Polyphenols of Honeybee Origin with Applications in Dental Medicine
Abstract
1. Introduction
2. Polyphenols in Honeybee Products
3. Bioactivities of Polyphenols
3.1. Cardio-Protective and Anti-Ulcer Properties
3.2. Antitumor Activity
3.3. Antidiabetic Activity
3.4. Neurological Diseases
3.5. Wound Healing
3.6. Anti-Oxidant Activity
3.7. Antimicrobial Effect
4. Polyphenols in Dental Medicine
4.1. Dental Caries
4.2. Periodontitis
4.3. Dental Plaque
4.4. Enamel Strengthening
4.5. Oral Cancers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Kenis, P.; Schol, L.G.C.; Kraaij-Dirkzwager, M.M.; Timen, A. Appropriate Governance Responses to Infectious Disease Threats: Developing Working Hypotheses. Risk Hazards Crisis Public Policy 2019, 10, 275–293. [Google Scholar] [CrossRef]
- Dos Santos, L.D.R.; Dos Santos, A.E.S.; Cerávolo, I.P.; Figueiredo, F.J.B.; Dias-Souza, M.V. Antibiofilm activity of black tea leaf extract, its cytotoxicity and interference on the activity of antimicrobial drugs. Biointerface Res. Appl. Chem. 2018, 8, 3565–3569. [Google Scholar]
- Genilloud, O. Natural products discovery and potential for new antibiotics. Curr. Opin. Microbiol. 2019, 51, 81–87. [Google Scholar] [CrossRef]
- Dias-Souza, M.V.; Dias, C.G.; Ferreira-Marçal, P.H. Interactions of natural products and antimicrobial drugs: Investigations of a dark matter in chemistry. Biointerface Res. Appl. Chem. 2018, 8, 3259–3264. [Google Scholar]
- Darwesh, O.M.; Barakat, K.M.; Mattar, M.Z.; Sabae, S.Z.; Hassan, S.H. Production of antimicrobial blue green pigment pyocyanin by marine Pseudomonas aeruginosa. Biointerface Res. Appl. Chem. 2019, 9, 4334–4339. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.-Y.; Zhang, D.-F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.-J. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Machado De-Melo, A.A.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- De-Melo, A.; Almeida-Muradian, L.; Sancho, M.; Pascual Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2017, 1–33. [Google Scholar] [CrossRef]
- Soto-Chilaca, G.A.; Mejía-Garibay, B.; Navarro-Amador, R.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Cinnamaldehyde-loaded chitosan nanoparticles: Characterization and antimicrobial activity. Biointerface Res. Appl. Chem. 2019, 9, 4060–4065. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef]
- Tauber, J.P.; Collins, W.R.; Schwarz, R.S.; Chen, Y.; Grubbs, K.; Huang, Q.; Lopez, D.; Peterson, R.; Evans, J.D. Natural Product Medicines for Honey Bees: Perspective and Protocols. Insects 2019, 10, 356. [Google Scholar] [CrossRef]
- Lewkowski, O.; Mureșan, C.I.; Dobritzsch, D.; Fuszard, M.; Erler, S. The Effect of Diet on the Composition and Stability of Proteins Secreted by Honey Bees in Honey. Insects 2019, 10, 282. [Google Scholar] [CrossRef]
- Münstedt, K.; Männle, H. Using Bee Products for the Prevention and Treatment of Oral Mucositis Induced by Cancer Treatment. Molecules 2019, 24, 3023. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical Composition of Honey. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 43–82. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, H.R. Composition and Synthesis of Beeswax. In Honeybees and Wax: An Experimental Natural History; Hepburn, H.R., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 44–56. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Argüelles, S. Chapter 4.1—Bee Products: Royal Jelly and Propolis. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 475–484. [Google Scholar] [CrossRef]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef]
- Shi, Z.; Enayatullah, H.; Lv, Z.; Dai, H.; Wei, Q.; Shen, L.; Karwand, B.; Shi, F. Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice. Animals 2019, 9, 977. [Google Scholar] [CrossRef]
- Jibril, F.I.; Hilmi, A.B.M.; Manivannan, L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Ding, Y.; Yao, H.; Yao, Y.; Fai, L.Y.; Zhang, Z. Protection of Dietary Polyphenols against Oral Cancer. Nutrients 2013, 5, 2173–2191. [Google Scholar] [CrossRef]
- Chatterjee, A.; Saluja, M.; Agarwal, G.; Alam, M. Green tea: A boon for periodontal and general health. J. Indian Soc. Periodontol. 2012, 16, 161–167. [Google Scholar] [CrossRef]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Shah, M.H. Assessment of phenolic contents, essential/toxic metals and antioxidant capacity of fruits of viburnum foetens decne. Biointerface Res. Appl. Chem. 2018, 8, 3178–3186. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kennedy, D.O. Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. Ptr. 2019, 33. [Google Scholar] [CrossRef]
- Uslu, M.E.; Mele, A.; Bayraktar, O. Evaluation of the hemostatic activity of Equisetum arvense extract: The role of varying phenolic composition and antioxidant activity due to different extraction conditions. Biointerface Res. Appl. Chem. 2019, 9, 4157–4163. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.X. Chemical composition, characterization, and differentiation of honey botanical and geographical origins. Adv. Food Nutr. Res. 2011, 62, 89–137. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Duman, A.; Mogulkoc, R.; Baltaci, A.K.; Sivrikaya, A. The effect of 3′,4′-dihydroxyflavonol on plasma oxidant and antioxidant systems in testis ischemia-reperfusion injury in rats. Biointerface Res. Appl. Chem. 2018, 8, 3441–3445. [Google Scholar]
- Moniruzzaman, M.; Sulaiman, S.A.; Azlan, S.A.; Gan, S.H. Two-year variations of phenolics, flavonoids and antioxidant contents in acacia honey. Molecules 2013, 18, 14694–14710. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Zhao, C.-N.; Xu, X.-Y.; Gan, R.-Y.; Cao, S.-Y.; Liu, Q.; Shang, A.; Mao, Q.-Q.; Li, H.-B. Phytochemical Composition and Antioxidant Capacity of 30 Chinese Teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A. The potential role of honey and its polyphenols in preventing heart diseases: A review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef]
- Uthurry, C.; Hevia, D.; Gomez-Cordoves, C. Role of honey polyphenols in health. J. Apiprod. Apimed. Sci. 2011, 3, 141–159. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insight (review). Int. J. Funct. Nutr. 2020. [Google Scholar] [CrossRef]
- Nasir, A.; Bullo, M.M.H.; Ahmed, Z.; Imtiaz, A.; Yaqoob, E.; Jadoon, M.; Ahmed, H.; Afreen, A.; Yaqoob, S. Nutrigenomics: Epigenetics and cancer prevention: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1375–1387. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X. Interactions of tea polyphenols with intestinal microbiota and their implication for anti-obesity. J. Sci. Food Agric. 2020, 100, 897–903. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Y.J.; Chang, W.A.; Li, W.M.; Ke, H.L.; Wu, W.J.; Kuo, P.L. Effects of Epigallocatechin Gallate (EGCG) on Urinary Bladder Urothelial Carcinoma-Next-Generation Sequencing and Bioinformatics Approaches. Medicina 2019, 55, 768. [Google Scholar] [CrossRef]
- ElAttar, T.M.; Virji, A.S. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. AntiCancer Drugs 1999, 10, 187–193. [Google Scholar] [CrossRef]
- Kim, J.S.; Saengsirisuwan, V.; Sloniger, J.A.; Teachey, M.K.; Henriksen, E.J. Oxidant stress and skeletal muscle glucose transport: Roles of insulin signaling and p38 MAPK. Free Radic. Biol. Med. 2006, 41, 818–824. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [PubMed]
- Syed-Badrul, S.-N.; Isa, N.-M.; Mohamed, S.Z.; Qodriyah, H.M.S. Natural Polyphenols in the Treatment of Alzheimer’s Disease. Curr. Drug Targets 2018, 19, 927–937. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with High Levels of Antioxidants Can Provide Protection to Healthy Human Subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compreshensive Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Basu, A.; Masek, E.; Ebersole, J.L. Dietary Polyphenols and Periodontitis-A Mini-Review of Literature. Molecules 2018, 23, 1786. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Toczewska, J.; Maciejczyk, M.; Konopka, T.; Zalewska, A. Total Oxidant and Antioxidant Capacity of Gingival Crevicular Fluid and Saliva in Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 450. [Google Scholar] [CrossRef]
- Özkök, D.; Silici, S. Antioxidant activities of honeybee products and their mixtures. Food Sci. Biotechnol. 2017, 26, 201–206. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of Gallic Acid on the Growth and Biofilm Formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Ferulic acid potentiates the antibacterial activity of quinolone-based antibiotics against Acinetobacter baumannii. Microb. Pathog. 2019, 126, 393–398. [Google Scholar] [CrossRef]
- Dias da Costa Júnior, S.; de Oliveira Santos, J.V.; de Almeida Campos, L.A.; Araújo Pereira, M.; Santos Magalhães, N.S.; Macário Ferro Cavalcanti, I. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1948–1958. [Google Scholar] [CrossRef]
- Hellewell, L.; Bhakta, S. Chalcones, stilbenes and ketones have anti-infective properties via inhibition of bacterial drug-efflux and consequential synergism with antimicrobial agents. Access Microbiol. 2020, 2, acmi000105. [Google Scholar] [CrossRef]
- Khan, M.S.; Agrawal, R.; Ubaidullah, M.; Hassan, M.I.; Tarannum, N. Design, synthesis and validation of antimicrobial coumarin derivatives: An efficient green approach. Heliyon 2019, 5, e02615. [Google Scholar] [CrossRef]
- Kayser, O.; Kolodziej, H. Antibacterial activity of simple coumarins: Structural requirements for biological activity. Z. Nat. CJ. Biosci. 1999, 54, 169–174. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Parages, M.L.; O’Gara, F. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed. Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef]
- Sankari, S.L.; Babu, N.A.; Rani, V.; Priyadharsini, C.; Masthan, K.M.K. Flavonoids—Clinical effects and applications in dentistry: A review. J. Pharm. Bioallied Sci. 2014, 6, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Mokhtarpour, M.; Jahangiri, A.; Dehghanzadeh, S.; Maleki-Dizaj, S.; Shahi, S. Hydroxyapatite nanofibers as beneficial nanomaterial in dental sciences. Biointerface Res. Appl. Chem. 2018, 8, 3695–3699. [Google Scholar]
- Ferrazzano, G.F.; Roberto, L.; Amato, I.; Cantile, T.; Sangianantoni, G.; Ingenito, A. Antimicrobial properties of green tea extract against cariogenic microflora: An in vivo study. J. Med. Food 2011, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Takada, K.; Otake, S. Inhibition of acid production in dental plaque bacteria by green tea catechins. Caries Res. 2006, 40, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Facchiano, S.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Paolucci, M.; Volpe, M.G.; Salvatore, P.; Pagliarulo, C. In vitro Synergy of Polyphenolic Extracts From Honey, Myrtle and Pomegranate Against Oral Pathogens, S. mutans and R. dentocariosa. Front. Microbiol. 2020, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.D.; Wu, C.D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch. Oral Biol. 2012, 57, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Ohara, M.; Hayashi, I.; Hino, T.; Nishimura, R.; Iwasaki, Y.; Ogawa, T.; Ohyama, Y.; Sugiyama, M.; Amano, H. The green tea polyphenol (-)-epigallocatechin gallate precipitates salivary proteins including alpha-amylase: Biochemical implications for oral health. Eur. J. Oral Sci. 2012, 120, 132–139. [Google Scholar] [CrossRef]
- Habluetzel, A.; Schmid, C.; Carvalho, T.S.; Lussi, A.; Eick, S. Impact of honey on dental erosion and adhesion of early bacterial colonizers. Sci. Rep. 2018, 8, 10936. [Google Scholar] [CrossRef] [PubMed]
- Akca, A.E.; Akca, G.; Topçu, F.T.; Macit, E.; Pikdöken, L.; Özgen, I. The Comparative Evaluation of the Antimicrobial Effect of Propolis with Chlorhexidine against Oral Pathogens: An In Vitro Study. Biomed. Res. Int. 2016, 2016, 3627463. [Google Scholar] [CrossRef]
- Jeon, J.G.; Pandit, S.; Xiao, J.; Gregoire, S.; Falsetta, M.L.; Klein, M.I.; Koo, H. Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. Int. J. Oral Sci. 2011, 3, 98–106. [Google Scholar] [CrossRef]
- Koo, H.; Jeon, J.G. Naturally occurring molecules as alternative therapeutic agents against cariogenic biofilms. Adv. Dent. Res. 2009, 21, 63–68. [Google Scholar] [CrossRef]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A. Propolis--based chitosan varnish: Drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complementary Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Dudonné, S.; Desjardins, Y.; Grenier, D. Wild Blueberry (Vaccinium angustifolium Ait.) Polyphenols Target Fusobacterium nucleatum and the Host Inflammatory Response: Potential Innovative Molecules for Treating Periodontal Diseases. J. Agric. Food Chem. 2015, 63, 6999–7008. [Google Scholar] [CrossRef]
- De Assis, J.S.; Lima, R.A.; Marques Lima, J.P.; Azevedo Rodrigues, L.K.; Santiago, S.L. Effect of epigallocatechin-3-gallate application for remaining carious dentin disinfection. J. Conserv. Dent. 2015, 18, 51–55. [Google Scholar] [CrossRef]
- De Oliveira Caleare, A.; Hensel, A.; Mello, J.C.P.; Pinha, A.B.; Panizzon, G.P.; Lechtenberg, M.; Petereit, F.; Nakamura, C.V. Flavan-3-ols and proanthocyanidins from Limonium brasiliense inhibit the adhesion of Porphyromonas gingivalis to epithelial host cells by interaction with gingipains. Fitoterapia 2017, 118, 87–93. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/oral_health/disease_burden/global/en/ (accessed on 30 September 2020).
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Ikegaki, M.; Sattler, A. Effect of Apis mellifera propolis from two Brazilian regions on caries development in desalivated rats. Caries Res. 1999, 33, 393–400. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Koo, H.; Pearson, S.K.; Scott-Anne, K.; Abranches, J.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Marquis, R.E.; Bowen, W.H. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immunol. 2002, 17, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Bowen, W.H.; Burne, R.A.; Kuramitsu, H.K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 1993, 61, 3811–3817. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Periodontal Disease. Available online: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html (accessed on 30 September 2020).
- Bodet, C.; Piché, M.; Chandad, F.; Grenier, D. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J. Antimicrob. Chemother. 2006, 57, 685–690. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.Z.; Casarin, R.C.; Cirano, F.R.; Tenenbaum, H.T.; Casati, M.Z. Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal Res. 2017, 52, 201–209. [Google Scholar] [CrossRef]
- Yoshinaga, Y.; Ukai, T.; Nakatsu, S.; Kuramoto, A.; Nagano, F.; Yoshinaga, M.; Montenegro, J.L.; Shiraishi, C.; Hara, Y. Green tea extract inhibits the onset of periodontal destruction in rat experimental periodontitis. J. Periodontal Res. 2014, 49, 652–659. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Ding, Y.; Bao, C.; Li, W. Mangiferin ameliorates Porphyromonas gingivalis-induced experimental periodontitis by inhibiting phosphorylation of nuclear factor-κB and Janus kinase 1-signal transducer and activator of transcription signaling pathways. J. Periodontal Res. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Hamzah, N.; Aziz, S.; Fauzi, A.-R.; Mohd Yusof, Y.; Razali, M.; Ibrahim, N.; Baharin, B. Effects of Gelam Honey (Melaleuca cajuputi) on Alveolar Bone Loss in Experimental Periodontitis. J. Dent. Surg. 2014, 2014. [Google Scholar] [CrossRef]
- Coutinho, A. Honeybee propolis extract in periodontal treatment: A clinical and microbiological study of propolis in periodontal treatment. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2012, 23, 294. [Google Scholar] [CrossRef]
- Halboub, E.; Al-Maweri, S.A.; Al-Wesabi, M.; Al-Kamel, A.; Shamala, A.; Al-Sharani, A.; Koppolu, P. Efficacy of propolis-based mouthwashes on dental plaque and gingival inflammation: A systematic review. BMC Oral Health 2020, 20, 198. [Google Scholar] [CrossRef]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Dental Biofilm and Laboratory Microbial Culture Models for Cariology Research. Dent. J. 2017, 5, 21. [Google Scholar] [CrossRef]
- Dental Plaque and Plaque as Biofilm. Available online: https://periobasics.com/dental-plaque/ (accessed on 30 September 2020).
- Farkash, Y.; Feldman, M.; Ginsburg, I.; Steinberg, D.; Shalish, M. Polyphenols Inhibit Candida albicans and Streptococcus mutans Biofilm Formation. Dent. J. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Furiga, A.; Roques, C.; Badet, C. Preventive effects of an original combination of grape seed polyphenols with amine fluoride on dental biofilm formation and oxidative damage by oral bacteria. J. Appl. Microbiol. 2014, 116, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; Souza, N.O.; Sousa, R.S.; Freitas, D.Q.; Lemos, M.V.; De Paula, D.M.; Maia, F.J.N.; Lomonaco, D.; Mazzetto, S.E.; Feitosa, V.P. Efficacy of new natural biomodification agents from Anacardiaceae extracts on dentin collagen cross-linking. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 1103–1109. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.B.; Yoo, K.J.; Ema, K.C.; Pashley, D.H. Mechanical properties of tannic-acid-treated dentin matrix. J. Dent. Res. 2009, 88, 807–811. [Google Scholar] [CrossRef]
- Oh, S.; Gu, Y.; Perinpanayagam, H.; Yoo, Y.-J.; Lee, Y.; Kim, R.K.; Chang, S.W.; Lee, J.; Zhu, Q.; Kum, K.Y. Dentinal tubule sealing effects of 532-nm diode-pumped solid-state laser, gallic acid/Fe3+ complex, and three commercial dentin desensitizers. Lasers Med. Sci. 2018, 33, 1237–1244. [Google Scholar] [CrossRef]
- Orsolić, N.; Knezević, A.; Sver, L.; Terzić, S.; Hackenberger, B.K.; Basić, I. Influence of honey bee products on transplantable murine tumours. Vet. Comp. Oncol. 2003, 1, 216–226. [Google Scholar] [CrossRef]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complementary Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef]
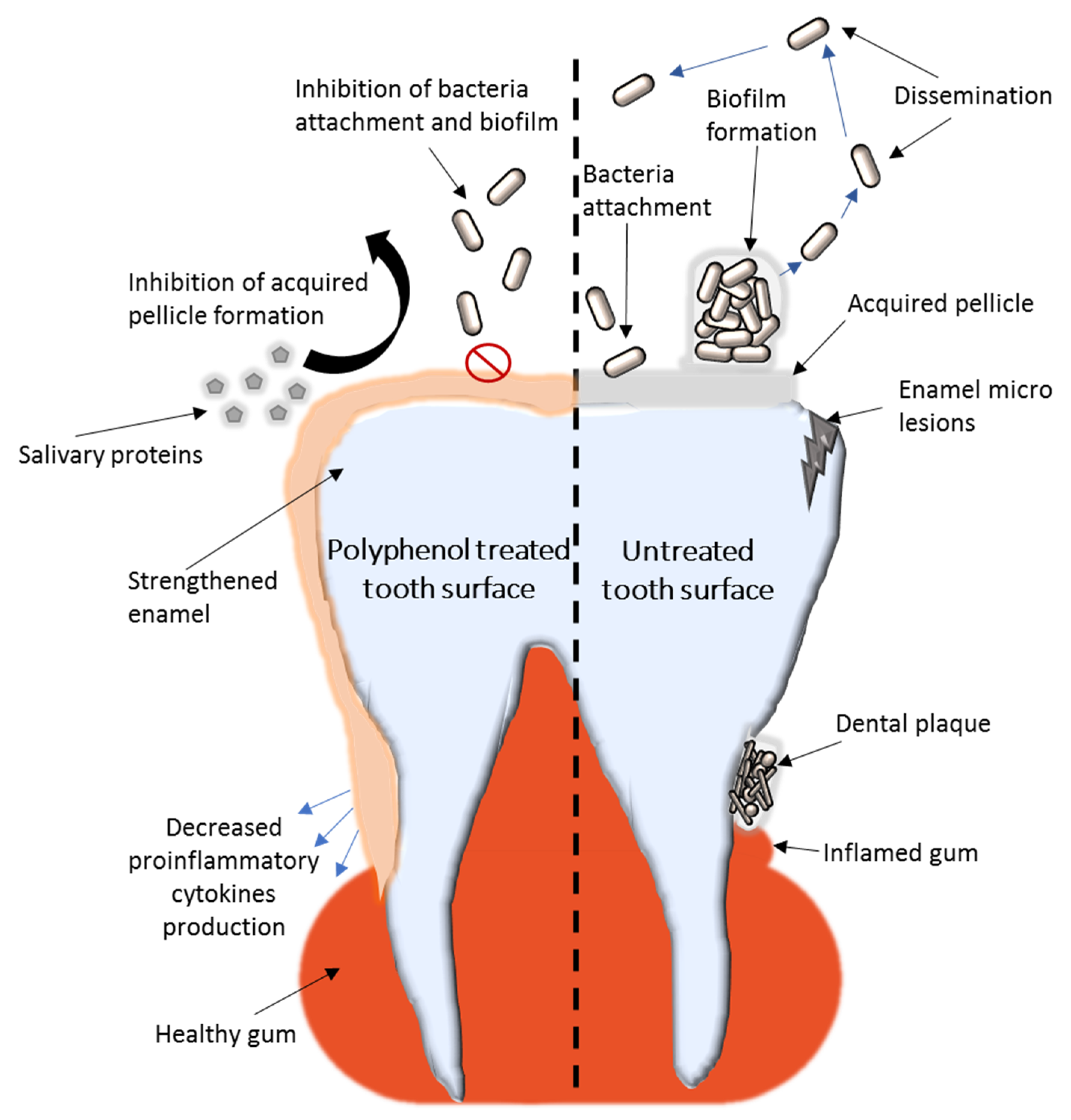
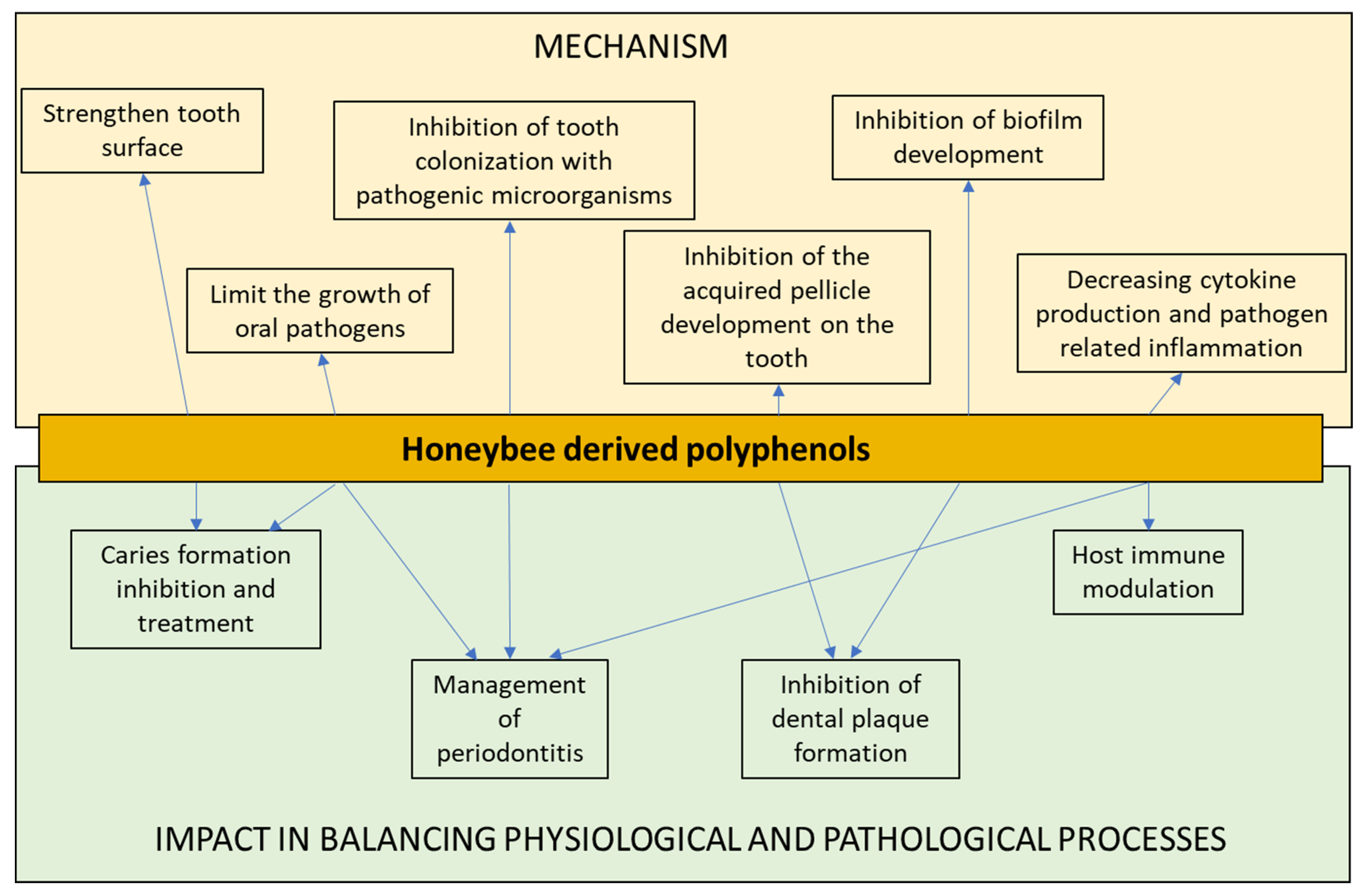
| Oral Pathology | Type of Polyphenols | Antimicrobial Mechanism | Microbial Pathogen | Reference |
|---|---|---|---|---|
| Dental caries | mixture of polyphenols | inhibition of bacterial growth | Streptococcus mutans and Lactobacilli species | [88] |
| inhibition of bacterial growth, adherence, and acid production | acidogenic oral streptococci | [89] | ||
| polyphenolic acid extracts form honey | growth inhibition | Streptococcus mutans and Rothia dentocariosa | [90] | |
| catechins | suppression of GtfB/C/D genes (responsible for biofilm formation and production of soluble virulence factors) | S. mutans and Enterococcus faecalis | [91] | |
| catechins | inhibit the activity of salivary amylase and bacterial attachment | inhibits adherence of dental colonizers | [92] | |
| Mixture of polyphenols from propolis | inhibition of bacterial growth and adherence | S. mutans and S. gordonii | [93] | |
| Lactobacilli, Prevotella intermedia, Porphyromonas gingivalis, Actinomyces israelii, and Candida albicans | [94] | |||
| apigenin | inhibit some virulence-related genes, such as GtfB and C | S. mutans | [85,95] | |
| trans-trans farnesol | reduce cell viability | |||
| trans-trans farnesol | inhibition of biofilms | S. mutans | [96] | |
| apigenin and tt-farnesol from propolis | inhibit bacterial growth and biofilm | Biofilm oral pathogens (bulk) | [97] | |
| proanthocyanins, flavonols, and myricetin | disrupt biofilm formation, inhibit attachment, and diminishes the acidogenicity | S. mutans | [98] | |
| Periodontitis | curcumin (followed by pyrogallol, pyrocatechol, and quercetin) | inhibition of growth | P. gingivalis | [99] |
| epigallocatechin-3-gallate | growth inhibition Inhibition of cytokine production in a host | S. mutans | [100,101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curuțiu, C.; Dițu, L.M.; Grumezescu, A.M.; Holban, A.M. Polyphenols of Honeybee Origin with Applications in Dental Medicine. Antibiotics 2020, 9, 856. https://doi.org/10.3390/antibiotics9120856
Curuțiu C, Dițu LM, Grumezescu AM, Holban AM. Polyphenols of Honeybee Origin with Applications in Dental Medicine. Antibiotics. 2020; 9(12):856. https://doi.org/10.3390/antibiotics9120856
Chicago/Turabian StyleCuruțiu, Carmen, Lia Mara Dițu, Alexandru Mihai Grumezescu, and Alina Maria Holban. 2020. "Polyphenols of Honeybee Origin with Applications in Dental Medicine" Antibiotics 9, no. 12: 856. https://doi.org/10.3390/antibiotics9120856
APA StyleCuruțiu, C., Dițu, L. M., Grumezescu, A. M., & Holban, A. M. (2020). Polyphenols of Honeybee Origin with Applications in Dental Medicine. Antibiotics, 9(12), 856. https://doi.org/10.3390/antibiotics9120856








