Survival of SARS-CoV-2 on Non-Porous Materials in an Experimental Setting Representative of Fomites
Abstract
1. Introduction
2. Materials and Methods
2.1. Inert and Activated Non-Porous Materials
2.2. Experimental Settings
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghinai, I.; McPherson, T.D.; Hunter, J.C.; Kirking, H.L.; Christiansen, D.; Joshi, K.; Rubin, R.; Morales-Estrada, S.; Black, S.R.; Pacilli, M.; et al. First Known Person-to-Person Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in the USA. Lancet Lond. Engl. 2020, 395, 1137–1144. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Bahl, P.; de Silva, C.M.; Chughtai, A.A.; MacIntyre, C.R.; Doolan, C. An Experimental Framework to Capture the Flow Dynamics of Droplets Expelled by a Sneeze. Exp. Fluids 2020, 61, 176. [Google Scholar] [CrossRef]
- Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. Available online: https://www.who.int/publications-detail-redirect/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 18 December 2020).
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T. Stability of SARS-CoV-2 on Critical Personal Protective Equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The Effect of Temperature on Persistence of SARS-CoV-2 on Common Surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in Different Environmental Conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Jin, X.; Tian, J.; Liu, J.; Mao, Y. Environmental Contamination by SARS-CoV-2 in a Designated Hospital for Coronavirus Disease 2019. Am. J. Infect. Control 2020, 48, 910–914. [Google Scholar] [CrossRef]
- Guo, Z.-D.; Wang, Z.-Y.; Zhang, S.-F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.-B.; Dong, Y.-Z.; Chi, X.-Y.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Coupeau, D.; Burton, N.; Lejeune, N.; Loret, S.; Petit, A.; Pejakovic, S.; Poulain, F.; Bonil, L.; Trozzi, G.; Wiggers, L.; et al. SARS-CoV-2 Detection for Diagnosis Purposes in the Setting of a Molecular Biology Research Lab. Methods Protoc. 2020, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic Inactivation of Influenza Virus by Titanium Dioxide Thin Film. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2012, 11, 1293–1298. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Ishihara, R.; Omiya, D.; Ishitsuka, M.; Hirano, S.; Suzuki, T. Application of a Photocatalyst as an Inactivator of Bovine Coronavirus. Viruses 2020, 12, 1372. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and UVC Radiation: Throwing Light on SARS-CoV-2. Viruses 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- EN 1096-2:2012—Glass in Building—Coated Glass—Part 2: Requirements and Test Methods for Class A, B and S Coatings. Available online: https://standards.iteh.ai/catalog/standards/cen/9f57ae4a-bee6-40cb-8af1-bb72f200d505/en-1096-2-2012 (accessed on 9 March 2021).
- Lavielle, M. Mixed Effects Models for the Population Approach: Models, Tasks, Methods and Tools; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-15718-9. [Google Scholar]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Sizun, J.; Yu, M.W.; Talbot, P.J. Survival of Human Coronaviruses 229E and OC43 in Suspension and after Drying Onsurfaces: A Possible Source Ofhospital-Acquired Infections. J. Hosp. Infect. 2000, 46, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Pyke, A.; Nair, N.; Yarlagadda, T.; Will, G.; Spann, K.; Yarlagadda, P.K.D.V. Antiviral Nanostructured Surfaces Reduce the Viability of SARS-CoV-2. ACS Biomater. Sci. Eng. 2020, 6, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Sheel, D.W. Photoactive and Antibacterial TiO2 Thin Films on Stainless Steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Bueckert, M.; Gupta, R.; Gupta, A.; Garg, M.; Mazumder, A. Infectivity of SARS-CoV-2 and Other Coronaviruses on Dry Surfaces: Potential for Indirect Transmission. Mater. Basel Switz. 2020, 13, 5211. [Google Scholar] [CrossRef]
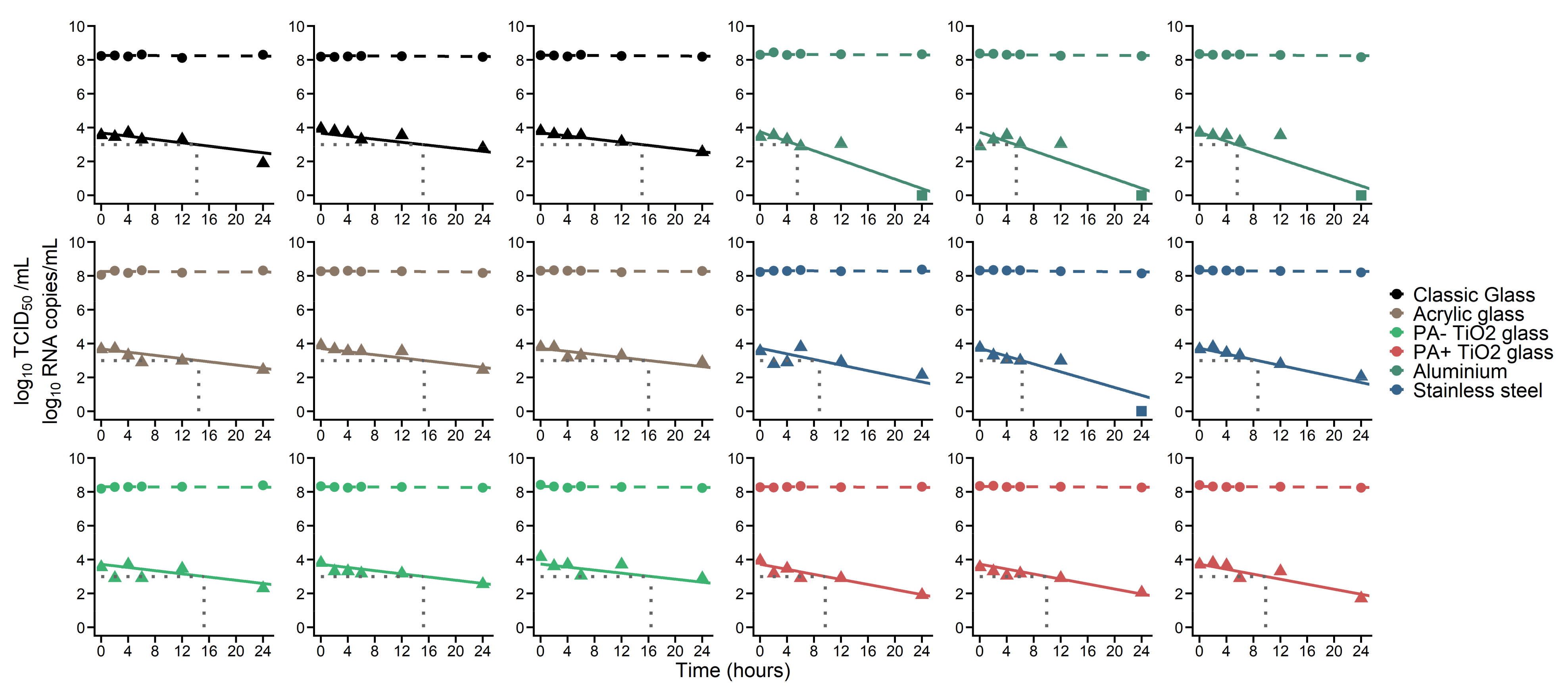
| Material | Half-Life (h) | Time to 90% Decrease of TCID50 (Median, h) | Time to Reach 1000 TCID50/mL (Median, h) |
|---|---|---|---|
| Classic glass | 6.9 1 | 21.6 | 15.2 |
| Acrylic glass | |||
| PA − TiO2 glass | |||
| PA + TiO2 glass | 4.1 | 13.5 | 9.8 |
| Aluminium | 2.3 | 7.3 | 5.5 |
| Stainless Steel | 3.5 | 11.9 | 8.7 |
| Viral dynamics parameters common to all materials | (RNA copies. mL−1) | c (d−1) | (%) |
| 5.89 | 0.0038 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonil, L.; Lingas, G.; Coupeau, D.; Lucet, J.-C.; Guedj, J.; Visseaux, B.; Muylkens, B. Survival of SARS-CoV-2 on Non-Porous Materials in an Experimental Setting Representative of Fomites. Coatings 2021, 11, 371. https://doi.org/10.3390/coatings11040371
Bonil L, Lingas G, Coupeau D, Lucet J-C, Guedj J, Visseaux B, Muylkens B. Survival of SARS-CoV-2 on Non-Porous Materials in an Experimental Setting Representative of Fomites. Coatings. 2021; 11(4):371. https://doi.org/10.3390/coatings11040371
Chicago/Turabian StyleBonil, Laura, Guillaume Lingas, Damien Coupeau, Jean-Christophe Lucet, Jérémie Guedj, Benoît Visseaux, and Benoît Muylkens. 2021. "Survival of SARS-CoV-2 on Non-Porous Materials in an Experimental Setting Representative of Fomites" Coatings 11, no. 4: 371. https://doi.org/10.3390/coatings11040371
APA StyleBonil, L., Lingas, G., Coupeau, D., Lucet, J.-C., Guedj, J., Visseaux, B., & Muylkens, B. (2021). Survival of SARS-CoV-2 on Non-Porous Materials in an Experimental Setting Representative of Fomites. Coatings, 11(4), 371. https://doi.org/10.3390/coatings11040371






