Q.; Zhang, D.; Wang, B. Hydrothermal synthesis of 3D hierarchical ordered porous carbon from yam biowastes for enhanced supercapacitor performance. Chem. Eng. Sci. 2022, 252, 117514. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. Biomass derived nitrogen-doped hierarchical porous carbon sheets for supercapacitors with high performance. J. Colloid Interface Sci. 2018, 523, 133–143. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. “Salt templating”: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef]
- Long, C.; Chen, X.; Jiang, L.; Zhi, L.; Fan, Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. [Google Scholar] [CrossRef]
- Tran, C.D.; Ho, H.C.; Keum, J.K.; Chen, J.; Gallego, N.C.; Naskar, A.K. Sustainable Energy-Storage Materials from Lignin-Graphene Nanocomposite-Derived Porous Carbon Film. Energy Technol. 2017, 5, 1927–1935. [Google Scholar] [CrossRef]
- Taer, E.; Afdal Yusra, D.; Amri, A.; Awitdrus; Taslim, R.; Apriwandi; Agustino; Putri, A. The synthesis of activated carbon made from banana stem fibers as the supercapacitor electrodes. Mater. Today Proc. 2021, 44, 3346–3349. [Google Scholar] [CrossRef]
- Zhou, W.; Lei, S.; Sun, S.; Ou, X.; Fu, Q.; Xu, Y.; Xiao, Y.; Cheng, B. From weed to multi-heteroatom-doped honeycomb-like porous carbon for advanced supercapacitors: A gelatinization-controlled one-step carbonization. J. Power Sources 2018, 402, 203–212. [Google Scholar] [CrossRef]
- Huang, J.; Wu, J.; Dai, F.; Li, C.M. 3D honeycomb-like carbon foam synthesized with biomass buckwheat flour for high-performance supercapacitor electrodes. Chem. Commun. 2019, 55, 9168–9171. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Wang, L.; Chen, Y.; Gao, S. Hierarchical porous biomass-derived carbon framework with ultrahigh surface area for outstanding capacitance supercapacitor. Renew. Energy 2021, 179, 1826–1835. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, J.; Guo, Z.; Wong, S.I.; Sunarso, J.; Zhao, Y.; Xing, W.; Zhou, J.; Zhuo, S. Watermelon Peel-Derived Heteroatom-Doped Hierarchical Porous Carbon as a High-Performance Electrode Material for Supercapacitors. ChemElectroChem 2021, 8, 1196–1203. [Google Scholar] [CrossRef]
- Wu, L.; Cai, Y.; Wang, S.; Li, Z. Doping of nitrogen into biomass-derived porous carbon with large surface area using N2 non-thermal plasma technique for high-performance supercapacitor. Int. J. Hydrogen Energy 2021, 46, 2432–2444. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Zhai, X.; Xie, M.; Sun, Y.; Kang, H.; Tian, Q.; Qiu, H. Hierarchical porous carbon microrods derived from albizia flowers for high performance supercapacitors. Carbon 2019, 147, 242–251. [Google Scholar] [CrossRef]
- Ma, F.; Ding, S.; Ren, H.; Liu, Y. Sakura-based activated carbon preparation and its performance in supercapacitor applications. RSC Adv. 2019, 9, 2474–2483. [Google Scholar] [CrossRef]
- Wu, F.; Ren, X.; Tian, F.; Han, G.; Sheng, J.; Yu, Y.; Liu, Y.; Yang, W. O and N co-doped porous carbon derived from crop waste for a high-stability all-solid-state symmetric supercapacitor. New J. Chem. 2022, 46, 19667–19674. [Google Scholar] [CrossRef]
- Bian, Z.; Wu, C.; Yuan, C.; Wang, Y.; Zhao, G.; Wang, H.; Xie, Y.; Wang, C.; Zhu, G.; Chen, C. One-step production of N-O-P-S co-doped porous carbon from bean worms for supercapacitors with high performance. RSC Adv. 2020, 10, 30756–30766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-H.; Chen, M.-H.; Liang, S.-X.; Lü, Q.-F. Oxygen-rich hierarchical porous carbon derived from biomass waste-kapok flower for supercapacitor electrode. Diam. Relat. Mater. 2021, 113, 108267. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Li, A.; Li, H.; Cui, D.; Dong, M.; Lin, J.; Fan, J.; Zhang, J.; Hou, H.; et al. Advanced porous hierarchical activated carbon derived from agricultural wastes toward high performance supercapacitors. J. Alloys Compd. 2020, 820, 153111. [Google Scholar] [CrossRef]
- Nie, Z.; Wang, Y.; Li, X.; Wang, R.; Zhao, Y.; Song, H.; Wang, H. Heteroatom-doped hierarchical porous carbon from corn straw for high-performance supercapacitor. J. Energy Storage 2021, 44, 103410. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, T.; Wang, H.; Yang, F.; Yan, J.; Zhu, K.; Ye, K.; Wang, G.; Zhou, L.; Cheng, K.; et al. High-throughput fabrication of porous carbon by chemical foaming strategy for high performance supercapacitor. Chem. Eng. J. 2018, 352, 459–468. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef]
- Tong, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).