Abstract
Pd/B4C multilayers with 2.5 nm of d-spacing and thick Si and B4C capping layers were fabricated to study temporal stability under storage in different environments with relative humidity of 10% and 50%. The two stored samples were investigated using grazing incidence X-ray reflectometry (GIXR), X-ray scattering (XRS), an optical microscope, transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). The GIXR results showed that the reflectivity of the samples under 10% humidity and 50% humidity dropped by 3% and 8%, respectively, after 13 months. The optical microscope showed that the surface of the 10% humidity sample was smooth and undamaged, whereas the surface of the 50% humidity sample significantly eroded. TEM showed that the internal multilayer structure of the sample stored in 10% humidity was well protected by the capping layers. For the sample stored in 50% humidity, a major part of the Si and B4C capping layers were wrinkled and delaminated, and some surface layers of the multilayer structure were degraded with severe diffusion of boron. The XPS results showed a relatively large amount of oxygen in the B4C capping layer of the 50% humidity sample, and an obvious oxidation of the boron was found in the B4C capping layer and the surface of the multilayer. The severe oxidation and diffusion of boron and the delamination of the capping layers caused the degradation of the Pd/B4C multilayers stored in 50% humidity.
1. Introduction
X-ray monochromator is a key optical element used in synchrotron radiation light sources, astronomical telescopes, and other lab-based X-ray instruments. It can select the desired X-ray spectrum with an exact wavelength for spectroscopy, imaging, and other applications. The existing monochromators working in the hard X-ray region include crystal monochromators and multilayer monochromators. Crystals have excellent performance for narrow bandwidth and high-energy resolution, but the integral flux is limited. Compared with those of crystals, the bandwidths of multilayers are about two orders of magnitude larger [1]. Thus, the multilayer monochromator is a useful alternative to crystal monochromators when high flux is required rather than high-energy resolution [2,3,4]. It has been widely used in X-ray nano diffraction imaging analysis, fluorescence analysis, small angle scattering, and other applications [5,6,7].
Despite the optical performance, the lifetime of multilayer monochromators is a matter of great concern. Given the small d-spacing (total thickness of each bilayer) of only a few nanometers, the surface and internal structure of a multilayer can significantly change with time under the exposure to ambient environment or irradiation [8,9,10]. It can cause significant degradation of the performance of the monochromator.
Pd/B4C multilayers have a high reflectance in the 6–20 keV region. It has been used in several synchrotron radiation light sources, e.g., the micro-imaging beamline in ANKA [11,12] and the protein crystallography beamline in Advanced Light Source [13]. However, it is not very stable after exposure to an ambient environment. Morawe et al. [14,15] reported the temporal stability of Pd/B4C multilayers, which were prepared via pure Ar sputtering. The study found that if the d-spacing was less than 4 nm, the multilayer with Pd as the topmost layer would dramatically deteriorate after a few days stored under atmospheric and room temperatures. The reason was found to be the surface oxidation of boron, especially under the catalysis effect of Pd. Morawe also improved the stability of the Pd/B4C multilayer by optimizing the cap layer. A B4C capping layer with a thickness of 1.4 to 4.7 nm could improve the lifetime of a multilayer up to two weeks or even up to three months. Multilayers with a Si(O2) cap showed the weakest and slowest evolution with time. After two years of storage, multilayers with thick Si(O2) caps still produced well-ordered Bragg peaks. Huang et al. [16] studied the temporal stability of the nitridated Pd/B4C multilayers with relatively large d-spacing of 5 nm and found that the reflectivity of the nitridated Pd/B4C multilayers decreased rapidly within a half-year in storage conditions with 20% humidity and at room temperature, which was even worse than the pure Ar-made sample. The sample deposited with a mixture gas of 96% Ar and 4% N2 degraded faster than the sample deposited with 15% N2.
Humidity is an important cause of the degradation of nanoscale multilayers [8]. Although the temporal stability of ultrathin Pd/B4C multilayers can be improved by adding capping layers, the effect of humidity on multilayers (with or without capping layers) has not yet been reported. This is important for the long-term application and storage of such multilayer mirrors. On the other hand, our group recently developed Pd/B4C multilayers (d-spacing equal to 2.5 nm) by using heavier Kr sputtering ions, and we compared their layer structures and X-ray performance with the conventional ones fabricated by Ar sputtering. The Kr-sputtered multilayer also showed a high reflectance with less incorporation of sputtering gas atoms in the multilayers [17]. In this work, we further studied the temporal stability of the Kr-sputtered multilayer to check its feasibility for applications. A long-time investigation of the multilayer structure and its X-ray performance under storage in different humidity environments was performed. Even with B4C and Si capping layers, we found that the Kr-sputtered Pd/B4C multilayers still began to degrade after three months if stored in an air environment with 50% humidity. The one stored in a 10% humidity environment remained almost intact after 13 months. The physical reasons for the degradation were also studied and discussed.
2. Materials and Methods
Pd/B4C multilayers were fabricated using a linear deposition facility [18] based on a direct current (DC) magnetron sputtering technique (designed by our group). The base pressure used in the deposition chamber was 9.6 × 10−5 Pa. High-purity Krypton (99.997%) was used as the working gas with a sputtering pressure of 0.10 Pa. The multilayers were deposited on super polished Si wafers with a surface roughness of ~0.2 nm. The multilayer was designed with a d-spacing of 2.50 nm and a thickness ratio (layer thickness of B4C to d-spacing) of ∼0.5. In order to obtain the saturated reflectivity of the multilayer, 150 bilayers were deposited. We added two thick B4C and Si layers on top of the multilayer to improve its stability. The B4C layer with 28 nm thickness was first deposited by Kr working gas on top of the multilayer, and the Si layer with 23 nm thickness was deposited by Ar on top of B4C. It only induced about a 1.7% (absolute) reduction of the theoretical reflectance of the multilayers, as calculated by IMD software (Version:5.0) [19]. Two identical Pd/B4C multilayers were fabricated according to the design parameters. Both samples were stored inside the clean room. One sample was further stored in a low-humidity cabinet and the relative humidity was controlled automatically to stabilize at around 10% (S1 sample). The other sample was stored in a normal cabinet and its humidity was the same as the clean room, which fluctuated from 45% to 55% over the storage time. For simplicity, we describe the relative humidity of the storage condition of this sample as 50% (S2 sample). Both samples were stored at room temperature. The two humidity values were selected to mimic the real storage conditions of the multilayer mirror.
The samples’ layer structure was measured via grazing incidence X-ray reflectometry and X-ray scattering (X-ray diffractometer, Bede D1, UK) at the Cu Ka emission line (λ = 0.154 nm) during the storage period. The scattering measurements were performed using the detector scan mode at a fixed incidence angle, which corresponded to the 1st Bragg peak angular position. The surface of the two samples was observed using an optical microscope after 13 months of storage. In order to further obtain the morphology and internal structure of the multilayers, the multilayer sample was characterized using transmission electron microscopy (TEM). The samples were prepared with the focused ion beam (FIB) technique and the measurements were performed with Talos F200X equipment (FEI, Hillsboro, OR, USA). High-resolution bright field images and selected area electron diffraction (SAED) patterns were taken to study the layer structure. Electron energy loss spectroscopy (EELS) was used to analyze the depth distribution of different elements. In order to study the elemental composition and their chemical states of the two multilayers quantitatively, an X-ray photoelectron spectroscopy (XPS) depth-profile analysis was performed. The multilayers were etched by 2 keV Ar+ ions and the excitation energy was 1.486 keV (Al Kα emission line). The scanning area was 400 × 400 μm2. CasaXPS software (Version:2.3.16) was used for fitting. A Shirley background type was adopted.
3. Results
3.1. GIXR Measurements
To monitor the changes, GIXR measurements were performed at different times for both samples during their 13 months of storage. The measurement results are depicted as Figure 1. The general trend of the GIXR curves, including the angular positions of different Bragg peaks for both multilayers, remained almost the same for 13 months. This indicates that the main part of the periodic layer structure was mostly unchanged for the two samples. However, the variation of the first order peak reflectivity was different for the two samples, as shown in Figure 2. For the S1 sample, the reflectivity did not change within seven months, considering the measurement error. From seven months to 13 months, its reflectance dropped by about 3%. For the S2 sample, the reflectivity was maintained for the first three months. From three months to seven months, a small decrease was observed. The degradation became faster after seven months, which eventually showed an 8% drop in reflectance after 13 months of storage. This means that humidity was an important factor affecting the temporal stability of the Pd/B4C multilayers, even with thick capping layers. Considering the diminished oscillations between the total reflection area and the 1st Bragg peak of the GIXR curves, especially for the S2 sample, the degradation can occur mostly in the surface layers.
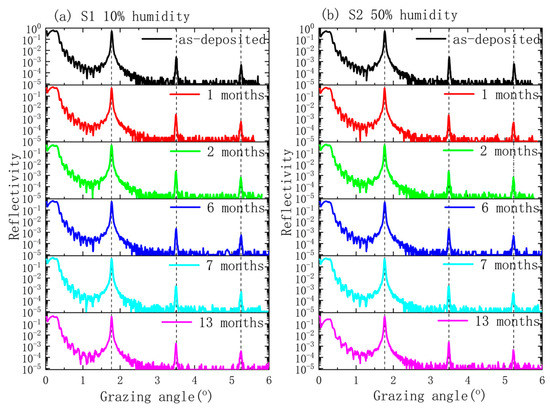
Figure 1.
X-ray reflectometry (GIXR) curves of the Pd/B4C multilayers: (a) sample S1 stored at 10% humidity and (b) sample S2 stored at 50% humidity.
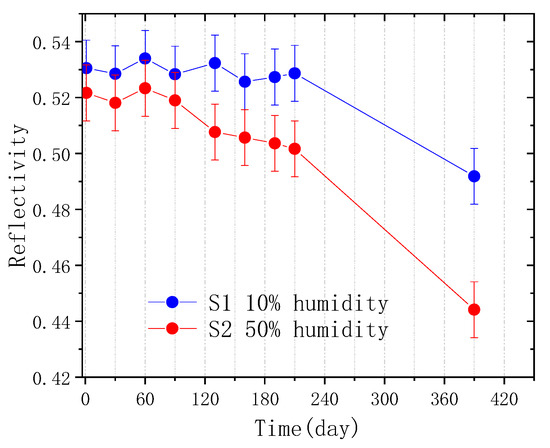
Figure 2.
The changes in the 1st order peak reflectance in the two Pd/B4C multilayers with storage time.
3.2. Surface Morphology Measured Using an Optical Microscope
The degradation effect can be seen more directly from the surface morphology. Optical microscope images of the two Pd/B4C multilayers after 13 months of storage are displayed in Figure 3. The S1 sample showed an intact and smooth surface (Figure 3a). However, severe corrosion with cracking and wrinkling of the layers was observed on the surface of the S2 sample, and some surface layers even fell off (Figure 3b). These damage spots began to appear after about three months of storage, and became more and more severe with time.
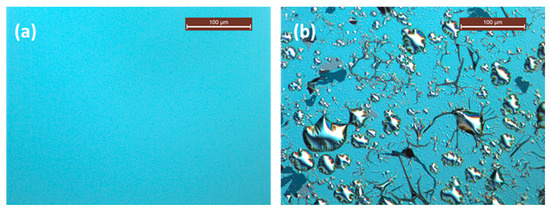
Figure 3.
Optical microscope image for the two samples: (a) sample S1 stored at 10% humidity and (b) sample S2 stored at 50% humidity.
3.3. X-ray Scattering Measurements
The scattering results for the two samples are shown in Figure 4, where the scattering angle was counted from the multilayer surface. For the S1 sample, the scattering curve after 13 months of storage was basically the same as the as-deposited state, including the scattering wings from non-conformal roughness and the resonant non-specular scattering (RNS) peak. This means that the interface roughness remained unchanged. For the S2 sample, the scattering intensity near the specular reflection peak significantly increased, and several small peaks appeared besides the RNS peak. These may have been caused by severe corrosion in the surface layers.
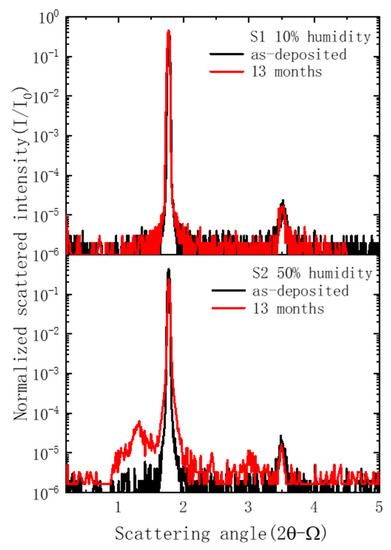
Figure 4.
X-ray scattering measurement results of the two Pd/B4C multilayers.
3.4. TEM Characterization of Internal Multilayer Structures
The sample cross-sections were observed via TEM to thoroughly characterize the internal structure changes of the multilayers and capping layers after 13 months of storage. The TEM and SAED images of the S1 sample are shown in Figure 5a–d. Figure 5a,b shows the Si and B4C protective layers, wherein the multilayer remained intact. The high-resolution bright field image in the middle of the Pd/B4C multilayers is shown in Figure 5c, where the Pd layers appear dark, and the B4C layers bright. The multilayer exhibited a clear periodic layered structure with sharp interfaces. The measured d-spacing was around 2.56 nm, which was the same as the GIXR. Figure 5d shows that there was a weak diffraction ring that corresponded to Pd (111) [16,17]. This means that the Pd layers in the multilayer had weak polycrystallization.
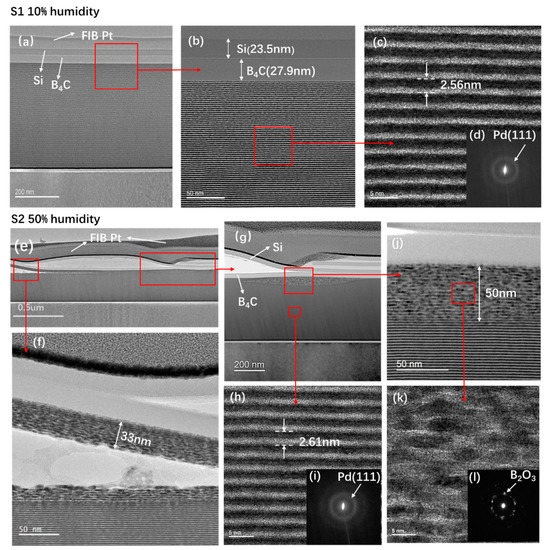
Figure 5.
Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) images of the Pd/B4C multilayers stored at 10% humidity and 50% humidity after 13 months. Figures (a–d) are the S1 sample at 10% humidity, whereas Figures (e–l) are the S2 sample at 50% humidity.
The TEM and SAED images of the S2 sample are shown in Figure 5e–l. A damaged area was specifically selected for the observation. Figure 5e shows the bright field view of the multilayer, and it can be seen that the Si and B4C capping layers were significantly degraded. A major part was delaminated from the multilayer and even cracked into several parts. In some areas, the surface multilayer was damaged and delaminated with the capping layers. The damaged areas of the multilayer were further magnified and shown in Figure 5f–k. Figure 5g shows an initially degraded area with a maximum 50 nm thickness, i.e., ~20 bilayers affected at the surface. The surface multilayer in Figure 5f also detached from the bottom stack. Some blisters were even observed in the detached area, which indicates that delamination can be caused by severe diffusion of gaseous atoms.
In order to study the changes in the damaged area of the multilayers, a high-resolution image of the damaged area is shown in Figure 5k. The layers were heavily crystallized with relatively large grains randomly distributed, and the interfaces completely disappeared. The SAED image of the damaged layers is shown in Figure 5l. Strong diffraction spots can be observed, which mainly corresponded to B2O3. This is consistent with the Morawe’s result that boron atoms in the surface layers oxidized and formed B2O3 crystals. Nevertheless, below the surface degraded layers (Figure 5h), the multilayer structure was still intact with smooth and sharp interfaces. The SAED image of the internal multilayer (Figure 5i) also showed a weak diffraction ring similar to the multilayers of the S1 sample. It is worth noting that these TEM images were specifically measured in the damaged area, and the film in the undamaged area of the S2 sample was still intact. This explains the relatively small drop of reflectance and minor changes in the GIXR result of S2.
To study the physical reason for structural deterioration, the elemental distribution in the intact and degraded area of the S2 sample was measured using EELS analysis in the TEM setup. Several line scans were performed continuously from the intact layers to the surface degraded area of the multilayer to study the relative changes. Figure 6a shows the TEM image of the near surface area of the S2 sample, as well as the number (20) of the degraded bilayers. Figure 6b shows an EELS line scan performed along the red line area indicated by the arrow shown in Figure 6a. Given the lack of standard calibration in the sample, the absolute concentration of different elements could not be determined. Nevertheless, the relative variations of signal intensity of each element with depth were deemed to be reliable. In the good layer structure area, clear oscillations of Pd, C, and B profiles were observed, wherein the depth distributions of C and B were the same and that of Pd was the opposite. In the degraded area, the periodic oscillation disappeared and a relative constant concentration with random fluctuations over the depth was observed for different elements, indicating severe interdiffusion. The B content in the degraded area was significantly reduced compared to the good area, which implied that the B atoms had diffused to other areas. This could play an important role in the degradation process. The reduced B content in the damaged area was also observed in the nitridated Pd/B4C multilayers after several months of aging [16]. The oxygen content in the damaged area seemed to slightly increase compared to the good area, which is further discussed in the following section.
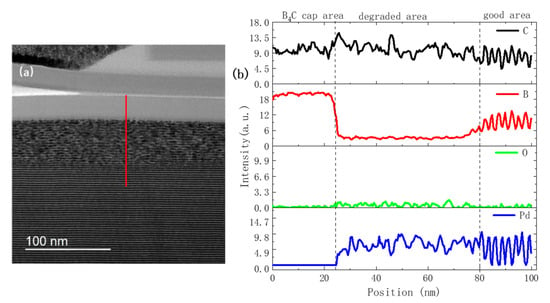
Figure 6.
TEM image (a) and electron energy loss spectroscopy (EELS) line scan (b) of the surface area of the 50% humidity multilayer after 13 months of storage.
3.5. XPS Measurement and Analysis
The exact content and chemical state of different elements in the surface area for the two samples were further studied via XPS. Besides the surface area, we analyzed three different depth positions etched from the surface (10 nm (middle of Si protective layer), 35 nm (middle of B4C protective layer), and 54 nm (first layers of Pd/B4C multilayers)). Figure 7a–e shows the atomic concentration of Si, B, Pd, C, and O at different depths.
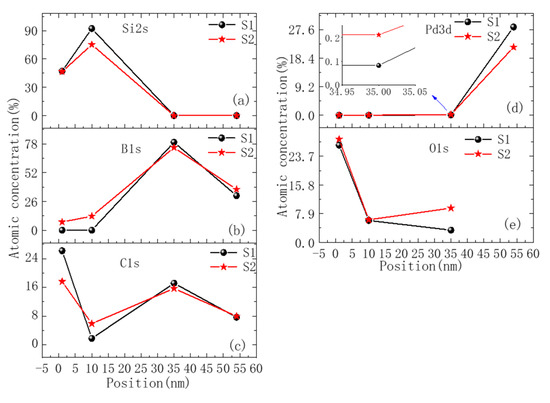
Figure 7.
The relative content distribution of each element along the depth of the sample as measured using XPS. Figures (a–e) are the relative contents of Si, B, C, Pd, and O, respectively.
For the surface area, there was no B element detected for the S1 sample, whereas a relatively large B content of 7.5% was found for the S2 sample. There could be two reasons for this. First, there could have been a cracking area in which the surface layer peeled off and the bottom B4C capping layer or multilayer was exposed, thus providing the B signal. Second, a small part of B atoms might have diffused from the B4C capping layer or even the multilayer upward to the outmost surface. For the carbon concentration, it is difficult to compare two samples given the natural adhesion of hydrocarbon on the surface. No Pd element was detected in the surface of both samples.
A similar trend was found in the Si capping layer. For the S1 sample, no B element was detected, whereas the concentration of B in the Si capping layer of the S2 sample was 12.7%. The C content at the S2 sample position was 5.9%, which was larger than the S1 sample (1.8%). No Pd element was detected in the Si capping layer of either sample.
For the middle position inside the B4C capping layer, the atomic concentration of the B and C elements in the S2 sample was slightly less than the S1 sample. This can indicate a stronger diffusion of B and C in the S2 sample and explains the higher content of B and C in the Si layer. The oxygen content in the S2 sample was 9.4%, which was much higher than the value of 3.3% in the S1 sample. No Si element was detected in either sample, indicating that the Si element is not the main diffusing species.
For the position at the multilayer surface, as the Pd3p spectral peak overlaps with the O1s peak [20], it is difficult to quantitatively analyze O and Pd content. The relative content for B content in the S2 sample was slightly larger than the S1 sample. This was not consistent with the EELS result, which could be due to the intermixing effect induced by ion etching during XPS measurements, which changed the relative content of elements on the multilayer’s surface.
The B1 peaks measured at the B4C capping layer and multilayer positions of the two samples were further fitted and shown in Figure 8. For the S1 sample, the B1 peak of the B4C capping layer is shown in Figure 8a. Its peak was located at 188.1 eV [21,22,23], which corresponded to the B–C bond in the B4C icosahedral unit structure [24,25]. It also contained a small peak at 190.5 eV, which likely corresponded to the B–C bond in the C–B–C chains that connected the icosahedral [22,23] or the B–O bond [26]. The B1s peak of the multilayer is shown in Figure 8b. The chemical state of B in the multilayer was similar to the B4C capping layer, whereas the peak at 190.5 eV was a little enhanced.
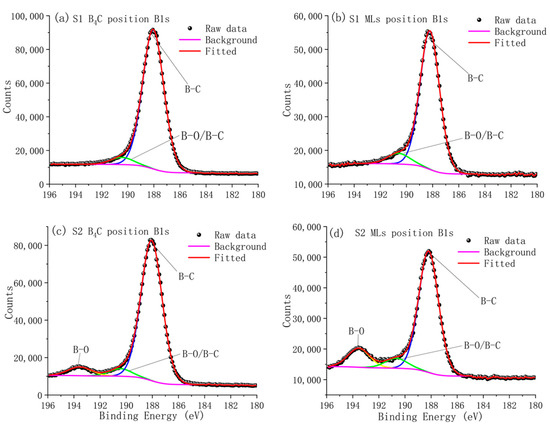
Figure 8.
Measured and fitted B1s photoelectron spectra from the S1 and S2 sample. Figures (a,c) show the chemical state of boron in the B4C capping layer in samples S1 and S2, respectively. Figures (b,d) show the chemical state of boron in the multilayer in samples S1 and S2, respectively.
For the S2 sample, the B1s peak in the B4C capping layer is shown in Figure 8c. A new peak appeared at the binding energy of 193.5 eV, which corresponded to B–O bond in the B2O3 [27,28]. The B1s peak of the multilayer is shown in Figure 8d. The peak at 193.5 eV was even stronger, indicating a further enhanced oxidation of B. This was also consistent with the presence of B2O3, as observed by SAED in the damaged area. According to the fitting results, about 6% of boron atoms in the B4C layer reacted with oxygen and formed B2O3 while the percentage in the multilayer was about 13%. This could be explained by the much higher content of Pd in the multilayer, which could further enhance the oxidation of boron due to its catalytic effect [15].
4. Discussion
Based on the above characterization results, a tentative explanation for the degradation of the Pd/B4C multilayer stored in a 50% humidity environment is provided as follows. Despite the presence of B4C and Si protective layers, during 13 months of long-term storage in high humidity conditions, a relatively large amount of reactive oxygen atoms and ions, as compared to the sample stored in 10% humidity environment, slowly permeated through the first Si layer, reaching the B4C capping layer and the surface of the multilayer [29]. This was evidenced by the significantly higher content of oxygen in the B4C capping layer of the S2 sample, as measured via XPS. The higher concentration of oxygen can significantly increase the reaction rate of boron with oxygen and form boron oxide, as seen in Figure 8c,d. The oxidation could be further enhanced by the catalytic effect of Pd. Part of the boron atoms also diffused upwards, driven by the oxidation process [30]. Moreover, the oxidation and diffusion seemed to cause increased stress at the surface [31], leading to a large amount of wrinkling and delamination of capping layers from the surface, as observed in the TEM and optical microscopes. The delamination created more channels for oxygen diffusion, which could make the degradation more severe. Nevertheless, despite the severe degradation of the capping layers, the multilayer structure underneath was not significantly changed except for a few areas at the surface. Therefore, a relatively small drop of reflectivity was observed in the GIXR measurements after 13 months. These results show that even with a thick Si and B4C capping layer, the Pd/B4C multilayers could not be fully protected under a high-humidity (50%) environment. Lower humidity (10%) was more favorable for storage. On the other hand, the capping layers’ cracking and delamination issues required more investigations and could be further optimized. It is worth noting that the Pd/B4C multilayers used in this paper were fabricated via Kr gas instead of conventional Ar. According to our previous analysis, the Kr-sputtered Pd/B4C multilayer had stronger polycrystallization and a larger amount of grain boundaries, which could further facilitate oxygen and boron diffusion. To confirm this, a detailed comparison of the stability of multilayers deposited by Kr and Ar would be needed.
5. Conclusions
Pd/B4C multilayers with capping layers of B4C and Si were fabricated via the Kr sputtering process and stored under 10% (S1) and 50% (S2) humidity conditions, respectively. After 13 months, the reflectance of the S1 and S2 samples decreased by 3% and 8%, respectively. Severe corrosion was observed on the S2 sample’s surface, whereas the S1 sample’s surface remained intact. TEM measurements found that after 13 months of storage, a major part of the B4C and Si protective layers of the S2 sample cracked and delaminated from the surface, and a few areas of the surface multilayer were degraded. Severe intermixing and the formation of B2O3 nanocrystals were observed in the degraded multilayer structure. According to the XPS analysis results, oxygen diffusion through the capping layer and a possible upward diffusion of boron together with carbon were indicated in the S2 sample. A new compound B2O3 formed in the B4C capping layer and the surface layers of the multilayer of the S2 sample. In the high-humidity environment, the strong diffusion and oxidation, as well as the delamination of the capping layers, contributed together to the sample’s deterioration. It was proven that even with thick Si and B4C capping layers, the ultrathin Pd/B4C multilayer could not be stored and applied in an environment with relative humidity above 50% for a long time. This work demonstrated a comprehensive understanding of the degradation process for the ultrathin Pd/B4C multilayers stored in high-humidity conditions. It also provided useful guidance for the future development and application of this and other related multilayer structures.
Author Contributions
Conceptualization, G.L., Q.H., and Z.W.; methodology, Q.H.; software, G.L. and Q.H.; validation, Q.H. and Z.W.; formal analysis, G.L.; investigation, G.L., R.Q., H.N., and Z.Z.; resources, Q.H., Z.Z., and Z.W.; data curation, G.L. and Y.F.; writing original draft preparation, G.L.; writing review and editing, Q.H.; visualization, G.L.; supervision, Q.H., Z.Z., and Z.W.; project administration, Q.H.; funding acquisition, Q.H. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC), grant number 61621001,12075170; the National Key R&D Program of China, grant number 2016YFA0401304; the Shanghai Rising Star Program, grant number 19QA1409200; and Major projects of Science and Technology Commission of Shanghai, grant number 17JC1400800.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ziegler, E. Multilayers for high heat load synchrotron applications. Opt. Eng. 1995, 34, 445–452. [Google Scholar] [CrossRef]
- Kazimirov, A.; Smilgies, D.M.; Shen, Q.; Xiao, X.; Hao, Q.; Fontes, E.; Bilderback, D.H.; Gruner, S.M.; Platonov, Y.; Martynov, V.V. Multilayer X-ray optics at CHESS. J. Synchrotron Radiat. 2006, 13, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Morawe, C. Graded multilayers for synchrotron optics. AIP Conf. Proc. 2006, 879, 764. [Google Scholar]
- Morawe, C. Multilayer based X-ray optics at the ESRF. In Synchrotron Radiation Instrumentation (SRI); AIP: Taipei, Taiwan, 2018; p. 060002. [Google Scholar]
- Rack, A.; Weitkamp, T.; Riotte, M.; Rack, T.; Dietsch, R.; Holz, T.; Krämer, M.; Siewert, F.; Meduna, M.; Morawe, C.; et al. Micro-imaging performance of multilayers used as monochromators for coherent hard X-ray synchrotron radiation. In Advances in X-Ray/EUV Optics and Components; SPIE: San Diego, CA, USA, 2010; p. 78020M. [Google Scholar]
- Knoth, J.; Prange, A.; Schneider, H.; Schwenke, H. Variable X-ray excitation for total reflection X-ray fluorescence spectrometry using an Mo/W alloy anode and a tunable double multilayer monochromator. Spectrochim. Acta Part B 1997, 52, 907–913. [Google Scholar] [CrossRef]
- Hexemer, A.; Bras, W.; Glossinger, J.; Schaible, E.; Gann, E.; Kirian, R.; MacDowell, A.; Church, M.; Rude, B.; Padmore, H. A SAXS/WAXS/GISAXS beamline with multilayer monochromator. In Journal of Physics: Conference Series, Proceedings of the XIV International Conference on Small-Angle Scattering (SAS09), Oxford, UK, 13–18 September 2009; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 247, p. 012007. [Google Scholar]
- Zhu, J.T.; Song, Z.Q.; Ding, T.; Ma, S.; Li, H. Stability of Mg/SiC,Mg/Co EUV multilayer. Opt. Precis. Eng. 2013, 21, 1380–1386. [Google Scholar]
- Windt, D.L.; Gullikson, E.M. Pd/B4C/Y multilayer coatings for extreme ultraviolet applications near 10 nm wavel. Appl. Opt. 2015, 54, 5850–5860. [Google Scholar] [CrossRef]
- Feng, J.; Huang, Q.; Qi, R.; Xu, X.; Zhou, H.; Huo, T.; Giglia, A.; Yang, X.; Wang, H.; Zhang, Z.; et al. Stability of Cr/C multilayer during synchrotron radiation exposure and thermal annealing. Opt. Express 2019, 27, 38493. [Google Scholar] [CrossRef]
- Rack, A.; Weitkamp, T.; Trabelsi, S.B.; Modregger, P.; Cecilia, A.; dos Santos Rolo, T.; Rack, T.; Haas, D.; Simon, R.; Heldele, R.; et al. The micro-imaging station of the TopoTomo beamline at the ANKA synchrotron light source. Nucl. Instrum. Methods Phys. Res. 2009, 267, 1978–1988. [Google Scholar] [CrossRef]
- Rack, A.; Riesemeier, H.; Vagovič, P.; Weitkamp, T.; Siewert, F.; Dietsch, R.; Diete, W.; Trabelsi, S.B.; Waterstradt, T.; Baumbach, T. Fully automated fixed exit in vacuum double-multilayer monochromator for synchrotron-based hard X-ray micro-imaging applications. AIP Conf. Proc. 2009, 1234, 734–737. [Google Scholar]
- Zwart, P.; Taylor, J.; Morton, S.; Cayford, R.; Fontenay, G.; Allaire, M.; Sankaran, B.; Dickert, J.; Royal, K.; Rozales, A.; et al. The berkeley center for structural biology at the advanced light source. Synchrotron Radiat. News 2015, 28, 22–27. [Google Scholar] [CrossRef]
- Morawe, C.; Supruangnet, R.; Peffen, J.C. Structural modifications in Pd/B4C multilayers for X-ray optical applications. Thin Solid Film. 2015, 588, 1–10. [Google Scholar] [CrossRef]
- Supruangnet, R.; Morawe, C.; Peffen, J.C.; Nakajima, H.; Rattanasuporn, S.; Photongkam, P.; Jearanaikoon, N.; Busayaporn, W. Chemical modification of B4C cap layers on Pd/B4C multilayers. Appl. Surf. Sci. 2016, 367, 347–353. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Yi, Q.; Kozhevnikov, I.V.; Qi, R.; Wen, M.; Jonnard, P.; Zhang, J.; Giglia, A.; Zhang, Z.; et al. Nitridated Pd/B4C multilayer mirrors for soft X-ray region: Internal structure and aging effects. Opt. Express 2017, 25, 7749. [Google Scholar] [CrossRef]
- Ni, H.; Huang, Q.; Liu, G.; Qi, R.; Zhang, Z.; Li, X.; Li, Z.; Wang, J.; Wang, Z. Comparative study of Pd/B4C X-ray multilayer mirrors fabricated by magnetron sputtering with Kr and Ar gas. Materials 2020, 13, 4504. [Google Scholar] [CrossRef]
- Ni, H.; Huang, Q.; Shi, Y.; Qi, R.; Zhang, Z.; Feng, Y.; Xu, X.; Zhang, Z.; Wang, J.; Li, Z.; et al. Development of large-size multilayer mirrors with a linear deposition facility for X-ray applications. Opt. Eng. 2019, 58, 1. [Google Scholar] [CrossRef]
- Windt, D.L. IMD-Software for modeling the optical properties of multilayer films. Comput. Phys. 1998, 12, 360–370. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J.C. XPS, AES and Auger parameter of Pd and PdO. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Postel, O.; Heberlein, J. Deposition of boron carbide thin film by supersonic plasma jet CVD with secondary discharge. Surf. Coat. Technol. 1998, 108, 247–252. [Google Scholar] [CrossRef]
- Ling, H.; Wu, J.D.; Sun, J.; Shi, W.; Ying, Z.F.; Li, F.M. Electron cyclotron resonance plasma-assisted pulsed laser deposition of boron carbon nitride films. Diam. Relat. Mater. 2002, 11, 1623–1628. [Google Scholar] [CrossRef]
- Zhou, M.J.; Wong, S.F.; Ong, C.W.; Li, Q. Microstructure and mechanical properties of B4C films deposited by ion beam sputtering. Thin Solid Film. 2007, 516, 336–339. [Google Scholar] [CrossRef]
- Vast, N.; Lazzari, R.; Besson, J.M.; Baroni, S.; Dal Corso, A. Atomic structure and vibrational properties of icosahedral B4C boron carbide. Comput. Mater. Sci. 2000, 17, 127–132. [Google Scholar] [CrossRef]
- Jiménez, I.; Sutherland, D.G.J.; Van Buuren, T.; Carlisle, J.A.; Terminello, L.J.; Himpsel, F.J. Photoemission and X-ray-absorption study of boron carbide and its surface thermal stability. Phys. Rev. B 1998, 57, 13167. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Yang, Y.; Qi, R.; Feng, Y.; Kozhevnikov, I.V.; Li, W.; Zhang, Z.; Jiang, H.; Zhang, L.; et al. Nitridated Ru/B4C multilayer mirrors with improved interface structure, zero stress, and enhanced hard X-ray reflectance. Opt. Express 2018, 26, 21803. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/EngElmSrchQuery.aspx?EType=PE&CSOpt=Retri_ex_dat&Elm=B (accessed on 20 December 2020).
- Cermignani, W.; Onneby, C.; Pantano, C.G.; Paulson, T.E. Synthesis and characterization of boron-doped carbons. Carbon 1995, 33, 367–374. [Google Scholar] [CrossRef]
- Li, Y.Q.; Qiu, T. Oxidation behaviour of boron carbide power. Mater. Sci. Eng. A 2007, 444, 184–191. [Google Scholar] [CrossRef]
- Taniguchi, K.; Kurosawa, K.; Kashiwagi, M. Oxidation enhanced diffusion of boron and phosphorus in (100) silicon. J. Electrochem. Soc. 1980, 127, 2243–2248. [Google Scholar] [CrossRef]
- Windt, D.L.; Donguy, S.; Seely, J.; Kjornrattanawanich, B. Experimental comparison of extreme-ultraviolet multilayers for solar physics. Appl. Opt. 2004, 43, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).