Abstract
Background/Objectives: The present study investigates the antifungal potential of 1,5-diarylidene-4-piperidones. Methods: These compounds were synthesized via Claisen–Schmidt condensation, and their antifungal efficacy was tested against Cryptococcus neoformans, a yeast recently qualified as a critical priority pathogen by the World Health Organization, through determination of their minimum inhibitory concentration (MIC). We designed and synthesized a series of piperidones to explore structure–activity relationships. Results: Systematic modification of the substituent pattern revealed that tetrabutoxy groups exhibited potent activity (MIC of 7.8 µM), surpassing standard antifungals like fluconazole. The selectivity index (SI) values confirmed their safety profile across various human cells. Docking analysis demonstrated that these compounds target sterol 14-demethylase, suggesting potential inhibition of ergosterol synthesis as a mechanism of action. Interestingly, the compounds also demonstrated broad-spectrum activity against other pathogenic yeasts and fungi, including Candida and Aspergillus species, and against fluconazole-resistant strains. Conclusions: These findings underscore the potential of 1,5-diarylidene-4-piperidones as promising antifungal candidates with a favorable safety profile.
1. Introduction
The infectious agent Cryptococcus neoformans is a capsulated yeast present in soils and organic debris that is transmitted by air. Invasive fungal infection with C. neoformans is a serious cause of morbidity in HIV/AIDS immunocompromised patients. The most common clinical forms of infection are septicemia, meningoencephalitis, and pneumonia [1]. In HIV/AIDS immunocompromised patients, infection by C. neoformans is life-threatening, mainly due to meningoencephalitis, accounting for approximately 10–15% of AIDS-associated deaths worldwide, with 1,000,000 new cases and over 600,000 deaths each year [2,3]. Regarding immunocompetent patients, although infection by C. neoformans is frequent, with up to 70% of healthy children having serum antibodies against C. neoformans confirming exposure, fatal outcome is less frequent, the infection being either naturally cleared or persisting as a latent and asymptomatic form [4,5,6]. C. neoformans was recently identified as a top-priority fungal pathogen, and even as a “critical priority” by the World Health Organization (WHO) due to its high mortality and morbidity and its resistance to treatment [7,8,9]. Indeed, the mortality rates due to C. neoformans infection have been reported to be as high as 41–61% and 8–20% for HIV-positive and HIV-negative patients, respectively, despite antifungal therapy [10]. The standard treatment is amphotericin B combined with flucytosine [11]. However, both agents are toxic, and laboratory monitoring of these patients is needed. Moreover, intravenous administration of amphotericin B is required, limiting, therefore, the access of patients from resource-constrained countries [12,13]. In infections with no meningoencephalitis, fluconazole has been a primary antifungal agent for managing cryptococcosis; however, excessive use of azole drugs has led to drug resistance. Indeed, the ability of Cryptococcus to alter its genomic architecture in the face of antifungal stress primarily occurs through a phenomenon referred to as heteroresistance. Bongomin et al. [14] reported in 2018 that the resistance to fluconazole in clinical strains of C. neoformans was present in about 18% of the 4995 clinical isolates described in 29 studies from 1988 to 2017 included in the EMBASE and MEDLINE databases. More recently, an analysis of heteroresistance conducted in Brazil on clinical strains of C. neoformans revealed that 85% of the isolates showed a moderate level of resistance to fluconazole (≥16 µg/mL) and 40% a high level (≥32 µg/mL), underlying the importance of this phenomenon and its increase over time [15]. The toxicity of the used molecules, their poor ability to cross the blood–brain barrier, and their ability to cause the development of resistance explain why mortality and morbidity caused by C. neoformans remain high despite actual antifungal therapy, necessitating the search and identification of novel antifungal drugs directed toward C. neoformans.
In this context, curcumin-derived 1,5-diarylidene-4-piperidones have attracted attention for their broad biological activities, including anti-cancer [16], anti-bacterial, anti-inflammatory, and antifungal [17,18,19]. Their structural features make them promising candidates for overcoming current therapeutic limitations. The α,β-unsaturated carbonyl moiety can act as a Michael acceptor, particularly to thiol nucleophiles, enabling specific interactions with fungal targets [20,21,22]. This assumption prompted us to design a new piperidone library to investigate their potential antifungal activities. The synthesis of a curcumin-derived drug is straightforward and based on a Claisen–Schmidt reaction, in which a ketone reacts with two molecules of benzaldehyde analogs in the presence of sodium hydroxide to reach benzylidene structures. Motivated by the simplicity and the potential of such molecules, we aimed to target a series of benzylidene compounds, given the urgent need for the development of new antifungal treatments in the face of drug resistance. We focused on structural modifications of piperidone compounds, including aryl and amine substitutions, to investigate their structure–activity relationships (SARs) and identify potent antifungal candidates against C. neoformans and other clinically relevant fungi.
2. Results
2.1. Synthesis of Piperidones
To target the structure–activity relationship (SAR) of novel curcumin analogs, preparation of several 1,5-diarylidene-4-piperidones (Scheme 1) was planned by varying their electronic and/or hydrophobic properties (Figure 1). The Claisen–Schmidt condensation of piperidones and aldehyde derivatives yielded twenty-six compounds in a one-step procedure without the need for column chromatography, as precipitation in ethanol is conclusive to obtaining pure products with yields ranging from 48 to 86% [23,24]. We introduced structural diversity through the modification of alkoxy groups, N-alkyl functions, and lateral moieties. Except for 3h and 3iso-h (Figure 1), all new compounds were isolated through recrystallization from ethanol as intensely colored solids. The structural identity and substitution patterns were confirmed by the 1H NMR and 13C NMR techniques. Mass spectroscopy confirmed the exact masses, while 1H NMR showed a purity of >95%. While the general procedure is reported in the Materials and Methods, the structural analyses of the new piperidones are described in the Supplementary Data.
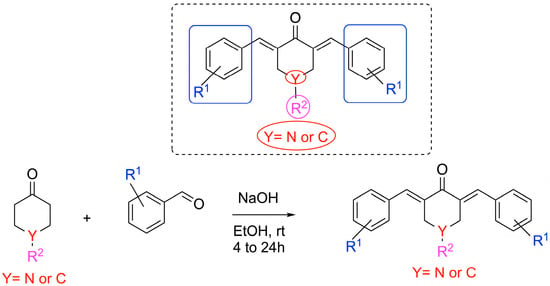
Scheme 1.
Chemical syntheses of 1,5-diarylidene-4-piperidinones.
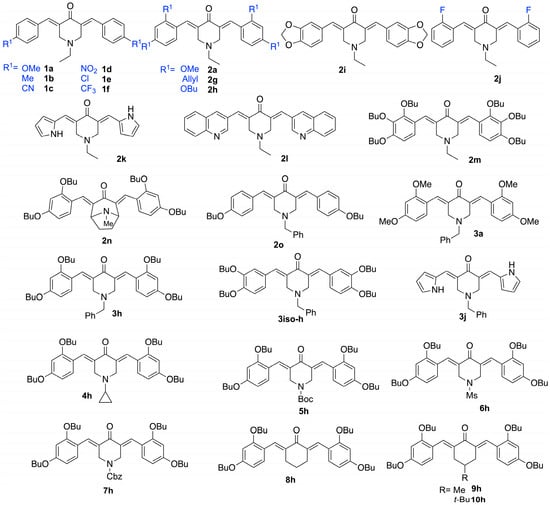
Figure 1.
Chemical structures of tested 1,5-diarylidene-4-piperidinones.
2.2. Antifungal Activities and Toxicity of Synthesized Piperidones
The antifungal activities of the twenty-three piperidone compounds were first determined against C. neoformans using the minimum inhibitory concentration (MIC) assay and are reported in Table 1. The antifungal potency was found to be modest for the monosubstituted aryl series, 1a–1f, as well as for compound 2j, with MICs ranging from 125 to 250 μM, regardless of the substitution (R1 = OMe, Me, CN, NO2, Cl, F, CF3). Disubstituted methoxy and allyloxy groups (2a and 2g) afforded even worse results. However, dibutoxy compound 2h exhibited a significant ability to inhibit the growth of C. neoformans (MIC of 31.2 μM). Compound 2i, derived from piperonal, showed weak activity with an MIC of 250 μM. Piperidone 2l, possessing a quinoline scaffold analogous to those reported by Shingate [25], was tested but showed lower efficiency (MIC = 250 μM). Since butoxy groups appeared to exhibit the best activity against C. neoformans, we decided to synthesize and evaluate compound 2m, which contains six butoxy groups, thereby enhancing the hydrophobic ability of the piperidone. Compound 2m displayed a good MIC of 62.5 µM, but it was less efficient than 2h. Bicyclic piperidone 2n was found to be inactive against C. neoformans. We then varied the nature of N-alkyl substitution. The results indicated that the cyclopropyl, Boc, mesyl, and Cbz groups were detrimental to the activity. Benzyl amines (e.g., butenafine) are known to be antifungal agents [26] with activity on the cell membrane through squalene epoxidase binding [27]. We decided, therefore, to synthesize compound 3h, which significantly improved activity, presenting an MIC as low as 7.8 μM. To confirm this effect, we compared the activity of 2k and 3j by switching from ethyl alkyl to the benzyl group on the nitrogen atom. Again, the results showed that benzyl substitution (compound 3j) was beneficial, presenting a lower MIC (62.5 μM) compared to the ethyl-substituted piperidone, 2k (MIC = 250 μM). Testing compound 2o, which contains one butoxy group on each aromatic moiety, again proved detrimental, with an MIC of 125 μM (vs. 7.8 μM for 3h), highlighting the importance of the presence of two butoxy groups on each aromatic moiety. A similar trend was observed when the position of the alkoxy group was changed from ortho to meta (3iso-h being less active than 3h, with MICs of 62.5 and 7.8 µM, respectively). The comparison between compound 3a (MIC > 250 µM), with methoxy instead of butoxy groups, and compound 3h strongly suggests that the hydrophobic nature and the length of the alkyl chains are crucial for the activity of these piperidone derivatives. The longer butyl chains in 3h likely facilitate stronger hydrophobic interactions with a specific region (hydrophobic pocket) on the target protein, leading to enhanced biological activity. In addition, to underscore the significance of the piperidone core, we synthesized and evaluated 1,5-diarylidene cyclohexanone analogs 8–10h, which demonstrated no activity with MIC > 250 µM. These results suggest that the amphiphilic character of piperidones—due to the presence of four alkyl chains and a nitrogen atom—plays an important role. In addition to the hydrophobic interactions identified in our docking study, the benzyl group in 3h may enhance antifungal potency through a mechanism similar to that of known benzylamine antifungals such as butenafine. Indeed, benzylamines are reported to interact with fungal membranes and enzymes such as squalene epoxidase, leading to potent antifungal effects [26,27]. In 3h, the benzyl substitution may, therefore, provide a dual advantage. Interestingly, a control test revealed that compound 3h was more active than fluconazole (MIC of 25 µM), an antifungal recommended to treat C. neoformans infection [28], particularly in low-income and middle-income countries [29].

Table 1.
Anti-C. neoformans activity and cytotoxicity of piperidone compounds. The MIC values (in µM) of piperidone compounds and fluconazole were measured on C. neoformans as explained in the Materials and Methods section. Their cytotoxic concentration 50 (CC50) (in µM) values were calculated from Figure 2 and Figure 3 after a 48 h incubation of human cells with increasing concentrations of molecules (n = 3). Selectivity indexes (SIs) were calculated by dividing CC50 by MIC values. Molecules with MICs superior to or equal to 250 µM were not tested in the toxicity assay and are indicated as “NT”.
To better evaluate the therapeutic potential of the active compounds, their toxicity was then measured using various human cells, i.e., A498 (kidney), BEAS-2B (lung), Caco-2 (intestine), HaCaT (skin), and HepG2 (liver) cells (Figure 2). For comparison, the toxicity of fluconazole was also determined (Figure 3). The CC50 values, i.e., the concentrations causing a 50% reduction in the cell viability, were determined from graphs and are provided in Table 1.
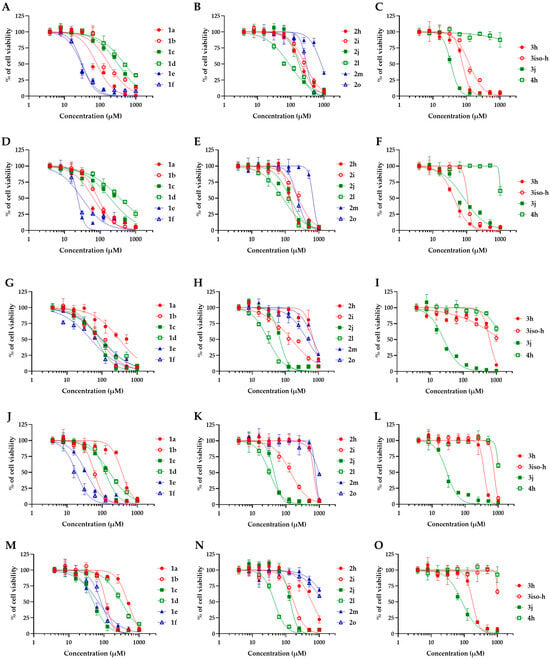
Figure 2.
Toxicity of piperidone compounds against human cells. Human cells were exposed to increasing concentrations of piperidone compounds active against C. neoformans (1a, 1b, 1c, 1d, 1e, 1f, 2h, 2i, 2j, 2m, 2o, 3h, 3iso-h, 3j, and 4h) for 48h before measurement of the cell viability using the resazurin assay. (A–C) A498 cells (kidney), (D–F) BEAS-2B cells (lung), (G–I) Caco-2 cells (intestine), (J–L) HaCaT cells (skin), and (M–O) HepG2 cells (liver). Curves were fitted using GraphPad® Prism 8 software (means ± SD, n = 3).
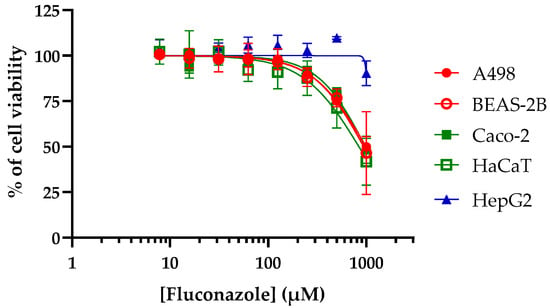
Figure 3.
Toxicity of fluconazole against human cells. Human cells were exposed to increasing concentrations of fluconazole for 48 h before measurement of the cell viability using resazurin assay. Curves were fitted using GraphPad® Prism 8 software (means ± SD, n = 3).
The CC50 values of the compounds range from 18.3 to >1000 µM, depending on the compound and the cell model tested. Compounds with monosubstituted aryl groups in para-positions 1b, 1c, 1e, 1f, and 2j showed the highest toxicity (CC50 of 18.3 to 313.9 µM). Introducing a substituted alkoxy group, like in 1a and 2o, was beneficial, providing lower toxicity (CC50 of 64.9 to >1000 µM). In addition to higher antifungal activity, the disubstituted butoxy groups (2h) revealed low toxicity (CC50 of 140 to 696.6 µM). When the aryl groups were connected to acetal, a detrimental effect was observed on the toxicity of compound 2l (CC50 of 30.0 to 89.5 µM). However, incorporating a third butoxy group in the 2m drug afforded an even lower toxicity (CC50 of 663 to >1000 µM). We then evaluated the effect of the substitution on the nitrogen atom by introducing alkyl groups. Benzyl group 3h revealed a small elevation in toxicity when it was compared to 2h (CC50 of 42.4 to 628.6 µM), whereas compound 4h, with the cyclopropyl group, showed the lowest toxicity regarding all the cell lines tested (>1000 µM).
For comparison, fluconazole provided a CC50 ranging from 841.2 to >1000 µM (Figure 3 and Table 1). In order to further identify the most promising candidates, the selectivity indexes (SIs) of each compound (i.e., the ratios of CC50 to MIC values) were calculated (Table 1).
These investigations revealed that 2h, 2m, 3iso-h, and 3h have the best safety/activity profile with very interesting SIs of up to 80.5. Importantly, 3h gave the highest SI values, ranging from 5.4 to 80.5, depending on the human cell model considered. Although less active than 3h (with MIC values on C. neoformans of 7.8 and 62.5 µM, respectively), 2m was found to be the least toxic of the active compounds, with CC50 values ranging from 663.7 to >1000 µM and SIs ranging from 10.6 to >16. For comparison, fluconazole yielded SIs ranging from 33.6 to >40, close to the ones obtained with the safest compounds of this study.
The spectrum of antifungal activity of the more active compounds (i.e., 2h, 2m, 3iso-h, and 3h) was further screened using various fungal strains (yeasts and filamentous species) infecting humans or plants (Table 2 and Table 3). Regarding human pathogenic yeasts (Table 2), 2h, 2m, 3iso-h, and 3h showed MIC values similar to the ones obtained on C. neoformans when tested against Candida auris and C. glabrata (MIC of 7.8 to 31.2 µM) but were found to be less active against C. albicans and C. tropicalis (MIC of 62.5 to 250 µM). Regarding filamentous fungi (Table 3), for the human pathogen Aspergillus fumigatus and the plant pathogen A. flavus, 3h was the only active compound (MIC of 31.2 µM), with 2h, 2m, and 3iso-h being found to be less active or not active (MIC of 125 to >250 µM). 2h, 2m, 3iso-h, and 3h were also found to be less active or not active against the plant pathogens Colletotrichum graminicola, Fusarium graminearum, and Penicillium verrucosum (MIC of 125 to >250 µM), except for 3h, yielding an MIC of 62.5 µM on P. verrucosum. Regarding the plant pathogens Magnaporthe oryzae and Microdochium bolleyi and the human pathogen Trichophyton rubrum, 2h, 2m, 3iso-h, and 3h displayed good activities (MIC of 7.8 to 62.5 µM), except for 2m on M. bolleyi (MIC > 250 µM) and 3iso-h on T. rubrum (MIC of 125 µM), with 3h yielding the lowest MIC on those strains (i.e., 7.8 µM). Interestingly, it must be noted that yeasts and filamentous fungi found to be resistant to fluconazole (MIC of 250 to >1000 µM) were still sensitive to 2h, 2m, 3iso-h, and 3h. Thus, once again, 3h showed the strongest antifungal activity, with the lowest MIC values across all strains.

Table 2.
Activity of piperidone compounds against pathogenic yeasts. The MIC values (in µM) of piperidone compounds 2h, 2m, 3h, and 3iso-h were measured using various yeast models as explained in the Materials and Methods section. Fluconazole was used as an antifungal control. Yeast strains tested were Candida albicans (DSM 10697), Candida auris (DSM 21092), Candida glabrata (DSM 11226), Candida tropicalis (DSM 9419), and Cryptococcus neoformans (DSM 11959).

Table 3.
Activity of piperidone compounds against pathogenic filamentous fungi. The MIC values (in µM) of piperidone compounds 2h, 2m, 3h, and 3iso-h were measured using various filamentous fungi models (human pathogens or plant pathogens) as explained in the Materials and Methods section. Fluconazole was used as an antifungal control. Filamentous fungi tested were either human pathogens, i.e., A. fumigatus (DSM 819) and Trichophyton rubrum (DSM16111), or plant pathogens, i.e., Aspergillus flavus (DSM 1959), Colletotrichum graminicola (DSM 63127), Fusarium graminearum (DSM 1095), Magnaporthe oryzae (gift from Richard O’Connell, UMR Bioger, Paris Saclay), Microdochium bolleyi (DSM 62073), and Penicillium verrucosum (DSM 12639).
Importantly, the twenty-three piperidone compounds were tested against Gram-positive and Gram-negative bacteria belonging to the ESKAPE group and were found to be inactive with MICs > 250 µM for all compounds, except 2k, yielding an MIC of 250 µM on the Gram-negative bacteria A. baumannii and E cloacae, and 2l and 3j, yielding an MIC of 250 µM on the Gram-positive bacterium S. aureus. This demonstrates that the compounds possess antimicrobial activity selectively directed against yeasts and fungi that can be explained by the specific targeting of fungal enzyme(s).
2.3. Molecular Modeling
It has been demonstrated that similar compounds are able to interact and inhibit cytochrome P450 14α-sterol 14-demethylase (CYP51), an enzyme involved in ergosterol biosynthesis in fungi [25]. Docking analysis was, therefore, performed to evaluate the affinity of the best piperidone molecules with the same sterol 14-demethylase (the one from T. cruzi) using its crystal structure (PDB code: 3KHM). Except for piperidone 2m, which contains three butoxy groups, the results indicated that compounds 2h, 3h, and 3iso-h effectively interacted within the active site of the CYP51 complex. The docking binding energy for 3h was found to be −8.2 kcal/mol, similar to that of fluconazole (binding energy = −8.1 kcal/mol), which supports the fact that 3h may act through the inhibition of ergosterol synthesis, as previously shown for other piperidone compounds described in the literature [22,25]. In this molecular docking study, all docked compounds (2m, 2h, 3h, and 3iso-h) formed interactions with Hem300 and hydrophobic interactions with numerous amino acid residues (Table 4 and Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9). These hydrophobic interactions with butoxy chains may explain the difference in activity between 3h (MIC = 7.8 µM) and its methyl analog, 3a (MIC > 250 µM). In addition, piperidones 2h and 3h showed T-shaped interactions with Tyr103, whereas 3iso-h exhibited T-shaped interactions with His294 and Phe290.

Table 4.
Interaction parameters. Interaction analysis of the sterol 14 α-demethylase protein on the basis of molecular docking studies with piperidones 2h, 3h, 2m, and 3iso-h compared with fluconazole.
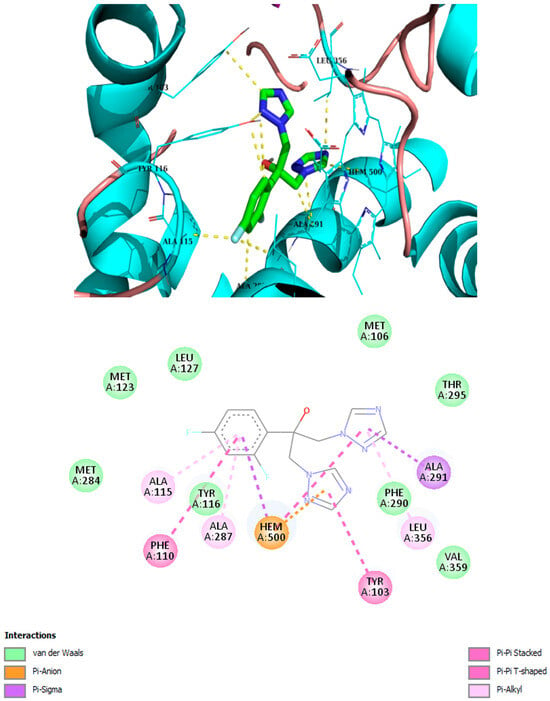
Figure 4.
Best binding mode of fluconazole into the active site of CYP51. Docking score: −8.1 kcal/mol.
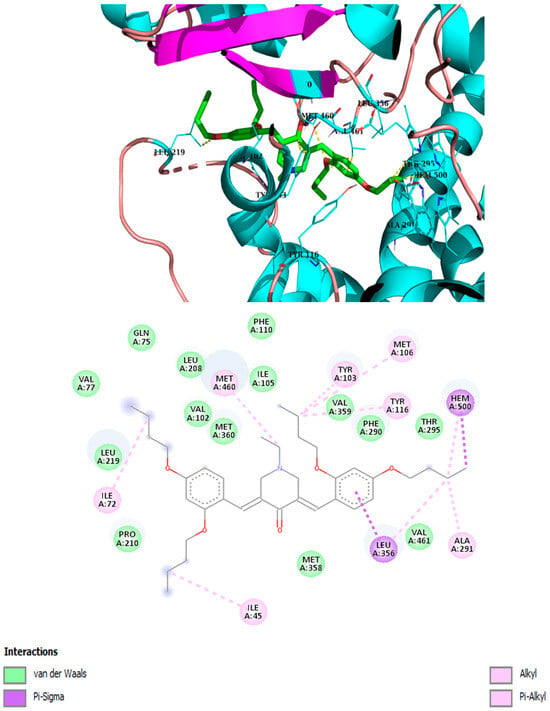
Figure 5.
Best binding mode of 2h into the active site of CYP51. Docking score: −7.8 kcal/mol.
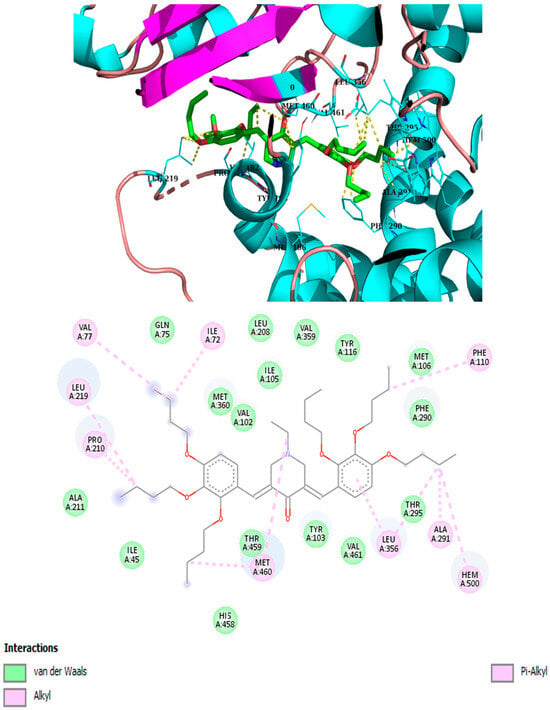
Figure 6.
Best binding mode of 2m into the active site of CYP5. Docking score: −7.2 kcal/mol.
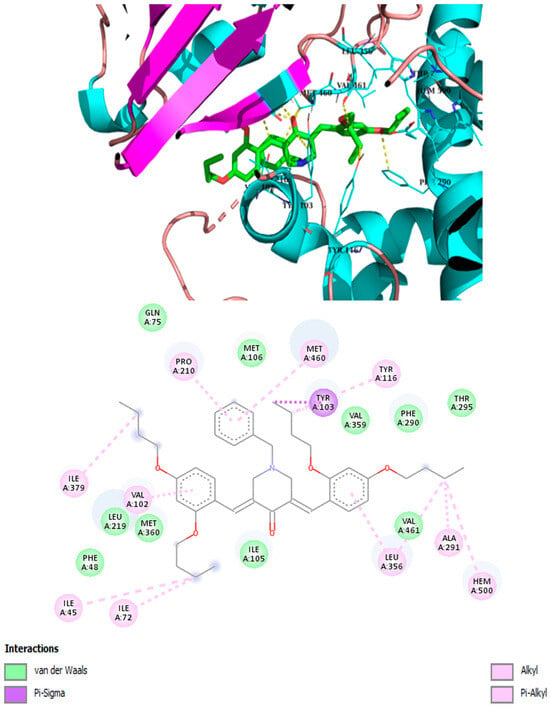
Figure 7.
Best binding mode of 3h into the active site of CYP51. Docking score: −8.2 kcal/mol.
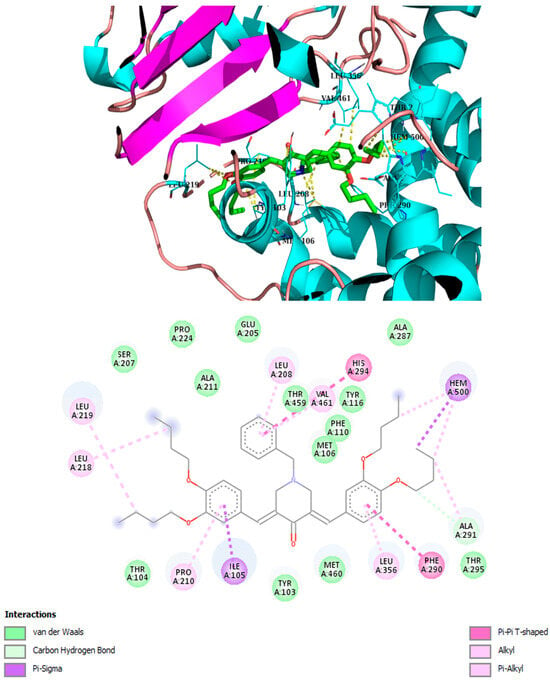
Figure 8.
Best binding mode of 3iso-h into the active site of CYP51. Docking score: −8.8 kcal/mol.
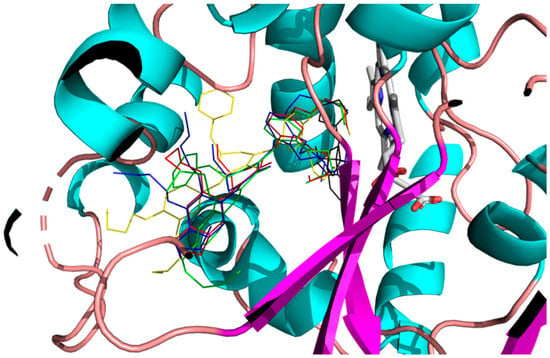
Figure 9.
Best binding mode of 2h (red), 2m (blue), 3h (green), 3iso-h (yellow), and fluconazole (black) into the active site of CYP51.
In order to study the stability of the ligands in the active sites, we performed Molecular Dynamics (MD) calculations on the best positions of fluconazole, 2h, 2m, 3h, and 3iso-h in CYP51. We calculated the Root Mean Square Deviation (RMSD) of the ligands with respect to starting geometries during the MD trajectories. The results are presented in Figure 10 and Table 5. The MD results show that all complexes are stable, with low RMSD mean values (less than 0.3 nm). 2m seems to be the least stable, as suggested by the docking results.
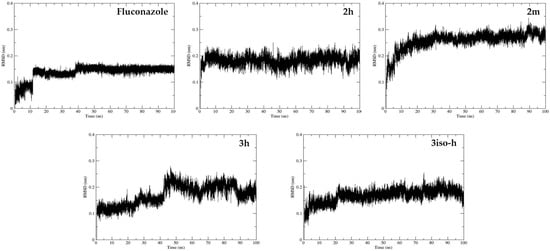
Figure 10.
Molecular Dynamics (MD) calculations using Gromacs 2021 on the best positions of fluconazole, 2h, 2m, 3h, and 3iso-h in CYP51. Root Mean Square Deviation (RMSD) values of the ligands were calculated with respect to starting geometries during the MD trajectories.

Table 5.
RMSD mean values and standard deviations in nm for fluconazole, 2h, 2m, 3h, and 3iso-h.
3. Discussion
C. neoformans is a significant human pathogen, but other fungi should also be considered when developing antifungal drugs. Candida spp. infect mainly immunocompromised patients but also immunocompetent ones, leading to more than 1.5 million cases of bloodstream infection or invasive candidiasis and around 900,000 deaths every year [30]. In addition to C. albicans, among the various Candida spp. that infect humans, C. auris, C. glabrata, and C. tropicalis have attracted more attention due to their increasing prevalence and their ability to develop resistance to antifungal drugs used in medicine, including fluconazole [31,32,33,34,35]. Similarly, Aspergillus fumigatus infects mainly immunocompromised patients and is estimated to cause over 600,000 deaths annually [36]. Although they do not infect humans, fungi that infect plants and crops are also important to consider when developing antifungals, as they cause massive losses (for example, every year, Fusarium graminearum causes losses of around 28 million metric tons of wheat grain valued at USD 5.6 billion [37] and/or produces mycotoxins harmful for humans and animals) [38].
This study demonstrates that 1,5-diarylidene-4-piperidones represent a promising scaffold for antifungal drug development, particularly against Cryptococcus neoformans. The structure–activity relationship analysis (Figure 11) revealed that substitution with butoxy groups significantly enhanced antifungal potency, as observed for compounds 2h and 3h, with the latter showing MIC values as low as 7.8 µM. The comparison between methoxy and butoxy analogs highlights the critical role of hydrophobic chain length in driving activity, likely through enhanced interactions within hydrophobic pockets of the target enzyme.
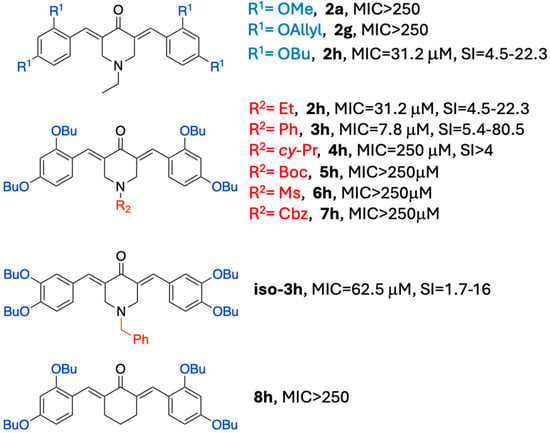
Figure 11.
Structure–activity relationship (SAR) for 2a–8h; comparison of MIC and safety index.
When testing the spectrum of antifungal activity in the best piperidone compounds, the data showed that in addition to possessing good activity against C. neoformans, 2h, 2m, 3iso-h, and 3h also demonstrated antifungal activity against significant human and plant pathogens, such as C. albicans, C. auris, C. glabrata, A. fumigatus, and T. rubrum, as well as A. flavus, M. oryzae, M. bolleyi, and P. verrucosum. In all cases, 3h was identified as the most active piperidone compound, with MIC ranging from 7.8 to 62.5 µM. Docking analysis and Molecular Dynamics (MD) calculations suggest that 2h, 2m, 3h, and 3iso-h, as with fluconazole, interact with CYP51, suggesting that these molecules act through the inhibition of this enzyme, altering the synthesis of ergosterol. Although docking analysis and MD calculation strongly indicate that the mechanism of action of piperidone involves CYP51 inhibition, future studies are required to confirm this hypothesis through enzyme inhibition assays and/or ergosterol level measurements. Importantly, fungi resistant to fluconazole were found to still be sensitive to 2h, 2m, 3h, and/or 3iso-h. This observation is not in contradiction with the docking data, indicating that these molecules target ergosterol biosynthesis like fluconazole. Indeed, the resistance to fluconazole is not due to a mutation in its target but typically involves a reduction in its uptake and/or an increase in its efflux in resistant strains [39]. The fact that fluconazole-resistant strains remain sensitive to 2h, 2m, 3h, and/or 3iso-h is highly significant due to the growing incidence of fluconazole resistance in fungal strains infecting humans, including Candida species and A. fumigatus [40,41].
4. Materials and Methods
4.1. Chemistry
All reagents and solvents were purchased from Sigma Aldrich (Saint-Quentin-Fallavier, France), FluoroChem (Penrose Dock, Ireland) or TCI (Zwijndrecht, Bengium) and used as received without further purification. Mass spectroscopy was performed by the Spectropole of Aix-Marseille University. ESI mass spectral analyses were recorded with a 3200 QTRAP (Applied Biosystems SCIEX, Nottingham, UK) mass spectrometer. The HRMS mass spectral analysis was performed with a QStar Elite (Applied Biosystems SCIEX, Framingham, MA, USA) mass spectrometer. Elemental analyses were recorded with a Thermo Finnigan EA 1112 (Thermo Fisher Scientific, Waltham, MA, USA) elemental analysis apparatus driven by the Eager 300 software. 1H and 13C NMR spectra were determined at room temperature in 5 mm o.d. tubes on a Bruker Avance 400 spectrometer or a Bruker Avance 300 spectrometer (Bruker, Billerica, MA, USA) at the Spectropole: 1H (400 MHz), 1H (300 MHz), 13C (100 MHz), and 13C (75 MHz). The 1H chemical shifts were referenced to their solvent peak, CDCl3 (7.26 ppm) or DMSO_d6 (2.50 ppm), and the 13C chemical shifts were referenced to their solvent peak, CDCl3 (77.16 ppm) or DMSO_d6 (39.52 ppm). All compounds were prepared at analytical purity up to accepted standards for new organic compounds (>95%), which was checked by high-field NMR analysis.
N-alkylpiperidin-4-one (10 mmol) was dissolved in 20 mL of ethanol and benzaldehyde derivative (20 mmol), and 40% NaOH (2 equiv.) was then added at 0 °C. The solution was stirred at room temperature overnight. The yellow precipitate was filtered off, washed with cold ethanol, and dried under vacuum (48–86% yield). Spectral data, structure, and NMR spectra of the compounds are provided in the Supplementary Materials.
4.2. Antimicrobial Activity
The antifungal effect of the compounds was measured following the reference methods for yeasts and molds as previously described [42]. Reference yeast strains tested were Candida albicans (DSM 10697), C. auris (DSM 21092), C. glabrata (DSM 11226), C. tropicalis (DSM 9419), and Cryptococcus neoformans (DSM11959). Filamentous fungi tested were either human pathogens (i.e., A. fumigatus (DSM 819) and Trichophyton rubrum (DSM16111)) or plant pathogens (i.e., Aspergillus flavus (DSM 1959), Colletotrichum graminicola (DSM 63127), Fusarium graminearum (DSM 1095), Magnaporthe oryzae (gift from Richard O’Connell, UMR Bioger, Paris Saclay) [43], Microdochium bolleyi (DSM 62073), and Penicillium verrucosum (DSM 12639)). Yeast suspensions were prepared by resuspending colonies collected from PDA plates in sterile NaCl 0.9% solution. Yeasts were then diluted to 1–2 × 103 yeasts/mL in RMPI media supplemented with glucose (1.8%) buffered with MOPS (final concentration of 0.165 mol/L (pH 7.0)). For filamentous fungi, conidia were collected from mycelium grown on PDA plates using a sterile solution of 0.9% NaCl supplemented with Tween at 0.1%. After counting under a microscope, dilutions at 2–3 × 104 conidia/mL were also prepared in MOPS-buffered RMPI media supplemented with glucose. Diluted yeast or fungi were then exposed to increasing concentrations of compounds (1/2 serial dilution) in 96-well plates, with pure DMSO (maximal concentration of 1%) used as a negative control. Plates were incubated at 35 °C for 24–48 h for human-infectious yeasts and filamentous fungi (i.e., A. fumigatus and T. rubrum) or 25 °C for 48–72 h for the other filamentous fungi from the environment or plant-infectious fungi. Minimum Inhibiting Concentrations (MICs) were determined as the lowest concentrations of compounds that totally inhibited the growth of the fungi. The antibacterial effect of the compounds was measured through liquid MIC determination using Gram-negative (Acinetobacter baumannii (DSM 30007), Enterobacter cloacae (DSM 30054), Klebsiella pneumoniae (DSM 26371), and Pseudomonas aeruginosa (ATCC 9027)) and Gram-positive (Enterococcus faecalis (DSM 2570), Enterococcus faecium (DSM 20477), and Staphylococcus aureus (ATCC 6538)) bacteria from the ESKAPE group following the National Committee of Clinical Laboratory Standards procedure, as previously described [44]. In all cases, MICs were determined using both visual evaluation and optical density measurements (at 600 nm). Experiments were conducted at n = 2–3.
4.3. Cytotoxicity Studies
The toxicity of the compounds was tested using human cells (i.e., A498 (human kidney cell line), BEAS-2B (normal human airway epithelial cells), Caco-2 (human intestinal cell line), HaCaT (normal human skin cells), and HepG2 (human liver cell line)) as previously described [45]. Cells were obtained from ATCC (Molsheim Cedex France) except HaCaT cells that were obtained from (Creative Bioarray, Shirley, NY 11967, USA). Cells were maintained at 37 °C in a 5% CO2 incubator in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1% L-glutamine, and 1% antibiotics (all from Thermo Fisher Scientific, Illkirch–Graffenstaden, France). Cells grown in 75 cm2 flasks were detached using trypsin–EDTA solution (from Thermo Fisher), counted using Mallasez’s chamber, and seeded into 96-well cell culture plates (Greiner bio-one from Dominique Dutscher, Brumath, France) at approximately 104 cells per well. After 24–48 h, when the cells reached 80–90% confluence, wells were aspirated, and increasing concentrations of compounds (from 0 to 1000 µM, ½ dilution) were added to the cells, with DMSO (maximal concentration of 1%) used as a negative control. After 48 h of incubation at 37 °C in a 5% CO2 incubator, wells were aspirated, and the cell viability was measured by adding 100 µL of resazurin solution at 0.03 mg/mL to phosphate-buffered saline with calcium and magnesium chloride (PBS++). After 1 h of incubation at 37 °C, the fluorescence intensity (Ex 530 nm/Em 590 nm) of the wells was measured using a microplate reader (Biotek, Synergy Mx, Colmar, France). The fluorescence values were normalized by the negative controls (DMSO-treated cells) and expressed as the percentage of cell viability. The cytotoxic concentration 50 (i.e., CC50) values of compounds corresponding to the concentrations that caused a reduction of 50% in cell viability were calculated using the GraphPad® Prism 8 software. Experiments were performed at n = 3.
4.4. Molecular Docking
All the ligands were fully optimized with the Gaussian16 Rev. A03 package [46] at the HF/6-31G(d) level of theory. The atomic charges were computed at the HF/6-31G(d) level of theory with the RESP scheme. For docking studies, the X-ray crystal structure of sterol 14alpha-demethylase (CYP51) from Trypanosoma cruzi in complex with inhibitor fluconazole was obtained from the Protein Data Bank (PDB) (PDB code: 3KHM). The enzyme was prepared by removing fluconazole and then adding hydrogen atoms and atomic charges with the AutoDock Tools 1.5.7 software. The molecular docking studies were carried out with the AutoDock Vina 1.2.5 software [47] with default parameters. The docking box was set at 60 × 60 × 60 Å and was centered at x = 2.270 Å, y = −23.537 Å, and z = 16.924 Å (center of fluconazole). The position drawings and interaction calculations were created with ProteinPlus and BIOVIA Discovery Studio. The 3D active sites were drawn with PyMOL 3.1.4.1. Details of the molecular docking analysis are provided in the Supplementary Materials.
4.5. Molecular Dynamics
Molecular Dynamics (MD) calculations were performed with the Gromacs 2021 [48] package. The complexes were solvated in a quasi-cubic box containing ~16,000 water molecules. Chloride counterions were added to obtain a neutral system. Concerning the force fields used in the MD simulations, we used the GAFF [49] force field and TIP3P [50] model to describe the water molecules. In order to optimize the simulation box, we performed an NPT calculation at 300 K and 1 bar over 400 ps with a time step of 0.5 fs. After this first stage, we performed an NVT trajectory at 300 K over 100 ns with a time step of 0.5 fs. We kept the last 99.5 ns of the trajectory for data analysis calculations.
5. Conclusions
This study highlights the potential of 1,5-diarylidene-4-piperidones as effective antifungal agents, particularly against Cryptococcus neoformans and other clinically significant fungi. Among the synthesized compounds, 3h emerged as the most promising candidate, displaying superior antifungal potency. Its ability to inhibit fluconazole-resistant strains further underscores its therapeutic value. Docking studies suggest that these compounds act by targeting sterol 14α-demethylase, disrupting ergosterol biosynthesis in fungal cells. Additionally, their broad-spectrum antifungal activity and low cytotoxicity support their potential application in both medical and agricultural settings. Because of it is dual functions (benzylamine and curcumin), with potential dual activity, further in vivo studies and clinical evaluations are necessary for piperidone 3h to fully explore its therapeutic potential and to address the growing need for new antifungal drugs to combat resistant fungal pathogens.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics14090883/s1: Supplementary File S1: Spectral data, NMR spectra, and molecular docking for compounds. References [51,52,53,54,55,56,57,58,59,60] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.N., M.M. and D.S.; methodology, M.N., M.M., E.C.-D. and D.S.; validation, M.N., M.M. and D.S.; formal analysis, E.C.-D., H.R., A.G., L.D., Y.C., F.D., D.S., M.M. and M.N.; investigation, E.C.-D., H.R., A.G., L.D., Y.C., F.D., D.S., M.M. and M.N.; writing—original draft preparation, M.M., M.N. and D.S.; writing—review and editing, M.M. and M.N.; supervision, M.M. and M.N.; project administration, M.M. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Aix-Marseille Université and CNRS for their financial support. This work was supported by the computing facilities of the CRCMM, “Centre Régional de Compétences en Modélisation Moléculaire de Marseille”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Perfect, J.R. Efficiently Killing a Sugar-Coated Yeast. N. Engl. J. Med. 2013, 368, 1354–1356. [Google Scholar] [CrossRef]
- Francis, V.I.; Liddle, C.; Camacho, E.; Kulkarni, M.; Junior, S.R.S.; Harvey, J.A.; Ballou, E.R.; Thomson, D.D.; Brown, G.D.; Hardwick, J.M.; et al. Cryptococcus neoformans Rapidly Invades the Murine Brain by Sequential Breaching of Airway and Endothelial Tissues Barriers, Followed by Engulfment by Microglia. mBio 2024, 15, e03078-23. [Google Scholar] [CrossRef]
- Poley, M.; Koubek, R.; Walsh, L.; McGillen, B. Cryptococcal Meningitis in an Apparent Immunocompetent Patient. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619834578. [Google Scholar] [CrossRef]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.; Niang, R.; Casadevall, A. Serologic Evidence for Cryptococcus neoformans Infection in Early Childhood. Pediatrics 2001, 107, e66. [Google Scholar] [CrossRef]
- Coelho, C.; Farrer, R.A. Pathogen and Host Genetics Underpinning Cryptococcal Disease. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2020; Volume 105, pp. 1–66. ISBN 978-0-12-821685-9. [Google Scholar]
- Alanio, A. Dormancy in Cryptococcus Neoformans: 60 Years of Accumulating Evidence. J. Clin. Investig. 2020, 130, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Calegari-Alves, Y.P.; Costa, R.P.; Innocente-Alves, C.; Soares, G.D.N.; Lima, E.S.; Saciloto-de-Oliveira, L.R.; Alves, L.R.; Vainstein, M.H.; Beys-da-Silva, W.O.; Santi, L. A Review of Bioactive Plant Compounds against WHO Priority Fungal Pathogens. Microb. Pathog. 2025, 207, 107930. [Google Scholar] [CrossRef]
- Briner, S.L.; Doering, T.L. Cryptococcus. Curr. Biol. 2025, 35, R518–R522. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Shoham, S. Cryptococcosis: Update on Therapeutics and New Targets. Curr. Opin. Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Dao, A.; Kim, H.Y.; Garnham, K.; Kidd, S.; Sati, H.; Perfect, J.; Sorrell, T.C.; Harrison, T.; Rickerts, V.; Gigante, V.; et al. Cryptococcosis—A Systematic Review to Inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae043. [Google Scholar] [CrossRef] [PubMed]
- Day, J.N.; Chau, T.T.H.; Wolbers, M.; Mai, P.P.; Dung, N.T.; Mai, N.H.; Phu, N.H.; Nghia, H.D.; Phong, N.D.; Thai, C.Q.; et al. Combination Antifungal Therapy for Cryptococcal Meningitis. N. Engl. J. Med. 2013, 368, 1291–1302. [Google Scholar] [CrossRef]
- Hartland, K.; Pu, J.; Palmer, M.; Dandapani, S.; Moquist, P.N.; Munoz, B.; DiDone, L.; Schreiber, S.L.; Krysan, D.J. High-Throughput Screen in Cryptococcus neoformans Identifies a Novel Molecular Scaffold That Inhibits Cell Wall Integrity Pathway Signaling. ACS Infect. Dis. 2016, 2, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Beattie, S.R.; Schnicker, N.J.; Murante, T.; Kettimuthu, K.; Williams, N.S.; Gakhar, L.; Krysan, D.J. Benzothiourea Derivatives Target the Secretory Pathway of the Human Fungal Pathogen Cryptococcus neoformans. ACS Infect. Dis. 2020, 6, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A Systematic Review of Fluconazole Resistance in Clinical Isolates of Cryptococcus Species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.D.M.B.; Cortez, A.C.A.; De Souza, É.S.; Pinheiro, S.B.; De Souza Oliveira, J.G.; Sadahiro, A.; Cruz, K.S.; Matsuura, A.B.J.; Melhem, M.D.S.C.; Frickmann, H.; et al. Investigation of Fluconazole Heteroresistance in Clinical and Environmental Isolates of Cryptococcus neoformans Complex and Cryptococcus gattii Complex in the State of Amazonas, Brazil. Med. Mycol. 2022, 60, myac005. [Google Scholar] [CrossRef]
- Pati, H.N.; Das, U.; Das, S.; Bandy, B.; De Clercq, E.; Balzarini, J.; Kawase, M.; Sakagami, H.; Quail, J.W.; Stables, J.P.; et al. The Cytotoxic Properties and Preferential Toxicity to Tumour Cells Displayed by Some 2,4-Bis(Benzylidene)-8-Methyl-8-Azabicyclo[3.2.1] Octan-3-Ones and 3,5-Bis(Benzylidene)-1-Methyl-4-Piperidones. Eur. J. Med. Chem. 2009, 44, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a Component of Golden Spice: From Bedside to Bench and Back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Bazzaro, M.; Linder, S. Dienone Compounds: Targets and Pharmacological Responses. J. Med. Chem. 2020, 63, 15075–15093. [Google Scholar] [CrossRef]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Sehgal, P.; Tung, T.T.; Møller, J.V.; Nielsen, J.; Palmgren, M.; Christensen, S.B.; Fuglsang, A.T. Demethoxycurcumin Is a Potent Inhibitor of P-Type ATPases from Diverse Kingdoms of Life. PLoS ONE 2016, 11, e0163260. [Google Scholar] [CrossRef]
- Lawson, S.; Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Thangamani, S. Dispiropyrrolidine Tethered Piperidone Heterocyclic Hybrids with Broad-Spectrum Antifungal Activity against Candida Albicans and Cryptococcus Neoformans. Bioorganic Chem. 2020, 100, 103865. [Google Scholar] [CrossRef]
- Nagargoje, A.A.; Akolkar, S.V.; Subhedar, D.D.; Shaikh, M.H.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. Propargylated Monocarbonyl Curcumin Analogues: Synthesis, Bioevaluation and Molecular Docking Study. Med. Chem. Res. 2020, 29, 1902–1913. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Allyloxy Ketones as Efficient Photoinitiators with High Migration Stability in Free Radical Polymerization and 3D Printing. Dyes Pigment. 2021, 185, 108900. [Google Scholar] [CrossRef]
- Nagargoje, A.A.; Akolkar, S.V.; Siddiqui, M.M.; Subhedar, D.D.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. Quinoline Based Monocarbonyl Curcumin Analogs as Potential Antifungal and Antioxidant Agents: Synthesis, Bioevaluation and Molecular Docking Study. Chem. Biodivers. 2020, 17, e1900624. [Google Scholar] [CrossRef]
- Fioravanti, R.; Biava, M.; Porretta, G.; Landolfi, C.; Simonetti, N.; Villa, A.; Conte, E.; Porta-Puglia, A. Research on Antibacterial and Antifungal Agents. XI. Synthesis and Antimicrobial Activity of N-Heteroaryl Benzylamines and Their Schiff Bases. Eur. J. Med. Chem. 1995, 30, 123–132. [Google Scholar] [CrossRef]
- Hammoudi Halat, D.; Younes, S.; Mourad, N.; Rahal, M. Allylamines, Benzylamines, and Fungal Cell Permeability: A Review of Mechanistic Effects and Usefulness against Fungal Pathogens. Membranes 2022, 12, 1171. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Loyse, A.; Burry, J.; Cohn, J.; Ford, N.; Chiller, T.; Ribeiro, I.; Koulla-Shiro, S.; Mghamba, J.; Ramadhani, A.; Nyirenda, R.; et al. Leave No One behind: Response to New Evidence and Guidelines for the Management of Cryptococcal Meningitis in Low-Income and Middle-Income Countries. Lancet Infect. Dis. 2019, 19, e143–e147. [Google Scholar] [CrossRef]
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Lacy, A.J.; Koyfman, A.; Liang, S.Y. Candida auris: A Focused Review for Emergency Clinicians. Am. J. Emerg. Med. 2024, 84, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kinjo, Y.; Koshikawa, T.; Miyazaki, Y. Basic Research on Candida Species: Disease Mechanism, Virulence, and Relationship with Environmental Factors. Med. Mycol. J. 2024, 65, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, A.M.; Saied, S.A.; Torayah, M.M.; Ramadan, A.E.; Elaskary, S.A. Antifungal Susceptibility and Virulence Determinants Profile of Candida Species Isolated from Patients with Candidemia. Sci. Rep. 2024, 14, 11597. [Google Scholar] [CrossRef]
- Beardsley, J.; Kim, H.Y.; Dao, A.; Kidd, S.; Alastruey-Izquierdo, A.; Sorrell, T.C.; Tacconelli, E.; Chakrabarti, A.; Harrison, T.S.; Bongomin, F.; et al. Candida glabrata (Nakaseomyces glabrata): A Systematic Review of Clinical and Microbiological Data from 2011 to 2021 to Inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae041. [Google Scholar] [CrossRef]
- Keighley, C.; Kim, H.Y.; Kidd, S.; Chen, S.C.-A.; Alastruey, A.; Dao, A.; Bongomin, F.; Chiller, T.; Wahyuningsih, R.; Forastiero, A.; et al. Candida tropicalis—A Systematic Review to Inform the World Health Organization of a Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae040. [Google Scholar] [CrossRef]
- Dhingra, S.; Cramer, R.A. Regulation of Sterol Biosynthesis in the Human Fungal Pathogen Aspergillus fumigatus: Opportunities for Therapeutic Development. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Schmitt, M.; Nechab, M.; Graff, B.; Morlet-Savary, F.; Xiao, P.; Dumur, F.; Lalevée, J. Effect of Decarboxylation on the Photoinitiation Behavior of Nitrocarbazole-Based Oxime Esters. Macromolecules 2022, 55, 2475–2485. [Google Scholar] [CrossRef]
- Deligeorgakis, C.; Magro, C.; Skendi, A.; Gebrehiwot, H.H.; Valdramidis, V.; Papageorgiou, M. Fungal and Toxin Contaminants in Cereal Grains and Flours: Systematic Review and Meta-Analysis. Foods 2023, 12, 4328. [Google Scholar] [CrossRef]
- Maebashi, K.; Niimi, M.; Kudoh, M.; Fischer, F.J.; Makimura, K.; Niimi, K.; Piper, R.J.; Uchida, K.; Arisawa, M.; Cannon, R.D.; et al. Mechanisms of Fluconazole Resistance in Candida Albicans Isolates from Japanese AIDS Patients. J. Antimicrob. Chemother. 2001, 47, 527–536. [Google Scholar] [CrossRef]
- Janowski, M.; Demchuk, O.M.; Wujec, M. Fluconazole Analogs and Derivatives: An Overview of Synthesis, Chemical Transformations, and Biological Activity. Molecules 2024, 29, 2855. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N. Antifungal Resistance: Current Trends and Future Strategies to Combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure–Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef]
- Notteghem, J.L. Distribution of the Mating Type Alleles in Magnaporthe grisea Populations Pathogenic on Rice. Phytopathology 1992, 82, 421. [Google Scholar] [CrossRef]
- Benkhaled, B.T.; Hadiouch, S.; Olleik, H.; Perrier, J.; Ysacco, C.; Guillaneuf, Y.; Gigmes, D.; Maresca, M.; Lefay, C. Elaboration of Antimicrobial Polymeric Materials by Dispersion of Well-Defined Amphiphilic Methacrylic SG1-Based Copolymers. Polym. Chem. 2018, 9, 3127–3141. [Google Scholar] [CrossRef]
- Olleik, H.; Yacoub, T.; Hoffer, L.; Gnansounou, S.M.; Benhaiem-Henry, K.; Nicoletti, C.; Mekhalfi, M.; Pique, V.; Perrier, J.; Hijazi, A.; et al. Synthesis and Evaluation of the Antibacterial Activities of 13-Substituted Berberine Derivatives. Antibiotics 2020, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Pall, S.; Smith, J.C.; Hess, B.; Lindahk, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. Software X 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Madura, J.D. Investigating novel thiazolyl-indazole derivatives as scaffolds for SARS-CoV-2 MPro inhibitors. J. Am. Chem. Soc. 1983, 105, 1407–1473. [Google Scholar] [CrossRef]
- Schmitt, F.; Subramaniam, D.; Anant, S.; Padhye, S.; Begemann, G.; Schobert, R.; Biersack, B. Halogenated Bis(Methoxybenzylidene)-4-piperidone Curcuminoids with Improved Anticancer Activity. ChemMedChem 2018, 13, 1115–1123. [Google Scholar] [CrossRef]
- Andreani, A.; Cavalli, A.; Granaiola, M.; Guardigli, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Recanatini, M.; Roda, A. Synthesis and Screening for Antiacetylcholinesterase Activity of (1-Benzyl-4-Oxopiperidin-3-Ylidene)Methylindoles and -Pyrroles Related to Donepezil. J. Med. Chem. 2001, 44, 4011–4014. [Google Scholar] [CrossRef]
- Bayomi, S.M.; El-Kashef, H.A.; El-Ashmawy, M.B.; Nasr, M.N.A.; El-Sherbeny, M.A.; Abdel-Aziz, N.I.; El-Sayed, M.A.-A.; Suddek, G.M.; El-Messery, S.M.; Ghaly, M.A. Synthesis and Biological Evaluation of New Curcumin Analogues as Antioxidant and Antitumor Agents: Molecular Modeling Study. Eur. J. Med. Chem. 2015, 101, 584–594. [Google Scholar] [CrossRef]
- Girgis, A.S.; Panda, S.S.; Farag, I.S.A.; El-Shabiny, A.M.; Moustafa, A.M.; Ismail, N.S.M.; Pillai, G.G.; Panda, C.S.; Hall, C.D.; Katritzky, A.R. Synthesis, and QSAR Analysis of Anti-Oncological Active Spiro-Alkaloids. Org. Biomol. Chem. 2015, 13, 1741–1753. [Google Scholar] [CrossRef]
- Parlar, S. Synthesis and Cholinesterase Inhibitory Activity Studies of Some Piperidinone Derivatives. Org. Commun. 2019, 12, 202–209. [Google Scholar] [CrossRef]
- Hanachi, R.; Said, R.B.; Rahali, S.; Tangour, B.; Abdelwahab, S.I.; Farasani, A.; Taha, M.M.E.; Bidwai, A.; Koko, W.S.; Khan, T.A.; et al. P-Trifluoromethyl- and p-Pentafluorothio-Substituted Curcuminoids of the 2,6-Di[(E)-Benzylidene)]Cycloalkanone Type: Syntheses and Activities against Leishmania Major and Toxoplasma Gondii Parasites. Bioorg. Chem. 2021, 114, 105099. [Google Scholar] [CrossRef]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Microwave-Assisted Synthesis of Pyrimido[4,5-b][1,6]Naphthyridin-4(3H)-Ones with Potential Antitumor Activity. Eur. J. Med. Chem. 2013, 60, 1–9. [Google Scholar] [CrossRef]
- Schmitt, F.; Gold, M.; Begemann, G.; Andronache, I.; Biersack, B.; Schobert, R. Fluoro and Pentafluorothio Analogs of the Antitumoral Curcuminoid EF24 with Superior Antiangiogenic and Vascular-Disruptive Effects. Bioorg. Med. Chem. 2017, 25, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ding, N.; Zhao, S.; Li, D.; Van Doren, J.; Qian, Y.; Wei, X.; Zheng, X. Synthesis and Evaluation of Curcumin-Related Compounds Containing Inden-2-One for Their Effects on Human Cancer Cells. Biol. Pharm. Bull. 2014, 37, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.K.; Mathew, J.; Achalkumar, A.S.; Mathews, M. Synthesis and Liquid Crystalline Properties of Low Molecular Weight Bis-Chalcone Compounds. Curr. Org. Synth. 2022, 19, 463–475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).