Lubricating Properties of Cyano-Based Ionic Liquids against Tetrahedral Amorphous Carbon Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Lubricants and Materials
2.2. Sliding Tests
2.3. Raman Spectroscopy
2.4. MALDI-TOF/MS Analysis
2.5. Thermogravimetric Analysis
3. Results
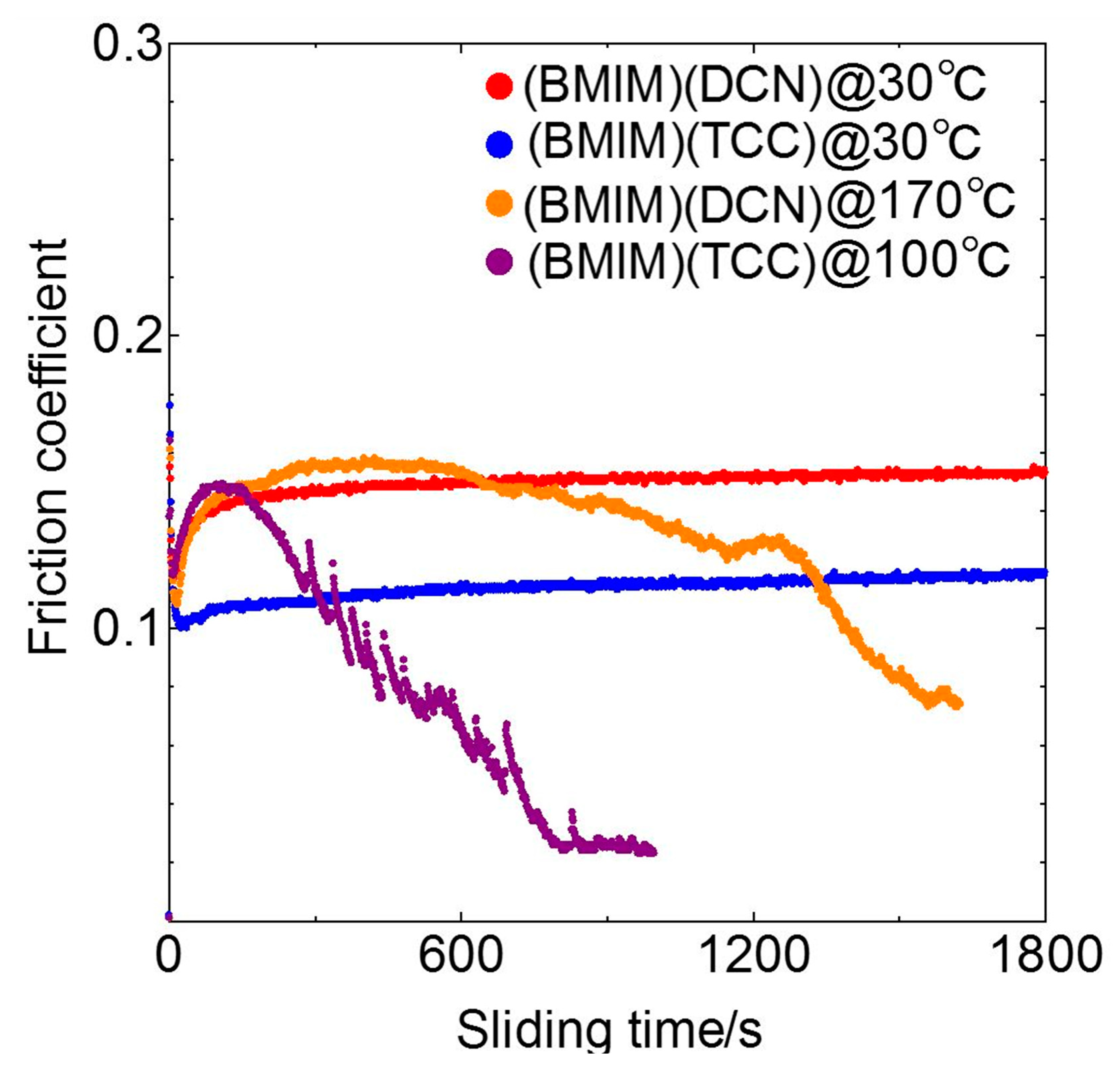
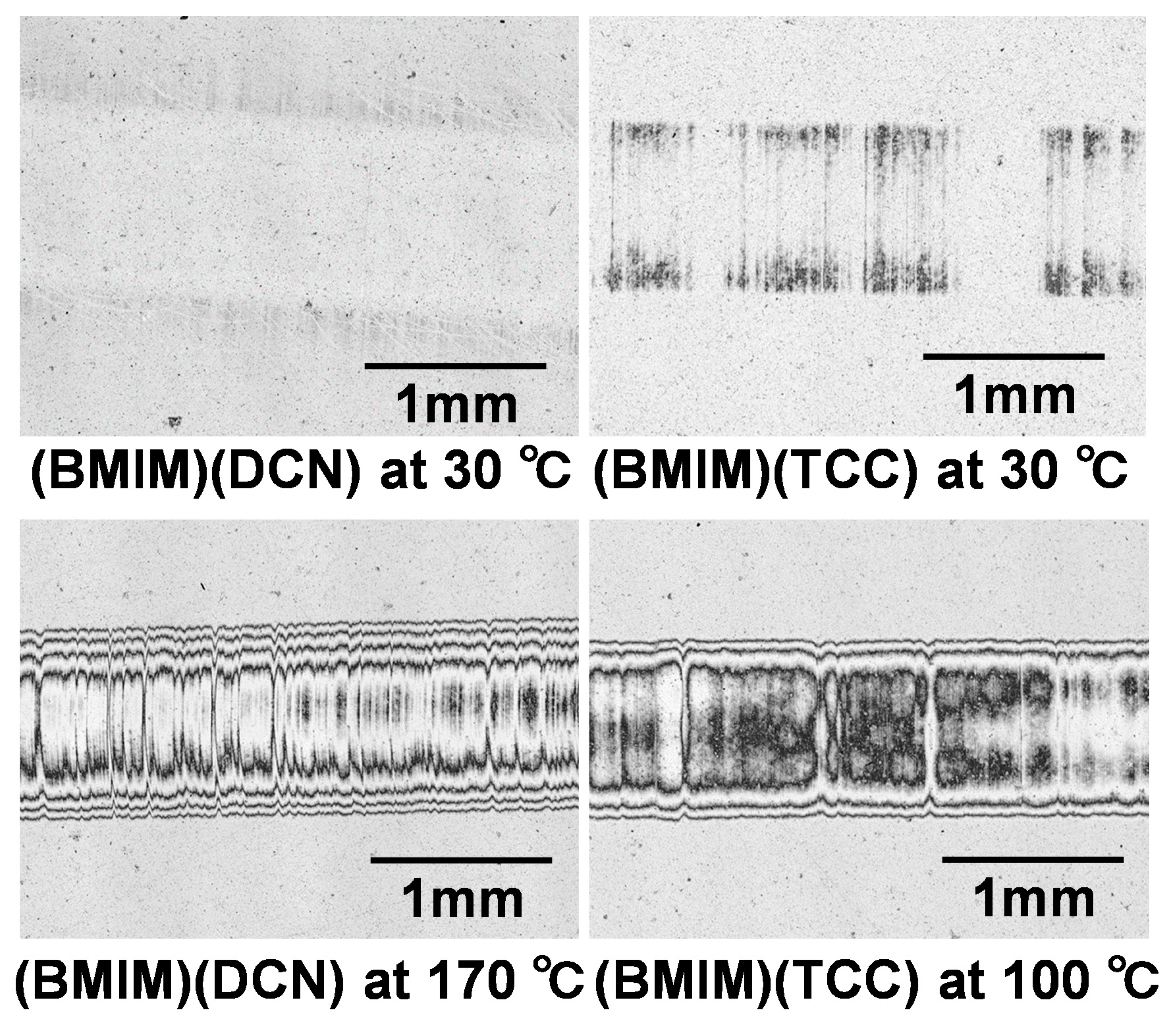
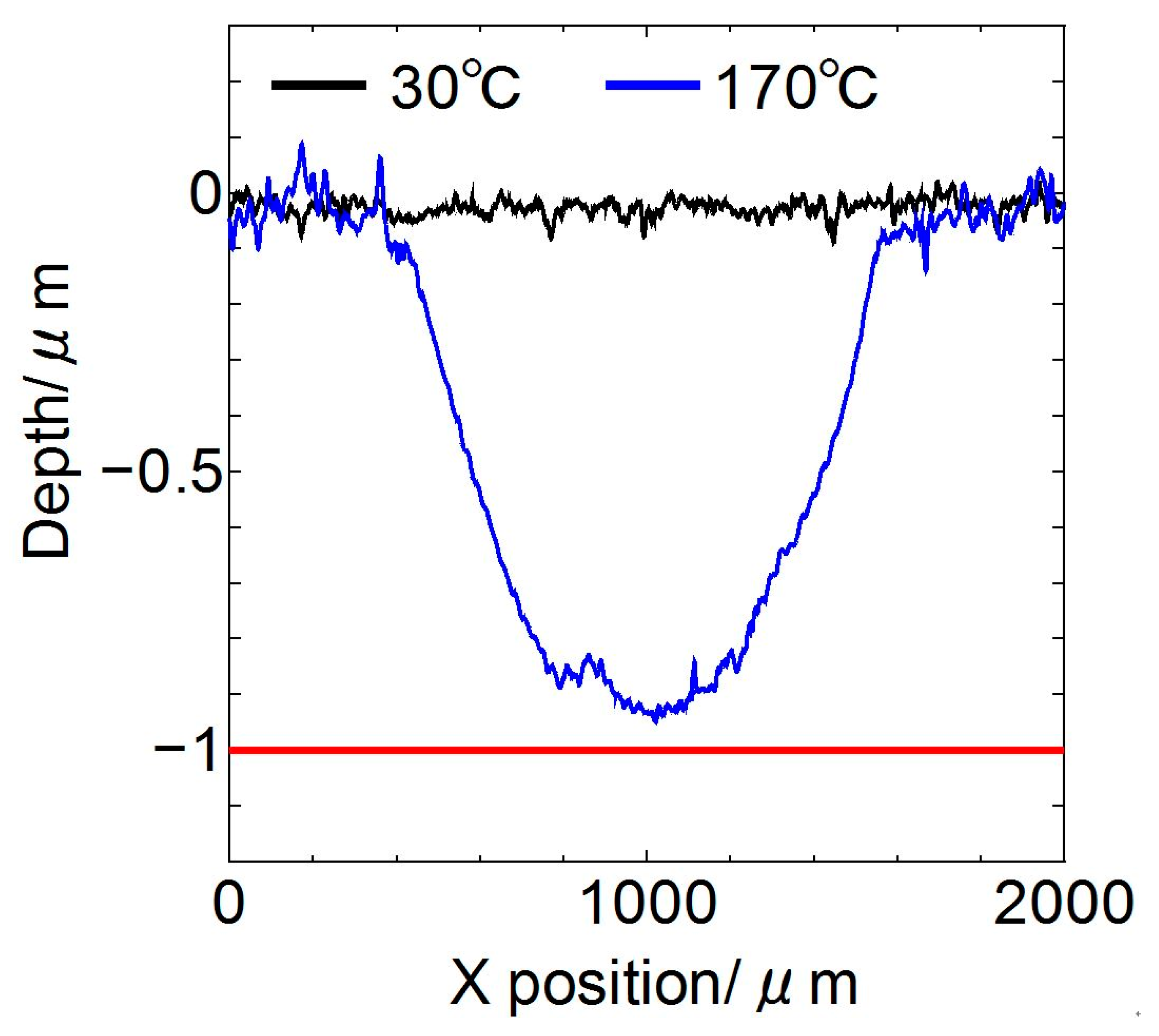
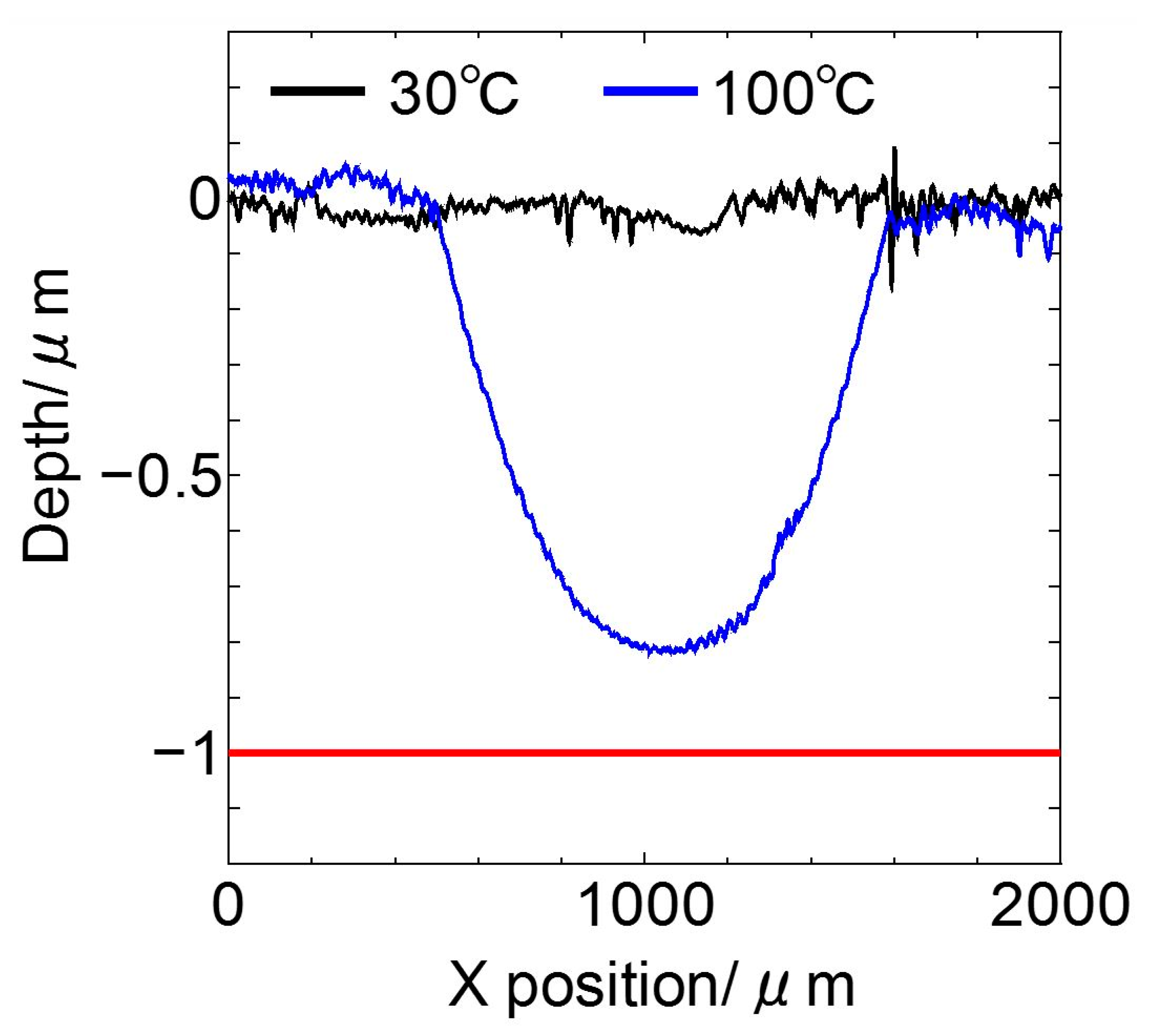
3.1. Friction Tests
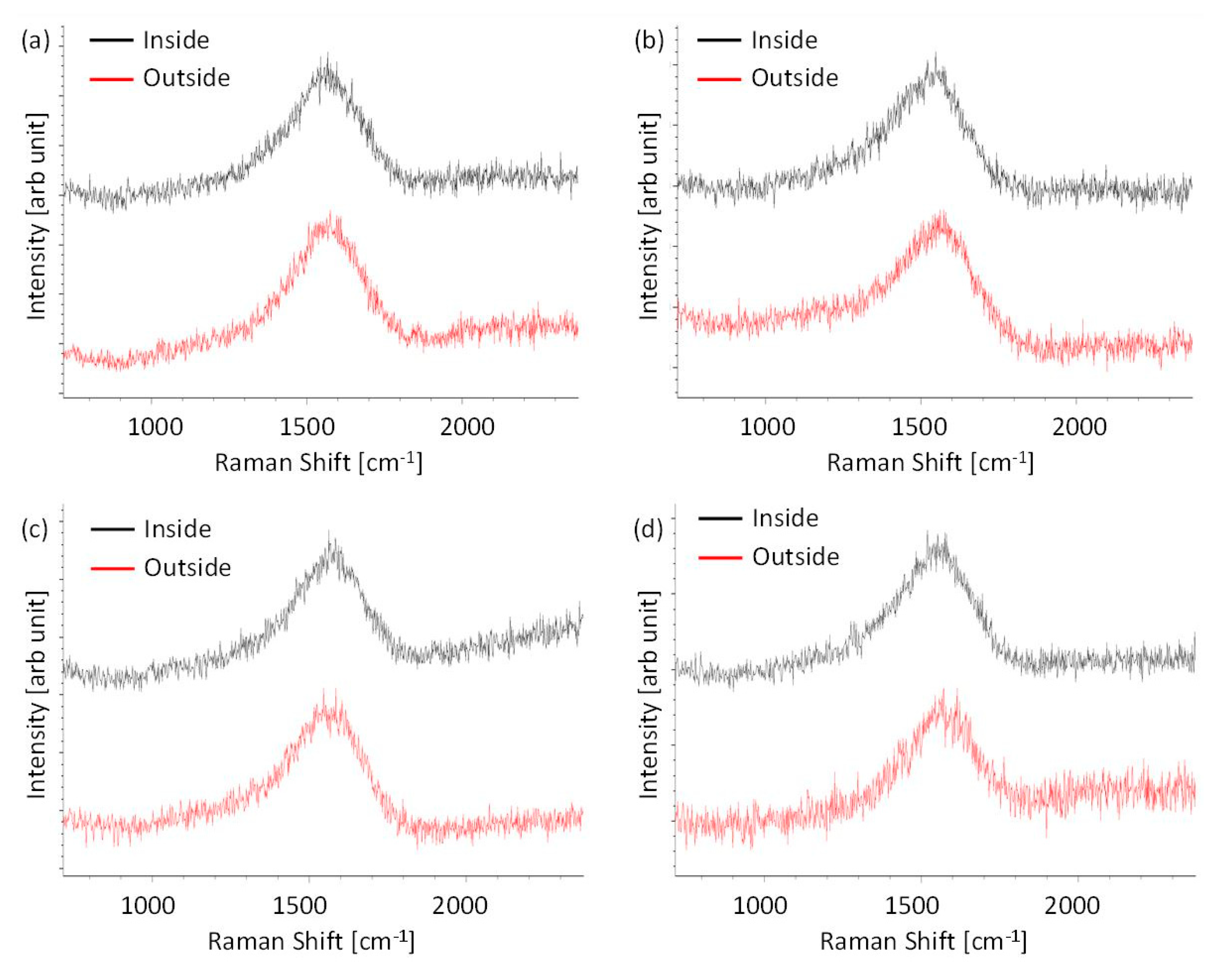
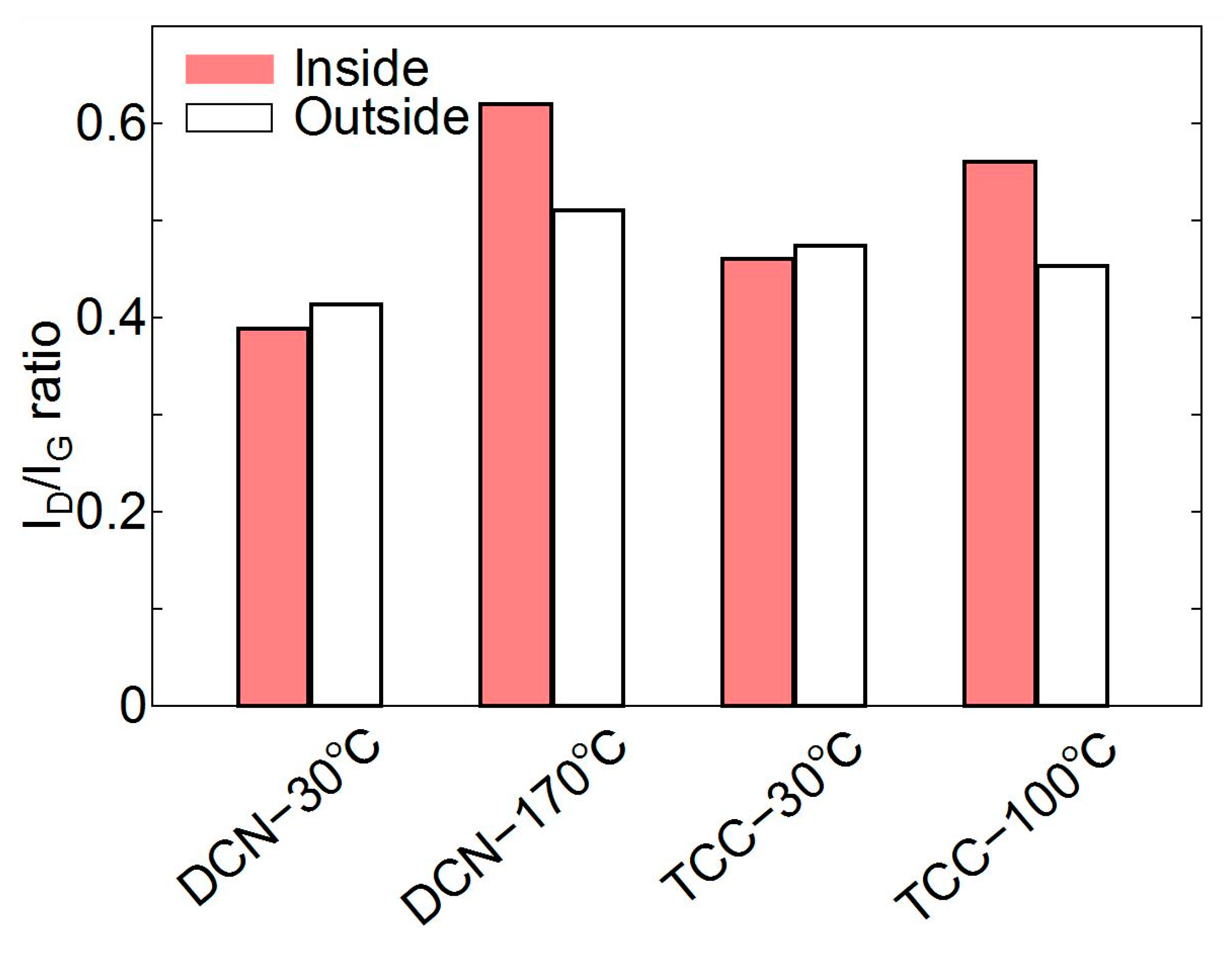
3.2. Raman Spectroscopy
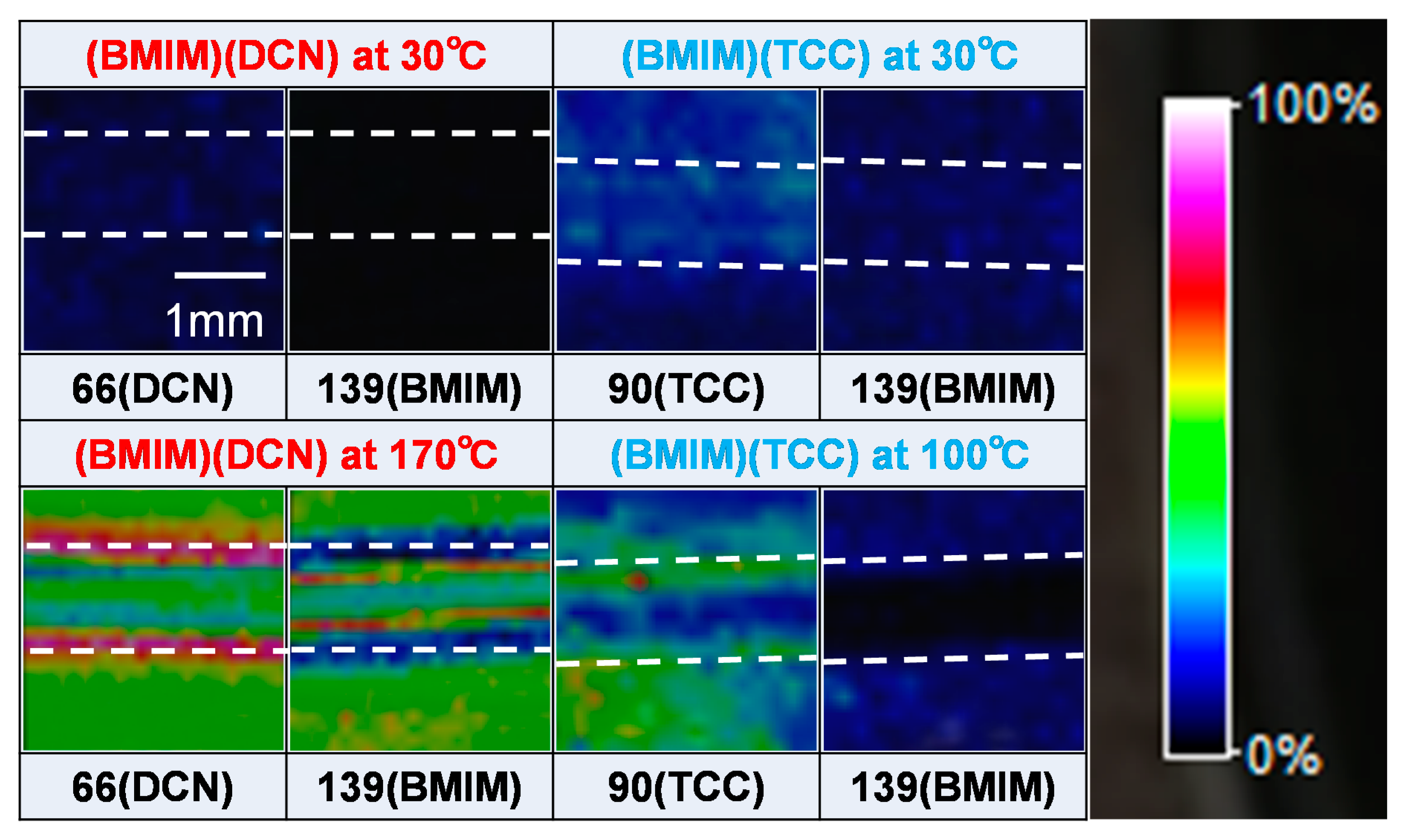
3.3. MALDI-TOF/MS Results
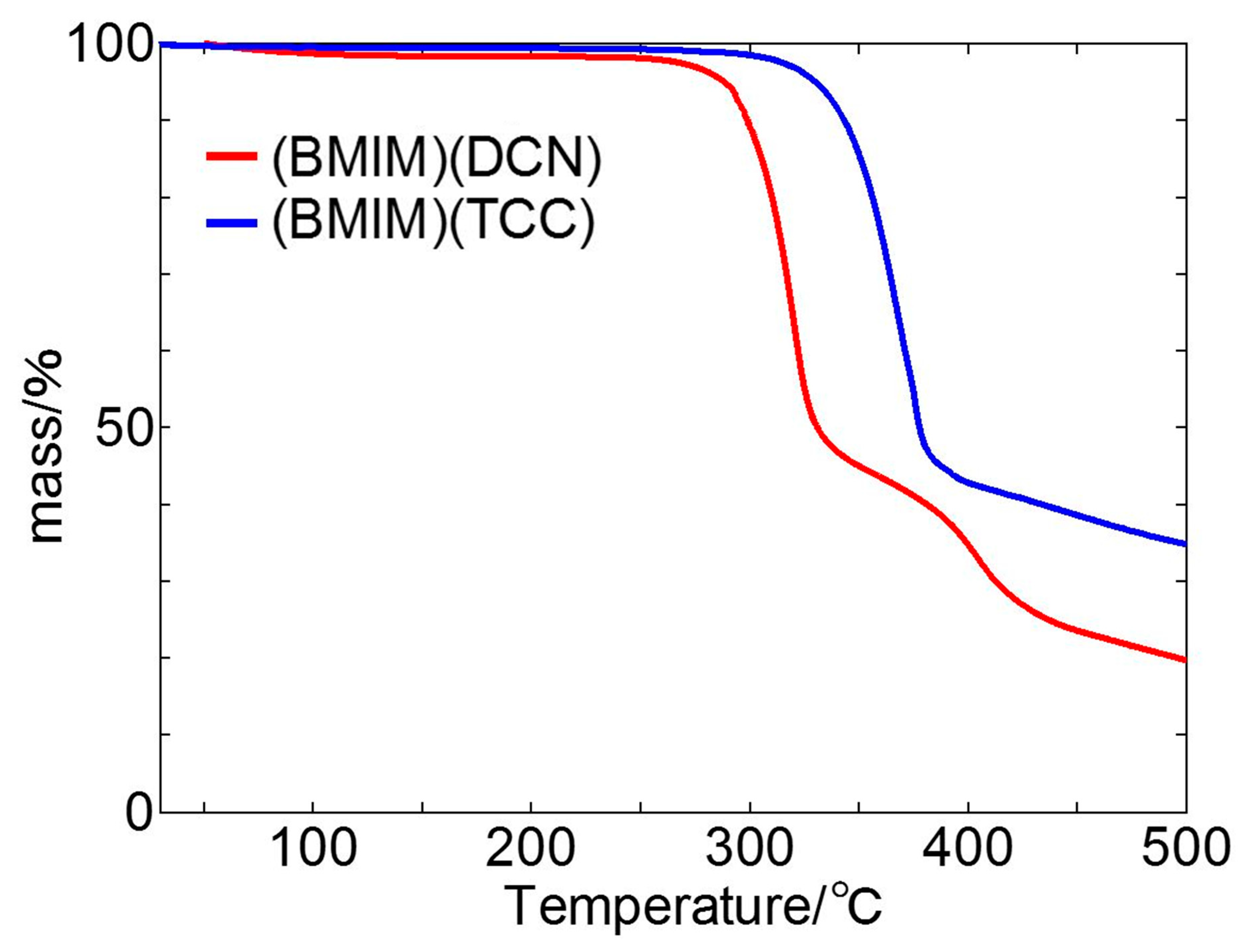
3.4. Thermogravimetric Analysis
4. Discussion
5. Conclusions
- The lubricating property of each cyano-based ionic liquid against the ta-C film was influenced by the anion structure and ambient temperature. (BMIM)(TCC) exhibited exceedingly low friction at 170 °C.
- Raman results indicated the occurrence of the graphitization of the ta-C films lubricated with (BMIM)(DCN) and (BMIM)(TCC) at the temperature corresponding to the low friction coefficient, which might have affected the friction behavior.
- MALDI-TOF/MS analyses indicated that anions were adsorbed on the worn surface at the temperature corresponding to the low friction coefficient. The cation content on the worn surface was small compared to the anion content. Therefore, it is speculated that the adsorption of anions also affects the friction behavior.
- We found a contrary trend to that of the sliding test at elevated temperatures with TGA. The ionic liquids might have experienced tribo-decomposition on the worn surface at 30 °C. Anion adsorption requires a high ambient temperature.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Seddon, K.R. A taste of the future. Nat. Mater. 2003, 2, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Endres, F.; Atkin, R. Effect of alkyl chain length and anion species on the interfacial nanostructure of ionic liquids at the Au (111)–ionic liquid interface as a function of potential. Phys. Chem. Chem. Phys. 2013, 15, 14624–14633. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, H.; Kubo, T.; Minami, I.; Mori, S. Effect and mechanism of additives for ionic liquids as new lubricants. Tribol. Int. 2007, 40, 620–625. [Google Scholar] [CrossRef]
- Gebbie, A.M.; Smith, M.A.; Dobbs, A.H.; Lee, A.A.; Warr, G.G.; Banquy, X.; Valtiner, M.; Rutland, W.M.; Israelachivili, N.J.; Perkin, S.; et al. Long range electrostatic forces in ionic liquids. Chem. Commun. 2017, 53, 1214–1224. [Google Scholar] [CrossRef]
- Motobayashi, K.; Nishi, N.; Inoue, Y.; Minami, K.; Sakka, T.; Osawa, M. Potential-induced restructuring dynamics of ionic liquids on a gold electrode: Steric effect of constituent ions studied by surface-enhanced infrared absorption spectroscopy. J. Electro. Chem. 2017, 800, 126–133. [Google Scholar] [CrossRef]
- Suzuki, A.; Shinka, Y.; Masuko, M. Tribological characteristics of imidazolium-based room temperature ionic liquids under high vacuum. Tribol. Lett. 2007, 27, 307–313. [Google Scholar] [CrossRef]
- Kondo, H.; Seto, J.; Ozawa, K.; Haga, S. Novel lubricants for magnetic thin film media. J. Magn. Soc. J. 1989, 13, 213–218. [Google Scholar] [CrossRef]
- Kondo, H.; Seki, A.; Watanabe, H.; Seto, J. Frictional properties of novel lubricants for magnetic thin film media. IEEE Trans. Magn. 1990, 26, 2691–2693. [Google Scholar] [CrossRef]
- Kondo, H.; Seki, A.; Kita, A. Comparison of an amide and amine salt as friction modifiers for a magnetic thin film medium. Tribol. Trans. 1994, 37, 88–104. [Google Scholar]
- Wamser, C.A. Hydrolysis of fluoboric acid in aqueous solution. J. Am. Chem. Soc. 1948, 70, 1209–1215. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Arias-Pardilla, J.; Espinosa, T.; Bermudez, M.D. Electrochemistry in Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 533–561. [Google Scholar]
- Kondo, Y.; Yagi, S.; Koyama, T.; Tsuboi, R.; Sasaki, S. Lubricity and corrosiveness of ionic liquids for steel-on-steel sliding contacts. Inst. Mech. Eng. J. Eng. Tribol. 2012, 226, 991–1006. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Tadokoro, C.; Sasaki, S. Effects of alkyl chaing length of sulfate and phosphate anion-based ionic liquids on Tribochemical reactions. Tribol. Lett. 2018, 66, 8. [Google Scholar] [CrossRef]
- Kondo, Y.; Koyama, T.; Tsuboi, R.; Nakano, M.; Miyake, K.; Sasaki, S. Tribological performance of halogen-free ionic liquids as lubricants of hard coatings and ceramics. Tribol. Lett. 2013, 51, 243–249. [Google Scholar] [CrossRef]
- Minami, I.; Inada, T.; Okada, Y. Tribological properties of halogen-free ionic liquids. Inst. Mech. Eng. J. Eng. Tribol. 2012, 226, 891–902. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Tadokoro, C.; Tsuboi, R.; Sasaki, S. Lubricating mechanism of cyano-based ionic liquids on nascent steel surface. Tribol. Int. 2018, 119, 474–480. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Sasaki, S.; Miyatake, M. Evaluation of friction behavior and surface interactions of cyano-based ionic liquids under different sliding contacts and high vacuum condition. Lubricatns 2018, 6, 69. [Google Scholar] [CrossRef]
- Shah, F.U.; Glavatskih, S.; Antzutkin, N.O. Boron in tribology: From borates to ionic liquids. Tribol. Lett. 2013, 51, 281–301. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. Appl. Mater. Interfaces. 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Y.; Su, H.; Li, W.; Wang, Z. Synthesis and evaluation of a protic ionic liquid as a multifunctional lubricant additive. Friction 2019, 1–9. [Google Scholar] [CrossRef]
- Avilés, M.D.; Pamies, R.; Sanes, J.; Carrión, F.J.; Bermúdez, M.D. Fatty acid-derived ionic liquid lubricants. Protic ionic liquid crystals as protic ionic liquid additives. Coatings 2019, 9, 710. [Google Scholar] [CrossRef]
- Guo, H.; Adukure, A.R.; Iglesias, P. Effect of ionicity of three protic ionic liquids as neat lubricants and lubricant additives to a biolubricant. Coatings 2019, 9, 713. [Google Scholar] [CrossRef]
- Tadokoro, C.; Sato, K.; Nagamine, T.; Nakano, K.; Sasaki, S.; Sato, T.; Sakakibara, K.; Tsujii, Y. Concentrated polymer brush as reciprocating sear material for low leakage and low friction. Tribol. Tans. 2019. [Google Scholar] [CrossRef]
- Tujii, Y.; Nomura, A.; Okayasu, K.; Gao, W.; Ohno, K.; Fukuda, T. AFM studies on microtribology of concentrated polymer brushes in solvents. J. Phys. Conf. Ser. 2009, 184, 012031. [Google Scholar]
- Sato, K.; Okubo, H.; Hirata, Y.; Tadokoro, C.; Nakano, K.; Tsujii, Y.; Sasaki, S. Macroscopic tribological properties of thick concentrated polymer brush on rough steel under lubrication with ionic liquid. J. Tribologi. 2019, 20, 97–113. [Google Scholar]
- Bouabibsa, I.; Lamri, S.; Sanchette, F. Structure, mechanical and tribological properties of Me-doped diamond-like carbon (DLC) (Me = Al, Ti, or Nb) hydrogenated amorphous carbon coatings. Coatings 2018, 8, 370. [Google Scholar] [CrossRef]
- Aijaz, A.; Ferreira, F.; Oliveira, J.; Kubart, T. Mechanical properties of hydrogen free diamond-like carbon thin films deposited by high power impulse magnetron sputtering with Ne. Coatings 2018, 8, 385. [Google Scholar] [CrossRef]
- Okubo, H.; Kawada, S.; Watanabe, S.; Sasaki, S. Tribological performance of halogen-free ionic liquids in steel–steel and DLC–DLC contacts. Tribol. Trans. 2018, 61, 71–79. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Tsuboi, R.; Sasaki, S.; Prakash, B. Lubrication mechanism of halogen-free ionic liquids. Tribol. Online 2017, 12, 155–161. [Google Scholar] [CrossRef][Green Version]
- Kawada, S.; Sato, K.; Watanabe, S.; Sasaki, S. Lubricating property of cyano-based ionic liquids against hard materials. J. Mech. Sci. Tech. 2017, 31, 5745–5750. [Google Scholar] [CrossRef]
- Ogwu, A.A.; Lamberton, R.W.; Morley, S.; Maguire, P.; McLaughlin, J. Characterization of thermally annealed diamond like carbon (DLC) and silicon modified DLC films by Raman spectroscopy. Physica B Condensed Matter 1999, 269, 335–344. [Google Scholar] [CrossRef]
- Vengudusamy, B.; Green, J.H.; Lamb, G.D.; Spikes, H.A. Tribological properties of tribofilms formed from ZDDP in DLC/DLC and DLC/steel contacts. Tribol. Int. 2011, 44, 165–174. [Google Scholar] [CrossRef]
- Liu, Y.; Erdemir, A.; Meletis, E.I. An investigation of the relationship graphitization and frictional behaviour of DLC coatings. Surf. Coat. Tech. 1996, 86–87, 564–568. [Google Scholar] [CrossRef]
- Christoph, G.; Ernst, P.; Nicole, D.; Gunter, A. Imaging of a Tribolayer Formed from Ionic Liquids by Laser Desorption/Ionization-Reflectron Time-of-Flight Mass Spectrometry. Anal. Chem. 2012, 84, 10708–10714. [Google Scholar]
- Liu, X.Q.; Zhou, F.; Liang, Y.; Liu, W. Tribological performance of phosphonium based ionic liquids for an aluminum-on-steel system and opinions on lubrication mechanism. WEAR 2006, 261, 1174–1179. [Google Scholar] [CrossRef]

| (BMIM)(DCN) |
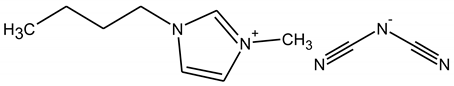 |
| (BMIM)(TCC) |
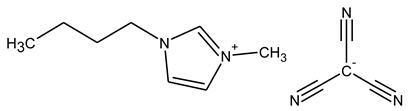 |
| Name | (BMIM)(DCN) | (BMIM)(TCC) |
|---|---|---|
| Viscosity (50 °C) | 11.6 [mPas] | 11.1 [mPas] |
| Melting point | <−50 [°C] | <−50 [°C] |
| Purity | >98 [%] | >98 [%] |
| Roughness | Ra = 0.01 [μm] |
|---|---|
| Hardness | 73 [GPa] |
| Film thickness | 1.0 [μm] |
| Hydrogen content | <1.0 [at%] |
| Interlayer | Cr |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawada, S.; Okubo, H.; Watanabe, S.; Tadokoro, C.; Tsuboi, R.; Sasaki, S.; Miyatake, M. Lubricating Properties of Cyano-Based Ionic Liquids against Tetrahedral Amorphous Carbon Film. Coatings 2020, 10, 153. https://doi.org/10.3390/coatings10020153
Kawada S, Okubo H, Watanabe S, Tadokoro C, Tsuboi R, Sasaki S, Miyatake M. Lubricating Properties of Cyano-Based Ionic Liquids against Tetrahedral Amorphous Carbon Film. Coatings. 2020; 10(2):153. https://doi.org/10.3390/coatings10020153
Chicago/Turabian StyleKawada, Shouhei, Hikaru Okubo, Seiya Watanabe, Chiharu Tadokoro, Ryo Tsuboi, Shinya Sasaki, and Masaaki Miyatake. 2020. "Lubricating Properties of Cyano-Based Ionic Liquids against Tetrahedral Amorphous Carbon Film" Coatings 10, no. 2: 153. https://doi.org/10.3390/coatings10020153
APA StyleKawada, S., Okubo, H., Watanabe, S., Tadokoro, C., Tsuboi, R., Sasaki, S., & Miyatake, M. (2020). Lubricating Properties of Cyano-Based Ionic Liquids against Tetrahedral Amorphous Carbon Film. Coatings, 10(2), 153. https://doi.org/10.3390/coatings10020153





