Abstract
Magnesium (Mg) is the most essential element constituent in chlorophyll molecules that regulates photosynthesis processes. The physiological response of ‘Superior Seedless’ grapes was evaluated under different foliar magnesium fertilization such as sulfate magnesium (MgSO4·7 H2O), magnesium disodium EDTA (Mg-EDTA), and magnesium nanoparticles (Mg-NPs) during the berry development stages (flowering, fruit set, veraison, and harvest). In general, the ‘Superior Seedless’ vine had a higher performance in photosynthesis with Mg-NPs application than other forms. The Fy/Fm ratio declined rapidly after the fruit set stage; then, it decreased gradually up until the harvesting stage. However, both MgSO4 and Mg-EDTA forms showed slight differences in Fv/Fm ratio during the berry development stages. The outcomes of this research suggest that the Fv/Fm ratio during the growth season of the ‘Superior Seedless’ vine may be a good tool to assess magnesium fertilization effects before visible deficiency symptoms appear. Mg-NPs are more effective at improving ‘Superior Seedless’ berry development than the other magnesium forms. These findings suggest that applying foliar Mg-NPs to vines grown on salinity-sandy soil alleviates the potential Mg deficiency in ‘Superior Seedless’ vines and improves bunches quality.
1. Introduction
Grapes are one of the most important fruit crops on the planet. Grape is a member of the Vitis genus, which is part of the Vitaceae family, which contains more than 60 genera. Grapes (Vitis vinifera L.) are cultivated in more than 100 countries throughout the world, with an estimated area of 7.8 million hectares in 2016. Wine, jam, juice, grape seed extract, dried grapes, vinegar, and grape seed oil are among the many goods made from grapes. In 2016, the world produced 75.8 million tons of grapes, with 39% produced in Europe, 34% produced in Asia, 18% produced in the Americas, and 9% produced in Africa []. Grapes are Egypt’s second most important fruit crop, after citrus. Egypt’s agriculture has succeeded in increasing vineyard area by 220,665 hectares over the past decade, yielding 1,586,342 tons of grapes []. The grapevine is one of the most important horticultural crops in the world. The high value of table grapes is primarily attributed to bio-compounds required for human health, such as antioxidants, anthocyanins, and phenolics, which include gallic acid, catechin, anthocyanins, and resveratrol [].
The fundamental issue with newly reclaimed and cultivated fields was that they were often sandy and calcareous soils with poor nutrient concentration, especially magnesium. Recently, research on magnesium nutrition has begun, with the goal of determining the Mg requirements of Egypt’s most important crops. Magnesium deficiency has been discovered in some Egyptian soils such as clay or newly reclaimed soils []. Therefore, magnesium (Mg) is the most essential element constituent in chlorophyll molecules that regulates the photosynthesis processes [,]. The deficiency of Mg during growth seasons limits photosynthesis performance []. The physiological functions of Mg in plants have also been characterized for flowering induction []. Mg is required for the growth and development of plants []. It is also a cofactor in the biosynthesis of various enzymes, including those involved in respiration and photosynthesis. It is a phloem-mobile nutrient that migrates between older and younger leaves []. Mg is also a significant component of the chlorophyll molecule’s ring structure []. Additionally, it alleviates abiotic stress conditions, such as dryness and heat, which can significantly enhance Mg deficit by inhibiting its absorption due to its mass flow transit []. Additionally, it mitigates aluminum toxicity in acid soils at micromolar concentrations, as opposed to calcium, which is required at millimolar concentrations []. A Mg shortage has been shown to adversely influence ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which is involved in CO2 fixation [], resulting in a decrease in photosynthetic performance [], which is correlated to a decrease in photosynthesis performance and stomatal mechanism []. Furthermore, it plays a role of metabolism nitrogen in plant []. The inhibitory influence of Mg loss on photosynthetic capacity and net CO2 absorption was marked in several plant species [,,]. As a result, in certain species, magnesium deprivation affects the structure and function of the PSI and PSII systems []. As a result, a decrease in the Fv/Fm ratio (maximum quantum efficiency of PSII) was observed in citrus seedlings []. Despite this, the Mg shortage had no effect on Fy/Fm and other fluorescence metrics in Helianthus annuus plants under Mg deficiency conditions. A rise in the chlorophyll a/chlorophyll b ratio is typically reported []. The decrease in light-harvesting complex II ( LHC-II) abundance in Mg the absence of Arabidopsis thaliana leaves is caused by thylakoid membrane dysfunction [].
Many researchers have begun to investigate magnesium nutrition and the determination of magnesium requirements for economically important crops [] such as ‘Washington navel’ orange trees [] and banana plants, and they have reported on the influence of magnesium on yield and fruit quality, stating that magnesium fertilization increased the yield and fruit quality of the aforementioned fruit species []. In addition, using the magnesium application can induce a state of magnesium deficiency during growing []. Furthermore, fertilizing “Grand Nain” bananas with 100 g/plant magnesium chelate plus a foliar spray of 2% magnesium sulfate increased growth metrics, yield, and fruit quality []. In addition, treating Le Conte pear plants with compost 45 kg/tree + biofertilizers 20 g/tree plus 1.5% magnesium sulfate produced the best production and fruit quality []. The foliar Mg (137.5 ppm) application boosted the growth characteristics and yield of Washington Navel orange trees []. Moreover, some studies were conducted to improve bunches of color quality of Crimson seedless by using foliar application of Mg [].
‘Superior seedless’ is one of the first seedless table grapes to be produced in the Mediterranean region, and it adapts well to and performs well in Egyptian circumstances as well. It was harvested when the meat was yellow-white and the skin was green, as requested by the European market []. ‘Superior Seedless’ is also considered as one of the most important international grape variety with a good economic return []. Consumers value this grape selection for its excellent nutritional value, great taste, versatile application, and higher economic returns []. The world’s vineyard area is growing as a result of a continual and unrelenting shift []. The purpose of this study is to determine the difference among foliar magnesium forms on ‘Superior Seedless’ vines grown in salinity sandy soil. Furthermore, this study also aims to determine the optimal magnesium form for vine nutrition under soil salinity conditions.
2. Materials and Methods
2.1. Vine and Experimental Setup
A commercial vineyard in the Nobaria area of Egypt (31.23° N, 29.96° E) was studied for two growth seasons (2020 and 2021). The soil was sandy in texture (Entisol-Typic Torripsamments), and the soil composition is described in Table 1. The farm consists of 6-year-old vines of the ‘Superior Seedless’ cv. grafted on 1103 Paulsen rootstock. Three-by-three-meter vines were planted in sandy soil using a drip watering system. The pruning level was done on all vines at 70 bud vines−1 (7 cans × 10 buds can−1 each on four cardons), and all vines were trained by the Y system. Table 1 summarizes the physical and chemical examination of the field experiment with ‘Superior Seedless’ vines [,]. All vines were pruned to a height of 60 buds’ vine−1, with the length of the cans ranging from 6 to 8 buds per can, and each can contain 12–14 buds and were produced until mid-July in European countries. Additionally, according to the Egyptian Agriculture Ministry, all vines received the same management program as for NPK fertilizer (300, 200, and 250 Kg were afforded on three portions from growth starting until harvesting (one portion was added at the vine dormancy stage) in sandy soil. Uniform vines (48) were chosen and treated with four different types of magnesium; each treatment consisted of three duplicates with four vines per replication. All treatments receive 750 g of magnesium sulfate per 600 L of irrigation water, which was employed to avoid magnesium shortages. It is distinguished by the yellowing of older leaves and a yellow tint between the veins of the leaves.

Table 1.
Soil and irrigation-water traits analysis.
2.2. Magnesium Fertilization Forms Treatment Protocol
The foliar magnesium application was laid out as control (0.5 g L−1), MgSO4·7H2O (0.5 g L−1), Mg-EDTA (Mg chelate 0.5 g L−1), and Mg-NPs powder (0.5 g L−1). Nanomaterials provided magnesium nanoparticles (MgO, 99%+ purity, 20 nm) in powder form at sundown. This optimal concentration was used for application. At sundown during the four stages of growth (flowers, fruit set, veraison, and harvest), foliar treatments were made (7:00 pm). In a bath of warm tap water, the magnesium compounds were melted. Using a knack-sap pump, the solutions were sprayed over the entire vine monthly until the leaves became saturated. The rest of the magnesium salts were acquired from EL-Gomhoria Co. Ltd. in Egypt from EL-Gomhoria Co. Ltd. In Mansoura city, Egypt.
2.3. Magnesium Deficiency Index
Magnesium deficiency (MD) results in interveinal yellowing or reddening on old leaves, beginning at the leaf edge and proceeding to the leaf veins’ petiole-connected point. These symptoms progress to necrotic brown patches, and in severe MD, the leaves exhibit necrosis, dray, and premature fall. The Mg deficiency was inspected and scored on a scale from 0 (no injury) to 5 (very severe injury) [].
2.4. Leaf Pigments Content and Chlorophyll Fluorescence
Total chlorophyll (Chls) and carotenoid (Car) content were determined spectrophotometrically [] on the 7th leaf (ten leaves) from the shoot base.
Individual dark-leaf CF data were recorded. The data were acquired using a commercial fluorimeter (Mini-PAM, Walz, Effeltrich, Germany) and data gathering software (Win Control, Walz, Effeltrich, Germany). These data included F0 (minimum fluorescence), Fm (light-saturated fluorescence), and the Fv/Fm ratio (the difference between maximum fluorescence and minimum fluorescence is Fv or variable fluorescence divided by maximum fluorescence). A fall in the Fv/Fm ratio below 0.75–0.78 suggests a decline in photosystem II photochemical transformation capability [,]. On the 7th leaf, CF parameters were determined.
2.5. Leaf Area, Total Carbohydrate Content, Ion Leakage Percentage, and Malondialdehyde (MDA)
On the 7th leaf, the Sokkia Planix 7 Digital Planimeter was used to quantify leaf area during four developmental stages. However, the vine canes’ cumulative carbohydrate content was assessed according to []. The leaf petiole cell permeability was also tested. After three washes with deionized water, the rachis samples were put in 10 mL of 0.4 M mannitol at 24 °C for three hours. After measuring the EC of the aqueous phase (M1), the rachis samples were killed in a water bath at 100 °C for 20 min. This was followed by room-temperature cooling. Then, it was estimated as a percentage of the relative electrolyte loss from M1 rachis samples using the equation: ion leakage percent = (M1M2)/M1 × 100 [,]. However, MDA was a by-product of lipid peroxidation that accumulated during salinity stress. They used 2.5 g of leaf petiole samples for MDA extraction [,]. This was done by measuring 0–3 mM of TBARS (equal to 0–1 mM MDA) in 1,3,3-tetraethoxypropane (Sigma, St. Louis, MO, USA). During the assay’s acid-heating halt, TEOP is stoichiometrically transformed to MDA.
2.6. Leaf Minerals Content
Leaf mineral content was measured on the 7th leaf from the base of the shoot during four vegetative growth stages. Nitrogen % [], phosphorous [], and potassium content [] as well as the magnesium, calcium, chloride, and sodium content percentages were demined [].
2.7. Yield and Berry Properties
At harvest, the number of clusters per vine, average cluster weight (Kg), and yield per vine (Kg) were determined. In addition, the pruned wood was weighted. The SSC % of berry juice was measured with a digital refractometer (PR32 ALA-GO Co., Tokyo, Japan) at lab temperature, and it was represented as a percentage. As for TA %, berry juice (20 mL) was used for titrating by NaOH (0.1N). The outcome was shown as a percentage. However, the SSC/TA-ratio was computed to judge bunch maturity [,].
2.8. Statistical Analysis
The experiment was designed as a randomized complete block in three-way ANOVA with three factors: seasons (2 levels), berry developmental phase (4 levels), and foliar magnesium forms (3 levels) with three replicates per treatment. The mean separations were run with Tukey’s HSD test (p ≤ 0.05). Pearson’s correlation matrix among the studied parameters and principal component analysis (PCA) were applied. Tukey’s HSD test was run using the JMP Pro 16 software, with p < 0.05 taken as indicating a statistically significant difference (SAS Institute, Cary, NC, USA).
3. Results
3.1. Magnesium Deficiency Index (MD-Index)
Figure 1 depicts the magnesium deficiency index (MD-index), which is a function of berry developmental stages (BDSs) for all magnesium types. When seasons, BDSs, and magnesium application forms are examined, the MD-index demonstrates a significant influence of p < 0.05. Considering the different magnesium forms, it is obvious that the Mg-NPs treatment produced fewer symptoms of magnesium deficiency than the other magnesium forms. Observably, the effect of ‘Mg-NPs’ was that there was no evidence of deficient symptoms prior to the veraison stage (berry change color) and that it rose somewhat until the harvesting stage was completed. For vines treated with Mg-EDTA, MgSO4, and control treatments, deficit symptoms were observed prior to fruit set, increased significantly during veraison, and persisted until harvesting. However, during the vegetative growth stages, the ‘Control’ treatment exhibited the most deficiency symptoms. The severity of Mg was noticed on ‘Control’ vines that were unaffected by the Mg forms, but the control vines had more symptoms throughout the vegetative growth stages. On sandy soils, symptoms of a magnesium deficit appear on vines during the growth season, necessitating monthly spraying of vines to compensate for the shortfall and thereby avoiding deficient occurrence. Regardless of the magnesium supply to the vines, 750 g of magnesium sulfate per 600 L of irrigation water is employed to avoid magnesium shortages. It is distinguished by the yellowing of older leaves and a yellow tint between the veins of the leaves.
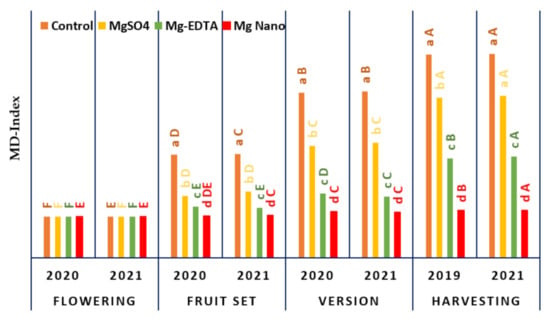
Figure 1.
The influence of various magnesium fertilizer forms on ‘Superior seedless’ vines throughout four berry development phases (flowering, fruit set, veraison, and harvesting) under soil salinity conditions on magnesium shortage during the four stages. The values represent the mean affect levels in each application plus standard error (n = 3). Tukey’s HSD test (p ≤ 0.05) used mean severance between blocks (capital letters) to detect significant differences between growing seasons and Mg applications (capital letters) to distinguish significant differences between Mg types.
3.2. Photosynthetic Pigments: Chlorophyll (Chls) and Carotene (Car)
Photosynthetic pigments as a function of BDSs for all foliar magnesium application forms are shown in Table 2 and Table 3. Leaf pigments show a significant interaction at p ≤ 0.05 when the seasons, BDSs, and foliar magnesium treatments were considered. Generally, chlorophyll compounds (Chl A and Chl B) and carotenoid (Car) were raised gradually during BDSs until the harvest stage for all Mg treatments, whereas the untreated vines (control) treatment presented the lowest decreases in Chls and Car until the end of the experiment. Despite this, there is a significant variance between Mg treatment on pigment content that was observed during both growing seasons. The obvious outcomes are that the Mg-NPs presented the highest amount of Chl A and Chl B and Car compared to the other Mg treatments and control vines. They were marked with the highest amount at the harvest stage. Moreover, the Car exhibited the highest content at the harvest time stage compared to other foliar treatments. Regarding the Chl A:b ratio, the lowest rates at the harvesting stage of the vegetative growth period decreased progressively until grape harvesting with all Mg treatments. Nevertheless, the Chl A:b ratio of Mg-NPs had more stable outcomes than those shown with other Mg treatments throughout the growing season.

Table 2.
The influence of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on leaf chlorophyll parameters pigment of ‘Superior seedless’ vines, which were used four times on various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).

Table 3.
The effect of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on leaf carotene pigment and the ratio of chlorophyll and carotenoid of ‘Superior seedless’ vines, which were used four times on various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).
3.3. Parameters of Chlorophyll Fluorescence (CF) (Fv/Fm, Fm, and F0)
A significant interaction between seasons and berry developmental stages was found as well as the influence of Mg treatments on Fm and F0 (p < 0.001). No significant variations in Fv/Fm ratio were observed for the interaction effect of seasons, berry developmental stages, and mg treatments, but significant differences in Fm and F0 were observed, whereas a significant difference (p < 0.01) was noted for the magnesium effect (p < 0.001). The Fv/Fm ratio of ‘Superior seedless’ vines was proposed as a function of BDSs; when seasons, BDSs, and foliar Mg form fertilization were considered, substantial results were obtained (Table 4). On average, untreated vines exhibit a higher decline in the Fv/Fm ratio than vines treated with other Mg compounds. It is drastically reduced until the harvest stage. Except for Mg-NPs treatment, the drop in the Fv/Fm ratio appears to be more gradual and progressive, including a trend toward a more inferior Fv/Fm ratio during vegetative growth stages.

Table 4.
The impact of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on chlorophyll fluorescence parameters of ‘Superior seedless’ vines, which were used four times on various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).
Both Fm and F0 rates increased significantly in overall Mg treatments from the initial stage (flowering) to the veraison stage (Table 4), and this increase was significant for both Fm and F0. It was discovered that the effect of Mg treatments on Fm and F0 varied according to the Mg forms. Then, both are steady until the experiment’s duration expires. In comparison to other treatments, the application of Mg-NPs resulted in the greatest Fm and F0 values. Thus, when the Fv/Fm ratio of the ‘Superior Seedless’ vine was changed, Mg-NPs enhanced CF parameters more than other Mg treatments. As a result, this sample fluorescence parameter can detect magnesium insufficiency.
3.4. Leaf Area, Shoot Carbohydrate, Ion Leakage, and Malondialdehyde Content
Table 5 presents the differences in leaf area, shoot carbohydrate, ion leakage, and malondialdehyde accumulation as a function of berry developmental stages. The interaction (p < 0.001) was significant between the berry developmental stages and the Mg foliar fertilization forms and season. The leaf area (cm2) and shoot carbohydrate content (%) have significantly (p < 0.008) higher values when vines receive the Mg-NPs form than other forms. Whereas, when considering the ion leakage percent and MDA content, there were significantly (p < 0.0005) lower values throughout the berry developmental stages. This implies that there is variability based on Mg type for previous variables.

Table 5.
The impact of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on leaf area (cm2), shoot carbohydrate content percentage, ion leakage percentage, and malondialdehyde of ‘Superior seedless’ vines, which were used four times on various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).
3.5. Mineral Content in Leaves
Table 6 and Table 7 exhibit the significant variances (p > 0.001) between seasons, BDSs, and Mg application foliar form treatments in the 7th leaf from the base of the shoot N, P, K+, Ca++, Mg++, Na+, and Cl− content when all were considered as experimental factors. Na+ and Cl− content significantly decreased with Mg-NPs application compared to other Mg forms. However, the rest of the mineral increased during the growth stages.

Table 6.
The effect of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on leaf mineral compositions of ‘Superior seedless’ vines, which were used four times on various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).

Table 7.
The influence of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on the leaf mineral compositions of ‘Superior seedless’ vines was studied for four terms in various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).
3.6. Yield and Berry Quality Properties
Table 8 presents the yield and berry quality properties. The quality variables were significantly affected by foliar fertilization at harvesting time by 5%. The yield was significantly affected more by using foliar Mg-NPs (9.13 kg vine−1) compared to other forms and control treatments.

Table 8.
The impact of various magnesium fertilization types (MgSO4, Mg-EDTA, and Mg-NPs) on ‘Superior seedless’ vines on yield, berries proprieties, and fruit quality of ‘Superior seedless’ vine. Treatments were used four times on various phases during berry growth (flowering, fruit set, version, and at harvest time) throughout two summers (2020 and 2021).
3.7. Multivariate Analysis of Leaf Parameters
A PCA for physiological and biochemical variables data obtained from leaves was conducted from the tested different foliar magnesium fertilization forms (MgSO4, Mg-EDTA, and Mg-NPs) applied four times on different fruit developmental stages (flowering, fruit set, version, and at harvest time) throughout two growth seasons (2020 and 2021) of ‘Superior Seedless’ vines. The PCA separated the effect of magnesium forms under each seasonal stage. The PC1 explained 70.9% of the variability in the data, while PC2 explained 16.1% of the variability (Figure 2A). Figure 2B displays the negative correlation between MD-index with all the parameters except for EL%, MDA, Na+ %, and Cl− %. Chlorophyll a and b and total chlorophyll contents were negatively correlated with chlorophyll fluorescence variables (Fv/Fm; Fm, and F0). These four valuables (MD, MDA, Na+ %, and Cl− %) had a negative correlation with the other variables. Chl B showed negative correlation with Chl A:B. Chl A:B was positively correlated with Chls:Caro and Fv/Fm, whereas it had a negative correlation with the other valuables. Pearson’s correlation matrix among the examined parameters shows the correlation and shows these results (Table 9).
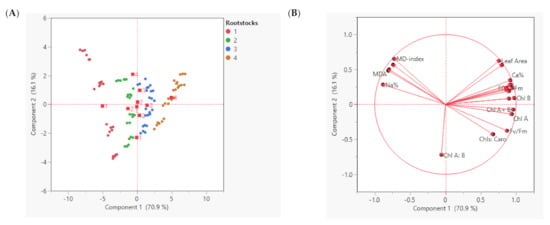
Figure 2.
Principal Component Analysis (PCA) representing seasons and magnesium application forms to ‘Superior seedless’ vine grown in sandy soil and salt conditions, plotted with the contribution of each parameter on the two PCA axes (A) and all the physiological and biochemical parameters measured in leaf during the growing season (B). Principal Component Analysis (PCA)-Variable correlation of 7th leaf.

Table 9.
Pearson’s correlation pattern among the considered variables of ‘Superior seedless’ vines under four levels of magnesium foliar application.
4. Discussion
Magnesium is involved in a number of biochemical and physiological processes that influence plant growth and development []. As a result, the wounded bunches’ early-stage leaves fall off throughout the growing season. However, under soil salinity conditions, a variety of mechanisms occur that result in Mg loss []. As a result, Mg insufficiency occurred on control vines earlier in the growth season than on vines treated with other Mg treatments. This can be seen in the slower transport of Mg through the soil profile, which results in more Mg adsorption []. In addition, changes in Ca and K content across Mg application rates suggest that Mg and two other cations interact throughout the season []. Foliar spraying is a common way for plants to adjust for nutritional deficiencies in the soil []. During the trial period, the efficiency of the nano-magnesium image revealed the fewest symptoms on the leaves. This result could be attributed to magnesium absorption being faster than the rest of the pictures, resulting in better photosynthetic efficiency []. These conclusions were reached because of the results shown in the graph. The presence of EDTA in chelated Mg form, on the other hand, has been shown to improve vine growth and biomass [], and the sulfate part plays a critical role in the catalytic or electrochemical functions of the biomolecules in the cells [].
Chlorophylls (Chls) are reputedly the most outstanding natural syntheses on the planet, as they are required for the photosynthesis process [,]. This method of vegetation occurs primarily based on gaining light rays by chlorophyll and especially chlorophyll A []. Photosynthesis is a very powerful method wherein it is supplied with 5 to 11 μmol CO2 m−2 s−1. This process is involved in the biosynthesis of essential organic molecules required for plant growth and development []. The photosynthesizing cells need a large amount of assimilatory pigment that reaches up to 5% of typical dry matter [,]. Most plant species have photosynthetic pigment content in their leaves (chlorophyll and carotene), which plays a fundamental function in the physiological overall performance of plants [,]. Mg participates in a variety of biochemical and physiological processes that contribute to vine growth. It is a critical component of the chlorophyll molecule, affecting both its structure and function []. Foliar magnesium fertilization compensates for deficits in the vines’ growth stages. Additionally, it reflected the quantity and activity of photosynthetic pigments []. Mg is a mineral activator constituent of the chlorophyll molecule, which is responsible for photosynthetic regulation []. As a result, as compared to other Mg forms, the usage of Mg-NPs increased the chlorophyll components and carotene content []. Our findings corroborated those published in Table 1 and Table 2. This comparison most likely reflects Mg-NPs’ superior mobility and absorption capacity when compared to other forms [].
Chlorophylls are critical functional and structural cofactors for all photosynthetic pigment proteins involved in oxygenic and anoxic photosynthesis, and so magnesium fertilization throughout the growing season contributes to photosynthesis’s efficacy. The pigments’ distinctiveness is owing to the porphyritic chromophore’s extensive electron system, which chelates the Mg2+ ion in the center []. The results in Table 3 can be clarified by the variation in the Fv/Fm ratios of the various forms of foliar magnesium fertilization applied at various growth stages. In comparison to other forms, Mg-NPs dramatically boosted nucleic acid and carbohydrate enzymes []. However, the onset of magnesium deficit during the growing season may result in a reduction in chlorophyll and carotene levels []. Our findings established that Mg-NPs boosted photosynthetic pigment in comparison to other Mg forms, and our findings corroborated those of [].
Since magnesium is required for carbohydrate accumulation in plants, its absence has an effect on the overall biomass production and distribution among plant sections [,]. This shows that three major factors could influence Mg effects. These are the magnesium forms, mobility, and absorption capacity of magnesium []. Our data indicated that the Mg-NPs increased the leaf area and carbohydrate content of the shoots during the growing season, owing to the higher photosynthesis performance. We observed reduced values for ion leakage and MDA quantity when vines were treated with Mg-NPs compared to other types. One may argue that increasing magnesium absorption in nano form [] resulted in a reduction in the size of the cell wall, which was most likely because of its role in ion transport across the membrane and involvement in membrane-center ATPase activity [,]. This conclusion was consistent with previous research on citrus [], banana [], and coffee []. On the current experiment, we discovered a similar pattern of carbohydrate accumulation in vines stressed with evident leaf symptoms in the presence of a magnesium deficiency.
Normally, in plants, an element’s uptake and distribution are controlled by both its supply conditions and interactions with other elements []. Mg, K, and Ca have been considered to exhibit opposing interactions as cation ions. Mg absorption was restricted when K or Ca concentrations increased and vice versa [,]. However, under salinity stress, the application of Mg-NPs increases the content of macro and micro-nutrients (Table 5 and Table 6). This may be explained by the inaction between Ca++ and K+ and Mg++, which increased the abortion of both cations by using Mg-NPs more than other forms []. The achieved outcomes regarding the effect of foliar Mg forms on leaf mineral content proved that the magnesium nano form has a pronounced effect on micronutrient status. The results agree with the findings of []. In addition, the foliar magnesium fertilizer improved the leaf mineral content of the mentioned fruit crop species.
This could be explained by the fact that the Mg-NPs enhanced photosynthesis during the growth stages []. As a result, the carbohydrate content of the product increased []. Our findings established that Mg-NPs raised carbohydrate content more than other forms (Table 4) and wood-trimmed weight more than other forms (Table 8). However, Mg-NPs had a considerably greater effect on berry quality features than other treatments, as measured by SSC percent (17.50%), TA percent (0.805%), and SSC:TA ratio (21.63%) (Table 7). The lowest SSC:TA ratio observed with Mg-NPs application might be read as indicating that bunches collected from vines treated with other Mg forms had a significantly longer shelf life. Additionally, magnesium has a role in protein synthesis as a bridge element that aids in ribosome assembly []. Additionally, it catalyzes about 300 enzymes, including phosphoenolpyruvate carboxylase, glutathione synthase, phosphatases, kinases, RNA polymerases, and ATPases [].
A negative connection was detected between Chl B and Chl A:B. Chls:Caro and Fv/Fm were positively linked with Chl A:B but negatively with the other assets. Our observations were acknowledged by both parties [].
5. Conclusions
The outcomes of this research recommend that the Fv/Fm ratio during the growth season of ‘Superior Seedless’ vines may be a good tool to assess magnesium fertilization effects before visible deficiency symptoms appear. Mg-NPs are more effective at improving ‘Superior Seedless’ vine growth than the other magnesium forms. Moreover, a comparison validated that the application of different forms of Mg foliar fertilization for ‘Superior Seedless’ vines does affect the yield and berry quality at harvest time as a final determination of the impact of Mg foliar fertilization. Overall, Mg-NPs are the most effective form for application to ‘Superior Seedless’ vines when compared to other Mg forms under saline soil. It enhanced biochemical and bunched quality variables.
Author Contributions
Conceptualization, S.M.A.-Q.; Data curation, S.F.A.E.-E; Formal analysis, H.M.A.; Funding acquisition, N.A.A.-H.; Investigation, Z.A.A.; Methodology, S.F.A.E.-E.; Project administration, S.M.A.-Q.; Resources, Z.A.A.; Software, L.A.A., N.A.A.-H. and H.M.A.; Supervision, L.A.A. and M.A.A.; Writing—original draft, M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Relevant data applicable to this research are within the paper.
Acknowledgments
The author Lo’ay, A.A. extend their thanks, appreciation, and gratitude to Sally F. Abo EL-Ezz for their constructive cooperation during the research stages. This research is presented as a tribute to the soul of our dead colleague, Sally F. Abo EL-Ezz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wen, J.; Lu, L.-M.; Nie, Z.-L.; Liu, X.-Q.; Zhang, N.; Ickert-Bond, S.; Gerrath, J.; Manchester, S.R.; Boggan, J.; Chen, Z.-D. A new phylogenetic tribal classification of the grape family (Vitaceae). J. Syst. Evol. 2018, 56, 262–272. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 6 December 2021).
- Asghari, M.; Rezaei-Rad, R. 24-Epibrassinolide enhanced the quality parameters and phytochemical contents of table grape. J. Appl. Bot. Food Qual. 2018, 91, 226–231. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium Fertilization Improves Crop Yield in Most Production Systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Delfani, M.; Baradarn Firouzabadi, M.; Farrokhi, N.; Makarian, H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 530–540. [Google Scholar] [CrossRef]
- Monteiro, A.I.; Malheiro, A.C.; Bacelar, E.A. Morphology, Physiology and Analysis Techniques of Grapevine Bud Fruitfulness: A Review. Agriculture 2021, 11, 127. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sunderland, UK, 2010. [Google Scholar]
- Grigore, M.N.; Boscaiu, M.; Llinares, J.; Vicente, O. Mitigation of salt stress-induced inhibition of Plantago crassifolia reproductive development by supplemental calcium or magnesium. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 58–66. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Ma, W.; Wei, J.; Mao, Y.; Peng, Z.; Zhang, J.; Kong, X.; Han, Q.; Fan, W.; Yang, Y.; et al. Magnesium promotes root growth and increases aluminum tolerance via modulation of nitric oxide production in Arabidopsis. Plant Soil 2020, 457, 83–95. [Google Scholar] [CrossRef]
- Pang, J.-J.; Shin, J.-S.; Li, S.-Y. The Catalytic Role of RuBisCO for in situ CO2 Recycling in Escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 543807. [Google Scholar] [CrossRef]
- Jamali Jaghdani, S.; Jahns, P.; Tränkner, M. The impact of magnesium deficiency on photosynthesis and photoprotection in Spinacia oleracea. Plant Stress 2021, 2, 100040. [Google Scholar] [CrossRef]
- Jamali Jaghdani, S.; Jahns, P.; Tränkner, M. Mg deficiency induces photo-oxidative stress primarily by limiting CO2 assimilation and not by limiting photosynthetic light utilization. Plant Sci. 2021, 302, 110751. [Google Scholar] [CrossRef] [PubMed]
- Pogłodziński, R.; Barłóg, P.; Grzebisz, W. Effect of nitrogen and magnesium sulfate application on sugar beet yield and quality. Plant Soil Environ. 2021, 67, 507–513. [Google Scholar] [CrossRef]
- Laing, W.; Greer, D.; Sun, O.; Beets, P.; Lowe, A.; Payn, T. Physiological impacts of Mg deficiency in Pinus radiata: Growth and photosynthesis. New Phytol. 2000, 146, 47–57. [Google Scholar] [CrossRef]
- Ling, L.L.; Peng, L.Z.; Cao, L.; Jiang, C.L.; Chun, C.P.; Zhang, G.Y.; Wang, Z.X. Effect of magnesium deficiency on photosynthesis characteristic of Beibei 447 Jinchen orange. J. Fruit Sci. 2009, 26, 275–280. [Google Scholar]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.; Yin, L. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-deficient banana seedlings and remedy potential by foliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current Understandings on Magnesium Deficiency and Future Outlooks for Sustainable Agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Sattari Vayghan, H.; Nawrocki, W.J.; Schiphorst, C.; Tolleter, D.; Hu, C.; Douet, V.; Glauser, G.; Giovanni, F.; Croce, R.; Wientjes, E.; et al. Photosynthetic light harvesting and thylakoid organization in a CRISPR/Cas9 Arabidopsis thaliana LHCB1 knockout mutant. bioRxiv 2021, 12, 22–473855. [Google Scholar] [CrossRef]
- El-Fouly, M.; Rezk, A.I.; Nofal, O.; Abou El-Nour, E.-Z. Depletion of magnesium in Egyptian soils, its content in crops and estimated needs. Afr. J. Agric. Res. 2010, 5, 1060–1067. [Google Scholar]
- Esteves, E.; Maltais-Landry, G.; Zambon, F.; Ferrarezi, R.S.; Kadyampakeni, D.M. Nitrogen, Calcium, and Magnesium Inconsistently Affect Tree Growth, Fruit Yield, and Juice Quality of Huanglongbing-affected Orange Trees. HortScience 2021, 56, 1269–1277. [Google Scholar] [CrossRef]
- Hu, W.; Yang, B.; He, Z.; Li, G. Magnesium may be a key nutrient mechanism related to Fusarium wilt resistance: A new banana cultivar (Zhongjiao No. 9). PeerJ 2021, 9, e11141. [Google Scholar] [CrossRef] [PubMed]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Iron, magnesium, nitrogen and potassium deficiency symptom discrimination by reflectance spectroscopy in grapevine leaves. Sci. Hortic. 2018, 241, 152–159. [Google Scholar] [CrossRef]
- Mostafa, E.A.M.; Sakeg, M.M.S.; El-Migeed Abd, M.M.M. Response of banana plants to soil and foliar application of magnesium. Am. Eurasian J. Agric. Environ. Sci. 2007, 2, 141–146. [Google Scholar]
- Fawzi, M.; Shahin, F.; Elham, A.; Kandil, E. Effect of organic, biofertilizers and magnesium sulfate on growth, yield, chemical composition and fruit quality of “Le Conte” pear trees. Nat. Sci. 2010, 8, 273–280. [Google Scholar]
- Hanafy, A.H.; Khalil, M.K.; El-Rahman, A.A.; Nadia, A.M. Effect of magnesium, copper and growth regulators on growth, yield and chemical composition of Washington Navel orange trees. J. Appl. Sci. Res. 2012, 8, 1271–1288. [Google Scholar]
- El-Badawy, H.E.M. Implication of Using Potassium and Magnesium Fertilization to Improve Growth, Yield and Quality of Crimson Seedless Grapes (Vitis vinifera L). J. Plant Prod. Mansoura Univ. 2019, 10, 133–141. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Tomás-Barberán, F.A.; Artés, F. Modified atmosphere packaging preserves quality of SO2-free ’Superior seedless’ table grapes. Postharvest Biol. Technol. 2006, 39, 146–154. [Google Scholar] [CrossRef]
- Menora, N.; Joshi, V.; Kumar, V.; Vijaya, D.; Debnath, M.; Pattanashe, S.; Padmavatha, A.S.; Variath, M.; Biradar, S.; Khadakabhavi, S. Influence of Rootstock on Bud Break, Period of Anthesis, Fruit Set, Fruit Ripening, Heat Unit Requirement and Berry Yield of Commercial Grape Varieties. Int. J. Plant Breed. Genet. 2015, 9, 126–135. [Google Scholar] [CrossRef]
- Gowda, V.N.; Keshava, S.A.; Shyamalamma, S. Growth, yield and quality of Bangalore Blue grapes as influenced by foliar applied Polyfeed and Multi-K. Acta Hortic. 2008, 785, 207–212. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taha, N.A. Evaluation rachis browning phenomena of ‘Superior Seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. 2020, 261, 109040. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India (Pvt.) Ltd.: New Delhi, India, 1973. [Google Scholar]
- Black, C.A. Method of Soil Analysis Part 2. Chem. Microbiol. Prop. 1965, 9, 1387–1388. [Google Scholar]
- Christensen, L.P.; Peacock, W.L. Mineral nutrition and fertilization. In Raisin. Production Manual; Christensen, L.P., Ed.; University of California: Oakland, CA, USA, 2000; pp. 102–114. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- DeEll, J.R.; Toivonen, P.M.A. Chlorophyll fluorescence as a nondestructive indicator of broccoli quality during storage in modified atmosphere packaging. HortScience 2000, 35, 256–259. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; El-Ezz, S.F.A.; Awadeen, A.A. Effect of different foliar potassium fertilization forms on vegetative growth, yield, and fruit quality of kaki trees grown in sandy soil. Sci. Hortic. 2021, 288, 110420. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Krauss, S.; Schnitzler, W.H.; Grassmann, J.; Woitke, M. The Influence of Different Electrical Conductivity Values in a Simplified Recirculating Soilless System on Inner and Outer Fruit Quality Characteristics of Tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; Ameer, N.M. Performance of calcium nanoparticles blending with ascorbic acid and alleviation internal browning of ‘Hindi Be-Sennara’ mango fruit at a low temperature. Sci. Hortic. 2019, 254, 199–207. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Doaa, M.H. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Pregl, F. Quantitative Organic Micro Analysis, 4th ed.; J. and A. Churchill Ltd.: London, UK, 1945; p. 203. [Google Scholar]
- Snell, F.D.; Snell, C.T. Colorimetric Method of Analysis; D. Van Nostrand Company: Princeton, NJ, USA, 1967; pp. 551–552. [Google Scholar]
- Wilde, A.A.; Corey, R.B.; Lyer, J.G.; Voigt, G.K. Soil and Plant Analysis for Tree Culture, 3rd ed.; Oxford IBH Publishing Co.: New Delhi, India, 1985; pp. 64–115. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- El-Banna, M.F.; Lo’ay, A.A. Evaluation berries shattering phenomena of ‘Flame seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. 2019, 246, 51–56. [Google Scholar] [CrossRef]
- Kirkby, E. Chapter 1—Introduction, Definition and Classification of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 3–5. [Google Scholar]
- Härdter, R.; Rex, M.; Orlovius, K. Effects of different Mg fertilizer sources on the magnesium availability in soils. Nutr. Cycl. Agroecosys. 2004, 70, 249–259. [Google Scholar] [CrossRef]
- Tang, M.K.; Mohd, N.; Loong, S.G. Oil palm responses to different sources of magnesium on an inland reworked soil in Peninsular Malaysia. In Proceedings of the 2001 PIPOC International Palm Oil Congress (Agriculture), Kuala Lumpur, Malaysia, 20–23 August 2001; pp. 261–271. [Google Scholar]
- Jayaganesh, S.; Venkatesan, S.; Senthurpandian, V.K. Impact of different sources and doses of magnesium fertilizer on biochemical constituents and quality parameters of black tea. Asian J. Biochem. 2011, 6, 273–281. [Google Scholar] [CrossRef][Green Version]
- Jezek, M.; Geilfus, C.-M.; Bayer, A.; Mühling, K.-H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef]
- Ali, E.A.M.; Aml, R.M.; Yousef, D.; Ahmed, M.M.; Abd El-Hady, M. Influence of foliar applications of magnesium sources on improving nutrients status, yield and fruit quality of murcott mandarins. Middle East J. Appl. Sci 2017, 7, 361–372. [Google Scholar]
- Székely, A.; Balota, D.A.; Duchek, J.M.; Nemoda, Z.; Vereczkei, A.; Sasvari Szekely, M. Genetic factors of reaction time performance: DRD47 repeat allele associated with slower responses. Genes Brain Behav. 2011, 10, 129–136. [Google Scholar] [CrossRef]
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Mochizuki, N.; Tanaka, R.; Grimm, B.; Masuda, T.; Moulin, M.; Smith, A.G.; Tanaka, A.; Terry, M.J. The cell biology of tetrapyrroles: A life and death struggle. Trends Plant Sci. 2010, 15, 488–498. [Google Scholar] [CrossRef]
- Harmatys, K.M.; Overchuk, M.; Zheng, G. Rational Design of Photosynthesis-Inspired Nanomedicines. Acc. Chem. Res. 2019, 52, 1265–1274. [Google Scholar] [CrossRef]
- Popescu, M.; Popescu, G.C. Diurnal changes in leaf photosynthesis and relative water content of grapevine. Curr. Trends Nat. Sci. 2014, 3, 74–81. [Google Scholar]
- Wang, J.; Lu, W.; Tong, X.Y.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, W.; Mou, S.; Wang, X.; Chen, D.; Hu, X.; Chen, L.; Bai, J. Plant growth and development of pepper seedlings under different photoperiods and photon flux ratios of red and blue LEDs. Trans. Chin. Soc. Agric. Eng. 2017, 33, 173–180. [Google Scholar] [CrossRef]
- Peter, E.; Rothbart, M.; Oelze, M.L.; Shalygo, N.; Dietz, K.J.; Grimm, B. Mg protoporphyrin monomethylester cyclase deficiency and effects on tetrapyrrole metabolism in different light conditions. Plant Cell Physiol. 2010, 51, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, L.; Kania, A.; Myśliwa-Kurdziel, B.; Orzeł, Ł.; Stochel, G. Understanding chlorophylls: Central magnesium ion and phytyl as structural determinants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S. Notes and amendments to the recommendations on manuring of tea in South India. In Handbook of Tea Culture; Section-11; UPASI Tea Research Institute: Valparai, India, 2006. [Google Scholar]
- Stagnari, F.; Onofri, A.; Pisante, M. Response of French bean (Phaseolus vulgaris L.) cultivars to foliar applications of magnesium. Ital. J. Agron. 2009, 3, 101–110. [Google Scholar] [CrossRef]
- Takacs-Hajus, M.; Kiss, A.S. The effect of Mg-sulphate foliar fertilization on economic qualities of different garden pea varieties. Acta Agron. 2004, 363, 44–50. [Google Scholar]
- Roca, M.; Chen, K.; Pérez-Gálvez, A. 6-Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 125–158. [Google Scholar]
- Broadley, M.R.; Hammond, J.P.; King, G.J.; Astley, D.; Bowen, H.C.; Meacham, M.C.; Mead, A.; Pink, D.A.C.; Teakle, G.R.; Hayden, R.M. Shoot calcium and magnesium concentrations differ between subtaxa, are highly heritable, and associate with potentially pleiotropic loci in Brassica oleracea. Plant Physiol. 2008, 146, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Haiadi, Y. Effects of magnesium availability on the activity of plasma membrane ion transporters and light-induced responses from broad bean leaf mesophyll. Planta 2005, 221, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; El-Ezz, S.F.A. Performance of ‘Flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Meireles da Silva, D.; Brandão, I.R.; Alves, J.D.; de Santos, M.O.; de Souza, K.R.D.; de Silveira, H.R.O. Physiological and biochemical impacts of magnesium-deficiency in two cultivars of coffee. Plant Soil 2014, 382, 133–150. [Google Scholar] [CrossRef]
- White, P.J. Chapter 2-Ion Uptake Mechanisms of Individual Cells and Roots: Short-distance Transport. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 7–47. [Google Scholar]
- Jakobsen, S.T. Interaction between Plant Nutrients: III. Antagonism between Potassium, Magnesium and Calcium. Acta Agric. Scand. Sect. B—Soil Plant Sci. 1993, 43, 1–5. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6-Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Bianchi, D.; Grossi, D.; Simone Di Lorenzo, G.; Zi Ying, Y.; Rustioni, L.; Brancadoro, L. Phenotyping of the “G series” Vitis hybrids: First screening of the mineral composition. Sci. Hortic. 2020, 264, 109155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).